Abstract
The current study examined regional frontal lobe volumes based on functionally relevant subdivisions in contemporaneously recruited samples of boys and girls with and without attention-deficit/hyperactivity disorder (ADHD). Forty-four boys (21 ADHD, 23 control) and 42 girls (21 ADHD, 21 control), ages 8–13 years, participated. Sulcal–gyral landmarks were used to manually delimit functionally relevant regions within the frontal lobe: primary motor cortex, anterior cingulate, deep white matter, premotor regions [supplementary motor complex (SMC), frontal eye field, lateral premotor cortex (LPM)], and prefrontal cortex (PFC) regions [medial PFC, dorsolateral PFC (DLPFC), inferior PFC, lateral orbitofrontal cortex (OFC), and medial OFC]. Compared to sex-matched controls, boys and girls with ADHD showed reduced volumes (gray and white matter) in the left SMC. Conversely, girls (but not boys) with ADHD showed reduced gray matter volume in left LPM; while boys (but not girls) with ADHD showed reduced white matter volume in left medial PFC. Reduced left SMC gray matter volumes predicted increased go/no–go commission rate in children with ADHD. Reduced left LPM gray matter volumes predicted increased go/no–go variability, but only among girls with ADHD. Results highlight different patterns of anomalous frontal lobe development among boys and girls with ADHD beyond that detected by measuring whole lobar volumes.
Keywords: Segmentation, Premotor, Prefrontal, Supplementary Motor Complex (SMC), Pre-SMA, Gender, Sex, Childhood
INTRODUCTION
Anatomic Neuroimaging in Children With ADHD
Neuroimaging and other methods that provide insight into the association between the brain and behavior are often applied to examining the neural underpinnings of neuropsychiatric disorders. Several lines of research suggest that indices of performance from tasks assessing response control may be robust intermediate endophenotypes of attention-deficit/hyperactivity disorder (ADHD) (Lijffijt, Kenemans, Verbaten, & Van Engeland, 2005). Supporting this assertion have been a proliferation of quantitative anatomic MRI (aMRI) studies of children with ADHD (Ellison-Wright, Ellison-Wright, & Bullmore, 2008; Kelly, Margulies, & Castellanos, 2007), which have revealed abnormalities in brain regions important for response control, including frontal cortex (Kates et al., 2002; Mostofsky, Cooper, Kates, Denckla, & Kaufmann, 2002; Shaw et al., 2007), and inter-connected subcortical structures, including the caudate, putamen (Wellington, Semrud-Clikeman, Gregory, Murphy, & Lancaster, 2006), ventral striatum (Carmona et al., 2009), globus pallidus (Basser & Pierpaoli, 1996), and cerebellum (Mackie et al., 2007; Mostofsky, Reiss, Lockhart, & Denckla, 1998). At the cerebral cortical level, decreased volume of several frontal (Mostofsky et al., 2002) and non-frontal (Kelly et al., 2007) regions, suggests that abnormalities may not be localized to a specific area.
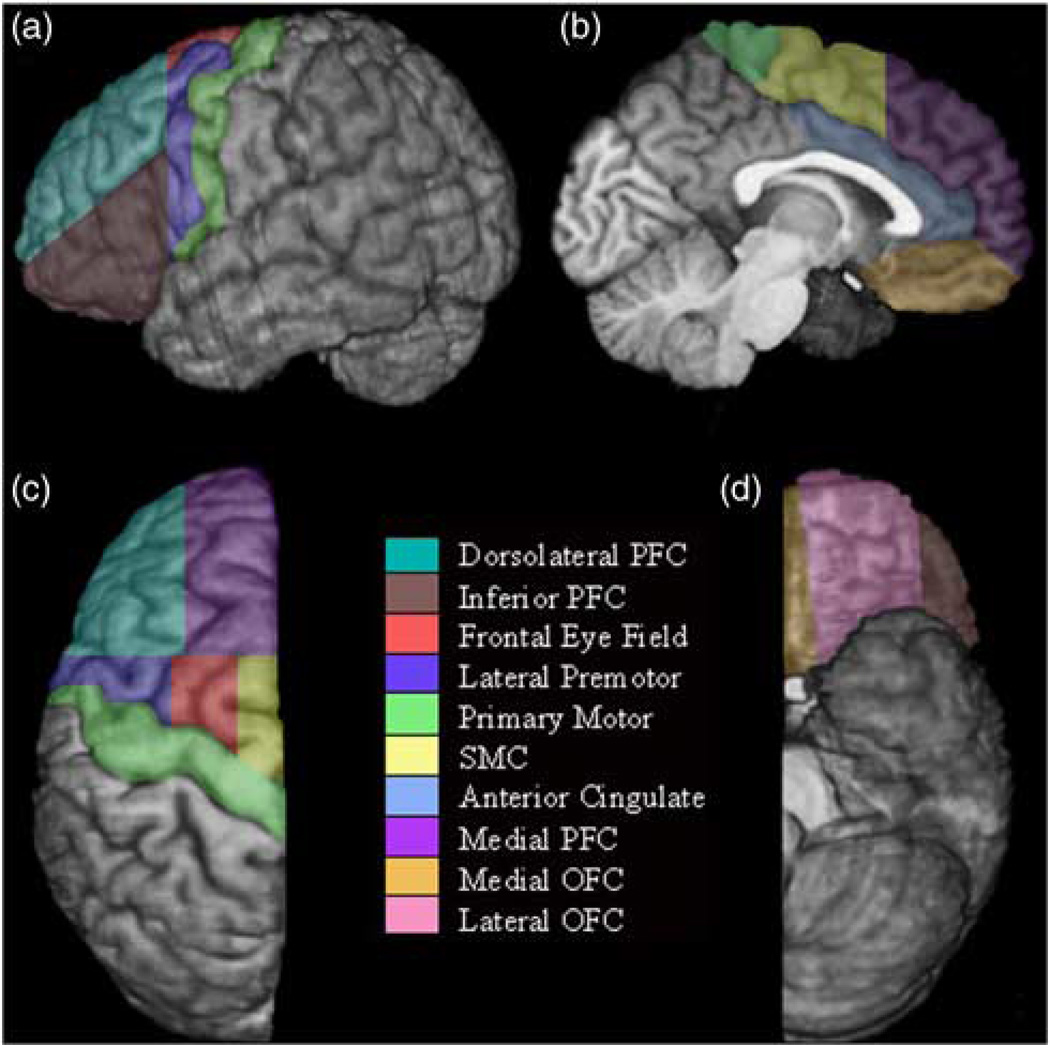
Among children with ADHD, converging evidence from aMRI studies has shown consistent reductions in total cerebral volume (3–8%) among children with ADHD, compared to typically developing (TD) children (Castellanos et al., 1996; Hill et al., 2003; Wolosin et al., 2009). Decreased regional frontal lobe volume has been a consistent finding since the early aMRI studies, with reductions observed in bilateral frontal (Fredericksen et al., 2002; Hynd, Semrud-Clikeman, Lorys, Novey, & Eliopulos, 1990), right anterior frontal (Castellanos et al., 1996), right anterior superior white matter (Filipek et al., 1997), right dorsolateral prefrontal cortex (Hill et al., 2003), premotor and prefrontal (Mostofsky et al., 2002), and left pre-frontal and deep white matter volumes (Kates et al., 2002). Conclusions about functional relevance of anomalous frontal cortex development in ADHD have been hindered, however, because the majority of studies examining regional frontal lobe volume have relied on protocols based primarily on structural landmarks (Desikan et al., 2007; Mostofsky et al., 2002). To address the latter issue, we recently expanded our larger-unit manual frontal lobe parcellation protocol (Kates et al., 2002) to delineate 11 functionally distinct regions (Ranta et al., 2009) (Figure 1) as indicated by cytoarchitectural, electro-physiological, magnetic stimulation, and functional imaging findings, and linked to circuitry outlined by Alexander and colleagues (Alexander & Crutcher, 1990).
Fig. 1.
Parcellation of the left frontal lobe on a) lateral, b) medial, c) dorsal, and d) ventral surfaces. The deep white matter region is not visible on the surface.
Regional Brain Anomalies in Boys Versus Girls With ADHD
The generalizability of published aMRI findings to girls with ADHD has been questioned because most published research on children with ADHD has been based on samples comprised primarily (or exclusively) of boys (Mahone & Wodka, 2008) and females have been underrepresented in aMRI studies of ADHD (Yang et al., 2008). A recent meta-analysis found that only 20% of participants studied were female and only 50% of the ADHD samples even included female subjects. This sampling disparity is problematic, since there is considerable published evidence of sexual dimorphism in brain development, with girls maturing earlier than boys. For example, from age 4 to 20 years, linear increases in white matter volume with age are observed in males and females, whereas age-related changes in gray matter are nonlinear, regionally specific, and different for boys and girls. In particular, frontal lobe gray matter volume increases during pre-adolescence, peaks around 10.5 years of age for boys (9.5 years for girls), and declines during post-adolescence resulting in an overall net decrease across the age span (Lenroot et al., 2007; Tiemeier et al., 2010).
When compared to female controls, frontal lobe findings among girls with ADHD have been equivocal (Castellanos et al., 2001), indicating a need for additional studies using contemporaneously collected scans among adequate samples of boys and girls with ADHD and a variety of aMRI methodologies to effectively contrast the frontal lobe anomalies of boys and girls with ADHD. Recently,Qiu et al. (2009) used large deformation diffeomorphic metric mapping to map ADHD-associated differences in basal ganglia shape. Within the striatum, boys with ADHD showed compression in several regions compared with control boys, including the left anterior and right ventral putamen, bilateral mid-body of the caudate, left dorsolateral and right ventromedial head of the caudate; however, no differences were observed in girls with ADHD compared to female controls. These identified regions are in circuit with the premotor cortex [including supplementary motor complex (SMC) and frontal eye fields (FEF)], dorsolateral prefrontal cortex (DLPFC) and anterior cingulate (AC). Also in boys, basal ganglia expansion was seen bilaterally in posterior putamen, which is in circuit with primary sensorimotor cortex (Nachev, Kennard, & Husain, 2008). The findings are consistent with a recently published ADHD study revealing delay in cortical thickening in prefrontal and premotor cortices, but earlier increased thickness in primary motor cortex (Shaw et al., 2007), and provide evidence for abnormalities in specific frontal-subcortical circuits among boys with ADHD; whereas female-specific anomalies underlying ADHD remain less clear.
Summary
These findings suggest that when studying neuroanatomic development in children with ADHD, data from girls and boys should be compared to sex-matched controls to elucidate the developmental anomalies unique to each sex, especially among earlier-maturing girls. The current study extends our previous work to examine volumes of functionally relevant frontal lobe sub-regions in a sample of contemporaneously recruited boys and girls with ADHD and TD children matched on age, sex, IQ, socioeconomic status (SES), handedness, and racial distribution. We hypothesized, based on prior research involving volumetric differences in children with ADHD, that reductions within both premotor and prefrontal regions would be observed in children with ADHD compared to controls. We further hypothesized, based on prior aMRI and developmental studies that within the age range of our sample (8–13 years), prefrontal and premotor reductions would be greater among boys than among girls with ADHD.
METHODS
Participants
Approval was granted from the Johns Hopkins Medicine Institution Review Board. A total of 86 children, ages 8–13 years, were included. Two groups were formed: TD controls (n=44) and children with ADHD (n=42). All children entering the study met the following the criteria: Full Scale IQ (FSIQ)>80 on the Wechsler Intellectual Scale for Children (WISC)–3rd edition (Wechsler, 1991) or the WISC–4 (Wechsler, 2003), no history of language, reading or neurological disorder, visual impairment, or hearing loss. Additionally, to minimize the effects of pubertal status on brain development, participants who (by parental report) had reached puberty were excluded from the sample.
Children with ADHD were recruited from outpatient programs and from local pediatricians, chapters of Children and Adults with ADHD (CHADD), schools, social organizations, and community advertisements. Diagnosis of ADHD was determined by a structured parent interview using DSM-IV criteria (Diagnostic Interview for Children and Adolescents, Fourth Edition; DICA-IV) (Reich, Welner, & Herjanic, 1997) and administration of behavior rating scales (Conners’ Parent Rating Scale-Revised—CPRS) (Conners, 1997). Children with ADHD with DSM-IV diagnoses other than Oppositional Defiant Disorder (n=13; 7 boys) and Specific Phobias (n=8; 5 boys) were excluded, as were those taking long-acting psychoactive medications other than stimulants.
Classification of ADHD subtype was made based on DICA-IV interview and rating scales. Children were classified as Inattentive subtype if they met criteria for inattentiveness but not hyperactivity/impulsivity on the DICA-IV and had a T-score >65 on the CPRS Scale L (DSM-IV Inattentive) and a T-score ≤60 on the CPRS Scale M (DSM-IV Hyperactive- Impulsive). Children were classified as Hyperactive-Impulsive subtype if they met criteria for hyperactivity/impulsivity but not inattention on the DICA-IV and had a T-score of ≥65 on the CPRS Scale M and T-score of ≤60 on CPRS Scale L. All other children who met criteria for ADHD were classified as Combined subtype. Within the ADHD group, 16 were classified as Inattentive (7 boys), 1 girl as Hyperactive-Impulsive, and 25 as Combined subtype (12 boys).
Controls were recruited through local schools and community flyers and were required to have no history of mental health services for behavior problems, no DICA-IV diagnoses, and no clinically significant elevation on the CPRS. Specific Phobias and ODD were not specifically excluded among controls; however, none met criteria for ODD, and only four (three girls) met criteria for Specific Phobia.
SES for the sample was assessed using the Hollingshead Index (Hollingshead, 1975). Scores were based on a weighted index, based on rating of parental job (score 1–9, multiplied by 5) and education (score 1–7, multiplied by 3). Scores for both parents were summed, and divided by two. When information from only one parent was available, that score was used.
Behavioral Measure of Response Control: Go/No–Go Test
Participants completed a computer-based go/no–go paradigm in which a series of red and green spaceships were presented. Each participant was instructed to use his/her dominant hand index finger to push a button immediately in response to green spaceships only. For detailed description of the paradigm, the reader is referred toRyan et al. (2010) and Wodka et al. (2007). Cues appeared on the screen for 300milliseconds (ms), and the interstimulus interval was fixed at 1500 ms. Presentation cues were weighted toward green spaceships at a ratio of 3:1 (162 “go” cues; 54 “no–go” cues), intensifying the need to inhibit a habituated motor response. Commission rate and intrasubject variability (ISV) were examined as measures of response control. ISV was calculated as [(standard deviation of response time for correct hits/mean response time for correct hits)×100].
MRI Acquisition and Measurement
High resolution T1-weighted three-dimensional magnetization prepared rapid gradient recalled echo (MPRAGE) images were acquired using a Philips 1.5 T Gyroscan NT system(MPRAGE parameters: TR=8 ms; TE=3.76 ms; flip angle=8°; matrix=256 × 256; 155 partitions; field of view=260 mm; voxel size=1×1×1.2 mm3). All image processing was conducted using the MIPAV (Medical Image Processing, Analysis and Visualization) software package from the Center for Imaging Technology of the National Institutes of Health (McAuliffe et al., 2001). Total cerebral volume was measured using Freesurfer (Fischl et al., 2004). Freesurfer used a fully automated method to perform pre-processing steps including Talairach alignment, intensity normalization, and removal of skull and non-brain tissue with a hybrid watershed/surface deformation procedure, separation of the cerebellum and brainstem from the cerebrum and splitting of the left and right hemispheres (Fischl, Liu, & Dale, 2001; Segonne et al., 2004; Segonne, Grimson, & Fischl, 2005).
Sulcal–gyral landmarks, some extended as cut planes, were used to manually delimit eleven functionally relevant regions within the frontal lobes, as indicated by cytoarchitectural, electrophysiological, magnetic stimulation, and functional imaging findings, using the protocol and methods previously published by our group (Ranta et al., 2009). To balance the competing priorities of validity and reliability, sulcal contours were used when appropriate for defining a functional area and cut planes were used where functional and sulcal divisions diverged or when the level of anatomical detail in images or degree of inter-subject variability made the use of standard anatomical landmarks excessively difficult.
Total tissue and segmented gray matter volumes for the protocol’s 10 (non-deep white matter) regions (Ranta et al., 2009) were measured in each hemisphere: primary motor cortex, anterior cingulate (AC), premotor cortex regions [supplementary motor complex (SMC), frontal eye field (FEF) and lateral premotor cortex (LPM)], and prefrontal cortex (PFC) regions [medial PFC, dorsolateral PFC (DLPFC), inferior PFC, lateral orbitofrontal cortex (OFC) and medial OFC]. White matter volumes were also obtained for these same 10 regions, plus 1 additional region: frontal deep white matter (DWM). Using the procedure outlined byKramer et al. (2007), each of these sub-regional volumes were normalized to correct for variance in overall head size by multiplying absolute sub-regional volumes by the average total cerebral volume of the analysis group and then dividing by the individual’s total cerebral volume.
The DWM region definition allowed a distinction between short association fibers (gyral white matter) and long or projecting association fibers (deep white matter). The (non-deep) white matter segmented from within each parcellated frontal lobe region was predominantly “superficial” (“radiate”) white matter (i.e., that found just below and between the gyri), as opposed to deep white matter. Superficial white matter contains the highest density of U fibers, whereas DWM contains longer fiber tracts (Makris et al., 1999). Thus, except for DWM, gray matter volume for each region was primarily (but not exclusively) cortical; whereas white matter volume was non-deep white, and otherwise determined by adjacent regions’ boundaries.
Total tissue segmentation into gray matter (GM), white matter (WM) and cerebrospinal fluid (CSF) components was carried out in MIPAV using a fuzzy segmentation algorithm (McAuliffe et al., 2001). Images were normalized to remove inhomogeneity artifacts and diffused to improve signal-to-noise ratio before segmentation. This segmentation method produced three images, one each for the GM, WM, and CSF components, with voxel intensity values ranging from zero to one, indicating the probability that an individual voxel belonged to each category. This value defined the proportion of each voxel that should be assigned to each tissue class (Ranta et al., 2009).
To determine reliability, frontal lobe isolation and parcellation components of the protocol were examined separately. For frontal lobe isolation reliability component, volumes of isolated frontal lobes of five boys with ADHD and five controls were measured. For the frontal lobe sub-parcellation component, the volumes of left and right hemisphere frontal sub-regions were measured for five boys, (two ADHD, three controls). Analyses of left and right hemisphere sub-regions were carried out independently. Raters were blind to diagnosis. A single rater (MER) completed the isolation and parcellation steps twice, using the second set of isolated frontal lobes as the starting point for both parcellation attempts. The time elapsed between the two measurements for each region was at least 2 weeks. Comparing unsegmented, absolute volume measurements for each sub-region in each hemisphere, 19 of 22 intra-rater intraclass correlation coefficients (ICC) were ≥0.9 (range, 0.778–0.997). A second rater (DC) independently isolated the frontal lobes of the same 10 boys, and results were compared with isolations by the first rater. Following this, the second rater carried out the parcellation of all frontal lobes in the five boys in the parcellation reliability group. Volumes of the parcellated regions produced by the second rater were compared with those of the first rater. Inter-rater ICC for the 22 frontal sub-regions ranged from 0.724–0.997, with all but seven measures >0.9 and all but two >0.8. Full results of the initial reliability analysis are reported inRanta et al. (2009).
Data Analyses
Data were analyzed in a sequential manner (from global to specific regions) to identify specific regions of anomalous development in children with ADHD. First, total cerebral volume and normalized left and right frontal lobe volumes (total tissue) were examined across the entire sample, using 2 (group)×2 (sex) analyses of variance (ANOVAs). Next, a 2 (hemisphere)×2 (gray vs. white matter)×2 (group)× 2 (sex) factorial multivariate ANOVA (MANOVA) was used, using the 10 sub-regions as dependent variables. To reduce the number of comparisons, univariate ANOVAs were only conducted following significant multivariate main or interaction effects (p <.05). DWM was analyzed separately, as there was no corresponding gray matter region. Finally, exploratory brain–behavior correlations (examining age, symptom severity, and response control) were used for frontal lobe sub-regions identified as anomalous among boys and girls with ADHD.
RESULTS
Demographic Information
Demographic information is listed in Table 1. The ADHD and control groups did not differ in age (p=.78), SES (p=.94), sex (χ2=0.04; p=.83), handedness (χ2=2.67; p=.26), racial distribution (χ2=3.38; p=.48), or Full Scale IQ (FSIQ) (p=.10). Within the ADHD group, there were no significant differences between boys and girls in age (p=.56), FSIQ (p=.25), SES (p=.87), handedness (χ2=2.10; p=.49), racial distribution (χ2=1.53; p=.68), or proportion of ADHD subtype (χ2=1.29; p=.53). Additionally, among those in the ADHD group, there were 24 children (10 boys) who were prescribed stimulant medications at the time of assessment; 8 children (5 boys) who had been prescribed stimulant medications in the past; and 10 children (6 boys) who were medication naïve. There were also no significant differences between boys with ADHD and male controls in age (p=.76), FSIQ (p=.49), SES (p=.99), handedness (χ2=4.02; p=.13), or racial distribution (χ2=0.88; p=.64), or between girls with ADHD and female controls in age (p=.95), FSIQ (p=.11), SES (p=.90), handedness (χ2=0.00; p=1.00), or racial distribution (χ2=3.03; p=.55).
Table 1.
Demographic and screening information
| Controls |
ADHD |
|||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Girls (n =21) |
Boys (n =23) |
Total (n =44) |
Girls (n =21) |
Boys (n =21) |
Total (n =42) |
|||||||||||||
| Mean | SD | Range | Mean | SD | Range | Mean | SD | Range | Mean | SD | Range | Mean | SD | Range | Mean | SD | Range | |
| Age | 10.3 | 1.5 | 8.2–13.2 | 10.6 | 1.3 | 8.2–13.0 | 10.5 | 1.4 | 8.2–13.2 | 10.3 | 1.3 | 8.2–12.8 | 10.5 | 1.2 | 8.7–12.6 | 10.4 | 1.3 | 8.2–12.8 |
| SES | 54.2 | 7.7 | 43–66 | 53.4 | 8.5 | 33–64 | 53.8 | 8.0 | 33–66 | 53.9 | 7.8 | 36–66 | 53.4 | 9.4 | 35–66 | 53.7 | 8.5 | 35–66 |
| FSIQ | 115.1 | 12.6 | 90–139 | 116.2 | 11.3 | 96–135 | 115.7 | 11.8 | 90–139 | 108.6 | 13.1 | 88–138 | 113.5 | 14.3 | 89–144 | 111.0 | 13.8 | 88–144 |
| Inattention | 46.3 | 5.0 | 42–59 | 43.4 | 3.1 | 40–50 | 44.7 | 4.3 | 40–59 | 80.7 | 9.5 | 63–90 | 67.7 | 6.4 | 55–77 | 73.8 | 10.3 | 55–90 |
| Hyp/Imp | 47.6 | 5.9 | 43–65 | 45.3 | 4.0 | 41–57 | 46.4 | 5.1 | 41–65 | 77.3 | 14.1 | 46–90 | 67.4 | 10.7 | 46–79 | 72.1 | 13.3 | 46–90 |
| DSM Total | 46.4 | 5.6 | 42–58 | 43.8 | 3.4 | 40–53 | 44.9 | 4.2 | 40–58 | 79.4 | 13.1 | 46–90 | 68.9 | 7.2 | 52–78 | 74.1 | 11.7 | 46–90 |
| n | % | n | % | n | % | n | % | n | % | n | % | |||||||
| Hand (R) | 19 | 90 | 19 | 90 | 38 | 86 | 19 | 90 | 21 | 0 | 40 | 95 | ||||||
| Hand (L/M) | 2 | 10 | 2 | 10 | 6 | 14 | 2 | 10 | 0 | 0 | 2 | 5 | ||||||
| Caucasian | 15 | 71 | 19 | 83 | 34 | 77 | 16 | 76 | 15 | 71 | 31 | 75 | ||||||
| AFA | 3 | 14 | 3 | 13 | 6 | 14 | 3 | 14 | 5 | 24 | 8 | 19 | ||||||
| Asian | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 5 | 0 | 0 | 1 | 2 | ||||||
| Nat. Amer. | 2 | 10 | 0 | 0 | 2 | 4.5 | 0 | 0 | 0 | 0 | 0 | 0 | ||||||
| Mixed Race | 1 | 5 | 1 | 4 | 2 | 4.5 | 1 | 5 | 1 | 5 | 2 | 4 | ||||||
| Phobia | 3 | 14 | 1 | 4 | 4 | 9 | 3 | 14 | 5 | 24 | 8 | 19 | ||||||
| ODD | 0 | 0 | 0 | 0 | 0 | 0 | 6 | 29 | 7 | 33 | 13 | 31 | ||||||
SES=Hollingshead Index; FSIQ=WISC-IV Full Scale IQ; Inattention=Conners’ Parent Rating Scale-Revised Scale L (DSM-IV Inattentive) T-Score; Hyp/Imp=Conners’ Parent Rating Scale-Revised Scale M (DSM-IV Hyperactive/Impulsive) T-Score; DSM Total=Conners’ Parent Rating Scale-Revised Scale N (DSM-IV Total) T-Score; Hand (R)=right-handed; Hand (L/M)=left- or mixed-handed; AFA=African-American; Nat. Amer.=Native American.
Total Cerebral and Frontal Lobe Volumes
For total cerebral volume, there were significant main effects for group (controls > ADHD) [F(1,82)=7.0; p=.01; η2 p=0.08)], and sex (boys > girls) [F(1,82)=30.9; p=.0003; η2 p=0.28)], but no significant group-by-sex inter-action (p=.65). Children with ADHD showed significantly reduced left [F(1,82)=10.7; p=.002; η2 p=0.116] and right [F(1,82)=15.4; p <.0001, η2 p=0.158] total frontal lobe gray matter volume, and significantly reduced left [F(1,82)=5.6; p=0.02; η2 p=0.064] and right [F(1,82)=7.6; p=.007; η2 p=0.084] total frontal lobe white matter volume. Main effects for sex (all p >.60) and group-by-sex interactions (all p >.70) were not significant for left or right frontal total gray or white matter volumes.
Analyses of Frontal Lobe Sub-regions
Factorial MANOVA yielded significant main effects (Pillai’s V) for tissue (gray > white; p <.00001; η2 p=0.98); hemisphere (right > left; p=0.0001; η2 p=0.15), and group (control > ADHD; p <.0001; η2 p=0.18), but not for sex (p=.36; η2 p=0.03). The group-by-sex interaction was also significant (p=.041; η2 p=0.06); however, none of the other two-way, three-way, or four-way interactions were significant. For DWM (analyzed separately), boys had significantly larger volumes than girls (p=.0001; η2 p=0.24); however, the effects for group, hemisphere, and the two-way and three-way interactions were not significant.
For frontal sub-region tissue, there were significant univariate differences (gray > white) in all 10 regions (all p <.001). In contrast, univariate analyses of regional volumes yielded significant hemispheric differences (collapsing data across groups, sex, and tissue type) in only two regions: AC (left > right; p=.01; η2 p=0.02) and medial OFC (right > left; p <.001; η2 p=0.08).
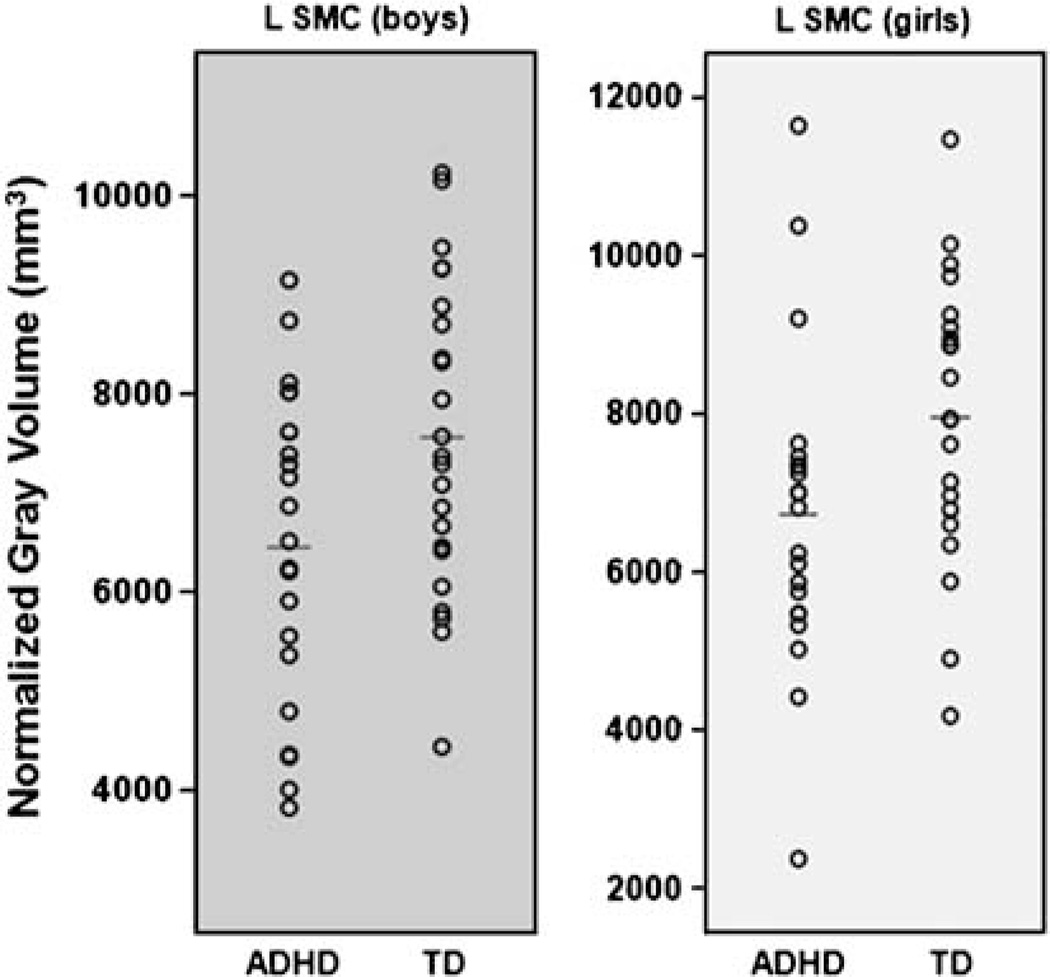
Further examination of the group differences revealed that children with ADHD showed significantly reduced volumes (collapsing data across hemispheres, sex, and tissue) compared to controls in 5 of 10 regions: AC (p=.033; η2 p=0.014), DLPFC (p=.029; η2 p=0.015), lateral OFC (p=.047; η2 p=0.012), medial PFC (p=.002; η2 p=0.028), and SMC (p=.0001; η2 p=0.054); however, in only two regions (medial PFC, SMC) were significant group differences observed when tissue types were examined separately (collapsing across sex and hemisphere)—medial PFC: gray (p=.046; η2 p=0.024), white (p=.005; η2 p=0.047); SMC: gray (p=.003; η2 p=0.052), white (p=.002; η2 p=0.058). Within these two regions, group differences were observed in the left hemisphere (collapsing across sex) for SMC: gray (p=.003; η2 p=0.105) and white matter (p <.001; η2 p=0.148), and medial PFC: white matter (p=.008; η2 p=0.082) although the group differences were not significant for these regions in the right hemisphere for gray or white matter. Within left SMC, there were significant reductions in gray matter for girls (p=.043; η2 p=0.099) and boys with ADHD (p=.025; η2 p=0.115), compared to sex-matched controls (Figure 2), and in white matter for girls (p=.047; η2 p=0.095) and boys with ADHD (p=.001; η2 p=0.216), compared to sex-matched controls. Within left medial PFC white matter, reductions were observed for boys with ADHD, compared to sex-matched controls (p=.014; η2 p=0.135), but not for girls with ADHD (p=.234; η2 p=0.035).
Fig. 2.
Scatterplots revealing significant reductions in supplemental motor complex (SMC) gray matter volume in both boys (left) and girls (right) with attention-deficit/hyperactivity disorder (ADHD) compared to typically developing (TD) children.
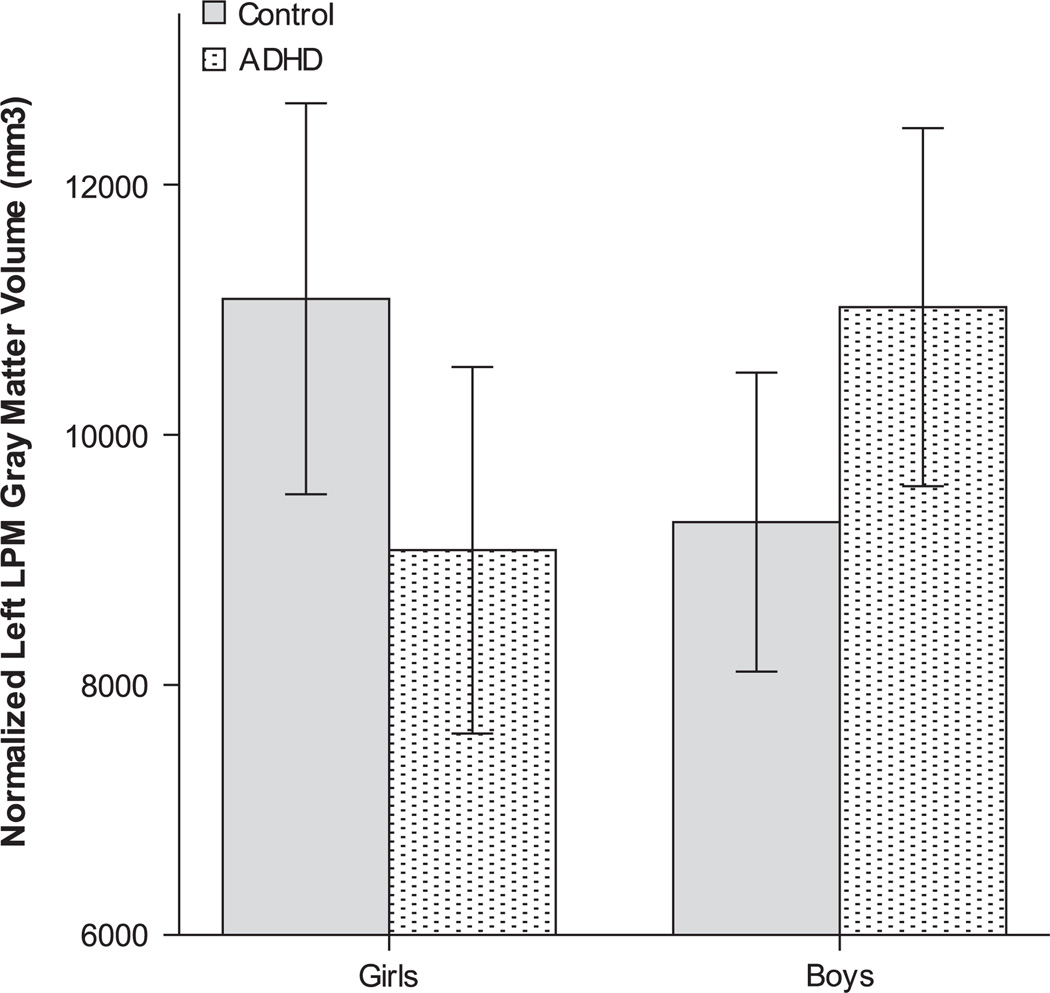
Univariate analyses of the significant multivariate group-by-sex interactions (collapsing data across hemispheres and tissue) yielded significant differences in two regions: LPM (p=.001; η2 p=0.034), and primary motor cortex (p=.005; η2 p=0.024). To explore the significant group-by-sex interactions, sex-specific univariate analyses were used to examine group differences in frontal lobe regions (collapsing across hemispheres and tissue). For LPM, girls with ADHD had significantly reduced volume, compared to female controls (p=.037; η2 p=0.026), while no significant differences were observed between boys with ADHD and male controls (p=.148; η2 p=0.012). Conversely, for primary motor cortex, no significant differences were observed between girls with ADHD and female controls (p=.084; η2 p=0.018), or between boys with ADHD and male controls (p=.38; η2 p=0.004). As follow-up to the significant reduction in LPM volume among girls with ADHD, univariate group comparisons were made among girls separately for gray matter and white matter, and then separately for each hemisphere. Compared to female controls, girls with ADHD showed reduced LPM gray matter (collapsing across hemispheres) [F(1,82)=5.0; p=.03; η2 p=0.057], but not white matter volumes [F(1,82)=2.6; p=.11; η2 p=0.031]. Within LPM gray matter, girls with ADHD showed reduced volumes compared to female controls for left LPM gray matter [F(1,40)=3.8; p=.05; η2 p=0.087], but not right LPM gray matter [F(1,40)=1.3; p=.27; η2 p=0.031] (Figure 3).
Fig. 3.
Significant group-by-sex interaction effect for left lateral premotor cortex (LPM) gray matter volume, corrected for total cerebral volume [F(1,84)=7.56; p=.007; η2 p=0.084].
In summary, the sequential analyses yielded six regions with significant sex-specific ADHD-related reductions: 1–4) left SMC gray and white matter (boys and girls); 5) left medial PFC white matter (boys); and 6) left LPM gray matter (girls). Of note, all these regions of interest were ones in which intra-rater and inter-rater measurement reliability (ICC) from the initial reliability analysis (Ranta et al., 2009) was greater than 0.90. Correlational analyses (below) were conducted only on these regions. Because left SMC volumes reductions were observed in boys and girls with ADHD, correlations across both sexes for left SMC gray and white matter were examined first, followed by sex-specific analyses in cases of significant combined sex associations. Conversely, correlations with left medial PFC white matter were only examined among boys; and correlations with left LPM gray matter were only examined among girls.
Frontal Lobe Correlations With Age
Across groups and sex, left SMC gray matter (r=−0.29; p=.027), but not white matter volumes (r=−0.12; p=.134) were associated with age. Left SMC gray matter volumes were significantly associated with age among controls (r=−0.51; p=.001), but not among children with ADHD (r=0.60; p=.354). Within controls, left SMC and age were significantly correlated in boys (r=−0.74; p=.001), but not girls (r=−0.30; p=.09).
Among girls, age was not significantly correlated with left SMC gray matter (r=−0.117; p=.459) or left LPM gray matter (r=−0.080; p=.614). Among boys, age was significantly correlated with left SMC gray matter only in controls (r=−0.737; p <.0001). Of note, among boys, the group difference (ADHD < control) in left SMC gray matter remained after covarying for age [F(1,41)=6.60; p=0.014; η2 p=.139]. Age was not significantly associated with left medial PFC white matter (r=0.168; p= =.277) among boys.
Frontal Lobe Regions and Associations With ADHD Symptoms
Across diagnostic groups and sex, left SMC gray matter (r=−0.256; p=.011) and white matter (r=−0.302; p=.003) volumes were significantly associated with ADHD symptom severity (CPRS-R Scale N: DSM-IV Total T-score); however, the associations were not significant in either of these region-tissue type combinations when examining diagnostic groups separately. Among girls, the association between left LPM gray matter volume and ADHD symptoms in girls was significant across groups (r=−0.292; p=.038), but not within either the ADHD or control group alone. Among boys, across groups, the associations between left SMC gray matter (r=−0.369; p=.008) and left SMC white matter (r=−0.486; p=.0005) were significantly associated with ADHD symptoms; however, these associations were not significant when ADHD and control groups were analyzed separately. The association between left medial PFC white matter and ADHD symptoms was significant only within control boys (r=0.353; p=.049).
Frontal Lobe Regions and Associations With Response Control
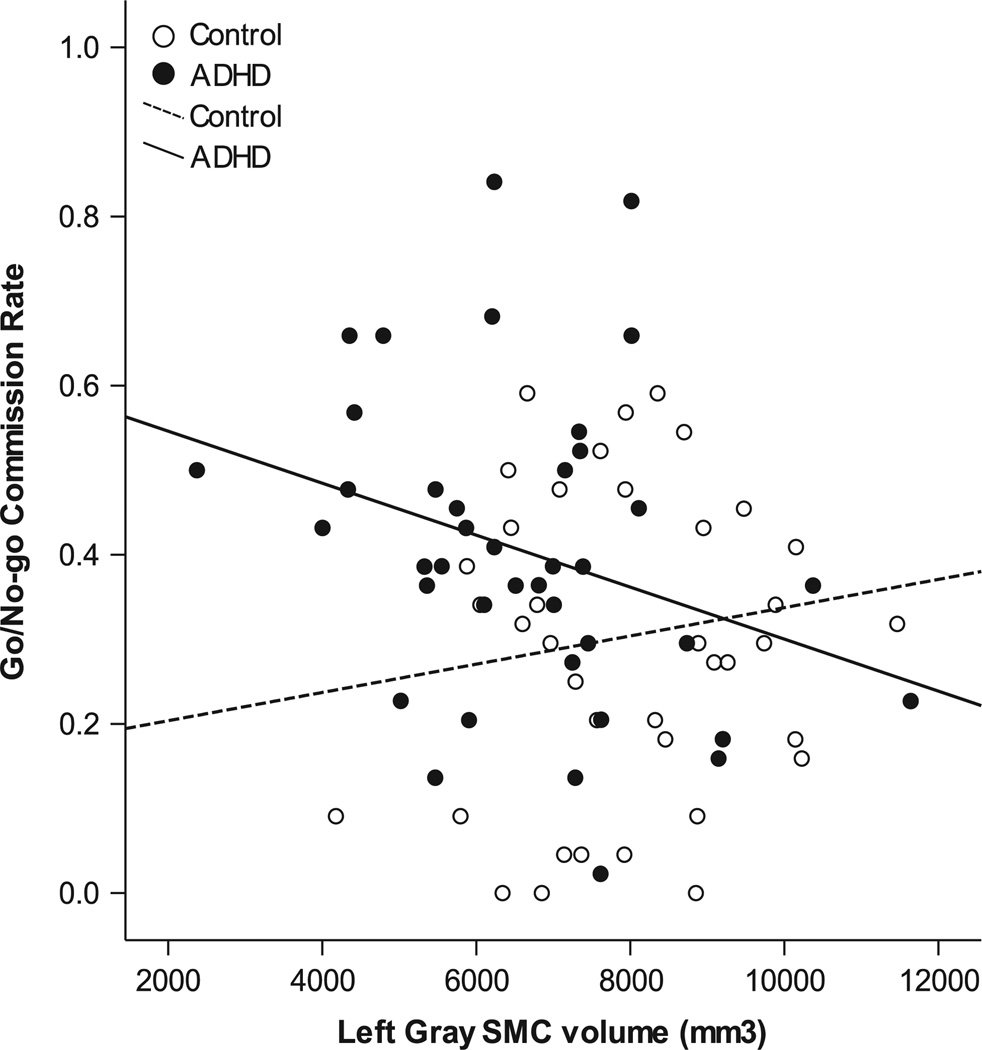
The relationships among the specific region-sex group combinations identified as anomalous among children with ADHD and performance on a go/no–go test were also examined. Across diagnostic groups and sex, left SMC gray matter (r=−0.183; p=.05) was significantly associated with commission rate. This association was also observed within children with ADHD (r=−0.30; p=.031), but not in controls (r=0.13; p=.210) (Figure 4). Conversely, left SMC white matter (r=−0.115; p=.16) was not associated with commission rate, and neither left SMC gray (r=−0.070; p=.269), nor left SMC white matter (r=0.05; p=.331) were significantly associated with go/no–go ISV.
Fig. 4.
Significant association between left SMC gray matter volume (boys and girls combined) and go/no–go commission rate (r2=0.09; p=.03) among children with ADHD, but not among controls r2=0.008; p=.21).
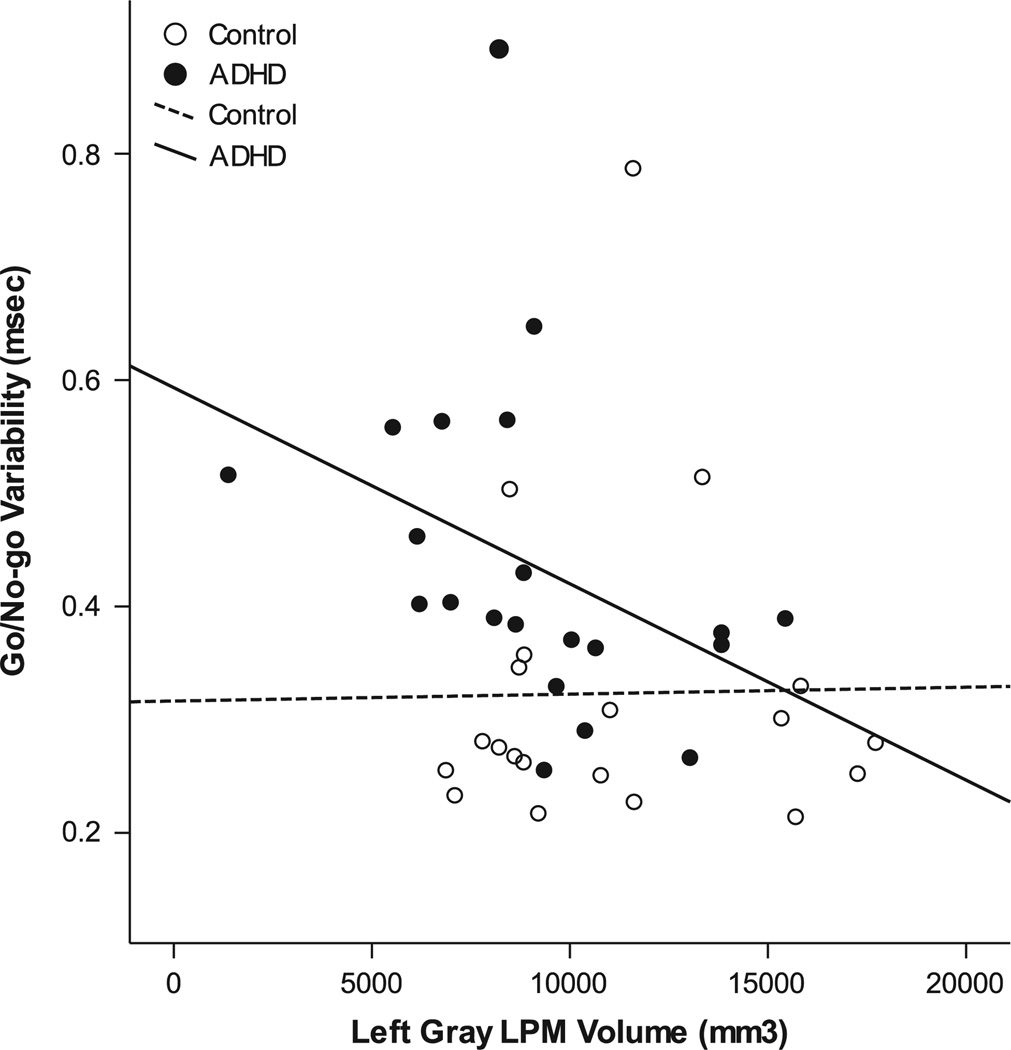
Among girls, across groups, neither left SMC gray matter (r=−0.153; p=.171), nor left LPM volumes (r=−0.07; p=.338) were associated with go/no–go commission rate. Conversely, across groups, left LPM gray matter volume was significantly associated with ISV (r=−0.29; p=.035). Within girls with ADHD, left LPM gray matter volume remained significantly associated with ISV, such that reduced left LPM volume was associated with greater variability (r=−0.41; p=.033); in contrast, the relationship between left LPM gray matter volume and ISV among female controls was not significant (r=−0.02; p=.474; Figure 5).
Fig. 5.
Normalized left lateral premotor cortex (LPM) volume predicts performance on a go/no-test among girls with ADHD (r2=0.17), but not among female controls (r2<0.001).
Among boys (groups combined), left SMC gray matter was not significantly correlated with commission rate (r=−0.21; p=.102), and left medial PFC white matter volumes were not significantly correlated with either commissions (r=−0.13; p=.212) or ISV (r=−0.17; p=.156).
DISCUSSION
New comprehensive frontal parcellation methods, such as the one used in the present study, provide important data about the anatomy of functionally relevant regions in comparison with more gyral–sulcal methods applied in the past (Kelly et al., 2007). Additionally, studying samples of boys and girls with ADHD simultaneously allows for comparison of the ADHD-related frontal lobe anomalies common to (and unique to) each sex. The present results highlight anomalous frontal lobe development among boys and girls with ADHD beyond that detected by measuring whole lobar volumes. By measuring functionally relevant frontal lobe sub-divisions, we identified ADHD-related reductions in multiple frontal lobe regions and identified those regions in which ADHD-related anomalies appear to differ by sex (medial prefrontal and lateral premotor cortex).
In this age range (8–13 years) the left SMC emerged as the most anomalous frontal lobe region (based on effect size) in children with ADHD, with reductions observed in both boys and girls, in both gray and white matter. Furthermore, reduced left SMC gray matter volume was significantly associated with increased commission rate on a go/no–go test among children with ADHD. This pattern of reduced SMC volume, and its link with impaired response control, suggests that abnormal development of circuits involving the SMC may be critical to the pathophysiology of ADHD. The SMC (in particular the rostral portion or “pre-SMA”) is considered to be critical to response control and selection, including consciously selecting to withhold (i.e., inhibit) responses (Mostofsky & Simmonds, 2008). Thus, it follows that abnormal SMC development may underlie impaired inhibitory control (Wodka et al., 2007), which has been identified as a core impairment in ADHD. Additionally, the basal ganglia, which serve as the nexus through which prefrontal, premotor and motor signals inhibit competing motor programs and disinhibit intended behaviors (Mink, 1996; Nachev et al., 2008),may also be involved in this process, since the number of cells that project from the basal ganglia to the SMC (reduced in boys and girls with ADHD) is 3–4 times the number that project from the cerebellum to the SMC, unlike the pattern for other cortical motor areas (Akkal, Dum, & Strick, 2007).
While boys and girls with ADHD showed similar patterns of reduced SMC volumes relative to controls, there were also sex-specific anomalies identified in left LPM (among girls) and left medial PFC (among boys). The finding of decreased left medial PFC white matter volumes only in boys with ADHD highlights dysfunction in circuits important for executive control of behavior. Since ADHD in both sexes is associated with reduced SMC volume, but persistent motor disinhibition (i.e., motor overflow) in this age range is observed primarily in boys (Cole, Mostofsky, Larson, Denckla, & Mahone, 2008; Macneil et al., 2011), the finding of spared prefrontal cortex suggests that girls with ADHD may be better able to recruit prefrontal regions for “top-down” control of behavior, including the inhibition of hyperactivity and more precise motor control; although, given the atypical development of the lateral premotor cortex and SMC among girls with ADHD, this recruitment may contribute to the emergence of more “inattentive” symptoms (including deficits associated with maintenance of response control) in girls by draining resources away from the cognitive tasks. The observed association between left lateral premotor cortex volume and go/no–go variability among girls with ADHD appears to support this hypothesis.
Quantitative aMRI techniques can also be used to examine mechanisms of effective compensation in childhood disorders. Cortical development progresses in a region-specific manner coinciding with functional maturation; regions that subserve primary sensorimotor functions mature earliest and higher-order association areas much later. Within the frontal lobe, the primary motor region matures earliest; after this, there is an anterior progression with premotor regions maturing next followed by prefrontal regions (DLPFC, medial PFC and orbitofrontal regions) maturing last in late adolescence/early adulthood (Gogtay et al., 2004). In ADHD, symptoms of impulsivity and hyperactivity tend to diminish with age in a manner parallel with frontal lobe maturation (Biederman, Mick, & Faraone, 2000). This pattern may be because by late elementary school age, the prefrontal cortex is thought to modulate activity in subcortical structures (Miller & D’Esposito, 2005), including limbic areas, giving rise to the ability to engage in inhibitory (i.e., top-down) control over behavior (Marsh, Gerber, & Peterson, 2008). Considering these patterns, some researchers have hypothesized that the prefrontal cortex and its interconnections may be primarily involved in the compensation for (and recovery from) ADHD, rather than in the primary cause of the disorder (Halperin & Schulz, 2006). The early childhood onset of ADHD would therefore appear to be inconsistent with PFC pathology and, in fact, early prefrontal lesions present a clinical picture very different from ADHD, with late childhood onset and worsening through adolescence (Anderson, Bechara, Damasio, Tranel, & Damasio, 1999). The observed patterns of brain development suggest that a core element in the pathophysiology of ADHD in boys and girls may involve abnormalities in earlier-maturing premotor/basal ganglia systems that subserve motor response control, with anomalous SMC development playing a key role. Given our present findings, alterations in the prefrontal development may also play a role in the pathogenesis and developmental trajectory of ADHD (Rubia et al., 2000; Shaw, Lalonde et al., 2009).
The different rates of development for boys and girls may affect group comparisons involving children with and without ADHD, especially in the pre-adolescent years. We addressed this potential confound by directly examining within-sex group differences in regional brain volumes. Sex-specific analyses are particularly important for examining gray matter differences, since gray matter volumes may plateau and decrease (especially in girls) during the age range of our sample (8–13 years), while white matter volumes are thought to increase throughout this age range for both boys and girls. The current findings, considered in light of prior studies, suggest that when studying disorders associated with anomalous regional brain development, differences in brain development and its timing, relative to one’s own sex, should be considered (Cahill, 2006).
Unique aspects of the current study design include the strict diagnostic procedures for ADHD (i.e., the exclusion of most comorbid conditions that could confound the interpretation of results), the over-sampling for girls with ADHD, and the matching of boys and girls with ADHD on subtype. While these procedures produce ADHD samples that are more “pure” diagnostically, they also tend to be associated with groups with slightly higher than average IQ. As such, the present findings may be less applicable to children with a wider range of comorbidities. Additionally, the study did not address the impact of length of stimulant medication treatment on frontal lobe development, and is a limitation, given the growing evidence suggesting more rapid “normalization” of volumes in those treated with stimulants (Shaw, Sharp et al., 2009). An additional limitation is the large number of variables obtained via the parcellation analysis and the use of a more liberal p <.05 significance level. Data analyses were similar to those from prior neuroimaging studies (Mostofsky et al., 2002; Wolosin et al., 2009), specifically designed to be sequential, beginning with an omnibus factorial MANOVA with additional analyses conducted only after significant multivariate effects. Future studies are indicated to replicate these findings with larger samples.
Given these considerations, future research should also continue to explore sex differences in brain–behavior relationships, emphasizing sexually dimorphic patterns of regional frontal lobe anomalies identified here. Given sex differences in brain maturation (Lenroot et al., 2007), it may be necessary to study children, in particular girls, with ADHD at younger ages to determine how these patterns of anomalous development (brain and behavior) emerge, how their trajectory affects later presentation (symptoms, subtype), and how these differences might be ameliorated with treatment. It will also be important to study regional brain development in children with ADHD beyond adolescence to examine the impact of post-pubertal growth patterns in boys and girls, and the relationship with progression of behavioral symptoms. The relationship between structural abnormality and dysfunction is complex. While structural abnormalities are almost invariably linked to functional abnormalities, the absence of structural abnormalities cannot be assumed to exclude dysfunction.
Supplementary Material
ACKNOWLEDGMENTS
Portions of this manuscript were presented at the 38th annual North American meeting of the International Neuropsychological Society in Atlanta, Georgia on February 13, 2009 and at the meeting for the Organization for Human Brain Mapping in San Francisco, CA (June 21, 2009). Supported by National Institutes of Health Grants R01 NS43480, R01 NS48527, R01 MH85328, R01 NSMH078160, P30, HD24061, and the Johns Hopkins Institute for Clinical and Translational Research, an NIH/NCRR CTSA Program, UL1 RR025005.
Footnotes
All authors report no competing interests.
Supplementary Materials
To review these additional data and analyses, please access the online-only supplementary Tables 1 and 2. Please visit journals.cambridge.org/INS, then click on the link “Supplementary Materials” at this article.
REFERENCES
- Akkal D, Dum RP, Strick PL. Supplementary motor area and presupplementary motor area: Targets of basal ganglia and cerebellar output. Journal of Neuroscience. 2007;27:10659–10673. doi: 10.1523/JNEUROSCI.3134-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander GE, Crutcher MD. Functional architecture of basal ganglia circuits: Neural substrates of parallel processing. Trends in Neuroscience. 1990;13:266–271. doi: 10.1016/0166-2236(90)90107-l. [DOI] [PubMed] [Google Scholar]
- Anderson SW, Bechara A, Damasio H, Tranel D, Damasio AR. Impairment of social and moral behavior related to early damage in human prefrontal cortex. Nature Neuroscience. 1999;2:1032–1037. doi: 10.1038/14833. [DOI] [PubMed] [Google Scholar]
- Basser PJ, Pierpaoli C. Microstructural and physiological features of tissues elucidated by quantitative-diffusion-tensor MRI. Journal of Magnetic Resonance B. 1996;111:209–219. doi: 10.1006/jmrb.1996.0086. [DOI] [PubMed] [Google Scholar]
- Biederman J, Mick E, Faraone SV. Age-dependent decline of symptoms of attention deficit hyperactivity disorder: Impact of remission definition and symptom type. American Journal of Psychiatry. 2000;157:816–818. doi: 10.1176/appi.ajp.157.5.816. [DOI] [PubMed] [Google Scholar]
- Cahill L. Why sex matters for neuroscience. Nature Reviews Neuroscience. 2006;7:477–484. doi: 10.1038/nrn1909. [DOI] [PubMed] [Google Scholar]
- Carmona S, Proal E, Haekzema EA, Gispert JD, Picado M, Moreno I, Vilarroya O. Ventro-striatal reductions underpin symptoms of hyperactivity and impulsivity in attention-deficit/hyperactivity disorder. Biological Psychiatry. 2009;66:972–977. doi: 10.1016/j.biopsych.2009.05.013. [DOI] [PubMed] [Google Scholar]
- Castellanos FX, Giedd JN, Berquin PC, Walter JM, Sharp W, Tran T, Rapoport JL. Quantitative brain magnetic resonance imaging in girls with attention-deficit/ hyperactivity disorder. Archives of General Psychiatry. 2001;58:289–295. doi: 10.1001/archpsyc.58.3.289. [DOI] [PubMed] [Google Scholar]
- Castellanos FX, Geidd JN, Marsh WL, Hamburger SD, Vaituzis AC, Dickstein DP, Rapoport JL. Quantitative brain magnetic resonance imaging in attention-deficit hyperactivity disorder. Archives of General Psychiatry. 1996;53:607–616. doi: 10.1001/archpsyc.1996.01830070053009. [DOI] [PubMed] [Google Scholar]
- Cole W, Mostofsky S, Larson J, Denckla M, Mahone EM. Age-related changes in motor subtle signs among girls and boys with ADHD. Neurology. 2008;71:1514–1520. doi: 10.1212/01.wnl.0000334275.57734.5f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conners CK. Conners’ Rating Scales - Revised. North Tonawanda, New York: Multi-Health Systems Inc.; 1997. [Google Scholar]
- Desikan RS, Segonne F, Fischl B, Quin BT, Dickerson BC, Blacker D, Killiany RJ. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage. 2007;31:968–980. doi: 10.1016/j.neuroimage.2006.01.021. [DOI] [PubMed] [Google Scholar]
- Ellison-Wright I, Ellison-Wright Z, Bullmore E. Structural brain change in attention deficit hyperactivity disorder identified by meta-analysis. BMC Psychiatry. 2008;8:51. doi: 10.1186/1471-244X-8-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filipek PA, Semrud-Clikeman M, Steingard RJ, Renshaw PF, Kennedy DN, Biederman J. Volumetric MRI analysis comparing subjects having attention-deficit hyperactivity disorder with normal controls. Neurology. 1997;48:589–601. doi: 10.1212/wnl.48.3.589. [DOI] [PubMed] [Google Scholar]
- Fischl B, Liu A, Dale AM. Automated manifold surgery: Constructing geometrically accurate and topologically correct models of the human cerebral cortex. IEEE Transactions on Medical Imaging. 2001;20:70–80. doi: 10.1109/42.906426. [DOI] [PubMed] [Google Scholar]
- Fischl B, van der Kouwe A, Destrieux C, Halgren E, Segonne F, Salat DH, Dale AM. Automatically parcellating the human cerebral cortex. Cerebral Cortex. 2004;14:11–22. doi: 10.1093/cercor/bhg087. [DOI] [PubMed] [Google Scholar]
- Fredericksen KA, Cutting LE, Kates WR, Mostofsky SH, Cooper KL, Lanham DC, Kaufman WE. Disproportionate increases of white matter in right frontal lobe in Tourette syndrome. Neurology. 2002;58:85–89. doi: 10.1212/wnl.58.1.85. [DOI] [PubMed] [Google Scholar]
- Gogtay N, Gied JN, Lusk L, Hayashi KM, Greenstein D, Vaituzis AC, Thompson PM. Dynamic mapping of human cortical development during childhood through early adulthood. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:8174–8179. doi: 10.1073/pnas.0402680101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halperin JM, Schulz KP. Revisiting the role of the prefrontal cortex in attention-deficit/hyperactivity disorder. Psychological Bulletin. 2006;132:560–581. doi: 10.1037/0033-2909.132.4.560. [DOI] [PubMed] [Google Scholar]
- Hill D, Yeo R, Campbell R, Hart B, Vigil J, Brooks W. Magnetic resonance imaging correlates of attention-deficit/hyperactivity disorder in children. Neuropsychology. 2003;17:496–506. doi: 10.1037/0894-4105.17.3.496. [DOI] [PubMed] [Google Scholar]
- Hollingshead AB. Four factor index of social status. New Haven, CT: Yale University, Department of Sociology; 1975. [Google Scholar]
- Hynd GW, Semrud-Clikeman M, Lorys A, Novey ES, Eliopulos D. Brain morphology in developmental dyslexia and attention deficit disorder/hyperactivity. Archives of Neurology. 1990;47:919–926. doi: 10.1001/archneur.1990.00530080107018. [DOI] [PubMed] [Google Scholar]
- Kates WR, Frederikse M, Mostofsky SH, Folley BS, Cooper K, Mazur-Hopkins P, Kaufman WE. MRI parcellation of the frontal lobe in boys with attention deficit hyperactivity disorder or Tourette syndrome. Psychiatry Research. 2002;116:63–81. doi: 10.1016/s0925-4927(02)00066-5. [DOI] [PubMed] [Google Scholar]
- Kelly AM, Margulies DS, Castellanos FX. Recent advances in structural and functional brain imaging studies of attention-deficit/hyperactivity disorder. Current Psychiatry Reports. 2007;9:401–407. doi: 10.1007/s11920-007-0052-4. [DOI] [PubMed] [Google Scholar]
- Kramer JH, Quitania L, Dean D, Neuhaus J, Rosen HJ, Halabi C, Miller BL. Magnetic resonance imaging correlates of set shifting. Journal of the International Neuropsychological Society. 2007;13:386–392. doi: 10.1017/S1355617707070567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenroot RK, Gogtay N, Greenstein DK, Wells EM, Wallace GL, Clasen LS, Gied JN. Sexual dimorphism of brain developmental trajectories during childhood and adolescence. Neuroimage. 2007;36:1065–1073. doi: 10.1016/j.neuroimage.2007.03.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lijffijt M, Kenemans JL, Verbaten MN, Van Engeland H. A meta-analytic review of stopping performance in attention-deficit/hyperactivity disorder: Deficient inhibitory motor control? Journal Abnormal Psychology. 2005;114:216–222. doi: 10.1037/0021-843X.114.2.216. [DOI] [PubMed] [Google Scholar]
- Mackie S, Shaw P, Lenroot R, Pierson R, Greenstein DK, Nugent TF, Rapoport JL. Cerebellar development and clinical outcome in attention deficit hyperactivity disorder. American Journal of Psychiatry. 2007;164:647–655. doi: 10.1176/ajp.2007.164.4.647. [DOI] [PubMed] [Google Scholar]
- Macneil LK, Xavier P, Garvey MA, Gilbert DL, Ranta ME, Denckla MB, Mostofksy SH. Quantifying excessive mirror overflow in children with attention-deficit/hyperactivity disorder. Neurology. 2011;76:622–628. doi: 10.1212/WNL.0b013e31820c3052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahone EM, Wodka EL. The neurobiological profile of girls with ADHD. Developmental Disabilities Research Reviews. 2008;14:276–284. doi: 10.1002/ddrr.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makris N, Meyer JW, Bates JF, Yeterian EH, Kennedy DN, Caviness VS. MRI based topographic parcellation of human cerebral white matter and nuclei II. Rationale and applications with systematics of cerebral connectivity. Neuroimage. 1999;9:18–45. doi: 10.1006/nimg.1998.0384. [DOI] [PubMed] [Google Scholar]
- Marsh R, Gerber AJ, Peterson BS. Neuroimaging studies of normal brain development and their relevance for understanding childhood neuropsychiatric disorders. Journal of the American Academy of Child and Adolescent Psychiatry. 2008;47:1233–1251. doi: 10.1097/CHI.0b013e318185e703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAuliffe M, Lalonde E, McGarry D, Gandler W, Csaky K, Trus B. Medical image processing, analysis and visualization in clinical research; Paper presented at the IEEE Symposium on computer-based medical systems; 2001. [Google Scholar]
- Miller BT, D’Esposito M. Searching for “the top” in top-down control. Neuron. 2005;48:535–538. doi: 10.1016/j.neuron.2005.11.002. [DOI] [PubMed] [Google Scholar]
- Mink JW. The basal ganglia: Focused selection and inhibition of competing motor programs. Progress in Neurobiology. 1996;50:381–425. doi: 10.1016/s0301-0082(96)00042-1. [DOI] [PubMed] [Google Scholar]
- Mostofsky S, Cooper K, Kates W, Denckla M, Kaufman W. Smaller prefrontal and premotor volumes in boys with ADHD. Biological Psychiatry. 2002;52:785–794. doi: 10.1016/s0006-3223(02)01412-9. [DOI] [PubMed] [Google Scholar]
- Mostofsky SH, Reis AL, Lockhart P, Denckla MB. Evaluation of cerebellar size in attention deficit hyperactivity disorder. Journal of Child Neurology. 1998;13:434–439. doi: 10.1177/088307389801300904. [DOI] [PubMed] [Google Scholar]
- Mostofsky SH, Simmonds DJ. Response inhibition and response selection: Two sides of the same coin. Journal of Cognitive Neuroscience. 2008;20:751–761. doi: 10.1162/jocn.2008.20500. [DOI] [PubMed] [Google Scholar]
- Nachev P, Kennard C, Husain M. Functional role of the supplementary and pre-supplementary motor areas. Nature Reviews Neuroscience. 2008;9:856–869. doi: 10.1038/nrn2478. [DOI] [PubMed] [Google Scholar]
- Qiu A, Crocetti D, Adler A, Mahone EM, Denckla MB, Miller MI, Mostofsky SH. Basal ganglia volume and shape in children with attention deficit hyperactivity disorder. American Journal of Psychiatry. 2009;166:74–82. doi: 10.1176/appi.ajp.2008.08030426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranta ME, Crocetti D, Claus JA, Kraut MA, Mostofsky SH, Kaufman WE. Manual MRI parcellation of the frontal lobe. Psychiatry Research. 2009;172(2):147–154. doi: 10.1016/j.pscychresns.2009.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reich W, Welner Z, Herjanic B. The diagnostic interview for children and adolescents-IV. North Tonawanda: Multi-Health Systems; 1997. [Google Scholar]
- Rubia K, Overmeyer S, Taylor E, Brammer M, Williams SC, Simmons A, Bullmore ET. Functional frontalisation with age: Mapping neurodevelopmental trajectories with fMRI. Neuroscience and Biobehavioral Reviews. 2000;24:13–19. doi: 10.1016/s0149-7634(99)00055-x. [DOI] [PubMed] [Google Scholar]
- Ryan M, Martin RB, Denckla MB, Mostofsky SH, Mahone EM. Interstimulus jitter facilitates response control in children with ADHD. Journal of the International Neuropsychological Society. 2010;16:388–393. doi: 10.1017/S1355617709991305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segonne F, Dale AM, Busa E, Glessner M, Salat D, Hahn HK, et al. A hybrid approach to the skull stripping problem in MRI. Neuroimage. 2004;22:1060–1075. doi: 10.1016/j.neuroimage.2004.03.032. [DOI] [PubMed] [Google Scholar]
- Segonne F, Grimson E, Fischl B. A genetic algorithm for the topology correction of cortical surfaces. Information Processing in Medical Imaging. 2005;19:393–405. doi: 10.1007/11505730_33. [DOI] [PubMed] [Google Scholar]
- Shaw P, Eckstrand K, Sharp W, Blumenthal J, Lerch JP, Greenstein D, Rapoport JL. Attention-deficit/ hyperactivity disorder is characterized by a delay in cortical maturation. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:19649–19654. doi: 10.1073/pnas.0707741104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw P, Lalonde F, Lepage C, Rabin C, Eckstrand K, Sharp W, Raporport J. Development of cortical asymmetry in typically developing children and its disruption in attention-deficit/ hyperactivity disorder. Archives of General Psychiatry. 2009;66:888–896. doi: 10.1001/archgenpsychiatry.2009.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw P, Sharp WS, Morrison M, Eckstrand K, Greenstein DK, Classen LS, Rapaport JL. Psychostimulant treatment and the developing cortex in attention deficit hyperactivity disorder. American Journal of Psychiatry. 2009;166:58–63. doi: 10.1176/appi.ajp.2008.08050781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiemeier H, Lenroot RK, Greenstein DK, Tran L, Pierson R, Gied JN. Cerebellum development during childhood and adolescence: A longitudinal morphometric MRI study. Neuroimage. 2010;49:63–70. doi: 10.1016/j.neuroimage.2009.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler DL. Wechsler intelligence scale for children, third edition. San Antonio, TX: The Psychological Corporation; 1991. [Google Scholar]
- Wechsler DL. Wechsler intelligence scale for children, fourth edition. San Antonio, TX: The Psychological Corporation; 2003. [Google Scholar]
- Wellington TM, Semrud-Clikeman M, Gregory AL, Murphy JM, Lancaster JL. Magnetic resonance imaging volumetric analysis of the putamen in children with ADHD: Combined type versus control. Journal of Attention Disorders. 2006;10:171–180. doi: 10.1177/1087054705284242. [DOI] [PubMed] [Google Scholar]
- Wodka EL, Mahone EM, Blankner JG, Larson JC, Fotedar S, Denckla MB, Mostofsky SH. Evidence that response inhibition is a primary deficit in ADHD. Journal of Clinical and Experimental Neuropsychology. 2007;29:345–356. doi: 10.1080/13803390600678046. [DOI] [PubMed] [Google Scholar]
- Wolosin SM, Richardson ME, Hennessey JG, Denckla MB, Mostofsky SH. Abnormal cerebral cortex structure in children with ADHD. Human Brain Mapping. 2009;30:175–184. doi: 10.1002/hbm.20496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang P, Wang PN, Chuang KH, Jong YJ, Chao TC, Wu MT. Absence of gender effect on children with attention-deficit/hyperactivity disorder as assessed by optimized voxel-based morphometry. Psychiatry Research. 2008;164:245–253. doi: 10.1016/j.pscychresns.2007.12.013. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.