Abstract
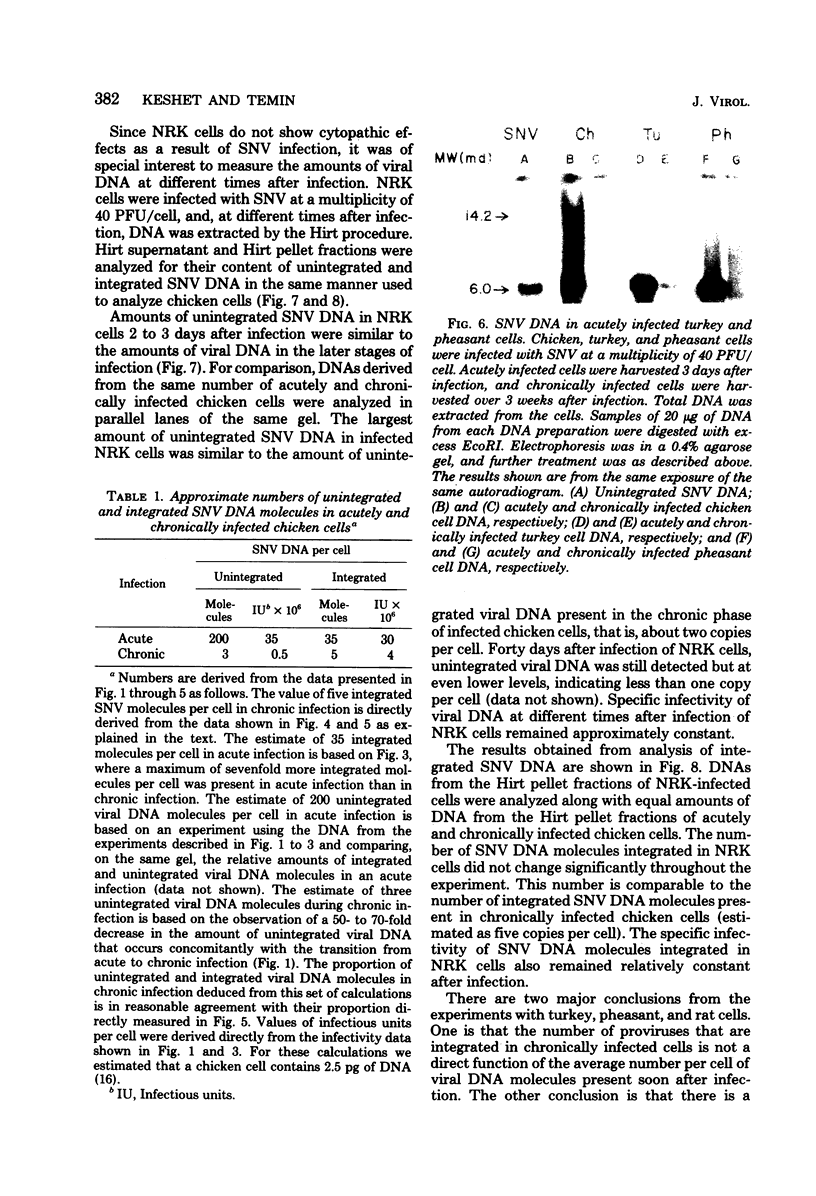
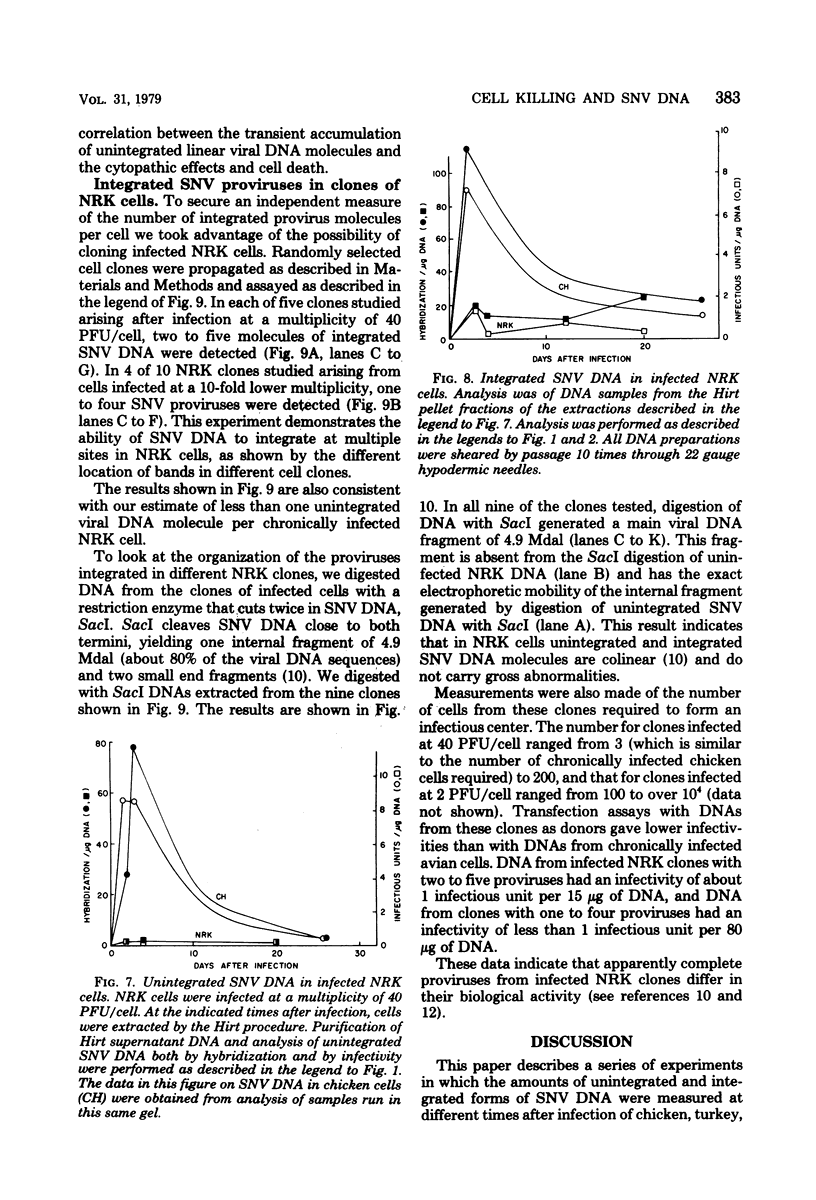
Spleen necrosis virus productively infects avian and rat cells. The average number of molecules of unintegrated and integrated viral DNA in cells at different times after infection was determined by hybridization and transfection assays. Shortly after infection, there was a transient accumulation of an average of about 150 to 200 molecules of unintegrated linear spleen necrosis virus DNA per chicken, turkey, or pheasant cell. No such accumulation was seen in infected rat cells. Soon after infection there was in chicken cells, but not inturkey, pheasant, or rat cells, also a transient integration of an average of 35 copies of viral DNA per cell. By 10 days after infection, the majority of this integrated viral DNA was lost from the population of infected chicken cells. At the same time, the majority of the unintegrated viral DNA was also lost from infected chicken, turkey, and pheasant cells. The transient cytopathic effect seen in these infected cells also occurred at this time. Late after infection about five copies of apparently nondefective spleen necrosis proviruses were stably integrated at multiple sites in chicken, turkey, pheasant, and rat DNA. These results demonstrate a correlation between the transient accumulation of large numbers of spleen necrosis virus DNA molecules and the transient occurrence of cytopathic effects.
Full text
PDF

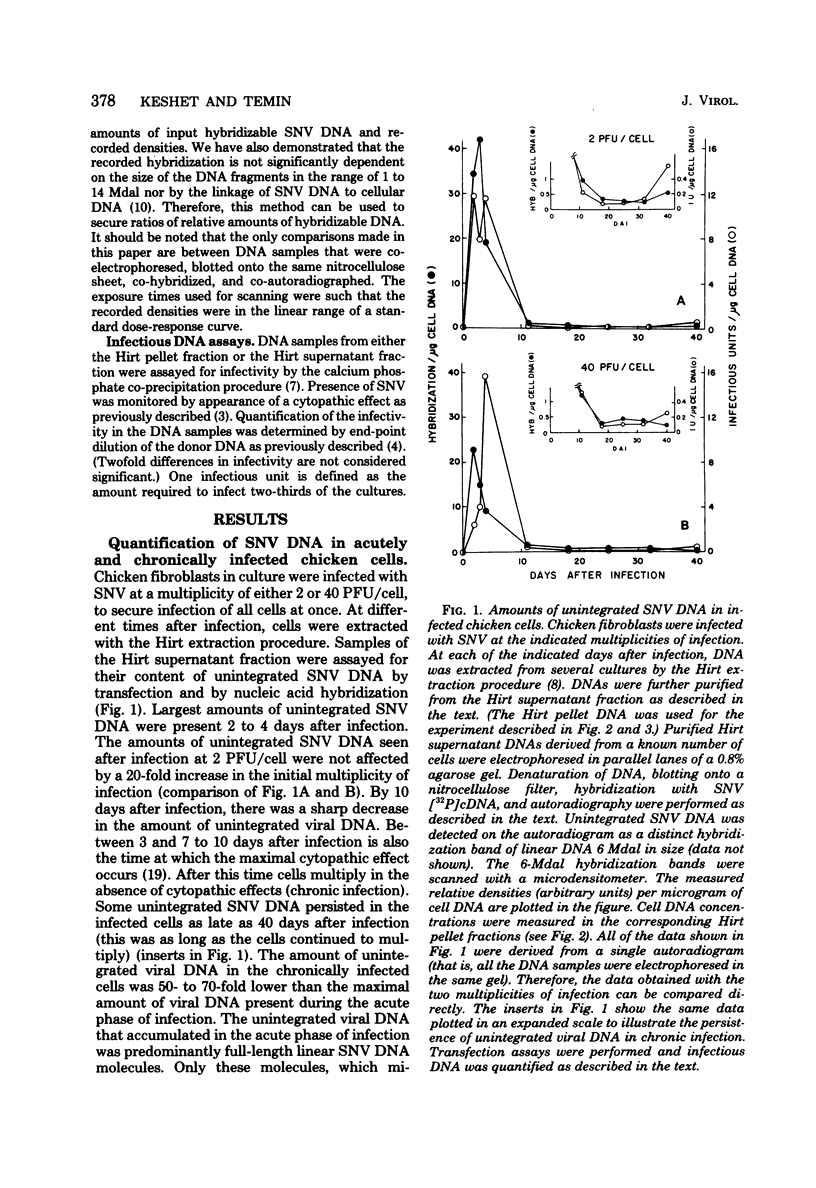

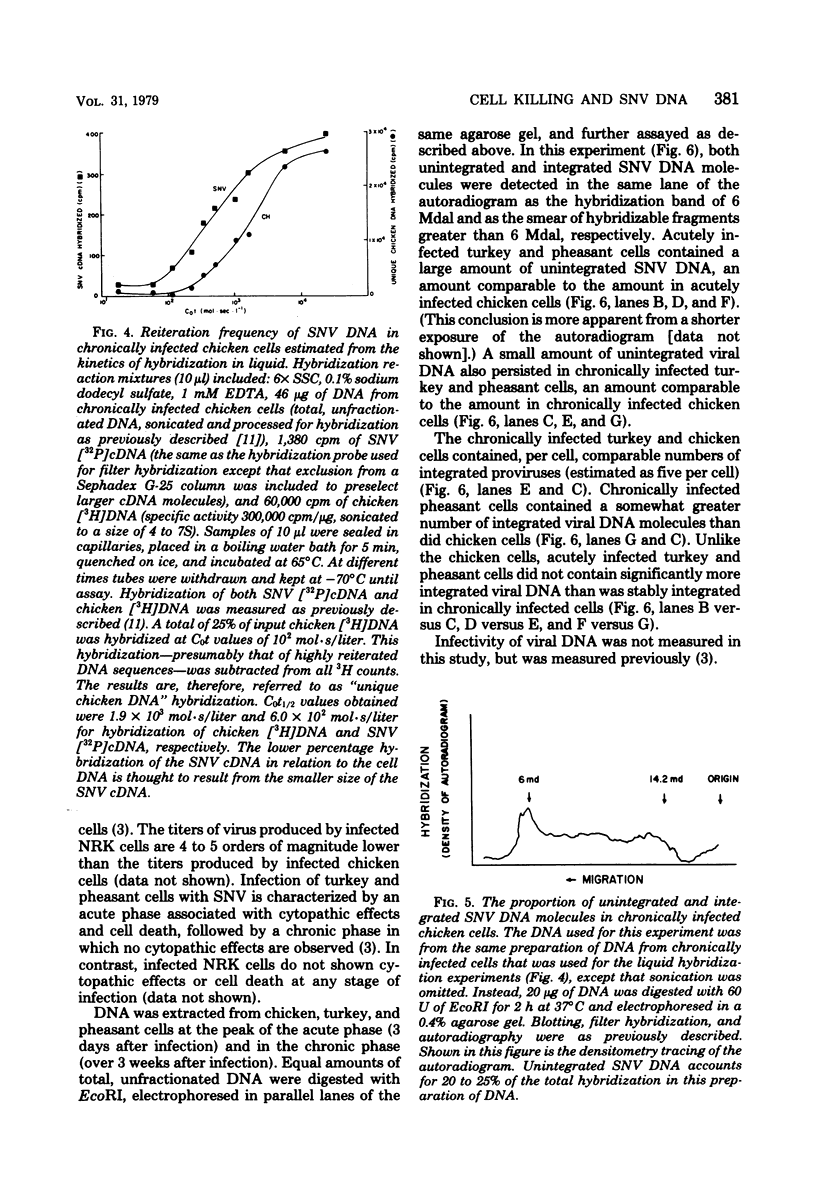





Images in this article
Selected References
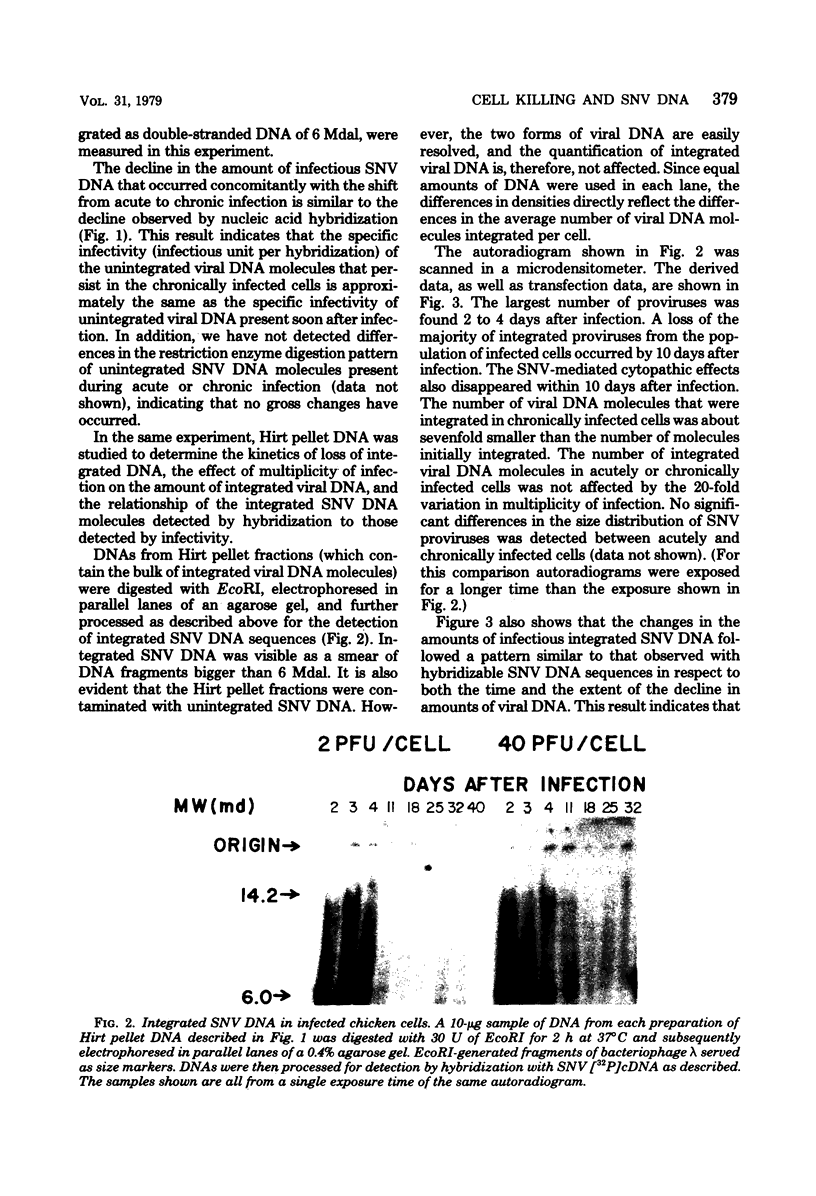
These references are in PubMed. This may not be the complete list of references from this article.
- Ali M., Baluda M. A. Synthesis of avian oncornavirus DNA in infected chicken cells. J Virol. 1974 May;13(5):1005–1013. doi: 10.1128/jvi.13.5.1005-1013.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Battula N., Temin H. M. Infectious DNA of spleen necrosis virus is integrated at a single site in the DNA of chronically infected chicken fibroblasts. Proc Natl Acad Sci U S A. 1977 Jan;74(1):281–285. doi: 10.1073/pnas.74.1.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Battula N., Temin H. M. Sites of integration of infectious DNA of avian reticuloendotheliosis viruses in different avian cellular DNAs. Cell. 1978 Feb;13(2):387–398. doi: 10.1016/0092-8674(78)90207-6. [DOI] [PubMed] [Google Scholar]
- Cooper G. M., Temin H. M. Infectious rous sarcoma virus and reticuloendotheliosis virus DNAs. J Virol. 1974 Nov;14(5):1132–1141. doi: 10.1128/jvi.14.5.1132-1141.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper G. M., Temin H. M. Lack of infectivity of the endogenous avian leukosis virus-related genes in the DNA of uninfected chicken cells. J Virol. 1976 Feb;17(2):422–430. doi: 10.1128/jvi.17.2.422-430.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritsch E., Temin H. M. Formation and structure of infectious DNA of spleen necrosis virus. J Virol. 1977 Jan;21(1):119–130. doi: 10.1128/jvi.21.1.119-130.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham F. L., van der Eb A. J. A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology. 1973 Apr;52(2):456–467. doi: 10.1016/0042-6822(73)90341-3. [DOI] [PubMed] [Google Scholar]
- Hirt B. Selective extraction of polyoma DNA from infected mouse cell cultures. J Mol Biol. 1967 Jun 14;26(2):365–369. doi: 10.1016/0022-2836(67)90307-5. [DOI] [PubMed] [Google Scholar]
- Kang C. Y., Temin H. M. Lack of sequence homology among RNAs of avian leukosis-sarcoma viruses, reticuloendotheliosis viruses, and chicken endogenous RNA-directed DNA polymerase activity. J Virol. 1973 Dec;12(6):1314–1324. doi: 10.1128/jvi.12.6.1314-1324.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keshet E., O'Rear J. J., Temin H. M. DNA of noninfectious and infectious integrated spleen necrosis virus (SNV) is colinear with unintegrated SNV DNA and not grossly abnormal. Cell. 1979 Jan;16(1):51–61. doi: 10.1016/0092-8674(79)90187-9. [DOI] [PubMed] [Google Scholar]
- Keshet E., Temin H. M. Nucleotide sequences derived from pheasant DNA in the genome of recombinant avian leukosis viruses with subgroup F specificity. J Virol. 1977 Nov;24(2):505–513. doi: 10.1128/jvi.24.2.505-513.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keshet E., Temin H. M. Sites of integration of reticuloendotheliosis virus DNA in chicken DNA. Proc Natl Acad Sci U S A. 1978 Jul;75(7):3372–3376. doi: 10.1073/pnas.75.7.3372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khoury A. T., Hanafusa H. Synethesis and integration of viral DNA in chicken cells at different time after infection with various multiplicities of avian oncornavirus. J Virol. 1976 May;18(2):383–400. doi: 10.1128/jvi.18.2.383-400.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neiman P. E. Rous sarcoma virus nucleotide sequences in cellular DNA: measurement by RNA-DNA hybridization. Science. 1972 Nov 17;178(4062):750–753. doi: 10.1126/science.178.4062.750. [DOI] [PubMed] [Google Scholar]
- Shoyab M., Baluda M. A., Evans R. Acquisition of new DNA sequences after infection of chicken cells with avian myeloblastosis virus. J Virol. 1974 Feb;13(2):331–339. doi: 10.1128/jvi.13.2.331-339.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Taylor J. M., Illmensee R., Summers J. Efficeint transcription of RNA into DNA by avian sarcoma virus polymerase. Biochim Biophys Acta. 1976 Sep 6;442(3):324–330. doi: 10.1016/0005-2787(76)90307-5. [DOI] [PubMed] [Google Scholar]
- Temin H. M., Kassner V. K. Replication of reticuloendotheliosis viruses in cell culture: acute infection. J Virol. 1974 Feb;13(2):291–297. doi: 10.1128/jvi.13.2.291-297.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Temin H. M., Kassner V. K. Replication of reticuloendotheliosis viruses in cell culture: chronic infection. J Gen Virol. 1975 Jun;27(3):267–274. doi: 10.1099/0022-1317-27-3-267. [DOI] [PubMed] [Google Scholar]
- Varmus H. E., Bishop J. M., Vogt P. K. Appearance of virus-specific DNA in mammalian cells following transformation by Rous sarcoma virus. J Mol Biol. 1973 Mar 15;74(4):613–626. doi: 10.1016/0022-2836(73)90052-1. [DOI] [PubMed] [Google Scholar]
- Varmus H. E., Shank P. R. Unintegrated viral DNA is synthesized in the cytoplasm of avian sarcoma virus-transformed duck cells by viral DNA polymerase. J Virol. 1976 May;18(2):567–573. doi: 10.1128/jvi.18.2.567-573.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varmus H. E., Vogt P. K., Bishop J. M. Integration of deoxyribonucleic acid specific for Rous sarcoma virus after infection of permissive and nonpermissive hosts. Proc Natl Acad Sci U S A. 1973 Nov;70(11):3067–3071. doi: 10.1073/pnas.70.11.3067. [DOI] [PMC free article] [PubMed] [Google Scholar]







