Abstract
High performance liquid chromatography (HPLC) and flow-injection mass spectrometric (FIMS) fingerprinting techniques were tested for their potential in differentiating organic and conventional peppermint samples. Ten organic and ten conventional peppermint samples were examined using HPLC-UV and FIMS methods. The principal component analysis (PCA) showed that both HPLC and FIMS fingerprints could determine the difference in the commercial organic and conventional peppermints. FIMS fingerprinting provided a rapid test to differentiate organic and conventional peppermints in 1 min analysis, and has potential for high-throughput applications. On the other hand, HPLC fingerprints provide more information about the chemical composition of the samples, but take a longer time to differentiate organic and conventional peppermint samples.
Keywords: HPLC fingerprint, flow injection, mass spectral fingerprint, principal component analysis, Peppermint (Mentha piperita), Kaempferol 7-O-rutinoside
INTRODUCTION
Consumer demand for organic foods has continuously increased because of the perception that they might contain greater amount of beneficial components than their conventionally produced counterparts. The organic industry grew to over $26 billion in 2010, increasing over four times from $6 billion in 2000.1 Conflicting findings on the quality and nutritional values of organic foods compared to their conventional counterparts have been reported. For instance, a human crossover intervention study involving 16 subjects demonstrated that organic and conventional fruits and vegetables differed in their concentrations of five selected flavonoids and resulted in different urinary excretions of the major dietary flavonoids.2 In contrast, a systematic review concluded that there was no evidence indicating the nutritional quality difference between organic and conventional foods.3
Peppermint, Mentha piperita L., is one of the most important and wildly used flavoring agents and spices. Peppermint extract exhibited several health properties, including antioxidant, radio protective and antitumorgenic activities.4-7 Peppermint oil and its primary component menthol might have antiemtic effect by acting on the 5-HT3 receptor of ion-channel complex,8 and induce Ca2+ influx in a subset of sensory neurons from dorsal root and trigeminal ganglia.9 Both conventional and organic peppermints are commercially available and differ significantly in their prices.
Our recent study showed that organic peppermint extract was more effective than that of conventional peppermint in suppressing IL-1β, IL-6 and COX-2 mRNA expressions at a 10 μg botanical equivalent per mL concentration in the LPS stimulated J774A.1 mouse macrophage cells, but they had no difference in inhibiting MCP-1 and TNF-α mRNA expressions.10 The organic peppermint also had greater p-coumaric acid content than the conventional counterpart, and both had same levels of gallic, caffeic and syringic acids, as well as catechin and epigallocatechin gallate (EGCG) contents, suggesting potential differences between organic and conventional peppermints or other edible botanicals in their chemical compositions and biological activities. It will be interesting to find out how organic and conventional peppermints may differ from each other in their chemical compositions, and if organic and conventional peppermints could be differentiated by instrumental analysis.
Our recent study successfully differentiated the tetra- and diploid Gynostemma pentaphyllum, and the different parts of same G. pentaphyllum genotype plant using HPLC-UV fingerprints and principle component analysis (PCA).11 Later in 2012, eleven commercial G. pentaphyllum samples were evaluated for their homogeneity and similarity using on-line HPLC-UV-MS technique combined with PCA.12 The eleven G. pentaphyllum samples were classified into three clusters or groups using the MS data for flavonoids and gypenosides collected under negative mode. In addition to chromatographic fingerprint analysis, flow injection mass spectrometric (FIMS) fingerprinting has also been used to differentiate botanicals from different growing conditions. In 2010, FIMS fingerprinting was used to differentiate between Rio Red grapefruits grown under organic and conventional farming practices, as well as from different years and times of harvest.13 HPLC fingerprinting provides detail information about every major chemical but requires more time for method development and HPLC analysis. FIMS fingerprinting does not supply the concentration information but quickly provides more information about the entire specific ion of the sample because of direct injection involving no chromatographic separation. These two methods examine different aspects of chemical information about the food samples because of their different chemical mechanisms. If used together, combined chromatographic and FIMS fingerprints may better determine the difference between conventional and organic foods.
In the present study, the chemical patterns represented by HPLC-UV chromatographic fingerprinting and flow-injection mass spectrometric (FIMS) fingerprints were analyzed using principal component analysis (PCA) to differentiate commercial organic and conventional peppermints.
MATERIALS AND METHODS
Standard Compounds and Other Chemicals
Optima grade water and acetonitrile were purchased from Fisher Scientific (Pittsburgh, PA, USA). MS grade formic acid was purchased from Sigma/Aldrich (St. Louis, MO, USA).
Plant Materials and Sample Preparation
Ten organic and ten conventional commercial dry peppermint spice samples were gifts from Frontier Natural Products Co-op (Norway, IA, USA). The organic peppermint samples were USDA certified. All peppermint samples were ground to 20 mesh particle size using an IKA A11 analytical grinding machine (IKA, Staufen, Baden-Württemberg, Germany), and stored at −20 °C before analysis. 100 mg of each peppermint powder were weighed and extracted with 10 mL H2O-MeOH (1:1, v:v) by ultra-sonication for 30 min using an fisherbrand ultrasonic machine (Fisher, Pittsburgh, PA, USA). The extracts were filtered through a 0.45 μm Nylon syringe filter (Alltech Associates, Deerfield, IL, USA). Each extract was analyzed in triplicate.
HPLC-UV and IT-MS Analyses
An Agilent 1100 HPLC system was used with a binary pump, a vacuum degasser, a column oven, and an auto-sampler (Agilent Technologies, Palo Alto, CA, USA). FIMS system consisted of the same HPLC system in combination with a LCQ Decaion-trap mass spectrometer (Thermo Fisher Scientific Inc., Waltham, MA, USA). Electrospray ionization (ESI) in positive ion mode was used.
The HPLC conditions were as follows: A Symmetry C18 column (2.1 mm i.d. × 150 mm, 3.5 μm) (Waters, Milford, MA, USA) was used with a column temperature set to 40 °C. Mobile phase A consisted of 0.1% formic acid in H2O and mobile phase B consisted of 0.1% formic acid in acetonitrile. The initial ratio of B was 5%; this was changed linearly to 22% B in 10 min, kept at 22% B until 20 min, then increased linearly to 30% B in 30 min, held constant 30% B to 35 min, to 35% B linearly at 45 min, kept at 35% B to 50 min, linear gradient to 95% B in 65 min and then washed at this ratio for 5 min. After that the ratio was returned to initial conditions for 5 min to re-equilibrate the column for the next injection. The flow rate was 0.2 mL/min, injection volume was 10 μL, and the detection wavelength was set to 330 nm.
The conditions for IT-MS were as follows: No analytical column was used but a guard column (Adsorbosphere All-Guard Cartridge, C18, 5 μm, 4.6 × 7.5 mm, Alltech Associates, Inc., Deerfield, IL, USA) was used to minimize potential contamination of the MS system. The mobile phase A consisted of 0.1% formic acid in H2O and mobile phase B consisted of 0.1% formic acid in acetonitrile with isocratic elution at 60:40 (v/v) at the flow rate of 0.5 mL/min. Peppermint extractions were diluted 10 times with water and the injection volume was 2 μL. MS Spectra were collected from 0.2 to 1 min and the mass range was from 120 to 1000 m/z. Sheath gas flow rate 80 L/min; aux gas flow rate 10 L/min; spray voltage, 4.5 kV; heated capillary temperature, 250 °C; capillary voltage, −4.0 V; and tube lens offset, 20 V. Triplicate analyses of 20 different peppermint samples provided 60 MS spectra.
UPLC-Q-TOF MS Analysis
A Waters UPLC-Xevo G2 Q-TOF MS system (Milford, MA, USA) was used to further confirm the compound identification using the accurate mass weight. The conditions were as follows: peppermint extractions were injection volume was 2 μL. A BEH C18 column (2.1 mm i.d. × 100 mm, 1.7 μm) (Waters, Milford, MA, USA) was used with a column temperature set to 40 °C. Flow rate was 0.2 mL/min. Mobile phase A consisted of 0.1% formic acid in H2O and mobile phase B consisted of 0.1% formic acid in acetonitrile. The initial ratio of B was 20%; this was changed linearly to 50% B in 20 min, then increased linearly to 95% B in 35 min and washed at this ratio for another 5 min. The ratio was returned to initial conditions for 5 min to re-equilibrate the column for the next injection. The flow rate was 0.2 mL/min, injection volume was 2 μL, and the detection wavelength was set at 330 nm. An ESI positive ion source was selected, capillary voltage was 3.00 KV; sampling cone voltage was 30 V; extraction cone voltage was 4.0 V. Source temperature was set at 120 °C, with a desolvation temperature at 450 °C. Cone gas flow rate was 50 L/h and the desolvation gas flow was 800 L/h. The mass range was from 100 to 1000 m/z; ramp collision energy was from 25 V to 35V.
Data Processing
A total of 32 peaks were selected for identification and quantitation in the HPLC fingerprints of conventional and organic peppermints, and their absolute peak area and relative peak area were analyzed by principal component analysis (PCA). For absolute peak area analyses, 32 peak areas in all the 60 chromatograms (20 samples with triplicate analyses each) were analyzed with PCA directly. For relative peak area analyses, the largest peak area was selected as the reference peak and other peak areas were converted to the relative peak areas against the reference peak in each chromatogram, and the relative peak areas were analyzed for PCA using the SIMCA-P software (Umetrics, Malmo, Skåne län, Sweden) based on UV data.
FIMS fingerprints obtained as one-dimensional spectra (m/z 120-1000) were used for comparison. The data were imported into Excel (Microsoft, Inc., Belleview, WA, USA) for data pre-processing and then to SIMCA-P 10.5 (Umetrics, Malmo, Skåne län, Sweden) for PCA. The processing in Microsoft Excel was to combine the 60 spectra, sort the data by sample names, and fill the mass matrix with zero for each missing m/z in the mass list so that the data points of each mass spectrum was aligned at 881. The resulting two-dimensional matrix was 60 × 881 (60 samples and 881 masses for sample wash). The matrix was exported to SIMCA-P and the mean centering was used before PCA.
RESULTS AND DISCUSSION
Chromatographic and MS fingerprints
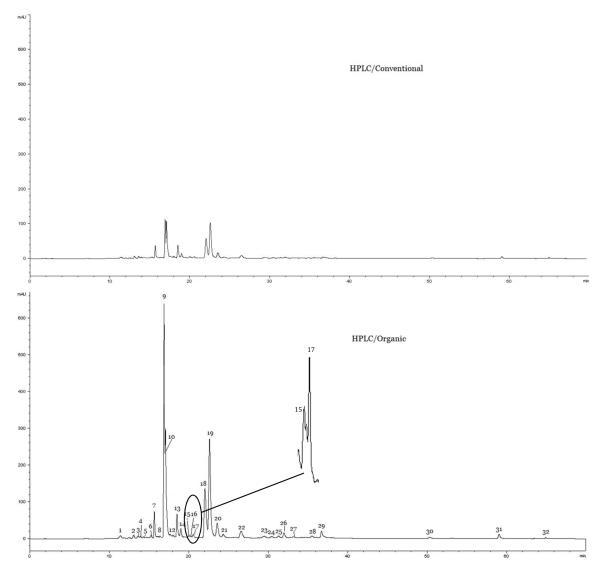
Fourteen components were identified using the accurate mass weight detected by UPLC-Q-TOF MS. These included pebrellin, eriodictyol 7-O-rutinoiside, tricetin 3′-O-glucoside 5′-O-rhamnoside, eriocitrin, kaempferol 7-O-rutinoside, luteolin 7-O-neohesperidoside, narirutin, 4′-methoxykaempferol 7-O-rutinoside, hesperidin, isosafrole, cyclohexanecarboxylic acid, gardenin D, 5,6-dihydroxy-4′,7,8-trimethoxyflavone and gardenin, which are corresponding to the Peaks 1, 3, 7, 9, 10, 11,13, 15, 17, 19, 26, 30, 31 and 32 in a typical were chromatographic fingerprints of peppermint (Figure 1), respectively. Typical chromatographic fingerprints for conventional and organic peppermints are shown in Figure 1. For each sample, a total of thirty-two major peaks were detected. Peak No. 9 was the greatest peak and was selected as the reference peak (RP) for calculating the relative peak area. Other major peaks were defined as at least 5% of the area of RP besides the last three peaks.
Figure 1. HPLC-DAD fingerprintings of conventional and organic peppermints.
Peaks 1, 3, 7, 9, 10, 11,13, 15, 17, 19, 26, 30, 31 and 32 were pebrellin, eriodictyol 7-O-rutinoiside, tricetin 3′-O-glucoside 5′-O-rhamnoside, eriocitrin, kaempferol 7-O-rutinoside, luteolin 7-O-neohesperidoside, narirutin, 4′-methoxykaempferol 7-O-rutinoside, hesperidin, isosafrole, cyclohexanecarboxylic acid, gardenin D, 5,6-dihydroxy-4′,7,8-trimethoxyflavoneand gardenin B, respectively.
As shown in Figure 1, the peaks in the organic peppermint were generally greater than the corresponding peaks in the conventional counterpart sample on a same per botanical weight basis. Peak No.15 was not detectable in any conventional peppermint samples. In addition, the average total area under the 32 peaks was 16581.26 ± 1322.06 for organic peppermint samples, which was two times more than that of 7388.28 ± 2741.41 for the ten conventional peppermints.
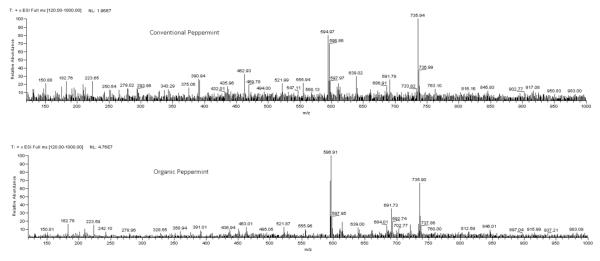
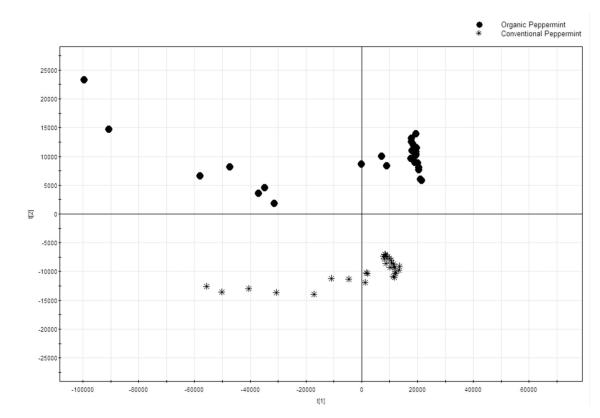
Typical FIMS fingerprints for the conventional and organic peppermints are shown in Figure 2. Although the mass spectrum had almost the same significant peaks between conventional and organic peppermint extracts, the natural abundance of their major peaks were different. In the spectrum of conventional peppermint, m/z 735 was the greatest natural abundance peak, followed by m/z 594 (Kaempferol 7-O-rutinoside). While for the organic peppermint, m/z 594 was generally the greatest peak and m/z 735 was the second. In addition, the average of total ion responses of FIMS spectra for organic samples was 4.75 × 107, more than 2 times greater than that of 1.96 × 107 for the conventional peppermint.
Figure 2.
MS fingerprinting for IT-MS; conventional and organicpeppermints.
PCA of chromatographic fingerprints
PCA uses a mathematical procedure to transform a number of correlated variables into a smaller group of uncorrelated variables, the principle components. PCA of fingerprints generally makes it easy to visually compare significant difference and avoids subjective decisions.
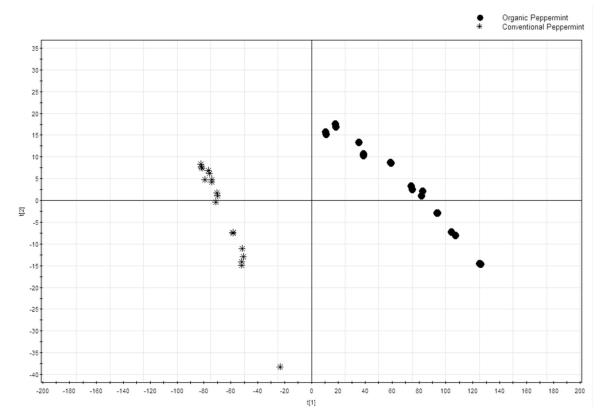
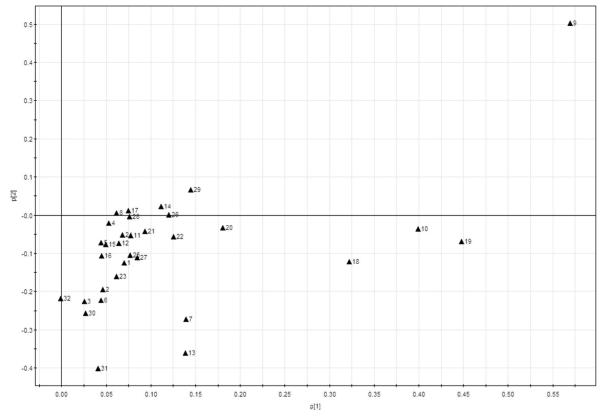
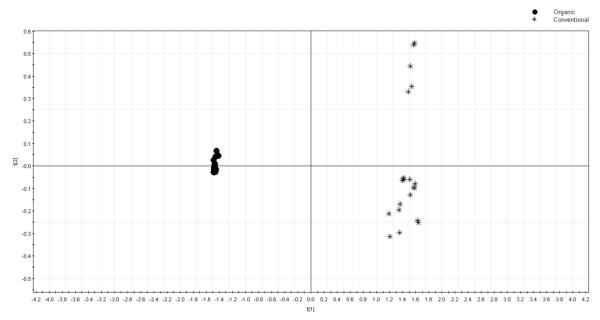
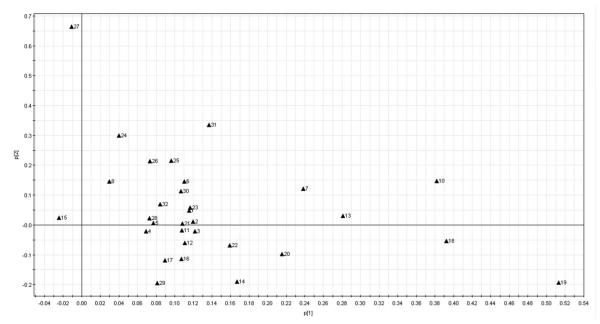
The PCA scores plot and loading plot of the chromatographic absolute peak areas are reported in Figure 3 and Figure 4, respectively. In these two figures, the absolute peak areas of the 32 peaks were selected for PCA. As shown in Figure 3, the conventional peppermint samples were all on the left of the PCA scores plot while all organic samples were located on the right side. In the loading plot of HPLC absolute peak areas (Figure 4), peaks 9, 19, 10 and 18 contributed significantly to the separation of organic samples from the conventional ones. Taking the HPLC chromatographic fingerprint (Figure 1) and PCA loading plot (Figure 4) into account, the organic peppermint samples contained greater concentrations of almost all the chemical compounds than its conventional counterpart.
Figure 3.
PCA scores plot for absolute peak area in different farming modes for HPLC fingerprinting.
Figuren 4.
PCA loading plot for absolute peak area in different farming modes for HPLC fingerprinting.
Figures 5 and 6 are the PCA scores plot and loading plot of the relative peak areas for the organic and conventional peppermints, respectively. In Figure 5, all the organic peppermint samples were clustered tightly in the left side of the scores plot, while conventional peppermint samples were all in the right side of the score plot. The tight cluster of the score plot from relative peak areas indicated that all the organic peppermint samples had relatively uniform chemical profiles, or the concentration of 31 major chemical components in all the organic peppermint samples had a similar ratio relative to peak 9. In the loading plot of relative peak areas (Figure 6), only Peaks 15 and 27 had negative PC1 values, indicating that the relative areas of these two peaks were greater in the organic peppermint samples, and the relative areas for the remaining peaks were greater in the conventional peppermint samples. Since peak No.9 was selected as the RP and the areas of all the other peaks were normalized against RP, the results also indicated that the peak areas of RP were greater in organic samples. Furthermore, Peaks 19, 10 and 18 contributed most to the organic peppermint in the loading plot, confirming that peaks 9, 10, 18 and 19 not only represented the four primary chemical components but also were the four most important peaks to decide if a peppermint was organically or conventionally produced using HPLC chromatographic fingerprinting.
Figure 5. PCA scores plot for relative peak area in different farming modes.
All peak areas were converted to their relative areas against that of peak No. 9 in the sample, and the relative peak areas were used for PCA.
Figure 6. PCA loading plot for relative peak area in different farming modes.
All peak areas were converted to their relative areas against that of peak No. 9 in the sample, and the relative peak areas were used for PCA.
PCA analysis of MS fingerprints
The FIMS PCA score plot and loading plot are shown in Figures 7 and 8, respectively. In the score plot of MS flow injection, all the organic peppermint samples were in the upper side, whereas the conventional peppermint samples were in the lower side (Figure 7). The organic and conventional peppermints were separated completely by PC2. The scores plot indicated that the FIMS fingerprinting technique might effectively differentiate conventional and organic peppermints in 1 minute.
Figure 7.
PCA scores plot for farming mode for IT-MS fingerprintings.
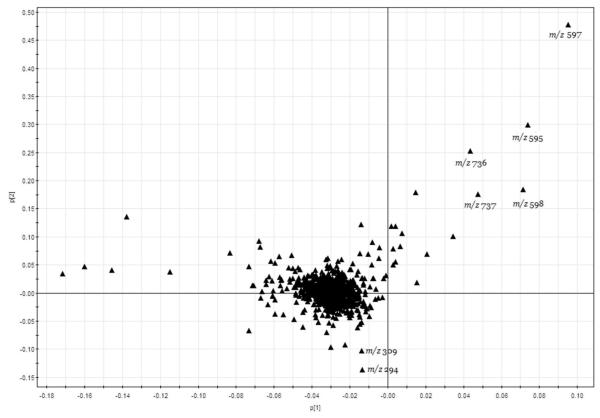
Figure 8.
PCA loading plot for farming mode for IT-MS fingerprintings.
In the loading plot of FIMS fingerprints, most of the ions were clustered near the central zero line (Figure 8). However, a few ions dispersed far away from the central, and these ions and their concentrations were most important to determine the farming practice of peppermint. In the upper right corner, ions m/z 595, 597 and 736 together with their isomer peaks m/z 596, 598 and 737 yield a higher score and would lead to positions to the upper side of PCA score plot. The most intense ions contributing to high scores were m/z 597 (M+H), the molecular ion of 598 for eriodictyol7-O-rutinoside. Another high score ion was m/z 736 (M+H) for molecular ion 737.
The most noticeable ions that contributed negatively to PC2 were ions at m/z 294 and 309. The other ions that also contributed positively to conventional peppermint samples were ions at m/z 266 and 280. The scores of these ions indicated that these compounds contributed most in the differentiation of organic and conventional peppermint samples.
In summary, the results from this study indicated that the commercial organic and conventional peppermints may have significantly different chemical profiles and nutrient composition. Both HPLC and FIMS fingerprints could effectively differentiate organic and conventional peppermints. FIMS may provide a rapid test and has potential for high-throughput applications. On the other hand, HPLC fingerprints provide more information about the chemical composition for the samples with a longer analysis time.
ACKNOWLEDGEMENT
This research was partially supported by SJTU 985-III disciplines platform and talent fund (Grant No. TS0414115001; TS0320215001), a special fund for Agro-scientific Research in the Public Interest (Grant No. 201203069), and a research gift from the Frontier Natural Products Co-op (Norway, IA, USA).
This research is also supported by the Agricultural Research Service of the United States Department of Agriculture and an Interagency Agreement with the Office of Dietary Supplements of the National Institutes of Health.
REFERENCES
- 1. [Obtained July 17, 2012];The organic trade association’s 2011 organic industry survey. Organic trade association. Website: http://www.ota.com/pics/documents/2011OrganicIndustrySurvey.pdf.
- 2.Grinder-Pedersen L, Rasmussen SE, Gel SB, Jorgensen LV, Dragsted LO, Gundersen V, Sandstrom B. Effect of Diets Based on Foods from Conventional versus Organic Production on Intake and Excretion of Flavonoids and Markers of Antioxidative Defense in Humans. J. Agric. Food Chem. 2003;51:5671–5676. doi: 10.1021/jf030217n. [DOI] [PubMed] [Google Scholar]
- 3.Dangour AD, Dodhia SK, Hayter A, Allen E, Lock K, Uauy R. Nutritional quality of organic foods: a systematic review. Am. J. Clin. Nutr. 2009;90:680–685. doi: 10.3945/ajcn.2009.28041. [DOI] [PubMed] [Google Scholar]
- 4.Baliga M, Rao S. Radioprotective potential of mint: a brief review. J. Cancer Res. Ther. 2010;6(3):255–262. doi: 10.4103/0973-1482.73336. [DOI] [PubMed] [Google Scholar]
- 5.Lopez V, Martin S, Gomez-Serranillos M, Carretero M, Jager A, Calvo M. Neuroprotective and neurochemical properties of mint extracts. Phytother. Res. 2010;24(6):869–874. doi: 10.1002/ptr.3037. [DOI] [PubMed] [Google Scholar]
- 6.Jain D, Pathak N, Khan S, Raghuram G, Bhargave A, Samarth R. Evaluation of cytotoxicity and anticarcinogenic potential of Mentha leaf extracts. Int. J. Toxicol. 2011;30(2):225–236. doi: 10.1177/1091581810390527. [DOI] [PubMed] [Google Scholar]
- 7.Rita P, Animesh DK. An Updated Overview on Peppermint (Mentha Piperital L.) International Research Jounarl of Pharmacy. 2011;2(8):1–10. [Google Scholar]
- 8.Haahr A, Bardow A, Thomsen C, Jensen S, Nauntofte B, Bakke M. Release of Peppermint flavour compounds from chewing gum: effect of oral functions. Physiol. Behav. 2004;82(283):531–540. doi: 10.1016/j.physbeh.2004.04.061. [DOI] [PubMed] [Google Scholar]
- 9.Pournemati P, Azarbayjani M, Rezaee M, Ziaee V, Pournemati P. The effect of inhaling peppermint odor and ethanol in women athletes. Bratisl Lek. Listy. 2009;110(12):782–787. [PubMed] [Google Scholar]
- 10.Lv J, Huang H, Yu L, Whent M, Niu Y, Shi H, Wang TTY, Luthria D, Charles D, Yu LL. Phenolic composition and nutraceutical properties of organic and conventional cinnamon and peppermint. Food Chemistry. 2012;132:1442–1450. doi: 10.1016/j.foodchem.2011.11.135. [DOI] [PubMed] [Google Scholar]
- 11.Xie Z, Zhao Y, Chen P, Jing P, Yue J, Yu LL. Chromatographic Fingerprint Analysis and Rutin and Quercetin Compositions in the Leaf and Whole-Plant Samples of Di- and Tetraploid Gynostemma pentaphyllum. J. Agric. Food Chem. 2011;59:3042–3049. doi: 10.1021/jf104329v. [DOI] [PubMed] [Google Scholar]
- 12.Zhao Y, Xie Z, Niu Y, Shi H, Chen P, Yu LL. Chemical compositions, HPLC/MS fingerprinting profiles and radical scavenging properties of commercial Gynostemma pentaphyllum (Thunb.) Makino samples. Food Chemistry. 2012;134:180–188. [Google Scholar]
- 13.Chen P, Harnly JM. Flow Injuection Mass Spectral Fingerprints Demonstrate Chemical Differents in Rio Red Grapefruit with Respect to Year, Harvest Time, and Conventioanl versus Organic Farming. J. Agric. Food Chem. 2010;58:4545–4553. doi: 10.1021/jf904324c. [DOI] [PMC free article] [PubMed] [Google Scholar]