Abstract
Introduction
Pompe disease is a rare, autosomal recessive disorder caused by deficiency of the glycogen-degrading lysosomal enzyme acid alpha-glucosidase. Late-onset Pompe disease is a multisystem condition, with a heterogeneous clinical presentation that mimics other neuromuscular disorders.
Methods
Objective is to propose consensus-based treatment and management recommendations for late-onset Pompe disease.
Methods
A systematic review of the literature by a panel of specialists with expertise in Pompe disease was undertaken.
Conclusions
A multidisciplinary team should be involved to properly treat the pulmonary, neuromuscular, orthopedic, and gastrointestinal elements of late-onset Pompe disease. Presymptomatic patients with subtle objective signs of Pompe disease (and patients symptomatic at diagnosis) should begin treatment with enzyme replacement therapy (ERT) immediately; presymptomatic patients without symptoms or signs should be observed without use of ERT. After 1 year of ERT, patients’ condition should be reevaluated to determine whether ERT should be continued.
Keywords: acid alpha-glucosidase, acid maltase deficiency, lysosomal storage disorder, neuromuscular disease, Pompe disease
Consensus statements are important for developing treatment recommendations when evidence-based medical guidelines and treatment recommendations based on controlled trials are sparse or nonexistent. In the case of late-onset Pompe disease, it is an appropriate time to assemble a group of experts in the field to review and analyze the medical literature and create recommendations for disease management (based on the best available evidence) until additional data from controlled studies become available to enable further recommendations. The American Association of Neuromuscular & Electrodiagnostic Medicine (AANEM) convened a consensus committee of specialists with expertise in the diagnosis and treatment of Pompe disease and, using a modified consensus development conference method,1 the committee worked to create consensus-based recommendations for the treatment of late-onset Pompe disease.
OVERVIEW OF POMPE DISEASE
History of Pompe Disease
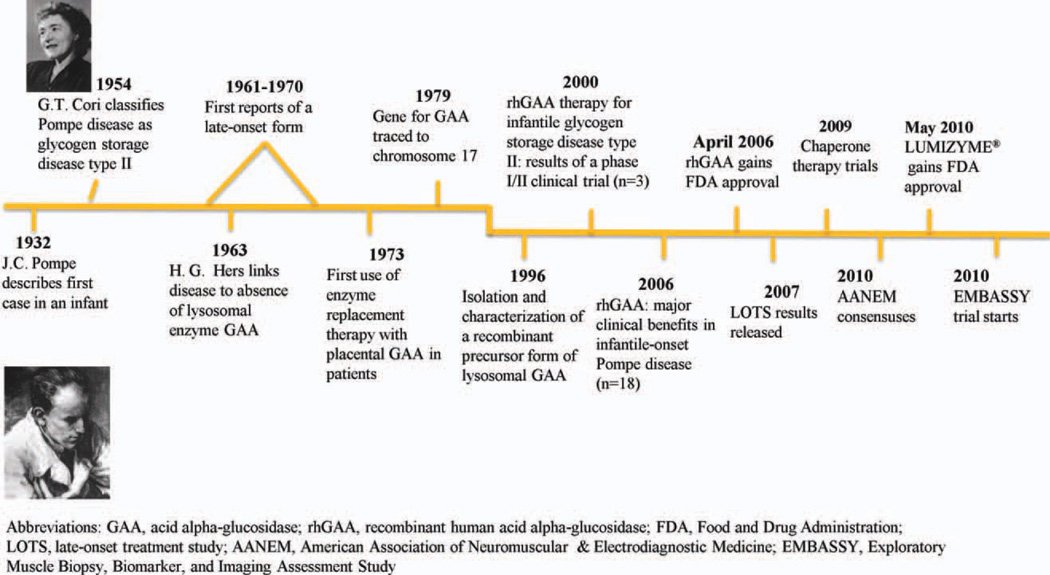
Pompe disease, also referred to as acid maltase deficiency, is a rare, autosomal recessive disorder that was first described in 1932 in a 7-month-old girl who died of cardiomyopathy. The disease was identified as a glycogen storage disorder in which glycogen had accumulated within vacuoles of all the examined tissue.2,3 In 1954, the disease was classified as glycogen storage disease type II,4 and in 1963 it became the first identified lysosomal storage disorder.5,6 During the 1960s and 1970s, cases of a milder phenotype that occurred in older patients (late-onset Pompe disease) were described by Engel and colleagues.7 The reported frequency of infantile-onset Pompe disease ranges from approximately 1 in 35,000 to 1 in 138,000 among Taiwanese and Dutch populations, respectively.8,9 The estimated frequency of late-onset Pompe disease is 1 in 57,000.10 The actual frequency of late-onset Pompe disease in the USA is not known, but one study suggested that the frequency of all forms may be as high as 1 in 40,000.11
There was no disease-specific treatment for Pompe disease until enzyme replacement therapy (ERT) was first attempted in 1973. Highly purified, placenta-derived acid alpha-glucosidase (GAA) enzyme was administered by intravenous infusion, taking advantage of the ability of lysosomes to internalize exogenous proteins by endocytosis.12 Initial attempts at ERT encountered problems of immunogenicity, and there was limited availability of purified GAA for enzyme delivery.13,14 In 1979, the 28-kb gene for GAA was identified on chromosome 17,15 and in the 1990s new recombinant technology became available, enabling the production of enough recombinant human acid alpha-glucosidase (rhGAA) to allow ERT clinical trials to be conducted. Figure 1 show the timeline for the development of ERT for Pompe disease.
FIGURE 1.
Timeline for Pompe disease: 75 years from description to availability of disease-modifying agent. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
In 2006, alglucosidase alfa (Myozyme; Genzyme Corporation, Cambridge, Massachusetts)16 was approved by the U.S. Food and Drug Administration (FDA) and the European Medicines Agency and became the first disease-specific treatment for Pompe disease. The approval was based on the results of a pivotal trial of ERT with alglucosidase alfa in 18 infants.17 The results of the first randomized, double-blind, placebo-controlled study of ERT, known as the Late-Onset Treatment Study (LOTS),18 led to the approval in 2010 of Lumizyme (Genzyme Corporation)19 for the treatment of late-onset Pompe disease in the USA. Along with its approval, the FDA mandated postmarketing surveillance of alglucosidase alfa. The manufacturer will conduct studies to evaluate the tests used for drug profiling and monitoring and for qualification, quantification, specification, and stability of alglucosidase alfa. Moreover, the FDA required that additional immunogenicity, safety, efficacy, pharmacokinetics, and long-term follow-up studies be conducted to better understand the role of alglucosidase alfa in the treatment of Pompe disease. These studies are currently ongoing.
Pathophysiology of Pompe Disease
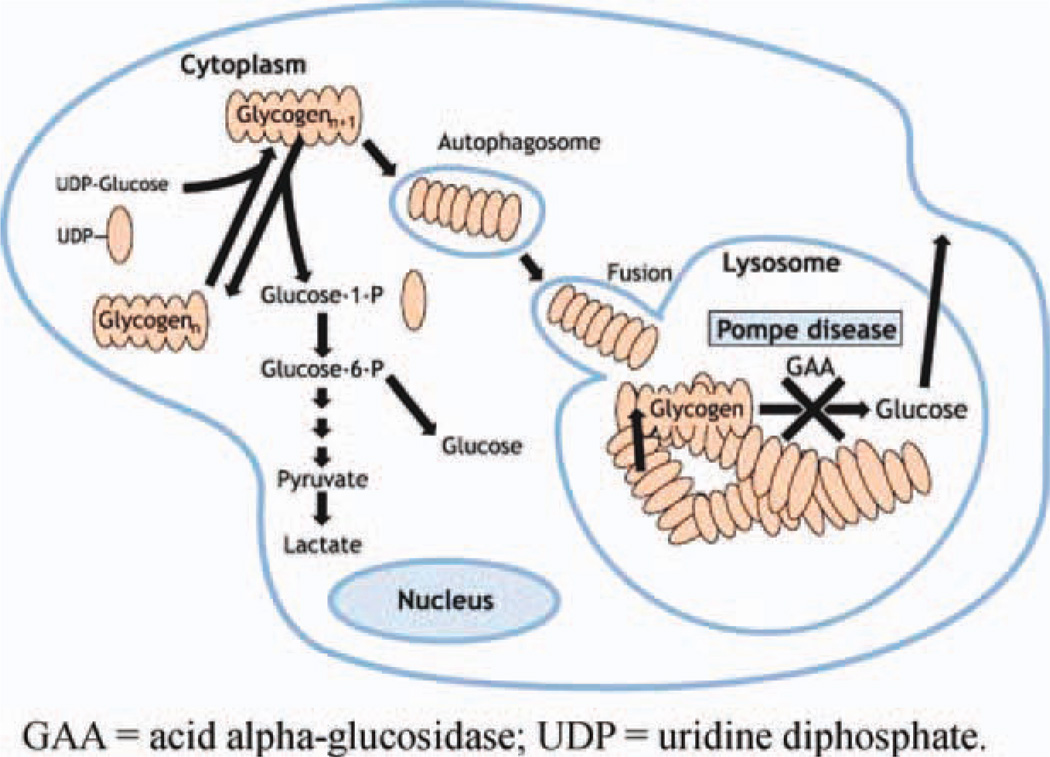
Lysosomal GAA catalyzes the breakdown of glycogen into glucose. Acid alpha-glucosidase deficiency in Pompe disease results in the accumulation of lysosomal and nonlysosomal glycogen in multiple tissues20–22 (Fig. 2). In infantile-onset Pompe disease, GAA enzyme activity is either completely or nearly completely absent (typically <1% of normal activity in skin fibroblasts). Some residual enzyme activity (approximately 2–40% of normal activity in skin fibroblasts) is present in most children and adults with the late-onset form.23,24 Recent evidence has shown a failure of productive autophagy in patients with Pompe disease and the progressive accumulation of autophagosomes that disrupt the contractile apparatus in muscle fibers.25 Moreover, currently unknown abnormalities in a subset of lysosomes interfere with the recycling of autophagosomes and their contents, which leads to skeletal muscle damage.25 The GAA gene is highly pleomorphic; 289 sequence variations have been reported to date that include 197 proven pathogenic mutations, 67 non-pathogenic mutations, and 25 sequence variants with unknown functional effect.26
FIGURE 2.
Pathophysiology of Pompe disease. GAA, acid alpha-glucosidase; UDP, uridine diphosphate. Based on Raben et al.108 with permission. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Clinical Presentation and Disease Progression
Infantile-onset Pompe disease is a progressive, multisystem disorder that causes hypotonia and feeding difficulties in the first year of life. The disease affects cardiac, skeletal and smooth muscles, the pulmonary and gastrointestinal systems, and anterior horn cells. Death due to cardiorespiratory failure typically occurs in the first year of life.3,24,27 Late-onset Pompe disease is also a multisystem disease; it may manifest at any time after 12 months of age. Its clinical presentation includes: progressive muscle weakness, especially in the trunk and lower limbs; respiratory symptoms; and progression to respiratory insufficiency due to diaphragmatic and intercostal muscle weakness.3,24 Respiratory complications are the most frequent cause of death in late-onset Pompe disease. To date, evidence regarding cardiac involvement in late-onset Pompe disease is inconclusive; however, Wolff–Parkinson–White syndrome has been reported.28
Diagnosis of Late-Onset Pompe Disease
The diagnosis of Pompe disease, particularly the late-onset form, is often difficult because it can clinically resemble a myriad of other neuromuscular disorders. A high level of clinical suspicion is necessary for a timely and accurate diagnosis.24 A complete discussion of the clinical presentation and diagnostic guidelines for late-onset Pompe disease was published in 2009.20 Briefly, physical examination usually reveals more proximal than distal weakness. The pelvic girdle is affected to a greater degree than the shoulder girdle, and weakness of the abdominal muscles and diaphragm may be present early on. Scapular winging, paraspinal muscle atrophy, scapuloperoneal weakness,29 and facial involvement with weakness or ptosis (unilateral or bilateral) have also been observed.30,31 Clinical myotonia is absent, but electrophysiological myotonia, especially in the paraspinal muscles, is detected frequently.20 Fatigue of the jaw muscles and difficulties with chewing and swallowing often result in inadequate intake of protein, calories, vitamins, and minerals, which leads to endogenous muscle protein breakdown.24 Swallowing dysfunction can be diagnosed in patients with late-onset Pompe disease by a videofluoroscopic swallowing assessment.24 Sensory, cerebellar, and cognitive involvement have not been reported.20
Laboratory testing usually reveals nonspecific elevation of serum creatine kinase.10 Needle electromyography (EMG) studies often indicate myopathic potentials with increased muscle membrane irritability and myotonia.20 Of note, even when needle EMG findings in limb muscles are normal, abnormalities may still be found in the paraspinal muscles alone.29 Pulmonary function testing may reveal decreased forced vital capacity (FVC); a detailed discussion of pulmonary involvement is provided in what follows.
Although the clinical phenotype of late-onset Pompe disease varies, genetic analysis of the GAA gene,24 or a determination of the level of GAA enzyme activity in blood, fibroblasts, or muscle tissue, can provide a definitive diagnosis.32 Although muscle biopsy often reveals a vacuolar myopathy with increased amounts of glycogen,31,33 a normal muscle biopsy does not exclude late-onset Pompe disease.20
METHODS
A literature search was conducted in the PubMed and Embase databases for clinical trials and relevant articles published before June 2010. The search terms included Pompe disease, acid alpha-glucosidase deficiency, acid maltase deficiency, glycogen storage disease type II, and glycogenosis type II. Panel members reviewed these articles individually and discussed them in small group sessions. Subsequently, the panel convened for further discussion and consensus development. In addition, articles published after the consensus meeting was held, references cited by the articles produced by the literature search, and references suggested by the authors are included in this article. Each panel member was assigned a section to write and review. Writing contributions are listed in the Appendix.
RESULTS
The literature search produced 105 articles relevant to Pompe disease. The AANEM consensus panel, consisting of several neuromuscular specialists and a pulmonologist, reviewed all articles. The most frequent signs and symptoms of late-onset Pompe disease are presented in what follows as musculoskeletal, pulmonary, and gastrointestinal tract manifestations. In addition, treatment recommendations for these signs and symptoms are discussed.
Musculoskeletal Manifestations and Management of Late-Onset Pompe Disease
Musculoskeletal Manifestations
Musculoskeletal involvement in late-onset Pompe disease is dominated by progressive muscle weakness affecting proximal more than distal muscles.24,34 In a recent observational study, weakness of the proximal extremities was the presenting symptom in 93% of patients with late-onset Pompe disease.35 In addition, the study demonstrated that strength assessed over a 1-year period by using quantitative muscle testing declined by 7.1% in the lower limbs and by 4% in the upper limbs.35 Subsequent secondary musculoskeletal complications include contractures, limb and spinal deformities, and osteopenia/osteoporosis.24 Scoliosis was found in approximately half of late-onset Pompe disease patients.36 Gross and fine motor functions required for daily living and ambulation are affected by primary and secondary musculoskeletal impairments, as well as by pulmonary (more than cardiac) dysfunction.20,37,38 In addition, general fatigue and poor endurance are common concerns, reported by 50–76% of patients,20,37,38 with pulmonary compromise as a likely contributing factor. Muscle pain and soreness are early symptoms that were reported in 46% of patients.37 Therefore, management of patients with late-onset Pompe disease should be individualized to account for the variations in musculoskeletal impairment observed between patients.38
Overall Management of Musculoskeletal Issues
General clinical management and rehabilitation principles to guide the care of patients with late-onset Pompe disease include preserving or optimizing motor function, preventing or minimizing secondary complications, maximizing the benefits of ERT or other therapies as they become available, promoting overall health while managing disease-associated symptoms, and improving quality of life. Rehabilitation and management efforts in late-onset Pompe disease should be comprehensive and preventive, based on individual assessment and appreciation of disease progression.24 To address quality-of-life issues, rehabilitation services should include physical therapy, occupational therapy, respiratory therapy, speech therapy, dysphagia evaluation and nutritional support, orthotics, and assistive technology, as well as vocational/avocational, mental health, and social work services.
Strengthening and Therapeutic Exercise
There are no established guidelines for muscle strengthening or therapeutic exercise for individuals with late-onset Pompe disease. Studies of the effects and treatment roles of exercise and nutrition in late-onset Pompe disease have been few and have had small sample sizes.34,39,40 Although more information is needed, these studies suggest that sub-maximal aerobic exercise may increase muscle strength and function through improved clearance of accumulated glycogen in the muscle cytosol.41 In contrast, there is insufficient evidence that resistance training improves strength in late-onset Pompe disease. Traditionally, excessively strenuous resistance exercises have been discouraged in muscle disorders because of the potential for exacerbating muscle degeneration,42–44 and in Pompe disease there is additional theoretical concern that excessive muscle contraction might lead to increased leakage of glycogen from lysosomes or cause lysosomal rupture, thereby hastening muscle damage.45 The favorable pattern of response in the strength of proximal and respiratory muscles in ERT studies of late-onset Pompe disease is encouraging, but further studies are needed.18,36 Therefore, at this time the authors recommend that the general precautions regarding strengthening exercises that are followed for other degenerative muscle diseases should be applied to late-onset Pompe disease46,47 (Table 1). Therapeutic exercise in late-onset Pompe disease may pose a risk for cardiopulmonary compromise in this population. Consequently, a pulmonologist should evaluate the patient before initiation of an exercise program.24
Table 1.
Treatment recommendations for the musculoskeletal element of late-onset Pompe disease.
| Provide patient with information on the following resources: |
| Muscular Dystrophy Association, Acid Maltase Deficiency Association, Pompe Registry, Association for Glycogen Storage Disease, International Pompe Association |
| Physical examination and assessments |
| Patients should be examined by a cardiologist and pulmonologist before beginning an exercise program |
| Screen all patients diagnosed with Pompe disease, regardless of age and wheelchair use, with dual-energy x-ray absorptiometry (DEXA); follow-ups can be considered on a yearly basis |
| Patients with late-onset Pompe disease and reduced bone density should undergo medical evaluation, including laboratory testing and medication review by an endocrinologist or bone density specialist |
| Conduct fall risk assessment followed by a formal evaluation for balance and safe gait training for patients at increased risk for osteoporosis and falls |
| Recommend adaptive equipment, such as a cane or walker, to reduce risk of falls |
| Physical/occupational therapy |
| A physical or occupational therapist should develop an exercise program that may include one or more of the following: walking, treadmill, cycling, pool-based program, swimming, submaximal aerobic exercise, or muscle strengthening, that follows the guidelines for other degenerative muscle diseases |
| Avoid overwork weakness, excessive fatigue, disuse, strenuous exercises, and eccentric contractions |
| Emphasize submaximal aerobic exercise |
| Incorporate functional activities when possible |
| Teach patient to monitor heart rate and breathing in relation to exertion |
| Integrate energy conservation techniques and biomechanical advantages |
| A preventive stretching regimen should be started early and performed as part of the daily routine to prevent or slow the development of muscle contractures and deformities |
| Management of contractures |
| Manage contractures by using orthotic devices, appropriate seating position in the wheelchair, and standing supports |
| Surgical intervention |
| Surgical intervention should be considered for scoliosis when the Cobb angle is between 30° and 40° |
| Vitamins and mineral supplements |
| Recommend vitamin D, calcium, and bisphosphonates, following the guidelines for other neuromuscular disorders |
Therapeutic exercise should start slowly, allowing for periods of rest, followed by a gradual increase in exercise intensity from mild to moderate, reaching aerobic levels of about 60–70% of maximal effort at a frequency of 3–5 days per week.24,40,43 Optimally, an experienced physical therapist should develop a structured program, monitor the patient, and remain in contact with the prescribing physician. Pulse oximetry, heart rate, and perceived exertional effort (e.g., Borg scale) should be monitored initially to guide progression of the program. Moreover, the physical therapist should teach the patient how to monitor heart rate and correlate it with perceived exertional effort and oxygen saturation information to prepare the patient for a self-monitored, home-based exercise program. A preliminary report of one late-onset Pompe disease patient demonstrated that side-alternating vibration training produced sustained improvement in mobility at 1 year of follow-up.48 Larger studies are needed before this form of training can be recommended.
Contracture and Limb Deformity
Because no established guidelines exist for management of secondary musculoskeletal impairments, including contracture and deformity, in late-onset Pompe disease, general principles established for the management of other neuromuscular disorders can be applied.49 These general principles include limiting contracture and deformity by gentle daily stretching, correction of improper positioning, judicious and timely use of splints and orthotic interventions, and provision of adequate support in all positions, including sitting and supported standing.49 Written instructional materials for the family are more effective than verbal instruction.49 Prevention of contracture and deformity is critical to preserve function and limit other secondary complications, such as skin breakdown and chronic musculoskeletal pain. A preventive stretching regimen should be implemented early and be performed as part of the daily routine. Such regimens are better tolerated and accepted if they are initiated before muscle tendon tightness and contractures develop, a point at which stretching often becomes painful. Aggressive stretching should be approached cautiously because, at least in severely affected children, a tendency for pathologic fracture has been observed.50
Adaptive Equipment and Orthotic Interventions
A variety of orthotic interventions are available for contracture management, such as ankle–foot orthoses (AFOs) to prevent plantarflexion contractures, thigh binders to prevent iliotibial band contractures, knee splints to prevent knee flexion contractures, resting wrist/hand/finger splints to prevent wrist and finger flexor contractures over multiple joints,34 and lumbar corsets for management of back pain. Nocturnal resting splints or AFOs to prevent plantarflexion contractures have not been adequately studied in Pompe disease.49,51 Wheeled walkers or quad canes may help with ambulation, depending on the degree and pattern of muscle weakness. As the disease progresses, adaptive equipment for bathroom needs and transfers will improve safety and independence while decreasing complications. A summary of rehabilitation strategies for musculoskeletal complications in Pompe disease is presented in Table 1. Other physical modalities (e.g., heat therapy) that have not been adequately studied in Pompe disease are not recommended. Appropriate seating systems for wheelchairs are important in the prevention of limb and spinal contractures and deformity. Prescribed equipment could include a head support, solid seat and back, lateral trunk support, and a leg rest/foot plate.52 Supported standing could promote dual treatment goals: it may achieve weight-activated stretching in multiple lower limb joints and may also provide benefit in the management of osteoporosis.53 Various supine, prone, vertical, or hydraulic standers are available, in addition to power standing functions on motorized wheelchairs. These measures can be considered after evaluation by a physical or occupational therapist.24 Prophylactic surgery for contracture management (e.g., tenotomy and tendon transfer) is rarely used and generally does not appear to improve function.49
Scoliosis and Spinal Deformity
Limited evidence is available to guide the management of scoliosis and spinal deformity in late-onset Pompe disease. As a consequence of proximal limb and truncal weakness, and paraspinal muscle atrophy, some patients with late-onset Pompe disease adopt a compensatory lumbar lordosis while standing, whereas others may experience rigid spine syndrome.54,55 Scoliosis progression can be monitored with serial radiography once transition to wheelchair use has occurred. The frequency of scoliosis screening depends on the degree and rate of disease progression and on the patient’s age.
Spinal deformities in Pompe disease may become very severe, and they have the potential to produce several problems. Severe scoliosis and pelvic obliquity lead to pain, poor sitting balance, more difficulty in attendant-provided care, skin ulcers, and potential exacerbation of restrictive lung disease.56 Information is limited regarding spinal bracing in Pompe disease, but in other neuromuscular disorders spinal bracing does not change the natural history of scoliosis.51,57,58 Recent studies in Duchenne dystrophy suggest that corticosteroids slow the progression of scoliosis59,60; it is unclear, however, whether ERT results in a similar benefit in late-onset Pompe disease. Although scoliosis was a common feature in an open-label ERT trial, it was not followed as an outcome measure.36
At present, surgical intervention remains the only effective treatment for progressive spinal deformity in neuromuscular disease. Drawing from experience in other neuromuscular disorders—particularly Duchenne dystrophy—the consensus is that surgical intervention is indicated when the Cobb angle is between 30°and 40°.60–64 As no outcome data are available for spinal corrective surgery in late-onset Pompe disease, the authors recommend that the general clinical guidelines for spinal intervention in Duchenne dystrophy be followed in late-onset Pompe disease and that these decisions be guided by the individual situation. Preoperative pulmonary function testing is essential, although a recent Duchenne dystrophy study showed no significant increase in operative and postoperative complications for patients with FVC >30% of predicted.65 Careful preoperative risk assessment and aggressive postoperative ventilatory support are recommended to minimize morbidity. In those patients at risk, preoperative mask-fitting and initiation of nocturnal noninvasive positive pressure ventilation can facilitate postoperative respiratory recovery.65 Postoperative management after corrective spinal surgery should focus on the early institution of physical therapies, mobilization when clinically stable, pain control, ventilatory support, and pulmonary hygiene. It remains unclear whether spinal surgery leads to sustained improvement in pulmonary function. It is important to keep in mind that a primary goal of either prevention or correction of scoliosis is to provide the patient with improved sitting balance; this, in itself, can help maintain use of a wheelchair, facilitate nursing care, and enhance quality of life.
Osteopenia and Osteoporosis
Low bone mineral density (osteoporosis) is a common feature in patients with Pompe disease. A recent study demonstrated that 67% of the patients tested had a bone mineral density z-score of −1 and that the decrease in bone density was present in both the infantile- and late-onset forms of Pompe disease.66 Therefore, the authors recommend that individuals with late-onset Pompe disease, including all children and adults who are wheelchair- or ventilator-dependent or who have decreasing muscle strength, be screened annually with dual-energy X-ray absorptiometry (DEXA).24,66 On the basis of a recent study showing a high prevalence of osteoporosis in patients with Pompe disease,66 the authors recommend that all patients with Pompe disease undergo fall risk assessment, followed by a more formal evaluation for balance testing and gait training in those individuals at increased risk of falls. Adaptive equipment such as a cane or walker may be recommended to reduce the risk of falls, and education for patients and family members regarding safe ambulation strategies should be provided. Vitamin D and calcium supplements should be taken by all patients with abnormal DEXA z-scores. Bisphosphonate use in late-onset Pompe patients should follow the same guidelines used for the general population. Pompe disease patients with reduced bone density should undergo routine medical evaluation by an endocrinologist or bone density specialist that includes laboratory testing and medication review. Standing frame and weight-bearing exercises can be incorporated into therapy sessions for bone density building.
Pulmonary Manifestations and Management
The respiratory concerns raised by Pompe disease center around progressive weakness of the diaphragm and the accessory muscles of respiration. In late-onset Pompe disease, trunk muscles and proximal muscles in the lower limbs are usually affected first, followed by involvement of the diaphragm and other respiratory muscles. This leads to pulmonary insufficiency and sleep-disordered breathing (SDB).24,67–69 Involvement of the respiratory system can be overlooked when overt respiratory symptoms are absent.67 Frequently, the earliest manifestation of respiratory system involvement is repeated episodes of tracheobronchitis and pneumonia due to expiratory muscle weakness and impaired cough.3 As the disease progresses, all respiratory muscles weaken, leading to lower-than-normal lung volumes.35,70 Sleep-disordered breathing may occur, including nocturnal hypoventilation and oxygen desaturation71 and, consequently, an increased need for ventilatory support.35 Although respiratory insufficiency is manageable with ventilatory assistance,68,69 respiratory insufficiency is the most common cause of death in patients with late-onset Pompe disease.67
Pulmonary Function Tests
Because of the high prevalence of respiratory complications and the progressive nature of Pompe disease, assessment of respiratory function is imperative and should be conducted on a regular basis.20,24,72 Objective assessment of respiratory muscle function is typically obtained by measurements of FVC and maximal inspiratory and expiratory muscle pressures (MIP and MEP, respectively).73,74 It is important to note that, even before an overt reduction in upright FVC occurs, diaphragmatic weakness may be detected by measurement of FVC in both the supine and seated positions.75 Diaphragmatic weakness is suggested if there is a ≥10% decrease of FVC in the supine compared with the upright position; a ≥30% decrease indicates severe weakness.74 In a recent observational study of patients with late-onset Pompe disease, approximately 65% of patients demonstrated reduction in upright FVC and, in the remaining patients, respiratory muscle dysfunction was identified either by reduction in FVC in the supine position or by reduction in MIP and MEP.35,76
Sleep-Disordered Breathing
Sleep-disordered breathing may occur in many neuromuscular disorders because of impaired respiratory muscle function in the supine position and alterations in respiratory control mechanisms during sleep.69,71,73 Ventilatory abnormalities during sleep include obstructive sleep apnea and sustained hypoventilation.68,69,71,73,77 Nocturnal hypoventilation may occur in parallel to or may precede daytime respiratory insufficiency. In neuromuscular disorders, SDB may occur when FVC falls below 30–50% predicted73,78; however, SDB in patients with Pompe disease may occur when upright FVC is only moderately abnormal, because of the disproportionate diaphragmatic involvement.71 Symptoms suggestive of SDB, such as apneas, gasping respirations, and restless sleep24,68 should be assessed as part of the clinical evaluation of all patients with Pompe disease. Until recently, full polysomnography was the preferred method for evaluation of upper airway obstruction; however, other diagnostic modalities are now available for respiratory disorders.78,79 Limited overnight monitoring with pulse oximetry to identify nocturnal hypoxemia and/or capnography to identify hypoventilation coupled with a sensitive airflow detector may be used. Capnography is a noninvasive test that can be used to evaluate hypercapnia; however, false-negative results may be obtained in patients at greatest risk for respiratory insufficiency (e.g., when either tidal volume is reduced or concomitant obstructive lung disease is present).80 Identification of ventilatory abnormalities during sleep is important, and it leads to timely initiation of the appropriate therapy; therefore, it is advisable that the managing physician order sleep studies if there is doubt that the patient might have SDB. Table 2 summarizes the treatment recommendations for the pulmonary aspects of late-onset Pompe disease.
Table 2.
Treatment recommendations for the respiratory element of late-onset Pompe disease.
|
Respiratory Insufficiency
Respiratory insufficiency in patients with late-onset Pompe disease develops because of progressive respiratory muscle weakness, with or without SDB. Because diaphragmatic involvement is an early and prominent finding, respiratory insufficiency can develop while patients are still ambulatory and, in rare cases, it can be the initial manifestation of the disease.28,71,72,81–86 Although assessment for carbon dioxide retention is best accomplished by analysis of arterial blood gases, this test may not be readily available in some outpatient settings. Alternatively, capnography may be used as a surrogate measure, or elevation of serum bicarbonate concentration in venous blood can be used as a screening test for chronic hypoventilation; an elevated serum bicarbonate concentration (>27 mEq/L) may be a marker of renal compensation for chronic carbon dioxide retention.87 Chronic respiratory insufficiency may progress to development of cor pulmonale if left untreated.
Treatment of Respiratory Issues
Maintenance of optimal functional status in patients with Pompe disease requires multiple interventions.24,88 Therapy should be titrated on the basis of the patient’s underlying abnormalities and pulmonary status. Patients benefit from the involvement of a pulmonologist experienced in managing patients with neuromuscular disorders to monitor and treat respiratory manifestations. A cardiologist should be consulted if the patient shows signs or symptoms of heart involvement, which are usually rare in late-onset Pompe disease.89 Chest radiographs should be obtained at baseline and as clinically indicated thereafter. All patients should receive regular vaccinations, including pneumococcus and influenza vaccinations.24
Impairment in secretion clearance due to weakened cough can be addressed by both manual and mechanical techniques.90 Inhaled bronchodilators can be used in conjunction with airway clearance techniques, assisted coughing maneuvers, and inspiratory muscle training.90 Bronchodilators and corticosteroids (inhaled and/or oral) may be useful if concurrent asthma is present. Treatment of both viral and bacterial infections should be early and aggressive.24,90 Treatment of SDB should target the underlying respiratory disorder before supplemental oxygen is given. For patients with obstructive sleep apnea, treatment can be limited to continuous positive airway pressure (CPAP); for patients with nocturnal hypoventilation, treatment with nocturnal noninvasive bilevel positive airway pressure (BiPAP) ventilation should be used.68,71 Pulmonary and sleep consultants are recommended. In the absence of sleep studies, BiPAP should be considered if arterial partial pressure of carbon dioxide is ≥45 mm Hg, supine FVC is <50% of predicted, negative inspiratory force is <60 cm H2O, or oxygen saturation falls to <88% for 5 continuous minutes during sleep.91,92 Supplemental oxygen can be used if these methods do not fully correct the hypoxia, but careful titration of the rate of oxygen administration coupled with close monitoring of arterial carbon dioxide levels are required to prevent oxygen-induced hypercapnia.93
Finally, results from the LOTS trial suggest that ERT may help to improve pulmonary function18; however, until the long-term effect of ERT on respiratory function is known, noninvasive ventilatory support should be pursued whenever the patient’s clinical history suggests SDB. Specific consideration should be given to patients with severely limited ventilatory reserve who are not able to sustain spontaneous breathing during wakefulness for even short periods. Although attention to pulmonary toilet combined with noninvasive ventilatory support may be successful for a prolonged time in selected patients,69 many patients who require continuous ventilatory support will need a tracheostomy to ensure a reliable connection to the airway and successful ventilation.24 Because of the morbidity and subsequent loss of cough associated with tracheostomy, this decision often presents as a therapeutic dilemma.
Gastrointestinal and General Medical Considerations for Late-Onset Pompe Disease
Patients with late-onset Pompe disease develop feeding and swallowing difficulties that can be attributed to facial hypotonia, facial muscle fatigue, tongue weakness and enlargement, fatigue and reduced range of motion of muscles of mastication, reduced tongue capping, and reduced lip seal. The difficulty in feeding and swallowing leads to inadequate intake of proteins and, consequently, endogenous protein breakdown.24 Swallowing dysfunction can be diagnosed by a videofluoroscopic swallowing test.24 An experienced dietitian, preferably one who specializes in metabolic disorders, should be involved in the development and management of a feeding plan for patients with Pompe disease. At this time, there are inadequate data to recommend any specific dietary regimen.40,94 However, a combination high-protein and low-carbohydrate diet has been suggested, which was shown to improve muscle strength in patients with late-onset Pompe disease.95 In addition, ERT may resolve gastrointestinal symptoms in patients with late-onset Pompe disease; however, additional studies are needed to further document the frequency and severity of gastrointestinal symptoms in patients and their rapid amelioration in response to ERT.96 If the risk of aspiration becomes high, oral feeding should be discontinued, and installation of a feeding tube becomes necessary.24 Table 3 lists the recommendations for treatment of the gastrointestinal aspects of late-onset Pompe disease.
Table 3.
Treatment recommendations for the gastrointestinal element of late-onset Pompe disease.24
|
DISCUSSION
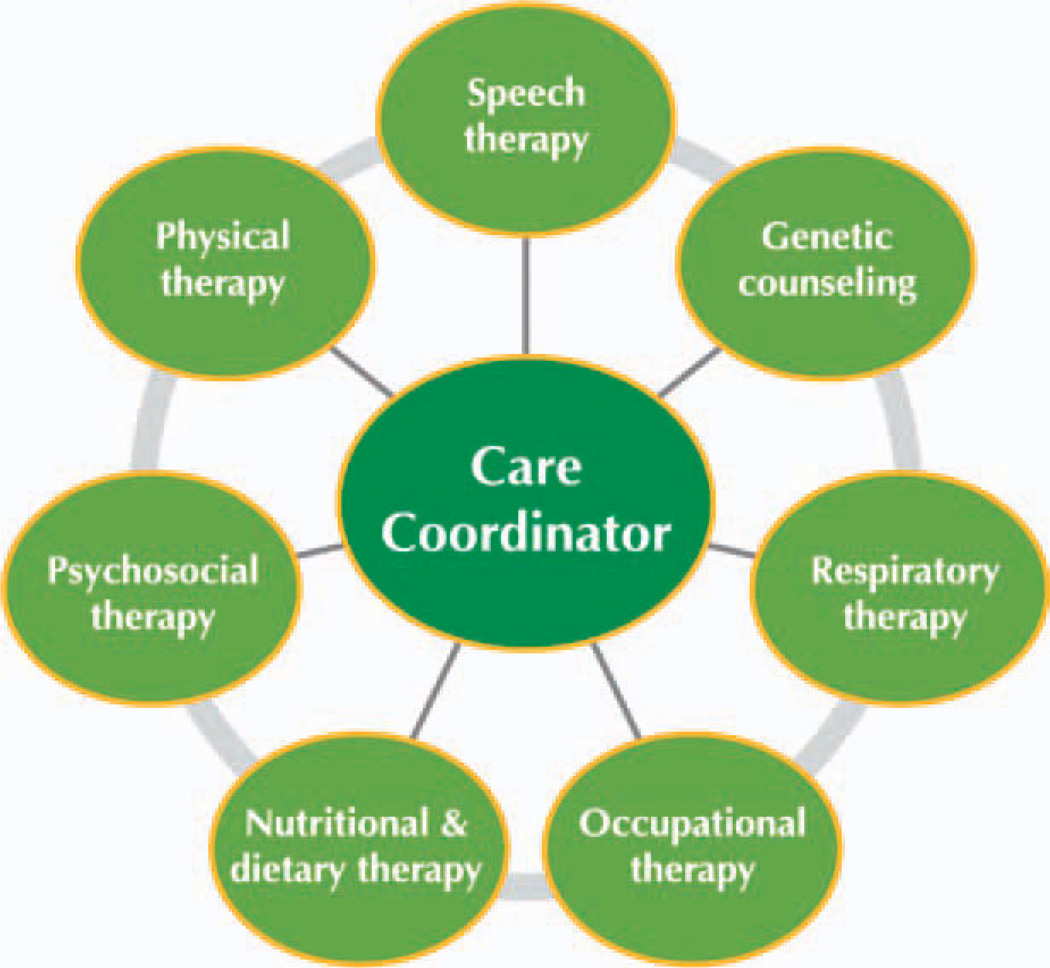
Late-onset Pompe disease is a multisystem disorder that requires the involvement of a multidisciplinary24 team for the proper management and treatment of the pulmonary, neuromuscular, and gastrointestinal elements of the disease (Fig. 3).
FIGURE 3.
Multidisciplinary approach for the management and care of Pompe disease. Based on Kishnani et al.24 [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
The Late-Onset Treatment Study
The success of alglucosidase alfa in the treatment of infantile-onset Pompe disease97 and the reported benefits in several small, open-label studies in late-onset Pompe disease36,98–101 led to the design and implementation of the LOTS trial in patients diagnosed with late-onset Pompe disease.18 Inclusion criteria included the ability to walk 40 meters during the 6-minute walk test (6MWT; with assistive devices permitted) and reproducible FVC 30–80% of predicted in the upright position with a postural drop in FVC of ≥10% after repositioning from upright to supine. Patients were excluded if they required any invasive ventilation or if they needed noninvasive ventilation while awake and upright.18
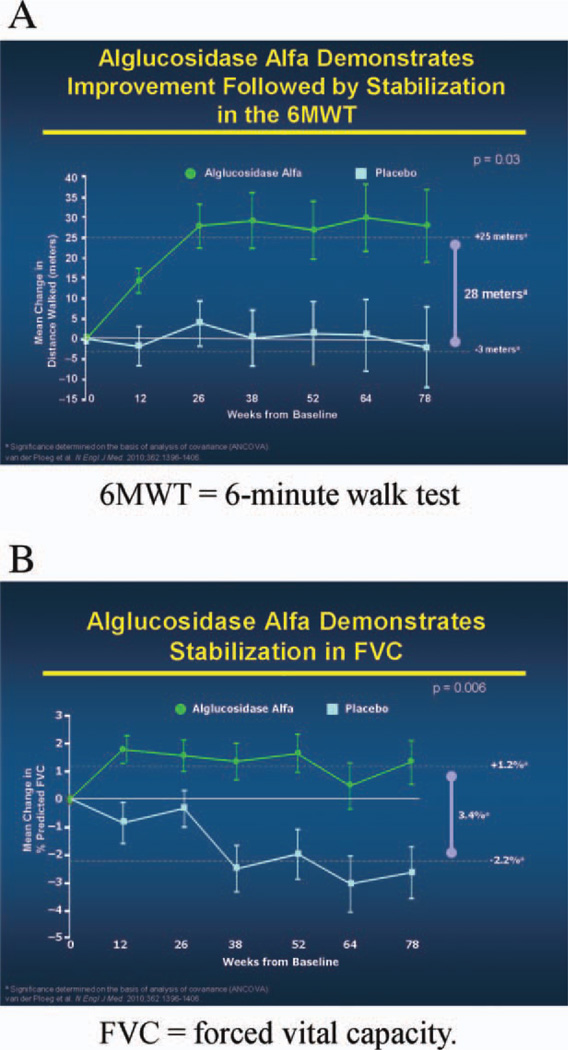
Those patients who qualified were randomly assigned in a ratio of 2:1 to receive biweekly infusions of alglucosidase alfa (20 mg/kg, based on body weight) or placebo. Patients were stratified by baseline distance walked on the 6MWT (<300 or >300 meters) and by baseline values recorded as percentage of predicted FVC in the upright position (<55% or >55%). The LOTS trial was conducted in 90 patients ≥8 years of age (range 10–70 years). The two coprimary endpoints were distance walked during a 6MWT and the percent of predicted FVC in the upright position. At week 78, statistically significant findings in favor of alglucosidase alfa were noted in both the 6MWT and percent of predicted upright FVC results (Fig. 4A and B). The increase in 6MWT distance occurred in the first 26 weeks of treatment and was maintained for the next 52 weeks (Fig. 4A). All of the secondary and tertiary endpoints favored the treatment group, but only the MEP results reached statistical significance.18 Patients in the treatment and placebo groups had similar frequencies of adverse events, serious adverse events, and treatment-related adverse events. Anaphylactic reactions occurred only in patients who received alglucosidase alfa (3 of 60 treated patients, or 5%). All alglucosidase alfa recipients tested negative for immunoglobulin G (IgG) anti-GAA antibodies at baseline, and all patients seroconverted by week 12. The data indicate that alglucosidase alfa has a positive effect on the disease process or processes that produce impaired ambulation and respiratory insufficiency in late-onset Pompe disease, but treatment carries a risk of serious potential complications, including anaphylactic reactions.18,19
FIGURE 4.
Changes from baseline in distance walked and in forced vital capacity according to study group. Based on van der Ploeg et al.14 (A) and van der Ploeg et al.18 (B) with permission. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Perspectives on the LOTS Trial
Currently ERT with alglucosidase alfa is the only specific treatment available for late-onset Pompe disease in adults and children. The greatest gains in all endpoints in the treatment group occurred during the first 26 weeks of therapy with alglucosidase alfa. Those gains were maintained during the 78 weeks of the study.18 The overall clinical response observed in the LOTS trial suggests that prevention of further loss of muscle function is the target treatment goal for ERT. A very modest, but statistically significant, benefit in the 6MWT was reported; however, it is not clear that this improvement is functionally significant. The 6MWT, although useful in clinical trials, does not reflect the routine functional walking employed by most individuals.102 This may explain why the improvements in 6MWT results documented in this study were not associated with subjective improvements in the quality-of-life measure (36-item Short Form [SF-36] Health Survey Questionnaire) that was employed in the same study. Of note, it is possible that the alternate-week, 20-mg/kg dose of alglucosidase alfa, which substantially altered the course of the disease in infantile-onset Pompe disease,97 might be insufficient for patients with the late-onset form of the disease. A longer-term study of alglucosidase alfa in children and adults with late-onset Pompe disease is warranted to fully understand the long-term potential of ERT in this population.
Anaphylactic reactions, a serious potential complication of treatment with any recombinant human protein, have been reported to occur with alglucosidase alfa.97 Because 5% of patients in the treatment group of the LOTS trial developed an anaphylactic reaction, the authors recommend caution during home-based infusion and that patients treated with alglucosidase alfa who have persistently high antibody titers should be closely monitored until the effect of the antibodies is more fully understood (Table 4).19
Table 4.
Treatment warning for alglucosidase alfa (Lumizyme; Genzyme Corporation, Cambridge, Massachusetts).19
| Warning | Life-threatening anaphylactic reactions, severe allergic reactions, and immune-mediated reactions have been observed in some patients during Lumizyme infusions.18 Therefore, appropriate medical support should be readily available when Lumizyme is administered. |
Practical Applications of the LOTS Trial and ERT
The decision making process to treat patients with late-onset Pompe disease may not completely mirror the strict inclusion criteria for the LOTS trial. More information is needed regarding the course and effect of long-term treatment of Pompe disease with alglucosidase alfa in children and adults. However, until such additional information is available, the authors have compiled a series of recommendations, as shown in Table 5.
Table 5.
Treatment recommendations based on the stage and severity of Pompe disease.
| Condition | Recommendations |
|---|---|
| Presymptomatic patients without objective signs |
|
| Presymptomatic patients with objective signs |
|
| Symptomatic patients |
|
| Severe symptoms |
|
| Length of ERT Monitoring |
|
Presymptomatic Patients without Objective Signs
Enzyme replacement therapy is not currently recommended for patients who have no symptoms or objective signs (proximal muscle weakness or reduced FVC in either upright or supine position) of Pompe disease. When a presymptomatic patient is confirmed to have Pompe disease through newborn screening, sibling screening, or another screening program, it is recommended that such patients be examined every 6 months and that muscle strength and pulmonary function (including FVC in both upright and supine positions) be monitored at these visits. Enzyme replacement therapy with alglucosidase alfa is recommended at the earliest onset of symptoms or objective signs of Pompe disease.103
Presymptomatic Patients with Objective Signs
Presymptomatic patients who were confirmed to have Pompe disease through any of the aforementioned screening programs and who have proximal muscle weakness detectable on the Medical Research Council scale or reduction in respiratory parameters, as evidenced by reduced FVC in either the upright or the supine position, should be treated with alglucosidase alfa.
Symptomatic Patients
Enzyme replacement therapy with alglucosidase alfa is recommended for patients who have symptoms or signs of Pompe disease, with demonstrable muscle weakness on physical examination or reduction in pulmonary parameters on pulmonary function testing. Treatment is recommended regardless of the use of noninvasive ventilation and irrespective of whether the patient meets the LOTS trial’s inclusion criteria of FVC <80% of predicted and lower extremity weakness. In addition, ERT is recommended for patients who are very weak physically (e.g., having difficulties completing activities of daily living).
Patients with Severe Symptoms
No randomized clinical trials have provided evidence regarding the effectiveness of ERT in patients who have severe signs and symptoms of Pompe disease and who use a wheelchair and require invasive ventilation during the day and at night. Until such evidence is available, the authors suggest that ERT with alglucosidase alfa be administered for at least 1 year to these patients. After 1 year, decisions regarding the continuation of ERT should be made on a case-by-case basis. Further studies are warranted to determine the utility of ERT in this patient population. Enzyme replacement therapy should be continued if, after 1 year of treatment, patients show no decline in signs and symptoms of Pompe disease or an alteration in the pretreatment course of clinical decline (an indication of prevention, stabilization, reversal, or slowing of disease progression) during routine neuromuscular clinic visits.
Length of ERT
The assumption is that ERT is a long-term, perhaps lifelong, commitment once a patient starts this therapy. Because the LOTS trial showed only modest improvement in the primary endpoints, it might be difficult for a patient or physician to perceive that the patient’s condition has stabilized or improved with ERT. In addition, because of the significant cost of ERT, and because the drug was FDA approved for LOTS on the basis of only one randomized, controlled study, it is reasonable to treat the patient with ERT for 1 year, after which the physician should discuss with the patient whether to continue ERT.
Lumizyme ACE Program
In the USA, Lumizyme is available only through a restricted distribution program—the Lumizyme ACE (Alglucosidase Alfa Control and Education) Program.104 The program is a risk evaluation and mitigation strategy program designed to mitigate the potential risk of rapid disease progression in infantile-onset Pompe disease patients and patients with late-onset disease who are <8 years of age, for whom the safety and efficacy of Lumizyme have not been evaluated in randomized, controlled studies. The Lumizyme ACE Program acts to ensure that …“the known risks of anaphylaxis and severe allergic reactions associated with the use of alglucosidase alfa are communicated to patients and prescribers and to ensure that potential risks of severe cutaneous and systemic immunomediated reactions to alglucosidase alfa are communicated to patients and prescribers.”104 Moreover, prescribers, health-care facilities, and patients treated in the USA must enroll in the Lumizyme ACE Program before alglucosidase alfa will be authorized for shipment, and prescribers and health-care facilities at which ERT infusions will be conducted must complete the Lumizyme ACE Program online certification (www.lumizyme.com/ace/default.asp).104
Newborn Screening Program
In the USA, the Newborn Screening Saves Lives Act was signed into law on April 24, 2008. The law allows for expansion of screenings for newborns and authorization of a grant program to increase funding to state and local health agencies to provide screening, counseling, and health-care services to newborns and children who have or are at risk of developing Pompe disease. Between 2007 and 2010, newborn screening programs for Pompe disease have been mandated by state legislatures in Illinois, Missouri, New Mexico, and New York. The legislation mandates that screening be performed for a panel of lysosomal storage disorders including Pompe, Gaucher, Fabry, Niemann–Pick, and Krabbe diseases. To date, no state has a fully functional program. The two main issues slowing the addition of Pompe disease screening to states’ newborn screening panels are cost and lack of a universal assay. Currently, four assays are being considered: tandem mass spectrometric, fluorometric, immunologic, and digital microfluidic assays. A pilot study of a digital microfluidic chip assay began in Illinois in November 2010.105
A study on the benefits of newborn screening in Taiwan showed that, between October 1, 2005 and December 31, 2007, 45% of all newborns had GAA activity measured in dried blood spots through the newborn screening program.106 Of 206,088 newborns screened, 6 cases of Pompe disease were diagnosed and treated immediately with ERT. After 14–32 months of treatment, infants who had early cardiac involvement demonstrated normalization of cardiac size, with normal physical growth and age-appropriate gains in motor development. The 1 infant without cardiac involvement also achieved normal motor development with treatment.106
RECOMMENDATIONS FOR FUTURE RESEARCH AND MANAGEMENT
The completion of the LOTS trial and the FDA approval of alglucosidase alfa as ERT for late-onset Pompe disease are tremendous achievements, with clear implications for other genetic metabolic disorders. However, much critical work remains to be completed in several areas, especially in relation to late-onset Pompe disease. Because Lumizyme was FDA approved on the basis of only one clinical trial, phase 4 studies are necessary to further evaluate the effect of this agent in the adult population. Additional clinical studies of alternative ERT strategies are urgently needed. Basic research is also needed to establish the links between GAA enzyme deficiency, glycogen deposition, lysosomal function, and skeletal muscle dysfunction. In addition, future research might build on this success and extend treatment benefits to all people living with Pompe disease. Potential studies may involve small-molecule pharmacological chaperones, gene therapy, and new-generation ERT.
Chaperone therapy explores the concept of protecting nascent but deficient enzyme from being targeted for degradation. Chaperone therapy is currently in clinical trials for a variety of lysosomal-storage diseases. Clinical trials of gene therapy for patients with Pompe disease have begun that use a recombinant adeno-associated virus GAA construct. Proof-of-concept trials performed in a mouse model for Pompe disease showed significant improvements in diaphragmatic function and respiratory parameters.107 Proof-of-concept trials that involve mannose-6-phosphate receptors or that target sarcolemmal endocytic lysosomal uptake, or that do both, may help to develop strategies that increase the benefits of alglucosidase alfa therapy.
From a clinical perspective, comprehensive screening studies are needed to confirm what initial studies have suggested—that Pompe disease, and especially late-onset Pompe disease, may be more common than previously thought. As discussed here, the clinical presentation of late-onset Pompe disease is so variable that prospective analyses of large groups of patients who have idiopathic myopathies with or without other organ involvement will be required to determine the frequency, as well as the phenotypic limits, of late-onset Pompe disease. Inherent in the goal of identifying all patients with Pompe disease are a precise determination of the sensitivity and specificity of currently used testing methodologies and a refinement and validation of currently recommended diagnostic algorithms.
Second, there is a need for the development of reliable newborn screening programs for Pompe disease that can be applied universally. Such programs will be invaluable for identifying, at the earliest stage possible, infants who have multiple signs of Pompe disease and are already suspected of having the disorder, as well as presymptomatic infants who can be monitored closely, so that ERT can be administered immediately or at the earliest sign of clinical involvement. Table 6 lists the recommendations for newborn screening programs. Accurate ascertainment programs, especially in late-onset Pompe disease, will also facilitate more robust natural history studies to define the progression and scope of the skeletal muscle and other organ involvement in the disease.
Table 6.
Treatment recommendations based on the experience in Taiwan.106
| The authors recommend the implementation of newborn screening programs in all states to diagnose and properly treat infants with or at risk of developing infantile-onset or late-onset Pompe disease. |
We suggest that the current information on managing the pulmonary, cardiac, and other general medical complications of Pompe disease is inadequate. Future studies should define the optimal methods for detecting early pulmonary involvement in patients by evaluating: (1) supine versus upright pulmonary function testing to assess diaphragmatic weakness; (2) the roles of nocturnal oximetry, capnography, and polysomnography; and (3) the optimal management of any detected respiratory abnormalities. Moreover, it is clear that additional studies are needed to better define the indications for ERT in late-onset Pompe disease, as well as the optimal ERT dose and the length of treatment needed in these cases. Currently, the Exploratory Muscle Biopsy, Biomarker, and Imaging Assessment Study (EMBASSY) of patients with late-onset Pompe disease treated with alglucosidase alfa is being developed and should provide useful information in this regard. However, the effects of ERT on strength, motor function, pulmonary and cardiac status, and quality-of-life measures in late-onset Pompe patients, at varying degrees of disease severity, need to be more fully documented.
Acknowledgments
The authors thank the AANEM and MedLogix Communications, LLC, for editorial support. This article was sponsored by an unrestricted grant from Genzyme Corporation.
Abbreviations
- 6MWT
6-minute walk test
- AANEM
American Association of Neuromuscular & Electrodiagnostic Medicine
- AFOs
ankle-foot orthoses
- BiPAP
bilevel positive airway pressure
- CPAP
continuous positive airway pressure
- DEXA
dual-energy X-ray absorptiometry
- EMBASSY
Exploratory Muscle Biopsy, Biomarker, and Imaging Assessment Study
- EMG
electromyography
- ERT
enzyme replacement therapy
- FDA
Food and Drug Administration
- FVC
forced vital capacity
- GAA
acid alpha-glucosidase
- LOTS
Late-Onset Treatment Study
- MEP
maximal expiratory pressure
- MIP
maximal inspiratory pressure
- rhGAA
recombinant human acid alpha-glucosidase
- SDB
sleep-disordered breathing
- SF-36
36-item Short Form Health Survey Questionnaire
APPENDIX
Author Contributions. Dr. Barohn and Dr. Kissel were the co-chairs of the AANEM Task Force. Dr. Barohn led the discussion of recommendations for future research. Dr. Berger led the discussion on pulmonary manifestations, pulmonary testing, and management in Pompe disease. Dr. Cupler provided expert opinion on the history, pathophysiology, and diagnosis (including a newborn screening program) of Pompe disease. Dr. Han and Dr. Wolfe led the discussion on the musculoskeletal component of Pompe disease. Dr. Kissel, along with Dr. Barohn, provided the recommendations for future research in Pompe disease and provided insightful practical applications of the LOTS trial data. Dr. Leshner provided detailed description and analysis of the LOTS trial and Lumizyme. All authors drafted, reviewed, and edited their designated section(s); provided critical revision of the full article; and approved the final draft of the article. Recommendations were developed by the authors based on a consensus of the group.
Authors’ Note. As recently reported in JAMA (May 11, 2011, Vol. 305, No. 18), providing “trustworthy guidelines” is important to society. The AANEM began the process of working on this report in early 2010 before the release of the Institute of Medicine’s (IOM’s) two reports creating standards for guideline development. The authors have addressed some of the eight recommendations in the IOM paper, which are included for the purpose of full disclosure. This paper was written by an AANEM Task Force. Dr. Barohn and Dr. Kissel were the co-chairs of the task force. They were chosen as chairs because they were free from any conflict. Every attempt was made to include authors without a conflict. Due to several physicians being unable to attend the initial meeting, the final panel involved three physicians without a conflict and four with a conflict (KIB, EJC, TRL, GIW). The papers underwent peer review by the Professional Practice Committee and the AANEM Board. It was believed that those with a conflict were important to include in the creation of the recommendations paper because they had the most experience with Pompe patients. The AANEM reviews the organization’s papers at least every 5 years. In addition, papers are updated when new evidence suggests the need for modification of recommendations included in the paper.
Footnotes
Publisher's Disclaimer: Disclaimer: This article was prepared and reviewed by the AANEM and did not undergo the separate review process of Muscle & Nerve. Approved by the American Association of Neuromuscular & Electrodiagnostic Medicine (AANEM) Board, September 2011.
Disclosures: Task force co-chairs: Dr. Barohn has nothing to disclose. Dr. Kissel serves as a consultant for Alexion Pharmaceuticals for a clinical trial in myasthenia gravis. Task force members: Dr. Han has nothing to disclose. Dr. Berger has served as a consultant to Genzyme Corporation for clinical trials in MPS I and Pompe disease, and he has served as a consultant to BioMarin Corporation for clinical trials in MPS IV and MPS VI. Dr. Cupler is a member of the speaker bureau for Genzyme Corporation and for Athena Diagnostics, Inc., and is a member of the Pompe Registry North American Board of Advisors for Genzyme Corporation. Dr. Leshner is a member of the Genzyme Global Advisory Board for Pompe disease and has received honoraria for speaking engagements and research support from Genzyme Corporation. Dr. Wolfe has served on the speaker bureau for Genzyme Corporation. None of the authors of this article is an inventor, and no authors are receiving royalty payments. All authors received travel support and honoraria for the initial consensus development meeting from MedLogix Communications, LLC, and the AANEM, through an educational grant from Genzyme Corporation.
REFERENCES
- 1.Murphy MK, Black NA, Lamping DL, McKee CM, Sanderson CF, Askham J, et al. Consensus development methods, and their use in clinical guideline development. Health Technol Assess. 1998;2:i–iv. 1–88. [PubMed] [Google Scholar]
- 2.Pompe JC. Over idioptische hypertrophie van het hart. Ned Tidschr Geneeskd. 1932;76:304–311. [Google Scholar]
- 3.Kishnani PS, Howell RR. Pompe disease in infants and children. J Pediatr. 2004;144(suppl):S35–S43. doi: 10.1016/j.jpeds.2004.01.053. [DOI] [PubMed] [Google Scholar]
- 4.Cori GT. Biochemical aspects of glycogen deposition disease. Mod Probl Paediat. 1957;3:344–358. [PubMed] [Google Scholar]
- 5.Hers HG. Alpha-glucosidase deficiency in generalized glycogen-storage disease (Pompe’s disease) Biochem J. 1963;86:11–16. doi: 10.1042/bj0860011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.de Duve C, Pressman B, Gianetto T, Wattiaux D, Appelmans F. Tissue fractionation studies. Biochem J. 1955;60:604–617. doi: 10.1042/bj0600604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Engel AG, Seybold ME, Lambert EH, Gomez MR. Acid maltase deficiency: comparison of infantile, childhood, and adult types. Neurology. 1970;20:382. [PubMed] [Google Scholar]
- 8.Chien YH, Chiang SC, Zhang XK, Keutzer J, Lee NC, Huang AC, et al. Early detection of Pompe disease by newborn screening is feasible: results from the Taiwan screening program. Pediatrics. 2008;122:e39–e45. doi: 10.1542/peds.2007-2222. [DOI] [PubMed] [Google Scholar]
- 9.Ausems MG, Verbiest J, Hermans MP, Kroos MA, Beemer FA, Wokke JH, et al. Frequency of glycogen storage disease type II in The Netherlands: implications for diagnosis and genetic counselling. Eur J Hum Genet. 1999;7:713–716. doi: 10.1038/sj.ejhg.5200367. [DOI] [PubMed] [Google Scholar]
- 10.Ausems MG, Lochman P, van Diggelen OP, Ploos van Amstel HK, Reuser AJ, Wokke JH. A diagnostic protocol for adult-onset glycogen storage disease type II. Neurology. 1999;52:851–853. doi: 10.1212/wnl.52.4.851. [DOI] [PubMed] [Google Scholar]
- 11.Martiniuk F, Chen A, Mack A, Arvanitopoulos E, Chen Y, Rom WN, et al. Carrier frequency for glycogen storage disease type II in New York and estimates of affected individuals born with the disease. Am J Med Genet. 1998;79:69–72. doi: 10.1002/(sici)1096-8628(19980827)79:1<69::aid-ajmg16>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 12.de Duve C, Wattiaux R. Functions of lysosomes. Annu Rev Physiol. 1966;28:435–492. doi: 10.1146/annurev.ph.28.030166.002251. [DOI] [PubMed] [Google Scholar]
- 13.de Barsy T, Jacquemin P, van Hoof F, Hers HG. Enzyme replacement in Pompe disease: an attempt with purified human acid alpha-glucosidase. Birth Defects Orig Artic Ser. 1973;9:184–190. [PubMed] [Google Scholar]
- 14.Van den Hout JM, Kamphoven JH, Winkel LP, Arts WF, de Klerk JB, Loonen MC, et al. Long-term intravenous treatment of Pompe disease with recombinant human alpha-glucosidase from milk. Pediatrics. 2004;113:e448–e457. doi: 10.1542/peds.113.5.e448. [DOI] [PubMed] [Google Scholar]
- 15.Honig J, Martiniuk F, D’Eustachio P, Zamfirescu C, Desnick R, Hirschhorn K, et al. Confirmation of the regional localization of the genes for human acid alpha-glucosidase (GAA) and adenosine deaminase (ADA) by somatic cell hybridization. Ann Hum Genet. 1984;48:49–56. doi: 10.1111/j.1469-1809.1984.tb00833.x. [DOI] [PubMed] [Google Scholar]
- 16.Myozyme [package insert] Cambridge, MA: Genzyme Corporation; 2010. [Google Scholar]
- 17.Kishnani PS, Nicolino M, Voit T, Rogers RC, Tsai AC, Waterson J, et al. Chinese hamster ovary cell-derived recombinant human acid alpha-glucosidase in infantile-onset Pompe disease. J Pediatr. 2006;149:89–97. doi: 10.1016/j.jpeds.2006.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.van der Ploeg AT, Clemens P, Corzo D, Escolar D, Florence J, Groeneveld G, et al. A randomized study of alglucosidase alfa in late-onset Pompe’s disease. N Engl J Med. 2010;362:1396–1406. doi: 10.1056/NEJMoa0909859. [DOI] [PubMed] [Google Scholar]
- 19.Lumizyme [package insert] Cambridge, MA: Genzyme Corporation; 2010. [Google Scholar]
- 20.American Association of Neuromuscular & Electrodiagnostic Medicine (AANEM) Diagnostic criteria for late-onset (childhood and adult) Pompe disease. Muscle Nerve. 2009;40:149–160. doi: 10.1002/mus.21393. [DOI] [PubMed] [Google Scholar]
- 21.Raben N, Roberts A, Plotz PH. Role of autophagy in the pathogenesis of Pompe disease. Acta Myol. 2007;26:45–48. [PMC free article] [PubMed] [Google Scholar]
- 22.Reuser AJ, Drost MR. Lysosomal dysfunction, cellular pathology and clinical symptoms: basic principles. Acta Paediatr Suppl. 2006;95:77–82. doi: 10.1111/j.1651-2227.2006.tb02395.x. [DOI] [PubMed] [Google Scholar]
- 23.Hirschhorn R, Reuser AJ. Glycogen storage disease type II: acid α-glucosidase (acid maltase) deficiency. [accessed August 9, 2011];Scriver’s OMMBID website. http://www.ommbid.com//OMMBID/the_online_metabolic_and_molecular_bases_of_inherited_disease/b/abstract/part16/ch135. [Google Scholar]
- 24.Kishnani PS, Steiner RD, Bali D, Berger K, Byrne BJ, Case LE, et al. Pompe disease diagnosis and management guideline. Genet Med. 2006;8:267–288. doi: 10.1097/01.gim.0000218152.87434.f3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Raben N, Takikita S, Pittis MG, Bembi B, Marie SK, Roberts A, et al. Deconstructing Pompe disease by analyzing single muscle fibers: to see a world in a grain of sand. Autophagy. 2007;3:546–552. doi: 10.4161/auto.4591. [DOI] [PubMed] [Google Scholar]
- 26.Kroos M, Pomponio RJ, van Vliet L, Palmer RE, Phipps M, van der Helm R, et al. Update of the Pompe disease mutation database with 107 sequence variants and a format for severity rating. Hum Mutat. 2008;29:E13–E26. doi: 10.1002/humu.20745. [DOI] [PubMed] [Google Scholar]
- 27.Kishnani PS, Hwu WL, Mandel H, Nicolino M, Yong F, Corzo D. A retrospective, multinational, multicenter study on the natural history of infantile-onset Pompe disease. J Pediatr. 2006;148:671–676. doi: 10.1016/j.jpeds.2005.11.033. [DOI] [PubMed] [Google Scholar]
- 28.Müller-Felber W, Horvath R, Gempel K, Podskarbi T, Shin Y, Pongratz D, et al. Late onset Pompe disease: clinical and neurophysiological spectrum of 38 patients including long-term follow-up in 18 patients. Neuromuscul Disord. 2007;17:698–706. doi: 10.1016/j.nmd.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 29.Barohn RJ, McVey AL, DiMauro S. Adult acid maltase deficiency. Muscle Nerve. 1993;16:672–676. doi: 10.1002/mus.880160614. [DOI] [PubMed] [Google Scholar]
- 30.Barnes D, Hughes RA, Spencer GT. Adult-onset acid maltase deficiency with prominent bulbar involvement and ptosis. J R Soc Med. 1993;86:50. [PMC free article] [PubMed] [Google Scholar]
- 31.Amato AA. Acid maltase deficiency and related myopathies. Neurol Clin. 2000;18:151–165. doi: 10.1016/s0733-8619(05)70182-1. [DOI] [PubMed] [Google Scholar]
- 32.Kallwass H, Carr C, Gerrein J, Titlow M, Pomponio R, Bali D, et al. Rapid diagnosis of late-onset Pompe disease by fluorometric assay of alpha-glucosidase activities in dried blood spots. Mol Genet Metab. 2007;90:449–452. doi: 10.1016/j.ymgme.2006.12.006. [DOI] [PubMed] [Google Scholar]
- 33.Engel AG, Hirschhorn R, Huie M. Acid maltase deficiency. In: Engel AG, Franzini AC, editors. Myology. 3rd ed. New York: McGraw-Hill; 2004. pp. 1559–1586. [Google Scholar]
- 34.Case LE, Kishnani PS. Physical therapy management of Pompe disease. Genet Med. 2006;8:318–327. doi: 10.1097/01.gim.0000217789.14470.c5. [DOI] [PubMed] [Google Scholar]
- 35.Wokke JH, Escolar DM, Pestronk A, Jaffe KM, Carter GT, van den Berg LH, et al. Clinical features of late-onset Pompe disease: a prospective cohort study. Muscle Nerve. 2008;38:1236–1245. doi: 10.1002/mus.21025. [DOI] [PubMed] [Google Scholar]
- 36.Strothotte S, Strigl-Pill N, Grunert B, Kornblum C, Eger K, Wessig C, et al. Enzyme replacement therapy with alglucosidase alfa in 44 patients with late-onset glycogen storage disease type 2: 12-month results of an observational clinical trial. J Neurol. 2010;257:91–97. doi: 10.1007/s00415-009-5275-3. [DOI] [PubMed] [Google Scholar]
- 37.Hagemans ML, Winkel LP, van Doorn PA, Hop WJ, Loonen MC, Reuser AJ, et al. Clinical manifestation and natural course of lateonset Pompe’s disease in 54 Dutch patients. Brain. 2005;128:671–677. doi: 10.1093/brain/awh384. [DOI] [PubMed] [Google Scholar]
- 38.van der Beek NA, Hagemans ML, Reuser AJ, Hop WC, van der Ploeg AT, van Doorn PA, et al. Rate of disease progression during long-term follow-up of patients with late-onset Pompe disease. Neuromuscul Disord. 2009;19:113–117. doi: 10.1016/j.nmd.2008.11.007. [DOI] [PubMed] [Google Scholar]
- 39.Slonim AE, Bulone L, Minikes J, Hays AP, Shanske S, Tsujino S, et al. Benign course of glycogen storage disease type IIb in two brothers: nature or nurture? Muscle Nerve. 2006;33:571–574. doi: 10.1002/mus.20473. [DOI] [PubMed] [Google Scholar]
- 40.Slonim AE, Bulone L, Goldberg T, Minikes J, Slonim E, Galanko J, et al. Modification of the natural history of adult-onset acid maltase deficiency by nutrition and exercise therapy. Muscle Nerve. 2007;35:70–77. doi: 10.1002/mus.20665. [DOI] [PubMed] [Google Scholar]
- 41.Bembi B, Ciana G, Martini C, Benettoni A, Gombacci A, Deganuto M, et al. Efficacy of multidisciplinary approach in the treatment of two cases of nonclassical infantile glycogenosis type II. J Inherit Metab Dis. 2003;26:675–681. doi: 10.1023/b:boli.0000005618.76542.ed. [DOI] [PubMed] [Google Scholar]
- 42.Sayers SP, Clarkson PM, Rouzier PA, Kamen G. Adverse events associated with eccentric exercise protocols: six case studies. Med Sci Sports Exerc. 1999;31:1697–1702. doi: 10.1097/00005768-199912000-00002. [DOI] [PubMed] [Google Scholar]
- 43.Abresch RT, Han JJ, Carter GT. Rehabilitation management of neuromuscular disease: the role of exercise training. J Clin Neuromuscul Dis. 2009;11:7–21. doi: 10.1097/CND.0b013e3181a8d36b. [DOI] [PubMed] [Google Scholar]
- 44.Clarkson PM. Exercise-induced muscle damage—animal and human models. Med Sci Sports Exerc. 1992;24:510–511. [PubMed] [Google Scholar]
- 45.Griffin J. Infantile acid maltase deficiency. I. Muscle fiber destruction after lysosomal rupture. Virchows Arch B Cell Pathol Incl Mol Pathol. 1984;45:23–36. doi: 10.1007/BF02889849. [DOI] [PubMed] [Google Scholar]
- 46.Fowler WM., Jr Role of physical activity and exercise training in neuromuscular diseases. Am J Phys Med Rehabil. 2002;81(suppl):S187–S195. doi: 10.1097/01.PHM.0000029726.80774.83. [DOI] [PubMed] [Google Scholar]
- 47.Eagle M. Report on the muscular dystrophy campaign workshop: exercise in neuromuscular diseases Newcastle, January 2002. Neuromuscul Disord. 2002;12:975–983. doi: 10.1016/s0960-8966(02)00136-0. [DOI] [PubMed] [Google Scholar]
- 48.Khan A, Ramage B, Robu I, Benard L. Side-alternating vibration training improves muscle performance in a patient with late-onset Pompe disease. Case Report Med. 2009 doi: 10.1155/2009/741087. 741087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.McDonald CM. Limb contractures in progressive neuromuscular disease and the role of stretching, orthotics, and surgery. Phys Med Rehabil Clin N Am. 1998;9:187–211. [PubMed] [Google Scholar]
- 50.Case LE, Hanna R, Frush DP, Krishnamurthy V, DeArmey S, Mackey J, et al. Fractures in children with Pompe disease: a potential long-term complication. Pediatr Radiol. 2007;37:437–445. doi: 10.1007/s00247-007-0428-y. [DOI] [PubMed] [Google Scholar]
- 51.Brooke MH, Fenichel GM, Griggs RC, Mendell JR, Moxley R, Florence J, et al. Duchenne muscular dystrophy: patterns of clinical progression and effects of supportive therapy. Neurology. 1989;39:475–481. doi: 10.1212/wnl.39.4.475. [DOI] [PubMed] [Google Scholar]
- 52.Sabol TP, Haley ES. Wheelchair evaluation for the older adult. Clin Geriatr Med. 2006;22:355–375. doi: 10.1016/j.cger.2005.12.013. [DOI] [PubMed] [Google Scholar]
- 53.Ward K, Alsop C, Caulton J, Rubin C, Adams J, Mughal Z. Low magnitude mechanical loading is osteogenic in children with disabling conditions. J Bone Miner Res. 2004;19:360–369. doi: 10.1359/JBMR.040129. [DOI] [PubMed] [Google Scholar]
- 54.Laforêt P, Doppler V, Caillaud C, Laloui K, Claeys KG, Richard P, et al. Rigid spine syndrome revealing late-onset Pompe disease. Neuromuscul Disord. 2010;20:128–130. doi: 10.1016/j.nmd.2009.11.006. [DOI] [PubMed] [Google Scholar]
- 55.Kostera-Pruszczyk A, Opuchlik A, Lugowska A, Nadaj A, Bojakowski J, Tylki-Szymanska A, et al. Juvenile onset acid maltase deficiency presenting as a rigid spine syndrome. Neuromuscul Disord. 2006;16:282–285. doi: 10.1016/j.nmd.2006.02.001. [DOI] [PubMed] [Google Scholar]
- 56.Hart DA, McDonald CM. Spinal deformity in progressive neuromuscular disease. Natural history and management. Phys Med Rehabil Clin N Am. 1998;9:213–232. [PubMed] [Google Scholar]
- 57.Cambridge W, Drennan JC. Scoliosis associated with Duchenne muscular dystrophy. J Pediatr Orthop. 1987;7:436–440. doi: 10.1097/01241398-198707000-00011. [DOI] [PubMed] [Google Scholar]
- 58.Merlini L, Granata C, Bonfiglioli S, Marini ML, Cervellati S, Savini R. Scoliosis in spinal muscular atrophy: natural history and management. Dev Med Child Neurol. 1989;31:501–508. doi: 10.1111/j.1469-8749.1989.tb04029.x. [DOI] [PubMed] [Google Scholar]
- 59.Alman BA, Raza SN, Biggar WD. Steroid treatment and the development of scoliosis in males with Duchenne muscular dystrophy. J Bone Joint Surg Am. 2004;86-A:519–524. doi: 10.2106/00004623-200403000-00009. [DOI] [PubMed] [Google Scholar]
- 60.Kinali M, Messina S, Mercuri E, Lehovsky J, Edge G, Manzur AY, et al. Management of scoliosis in Duchenne muscular dystrophy: a large 10-year retrospective study. Dev Med Child Neurol. 2006;48:513–518. doi: 10.1017/S0012162206001083. [DOI] [PubMed] [Google Scholar]
- 61.Cervellati S, Bettini N, Moscato M, Gusella A, Dema E, Maresi R. Surgical treatment of spinal deformities in Duchenne muscular dystrophy: a long term follow-up study. Eur Spine J. 2004;13:441–448. doi: 10.1007/s00586-002-0515-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Oda T, Shimizu N, Yonenobu K, Ono K, Nabeshima T, Kyoh S. Longitudinal study of spinal deformity in Duchenne muscular dystrophy. J Pediatr Orthop. 1993;13:478–488. doi: 10.1097/01241398-199307000-00012. [DOI] [PubMed] [Google Scholar]
- 63.Granata C, Merlini L, Cervellati S, Ballestrazzi A, Giannini S, Corbascio M, et al. Long-term results of spine surgery in Duchenne muscular dystrophy. Neuromuscul Disord. 1996;6:61–68. doi: 10.1016/0960-8966(95)00019-4. [DOI] [PubMed] [Google Scholar]
- 64.Miller F, Moseley CF, Koreska J. Spinal fusion in Duchenne muscular dystrophy. Dev Med Child Neurol. 1992;34:775–786. doi: 10.1111/j.1469-8749.1992.tb11516.x. [DOI] [PubMed] [Google Scholar]
- 65.Harper CM, Ambler G, Edge G. The prognostic value of pre-operative predicted forced vital capacity in corrective spinal surgery for Duchenne’s muscular dystrophy. Anaesthesia. 2004;59:1160–1162. doi: 10.1111/j.1365-2044.2004.03940.x. [DOI] [PubMed] [Google Scholar]
- 66.van den Berg LE, Zandbergen AA, van Capelle CI, de Vries JM, Hop WC, Van den Hout JM, et al. Low bone mass in Pompe disease: muscular strength as a predictor of bone mineral density. Bone. 2010;47:643–649. doi: 10.1016/j.bone.2010.06.021. [DOI] [PubMed] [Google Scholar]
- 67.Katzin LW, Amato AA. Pompe disease: a review of the current diagnosis and treatment recommendations in the era of enzyme replacement therapy. J Clin Neuromuscul Dis. 2008;9:421–431. doi: 10.1097/CND.0b013e318176dbe4. [DOI] [PubMed] [Google Scholar]
- 68.Mellies U, Stehling F, Dohna-Schwake C, Ragette R, Teschler H, Voit T. Respiratory failure in Pompe disease: treatment with noninvasive ventilation. Neurology. 2005;64:1465–1467. doi: 10.1212/01.WNL.0000158682.85052.C0. [DOI] [PubMed] [Google Scholar]
- 69.Perrin C, D’Ambrosio C, White A, Hill NS. Sleep in restrictive and neuromuscular respiratory disorders. Semin Respir Crit Care Med. 2005;26:117–130. doi: 10.1055/s-2005-864205. [DOI] [PubMed] [Google Scholar]
- 70.Laforêt P, Nicolino M, Eymard PB, Puech JP, Caillaud C, Poenaru L, et al. Juvenile and adult-onset acid maltase deficiency in France: genotype–phenotype correlation. Neurology. 2000;55:1122–1128. doi: 10.1212/wnl.55.8.1122. [DOI] [PubMed] [Google Scholar]
- 71.Mellies U, Ragette R, Schwake C, Baethmann M, Voit T, Teschler H. Sleep-disordered breathing and respiratory failure in acid maltase deficiency. Neurology. 2001;57:1290–1295. doi: 10.1212/wnl.57.7.1290. [DOI] [PubMed] [Google Scholar]
- 72.Pellegrini N, Laforêt P, Orlikowski D, Pellegrini M, Caillaud C, Eymard B, et al. Respiratory insufficiency and limb muscle weakness in adults with Pompe’s disease. Eur Respir J. 2005;26:1024–1031. doi: 10.1183/09031936.05.00020005. [DOI] [PubMed] [Google Scholar]
- 73.Ward NS, Hill NS. Pulmonary function testing in neuromuscular disease. Clin Chest Med. 2001;22:769–781. doi: 10.1016/s0272-5231(05)70065-4. [DOI] [PubMed] [Google Scholar]
- 74.American Thoracic Society/European Respiratory Society (ATS/ERS) statement on respiratory muscle testing. Am J Respir Crit Care Med. 2002;166:518–624. doi: 10.1164/rccm.166.4.518. [DOI] [PubMed] [Google Scholar]
- 75.van der Ploeg AT. Monitoring of pulmonary function in Pompe disease: a muscle disease with new therapeutic perspectives. Eur Respir J. 2005;26:984–985. doi: 10.1183/09031936.05.00112005. [DOI] [PubMed] [Google Scholar]
- 76.Berger KI, Skrinar A, Norman RG, et al. Ventilatory dysfunction in late onset Pompe disease. Proc Am Thorac Soc. 2005;171:A788. [Google Scholar]
- 77.Margolis ML, Howlett P, Goldberg R, Eftychiadis A, Levine S. Obstructive sleep apnea syndrome in acid maltase deficiency. Chest. 1994;105:947–949. doi: 10.1378/chest.105.3.947. [DOI] [PubMed] [Google Scholar]
- 78.Mellies U, Dohna-Schwake C, Voit T. Respiratory function assessment and intervention in neuromuscular disorders. Curr Opin Neurol. 2005;18:543–547. doi: 10.1097/01.wco.0000180662.03544.5f. [DOI] [PubMed] [Google Scholar]
- 79.Sleep-related breathing disorders in adults: recommendations for syndrome definition and measurement techniques in clinical research. The report of an American Academy of Sleep Medicine Task Force. Sleep. 1999;22:667–689. [PubMed] [Google Scholar]
- 80.Gay PC. Sleep and sleep-disordered breathing in the hospitalized patient. Respir Care. 2010;55:1240–1254. [PubMed] [Google Scholar]
- 81.Moufarrej NA, Bertorini TE. Respiratory insufficiency in adult-type acid maltase deficiency. South Med J. 1993;86:560–567. doi: 10.1097/00007611-199305000-00015. [DOI] [PubMed] [Google Scholar]
- 82.Keunen RW, Lambregts PC, Op de Coul AA, Joosten EM. Respiratory failure as initial symptom of acid maltase deficiency. J Neurol Neurosurg Psychiatry. 1984;47:549–552. doi: 10.1136/jnnp.47.5.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kim DG, Jung K, Lee MK, Hyun IG, Lim HJ, Song HG, et al. A case of juvenile form Pompe’s disease manifested as chronic alveolar hypoventilation. J Korean Med Sci. 1993;8:221–224. doi: 10.3346/jkms.1993.8.3.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Trikalinos TA, Ip S, Raman G, Cepeda MS, Balk EM, D’Ambrosio C, et al. Home diagnosis of obstructive sleep apnea–hypopnea syndrome. Rockville, MD: Agency for Healthcare Research and Quality; 2007. pp. 1–127. [PubMed] [Google Scholar]
- 85.Winkel LP, Hagemans ML, van Doorn PA, Loonen MC, Hop WJ, Reuser AJ, et al. The natural course of non-classic Pompe’s disease; a review of 225 published cases. J Neurol. 2005;252:875–884. doi: 10.1007/s00415-005-0922-9. [DOI] [PubMed] [Google Scholar]
- 86.Rosenow EC, Engel AG. Acid maltase deficiency in adults presenting as respiratory failure. Am J Med. 1978;64:485–491. doi: 10.1016/0002-9343(78)90235-8. [DOI] [PubMed] [Google Scholar]
- 87.Mokhlesi B, Tulaimat A, Faibussowitsch I, Wang Y, Evans AT. Obesity hypoventilation syndrome: prevalence and predictors in patients with obstructive sleep apnea. Sleep Breath. 2007;11:117–124. doi: 10.1007/s11325-006-0092-8. [DOI] [PubMed] [Google Scholar]
- 88.Mellies U, Lofaso F. Pompe disease: a neuromuscular disease with respiratory muscle involvement. Respir Med. 2009;103:477–484. doi: 10.1016/j.rmed.2008.12.009. [DOI] [PubMed] [Google Scholar]
- 89.Soliman OI, van der Beek NA, van Doorn PA, Vletter WB, Nemes A, van Dalen BM, et al. Cardiac involvement in adults with Pompe disease. J Intern Med. 2008;264:333–339. doi: 10.1111/j.1365-2796.2008.01966.x. [DOI] [PubMed] [Google Scholar]
- 90.Finder JD, Birnkrant D, Carl J, Farber HJ, Gozal D, Iannaccone ST, et al. Respiratory care of the patient with Duchenne muscular dystrophy: ATS consensus statement. Am J Respir Crit Care Med. 2004;170:456–465. doi: 10.1164/rccm.200307-885ST. [DOI] [PubMed] [Google Scholar]
- 91.LCD for Respiratory Assist Devices (L27228) National Government Services website. 2010;Vol. 2011 http://apps.ngsmedicare.com/applications/Content.aspx?DOCID=21448&CatID=3&RegID=51&ContentID=34363. [Google Scholar]
- 92.Clinical indications for noninvasive positive pressure ventilation in chronic respiratory failure due to restrictive lung disease, COPD, and nocturnal hypoventilation—a consensus conference report. Chest. 1999;116:521–534. doi: 10.1378/chest.116.2.521. [DOI] [PubMed] [Google Scholar]
- 93.Aubier M, Murciano D, Milic-Emili J, Touaty E, Daghfous J, Pariente R, et al. Effects of the administration of O2 on ventilation and blood gases in patients with chronic obstructive pulmonary disease during acute respiratory failure. Am Rev Respir Dis. 1980;122:747–754. doi: 10.1164/arrd.1980.122.5.747. [DOI] [PubMed] [Google Scholar]
- 94.Ravaglia S, Pichiecchio A, Rossi M, Filippi PD, Minelli A, Moglia A, et al. Dietary treatment in adult-onset type II glycogenosis. J Inherit Metab Dis. 2006;29:590. doi: 10.1007/s10545-006-0144-z. [DOI] [PubMed] [Google Scholar]
- 95.Schoser B, Hill V, Raben N. Therapeutic approaches in glycogen storage disease type II/Pompe disease. Neurotherapeutics. 2008;5:569–578. doi: 10.1016/j.nurt.2008.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Bernstein DL, Bialer MG, Mehta L, Desnick RJ. Pompe disease: dramatic improvement in gastrointestinal function following enzyme replacement therapy. A report of three later-onset patients. Mol Genet Metab. 2010;101:130–133. doi: 10.1016/j.ymgme.2010.06.003. [DOI] [PubMed] [Google Scholar]
- 97.Kishnani PS, Corzo D, Nicolino M, Byrne B, Mandel H, Hwu WL, et al. Recombinant human acid [alpha]-glucosidase: major clinical benefits in infantile-onset Pompe disease. Neurology. 2007;68:99–109. doi: 10.1212/01.wnl.0000251268.41188.04. [DOI] [PubMed] [Google Scholar]
- 98.Winkel LP, Van den Hout JM, Kamphoven JH, Disseldorp JA, Remmerswaal M, Arts WF, et al. Enzyme replacement therapy in late-onset Pompe’s disease: a three-year follow-up. Ann Neurol. 2004;55:495–502. doi: 10.1002/ana.20019. [DOI] [PubMed] [Google Scholar]
- 99.van Capelle CI, Winkel LP, Hagemans ML, Shapira SK, Arts WF, van Doorn PA, et al. Eight years experience with enzyme replacement therapy in two children and one adult with Pompe disease. Neuromuscul Disord. 2008;18:447–452. doi: 10.1016/j.nmd.2008.04.009. [DOI] [PubMed] [Google Scholar]
- 100.Ravaglia S, Moglia A, Costa A, Repetto A, Danesino C. Enzyme replacement therapy in late-onset type II glycogenosis. Eur J Neurol. 2009;16:e125. doi: 10.1111/j.1468-1331.2009.02667.x. [DOI] [PubMed] [Google Scholar]
- 101.Merk T, Wibmer T, Schumann C, Kruger S. Glycogen storage disease type II (Pompe disease)—influence of enzyme replacement therapy in adults. Eur J Neurol. 2009;16:274–277. doi: 10.1111/j.1468-1331.2008.02377.x. [DOI] [PubMed] [Google Scholar]
- 102.Orendurff MS, Schoen JA, Bernatz GC, Segal AD, Klute GK. How humans walk: bout duration, steps per bout, and rest duration. J Rehabil Res Dev. 2008;45:1077–1089. doi: 10.1682/jrrd.2007.11.0197. [DOI] [PubMed] [Google Scholar]
- 103.Laloui K, Wary C, Carlier RY, Hogrel JY, Caillaud C, Laforêt P. Making diagnosis of Pompe disease at a presymptomatic stage: to treat or not to treat? Neurology. 2011;77:594–595. doi: 10.1212/WNL.0b013e318228c0ea. [DOI] [PubMed] [Google Scholar]
- 104.Lumizyme ACE Program. Vol. 2011. Cambridge, MA: Genzyme Corporation; 2011. Genzyme. [Google Scholar]
- 105.Millington DS, Sista R, Eckhardt A, Rouse J, Bali D, Goldberg R, et al. Digital microfluidics: a future technology in the newborn screening laboratory? Semin Perinatol. 2010;34:163–169. doi: 10.1053/j.semperi.2009.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Chien YH, Lee NC, Thurberg BL, Chiang SC, Zhang XK, Keutzer J, et al. Pompe disease in infants: improving the prognosis by newborn screening and early treatment. Pediatrics. 2009;124:e1116–e1125. doi: 10.1542/peds.2008-3667. [DOI] [PubMed] [Google Scholar]
- 107.Mah CS, Falk DJ, Germain SA, Kelley JS, Lewis MA, Cloutier DA, et al. Gel-mediated delivery of AAV1 vectors corrects ventilatory function in Pompe mice with established disease. Mol Ther. 2010;18:502–510. doi: 10.1038/mt.2009.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Raben N, Plotz P, Byrne BJ. Acid alpha-glucosidase deficiency (glycogenosis type II Pompe disease) Curr Mol Med. 2002;2:145–166. doi: 10.2174/1566524024605789. [DOI] [PubMed] [Google Scholar]