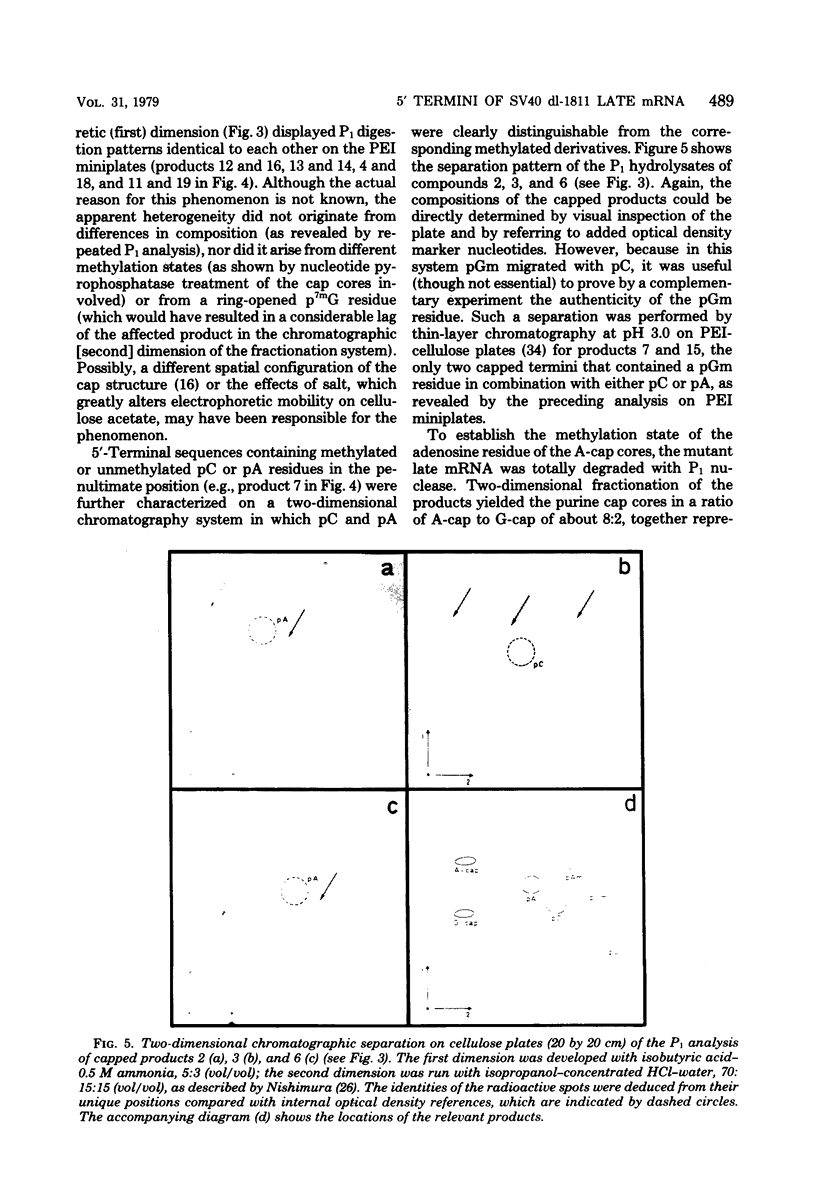
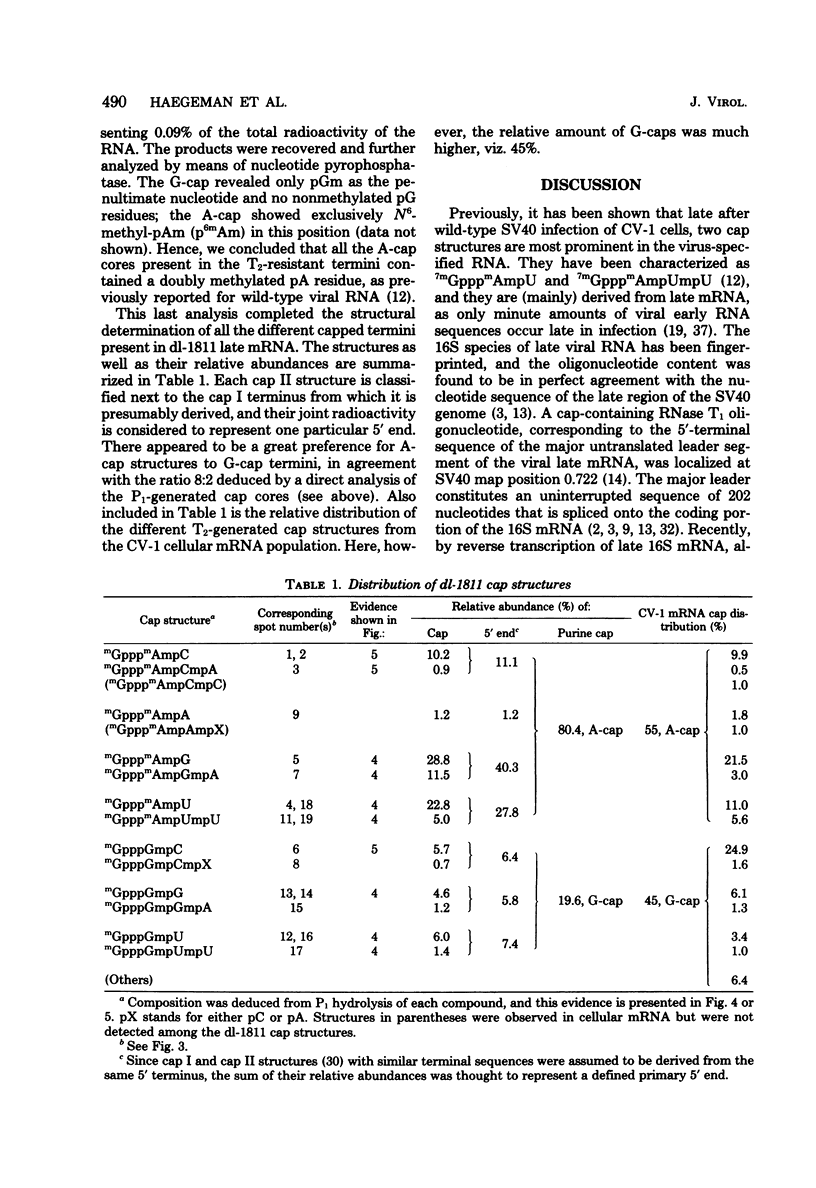
Abstract
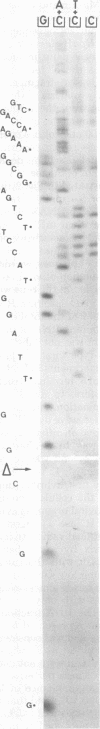
d1-1811 is a viable simian virus 40 deletion mutant which lacks the DNA region corresponding to the major capping site of the late viral RNA. The exact size of the deletion (40 base pairs) was determined by comparison of the mutant DNA sequence with the wild-type simian virus 40 (strain 776) DNA sequence. Although d1-1811 forms somewhat smaller plaques, the amount of viral RNA late after infection was not significantly reduced compared with that of the wild type. Virus-specific, polyadenylate-containing, 32P-labeled late RNA was purified from the cytoplasm and enzymatically degraded to characterize the 5' terminus. The cap-containing oligonucleotides were isolated, and their structures were analyzed by further digestion. Instead of a single cap structure, we found a variety of capped 5' termini, with adenosine caps occurring much more frequently than guanosine caps. Nevertheless, there was a remarkable homology between both types of terminal sequences. Conceivably, the minor cap population present in wild-type simian virus 40 late mRNA may correspond to the collection of capped termini identified in the d1-1811 late mRNA . Cellular cytoplasmic RNA shows a similar pattern of cap structures, but the relative abundance is quite different.
Full text
PDF


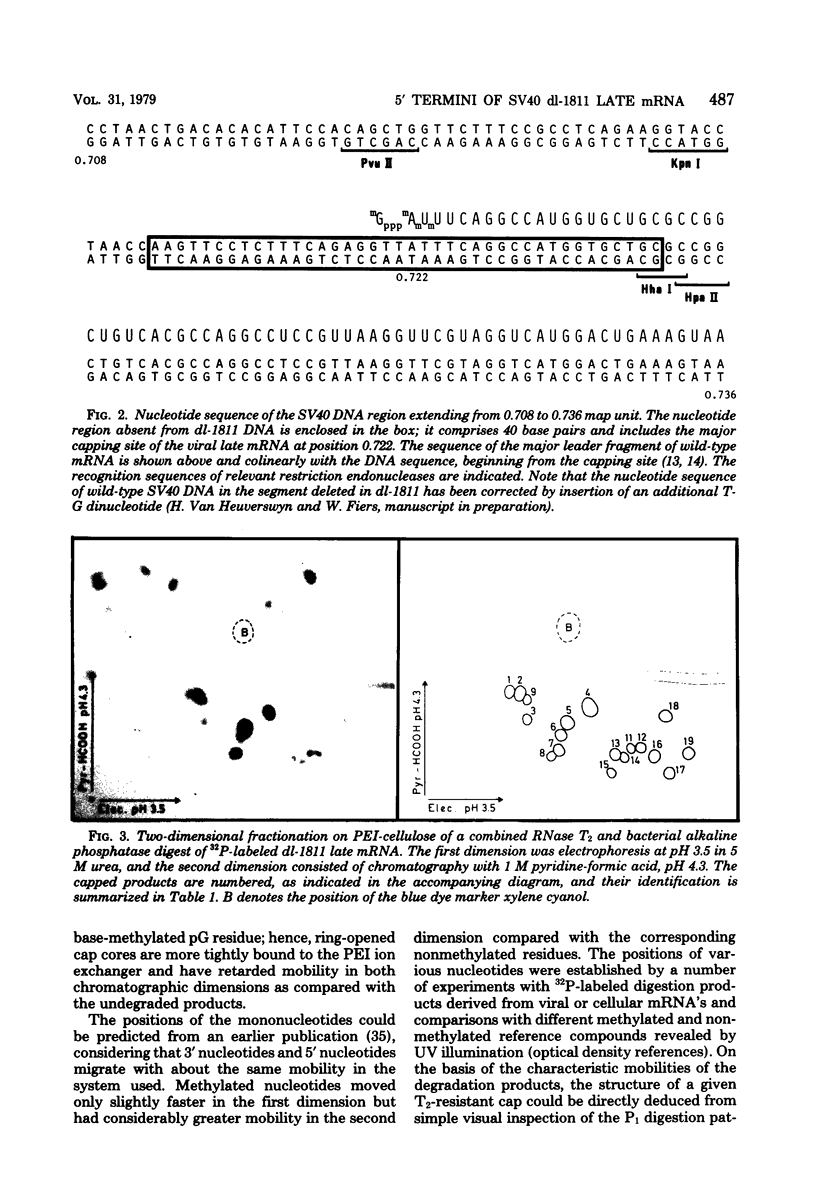
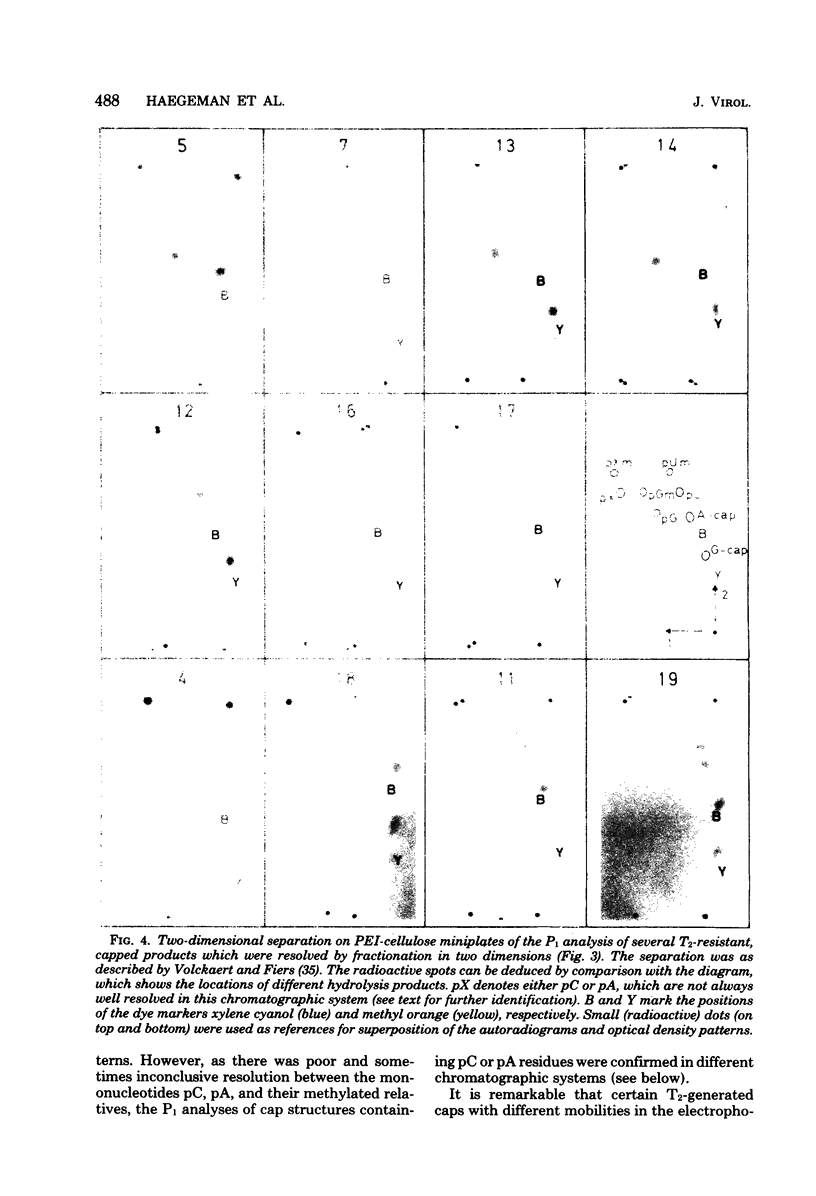



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aloni Y., Shani M., Reuveni Y. RNAs of simian virus 40 in productively infected monkey cells: kinetics of formation and decay in enucleate cells. Proc Natl Acad Sci U S A. 1975 Jul;72(7):2587–2591. doi: 10.1073/pnas.72.7.2587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bina-Stein M., Thoren M., Salzman N., Thomspon J. A. Rapid sequence determination of late simian virus 40 16S mRNA leader by using inhibitors of reverse transcriptase. Proc Natl Acad Sci U S A. 1979 Feb;76(2):731–735. doi: 10.1073/pnas.76.2.731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celma M. L., Dhar R., Pan J., Weissman S. M. Comparison of the nucleotide sequence of the messenger RNA for the major structural protein of SV40 with the DNA sequence encoding the amino acids of the protein. Nucleic Acids Res. 1977 Aug;4(8):2549–2550. [PMC free article] [PubMed] [Google Scholar]
- Chiu N. H., Radonovich M. F., Thoren M. M., Salzman N. P. Selective degradation of newly synthesized nonmessenger simian virus 40 transcripts. J Virol. 1978 Nov;28(2):590–599. doi: 10.1128/jvi.28.2.590-599.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filipowicz W. Functions of the 5,-terminal m7G cap in eukaryotic mRNA. FEBS Lett. 1978 Dec 1;96(1):1–11. doi: 10.1016/0014-5793(78)81049-7. [DOI] [PubMed] [Google Scholar]
- Flavell A. J., Cowie A., Legon S., Kamen R. Multiple 5' terminal cap structures in late polyoma virus RNA. Cell. 1979 Feb;16(2):357–371. doi: 10.1016/0092-8674(79)90012-6. [DOI] [PubMed] [Google Scholar]
- Furuichi Y. "Pretranscriptional capping" in the biosynthesis of cytoplasmic polyhedrosis virus mRNA. Proc Natl Acad Sci U S A. 1978 Mar;75(3):1086–1090. doi: 10.1073/pnas.75.3.1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuichi Y., Muthukrishnan S., Tomasz J., Shatkin A. J. Mechanism of formation of reovirus mRNA 5'-terminal blocked and methylated sequence, m7GpppGmpC. J Biol Chem. 1976 Aug 25;251(16):5043–5053. [PubMed] [Google Scholar]
- Ghosh P. K., Reddy V. B., Swinscoe J., Choudary P. V., Lebowitz P., Weissman S. M. The 5'-terminal leader sequence of late 16 S mRNA from cells infected with simian virus 40. J Biol Chem. 1978 May 25;253(10):3643–3647. [PubMed] [Google Scholar]
- Ghosh P. K., Reddy V. B., Swinscoe J., Lebowitz P., Weissman S. M. Heterogeneity and 5'-terminal structures of the late RNAs of simian virus 40. J Mol Biol. 1978 Dec 25;126(4):813–846. doi: 10.1016/0022-2836(78)90022-0. [DOI] [PubMed] [Google Scholar]
- Groner Y., Carmi P., Aloni Y. Capping structures of simian virus 40 19S and 16S mRNAs. Nucleic Acids Res. 1977 Nov;4(11):3959–3968. doi: 10.1093/nar/4.11.3959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haegeman G., Fiers W. Characterization of the 5'-terminal capped structures of late simian virus 40-specific mRNA. J Virol. 1978 Mar;25(3):824–830. doi: 10.1128/jvi.25.3.824-830.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haegeman G., Fiers W. Evidence for 'splicing' of SV40 16S mRNA. Nature. 1978 May 4;273(5657):70–73. doi: 10.1038/273070a0. [DOI] [PubMed] [Google Scholar]
- Haegeman G., Fiers W. Localization of the 5' terminus of late SV40 mRNA. Nucleic Acids Res. 1978 Jul;5(7):2359–2371. doi: 10.1093/nar/5.7.2359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heckle W. L., Jr, Fenton R. G., Wood T. G., Merkel C. G., Lingrel J. B. Methylated nucleosides in globin mRNA from mouse nucleated erythroid cells. J Biol Chem. 1977 Mar 10;252(5):1764–1770. [PubMed] [Google Scholar]
- Kim C. H., Sarma R. H. Spatial configuration of mRNA 5'-terminus. Nature. 1977 Nov 17;270(5634):223–227. doi: 10.1038/270223a0. [DOI] [PubMed] [Google Scholar]
- Kozak M. How do eucaryotic ribosomes select initiation regions in messenger RNA? Cell. 1978 Dec;15(4):1109–1123. doi: 10.1016/0092-8674(78)90039-9. [DOI] [PubMed] [Google Scholar]
- LAWLEY P. D., BROOKES P. FURTHER STUDIES ON THE ALKYLATION OF NUCLEIC ACIDS AND THEIR CONSTITUENT NUCLEOTIDES. Biochem J. 1963 Oct;89:127–138. doi: 10.1042/bj0890127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai C. J., Dhar R., Khoury G. Mapping the spliced and unspliced late lytic SV40 RNAs. Cell. 1978 Aug;14(4):971–982. doi: 10.1016/0092-8674(78)90351-3. [DOI] [PubMed] [Google Scholar]
- Laub O., Aloni Y. Transcription of simian virus 40. V. Regulattion of simian virus 40 gene expression. J Virol. 1975 Nov;16(5):1171–1183. doi: 10.1128/jvi.16.5.1171-1183.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavi S., Shatkin A. J. Methylated simian virus 40-specific RNA from nuclei and cytoplasm of infected BSC-1 cells. Proc Natl Acad Sci U S A. 1975 Jun;72(6):2012–2016. doi: 10.1073/pnas.72.6.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lockard R. E. Different cap 1 : cap 2 ratios in rabbit alpha and beta globin mRNA. Nature. 1978 Sep 14;275(5676):153–154. doi: 10.1038/275153a0. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. A new method for sequencing DNA. Proc Natl Acad Sci U S A. 1977 Feb;74(2):560–564. doi: 10.1073/pnas.74.2.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mertz J. E., Berg P. Viable deletion mutants of simian virus 40: selective isolation by means of a restriction endonuclease from Hemophilus parainfluenzae. Proc Natl Acad Sci U S A. 1974 Dec;71(12):4879–4883. doi: 10.1073/pnas.71.12.4879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muthukrishnan S., Moss B., Cooper J. A., Maxwell E. S. Influence of 5'-terminal cap structure on the initiation of translation of vaccinia virus mRNA. J Biol Chem. 1978 Mar 10;253(5):1710–1715. [PubMed] [Google Scholar]
- Noyes B. E., Stark G. R. Nucleic acid hybridization using DNA covalently coupled to cellulose. Cell. 1975 Jul;5(3):301–310. doi: 10.1016/0092-8674(75)90105-1. [DOI] [PubMed] [Google Scholar]
- Reddy V. B., Ghosh P. K., Lebowitz P., Weissman S. M. Gaps and duplicated sequences in the leaders of SV40 16S RNA. Nucleic Acids Res. 1978 Nov;5(11):4195–4213. doi: 10.1093/nar/5.11.4195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Revel M., Groner Y. Post-transcriptional and translational controls of gene expression in eukaryotes. Annu Rev Biochem. 1978;47:1079–1126. doi: 10.1146/annurev.bi.47.070178.005243. [DOI] [PubMed] [Google Scholar]
- Shenk T. E., Carbon J., Berg P. Construction and analysis of viable deletion mutants of simian virus 40. J Virol. 1976 May;18(2):664–671. doi: 10.1128/jvi.18.2.664-671.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villarreal L. P., White R. T., Berg P. Mutational alterations within the simian virus 40 leader segment generate altered 16S and 19S mRNA's. J Virol. 1979 Jan;29(1):209–219. doi: 10.1128/jvi.29.1.209-219.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volckaert G., Fiers W. A micromethod for base analysis of 32P-labeled oligoribonulcleotides. Anal Biochem. 1977 Nov;83(1):222–227. doi: 10.1016/0003-2697(77)90530-9. [DOI] [PubMed] [Google Scholar]
- Volckaert G., Fiers W. Micro thin-layer techniques for rapid sequence analysis of 32P-labeled RNA: double digestion and pancreatic ribonuclease analyses. Anal Biochem. 1977 Nov;83(1):228–239. doi: 10.1016/0003-2697(77)90531-0. [DOI] [PubMed] [Google Scholar]
- Wei C., Moss B. 5'-Terminal capping of RNA by guanylyltransferase from HeLa cell nuclei. Proc Natl Acad Sci U S A. 1977 Sep;74(9):3758–3761. doi: 10.1073/pnas.74.9.3758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg R. A., Ben-Ishai Z., Newbold J. E. Simian virus 40 transcription in productively infected and transformed cells. J Virol. 1974 Jun;13(6):1263–1273. doi: 10.1128/jvi.13.6.1263-1273.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winicov I., Perry R. P. Synthesis methylation, and capping of nuclear RNA by a subcellular system. Biochemistry. 1976 Nov 16;15(23):5039–5046. doi: 10.1021/bi00668a014. [DOI] [PubMed] [Google Scholar]