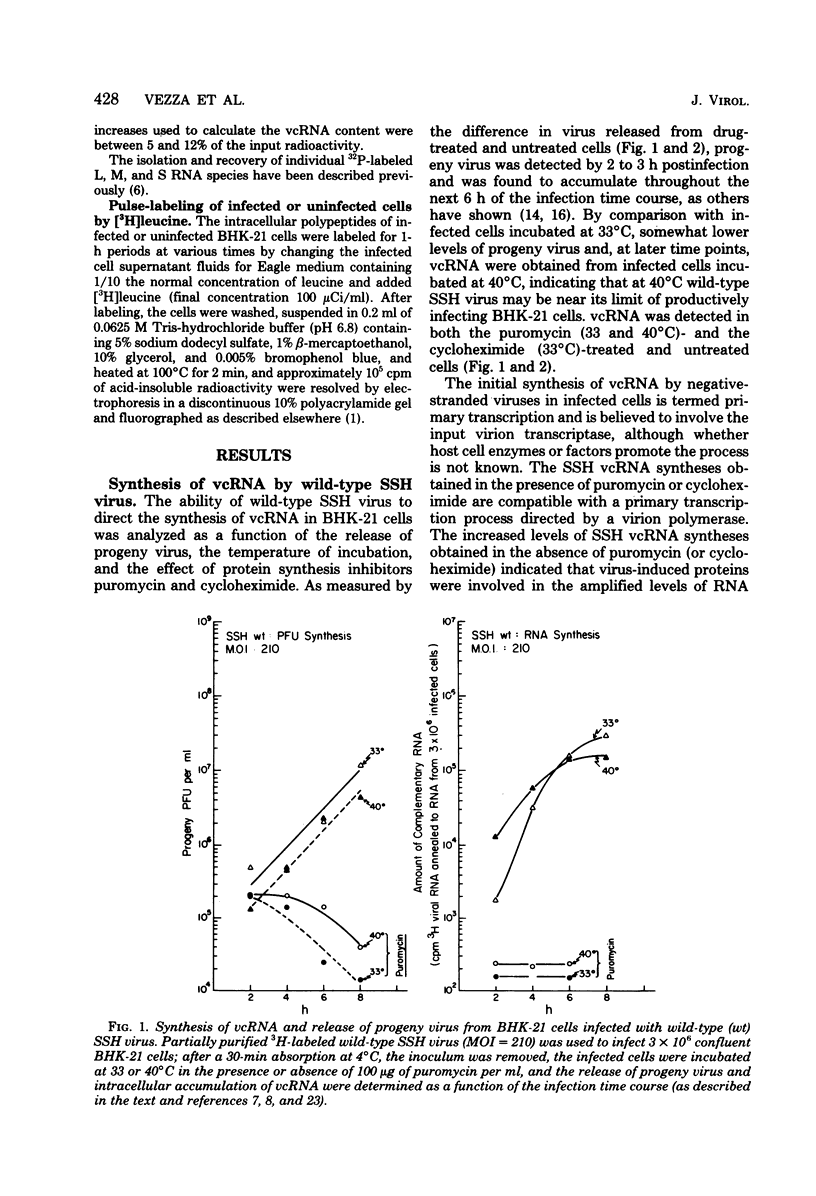
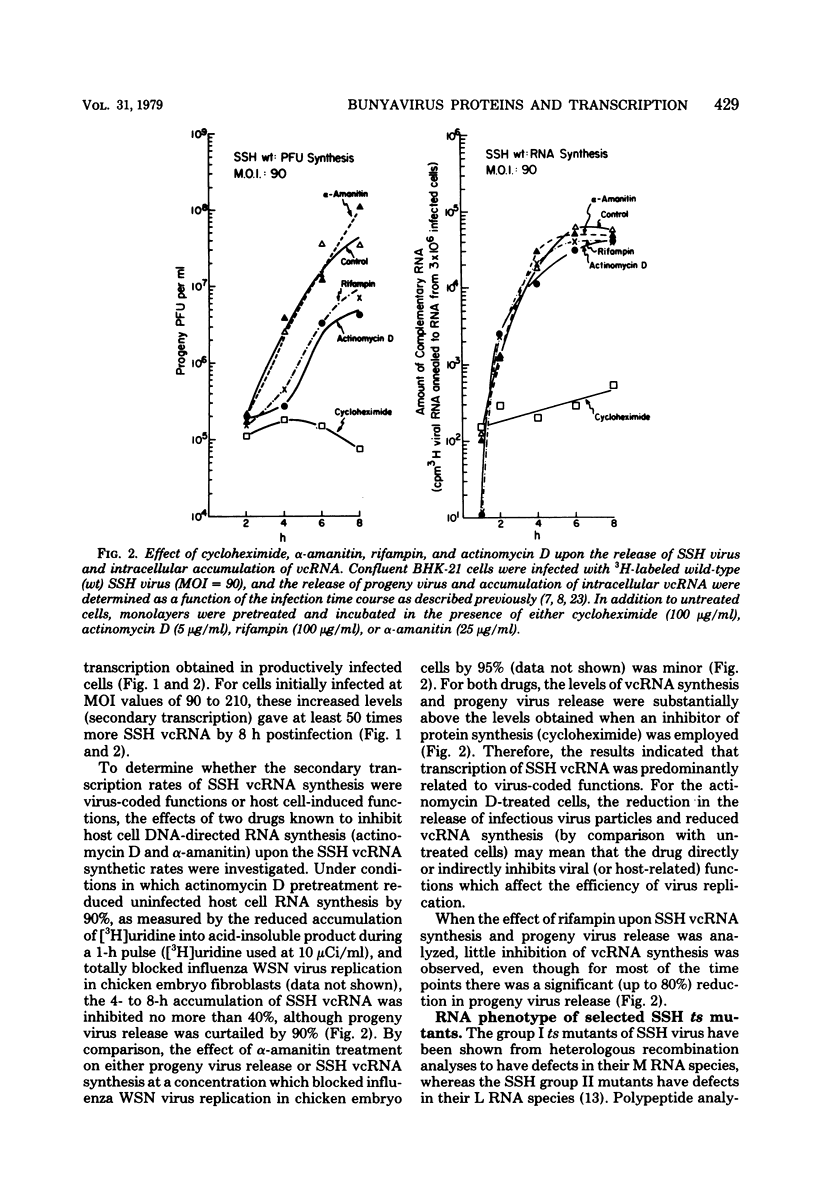
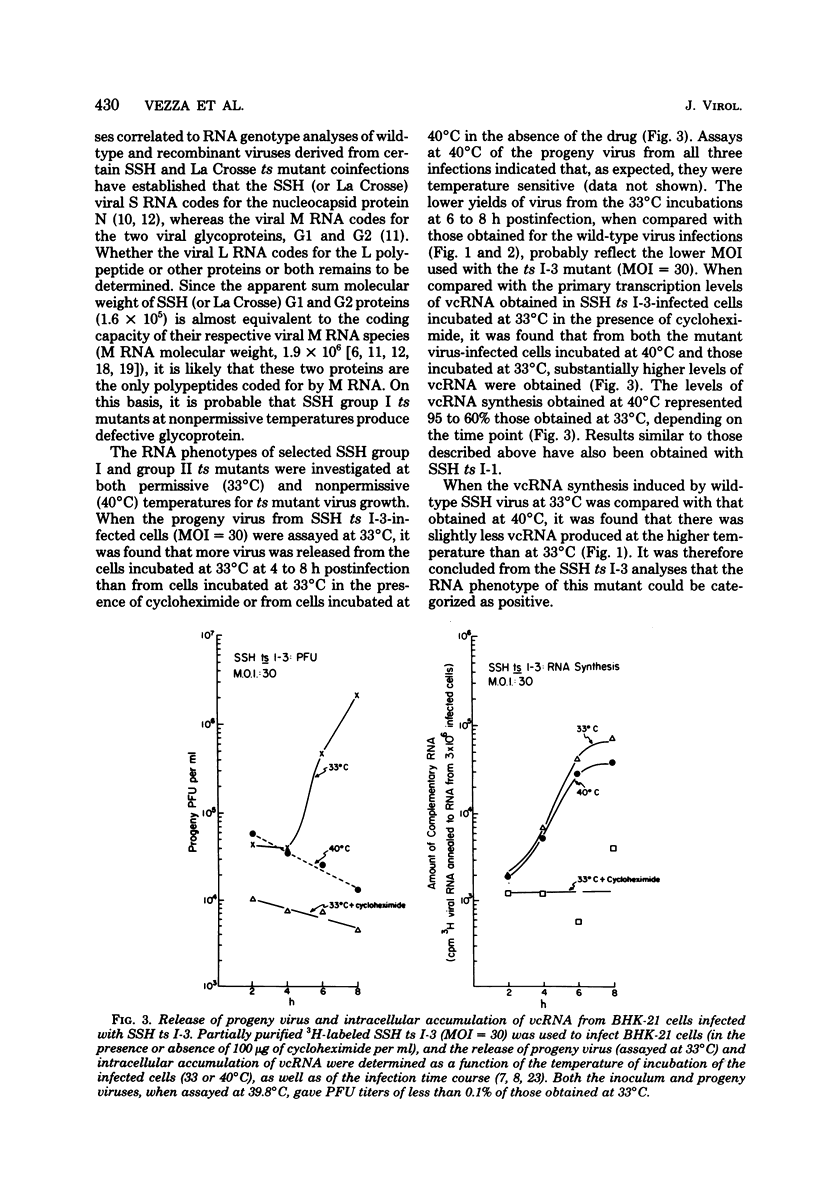
Abstract
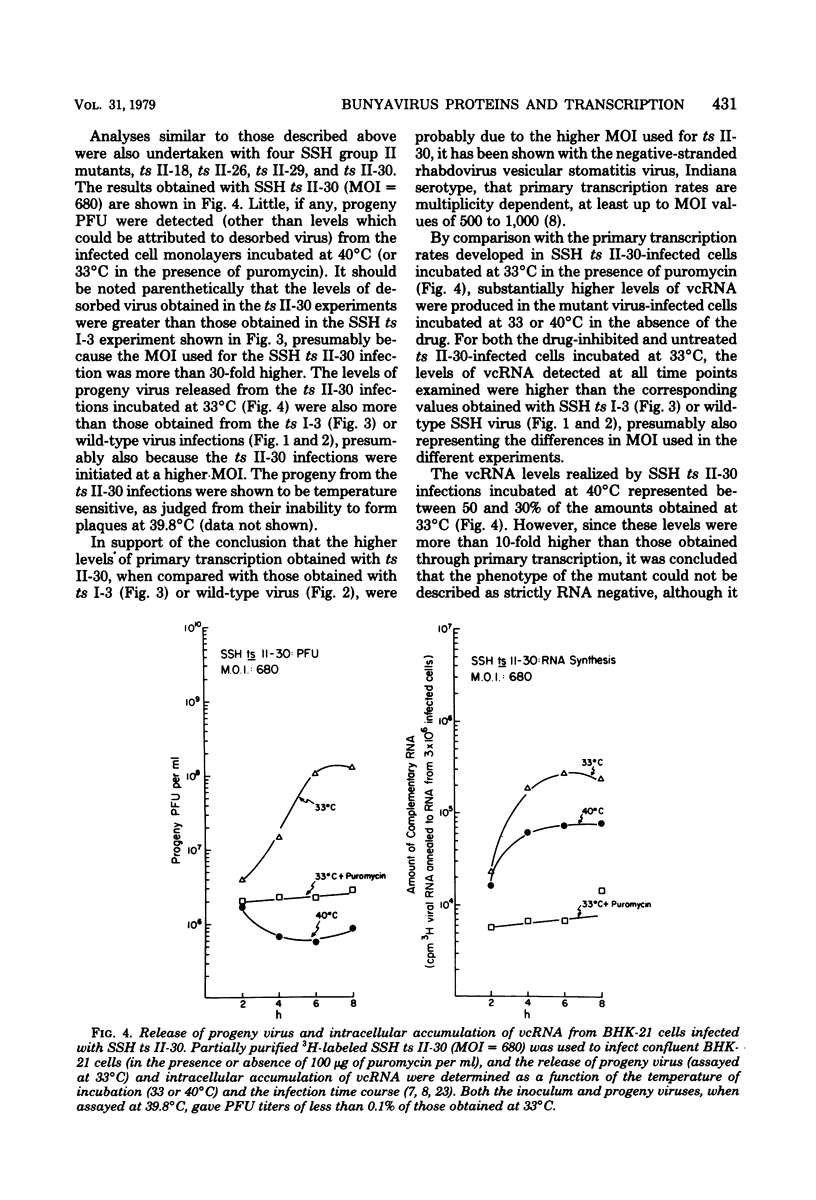
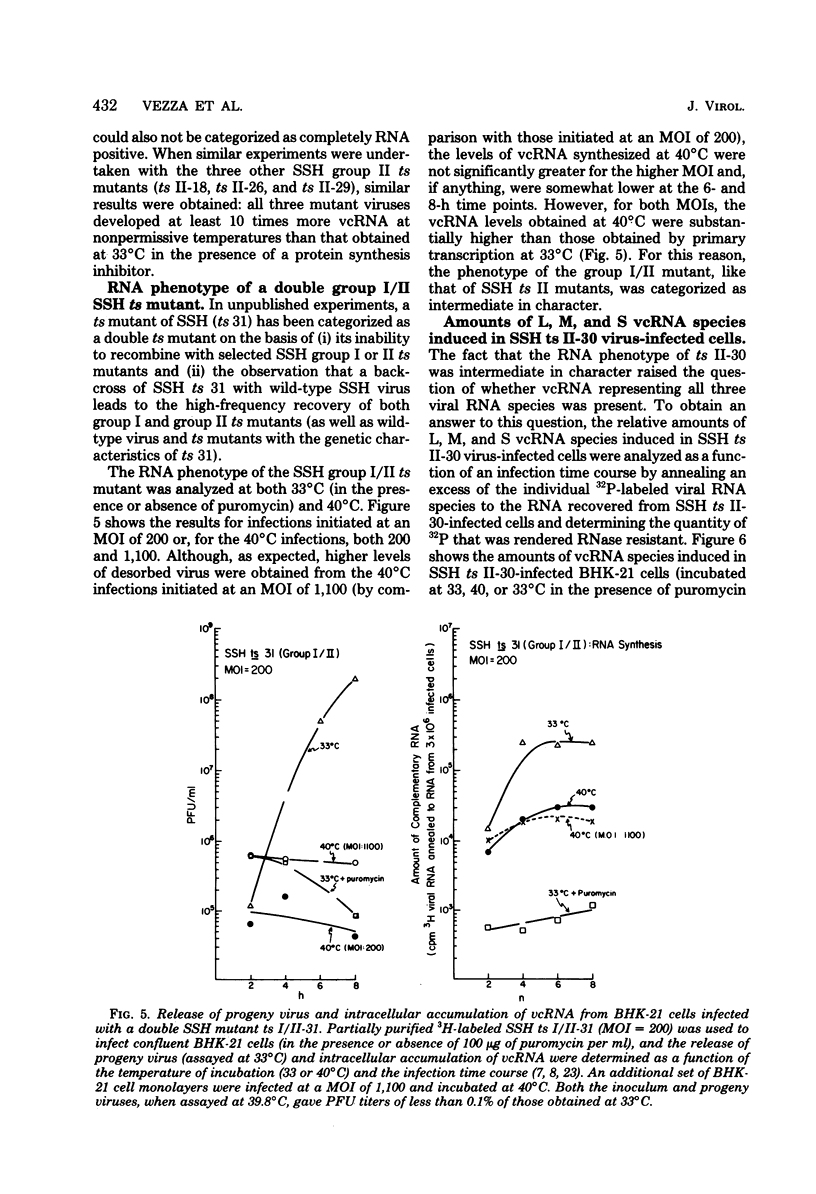
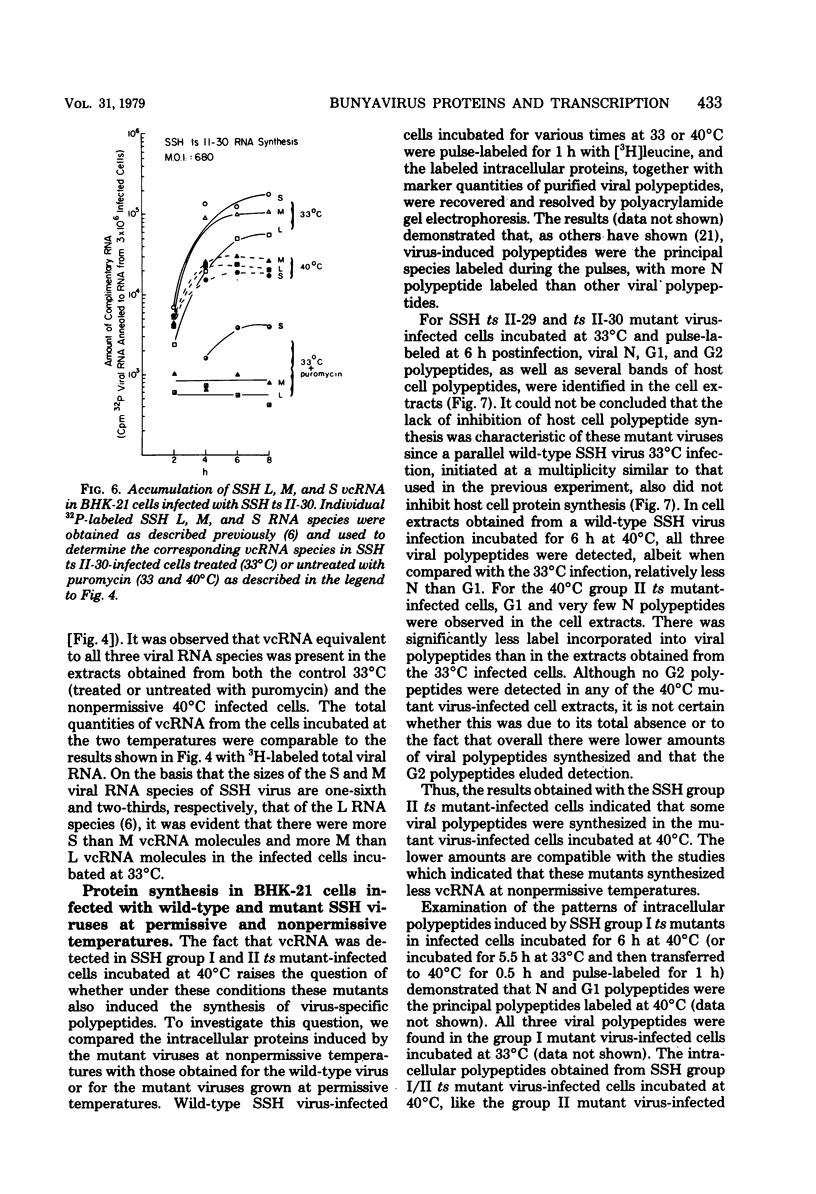
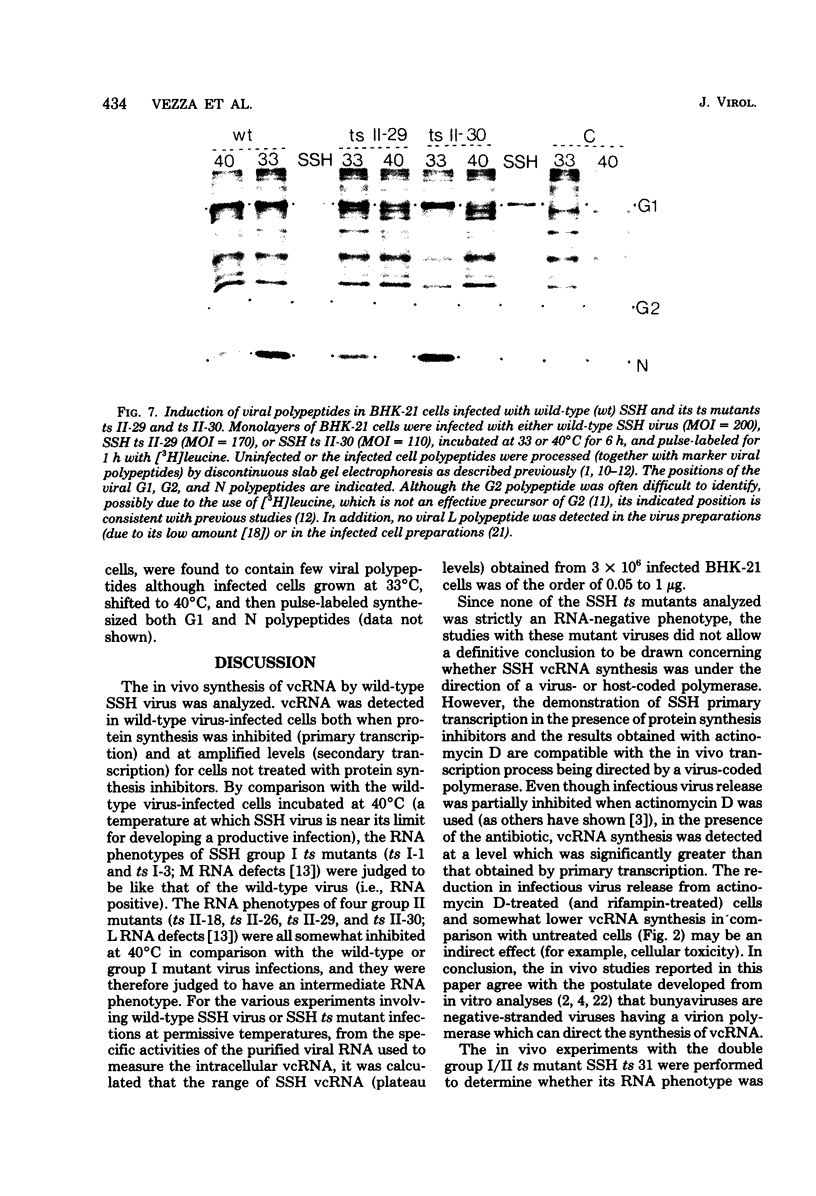
The in vivo primary and secondary transcription capabilities of wild-type snowshoe hare (SSH) virus and certain of its temperature-sensitive (ts) mutants have been analyzed. The results obtained agree with in vitro studies (Bouloy et al., C.R. Acad. Sci. Paris 280:213-215, 1975; M. Bouloy and C. Hannoun, Virology 69:258-264, 1976; M. Ranki and R. Pettersson, J. Virol. 16:1420-1425, 1975) which have shown that bunyaviruses are negative-stranded RNA viruses with a virion RNA-directed RNA polymerase. The in vivo transcription studies have demonstrated that in the presence of protein synthesis inhibitors (puromycin or cycloheximide) SSH virus can synthesize viral complementary RNA (primary transcription) throughout the infection cycle. The increased levels of viral complementary RNA obtained in the absence of protein synthesis inhibitors (secondary transcription) were not markedly reduced if cells were pretreated with actinomycin D (5 μg/ml), α-amanitin (25 μg/ml), or rifampin (100 μg/ml), although progeny virus yields were reduced by up to 80% in the actinomycin D- and rifampin-treated cells. The in vivo transcription capabilities of SSH group I ts mutants at temperatures which were nonpermissive (40°C) for virus replication gave values comparable to those obtained at permissive temperatures (33°C). The SSH group I mutants appear, therefore, to be RNA-positive mutant types. When compared with their transcription capabilities at 33°C, the in vivo transcription abilities of four SSH group II ts mutants (and one double group I/II ts mutant) were found to be more impaired at 40°C than those of the SSH group I ts mutants or wild-type SSH virus at 40°C, although the viral complementary RNA synthetic capabilities of these group II (and group I/II) mutants at 40°C were significantly higher than their primary transcription capabilities (as measured at 33°C in the presence of puromycin or cycloheximide). It was concluded, therefore, that these SSH group II (and double group I/II) ts mutants have an intermediate RNA phenotype. Hybridization studies using 32P-labeled individual L, M, and S viral RNA species of SSH virus have demonstrated the presence of viral complementary RNA to all three species in extracts of cells infected with SSH ts II-30 and incubated at 33°C (primary and secondary transcription) or 40°C, a nonpermissive temperature for its replication. The results of pulse-labeled in vivo protein analyses indicated that greater quantities of intracellular N protein (coded for by S RNA [J. R. Gentsch and D. H. L. Bishop, J. Virol. 28:417-419, 1978]) than G1 and G2 polypeptides (coded for by M RNA [J. R. Gentsch and D. H. L. Bishop, J. Virol. 30:767-776, 1979]) were present in extracts of cells infected with wild-type SSH virus. In extracts of SSH group I, II, or I/II ts mutant-infected cells incubated at 33°C, N and G1, and for the group II mutant-infected cells, G2, viral polypeptides were detected, whereas in extracts obtained from group I or II mutant virus-infected cells incubated at 40°C, low levels of N and G1 polypeptides were evident.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Bouloy M., Colbère F., Krams-Ozden S., Vialat P., Garapin A. C., Hannoun C., Lépine P. Activité RNA polymérasique à un bunyavirus (Lumbo) C R Acad Sci Hebd Seances Acad Sci D. 1975 Jan 13;280(2):213–215. [PubMed] [Google Scholar]
- Bouloy M., Hannoun C. Effet de l'actinomycine D sur la multiplication du virus Tahyna. Ann Microbiol (Paris) 1973 Dec;124(4):547–553. [PubMed] [Google Scholar]
- Bouloy M., Hannoun C. Studies on Lumbo virus replication. II. Properties or viral ribonucleoproteins and characterization of messenger RNAs. Virology. 1976 Jun;71(2):363–370. doi: 10.1016/0042-6822(76)90363-9. [DOI] [PubMed] [Google Scholar]
- Bouloy M., Hannoun C. Studies on lumbo virus replication. I. RNA-dependent RNA polymerase associated with virions. Virology. 1976 Jan;69(1):258–264. doi: 10.1016/0042-6822(76)90212-9. [DOI] [PubMed] [Google Scholar]
- Clewley J., Gentsch J., Bishop D. H. Three unique viral RNA species of snowshoe hare and La Crosse bunyaviruses. J Virol. 1977 May;22(2):459–468. doi: 10.1128/jvi.22.2.459-468.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flamand A., Bishop D. H. In vivo synthesis of RNA by vesicular stomatitis virus and its mutants. J Mol Biol. 1974 Jul 25;87(1):31–53. doi: 10.1016/0022-2836(74)90558-0. [DOI] [PubMed] [Google Scholar]
- Flamand A., Bishop D. H. Primary in vivo transcription of vesicular stomatitis virus and temperature-sensitive mutants of five vesicular stomatitis virus complementation groups. J Virol. 1973 Dec;12(6):1238–1252. doi: 10.1128/jvi.12.6.1238-1252.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gentsch J. R., Bishop D. L. M viral RNA segment of bunyaviruses codes for two glycoproteins, G1 and G2. J Virol. 1979 Jun;30(3):767–770. doi: 10.1128/jvi.30.3.767-770.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gentsch J., Bishop D. H., Obijeski J. F. The virus particle nucleic acids and proteins of four bunyaviruses. J Gen Virol. 1977 Feb;34(2):257–268. doi: 10.1099/0022-1317-34-2-257. [DOI] [PubMed] [Google Scholar]
- Gentsch J., Bishop D. H. Recombination and complementation between temperature-sensitive mutants of a Bunyavirus, snowshoe hare virus. J Virol. 1976 Oct;20(1):351–354. doi: 10.1128/jvi.20.1.351-354.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gentsch J., Wynne L. R., Clewley J. P., Shope R. E., Bishop D. H. Formation of recombinants between snowshoe hare and La Crosse bunyaviruses. J Virol. 1977 Dec;24(3):893–902. doi: 10.1128/jvi.24.3.893-902.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman N., Presser I., Sreevalsan T. California encephalitis virus: some biological and biochemical properties. Virology. 1977 Jan;76(1):352–364. doi: 10.1016/0042-6822(77)90308-7. [DOI] [PubMed] [Google Scholar]
- Huang A. S., Manders E. K. Ribonucleic acid synthesis of vesicular stomatitis virus. IV. Transcription by standard virus in the presence of defective interfering particles. J Virol. 1972 Jun;9(6):909–916. doi: 10.1128/jvi.9.6.909-916.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kascsak R. J., Lyons M. J. Bunyamwera virus. I. The molecular complexity of the virion RNA. Virology. 1977 Oct 1;82(1):37–47. doi: 10.1016/0042-6822(77)90030-7. [DOI] [PubMed] [Google Scholar]
- Marcus P. I., Engelhardt D. L., Hunt J. M., Sekellick M. J. Interferon action: inhibition of vesicular stomatitis virus RNA synthesis induced by virion-bound polymerase. Science. 1971 Nov 5;174(4009):593–598. doi: 10.1126/science.174.4009.593. [DOI] [PubMed] [Google Scholar]
- Obijeski J. F., Bishop D. H., Murphy F. A., Palmer E. L. Structural proteins of La Crosse virus. J Virol. 1976 Sep;19(3):985–997. doi: 10.1128/jvi.19.3.985-997.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obijeski J. F., Bishop D. H., Palmer E. L., Murphy F. A. Segmented genome and nucleocapsid of La Crosse virus. J Virol. 1976 Dec;20(3):664–675. doi: 10.1128/jvi.20.3.664-675.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obijeski J. F., Marchenko A. T., Bishop D. H., Cann B. W., Murphy F. A. Comparative electrophoretic analysis of the virus proteins of four rhabdoviruses. J Gen Virol. 1974 Jan;22(1):21–33. doi: 10.1099/0022-1317-22-1-21. [DOI] [PubMed] [Google Scholar]
- Pennington T. H., Pringle C. R., McCrae M. A. Bunyamwera virus-induced polypeptide synthesis. J Virol. 1977 Oct;24(1):397–400. doi: 10.1128/jvi.24.1.397-400.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranki M., Pettersson R. F. Uukuniemi virus contains an RNA polymerase. J Virol. 1975 Dec;16(6):1420–1425. doi: 10.1128/jvi.16.6.1420-1425.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Repik P., Flamand A., Bishop D. H. Synthesis of RNA by mutants of vesicular stomatitis virus (Indiana serotype) and the ability of wild-type VSV New Jersey to complement the VSV Indiana ts G I-114 transcription defect. J Virol. 1976 Oct;20(1):157–169. doi: 10.1128/jvi.20.1.157-169.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]