Abstract
Kisspeptins, and their G-protein coupled receptor 54 (GPR54), are key components in the regulation of gonadotropin-releasing hormone (GnRH) secretion in humans and other mammals. Several studies demonstrate that the central or systemic administration of kisspeptin increases GnRH and gonadotropin secretion in both prepubertal and adult animals; however, the cellular targets and intracellular mechanisms of action in the central reproductive axis are unclear. In this study, we documented the presence of GPR54 in two GnRH secreting neuronal cell lines (GT1-7 and GN11). Kisspeptin treatment increases GnRH secretion and GnRH mRNA levels in a dose and time dependent manner. 10−9M Kisspeptin maximally stimulated GnRH secretion by 2-fold and GnRH mRNA levels up to 4-fold after 4 hours of treatment in both cell lines. Negative regulation by 17β-estradiol of GnRH secretion and GnRH mRNA was antagonized by kisspeptin. Co-treatment with kisspeptin and 17β-estradiol increased GnRH secretion by 2-fold and GnRH mRNA by 4-fold over estradiol alone in both cell lines. Intracellular signaling pathway studies showed that an ERK1/2 MAPK inhibitor (PD98059) and a PI3K inhibitor, LY29402, attenuated the effects of kisspeptin on GnRH mRNA modulation. Furthermore, Western blot analysis showed that phosphorylation of both MAPK and Akt substrates increased with kisspeptin treatment. This work demonstrates that the kisspeptin-GPR54 system plays a significant role stimulating GnRH secretion and positive regulation of GnRH mRNA levels in GnRH neurons in culture, and also, demonstrates the activation of MAPK and Akt signaling pathways by kisspeptin in GT1-7 and GN11 cell lines.
Keywords: GPR54, GT1-7 cells, GN11 cells, Estradiol
1. Introduction
The hormonal network responsible for the control of reproduction is composed of three major hierarchical elements: the hypothalamic gonadotropin-releasing hormone (GnRH), the pituitary gonadotropins (LH and FSH) and the products of the gonads, principally, sex steroids. Reproductive capacity is attained at puberty as the end-point of a complex series of developmental and neuroendocrine events that lead to full activation of the GnRH pulse generator, enhanced gonadotropin secretion and complete gonadal maturation and function (Terasawa and Fernandez, 2001; Plant and Witchel, 2006). Since GnRH neurons play a critical central role in the regulation of pubertal development and reproduction, the central and peripheral signals that regulate these cells are important to elucidate.
Gonadal sex steroids are major regulators of GnRH secretion through negative and positive feedback loops. In addition, most recently, the physiological control of the reproductive axis was advanced by the identification of the essential role of kisspeptin, the peptide product of the KiSS-1 gene, and its receptor, G-protein coupled receptor 54 (GPR54), in the neuroendocrine regulation of reproduction (de Roux et al., 2003; Funes et al., 2003; Seminara et al., 2003; Castellano et al., 2005). Investigations by many laboratories over the last 5 years have led to the general concept that kisspeptin neurons activate GnRH neurons (Tena-Sempere, 2006; Kauffman et al., 2007; Caraty and Franceschini, 2008; Roa et al., 2008a; Roa, et al., 2008b; Seminara and Crowley, 2008).
Navarro et al. (Navarro et al., 2004) and Shahab et al. (Shahab et al., 2005) observed an increase of KiSS-1 and GPR54 mRNAs during pubertal development in experiments performed in intact rats and monkeys, suggesting that, these developmental changes in the expression of KiSS-1 mRNA and GPR54 may play a role in the onset of puberty, and could be considered proximal signaling events for pubertal maturation (Han et al., 2005; Gottsch et al., 2006). Central or systemic administration of kisspeptin leads to increased GnRH and gonadotropin secretion in both prepubertal and adult animals (Plant and Barker-Gibb, 2004; Navarro et al., 2005a; Gottsch et al., 2006; Roa et al., 2006; Pielecka-Fortuna et al., 2008; Roa et al., 2008c). Furthermore, GPR54 mutations in humans or targeted deletions in mice produce isolated hypogonadotropic hypogonadism and infertility, thereby demonstrating a required regulatory function in both sexes (Messager et al., 2005; Smith et al., 2006a; Tena-Sempere, 2006; Gottsch et al., 2006; d'Anglemont de Tassigny et al., 2007; Dungan et al., 2007; Clarkson et al., 2008).
Estradiol is one of the most important regulators of GnRH neuronal activity (Herbison, 1998; Wintermantel et al., 2006; Herbison, 2008). Irwig et al. (Irwig et al., 2004) and Navarro et al. (Navarro et al., 2004) have provided evidence in rats that kisspeptin-expressing neurons are targets for regulation by sex steroids, furthermore, this neurons are directly regulated by the negative and positive feedback actions of sex steroids in distinct regions of the forebrain (Gottsch et al., 2006; Smith et al., 2006a). These observations suggest that kisspeptin/GPR54 signaling provides tonic stimulatory input to GnRH neurons, which could be governed by the feedback effects of sex steroids acting on kisspeptin secreting neurons (Gottsch et al., 2006). In the female, the estradiol-dependent induction of KiSS-1 mRNA in the anteroventral periventricular nucleus (AVPV) may play a role in mediating the preovulatory GnRH/LH surge, that drives ovulation or the regulation of sexual behavior (Kinoshita et al., 2005; Smith et al., 2005a; Smith et al., 2005b; Smith et al., 2006b; Clarkson et al., 2008 Herbison, 2008). Unlike in the AVPV, kisspeptin neurons in the arcuate nucleus (Arc) play the same role in both sexes (negative feedback regulation of gonadotropin secretion by gonadal steroids). Recently, studies in GPR54 KO mice reported that GPR54 signaling is critical for the maintenance of tonic LH secretion, reflecting a lack of normal follicular development and resulting in infertility (Dungan et al., 2007). In addition to receiving input from estradiol sensitive presynaptic afferents, GnRH neurons have been shown to express estrogen receptors, although the studies have been difficult and controversial. In vivo evidence for the presence of ERβ has been demonstrated, suggesting that the effects of estrogen may be directly transmitted (Skynner et al., 1999; Hrabovszky et al., 2000; Herbison and Pape, 2001; Petersen et al., 2003; Skinner and Dufourny, 2005; Wintermantel et al., 2006). The model GnRH neuronal cell lines, GT1-7 and GN11 have been shown to also express functional ERα and ERβ (Radovick et al., 1991a; Roy et al., 1999; Ng et al., 2009).
The KiSS-1/GPR54 system revealed a fundamental role of in the control of puberty and/or maintenance of reproductive function; although the direct targets of kisspeptin, its specific pharmacokinetics and the cellular signaling mechanism remained obscure (Tena-Sempere, 2006). In the present studies both the GN11 and GT1-7 GnRH expressing cell lines were used as models to explore the direct regulation of the GnRH neuron by kisspeptin. The aim of this work is to determine whether the GnRH neuron can be directly regulated by kisspeptin; the potential mechanism of kisspeptin signaling in GnRH neurons, and whether GnRH neurons may be integrators of sex steroid feedback with kisspeptin regulation.
2. Materials and methods
2.1. Cell culture
GN11 cells were grown in Dulbecco’s modified Eagle’s medium (DMEM; Mediatech Inc., Herndon, VA, USA) supplemented with 10% fetal bovine serum (Hyclone, Logan, UT, USA) and 25 mM glucose, 5 mM L-glutamine, 100 U/ml penicillin and 100 µg/ml streptomycin (Gibco, Grand Island, NY, USA) in an atmosphere with 5% CO2 at 37°C. GT1-7 cells were grown in a similar manner, except supplemented with 10% heat-inactivated fetal bovine serum. Cells were placed in media supplemented with 10% dextran and charcoal stripped serum 24h before treatment. Cells were treated with kisspeptin-10 (EMD Biosciences, Inc., San Diego, CA, USA) at final concentrations of 10−12, 10−11, 10−10, 10−9, 10−8 10−7 and 10−6M for 4h or 16h. To study the effect of kisspeptin-10 in the presence of 17β-estradiol (Sigma, St. Louis, MO, USA), cells were treated with 10−8M 17β-estradiol for 12h and then with 10−9M kisspeptin-10 for 4h. To determine the role of the ERK1/2 MAPK and PI3K signaling pathway in GPR54 activation; cells were co-treated with 10µM PD98059, an ERK1/2 MAPK inhibitor, or with 50 µM LY29402 a specific PI3K inhibitor, and 10−9M kisspeptin-10 for 4 hours. Control wells were exposed in parallel to vehicle and are referred to as non-treated groups (NT).
2.2. RT-PCR
Total RNA was harvested from GT1-7 and GN11 cells using Trizol reagent (Invitrogen, Carlsbad, CA, USA) following the protocol suggested by the manufacturer. cDNA was synthesized from one microgram of RNA using the iScript cDNA kit (BioRad, Hercules, CA, USA). The negative control corresponds to 1 µg aliquot of total RNA used for cDNA synthesis in the absence of the RT enzyme referred to as RT(−).
Two µl of cDNA reaction were used in the presence of 2.5 units Taq polymerase (Gene Choice, Frederick, MD, USA), 0.2 µM of each primer, 0.2 µM of each nucleotide and standard reaction buffer (Gene Choice, Frederick, MD, USA). PCR was performed under the following conditions: initial denaturation at 94°C for 4 min, followed by 35 cycles of denaturation (94°C, 30 sec), annealing (59°C, 30 sec) and extension (72°C, 45 sec). The reaction was concluded with an extension for 10 min at 72°C. The primers used to amplify GPR54 were: sense primer: 5’ CTGCCACAGACGTCACTTTC 3’ and antisense primer: 5’ ACATACCAGCGGTCCACACT 3’, which produced a DNA segment of 175 bp (Jacobi et al., 2007). The identities of amplified products were observed by agarose gel electrophoresis (1.5% agarose in buffer containing 40 mM Tris/acetate and 1 mM EDTA) and visualized with ethidium bromide staining (0.5 µg/ml) under ultraviolet light.
2.3. GnRH Radioimmunoassays
One ml of media from each treated plate was harvested for quantification of GnRH secretion. RIA for GnRH was performed by the Ligand Assay Core of the Baltimore-Chicago Cooperative Center in Reproductive Research at Northwestern University (Dr. Jon Levine, Director). Samples collected in one experiment were assayed together. This assay exhibits inter- and intra-assay coefficient of variation of less than 10%. The lower limit of sensitivity of the assay is 0.1 pg/100uL. The total protein from lysates of each treated plate was used to normalize the results.
2.4. Quantitative RT-PCR
Two micrograms of RNA was reverse transcribed using the iScript cDNA kit (BioRad, Hercules, CA, USA). Real-time quantitative PCR was performed in duplicate using the SyberGreen MasterMix (BioRad, Hercules, CA, USA) and the ICycler quantitative PCR machine (BioRad). 18S RNA was used as internal control for cDNA input. The following primers were used: GnRH sense 5’ CCCTTTGACTTTCACATCC 3’; antisense 5’ GGGTTCTGCCATTTGATCCAC 3’; 18S sense 5’ TGGTTGATCCTGCCAGTAG 3’; and antisense 5’ CGACCAAAGGAACCATAACT 3’. To determine PCR efficiency, a 10-fold serial dilution of cDNA was performed as previously described (Wong and Medrano, 2005). PCR conditions were optimized to generate >95% PCR efficiency and only those reactions with between 95 and 105% efficiency were included in subsequent analyses. Relative differences in cDNA concentration between baseline and experimental conditions were then calculated using the comparative threshold cycle (Ct) method (Bustin et al., 2005). Briefly, for each sample, a ΔCt was calculated to normalize for the internal control using the equation: ΔCt = Ct(gene) − Ct(18S). To obtain differences between experimental and control conditions, ΔΔCt was calculated: ΔCt(sample) − ΔCt(control). Relative mRNA levels were then calculated using the equation fold difference =2ΔΔCt.
2.5. Western Blot Analysis
Immunoprecipitation and Western blot analysis were performed for GPR54 protein analysis in GT1-7 and GN11 cells. Cellular protein lysates were obtained and then 200 µL total protein was incubated with a rabbit polyclonal anti-G protein-couple receptor antibody diluted 1:100 (Novus Biologicals, Inc, Littleton, CO, USA). Immunoprecipitates were collected with protein A–agarose. After separating on 10% SDS/polyacrylamide gels proteins were transferred to Protran nitrocellulose membrane (Whatman GmbH, Hahnestrabe, Dassel, Germany) in Tris/glycine transfer buffer. The membranes were blocked for 1 h with 5% nonfat milk in Tris-buffered saline containing 0.05% Tween 20 (TTBS) at room temperature and then were incubated overnight with anti-GPR54 primary antibody (1:1000) in the blocking buffer. The membranes were washed three times for 10 min with TTBS and incubated for 1 h at room temperature with goat anti-rabbit IgG-HRP conjugate secondary antibody diluted 1:2000 (Bio-Rad, Hercules, CA, USA). After washing, the blots were visualized using ECL Plus Western blotting detection system (GE Healthcare, Amersham, USA).
To evaluate the activation of Akt and p42/44 MAPK protein, intermediate proteins involved in kisspeptin activation, GT1-7 and GN11 cells were incubated at different time points with 10−9M kisspeptin-10 and then Western blot assay was performed. Briefly, cellular protein lysates were obtained and then 20 µg total protein was separated on 10% SDS/polyacrylamide gels and transferred to nitrocellulose membrane. The blots were incubated overnight with Phospho-Akt (Ser473) and Phospho-p44/42 MAPK (Thr202/Tyr204) primary antibodies (1:1000) or for 1 h with total Akt and total p44/42 MAPK primary antibodies diluted 1:1000 (Cell Signaling Technology, Danvers, MA, USA) in the blocking buffer. The membranes were washed and incubated with their appropriate goat anti-rabbit or anti mouse IgG-HRP conjugate secondary antibody (1:2000). The density of the bands corresponding to the expected size of the protein was analyzed by Scion Image for Windows version Alpha 4.0.3.2 (Scion Corporation, Maryland, USA)
2.6. Statistical analysis
Statistical significance was assessed by one-way ANOVA followed by the Newman–Keuls multiple comparison tests. We used an unpaired Student’s t test to compare the secretion levels of GnRH between groups treated with kisspeptin-10 and vehicle. All results are expressed as mean ± SEM and p ≤ 0.05 assigned as significant using the GraphPad Prism 4 software (San Diego, CA, USA).
3. Results
3.1. GnRH mRNA and protein expression in GT1-7 and GN11 cells
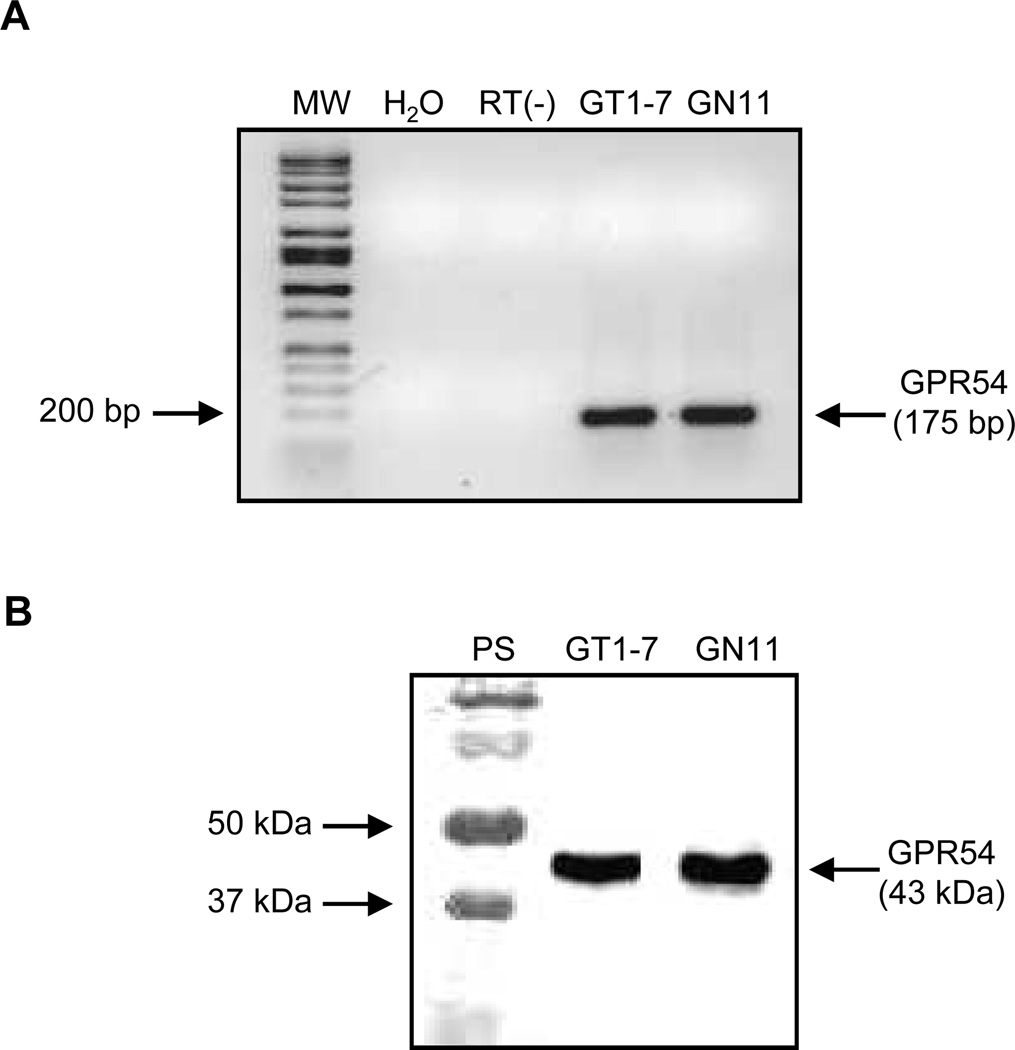
Initial studies using RT-PCR revealed the presence of GPR54 mRNA in both GT1-7 and GN11 neuronal cell lines (Fig. 1A). The detection of a 175 bp band corresponds to amplification of a region between exons 2 and 3 from murine genomic DNA. No product was detected in the lane containing no cDNA (H2O) or performed without RT (RT(−)). Furthermore, studies using immunoprecipitation and Western blot assay revealed the presence of GPR54 protein as a 43 kDa product (Prentice et al., 2007) in these cells (Fig. 1B).
Figure 1. Detection of GPR54 mRNA and Protein in GT1-7 and GN11 cells.
(A) Representative 1% agarose gel of GPR54 (175 bp) RT-PCR product. Lane 1, 100 bp molecular mass ladder; lane 2, H2O control; lane 3, RT(−) control; lane 4, GT1-7 cells and lane 5, GN11 cells. (B) Detection of GPR54 protein (43kDa) by Western blot analysis. Lane 1, protein standards; lane 2, GT1-7 cells and lane 3, GN11 cells.
3.2. The time course of GnRH secretion by GT1-7 and GN11 cells in response to kisspeptin
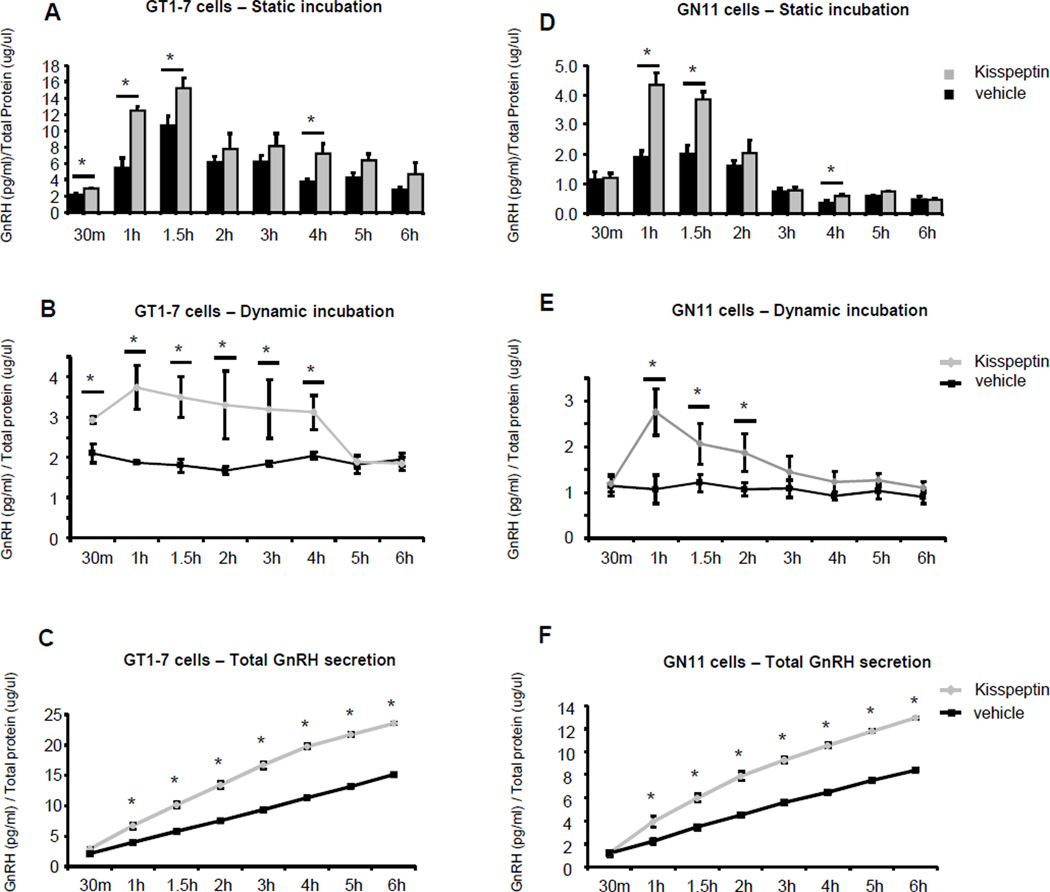
To determine GnRH secretion in response to kisspeptin, two different time course assays were performed. First, cells were treated for 30 min through 6 hours (static incubation) in the presence of 10−9M kisspeptin (10−9M kisspeptin concentration was chosen from parallel experiments evaluating mRNA expression) or vehicle. In GT1-7, we observed a maximum 5-fold response after 1.5 hours of treatment with kisspeptin compared to the NT 30 min group (n=4–11, p≤0.05, Fig. 2A). An increase in GnRH secretion was also observed at later time points in GT1-7 cells (n=4–11, p≤0.05, Fig. 2A) but declined at 2 hours of treatment, and returned to baseline by 3 hours. Similarly, static incubation of GN11 cells with kisspeptin showed a maximum 4-fold response after 1 hour of treatment compared to the NT 30 min group (n=4–10, p≤0.05, Fig. 2D). Like GT1-7 cells GnRH secretion decreased to reach baseline levels at 3 hours.
Figure 2. Effect of kisspeptin on GnRH secretion in GT1-7 and GN11 cells.
GT1-7 and GN11 cells were incubated with 10−9M kisspeptin (30min through 6 hours) and GnRH levels in the medium were determined by RIA. The cellular total protein lysates from each treated culture dish was used to normalize the results. (A and D) Static incubation, (B and E) Dynamic incubation and (C and F) Total GnRH secretion in both kisspeptin and NT groups in GT1-7 and GN11 cells. Graphic representation of the means±SE of GnRH levels. Student’s t test was used for statistical analysis. Asterisks indicate significant differences (P ≤0.05).
Given that static incubations cultures may represent both secretion and peptide accumulation, we performed the same treatment time course but replaced the medium at each time point with 10−9M kisspeptin or vehicle (dynamic incubation). In GT1-7, we observed an increase of GnRH secretion at 30 min of 1.5-fold with the kisspeptin treatment when compared to the NT group at the same time point (n=4–6, p≤0.05, Fig. 2B). A doubling of GnRH secretion compared with the NT group was observed from 1 through 4 hours (n=4–6, p≤0.05, Fig. 2B). Between 5 and 6 hours of treatment no differences in GnRH secretion were observed between the kisspeptin and NT groups. GnRH secretion in the NT group did not change at each time point assayed, demonstrating consistent GnRH secretion during the experimental time period (Fig. 2B). In Figure 2C, the total GnRH secretion in both kisspeptin and NT groups in this experiment is summarized as a continuous graphic. The dynamic incubation of GN11 with kisspeptin and vehicle showed a maximal 3-fold increase in GnRH secretion at 1 hour after treatment with kisspeptin when compared to the NT group (n=4–6, p≤0.05, Fig. 2D). Secretion began to decline by 1.5 hours; and no significant difference in response at 3 through 6 hours of treatment was seen when the secretion stimulated by kisspeptin was compared with the NT groups. GnRH secretion in the NT group again remained unchanged at all point times of this experiment, demonstrating a consistent GnRH secretion pattern, similar to the GT1-7 cells (Fig. 2E). In Figure 2F, the total GnRH secretion in both kisspeptin and NT groups in this experiment is summarized as a continuous graphic.
3.3. GnRH mRNA expression is increased by kisspeptin in GT1-7 and GN11 cells
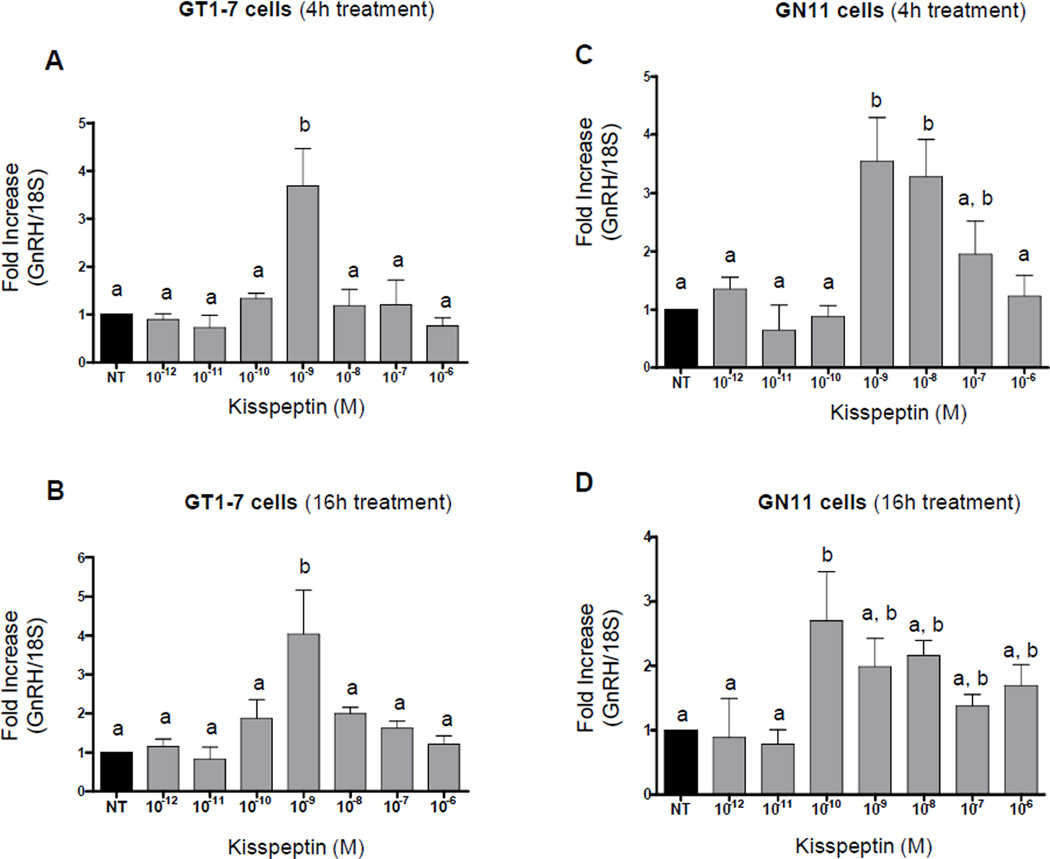
Changes in GnRH mRNA was evaluated by quantitative RT-PCR (qRT-PCR) obtained from both cell lines treated with varying kisspeptin concentrations for 4 or 16 hours. In GT1-7 cells 10−9M kisspeptin produced a maximal increase (~4-fold) in GnRH mRNA levels after 4 hours (n=3–8, p≤0.01, Fig. 3A) that continued through 16 hours (n=3–5, p≤0.05, Fig. 3B). As shown in Figure 3C, GnRH mRNA levels in GN11 cells were maximally stimulated by ~3.5-fold after 4 hours of treatment with 10−9M kisspeptin (n=3–5, p≤0.05) and by ~3-fold after 16 hours of treatment with 10−10M kisspeptin (n=3–9, p≤0.05, Fig. 3D). There was an increase in stimulation of GnRH mRNA levels with 10−10M kisspeptin at 16 hours when levels were compared with 4 hours of treatment. A decrease in fold stimulation with 10−9 and 10−8M kisspeptin treatment in GN11 cells was observed after 16 hours when compared to 4 hours of treatment. Interestingly, GnRH gene expression in GN11 cells continued to be stimulated at higher concentrations of kisspeptin (10−8 and 10−7M) at 4 hours and (10−8 through 10−6M) at 16 hours, in contrast to a decline in responsiveness in GT1-7 cells at doses higher than 10−9M kisspeptin.
Figure 3. Dose–response and time course of GnRH mRNA expression by kisspeptin in GT1-7 and GN11 cells (quantitative RT-PCR).
Graphic representation of the means±SE of relative mRNA and graphed as fold increasing of levels relative to non-treated cells. Cells were treated with 10−12, 10−11, 10−10, 10−9, 10−8, 10−7 and 10−6 M of kisspeptin for 4 hours (A and C) and 16 hours (B and D). There is no statistical difference between groups with the same letters (P≤0.05; ANOVA followed by Newman–Keuls test).
3.4. Kisspeptin modulates GnRH secretion in GT1-7 and GN11 cells in the presence of 17β-estradiol
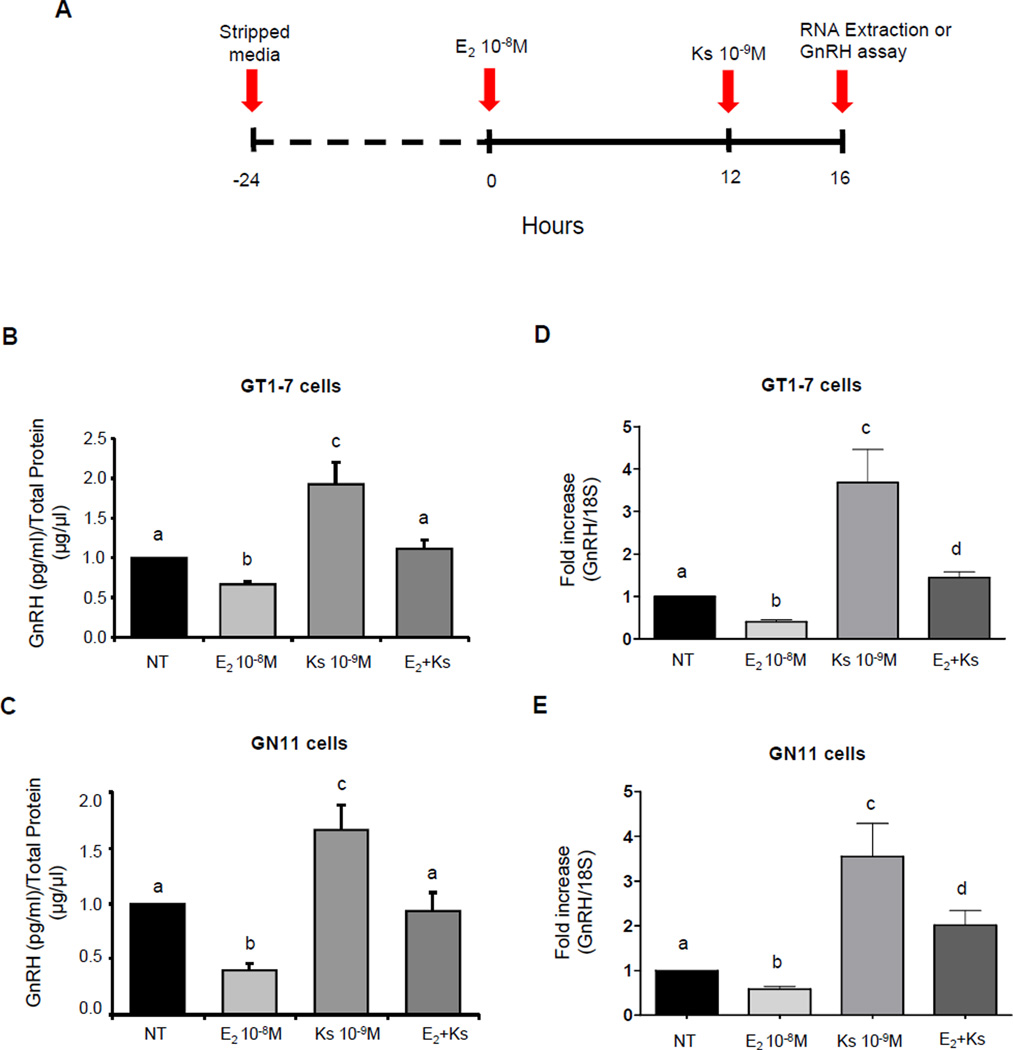
To examine the role of kisspeptin on GnRH secretion in the presence of suppressive doses of 17β-estradiol, these cell lines were treated with 10−8M 17β-estradiol for 16 hours, previously shown to maximally suppress GnRH mRNA expression (Ng et al., 2009). 10−9M kisspeptin was then added to the media after 12 hours of estradiol treatment; and the media of the cells was collected after 4 hours of co-treatment (Fig. 4A). 10−9M kisspeptin treatment increased the GnRH secretion after 4 hours by ~2-fold in GT1-7 (n=8–9, p≤0.05, Fig. 4B) and 1.7-fold in GN11 cells (n=7–8, p≤0.05, Fig. 4C) when compared with the NT group. The treatment with estradiol alone suppressed GnRH secretion in GT1-7 and GN11 cells by 44% and 60% respectively (p≤0.05, Fig. 4B and 4C). Co-treatment with kisspeptin in the presence of 17β-estradiol resulted in an increase of GnRH secretion of ~2-fold in GT1-7 cells (n=8–9, p≤0.05, Fig. 4B) and ~2.5-fold in GN11 cells (n=7–8, p≤0.05, Fig. 4C) compared to the group treated with 17β-estradiol alone. Furthermore, co-treatment resulted in GnRH secretion similar to basal levels of the non-treated group in both cell lines (Fig. 4B and 4C). Therefore, these results demonstrate that kisspeptin is able to increase GnRH secretion in the presence of 17β-estradiol reaching basal levels. In addition, kisspeptin treatment in the presence of 17β-estradiol reduced GnRH secretion by 50% in GT1-7 cells (n=8–11, p≤0.05, Fig. 4B) and to the same degree (50%) in GN11 cells (n=7–8, p≤0.05, Fig. 4C) compared to the group treated with kisspeptin alone.
Figure 4. Effect of kisspeptin on GnRH secretion and GnRH mRNA expression in GT1-7 and GN11 cells.
(A) Detailed representation of the cell treatment regimen paradigm. (B and C) Graphic representation of means±SE of relative secreted GnRH levels in GT1-7 and GN11 cells respectively, graphed as fold increases relative to non-treated cells. (D and E) Graphic representation of the means±SE of relative mRNA levels in GT1-7 and GN11 cells graphed as fold increasing of levels relative to non-treated cells. Cells were treated with 10−8M estradiol (E2 10−8M), 10−9M kisspeptin (Ks 10−9M) or co-treated with 10−9M kisspeptin in the presence of 10−8M estradiol (E2+Ks). There is no statistical difference between groups with the same letters (n=4–7, P≤0.05; ANOVA followed by Newman–Keuls test).
3.5. GnRH mRNA modulation by kisspeptin in GT1-7 and GN11 cells in the presence of 17β-estradiol
Further studies were performed to determine whether GnRH mRNA expression in the presence of suppressive doses of 17β-estradiol paralleled the changes seen in GnRH secretion levels. Estradiol (10−8M) alone suppressed GnRH mRNA expression in GT1-7 and GN11 by 60% and 42% respectively (Fig. 4D and 4E). In parallel with the previous results, treatment with kisspeptin in the presence of 17β-estradiol resulted in an increase of GnRH mRNA expression of ~4-fold in GT1-7 cells (n=4–7, p≤0.05, Fig. 4D) and ~3.5-fold in GN11 cells (n=3–6, p≤0.05, Fig. 4E) compared to the group treated with 17β-estradiol alone. In the same experiment, co-treatment increased the GnRH mRNA levels only ~1.5-fold in GT1-7 cells (n=4–7, p≤0.05, Fig. 4D) and ~2-fold in GN11 cells (n=3–6, p≤0.05, Fig. 4E) when compared to the non-treated group (NT). Thus, co-treatment with 17β-estradiol resulted in a decrease in GnRH mRNA expression of 2.5-fold in GT1-7 cells (n=4–7, p≤0.05, Fig. 4D) and 2-fold in GN11 cells (n=3–6, p≤0.05, Fig. 4E) compared to the group treated with kisspeptin alone for 4 hours. These results highlight an attenuated GnRH response to kisspeptin in the presence of 17β-estradiol.
3.6. GnRH mRNA modulation by kisspeptin in GT1-7 and GN11 cells involves the participation of ERK1/2 MAPK and PI3K in the signaling pathway
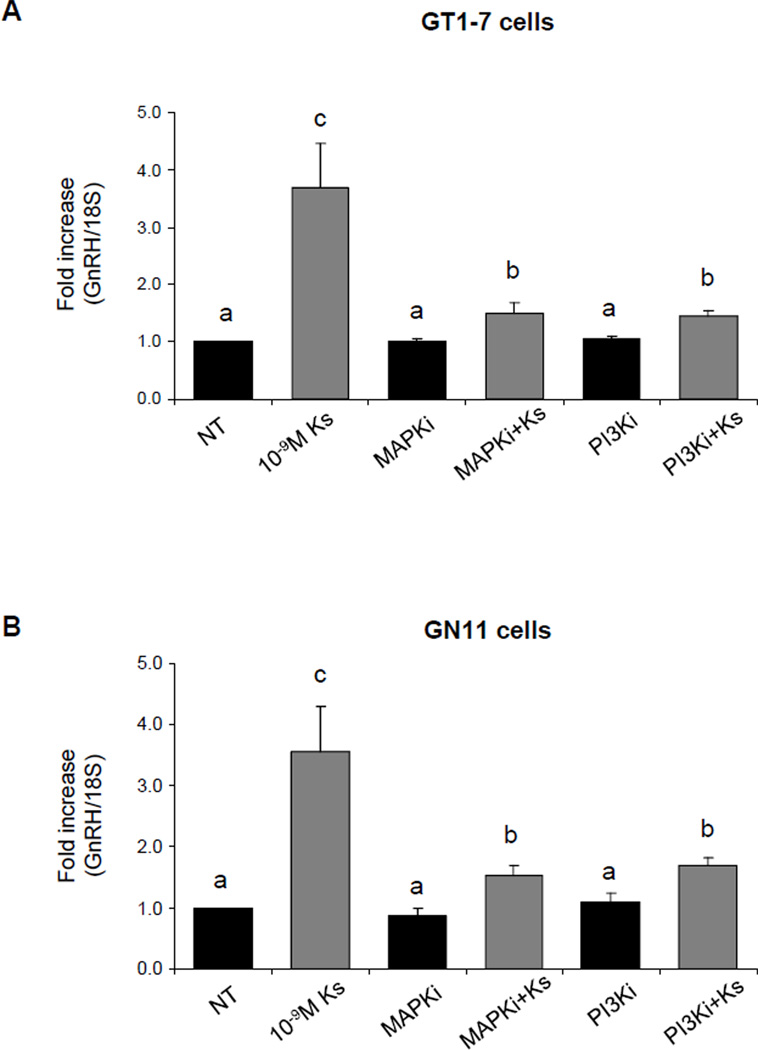
To determine if kisspeptin stimulation of GnRH neurons involved the activation of ERK 1/2 and PI3K, cells were pretreated with the ERK1/2 inhibitor (MAPKi), PD98059, or with a specific PI3K inhibitor (PI3Ki), LY29402, and expression of GnRH mRNA levels after 4 hours of treatment with 10−9M kisspeptin was examined by qRT-PCR. As shown in Figure 5A and 5B, pretreatment with MAPKi or PI3Ki drastically attenuated the GnRH mRNA levels induced by kisspeptin treatment in GT1-7 and GN11. A significant differences was observed when groups co-treated with MAPKi plus kisspeptin or PI3Ki plus kisspeptin, respectively, were compared to the NT group (n=3–6, p≤0.05). GT1-7 and GN11 cells incubated with the MAPKi or PI3Ki alone showed no change in GnRH mRNA levels when compared to the NT group (n=3–6, P≥0.05, Fig. 5A and 5B).
Figure 5. GnRH mRNA expression in GT1-7 cells treated with kisspeptin, MAPK inhibitor and PI3K inhibitor (quantitative RT-PCR).
(A) GT1-7 and (B) GN11 cells. Graphic representation of the means±SE of relative mRNA levels graphed as fold increasing of levels relative to non-treated cells. Cells were treated with 10−9M kisspeptin (Ks 10−9M), 10µM MAPK inhibitor or 50µM PI3K inhibitor. And co-treated with Ks 10−9M+MAPKi or Ks 10−9M+PI3Ki. There is no statistical difference between groups with the same letters (n=4–6, P ≤0.05; ANOVA followed by Newman–Keuls test).
3.7. Kisspeptin increases Akt and ERK1/2 phosphorylation in GT1-7 and GN11 cells
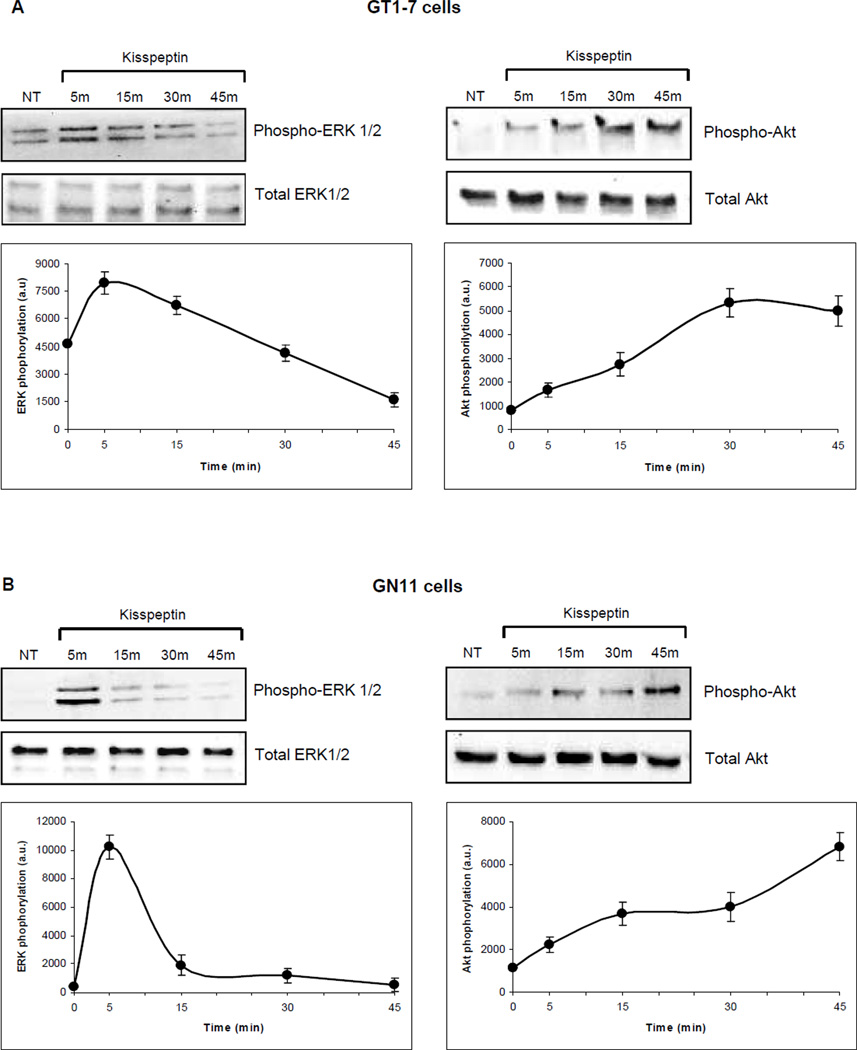
To evaluate further the signaling pathway activated by kisspeptin, GT1-7 and GN11 cells were incubated at different times (5, 15, 30 and 45 min) with 10−9M kisspeptin, and activation of Akt and ERK1/2 proteins was measured by Western blot analysis using primary antibodies against their respective phosphorylated and total protein levels (Fig. 6A and 6B). In both cell lines, ERK1/2 was maximally phosphorylated after 5 min of incubation with kisspeptin, which decreased over time (n=3, Fig. 6A and 6B). The phosphorylation of Akt in these cell lines showed a different pattern of activation when compared with MAPK signaling; a constant increases in a time dependent fashion from 5 min of treatment through 45 min (n=3, Fig. 6A and 6B) was observed. No significant changes in the total protein levels of Akt and ERK1/2 were identified.
Figure 6. Expression and activation of Akt and p42/44 MAPK in GT1-7 and GN11 cells.
(A) GT1-7 and (B) GN11 cells. Western blots were performed in a time course over 45 min after incubation with 10−9M kisspeptin. Protein was analyzed for expression and activation of Akt using primary antibodies against phospho-Akt at Ser473 and p42/44 MAPK using primary antibodies against phospho-p44/42 MAPK at Thr202/Tyr204. Total levels of Akt and p42/44 MAPK were not altered by kisspeptin. The data were quantitated and the means±SE as arbitrary units (a.u.) are shown in the lower panels (n=3).
4. Discussion
The onset of puberty is heralded by activation of neurons in the forebrain that produce GnRH. Although the central reproductive axis has been studied in many mammalian species, precise identification of the molecular and cellular events in the forebrain that initiate pubertal processes and maintain reproductive competence remains elusive (Plant, 2008).
The goal of our study was to define whether kisspeptin had a direct role in GnRH neuronal function, and if so, the pharmacology of its effect on the cellular action in GnRH neurons. Previous reports using incubations of whole hypothalamic explants demonstrated the stimulatory effect of kisspeptin on the gonadotropin-releasing hormone system (Messager et al., 2005; Castellano et al., 2006; Seminara et al., 2006; Clarkson et al., 2008; d'Anglemont de Tassigny et al., 2008), however, these studies could not discriminate between direct effects of kisspeptin upon GnRH neurons versus indirect actions mediated through intermediate pathways.
The molecular and biochemical analyses of kisspeptin regulation of GnRH neurons have been difficult to assess due to their scattered distribution and small number in the basal hypothalamus. GnRH neurons originate in the olfactory placode and migrate to their final destination during early embryonic development. Cell lines have been developed that constitute a homogeneous population that allows for the study of direct effects and to examine gene and protein expression in a defined cell population. GN11 and GT1-7 cells are immortalized GnRH expressing cell lines exhibiting the features of immature olfactory neurons and differentiated hypothalamic neurons, respectively. GN11 cells were obtained from an olfactory bulb tumor in mice containing a transgene consisting of a human GnRH promoter fused to the SV40 Large T-Antigen oncogene (Tag) (Radovick, et al., 1991b); in contrast, GT1-7 cells were derived from a hypothalamic tumor obtained from a mouse containing a transgene consisting of a rat GnRH promoter fused to Tag (Mellon et al., 1990). Thus, GN11 cells express the characteristic features of migrating neurons and GT1-7 cells are non-migrating, fully differentiated cells.
Our initial studies revealed the presence of GPR54 mRNA in GN11 neuronal cell lines, for the first time, as well as confirmed its presence in GT1-7 cells (Jacobi et al., 2007; Quaynor et al., 2007). We also demonstrated the presence of GPR54 protein in these GnRH neurons. Therefore both cell lines providing a model system in which to study the role of kisspeptin in the regulation of GnRH gene expression and secretion.
Studies of kisspeptin-stimulated GnRH secretion in time course assays showed similar patterns in both cell lines. We observed a maximum response after 1 and 1.5 hours of treatment and a decrease in GnRH secretion after 2 hours of treatment, lasting through 6 hours in both kisspeptin treated and NT cells. A similar study by Nazian (Nazian, 2006) performed in GT1-7 cells showed that kisspeptin treatment was unable to increase GnRH secretion after a 2½ hour and overnight incubation. This study did not include shorter incubation periods, making direct comparisons difficult. Conversely, in this same study, infantile male rat hypothalamic explants exposed to kisspeptin for 1 hour revealed an increase in GnRH secretion (Nazian, 2006). These data suggest the possibility of an indirect effect of kisspeptin, or potentially a differential in the time course of the responses. Therefore, we can consider several explanations for differences in the reported results. First, it is possible that with longer incubation, the increase of GnRH in the culture media was followed by degradation, masking an earlier response. Second, it is also possible that ligand-induced internalization of GnRH receptor (Suarez-Quian et al., 1986) or third, GnRH inhibition of its own release as shown in cultured hypothalamic explants (Bourguignon et al., 1987; Martinez-Fuentes et al., 2004) is responsible for the decrease in GnRH secretion by these cells.
To explore further these possibilities, dynamic incubation studies in GT1-7 and GN11 cells were performed by replacing medium at each time point with kisspeptin or vehicle. In this series of incubations, GnRH secretion was unchanged in the basal state suggesting that these cultured cell lines secreted similar quantities of GnRH over time. In contrast, GnRH protein levels steady increased in the static incubation cultures presumably reflecting accumulation of GnRH. During dynamic incubation, we observed an earlier and longer kisspeptin response in GT1-7 cells versus GN11 cells. Taken together, these data support a model of desensitization of GPR54 during long-term treatment with kisspeptin and are consistent with results from d’Anglemont de Tassigny and colleagues (d'Anglemont de Tassigny et al., 2008) who reported a desensitization of GnRH release directly on hypothalamic tissue treated after 5 hours of continuous kisspeptin stimulation (Seminara et al., 2006). Our study, however, has provided evidence for a direct effect.
In parallel experiments, GnRH neuronal cell lines were treated with kisspeptin at varying concentrations for 4 or 16 hours (Matsui et al., 2004; Navarro et al., 2005b). In GT1-7 cells, maximal GnRH mRNA expression was detected with 10−9M kisspeptin treatment after 4 and 16 hours with a dose-response and similar biphasic stimulation pattern at both time points. In GN11 cells, we detected a maximal increase in GnRH mRNA levels with 10−9M kisspeptin treatment after 4 hours and a maximal treatment response with 10−10M kisspeptin after 16 hours, demonstrating a dose and time-course response. These data demonstrated GnRH mRNA regulation in GN11 cells by kisspeptin as well as a differential response between GT1-7 and GN11 cells. The decrease in GnRH mRNA levels in the presence of higher concentrations of kisspeptin in both cell lines could be explained by desensitization or down-regulation of GPR54 to exogenous kisspeptin treatment, such as was observed in vivo in juvenile male monkeys (Seminara et al., 2006). These data provide evidence that GnRH mRNA expression is directly regulated by kisspeptin in both GT1-7 and GN11 neuronal cells lines. This is complementary to emerging evidence suggesting that one of the roles of kisspeptin/GPR54 signaling is as an afferent stimulatory input to GnRH neurons. In a previous report, kisspeptin also increased GnRH mRNA levels after 24 hours of treatment in GT1-7 cells (Jacobi et al., 2007). In contrast, GnRH mRNA expression was unchanged after 24 hours of kisspeptin treatment in human GnRH secreting neuronal cell line derived from fetal olfactory epithelium (FNC-B4), while GnRH secretion in these cells was induced after 24 hours of kisspeptin treatment (Morelli et al., 2008). These studies highlight the importance of evaluating different in vitro models to elucidate kisspeptin effects on GnRH neurons as well as to attempt to understand discrepancies between the in vitro and in vivo models.
In order to determine whether the direct negative feedback effect of estradiol on GnRH gene expression (El Majdoubi et al., 1998; Roy et al., 1999) and both negative and positive feedback effect on GnRH secretion (Sarkar and Fink, 1980; Moenter et al., 1990; Xia et al., 1992 Wintermantel et al., 2006; Herbison, 2008) may be affected by kisspeptin, we performed in vitro experiments using immortalized GnRH neuronal cell lines in the presence of 17β-estradiol. Studies by our laboratory and others have demonstrated the presence of estrogen receptors in immortalized GnRH neuronal cell lines, mediating direct negative regulation of GnRH (Radovick et al., 1991a; Radovick et al., 1994; Roy et al., 1999; Ng et al., 2009). Specifically, a maximal suppressive dose of 10−8M 17β-estradiol at 12–16 hours of treatment on GnRH mRNA levels was determined in both cell lines. The data provided by the current studies show that treatment with kisspeptin is able to antagonize the inhibition of estradiol on GnRH mRNA expression. Both cell lines responded to 4 hours of kisspeptin treatment after 16 hours of 17β-estradiol treatment, with a 4 fold (GT1-7) and 3.5 fold (GN11) increase in GnRH mRNA level expression compared to estradiol alone. Fold change in GnRH mRNA levels to kisspeptin are similar between groups either untreated or treated with estradiol, suggesting estradiol and kisspeptin act through independent signaling pathways. In addition, our data demonstrated that 17β-estradiol blunted the kisspeptin response in both cell lines. GnRH secretion measured in the same experimental treatment regimen showed a similar pattern but with a reduced magnitude of relative change. These results demonstrate that kisspeptin is able to increase GnRH secretion by acting directly on GnRH neurons in the presence of 17β-estradiol induced suppression. These data continue to suggest a connection between GnRH gene expression and secretion, since changes in GnRH mRNA levels parallel changes in protein secretion into the medium. These data also suggest a role for kisspeptin acting directly at the GnRH neuron to increase GnRH expression and secretion, as well as a potential mechanism for generation of the gonadotropin surge by kisspeptin in an estrogen milieu, as previously suggested (Dungan et al., 2007).
Strong evidence showed that kisspeptin plays an important role in GnRH gene modulation and GnRH secretion, therefore, the intracellular signaling cascade initiated by GPR54 activation in GnRH neurons is important to elucidate. Using the ERK1/2 kinase inhibitor, PD98059, and a specific PI3K inhibitor, LY29402, we observed that GnRH mRNA modulation by kisspeptin involved the activation of ERK1/2 and PI3K in the GnRH neuronal cell cultures. In addition, kisspeptin activated the MAPK and Akt signaling pathways in a time dependent manner by endogenous GPR54. These results are in agreement with previous studies using heterologous cell systems where GPR54 is overepxressed (Kotani et al., 2001). Moreover, kisspeptin incubation resulted in phosphorylation of Akt and p42/44 MAPK in another GPR54 overexpression model in GPR54-null thyroid cancer cells (ARO thyroid cancer cells) (Ringel et al., 2002; Stathatos et al., 2005) and more recent studies, using brain slice preparation showed that kisspeptin activates GPR54 to initiate a PLC-IP3K-calcium cascade that modulate both potassium and nonselective cation (NSC) channels to initiate depolarization in GnRH neurons (Liu et al., 2008)
In conclusion, we have demonstrated that kisspeptin-GPR54 system plays a significant role in positive regulation of GnRH expression and secretion acting directly on GnRH secreting neuronal cell lines with a time course consistent with activation of Akt and MAP kinase signaling pathways. This positive regulation of GnRH by kisspeptin is demonstrated even in the presence of suppressive doses of estradiol. Direct action of kisspeptin on the GnRH neuron may play an important role in physiologic processes such as the activation/maintenance of the GnRH pulse generator during pubertal development and/or the regulation of the GnRH surge during the estrous cycle.
Acknowledgements
This research was supported by NICHD/NIH through cooperative agreement [U54 HD 933067 (The Baltimore-Chicago Center for Reproductive Research)] as part of the Specialized Cooperative Centers Program in Reproduction and Infertility Research (SCCPIR) and R01 as NIH HD 370246. The authors would like to thank Dr Pamela Mellon for kindly providing the GT1-7 cell line and Dr. Jennifer Mammen for the assistance in the writing of the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bourguignon JP, Gerard A, Debougnoux G, Rose J, Franchimont P. Pulsatile release of gonadotropin-releasing hormone (GnRH) from the rat hypothalamus in vitro: Calcium and glucose dependency and inhibition by superactive GnRH analogs. Endocrinology. 1987;1213:993–999. doi: 10.1210/endo-121-3-993. [DOI] [PubMed] [Google Scholar]
- Bustin SA, Benes V, Nolan T, Pfaffl MW. Quantitative real-time RT-PCR--a perspective. Journal of Molecular Endocrinology. 2005;343:597–601. doi: 10.1677/jme.1.01755. [DOI] [PubMed] [Google Scholar]
- Caraty A, Franceschini I. Basic aspects of the control of GnRH and LH secretions by kisspeptin: Potential applications for better control of fertility in females. Reproduction in domestic animals = Zuchthygiene. 2008;43(Suppl 2):172–178. doi: 10.1111/j.1439-0531.2008.01158.x. [DOI] [PubMed] [Google Scholar]
- Castellano JM, Navarro VM, Fernandez-Fernandez R, Nogueiras R, Tovar S, Roa J, Vazquez MJ, Vigo E, Casanueva FF, Aguilar E, Pinilla L, Dieguez C, Tena-Sempere M. Changes in hypothalamic KiSS-1 system and restoration of pubertal activation of the reproductive axis by kisspeptin in undernutrition. Endocrinology. 2005;1469:3917–3925. doi: 10.1210/en.2005-0337. [DOI] [PubMed] [Google Scholar]
- Castellano JM, Navarro VM, Fernandez-Fernandez R, Castano JP, Malagon MM, Aguilar E, Dieguez C, Magni P, Pinilla L, Tena-Sempere M. Ontogeny and mechanisms of action for the stimulatory effect of kisspeptin on gonadotropin-releasing hormone system of the rat. Molecular and cellular endocrinology. 2006:257–258. 75–83. doi: 10.1016/j.mce.2006.07.002. [DOI] [PubMed] [Google Scholar]
- Clarkson J, d'Anglemont de Tassigny X, Moreno AS, Colledge WH, Herbison AE. Kisspeptin-GPR54 signaling is essential for preovulatory gonadotropin-releasing hormone neuron activation and the luteinizing hormone surge. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2008;2835:8691–8697. doi: 10.1523/JNEUROSCI.1775-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- d'Anglemont de Tassigny X, Fagg LA, Dixon JP, Day K, Leitch HG, Hendrick AG, Zahn D, Franceschini I, Caraty A, Carlton MB, Aparicio SA, Colledge WH. Hypogonadotropic hypogonadism in mice lacking a functional Kiss1 gene. Proceedings of the National Academy of Sciences of the United States of America. 2007;10425:10714–10719. doi: 10.1073/pnas.0704114104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- d'Anglemont de Tassigny X, Fagg LA, Carlton MB, Colledge WH. Kisspeptin can stimulate gonadotropin-releasing hormone (GnRH) release by a direct action at GnRH nerve terminals. Endocrinology. 2008;1498:3926–3932. doi: 10.1210/en.2007-1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Roux N, Genin E, Carel JC, Matsuda F, Chaussain JL, Milgrom E. Hypogonadotropic hypogonadism due to loss of function of the KiSS1-derived peptide receptor GPR54. Proceedings of the National Academy of Sciences of the United States of America. 2003;10019:10972–10976. doi: 10.1073/pnas.1834399100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dungan HM, Gottsch ML, Zeng H, Gragerov A, Bergmann JE, Vassilatis DK, Clifton DK, Steiner RA. The role of kisspeptin-GPR54 signaling in the tonic regulation and surge release of gonadotropin-releasing hormone/luteinizing hormone. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2007;2744:12088–12095. doi: 10.1523/JNEUROSCI.2748-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Majdoubi M, Sahu A, Plant TM. Effect of estrogen on hypothalamic transforming growth factor alpha and gonadotropin-releasing hormone gene expression in the female rhesus monkey. Neuroendocrinology. 1998;674:228–235. doi: 10.1159/000054318. [DOI] [PubMed] [Google Scholar]
- Funes S, Hedrick JA, Vassileva G, Markowitz L, Abbondanzo S, Golovko A, Yang S, Monsma FJ, Gustafson EL. The KiSS-1 receptor GPR54 is essential for the development of the murine reproductive system. Biochemical and biophysical research communications. 2003;3124:1357–1363. doi: 10.1016/j.bbrc.2003.11.066. [DOI] [PubMed] [Google Scholar]
- Gottsch ML, Clifton DK, Steiner RA. Kisspepeptin-GPR54 signaling in the neuroendocrine reproductive axis. Molecular and cellular endocrinology. 2006:254–255. 91–96. doi: 10.1016/j.mce.2006.04.030. [DOI] [PubMed] [Google Scholar]
- Han SK, Gottsch ML, Lee KJ, Popa SM, Smith JT, Jakawich SK, Clifton DK, Steiner RA, Herbison AE. Activation of gonadotropin-releasing hormone neurons by kisspeptin as a neuroendocrine switch for the onset of puberty. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2005;2549:11349–11356. doi: 10.1523/JNEUROSCI.3328-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbison AE. Multimodal influence of estrogen upon gonadotropin-releasing hormone neurons. Endocrine reviews. 1998;193:302–330. doi: 10.1210/edrv.19.3.0332. [DOI] [PubMed] [Google Scholar]
- Hrabovszky E, Shughrue PJ, Merchenthaler I, Hajszan T, Carpenter CD, Liposits Z, Petersen SL. Detection of estrogen receptor-beta messenger ribonucleic acid and 125I-estrogen binding sites in luteinizing hormone-releasing hormone neurons of the rat brain. Endocrinology. 2000;1419:3506–3509. doi: 10.1210/endo.141.9.7788. [DOI] [PubMed] [Google Scholar]
- Herbison AE, Pape JR. New evidence for estrogen receptors in gonadotropin-releasing hormone neurons. Frontiers in neuroendocrinology. 2001;224:292–308. doi: 10.1006/frne.2001.0219. [DOI] [PubMed] [Google Scholar]
- Herbison AE. Estrogen positive feedback to gonadotropin-releasing hormone (GnRH) neurons in the rodent: The case for the rostral periventricular area of the third ventricle (RP3V) Brain Research Reviews. 2008;572:277–287. doi: 10.1016/j.brainresrev.2007.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irwig MS, Fraley GS, Smith JT, Acohido BV, Popa SM, Cunningham MJ, Gottsch ML, Clifton DK, Steiner RA. Kisspeptin activation of gonadotropin releasing hormone neurons and regulation of KiSS-1 mRNA in the male rat. Neuroendocrinology. 2004;804:264–272. doi: 10.1159/000083140. [DOI] [PubMed] [Google Scholar]
- Jacobi JS, Martin C, Nava G, Jeziorski MC, Clapp C, Martinez de la Escalera G. 17-beta-estradiol directly regulates the expression of adrenergic receptors and kisspeptin/GPR54 system in GT1-7 GnRH neurons. Neuroendocrinology. 2007;864:260–269. doi: 10.1159/000107770. [DOI] [PubMed] [Google Scholar]
- Kauffman AS, Clifton DK, Steiner RA. Emerging ideas about kisspeptin-GPR54 signaling in the neuroendocrine regulation of reproduction. Trends in neurosciences. 2007;3010:504–511. doi: 10.1016/j.tins.2007.08.001. [DOI] [PubMed] [Google Scholar]
- Kinoshita M, Tsukamura H, Adachi S, Matsui H, Uenoyama Y, Iwata K, Yamada S, Inoue K, Ohtaki T, Matsumoto H, Maeda K. Involvement of central metastin in the regulation of preovulatory luteinizing hormone surge and estrous cyclicity in female rats. Endocrinology. 2005;14610:4431–4436. doi: 10.1210/en.2005-0195. [DOI] [PubMed] [Google Scholar]
- Kotani M, Detheux M, Vandenbogaerde A, Communi D, Vanderwinden JM, Le Poul E, Brezillon S, Tyldesley R, Suarez-Huerta N, Vandeput F, Blanpain C, Schiffmann SN, Vassart G, Parmentier M. The metastasis suppressor gene KiSS-1 encodes kisspeptins, the natural ligands of the orphan G protein-coupled receptor GPR54. The Journal of biological chemistry. 2001;27637:34631–34636. doi: 10.1074/jbc.M104847200. [DOI] [PubMed] [Google Scholar]
- Liu X, Lee K, Herbison AE. Kisspeptin excites gonadotropin-releasing hormone neurons through a phospholipase C/calcium-dependent pathway regulating multiple ion channels. Endocrinology. 2008;1499:4605–4614. doi: 10.1210/en.2008-0321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Fuentes AJ, Hu L, Krsmanovic LZ, Catt KJ. Gonadotropin-releasing hormone (GnRH) receptor expression and membrane signaling in early embryonic GnRH neurons: Role in pulsatile neurosecretion. Molecular endocrinology (Baltimore, Md.) 2004;187:1808–1817. doi: 10.1210/me.2003-0321. [DOI] [PubMed] [Google Scholar]
- Matsui H, Takatsu Y, Kumano S, Matsumoto H, Ohtaki T. Peripheral administration of metastin induces marked gonadotropin release and ovulation in the rat. Biochemical and biophysical research communications. 2004;3202:383–388. doi: 10.1016/j.bbrc.2004.05.185. [DOI] [PubMed] [Google Scholar]
- Mellon PL, Windle JJ, Goldsmith PC, Padula CA, Roberts JL, Weiner RI. Immortalization of hypothalamic GnRH neurons by genetically targeted tumorigenesis. Neuron. 1990;51:1–10. doi: 10.1016/0896-6273(90)90028-e. [DOI] [PubMed] [Google Scholar]
- Messager S, Chatzidaki EE, Ma D, Hendrick AG, Zahn D, Dixon J, Thresher RR, Malinge I, Lomet D, Carlton MB, Colledge WH, Caraty A, Aparicio SA. Kisspeptin directly stimulates gonadotropin-releasing hormone release via G protein-coupled receptor 54. Proceedings of the National Academy of Sciences of the United States of America. 2005;1025:1761–1766. doi: 10.1073/pnas.0409330102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moenter SM, Caraty A, Karsch FJ. The estradiol-induced surge of gonadotropin-releasing hormone in the ewe. Endocrinology. 1990;1273:1375–1384. doi: 10.1210/endo-127-3-1375. [DOI] [PubMed] [Google Scholar]
- Morelli A, Marini M, Mancina R, Luconi M, Vignozzi L, Fibbi B, Filippi S, Pezzatini A, Forti G, Vannelli GB, Maggi M. Sex steroids and leptin regulate the "first kiss" (KiSS 1/G-protein-coupled receptor 54 system) in human gonadotropin-releasing-hormonesecreting neuroblasts. The journal of sexual medicine. 2008;55:1097–1113. doi: 10.1111/j.1743-6109.2008.00782.x. [DOI] [PubMed] [Google Scholar]
- Navarro VM, Castellano JM, Fernandez-Fernandez R, Barreiro ML, Roa J, Sanchez-Criado JE, Aguilar E, Dieguez C, Pinilla L, Tena-Sempere M. Developmental and hormonally regulated messenger ribonucleic acid expression of KiSS-1 and its putative receptor, GPR54, in rat hypothalamus and potent luteinizing hormone-releasing activity of KiSS-1 peptide. Endocrinology. 2004;14510:4565–4574. doi: 10.1210/en.2004-0413. [DOI] [PubMed] [Google Scholar]
- Navarro VM, Castellano JM, Fernandez-Fernandez R, Tovar S, Roa J, Mayen A, Nogueiras R, Vazquez MJ, Barreiro ML, Magni P, Aguilar E, Dieguez C, Pinilla L, Tena-Sempere M. Characterization of the potent luteinizing hormone-releasing activity of KiSS-1 peptide, the natural ligand of GPR54. Endocrinology. 2005a;1461:156–163. doi: 10.1210/en.2004-0836. [DOI] [PubMed] [Google Scholar]
- Navarro VM, Castellano JM, Fernandez-Fernandez R, Tovar S, Roa J, Mayen A, Barreiro ML, Casanueva FF, Aguilar E, Dieguez C, Pinilla L, Tena-Sempere M. Effects of KiSS-1 peptide, the natural ligand of GPR54, on follicle-stimulating hormone secretion in the rat. Endocrinology. 2005b;1464:1689–1697. doi: 10.1210/en.2004-1353. [DOI] [PubMed] [Google Scholar]
- Nazian SJ. Role of metastin in the release of gonadotropin-releasing hormone from the hypothalamus of the male rat. Journal of andrology. 2006;273:444–449. doi: 10.2164/jandrol.05144. [DOI] [PubMed] [Google Scholar]
- Ng Y, Wolfe A, Novaira HJ, Radovick S. Estrogen regulation of gene expression in GnRH neurons. Molecular and cellular endocrinology. 2009;3031-2:25–33. doi: 10.1016/j.mce.2009.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen SL, Ottem EN, Carpenter CD. Direct and indirect regulation of gonadotropin-releasing hormone neurons by estradiol. Biology of reproduction. 2003;696:1771–1778. doi: 10.1095/biolreprod.103.019745. [DOI] [PubMed] [Google Scholar]
- Pielecka-Fortuna J, Chu Z, Moenter SM. Kisspeptin acts directly and indirectly to increase gonadotropin-releasing hormone neuron activity and its effects are modulated by estradiol. Endocrinology. 2008;1494:1979–1986. doi: 10.1210/en.2007-1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plant TM, Barker-Gibb ML. Neurobiological mechanisms of puberty in higher primates. Human reproduction update. 2004;101:67–77. doi: 10.1093/humupd/dmh001. [DOI] [PubMed] [Google Scholar]
- Plant TM, Witchel SF. Puberty in nonhuman primates and humans. In: Neil JD, editor. knobil and neill's physiology of reproduction. 3rd ed. San Diego: 2006. pp. 2177–2230. [Google Scholar]
- Plant TM. Hypothalamic control of the pituitary-gonadal axis in higher primates: Key advances over the last two decades. Journal of neuroendocrinology. 2008;206:719–726. doi: 10.1111/j.1365-2826.2008.01708.x. [DOI] [PubMed] [Google Scholar]
- Prentice LM, Klausen C, Kalloger S, Kobel M, McKinney S, Santos JL, Kenney C, Mehl E, Gilks CB, Leung P, Swenerton K, Huntsman DG, Aparicio SA. Kisspeptin and GPR54 immunoreactivity in a cohort of 518 patients defines favourable prognosis and clear cell subtype in ovarian carcinoma. BMC medicine. 2007;5:33. doi: 10.1186/1741-7015-5-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quaynor S, Hu L, Leung PK, Feng H, Mores N, Krsmanovic LZ, Catt KJ. Expression of a functional g protein-coupled receptor 54-kisspeptin autoregulatory system in hypothalamic gonadotropin-releasing hormone neurons. Molecular endocrinology (Baltimore, Md.) 2007;2112:3062–3070. doi: 10.1210/me.2007-0207. [DOI] [PubMed] [Google Scholar]
- Radovick S, Ticknor CM, Nakayama Y, Notides AC, Rahman A, Weintraub BD, Cutler GB, Jr, Wondisford FE. Evidence for direct estrogen regulation of the human gonadotropin-releasing hormone gene. The Journal of clinical investigation. 1991a;885:1649–1655. doi: 10.1172/JCI115479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radovick S, Wray S, Lee E, Nicols DK, Nakayama Y, Weintraub BD, Westphal H, Cutler GB, Jr, Wondisford FE. Migratory arrest of gonadotropin-releasing hormone neurons in transgenic mice. Proceedings of the National Academy of Sciences of the United States of America. 1991b;888:3402–3406. doi: 10.1073/pnas.88.8.3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radovick S, Wray S, Muglia L, Westphal H, Olsen B, Smith E, Patriquin E, Wondisford FE. Steroid hormone regulation and tissue-specific expression of the human GnRH gene in cell culture and transgenic animals. Hormones and behavior. 1994;284:520–529. doi: 10.1006/hbeh.1994.1050. [DOI] [PubMed] [Google Scholar]
- Ringel MD, Hardy E, Bernet VJ, Burch HB, Schuppert F, Burman KD, Saji M. Metastin receptor is overexpressed in papillary thyroid cancer and activates MAP kinase in thyroid cancer cells. The Journal of clinical endocrinology and metabolism. 2002;875:2399. doi: 10.1210/jcem.87.5.8626. [DOI] [PubMed] [Google Scholar]
- Roa J, Vigo E, Castellano JM, Navarro VM, Fernandez-Fernandez R, Casanueva FF, Dieguez C, Aguilar E, Pinilla L, Tena-Sempere M. Hypothalamic expression of KiSS-1 system and gonadotropin-releasing effects of kisspeptin in different reproductive states of the female rat. Endocrinology. 2006;1476:2864–2878. doi: 10.1210/en.2005-1463. [DOI] [PubMed] [Google Scholar]
- Roa J, Aguilar E, Dieguez C, Pinilla L, Tena-Sempere M. New frontiers in kisspeptin/GPR54 physiology as fundamental gatekeepers of reproductive function. Frontiers in neuroendocrinology. 2008a;291:48–69. doi: 10.1016/j.yfrne.2007.07.002. [DOI] [PubMed] [Google Scholar]
- Roa J, Castellano JM, Navarro VM, Handelsman DJ, Pinilla L, Tena-Sempere M. Kisspeptins and the control of gonadotropin secretion in male and female rodents. Peptides. 2008b doi: 10.1016/j.peptides.2008.08.009. [DOI] [PubMed] [Google Scholar]
- Roa J, Vigo E, Castellano JM, Gaytan F, Navarro VM, Aguilar E, Dijcks FA, Ederveen AG, Pinilla L, van Noort PI, Tena-Sempere M. Opposite roles of estrogen receptor (ER)-alpha and ERbeta in the modulation of luteinizing hormone responses to kisspeptin in the female rat: Implications for the generation of the preovulatory surge. Endocrinology. 2008c;1494:1627–1637. doi: 10.1210/en.2007-1540. [DOI] [PubMed] [Google Scholar]
- Roy D, Angelini NL, Belsham DD. Estrogen directly respresses gonadotropin-releasing hormone (GnRH) gene expression in estrogen receptor-alpha (ERalpha)- and ERbeta-expressing GT1-7 GnRH neurons. Endocrinology. 1999;14011:5045–5053. doi: 10.1210/endo.140.11.7117. [DOI] [PubMed] [Google Scholar]
- Sarkar DK, Fink G. Luteinizing hormone releasing factor in pituitary stalk plasma from long-term ovariectomized rats: Effects of steroids. The Journal of endocrinology. 1980;863:511–524. doi: 10.1677/joe.0.0860511. [DOI] [PubMed] [Google Scholar]
- Seminara SB, Messager S, Chatzidaki EE, Thresher RR, Acierno JS, Jr, Shagoury JK, Bo-Abbas Y, Kuohung W, Schwinof KM, Hendrick AG, Zahn D, Dixon J, Kaiser UB, Slaugenhaupt SA, Gusella JF, O'Rahilly S, Carlton MB, Crowley WF, Jr, Aparicio SA, Colledge WH. The GPR54 gene as a regulator of puberty. The New England journal of medicine. 2003;34917:1614–1627. doi: 10.1056/NEJMoa035322. [DOI] [PubMed] [Google Scholar]
- Seminara SB, Dipietro MJ, Ramaswamy S, Crowley WF, Jr, Plant TM. Continuous human metastin 45–54 infusion desensitizes G protein-coupled receptor 54-induced gonadotropin-releasing hormone release monitored indirectly in the juvenile male rhesus monkey (macaca mulatta): A finding with therapeutic implications. Endocrinology. 2006;1475:2122–2126. doi: 10.1210/en.2005-1550. [DOI] [PubMed] [Google Scholar]
- Seminara SB, Crowley WF., Jr Kisspeptin and GPR54: Discovery of a novel pathway in reproduction. Journal of neuroendocrinology. 2008;206:727–731. doi: 10.1111/j.1365-2826.2008.01731.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahab M, Mastronardi C, Seminara SB, Crowley WF, Ojeda SR, Plant TM. Increased hypothalamic GPR54 signaling: A potential mechanism for initiation of puberty in primates. Proceedings of the National Academy of Sciences of the United States of America. 2005;1026:2129–2134. doi: 10.1073/pnas.0409822102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skynner MJ, Sim JA, Herbison AE. Detection of estrogen receptor alpha and beta messenger ribonucleic acids in adult gonadotropin-releasing hormone neurons. Endocrinology. 1999;14011:5195–5201. doi: 10.1210/endo.140.11.7146. [DOI] [PubMed] [Google Scholar]
- Skinner DC, Dufourny L. Oestrogen receptor beta-immunoreactive neurones in the ovine hypothalamus: Distribution and colocalisation with gonadotropin-releasing hormone. Journal of neuroendocrinology. 2005;171:29–39. doi: 10.1111/j.1365-2826.2005.01271.x. [DOI] [PubMed] [Google Scholar]
- Smith JT, Dungan HM, Stoll EA, Gottsch ML, Braun RE, Eacker SM, Clifton DK, Steiner RA. Differential regulation of KiSS-1 mRNA expression by sex steroids in the brain of the male mouse. Endocrinology. 2005a;1467:2976–2984. doi: 10.1210/en.2005-0323. [DOI] [PubMed] [Google Scholar]
- Smith JT, Cunningham MJ, Rissman EF, Clifton DK, Steiner RA. Regulation of Kiss1 gene expression in the brain of the female mouse. Endocrinology. 2005b;1469:3686–3692. doi: 10.1210/en.2005-0488. [DOI] [PubMed] [Google Scholar]
- Smith JT, Clifton DK, Steiner RA. Regulation of the neuroendocrine reproductive axis by kisspeptin-GPR54 signaling. Reproduction (Cambridge, England) 2006a;1314:623–630. doi: 10.1530/rep.1.00368. [DOI] [PubMed] [Google Scholar]
- Smith JT, Popa SM, Clifton DK, Hoffman GE, Steiner RA. Kiss1 neurons in the forebrain as central processors for generating the preovulatory luteinizing hormone surge. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2006b;2625:6687–6694. doi: 10.1523/JNEUROSCI.1618-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stathatos N, Bourdeau I, Espinosa AV, Saji M, Vasko VV, Burman KD, Stratakis CA, Ringel MD. KiSS-1/G protein-coupled receptor 54 metastasis suppressor pathway increases myocyte-enriched calcineurin interacting protein 1 expression and chronically inhibits calcineurin activity. The Journal of clinical endocrinology and metabolism. 2005;909:5432–5440. doi: 10.1210/jc.2005-0963. [DOI] [PubMed] [Google Scholar]
- Suarez-Quian CA, Wynn PC, Catt KJ. Receptor-mediated endocytosis of GnRH analogs: Differential processing of gold-labeled agonist and antagonist derivatives. Journal of steroid biochemistry. 1986;241:183–192. doi: 10.1016/0022-4731(86)90049-x. [DOI] [PubMed] [Google Scholar]
- Tena-Sempere M. GPR54 and kisspeptin in reproduction. Human reproduction update. 2006;125:631–639. doi: 10.1093/humupd/dml023. [DOI] [PubMed] [Google Scholar]
- Terasawa E, Fernandez DL. Neurobiological mechanisms of the onset of puberty in primates. Endocrine reviews. 2001;221:111–151. doi: 10.1210/edrv.22.1.0418. [DOI] [PubMed] [Google Scholar]
- Wintermantel TM, Campbell RE, Porteous R, Bock D, Grone HJ, Todman MG, Korach KS, Greiner E, Perez CA, Schutz G, Herbison AE. Definition of estrogen receptor pathway critical for estrogen positive feedback to gonadotropin-releasing hormone neurons and fertility. Neuron. 2006;522:271–280. doi: 10.1016/j.neuron.2006.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong ML, Medrano JF. Real-time PCR for mRNA quantitation. BioTechniques. 2005;391:75–85. doi: 10.2144/05391RV01. [DOI] [PubMed] [Google Scholar]
- Xia L, Van Vugt D, Alston EJ, Luckhaus J, Ferin M. A surge of gonadotropin-releasing hormone accompanies the estradiol-induced gonadotropin surge in the rhesus monkey. Endocrinology. 1992;1316:2812–2820. doi: 10.1210/endo.131.6.1446619. [DOI] [PubMed] [Google Scholar]