Abstract
In 2002, section B of the Larsen ice shelf, off of the Eastern Antarctic Peninsula, collapsed and created the opportunity to study whether the changes at the sea surface left evidence in the sedimentary record. Biogenic silica is major constituent of Antarctic marine sediment, and its presence in the sediment column is associated with diatom production in the euphotic zone. The abundance of diatom valves and the number of sponge spicules in the biogenic silica was analyzed to determine how the origin of the biogenic silica in the upper layers of the sediment column responded to recent environmental changes. Diatom valves were present only in the upper 2 cm of sediment, which roughly corresponds to the period after the collapse of the ice shelf. In contrast, sponge spicules, a more robust form of biogenic silica, were also found below the upper 2 cm layer of the sediment column. Our results indicate that in this region most of the biogenic silica in the sedimentary record originated from sponge spicules rather than diatoms during the time when the sea surface was covered by the Larsen ice shelf. Since the collapse of the ice shelf, the development of phytoplankton blooms and the consequent influx of diatom debris to the seabed have shifted the biogenic silica record to one dominated by diatom debris, as occurs in most of the Antarctic marine sediment. This shift provides further evidence of the anthropogenic changes to the benthic habitats of the Antarctic and will improve the interpretation of the sedimentary record in Polar Regions where these events occur.
Introduction
Over the last 60 years, atmospheric and oceanic temperatures in the Antarctic Peninsula have increased more than the global average [1], [2]. This increase is responsible for the retreat of the ice shelf on both sides of the peninsula [3] and for the collapse of the Larsen B ice shelf, which disintegrated in 2002 after millennia of stability [4]. The collapse of the Larsen B ice shelf on the east side of the Antarctic Peninsula must have changed the conditions in the water column, which now favor increased primary production in the euphotic zone [5]. The sediment cores collected on the continental shelf below the collapsed sections of the ice shelf contained phytopigments and diatom valves together with excess 210Pb activity, only in the upper 2 cm of the sediment column, which were attributed to a recent flux of biogenic material to the seafloor [6], [7]. 210Pb reaches the marine environment by atmospheric precipitation and in-situ decay of its parent, 226Ra. 210Pb is insoluble in sea water and is scavenged from the water column by settling particles during their transit to the seabed. Upon settlement, these particles produce “excess” 210Pb activity which adds to the supported activity levels found deeper in the sediment column, where the older material has been stabilized.
Along with diatoms, radiolaria, siliceous sponges and silicoflagellates contribute their siliceous hard parts to the biogenic silica that reaches the seafloor [8], [9], [10]. In general, diatom valves, radiolaria and sponge spicules are the principal sources of biogenic silica in marine sediments [11], [9], [12], [13]. Southern Ocean sediments account for ∼50% of the biogenic silica deposited in the marine environment, and most of this siliceous material has been attributed to diatoms [10]. Diatoms can have high biomass in Antarctic coastal regions [14], [15] and can represent as much as 40% of the total primary productivity in the Southern Ocean [16]. Despite partial degradation during grazing [17] and dissolution in the water column and seabed [18], siliceous diatom frustules are often well-preserved in the sediment column and can serve as an indicator of the environmental conditions at the time that they were formed [19], [20]. Siliceous sponges are often important components of Antarctic benthic communities [21]. Most Demospongiae and Hexactinellida sponges produce siliceous skeletons composed of individualized elements (spicules) with lengths ranging from micrometers to centimeters [22]. Hexactinellids are one of the dominant groups in the benthic communities of the Weddell Sea [23] and play an important role in forming substrata by generating spicule mats [24], [23]. Some spicules are too fragile to survive intact in the sedimentary record, which prevents them from serving as accurate oceanographic proxies of the paleoenvironmental record. However, sponges can be important contributors to biogenic silica in the sedimentary column because they are relatively long-lived and their spicules dissolve more slowly than diatom frustules [13], [25]. Earlier observations in the Larsen region suggested that hexactinellid sponges dwelled on the continental shelf before the collapse of the Larsen ice shelf [26]. Thus, it is possible that sponge spicules could have constituted an important fraction of the biogenic silica in the sediment column of that region during the period when diatom development was restricted by the ice shelf [7]. The recent disintegration of the Larsen ice shelf offers a unique opportunity for research. The collapse of the ice shelf and the development of diatom blooms may have changed the sedimentary regime of biogenic silica from one dominated by sponge spicules to one in which diatom frustules play a greater role.
Methods
1. Sediment sampling
In the austral summer of 2006–2007, during ANT-XXIII/8, the R/V Polarstern reached the area off the Eastern Antarctic Peninsula (EAP) that was previously occupied by section B of the Larsen ice shelf. Four sediment cores were collected using a multi- corer with a diameter of 10 cm [27] at the Larsen B South (LBS), Larsen B West (LBW), Larsen B Central (LBC) and Larsen B North (LBN) stations ( Fig. 1 ). Before laboratory and microscopic analyses, the samples were freeze dried (at 0.1 mbar and −80°C) for 24 hours. Permission to collect samples from the prospected areas was obtained from the Spanish Polar Committee (Ref. CPE-EIA-2006-16) observing the guidelines of the Antarctic Treaty. None of the sampling stations occupied specially protected areas in the Antarctic.
Figure 1. Study area with the four sampling stations.
2. Biogenic silica
The percentage of biogenic silica (SiO2) in the sediment (expressed as dry weight %) was measured following established procedures [28], [9]. Five milliliters of a 10% hydrogen peroxide (H2O2) solution was added to approximately 100–200 mg of dry sediment to remove the organic matter. After 30 minutes (min), 5 ml of a 10% hydrochloric acid (HCl) solution was added to dissolve the calcium carbonate (CaCO3). The samples were rinsed with bi-distilled water and, after centrifugation (5 min at 5000 revolutions per minute), were dried in the oven before alkaline extraction. After sonication for 5 min in 40 ml of a 2 M sodium carbonate (Na2CO3) solution, the samples were placed in a bath (T = 85°C) for 5 h. Five milliliters of solution were collected twice, after 2 h and again after 5 h of extraction. The extraction after 2 h primarily targeted biogenic SiO2, whereas the extraction after 5 h primarily targeted the lithogenic silica minerals that took longer to dissolve in Na2CO3 [9]. After extraction, 17.5 ml of a ammonium molybdate tetrahydrate (NH4)6Mo7O24 4H2O) solution was added to every aliquot, and after 20 min, 7.5 ml of a reducing solution with sodium metol-sulfite (C7H10NO)2SO4 Na2SO3), oxalic acid (C2H2O4) and sulfuric acid (H2SO4) were added to produce a blue chromophore, which was read using a spectrophotometer at 815 nm. A regression line was plotted through the two SiO2 concentration values corresponding to the 2 h and 5 h extractions. The intercept of the regression line with the y-axis corresponded to the percent weight of biogenic SiO2 in the sample [9].
3. Diatom valves and sponge spicules
Diatom valves and sponge spicules were counted using an optical microscope. HCl and H2O2 were added to the dry sediment to dissolve carbonates and attack organic matter, respectively. The sediment was rinsed several times with bi-distilled water, the slides were mounted, and diatom valve counts were performed at 1000 magnification using a Leica DMLB with phase-contrast illumination. The counts were carried out on permanent slides of acid-cleaned material (Permount mounting medium). The recommendations of [29] were followed for counting microfossil valves. Several transects along each cover slip were examined according to the abundance of the diatoms. A minimum of 350 valves, including at least 100 valves from non-dominant taxa, were counted per sample. In addition to diatoms, sponge spicules were also counted.
To calculate the contribution of diatoms and siliceous sponges to the biogenic silica, we estimated the weight of the main groups of diatom taxa and siliceous spicules. We calculated the volume of specimen types of Chaetoceros resting spores (RS) (approximately 43% of the diatom assemblage), Fragilariopsis curta (the primary species of the Sea-ice taxa group, representing approximately 45% of the assemblage) and of Thalassosira antarctica RS (a minor component of the assemblage). For this task, we used NIS- Element BR3.1 software for microscope image analyses with a NIKON Eclipse 80 at x1000 magnification. The mean volume of a diatom valve and a sponge spicule fragment were calculated by measuring 100 specimens for each sample. The volumes were multiplied by the biogenic silica density, which was assumed to be ∼2 g cm−3 [30]. For Chaetoceros RS, the mean value of the volume occupied by biogenic SiO2 was 3.7×10−11 cm3 diatom−1, and the weight of the biogenic SiO2 was 7.4×10−11 grams per diatom. Assuming that approximately 30% of the surface of a F. curta specimen-type was empty due to the perforations in the structure of the valve, the mean volume occupied by biogenic silica was 4.41×10−11 cm3 diatom−1. The estimated weight of the F. curta specimen-type was 8.82×10−11 grams of biogenic SiO2 per diatom. In the case of T. antarctica RS, assuming that approximately 50% of the valve surface was perforated, the silica-volume for the specimen-type was 9.82×10−11 cm3 diatom−1. Consequently, the estimated weight of the specimen type was 1.96×10−11 grams of biogenic SiO2 per diatom.
The morphometrics for sponge spicules consisted of the length, external diameter and internal diameter of the fragments. The difference between the external and internal cylinders was considered to be the silica-volume of the fragment type, which was 2.39×10−9 cm3 spicule−1. Using the same value of 2 g cm−3 for biogenic silica, we determined that the weight of the biogenic SiO2 of a sponge spicule fragment was 4.78×10−9 grams. To obtain the total amount of biogenic silica originating from diatoms and sponge spicules, the weight in grams of the biogenic SiO2 per diatom and sponge spicule fragments was multiplied by the abundance of diatoms and spicule fragments, respectively.
Results
1. Biogenic silica
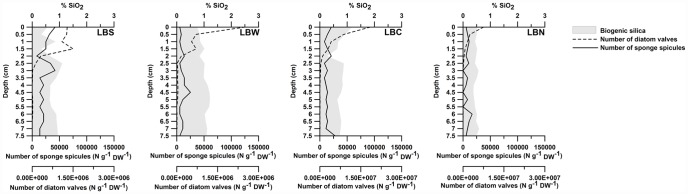
In the surface sediment (upper 0.5 cm), the biogenic SiO2 content varied between 0.53% (LBN) and 1.2% (LBC) ( Fig. 2 ). The percentage of biogenic silica did not decrease with depth in any of the four cores ( Fig. 2 ).
Figure 2. In the superior x-axis, percentage of biogenic silica from silica extractions.
In the inferior x-axis, number of diatom valves and number of sponge spicules. Axis scale for diatom valves is different for Larsen B Central (LBC) and North (LBN).
2. Diatom valves and sponge spicules
The number of diatom valves in the surface sediment varied between ∼1.3×106 valves per gram of dry sediment (N g DW−1) (station LBS) and ∼18×106 valves g DW−1 (station LBC) ( Fig. 2 ). In all of the cores, the diatom valve abundances decreased with depth and were negligible at depths greater than 2 cm ( Fig. 2 ).
The number of sponge spicules in the upper 0.5 cm varied between ∼5600 (LBW and LBN) and ∼42000 (LBS) spicules g DW−1 ( Fig. 2 ). The number of sponge spicules did not decrease with depth in any of the cores ( Fig. 2 ).
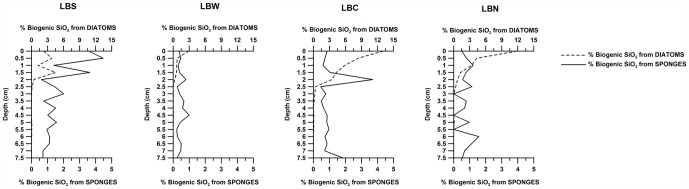
In the surface sediment (0–0.5 cm depth), the biogenic silica content from diatoms varied between 0.1×10−3 (LBS) and 1.5×10−3 (LBC) grams of biogenic silica per gram of dry sediment ( Fig. 3 ). Below 2 cm, the concentration of biogenic silica from diatoms was negligible ( Fig. 3 ). The contribution of sponge spicules to the biogenic silica varied between 0.03×10−3 (LBW) and 0.2×10−3 (LBS) grams of biogenic silica per gram of dry sediment ( Fig. 3 )
Figure 3. In the superior x-axis, percentage of biogenic silica from diatoms (calculated from the number of frustules and associated weight) and, in the inferior x-axis, percentage of biogenic silica from sponges (calculated from the number of spicules and associated weight), as a function of depth.
Discussion
1. Biogenic silica under ice shelves
Overall, the biogenic silica percentages obtained in the present study were rather low ( Fig. 2 ) for the Antarctic context (including the Weddell and Ross Seas and the Bransfield and Gerlache Straits), where dry weight percentages varied between 1% and 49% in surface sediments [31], [32], [33], [34]. Based on the frustule and spicule volumes and the biogenic silica density, less than ∼14% (LBC station, from 0 to 0.5 cm depth) of the total biogenic silica in our samples was from diatoms or sponge spicules ( Fig. 3 ). These low values suggest that significant amounts of silica leached from coexisting aluminosilicates in the lithogenic fraction of the sample, which could not be identified with the alkaline extraction method. However, alkaline extraction is the standard technique used to measure biogenic silica in marine sediments and therefore allows a direct comparison of the biogenic silica contents of our samples to those of different Antarctic regions [31], [32], [33], [34]. The methodological restrictions led us to use the diatom frustule and spicule concentrations ( Fig. 2 and Fig. 3 ) to establish reliable comparisons between the sites and to identify changes in the sedimentary regime of biogenic silica within the study area. Even given the potential overestimation from the aluminum silicates, there was relatively little biogenic silica in the continental shelf sediments below the extinct Larsen ice shelf. The concentrations indicate that the supply of biogenic silica to the seabed below the ice shelves is comparatively low and that this condition persists a decade after the collapse of the Larsen ice shelf. This persistence most likely occurs because the sea surface in the Larsen region still has heavy sea ice during most of the year, which prevents primary productivity from reaching the levels that are reached truly open water. However, the exponential increase in diatomaceous material ( Fig. 2 ) towards the surface of the sediment cores indicates that the production of diatomaceous biogenic silica is increasing. It is worth mentioning that station LBC is found in the axis of a glacial trough, where sediment focusing produces higher concentrations of diatom valves and biogenic silica percentages ( Fig. 2 ).
2. Spicules as source of biogenic silica
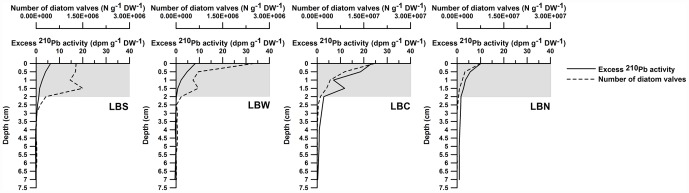
Based on the 14C sediment accumulation rate (SAR) obtained for the Larsen sediment cores (∼0.04 cm y−1), only the upper millimeters of sediment were related to the post- ice-shelf collapse period [6], [7]. This low SAR, taken together with the correlation between the excess 210Pb activity and the abundance profiles of the diatom valves ( Fig. 4 ) [6], indicates that diatoms reached the seabed after the collapse of the ice shelf and then were incorporated deeper into the upper 2 cm layer of the sediment column via diffusion (presumably bioturbation) rather than advection ( Fig. 2 ). The presence of few valves below this layer is consistent with the theory that the biogenic silica reaching the seabed before the collapse of the ice shelf did not originate from diatoms.
Figure 4. Number of diatom valves and excess 210Pb activity.
Axis scale for diatom valves is different for Larsen B Central (LBC) and North (LBN).
In addition to diatoms, radiolarians, silicoflagellates and sponges are sources of biogenic silica in the marine environment [9]. In high-latitude sediments, diatom valves and sponge spicules can constitute major sources of this biogenic material on the seafloor [11], [9], [12], [13]. In the Antarctic continental shelf, sponges can show high abundances and biomasses [35], [23]. Eighty-one percent of Antarctic sponges belong to class Demospongiae, which are characterized by slow growth rates [36] and by a well-developed siliceous skeleton [37]. The absence of grounding ice under the Larsen B ice shelf most likely favored the development of Demospongiae in the EAP region [38], [26], leading to the relatively high percentage of this class in terms of biomass that was observed in previous studies of the Larsen regions [26], [39]. Sponges, similar to other suspension feeders, may develop under ice shelves [40]. Therefore, it is likely that sponge spicules from Hexactinellidae and Demospongiae accumulated in the Larsen B seafloor during the years of permanent shelf ice coverage. However, it has been observed that biogenic silica-rich sediments in regions with low productivity can be the result of the lateral transport of particles from adjacent areas [41], [10], [34]. Siliceous particles, including sponge spicule fragments, could have reached the Larsen region before the collapse of the ice shelf via lateral transport driven by the Weddell Gyre, which carries mud-like particles from the sponge-rich southeastern Weddell Sea [42] to the tip of the Antarctic Peninsula [43]. Further evidence of lateral transport into the Larsen region is provided by analyses of fatty acids, which were found below the upper 2 cm of the sediment column and had signals correlated to relatively older, refractory material [7].
Our results demonstrate that under ice shelves, the supply of biogenic silica from sponges (spicules) to the sedimentary record may be greater than that from diatomaceous sources ( Fig. 3 ). However, these conditions are changing in the continental shelf below the extinct Larsen ice shelf. The concentration of biogenic silica originating from diatoms is becoming larger than that originating from spicules in the upper cm of the sediment column ( Fig. 3 ), and this change is related to ongoing global warming, which is especially dramatic in the Antarctic Peninsula. This feature of the sedimentary record leaves unambiguous evidence of the disintegration of the ice shelf and provides further evidence of the effects of ongoing global warming in the Antarctic.
Conclusions
The disintegration of the Larsen ice shelves produced a drastic change in the upper water column: it increased primary production and consequently increased the diatomaceous material. The arrival of this material shifted the sedimentary regime of biogenic silica from one dominated by sponge spicules to one in which diatom debris plays a more important role. This change will eventually integrate the region into the area of the Southern Ocean, where open water conditions during the austral summer create high levels of biogenic silica containing significant proportions of diatomaceous material. These results provide further evidence of the profound changes experienced in the benthic realm of the Antarctic as a consequence of ongoing climate change.
Acknowledgments
The authors wish to thank the Captain and the crew of the R/V Polarstern. The comments of J. Gutt improved the earlier version of the manuscript.
Funding Statement
This study was supported by the Spanish Ministry of Science and Innovation, through the project CLIMANT (POL2006-06399) and the graduate program fellowship FPU AP 2005-5060. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Vaughan DG, Marshall GJ, Connolley WM, King JC, Mulvaney R (2001) Devil in the Detail. Science 293: 1777–1779. [DOI] [PubMed] [Google Scholar]
- 2. Gille ST (2002) Warming of the Southern Ocean Since the 1950s. Science 295: 1275–1277. [DOI] [PubMed] [Google Scholar]
- 3. Cook AJ, Fox AJ, Vaughan DG, Ferrigno JG (2005) Retreating Glacier Fronts on the Antarctic Peninsula over the Past Half-Century. Science 308: 541–544. [DOI] [PubMed] [Google Scholar]
- 4. Domack E, Duran D, Leventer A, Ishman S, Doane S, et al. (2005) Stability of the Larsen B ice shelf on the Antarctic Peninsula during the Holocene epoch. Nature 436: 681–685. [DOI] [PubMed] [Google Scholar]
- 5. Bertolin ML, Schloss IR (2009) Phytoplankton production after the collapse of the Larsen A Ice Shelf, Antarctica. Polar Biology 32: 1435–1446. [Google Scholar]
- 6. Sañé E, Isla E, Grémare A, Gutt J, Vétion G, et al. (2011) Pigments in sediments beneath a recently collapsed ice shelves: the case of Larsen A and B shelves, Antarctic Peninsula. Journal of Sea Research 65: 94–102. [Google Scholar]
- 7. Sañé E, Isla E, Pruski AM, Bárcena MA, Vétion G, et al. (2011) Diatom valve distribution and sedimentary fatty acid composition in Larsen Bay, Eastern Antarctic Peninsula. Continental Shelf Research 31: 1161–1168. [Google Scholar]
- 8. Abelmann A, Gersonde R (1991) Biosiliceous particle flux in the Southern Ocean. Marine Chemistry 35: 503–536. [Google Scholar]
- 9. DeMaster DJ (1981) The supply and accumulation of silica in the marine environment. Geochimica et Cosmochimica Acta 45: 1715–1732. [Google Scholar]
- 10. DeMaster DJ (2002) The accumulation and cycling of biogenic silica in the Southern Ocean: revisiting the marine silica budget. Deep Sea Research II 49: 3155–3167. [Google Scholar]
- 11. Rützler K, Macintyre IG (1978) Siliceous sponge spicules in coral reef sediments. Marine Biology 49: 147–159. [Google Scholar]
- 12. Treguer P, Nelson DM, Van Bennekom AJ, DeMaster DJ, Leynaert A, et al. (1995) The Silica Balance in the World Ocean: A Reestimate. Science 268: 375–379. [DOI] [PubMed] [Google Scholar]
- 13. Bavestrello G, Cattaneo-Vietti R, Cerrano C, Cerutti S, Sará M (1996) Contribution of sponge spicules to the composition of biogenic silica in the Ligurian Sea. Marine Ecology 17: 41–50. [Google Scholar]
- 14. Wright SW, van den Enden RL (2000) Phytoplankton community structure and stocks in the East Antarctic Marginal ice zone (BROKE survey, January-March 1996) determined by CHEMTAX analysis of HPLC pigment signatures. Deep Sea Research II 47: 2363–2400. [Google Scholar]
- 15. Arrigo KR, van Dijken GL, Bushinsky E (2008) Primary production in the Southern Ocean, 1997–2006. Journal of Geophysical Research 113: C08004 doi:10.1029/2007JC004551. [Google Scholar]
- 16. Cortese G, Gersonde R (2007) Morphometric variability in the diatom Fragilariopsis kerguelensis: implications for Southern Ocean paleoceanography. Earth and Planetary Science Letters 257: 526–544. [Google Scholar]
- 17. Crosta X (2009) Holocene size variations in two diatoms species, East Antarctica: productivity vs environmental conditions. Deep Sea Research I 56: 1983–1993. [Google Scholar]
- 18. Buffen A, Leventer A, Rubin A, Hutchins T (2007) Diatom assemblages in surface sediments of the north western Weddell Sea, Antarctic Peninsula. Marine Micropaleontology 62: 7–30. [Google Scholar]
- 19. Bárcena MA, Isla E, Plaza A, Flores JA, Sierro FJ, et al. (2002) Bioaccumulation record and its relation with paleoclimatic evolution in the western Bransfield Strait. Deep Sea Research 49: 935–950. [Google Scholar]
- 20. Tsoy IB, Obrezkova MS, Artemova AV (2009) Diatoms in Surface Sediments of the Sea of Okhotsk and the Northwest Pacific Ocean. Marine Geology 49: 141–150. [Google Scholar]
- 21. Gerdes D, Hilbig B, Montiel A (2003) Impact of iceberg scouring on macrobenthic communities in the high Antarctic Weddell Sea. Polar Biology 26: 295–301. [Google Scholar]
- 22. Uriz MJ, Turon X, Becerro MA, Agell G (2003) Siliceous spicules and skeleton frameworks in sponges: origin, diversity, ultrastructural patterns, and biological functions. Microscopy Research and Technique 62: 279–299. [DOI] [PubMed] [Google Scholar]
- 23. Barthel D, Gutt J (1992) Sponge associations in the eastern Weddell Sea. Antarctic Science 4: 137–150. [Google Scholar]
- 24. Barthel D, Gutt J, Tendal OS (1991) New information on the biology of Antarctic deep-water sponges derived from underwater photography. Marine Ecology Progress Series 69: 303–307. [Google Scholar]
- 25. Maldonado M, Carmona MC, Velásquez Z, Puig A, Cruzado A, et al. (2005) Siliceous sponges as a silicon sink: an overlooked aspect of benthopelagic coupling in the marine silicon cycle. Limnology and Oceanography 50: 799–809. [Google Scholar]
- 26. Gutt J, Barratt I, Domack E, d'Udekem d'Acoz C, Dimmler W, et al. (2011) Biodiversity change after climate-induced ice-shelf collapse in the Antarctic. Deep Sea Research II 58: 74–83. [Google Scholar]
- 27. Barnett PRO, Watson J, Connelly D (1984) A multiple corer for taking virtually 410 undisturbed samples from shelf, bathyal and abyssal sediments. Oceanologica Acta 7: 399–408. [Google Scholar]
- 28. Mortlock RA, Froelich PN (1989) A simple method for the rapid determination of biogenic opal in pelagic marine sediments. Deep Sea Research 36: 1415–1426. [Google Scholar]
- 29. Schrader HJ, Gersonde R (1978) Diatoms and silicoflagellates. In: Micropaleontological Counting Methods and Techniques: An Exercise of an Eight Metres Section of the Lower Pliocene of Cap Rossello, Sicily Zachariasse WJ, et al. , editors. Utrecht Micropaleontology Bulletin; 17: 129–176. [Google Scholar]
- 30.DeMaster DJ (2003) Organic carbon, calcium carbonate and biogenic silica of sediment core TT013_41. doi:10.1594/PANGAEA.124976.
- 31. Dunbar RB, Leventer AR, Stockton WL (1989) Biogenic sedimentation in McMurdo Sound, Antarctica. Marine Geology 85: 155–179. [Google Scholar]
- 32. DeMaster DJ, Smith W, Nelson DM, Aller JY (1996) Biogeochemical processes in Amazon shelf waters: chemical distributions and uptake rates of silicon, carbon and nitrogen. Continental Shelf Research 16: 617–643. [Google Scholar]
- 33. Schlüter M, Rutgers van der Loeff MM, Holby O, Kuhn G (1998) Silica cycle in surface sediments of the South Atlantic. Deep Sea Research 45: 1085–1109. [Google Scholar]
- 34. Isla E, Masqué P, Palanques A, Guillén J, Puig P, et al. (2004) Sedimentation of biogenic constituents during the last century in western Bransfield and Gerlache Straits, Antarctica: a relation to currents, primary production, and sea floor relief. Marine Geology 209: 265–277. [Google Scholar]
- 35. Gerdes D, Klages M, Arntz WE, Herman RL, Galerón J, et al. (1992) Quantitative investigations on macrobenthos communities of the southeastern Weddell Sea shelf based on multibox corer samples. Polar Biology 12: 291–301. [Google Scholar]
- 36. Post AL, Hemer MA, O'Brien EO, Roberts D, Craven M (2007) History of benthic colonisation beneath the Amery Ice Shelf, East Antarctica. Marine Ecology Progress Series 344: 29–37. [Google Scholar]
- 37. McClintock JB, Amsler CD, Baker BJ, Van Soest RWM (2005) Ecology of Antarctic Marine Sponges: An Overview. Integrative and Comparative Biology 45: 359–368. [DOI] [PubMed] [Google Scholar]
- 38. Gutt J (2001) On the direct impact of ice on marine benthic communities, a review. Polar Biology 24: 553–564. [Google Scholar]
- 39. Sañé E, Isla E, Gerdes D, Montiel A, Gili JM (2012) Benthic macrofauna assemblages and biochemical properties of sediments in two Antarctic regions differently affected by climate change. Continental Shelf Research 35: 53–63. [Google Scholar]
- 40. Riddle MJ, Craven M, Goldsworthy PM, Carsey F (2007) A diverse benthic assemblage 100 km from open water under the Amery Ice Shelf. Paleoceanography 22 doi:10.1029/2006PA001327. [Google Scholar]
- 41. Nelson DM, DeMaster DJ, Dunbar R, Smith WO Jr (1996) Cycling of organic matter and biogenic silica in the Southern Ocean: estimates of water-column and sedimentary fluxes on the Ross Sea continental shelf. Journal of Geophysical Research 101: 18519–18532. [Google Scholar]
- 42. Brey T, Gerdes D (1997) Is Antarctic benthic biomass really higher than elsewhere? Antarctic Science 9: 266–267. [Google Scholar]
- 43. Diekmann B, Kuhn G (1999) Provenance and dispersal of glacial–marine surface sediments in the Weddell Sea and adjoining areas, Antarctica: ice -rafting versus current transport. Marine Geology 158: 209–231. [Google Scholar]