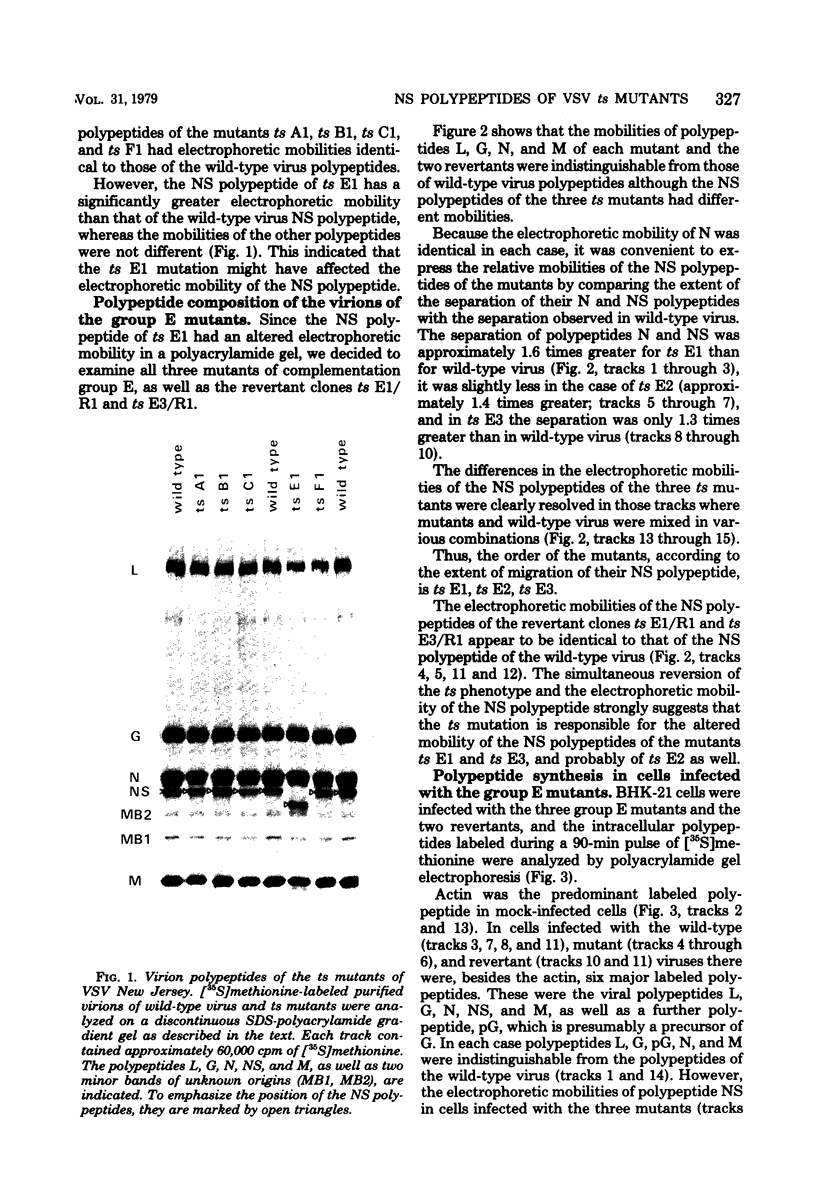
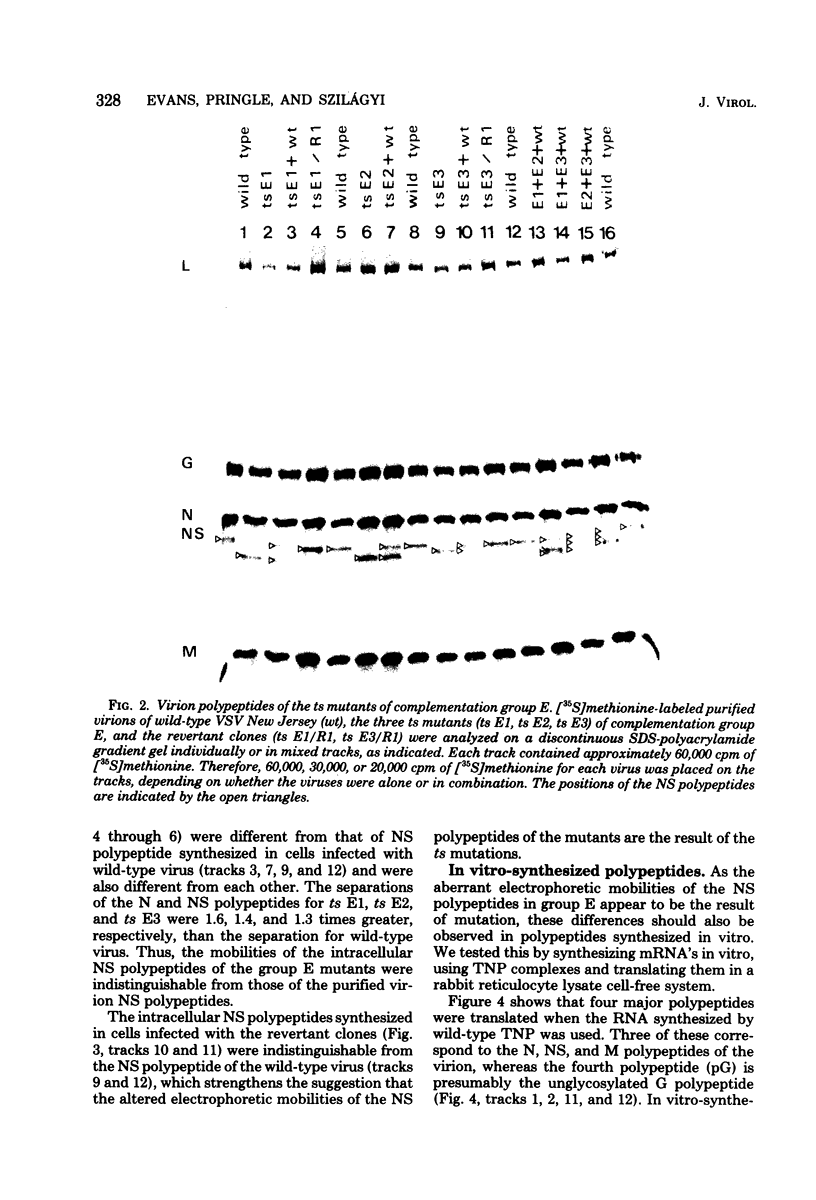
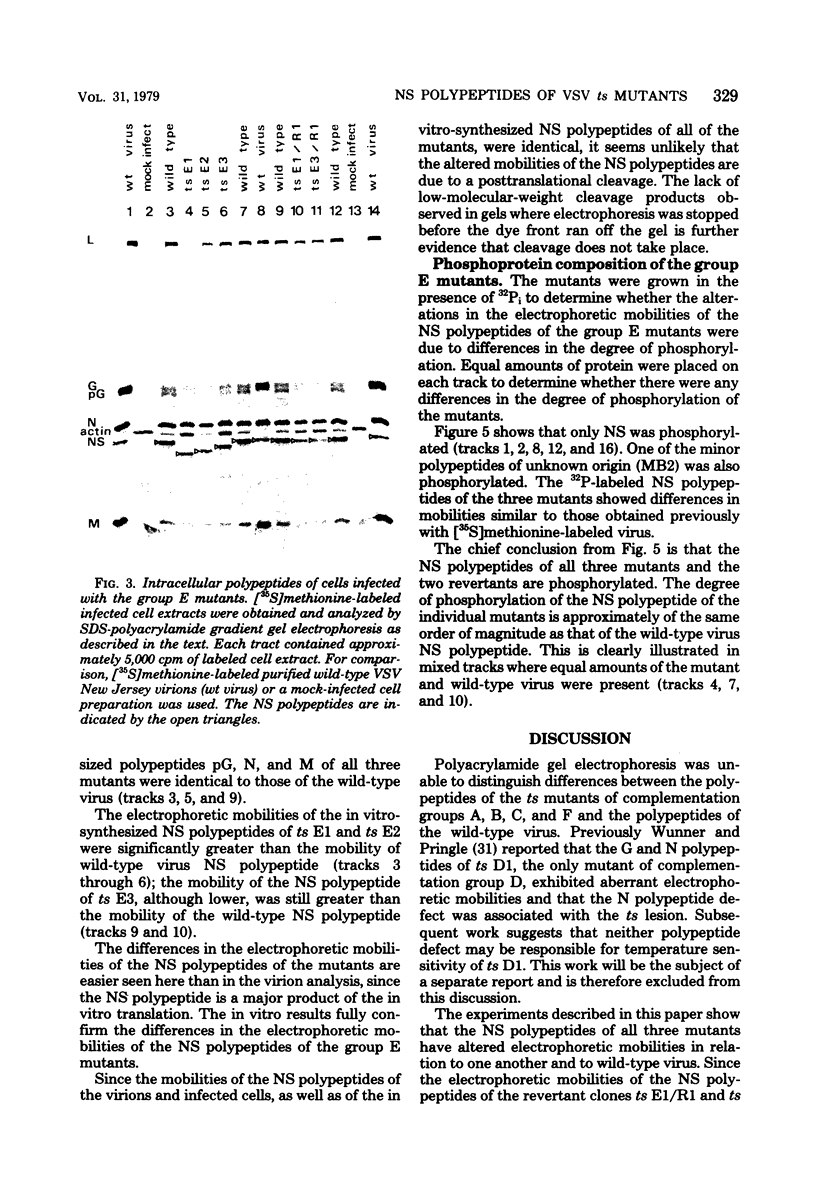
Abstract
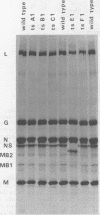
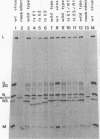
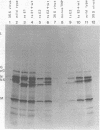
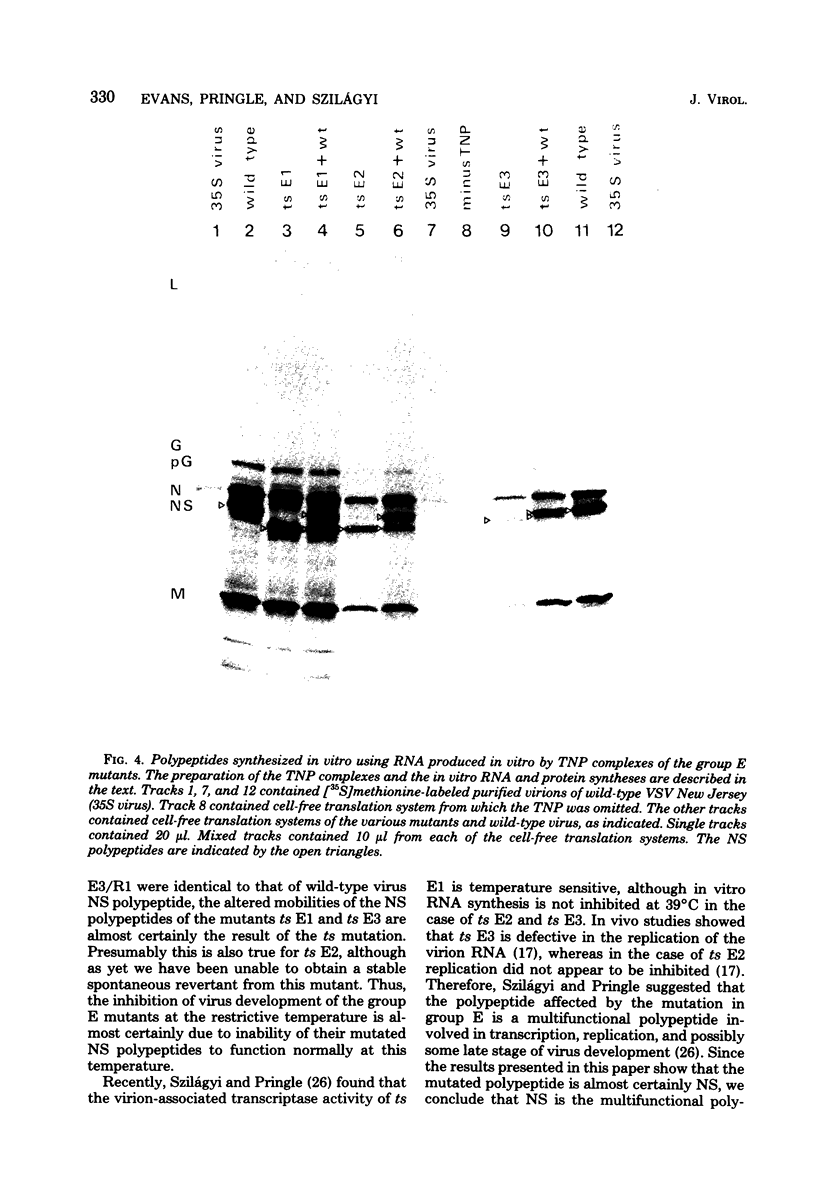
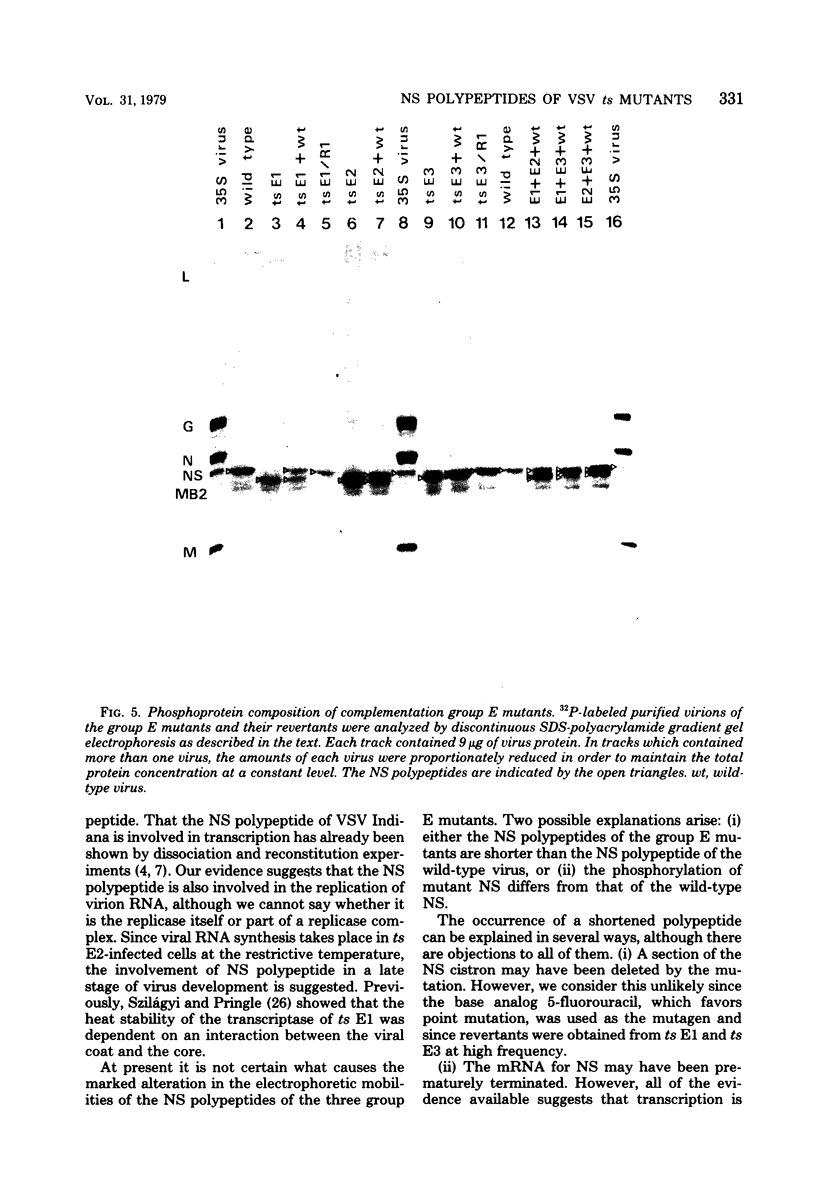
In vesicular stomatitis virus New Jersey serotype polyacrylamide gel electrophoresis was unable to distinguish the polypeptides of the temperature-sensitive (ts) mutants of complementation groups A, B, C, and F from those of the wild-type virus. However, the NS polypeptide of the representative mutant of group E, ts E1, had a significantly greater electrophoretic mobility than that of the wild-type virus NS polypeptide. The electrophoretic mobilities of the NS polypeptides of the three mutants of complementation group E varied, being greatest in the case of ts E1, slightly less for ts E2, and only a little greater than that of wild-type virus NS polypeptide in the case of ts E3. Since the NS polypeptides of the revertant clones ts E1/R1 and ts E3/R1 have mobilities identical to that of wild-type NS polypeptide, the observed altered mobilities of the group E mutants are almost certainly the direct result of the ts mutations in the E locus. The electrophoretic mobilities of the intracellular NS polypeptides of the group E mutants were indistinguishable from those of their virion NS polypeptides. The electrophoretic mobilities of the NS polypeptides of the group E mutants synthesized in vitro using mRNA synthesized in vitro by TNP were identical to those of the NS polypeptides of their purified virions. The NS polypeptides of all three mutants were labeled with 32Pi to approximately the same extent as wild-type virus NS polypeptide, indicating that gross differences in phosphorylation of this polypeptide are unlikely to account for the altered mobilities. We propose a model in which the NS polypeptide consists of at least three loops held in this configuration by hydrophobic or ionic forces or both and stabilized by phosphodiester bridges. If a mutation affects one of the amino acids to which the phosphate is covalently linked, the phosphodiester bridge cannot be formed, and, as a result, in the presence of sodium dodecyl sulfate the affected loop opens and thus the NS polypeptide migrates further into the gel. Such a configuration may also explain the multifunctional nature of the NS polypeptide.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abraham G., Banerjee A. K. Sequential transcription of the genes of vesicular stomatitis virus. Proc Natl Acad Sci U S A. 1976 May;73(5):1504–1508. doi: 10.1073/pnas.73.5.1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ball L. A., White C. N. Order of transcription of genes of vesicular stomatitis virus. Proc Natl Acad Sci U S A. 1976 Feb;73(2):442–446. doi: 10.1073/pnas.73.2.442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cancedda R., Schlesinger M. J. Formation of Sindbis virus capsid protein in mammalian cell-free extracts programmed with viral messenger RNA. Proc Natl Acad Sci U S A. 1974 May;71(5):1843–1847. doi: 10.1073/pnas.71.5.1843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emerson S. U., Yu Y. Both NS and L proteins are required for in vitro RNA synthesis by vesicular stomatitis virus. J Virol. 1975 Jun;15(6):1348–1356. doi: 10.1128/jvi.15.6.1348-1356.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman G. J., Rose J. K., Clinton G. M., Huang A. S. RNA synthesis of vesicular stomatitis virus. VII. Complete separation of the mRNA's of vesicular stomatitis virus by duplex formation. J Virol. 1977 Mar;21(3):1094–1104. doi: 10.1128/jvi.21.3.1094-1104.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HEPPEL L. A., HARKNESS D. R., HILMOE R. J. A study of the substrate specificity and other properties of the alkaline phosphatase of Escherichia coli. J Biol Chem. 1962 Mar;237:841–846. [PubMed] [Google Scholar]
- Imblum R. L., Wagner R. R. Inhibition of viral transcriptase by immunoglobulin directed against the nucleocapsid NS protein of vesicular stomatitis virus. J Virol. 1975 Jun;15(6):1357–1366. doi: 10.1128/jvi.15.6.1357-1366.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knipe D., Rose J. K., Lodish H. F. Translation of individual species of vesicular stomatitis viral mRNA. J Virol. 1975 Apr;15(4):1004–1011. doi: 10.1128/jvi.15.4.1004-1011.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lesnaw J. A., Reichmann M. E. RNA synthesis by temperature-sensitive mutants of vesicular stomatitis virus, New Jersey serotype. Virology. 1975 Feb;63(2):492–504. doi: 10.1016/0042-6822(75)90322-0. [DOI] [PubMed] [Google Scholar]
- Marsden H. S., Crombie I. K., Subak-Sharpe J. H. Control of protein synthesis in herpesvirus-infected cells: analysis of the polypeptides induced by wild type and sixteen temperature-sensitive mutants of HSV strain 17. J Gen Virol. 1976 Jun;31(3):347–372. doi: 10.1099/0022-1317-31-3-347. [DOI] [PubMed] [Google Scholar]
- Moyer S. A., Summers D. F. Phosphorylation of vesicular stomatitis virus in vivo and in vitro. J Virol. 1974 Feb;13(2):455–465. doi: 10.1128/jvi.13.2.455-465.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obijeski J. F., Marchenko A. T., Bishop D. H., Cann B. W., Murphy F. A. Comparative electrophoretic analysis of the virus proteins of four rhabdoviruses. J Gen Virol. 1974 Jan;22(1):21–33. doi: 10.1099/0022-1317-22-1-21. [DOI] [PubMed] [Google Scholar]
- Pelham H. R., Jackson R. J. An efficient mRNA-dependent translation system from reticulocyte lysates. Eur J Biochem. 1976 Aug 1;67(1):247–256. doi: 10.1111/j.1432-1033.1976.tb10656.x. [DOI] [PubMed] [Google Scholar]
- Preston C. M., Szilagyi J. F. Cell-free translation of RNA synthesized in vitro by a transcribing nucleoprotein complex prepared from purified vesicular stomatitis virus. J Virol. 1977 Mar;21(3):1002–1009. doi: 10.1128/jvi.21.3.1002-1009.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pringle C. R., Duncan I. B., Stevenson M. Isolation and characterization of temperature-sensitive mutants of vesicular stomatitis virus, New Jersey serotype. J Virol. 1971 Dec;8(6):836–841. doi: 10.1128/jvi.8.6.836-841.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purchio A. F., Erikson E., Erikson R. L. Translation of 35S and of subgenomic regions of avian sarcoma virus RNA. Proc Natl Acad Sci U S A. 1977 Oct;74(10):4661–4665. doi: 10.1073/pnas.74.10.4661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhodes D. P., Abraham G., Colonno R. J., Jelinek W., Banerjee A. K. Characterization of vesicular stomatitis virus mRNA species synthesized in vitro. J Virol. 1977 Mar;21(3):1105–1112. doi: 10.1128/jvi.21.3.1105-1112.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons D. T., Strauss J. H. Translation of Sindbis virus 26 S RNA and 49 S RNA in lysates of rabbit reticulocytes. J Mol Biol. 1974 Jun 25;86(2):397–409. doi: 10.1016/0022-2836(74)90027-8. [DOI] [PubMed] [Google Scholar]
- Sokol F., Clark H. F. Phosphoproteins, structural components of rhabdoviruses. Virology. 1973 Mar;52(1):246–263. doi: 10.1016/0042-6822(73)90413-3. [DOI] [PubMed] [Google Scholar]
- Stampfer M., Baltimore D. Identification of the vesicular stomatitis virus large protein as a unique viral protein. J Virol. 1973 Apr;11(4):520–526. doi: 10.1128/jvi.11.4.520-526.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szilágyi J. F., Pringle C. R. Effect of temperature-sensitive mutation on activity of the RNA transcriptase of vesicular stomatitis virus New Jersey. J Virol. 1979 Jun;30(3):692–700. doi: 10.1128/jvi.30.3.692-700.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szilágyi J. F., Pringle C. R. Effect of temperature-sensitive mutations on the virion-associated RNA transcriptase of vesicular stomatitis virus. J Mol Biol. 1972 Nov 14;71(2):281–291. doi: 10.1016/0022-2836(72)90351-8. [DOI] [PubMed] [Google Scholar]
- Szilágyi J. F., Pringle C. R. Virion trascriptase activity differences in host range mutants of vesicular stomatitis virus. J Virol. 1975 Oct;16(4):927–936. doi: 10.1128/jvi.16.4.927-936.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szilágyi J. F., Uryvayev L. Isolation of an infectious ribonucleoprotein from vesicular stomatitis virus containing an active RNA transcriptase. J Virol. 1973 Feb;11(2):279–286. doi: 10.1128/jvi.11.2.279-286.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner R. R., Prevec L., Brown F., Summers D. F., Sokol F., MacLeod R. Classification of rhabdovirus proteins: a proposal. J Virol. 1972 Dec;10(6):1228–1230. doi: 10.1128/jvi.10.6.1228-1230.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wunner W. H., Pringle C. R. A temperature-sensitive mutant of vesicular stomatitis virus with two abnormal virus proteins. J Gen Virol. 1974 Apr;23(1):97–106. doi: 10.1099/0022-1317-23-1-97. [DOI] [PubMed] [Google Scholar]
- Wunner W. H., Pringle C. R. Protein synthesis in BHK21 cells infected with vesicular stomatitis virus. II. ts Mutants of the New Jersey serotype. Virology. 1972 Oct;50(1):250–253. doi: 10.1016/0042-6822(72)90365-0. [DOI] [PubMed] [Google Scholar]