Summary
Despite decades of successful use of cytotoxic chemotherapy in acute myelogenous leukemia (AML), the biological basis for its differential success among individuals and for the existence of a therapeutic index has remained obscure. Rather than taking a genetic approach favored by many, we took a functional approach to ask how differential mitochondrial readiness for apoptosis (“priming”) might explain individual variation in clinical behavior. We found that mitochondrial priming measured by BH3 profiling was a determinant of initial response to induction chemotherapy, relapse following remission, and requirement for allogeneic bone marrow transplantation. Differential priming between malignant myeloblasts and normal hematopoietic stem cells supports a mitochondrial basis to the therapeutic index for chemotherapy. BH3 profiling identified BCL-2 inhibition as a targeted strategy likely to have a useful therapeutic index. BH3 profiling refines predictive information provided by conventional biomarkers currently in use, and thus may itself have utility as a clinical predictive biomarker.
Introduction
Though the majority of current cancer research has focused on novel targeted therapies, empirically derived conventional chemotherapy, largely targeting DNA and microtubules, has cured millions of cancer patients over the last 5 decades. A better understanding of why these therapies work can help us more wisely utilize them presently and better exploit targeted therapies in the future. Acute myeloid leukemia (AML) is a malignancy primarily of adults in which a malignant myeloid clone in the bone marrow is arrested in development and proliferates abnormally. A highly successful empirically derived treatment scheme combining cytarabine with an anthracycline has yielded a 70% remission rate, greater overall survival and even cures for what is otherwise a fatal disease (Fernandez et al., 2009). The only curative option for patients who are resistant to or relapse following this induction regimen is allogeneic bone marrow or stem cell transplantation (Allo-SCT), which consists of an intensive preparatory chemotherapeutic regimen followed by introduction of donor hematopoietic stem cells (HSCs) (Schlenk et al., 2008). The success of the allogeneic approach is thought to depend on an immunologic graft-versus-leukemia effect rather than direct chemotherapeutic cytotoxicity for success.
The risk of induction-related death increases with age, yet alternatives to high-dose chemotherapy have modest efficacy (Appelbaum et al., 2006; Sekeres and Stone, 2002). Patients at high risk of relapse after induction of a complete remission are typically referred for allogeneic transplantation since chemotherapy alone is usually insufficient to ensure a durable remission in those cases (Schlenk et al., 2008). However, due to treatment related mortality and graft versus host disease, allogeneic transplantation bears considerable risks and should be used only for patients who are at high risk of relapse with standard chemotherapy. Thus predicting how well a patient will respond to chemotherapy and the risk of relapse is essential in deciding the best treatment course for each individual patient. Currently, prognostic factors based on cytogenetic abnormalities and gene mutations govern the use of allogeneic transplantation (Dohner et al., 2010). Current strategies in AML treatment are based on meticulous clinical observations rather than on a biological understanding of differential response to standard chemotherapeutic regimens. We propose here that the basis of differential response and clinical outcome following chemotherapy in AML lies in the intrinsic mitochondrial priming of the AML cells.
Mitochondrial priming is controlled by the BCL-2 family of proteins (Brunelle and Letai, 2009; Brunelle et al., 2009; Certo et al., 2006; Deng et al., 2007; Letai, 2008; Ni Chonghaile et al., 2011; Ryan et al., 2010). This family consists of pro-apoptotic and anti-apoptotic members. If pro-apoptotic members overwhelm the anti-apoptotic members, the threshold of death is crossed and the cell dies. The BCL-2 family consists of four groups of proteins containing at least one of four homology domains called the BH domains (BH1-BH4) (Brunelle and Letai, 2009; Danial and Korsmeyer, 2004). The first group consists of pro-apoptotic multi-domain “effector” members Bax and Bak. Once activated these proteins homo-oligomerize to induce mitochondrial outer membrane permeabilization (MOMP) (Wei et al., 2000; Wei et al., 2001), which results in the release of cytochrome c (and other pro-apoptotic factors) from the mitochondria and loss of mitochondrial transmembrane potential (Kluck et al., 1997). In the cytosol, cytochrome c cooperates in the formation of a multi-molecular apoptosome complex that initiates a cascade of proteolysis executed by caspases (Zou et al., 1999). Bim and Bid (and perhaps PUMA) proteins contain the BH3 domain (“BH3-only”) and are pro-apoptotic “activators” of Bax and Bak (Gavathiotis et al., 2008; Wei et al., 2000). Anti-apoptotic members like BCL-2, BCL-XL, BCL-w, BFL-1 and MCL-1 contain multiple BH domains and can inhibit by sequestration both the multi-domain effectors and BH3-only activator proteins (Certo et al., 2006; Cheng et al., 2001; Willis et al., 2005). The last class consists of BH3-only proteins (Puma, Bmf, Bad, Noxa, Hrk) referred to as “sensitizers” since they lack the ability to directly activate Bax/Bak (Certo et al., 2006; Letai et al., 2002). However, they sensitive cells to death by antagonizing antii-apoptotic members. Sensitizer proteins have unique binding specificity to the anti-apoptotic proteins and thus can only inhibit certain anti-apoptotic members (Certo et al., 2006; Chen et al., 2005; Kuwana et al., 2005; Opferman et al., 2003). Cellular stress caused by chemotherapeutic agents induces the relative increase of BH3-only proteins. In cells highly primed for death, this overwhelms anti-apoptotic members and results in cell death. In less primed cells, anti-apoptotic reserves prevent death.
We define priming functionally as the magnitude of response of mitochondria to pro-apoptotic peptides derived from the BH3 domains of BH3-only proteins (Deng et al., 2007; Ni Chonghaile et al., 2011; Ryan et al., 2010). In practice, we measure this as the release of cytochrome c or the loss of mitochondrial transmembrane potential caused by standardized doses of BH3 peptides in an assay we call BH3 profiling. The greater the loss of mitochondrial transmembrane potential caused by the BH3-only peptides, the more cells are primed for death. Loss of potential caused by BH3 peptides like BIM that promiscuously inhibits all the anti-apoptotic members provides a measure of overall priming (Ni Chonghaile et al., 2011). Sensitizer BH3 peptides like BAD, NOXA and HRK inhibit only specific antiapoptotic members and thus provide a measurement of dependence on the anti-apoptotic proteins they inhibit (Brunelle et al., 2009; Certo et al., 2006). For instance, mitochondrial response to the NOXA BH3 peptide is an indication of dependence on MCL-1, while mitochondrial response to BAD BH3 peptide is an indication of dependence on BCL-2, BCL-w or BCL-XL.
Conventional chemotherapeutic agents generally kill via the mitochondrial apoptotic pathway. We have previously found that increased priming is the basis for differential clinical response in several cancers, including multiple myeloma, acute lymphocytic leukemia, ovarian cancer, and AML (Ni Chonghaile et al., 2011). In the case of AML, we found that in a preliminary series of 15 patients, achieving remission was related to the degree of mitochondrial priming. Here we expand upon those results to explain a wide range of clinical phenomena associated with AML that have previously lacked elucidation of a biological mechanism. We find that differential priming determines not only the initial response rate, but also risk of relapse. Using AML cell lines, we demonstrate that increasing mitochondrial priming enhances chemosensitivity. We determine that the source of a therapeutic index in AML depends upon AML cells being more primed than normal hematopoietic stem cells (HSC). We find that low priming identifies a subset of patients with a very high risk of relapse for whom allogeneic stem cell transplantation is likely required for cure. Finally, we use BH3 profiling to identify dependence on BCL-2 present even in chemorefractory myeloblasts but not in normal HSC, suggesting another potential avenue to rescue low primed AML cases. Our results demonstrate that the functional information about mitochondrial apoptotic priming provided by BH3 profiling not only is useful in defining biological mechanisms in AML, but also can be potentially exploited as a clinical predictive biomarker in AML.
Results
Mitochondrial Priming is a Major Determinant of Topoisomerase II Inhibitor Efficacy
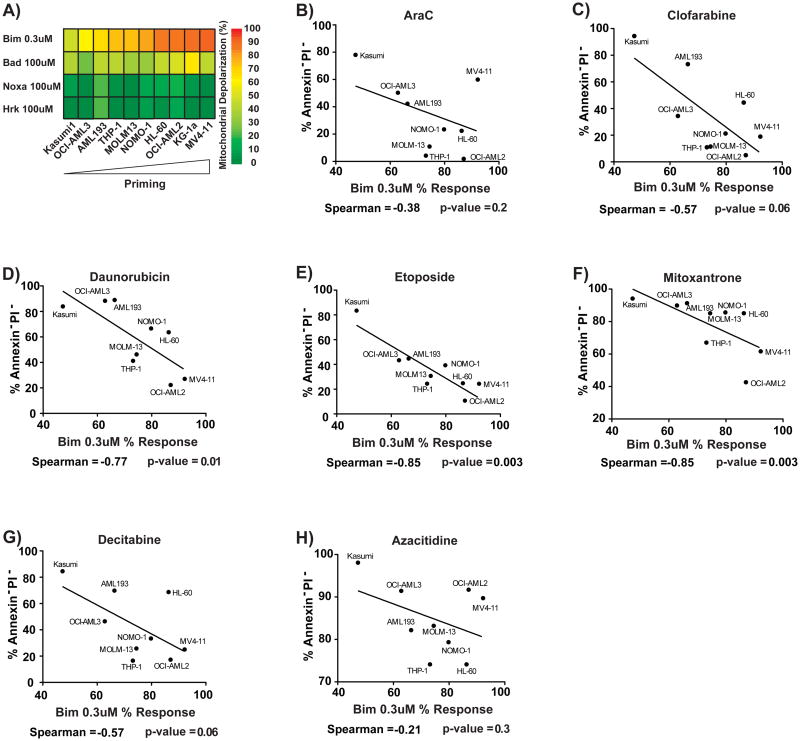
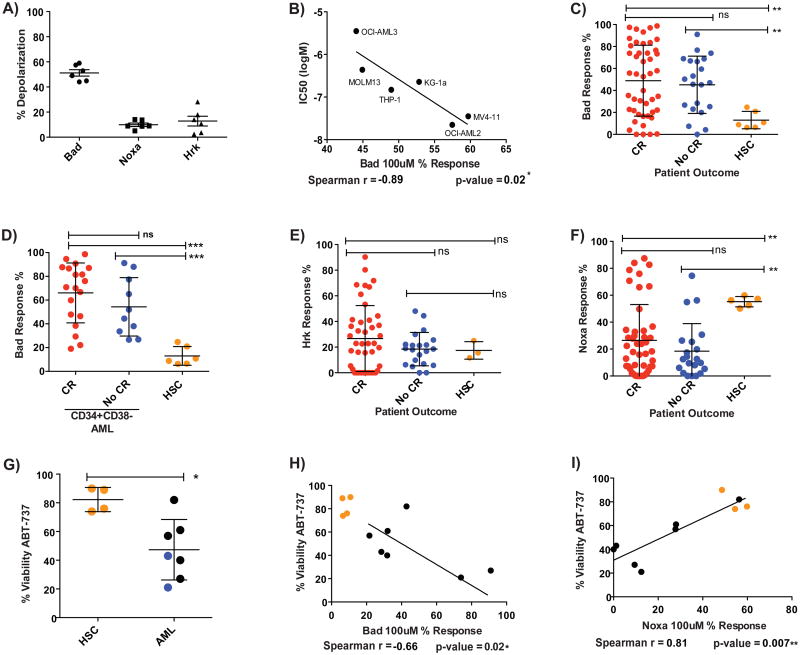
We first asked whether there was a relationship between pretreatment mitochondrial priming and cellular response to chemotherapeutic drugs used clinically in AML. To answer this question, we BH3 profiled AML cell lines to determine their priming (Figure 1A) and treated each line with the drugs. We noted that one cell line KG-1, had potent drug efflux activity that was not inhibited by verapamil, so we excluded it from further analysis (Figure S1A). We denote priming as the percentage of mitochondrial charge loss caused by the BIM BH3 peptide. We found that 0.3 uM of BIM BH3 peptide gave the appropriate dynamic range to compare the priming of the various AML lines (Figure 1A). Mitochondrial priming best correlated with cellular response to the drugs etoposide, daunorubicin and mitoxantrone (Figure 1B-H, S1D-F). It is notable that these three drugs are all topoisomerase II inhibitors, suggesting that reliance on mitochondrial priming for killing is a property of this class of drug (Nitiss, 2009). In contrast, araC and clofarabine are nucleoside analogs, and azacitidine and decitabine are hypomethylating agents, suggesting that killing of AML cells by these classes of agents may be less reliant on mitochondrial priming. Notably, proliferation rate, often cited as a key determinant of chemotherapy response, did not correlate with chemosensitivity (Figure S1G-M). We tested whether expression of individual BCL-2 family proteins correlated with chemosensitivity. Of the seven tested, correlation with BAX levels achieved statistical significance, though not if a Bonferroni correction for multiple hypothesis testing were applied. (Figure S1H-N) Note that the correlation of BAX with etoposide response is not as good as it is for BH3 profiling. Finally, there is not a good correlation between BAX expression and BH3 profiling (Figure S1O), suggesting that variability in BAX expression alone cannot explain differences in priming. Overall, these results suggest that mongenic predictors provide less predictive information than the functional approach of BH3 profiling.
Figure 1. Mitochondrial Priming Predicts Response to Topoisomerase II Inhibitors.
(A) BH3 profiling response of each AML cell line to the BH3 peptides. Priming is measured using 0.3 μM Bim response, the percent mitochondrial depolarization induced by the Bim BH3 peptide. Priming is compared with killing by nucleoside analogs (A) araC and (B) clofarabine, topoisomerase II inhibitors (D) daunorubicin, (E) etoposide and (F) mitoxantrone and DNA demethylating agents (G) azacytidine and (H) decitabine. All are one-tailed Spearman correlations. Treatment with topoisomerase II inhibitors were done in the presence of 20 μM verapamil to exclude the effects of drug pumps. See also Figure S1 and Table S1.
Perturbation of mitochondria alters chemosensitivity of AML cells
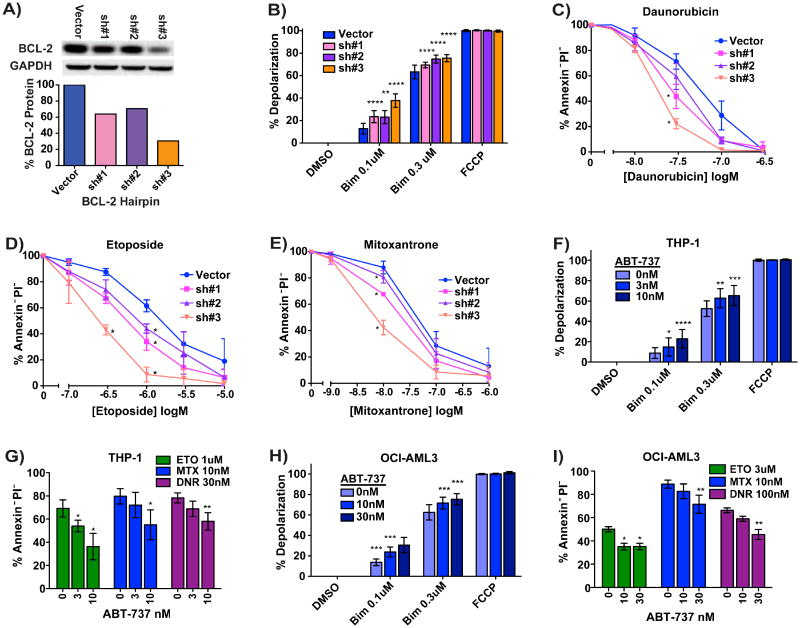
If priming truly causes as well as correlates with chemosensitivity, then increasing mitochondrial priming should increase chemosensitivity. To test this, we lowered anti-apoptotic BCL-2 expression in the MOLM13 AML lines using shRNA knockdown (Figure 2A). The MOLM13 BCL-2 partial knockdown produced increased priming as measured by BH3 profiling (Figure 2B). The increase in priming was associated with increased killing by etoposide, daunorubicin and mitoxantrone in proportion to the quality of the knockdown (Figure 2C-E).
Figure 2. Specifically Increasing Mitochondrial Priming Increases Sensitivity to Chemotherapy.
(A) BCL-2 protein levels after lenti-viral introduction of shRNAs into the MOLM13 cell line. (B) Priming as measured by depolarization induced by BIM BH3 at two concentrations. BCL-2 knockdown increased cellular sensitivity to (C) daunorubicin, (D) etoposide and (E) mitoxantrone. Alternatively, priming was increased using the BCL-2 antagonist ABT-737 in the (F) THP-1 and (H) OCI-AML3 cell lines. (G) and (I) Increased priming by ABT-737 also increased these cell lines' sensitivity to etoposide, mitoxantrone and daunorubicin.
In an alternative approach to directly altering mitochondrial priming, we used the small molecule BCL-2 antagonist ABT-737 (Oltersdorf et al., 2005). Only the two lines that are least sensitive to the BAD BH3 peptide, OCI-AML2 and THP-1, could be primed by ABT-737 without being killed by the inhibitor. As expected, treatment of OCI-AML2 and THP-1 cell lines with ABT-737 caused an increase in priming (Figure 2F,H) and this increase in priming resulted in a concomitant increase in chemosensitivity (Figure 2G,I). Thus specific perturbation of mitochondrial priming by two distinct methods resulted in a change in chemosensitivity. These results supports the concept that mitochondrial priming is not merely correlative, but is actually causative of chemosensitivity.
BH3 profiling of primary blood and bone marrow samples
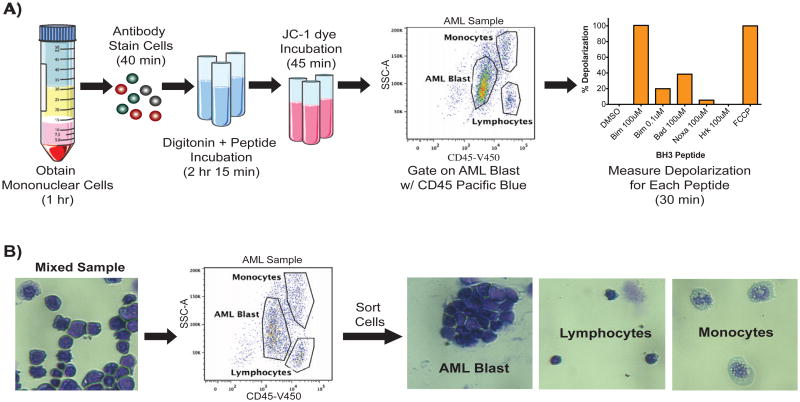
We next wanted to test if priming was similarly deterministic in the clinical setting. We first needed to develop a reliable method of BH3 profiling normal and malignant cells in heterogeneous patient blood and bone marrow samples. Often, only a minority of nucleated cells in the bone marrow are AML myeloblasts. For this reason we adapted our BH3 profiling method to identify cells of interest by FACS analysis (Lacombe et al., 1997; Ni Chonghaile et al., 2011; Ryan et al., 2010) (Figure 3A). We combined a patient AML sample with normal leukocytes to establish the AML blast gate using CD45 staining and side scatter (Figure S2A). We validated that AML myeloblasts are CD45lo and SSClo/mid by FACS sort followed by histological verification (Figure 3B). The digitonin used to permit peptide access to mitochondria did not affect the gating of our AML blasts (Figure S2B). JC-1 dye was used to detect mitochondrial charge loss caused by BH3 peptides upon initiation of MOMP on the PE channel (Cossarizza et al., 1993)(Figure S2C). Most patient bone marrow and blood samples were available only as viably frozen cells in 10% DMSO. We found that the freezing process in 10% DMSO does not change the priming of the cells as long as cell viability is high after thawing (Figure S2D). Moreover, the same patient sample frozen in two different media also showed comparable priming readouts (Figure S2E).
Figure 3. BH3 Profiling by FACS Identifies AML Myeloblasts.
(A) The FACS based BH3 profiling method for primary AML starts with Ficoll gradient purification of mononuclear cells from patient blood or bone marrow. Mononuclear cells are stained with antibodies recognizing CD45, CD34 and CD38. Cells are then incubated in digitonin and peptide for 3 hours to allow the BH3 peptide time to induce mitochondrial depolarization. JC-1 dye is added to measure the remaining mitochondrial charge. AML myeloblasts are identified as CD45lo and SSClo/mid. Mitochondrial depolarization induced by peptide was detected for the AML gated cells and graphed. (B) To validate our gating, a mixed patient sample was stained with CD45 and the AML, lymphocyte and monocyte populations were gated. These populations were sorted and verified by histology. See also Figure S2.
Higher Pretreatment Mitochondrial Priming of Patient AML Correlates With Clinical Induction Success
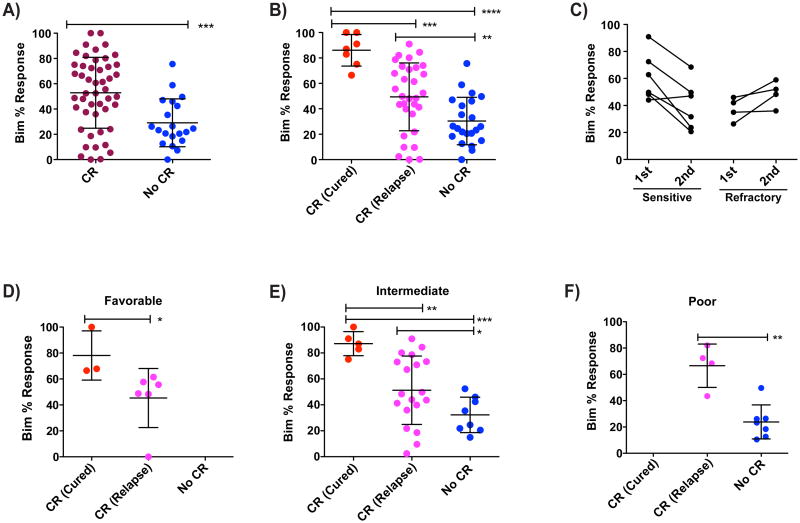
We used two different sources of clinically annotated AML samples: Dana-Farber Cancer Institute and Memorial Sloan Kettering. All samples were frozen prior to BH3 profiling. Investigators performing BH3 profiling were blinded to clinical response. In the primary test of our hypothesis that mitochondrial priming determines response to chemotherapy in AML, the myeloblasts of patients who subsequently attained a complete remission (CR) in response to induction therapy were more primed than patients who did not achieve a CR (Figure 4A, S3A). We considered induction therapies to be those that included a topisomerase II inhibitor like daunorubicin, idarubicin, mitoxantrone, or etoposide, essential components of most standard AML induction regimens.
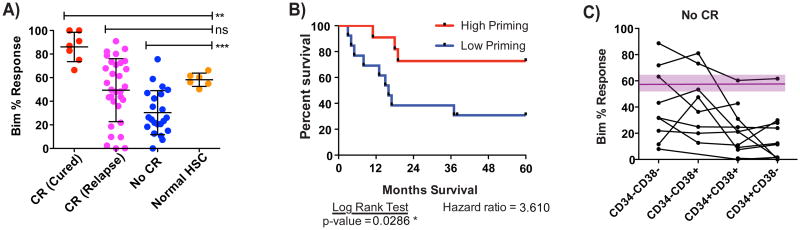
Figure 4. Relative priming of AML determines clinical outcome.
(A) Pre-induction priming as measured by Bim 0.1 μM depolarization of patient AML samples compared with clinical response to induction therapy specified as CR (complete response) or no CR. (B) Patients who achieve CR are separated into Cured (no relapse and no transplantation) or Relapse (relapse after initial CR). Highly primed patients having better clinical outcome. (C) Sequential samples were obtained from the same patients at two different dates. Patients who were initially obtained a CR and then relapsed and patients who never achieved a CR and had a second biopsy taken after induction. Decrease in priming for after relapse is statistically significant based on a one-tailed Wilcoxon matched-pair signed rank test with p-value 0.03. No such significant trend was observed for the refractory sequential samples. (D-F) Patients were grouped based on their ELN relapse risk factors and clinical outcome was compared with priming of primary AML samples. Patients with normal cytogenetics lacking NPM1 and FLT3 information were classified as Intermediate Risk. Significant differences were calculated using a nominal one-tailed Mann-Whitney test. (* p-value < 0.05, ** p < 0.005, *** p < 0.0005). See also Figure S3 and Table S2.
In those patients where sufficient followup was available, we examined long term duration of response, defined as 5 year disease free survival following completion of induction therapy (Figure 4B). Since we wanted to evaluate outcome following chemotherapy alone, we excluded patients who received an allogeneic bone marrow or stem cell transplant following induction therapy. We found that those who obtained a CR and were cured were more primed than those that underwent a CR and then relapsed. The latter were in turn more primed than those who did not achieve CR. Thus, mitochondrial priming appears to determine both the rate of clinical remission as well as its duration. We found no difference in proliferation rate in vivo of myeloblasts in patients who attained CR and were cured with those of patients who did not achieve CR. This suggests that differences in proliferation rate in vivo did not drive the differences in response.
If priming is a determinant of response, we would expect to see selection for decreased priming of AML cells following relapse. In our cohort, we identified cases for which there was a CR followed by a relapse. Of these, six cases were identified for which paired samples were available, one obtained before treatment and one after relapse (Figure 4C). We indeed observed in vivo selection for decreased mitochondrial priming in cases of relapsed AML where the relapsed samples tended to be less primed than the initial samples (p= 0.031). As a control for time or chemotherapy causing these changes in the absence of selection, we examined myeloblast samples before and after chemotherapy in patients who did not enter a complete remission, and found no evidence of decreased priming. This suggests that in the relapsed samples we are observing true selection rather than a paradoxical direct chemotherapy effect, since response is required to observe the selection for decreased priming.
BH3 Profiling Further Refines Prognostic Information from Cytogenetics and Genetics
The most widely used prognostic system currently in use for AML is that of the European LeukemiaNet (Dohner et al., 2010). This classification system is based on classical cytogenetic results as well as possession of a nucleophosmin (NPM1) or FLT-3 internal tandem duplication (ITD) mutation to classify patients in one of four prognostic groups: Favorable, Intermediate I, Intermediate II, and Poor. While of demonstrated prognostic utility, the ELN criteria leave considerable dispersion in outcome in each of the groups. Restricting our analysis to those patients from 4A for whom we had cytogenetic data, we segregated patients as Favorable, Intermediate (combining Intermediate I and II) or Poor risk according to this system. As expected, clinical outcome was best in the Favorable group, worse in the Intermediate and worst in the Poor risk group. However, as expected, even within each of these groups, there was a mixture of the clinical responses: CR (cured), CR (relapse), and No CR. We asked whether BH3 profiling could better refine the prognostic information provided by the ELN criteria, even within these genetically defined groups. In each case it could. In the favorable group, BH3 profiling could distinguish between CR (cured) and CR (relapse) (Figure 4D). In the Intermediate group, BH3 profiling could distinguish between those destined for Cure, Relapse, or No CR (Figure 4E). Finally, BH3 profiling could distinguish between those destined to relapse and those who would be refractory to initial therapy (Figure 4F).
Cytogenetic abnormalities have long been demonstrated to provide prognostic information about both short and long term clinical outcomes in AML (Mrozek et al., 2004). However, the important physiological effects of major chromosomal alterations that confer altered prognosis and chemotherapy response are obscure. We hypothesize that poor risk cytogenetic abnormalities are related to low mitochondrial priming, resulting in relative chemoresistance. Monosomy 7 is the one cytogenetic abnormality present in sufficient abundance in our sample for us to test this hypothesis. We therefore examined the mitochondrial priming of myeloblasts containing monosomy 7 and found that they were significantly less primed than responsive AML samples lacking monosomy 7 (Figure S3B). Low mitochondrial priming may provide for the first time a physiological mechanism to connect this poor risk cytogenetic finding to poor chemotherapy response in AML.
Priming of Myeloblast Relative to Hematopoietic Stem Cell Determines Therapeutic Index
If poorly primed AML is less sensitive to chemotherapy, what is limiting oncologists from just giving higher doses of chemotherapy to the poorly primed population? In the clinic, drug dosing is limited by the tolerance of critical normal cells. Induction chemotherapy regimens for AML have evolved over the past few decades based mainly on empiric observations of what drugs were effective and what doses were tolerable. By far the most common dose limiting toxicity is bone marrow toxicity. Since HSCs are responsible for re-growth of the ablated bone marrow, we wondered whether the therapeutic index of induction chemotherapy was dependent on the difference in chemosensitivity between normal HSCs and AML myeloblasts.
We proceeded to test the hypothesis that the therapeutic index of conventional induction chemotherapy in AML is based on the relative mitochondrial priming of normal HSCs and AML myeloblasts. That is, AML patients whose myeloblasts are less primed than normal HSCs would have a lower rate of clinical success, while those whose AML are more primed than HSCs would have a higher rate of success. To test this, we measured the priming of normal human HSCs similarly to our FACS BH3 profile of AML, except that we identified HSCs as Lin-CD34+CD38-CD45RA-CD90+ (Majeti et al., 2007)(Figure S4).
We indeed found that the priming of HSCs represents the boundary between the priming of myeloblasts from AML patients cured by chemotherapy and those refractory to it (Figure 5A). We therefore used the priming of HSCs as an index to categorize AML cells into highly primed (more primed than HSCs) or low primed (less primed than HSCs). In patients for whom we have at least 3 years clinical followup, we compared overall survival based on whether the pre-induction myeloblasts were more or less primed than the mean of normal HSCs. Patients with highly primed AML achieve a much greater overall survival than patients with low primed AML (Figure 5B). This finding supports the concept that the mitochondrial priming of HSC may be important in establishing the maximum tolerated doses of induction therapy, and hence the therapeutic index of induction therapy in AML.
Figure 5. Priming of AML relative to HSC priming determines clinical outcome.
(A) The priming of normal hematopoietic stem cells (HSCs) is compared with priming of Cured, Relapse, and No CR patients using a one-tailed Mann-Whitney test. (B) Kaplan-Meier survival curves based on pretreatment priming. Using the priming of normal HSCs as a cut-off, patients were categorized as high primed or low primed. Patients with AML that are high primed have significantly better overall survival than low primed patients. (C) Priming of AML subsets based on CD34 and CD38 staining of patients who did not achieve a CR. Purple region represents the average and standard deviation of normal HSC priming. All samples had a CD34+ progenitor population that was as primed or less primed than the HSCs. (** p < 0.005, *** p < 0.0005). See also Figure S4.
Myeloblasts can comprise a heterogeneous population as demonstrated by differential expression of cell surface antigens CD34 and CD38 (Lapidot et al., 1994). We noted that some AML cases that failed to achieve CR were relatively highly primed. We examined the distribution of mitochondrial priming among subpopulations based on CD34 and CD38 expression. We noted that for all of these cases, the least mature CD34+ CD38- population was the least primed, often much less primed than more mature populations (Figure 5C). This is particularly significant as the CD34+CD38- subpopulation is thought to most commonly harbor AML stem cells. While it would take a much greater sample to test this thoroughly, this raises the question of whether there are subpopulations that are of dominant importance in determining clinical outcome.
Poorly Primed AML Requires Allogeneic Transplantation for Long-term Survival
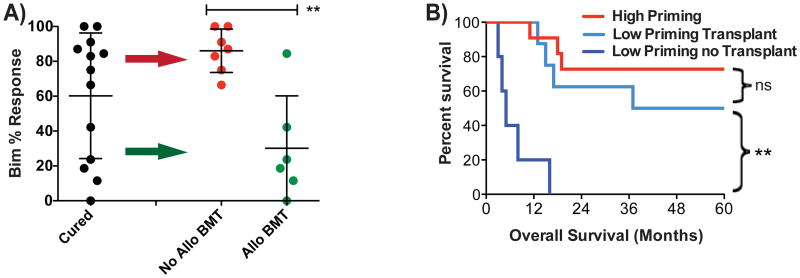
When we examined the patients who attained long-term survival after induction therapy without discriminating based on post-induction therapy, it became clear that some patients were cured despite presenting with very poorly primed myeloblasts (Figure 6A). To understand this observation, we examined post-induction therapy. It became clear that all poorly primed patients that were cured underwent allogeneic transplantation. The biological significance of this finding is that it suggests that apoptosis is irrelevant to the mechanism of graft-versus-leukemia killing of myeloblasts in vivo in humans. Furthermore, it suggests that given the otherwise poor prognosis of AML patients presenting with poorly primed myeloblasts, allogeneic bone marrow transplant may be required in this setting. To test this, we compared the overall survival of low primed AML patients (myeloblasts less primed than the normal HSC mean) according to whether or not they received an allogeneic transplant (Figure 6B). The difference in survival is dramatic, with all low-primed AML patients not receiving allogeneic transplant dying in less than 18 months, while half of the transplanted patients were alive at 5 years. In fact, the survival of the low priming patients receiving transplant was not significantly different from that of the high priming patients. Thus, while allogeneic transplant is not a guarantee of long term survival in patients presenting with poorly primed AML, it seems to be a requirement thereof.
Figure 6. Allogeneic transplantation benefits patients with low priming.
(A) Comparison of priming of Cured patients based on post-remission therapy. A one-tailed Mann-Whitney test is used. (B) Kaplan-Meier Survival curves based on priming and post-remission therapy. Low primed patients who received a transplant had better overall survival than no-transplanted low primed patients. Log-rank test. (**p-value<0.005)
Chemosensitive and Chemoresistant Myeloblasts are More Sensitive to BCL-2 Antagonism than Normal HSCs
While allogeneic bone marrow or stem cell transplantation apparently is an attractive option for low primed chemoresistant AML patients, its application is often limited by the age and comorbidities of the patient, as well as the availability of a suitable allograft. We therefore asked if BH3 profiling could detect other differences between AML myeloblasts and HSCs that would suggest alternative, less toxic therapeutic strategies that could be exploited. While we have so far focused on priming measures based on the promiscuously interactive BIM BH3 peptide, other BH3 peptides used in the BH3 profile inhibit only specific anti-apoptotic BCL-2 family proteins. Mitochondrial dysfunction induced by such peptides is therefore a measure of specific dependence on the anti-apoptotic protein with which they inhibit (Certo et al., 2006). For instance, we have previously shown that MOMP induced by the BAD BH3 peptide indicates dependence on BCL-2, BCL-XL or BCL-w.
When we examined BH3 profiling results for our AML cell lines, we found that all AML cell line mitochondria were more sensitive to the BAD BH3 peptide than the NOXA BH3 (which antagonizes MCL-1) or HRK BH3 (which antagonizes BCL-XL) peptides (Figure 7A). This suggests a specific dependence on BCL-2 or BCL-w for these cells' survival. Prior work has indicated that BCL-2 is the key protein (Konopleva et al., 2006). To directly test whether this observed mitochondrial dependence corresponded to a cellular dependence, we exposed the cell lines to ABT-737, which antagonizes function of BCL-2, BCL-w and BCL-XL (Oltersdorf et al., 2005). We found that the mitochondrial response to the BAD BH3 peptide correlated well with cellular killing by ABT-737 (Figure 7B, Figure S5A). This result demonstrates that BH3 profiling can detect anti-apoptotic dependencies that we can exploit pharmacologically to predictably kill AML cells.
Figure 7. BCL-2 independence of HSCs provides a therapeutic window for ABT-737.
BH3 profiling responses to BH3 peptides show BCL-2 dependency in all AML lines. (B) Comparison of IC50 killing by ABT-737 with BAD peptide response for each line after 24 hour treatment. Correlation determined by a one-tailed Spearman correlation. (C) Most AML cells from both sensitive and refractory patients are responsive to the BAD peptide, while HSCs are not. (D) BAD peptide response of primary CD34+CD38- AML population is more pronounced than HSC response. (E) HSCs and most primary AML are not responsive to the HRK peptide. (F) HSCs are responsive to the NOXA peptide but most primary AML are not. (G) Primary AML cells are significantly more sensitive to 1 μM ABT-737 than HSCs after 9 hours treatment. Blue dots represent to low primed AML refractory to standard induction. (H) Greater ABT-737 sensitivity correlates with greater BAD BH3 peptide sensitivity and also (I) correlates with less NOXA BH3 peptide sensitivity. (* p-value <0.05, **p-value<0.005, ***p-value<0.0005; viability = Annexin V-/PI- population). See also Figure S5.
Next we compared the response of mitochondria of primary AML cells from both sensitive and refractory AML patients with the response of HSCs to BAD, NOXA and HRK BH3 peptides. Most AML mitochondria from both refractory and sensitive patients responded significantly more to the BAD BH3 peptide than did that of the HSCs (Figure 7C). It is noteworthy that increased mitochondrial sensitivity to BAD BH3 was also found in the CD34+ CD38- subpopulation of myeloblasts, the subpopulation most commonly thought to harbor AML stem cells (Figure 7D). Taken in conjunction with an overall weak response to the HRK BH3 peptide (Figure 7E), these results suggest that AML cells, both bulk and CD34+CD38-, regardless of their clinical response to standard induction chemotherapy, are usually more dependent on BCL-2 than are normal HSCs.
In contrast, mitochondria from HSCs were significantly more sensitive to the NOXA BH3 peptide than were the majority of the AML cells (Figure 7F). This suggests that normal human HSCs are selectively more dependent on MCL-1 for survival than are most AML cells. These results are congruent with the demonstration of MCL-1 dependence of murine HSCs obtained from mouse genetic models (Opferman et al., 2005). However, this represents the first demonstration of MCL-1 dependence in human HSCs. Note that BH3 profiling also identified a subset of AML cases that were MCL-1 dependent, and that would therefore likely benefit from MCL-1 directed therapy should such a drug become available. This illustrates a strength of the BH3 profiling tool, that it can provide important information about genetic dependencies and the apoptotic pathway without requiring the sort of genetic manipulation used in mice that is impossible in primary human tissues.
Recall that all of the refractory AML patient cells were poorly primed. Since most of these refractory cells are sensitive to the BAD BH3 peptide, this suggests that BCL-2 inhibition might benefit even refractory low-primed AML, offering a potential non-transplant alternative to these poor prognosis patients. These results prompted us to compare sensitivity of AML and HSCs to treatment with ABT-737 (Figure 7G, Figure S5B). As predicted by the BH3 profiling results, primary AML cells, whether sensitive or refractory to induction, were more sensitive to ABT-737 than were normal HSCs. Two of the AML samples we tested were poorly primed, chemo-refractory AML cells (blue dots), both of which were highly sensitive to ABT-737 killing. We also found that cellular response to ABT-737 correlated with mitochondrial sensitivity to the BAD BH3 peptide (Figure 7H). Interestingly, cellular sensitivity to ABT-737 correlated inversely to mitochondrial dependency on MCL-1 as measured by the response to out Noxa peptide (Figure 7I). Various groups have observed MCL-1 can promote resistance to ABT-737 (Konopleva et al., 2006; van Delft et al., 2006). Thus BH3 profiling can detect resistance as well as sensitivity to ABT-737. In summary, these results suggest that there is a useful therapeutic index for BCL-2 inhibition between malignant myeloblasts and normal HSCs that can be exploited. Significantly, BCL-2 inhibition offers a therapeutic index even in those patients who are poorly primed and respond poorly to conventional chemotherapy. These results suggest a promising therapeutic intervention for AML patients with poor conventional options, and furthermore, BH3 profiling could provide a predictive biomarker of potential utility in guiding BCL-2 directed therapy.
Discussion
Over the past 4 decades since effective chemotherapy regimens for AML were introduced, several common observations have dominated thinking about AML therapy. These observations include: 1)some cases have excellent responses to chemotherapy while others do not; 2) patients who relapse after initial response are unlikely to be cured by chemotherapy alone; 3) there is an unexplained therapeutic index for induction chemotherapy; and 4)allogeneic transplant is required to rescue relapsed and poor risk patients. Our results suggest that differences in pre-treatment mitochondrial priming, as measured by BH3 profiling, provide a novel biological explanation for these heretofore unexplained clinical observations.
The observation that some patients achieve a complete remission and maintain it after chemotherapy alone, while others do not achieve remission, or relapse, has lacked a biological explanation. While cytogenetics have proven valuable as prognostic indicators of this behavior, it should be noted that cytogenetics have behaved mainly as empirically derived markers, with little power to explain the biological mechanism underlying differential killing of myeloblasts between patients. In this study, we tested the hypothesis that the differential mitochondrial tendency to apoptosis, or priming, measured by BH3 profiling explains the differential cellular and clinical response to cytotoxic chemotherapy. Our results support this hypothesis, as reflected in both initial response (Figure 4A) and longer term freedom from relapse (Figure 4B). This lends additional support to the concept that mitochondrial priming is an important determinant of clinical chemosensitivity that we supported in prior work in other cancers including multiple myeloma, acute lymphoblastic leukemia, and ovarian cancer (Ni Chonghaile et al., 2011).
Another important observation is that patients who relapse following an initial complete remission to induction chemotherapy are unlikely to achieve long-term remissions from subsequent chemotherapy, no matter what combination of agents is used. It is known that relapsed AML tends to be broadly more chemoresistant than that of the initial presentation, but the mechanism underlying this chemoresistance has been unclear. Our results suggest that selection for reduced mitochondrial priming in relapsed AML may well be an important determinant of this chemoresistant phenotype (Figure 4C).
Success of induction chemotherapy depends on a therapeutic index. That is, there must be a feature of myeloblasts that renders them selectively more chemosensitive than critical normal tissues. Perhaps surprisingly, given its centrality to the treatment of AML, the biologic basis of this feature remains poorly understood. There is no obvious AML-specific target exploited by standard induction chemotherapy, as it acts primarily to damage DNA, a target present in normal as well as malignant cells. We found that mitochondrial priming was a key determinant of the therapeutic index between myeloblasts and normal cells (Figure 5) and therefore one answer to the question, “Why does chemotherapy work?” We could not identify a BCL-2 family protein whose level replicated the performance of BH3 profiling, supporting the concept that priming is likely the result of the simultaneous contribution of many proteins, perhaps even including some outside the BCL-2 family.
Two important points go beyond the elucidation of biological mechanisms of clinical behavior of AML and into potential clinical application. The first is the identification of a therapeutic index and a potential predictive biomarker for BCL-2 inhibition. We have made the observation not only of myeloblast sensitivity, but also relative HSC insensitivity to BCL-2 inhibition. Others have previously made a similar observation (Konopleva et al., 2006). However, we demonstrated, based on our mitochondrial BH3 profiling studies, that this is an on-target effect, based in the mitochondrion, and, further, that BH3 profiling is a potential predictive biomarker. Significantly, we found that BCL-2 dependence is observed even in cases where there was a poor response to conventional induction chemotherapy, indicating a potential strategy to rescue this difficult to treat population. Notably, identifying this therapeutic index and validating a predictive biomarker is of more than purely scientific interest, since clinical BCL-2 inhibition is now a practicable clinical approach. Currently, Abbott Laboratories has two drugs in clinical trials that directly target BCL-2, ABT-263 and ABT-199 (Roberts et al., 2012; Tse et al., 2008; Wilson et al., 2010). Both are orally available counterparts of ABT-737. An on-target toxicity, thrombocytopenia, due to high affinity binding to BCL-XL on which platelets depend, may limit testing of ABT-263 in AML, since patients very commonly present with existing thrombocytopenia. However, ABT-199 has greater selectivity for BCL-2, and lower affinity for BCL-XL, so the chances are better for achieving in vivo BCL-2 antagonism without exacerbating thrombocytopenia.
The second important advance is our identification of BH3 profiling as a potential predictive biomarker in AML, not only for BCL-2 inhibition but also for conventional chemotherapy. The understandable enthusiasm for BCL-2-targeted therapy notwithstanding, we expect that conventional chemotherapy and allogeneic bone marrow and stem cell transplantation, with their demonstrated curative potential, will remain a mainstay of AML therapy for many years to come. Therefore, it is worth considering how our findings could be used to better direct use of these modalities. There are two important dilemmas often encountered in the treatment of AML patients. In those under 60, what is the best post-remission strategy? In other words, who should receive an Allo-SCT in first complete remission? Allo-SCT has the potential to cure patients who are at high risk of relapse, but it is bears a higher treatment related mortality, and can be accompanied by years of chronic graft versus host disease. Therefore, the optimal strategy is to identify those that are most likely to relapse following complete remission, and selectively direct them to Allo-SCT. Currently, predictive tools such as those used by the ELN employ a combination of genetic and cytogenetic markers to perform such prediction. While useful, these still appear to be imprecise tools, and we have found that BH3 profiling can actually improve the prognostic capabilities of the conventional prognostic approach (Figure 4 D-F) and by itself identify a subpopulation that apparently requires ALLO-SCT for cure (Figure 6).
Another decision-making dilemma faced by clinicians is whether to administer high-dose induction chemotherapy to newly diagnosed patients over 60 years of age. In older patients, treatment related mortality is higher and complete remission rates are lower. Clinical benefit appears to be restricted to those who attain complete remission. Therefore, it would be useful to be able to predict which patients are most likely to achieve a complete remission, and direct them to standard induction chemotherapy, sparing those patients unlikely to achieve remission the significant side effects. BH3 profiling apparently can identify those patients most likely to achieve a complete remission following induction chemotherapy (Figure 4). We will be testing the predictive utility of BH3 profiling in these two clinical settings in followup prospective clinical trials.
Our studies here do not directly demonstrate what upstream factors determine the relative priming of different cells. It is likely that activation of different oncogenes contributes, as this by itself can affect sensitivity to apoptosis. It is also likely that differential activation of any of a number of tyrosine kinase driven pathways could affect priming, since it is clear that killing via inhibition of these pathways proceeds by perturbation of BCL-2 family proteins and utilization of the mitochondrial pathway of apoptosis. One strategy for improving response to chemotherapy in poorly primed AML might be to selectively increase priming in AML cells, perhaps with an agent that is highly selective, but less potent that conventional chemotherapy. Once the AML is primed into a range consistent with good clinical response, then chemotherapy might be added. We tested such a strategy in vitro, and showed it to work using BCL-2 inhibition to prime AML cells and render them more sensitive to chemotherapy (Figure 2 E-H). We propose that such an approach merits testing in clinical trials, and that guidance by BH3 profiling might assist with identifying useful priming agents.
There is considerable and appropriate interest in better personalization of therapy in cancer patients, including in those with AML. The vast majority of these personalization strategies are based on genetics, and are directed toward targeted therapies. Even in these therapies, the gulf between genotype and phenotype can be difficult to bridge. Here we demonstrate that BH3 profiling, an assay of mitochondrial apoptotic function, can provide information that can potentially be exploited for personalization of AML therapy in the application of BCL-2 antagonists, allogeneic bone marrow transplant, and, likely to be a therapeutic remain a mainstay for many years, conventional chemotherapy.
Experimental Procedures
Cell lines and drug treatments
AML cell lines were grown in 20% FBS in standard RPMI media for BH3 profiling and AML drug treatments. For details see Extended Experimental Procedures.
BH3 Profiling of Cell Lines
Cell lines were profiled using the plate-based JC-1 BH3 profiling assay previously described (Ryan et al., 2010). Cells were permeabilized with digitonin, exposed to BH3 peptides, and mitochondrial transmembrane potential loss was monitored using the ratiometric dye JC-1. For details see Extended Experimental Procedures.
JC-1 FACS-based BH3 profiling of AML Primary Samples
Primary AML cells were obtained from the Leukemia Group and Pasquerrelo Tissue Bank at the Dana-Farber Cancer Institute under IRB-approved protocol 01-206. Samples were also obtained from through Dr. Mark Frattini at Memorial Sloan-Kettering Cancer Center under IRB-approved protocols 95-091, 06-107, and 09-141. BH3 profiling was performed as previously described (Chonghaile et al., 2011). For details see Extended Experimental Procedures.
Human HSC FACS
Normal human bone marrow was obtained from discarded de-identified bone marrow filters used at the Dana-Farber Cancer Institute during bone marrow isolation from healthy donors. Mononuclear cells were obtained by Ficoll. Lineage depletion was done using Miltenyi's Human Lineage Depletion Kit to enrich for progenitor cells. The cells were further enriched using Miltenyi's CD34 Enrichment Kit. Cells were then stained with biotin-lineage cocktail (Miltenyi), CD34-PECy7 (clone 8G12), CD38-V450 (clone HB7), CD90-APC (clone 5E10), CD45RA-biotin (clone HI100). Biotinylated cells are detected using streptavidin-APC-AlexaFluor750 (Invitrogen). The depletion, enrichment and antibody staining were all done in FACS buffer on ice. Stained cells were BH3 profiled as described for primary AML. Human HSCs were identified as cells in the Lin-CD34+CD38-CD90+CD45RA- subpopulation (Majeti et al., 2007).
Supplementary Material
Highlights.
Mitochondrial priming of AML vs. HSCs determines the chemotherapeutic index.
Pretreatment BH3 profiling may have utility as a clinical decision-making tool.
Myeloblasts tend to be BCL-2 dependent while human HSCs tend to be MCL-1 dependent.
Targeting BCL-2 is selectively toxic to even chemorefractory myeloblasts over HSCs.
Acknowledgments
The authors gratefully acknowledge support from the following sources: NIH grants F31 CA150562-01, R01 CA129974, and P01 CA139980, as well as Gabrielle's Angel Foundation for Cancer Research. AL is a Leukemia and Lymphoma Society Scholar. We thank Abbott Laboratories for providing ABT-737. We thank Martha Wadleigh, MD, for help with clinical data. AL is a cofounder and until recently served on the scientific advisory board of Eutropics Pharmaceuticals, which has a license for BH3 profiling.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Appelbaum FR, Gundacker H, Head DR, Slovak ML, Willman CL, Godwin JE, Anderson JE, Petersdorf SH. Age and acute myeloid leukemia. Blood. 2006;107:3481–3485. doi: 10.1182/blood-2005-09-3724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunelle JK, Letai A. Control of mitochondrial apoptosis by the Bcl-2 family. J Cell Sci. 2009;122:437–441. doi: 10.1242/jcs.031682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunelle JK, Ryan J, Yecies D, Opferman JT, Letai A. MCL-1-dependent leukemia cells are more sensitive to chemotherapy than BCL-2-dependent counterparts. J Cell Biol. 2009;187:429–442. doi: 10.1083/jcb.200904049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Certo M, Del Gaizo Moore V, Nishino M, Wei G, Korsmeyer S, Armstrong SA, Letai A. Mitochondria primed by death signals determine cellular addiction to antiapoptotic BCL-2 family members. Cancer Cell. 2006;9:351–365. doi: 10.1016/j.ccr.2006.03.027. [DOI] [PubMed] [Google Scholar]
- Chen L, Willis SN, Wei A, Smith BJ, Fletcher JI, Hinds MG, Colman PM, Day CL, Adams JM, Huang DC. Differential targeting of prosurvival Bcl-2 proteins by their BH3-only ligands allows complementary apoptotic function. Mol Cell. 2005;17:393–403. doi: 10.1016/j.molcel.2004.12.030. [DOI] [PubMed] [Google Scholar]
- Cheng EH, Wei MC, Weiler S, Flavell RA, Mak TW, Lindsten T, Korsmeyer SJ. BCL-2, BCL-X(L) sequester BH3 domain-only molecules preventing BAX- and BAK-mediated mitochondrial apoptosis. Mol Cell. 2001;8:705–711. doi: 10.1016/s1097-2765(01)00320-3. [DOI] [PubMed] [Google Scholar]
- Cossarizza A, Baccarani-Contri M, Kalashnikova G, Franceschi C. A new method for the cytofluorimetric analysis of mitochondrial membrane potential using the J-aggregate forming lipophilic cation 5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethylbenzimidazolcarbocyanine iodide (JC-1) Biochem Biophys Res Commun. 1993;197:40–45. doi: 10.1006/bbrc.1993.2438. [DOI] [PubMed] [Google Scholar]
- Danial NN, Korsmeyer SJ. Cell death: critical control points. Cell. 2004;116:205–219. doi: 10.1016/s0092-8674(04)00046-7. [DOI] [PubMed] [Google Scholar]
- Deng J, Carlson N, Takeyama K, Dal Cin P, Shipp M, Letai A. BH3 profiling identifies three distinct classes of apoptotic blocks to predict response to ABT-737 and conventional chemotherapeutic agents. Cancer Cell. 2007;12:171–185. doi: 10.1016/j.ccr.2007.07.001. [DOI] [PubMed] [Google Scholar]
- Dohner H, Estey EH, Amadori S, Appelbaum FR, Buchner T, Burnett AK, Dombret H, Fenaux P, Grimwade D, Larson RA, et al. Diagnosis and management of acute myeloid leukemia in adults: recommendations from an international expert panel, on behalf of the European LeukemiaNet. Blood. 2010;115:453–474. doi: 10.1182/blood-2009-07-235358. [DOI] [PubMed] [Google Scholar]
- Fernandez HF, Sun Z, Yao X, Litzow MR, Luger SM, Paietta EM, Racevskis J, Dewald GW, Ketterling RP, Bennett JM, et al. Anthracycline dose intensification in acute myeloid leukemia. N Engl J Med. 2009;361:1249–1259. doi: 10.1056/NEJMoa0904544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavathiotis E, Suzuki M, Davis ML, Pitter K, Bird GH, Katz SG, Tu HC, Kim H, Cheng EH, Tjandra N, et al. BAX activation is initiated at a novel interaction site. Nature. 2008;455:1076–1081. doi: 10.1038/nature07396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kluck RM, Bossy-Wetzel E, Green DR, Newmeyer DD. The release of cytochrome c from mitochondria: a primary site for Bcl-2 regulation of apoptosis. Science. 1997;275:1132–1136. doi: 10.1126/science.275.5303.1132. [DOI] [PubMed] [Google Scholar]
- Konopleva M, Contractor R, Tsao T, Samudio I, Ruvolo PP, Kitada S, Deng X, Zhai D, Shi YX, Sneed T, et al. Mechanisms of apoptosis sensitivity and resistance to the BH3 mimetic ABT-737 in acute myeloid leukemia. Cancer Cell. 2006;10:375–388. doi: 10.1016/j.ccr.2006.10.006. [DOI] [PubMed] [Google Scholar]
- Kuwana T, Bouchier-Hayes L, Chipuk JE, Bonzon C, Sullivan BA, Green DR, Newmeyer DD. BH3 Domains of BH3-Only Proteins Differentially Regulate Bax-Mediated Mitochondrial Membrane Permeabilization Both Directly and Indirectly. Mol Cell. 2005;17:525–535. doi: 10.1016/j.molcel.2005.02.003. [DOI] [PubMed] [Google Scholar]
- Lacombe F, Durrieu F, Briais A, Dumain P, Belloc F, Bascans E, Reiffers J, Boisseau MR, Bernard P. Flow cytometry CD45 gating for immunophenotyping of acute myeloid leukemia. Leukemia. 1997;11:1878–1886. doi: 10.1038/sj.leu.2400847. [DOI] [PubMed] [Google Scholar]
- Lapidot T, Sirard C, Vormoor J, Murdoch B, Hoang T, Caceres-Cortes J, Minden M, Paterson B, Caligiuri MA, Dick JE. A cell initiating human acute myeloid leukaemia after transplantation into SCID mice. Nature. 1994;367:645–648. doi: 10.1038/367645a0. [DOI] [PubMed] [Google Scholar]
- Letai A, Bassik MC, Walensky LD, Sorcinelli MD, Weiler S, Korsmeyer SJ. Distinct BH3 domains either sensitize or activate mitochondrial apoptosis, serving as prototype cancer therapeutics. Cancer Cell. 2002;2:183–192. doi: 10.1016/s1535-6108(02)00127-7. [DOI] [PubMed] [Google Scholar]
- Letai AG. Diagnosing and exploiting cancer's addiction to blocks in apoptosis. Nat Rev Cancer. 2008;8:121–132. doi: 10.1038/nrc2297. [DOI] [PubMed] [Google Scholar]
- Majeti R, Park CY, Weissman IL. Identification of a hierarchy of multipotent hematopoietic progenitors in human cord blood. Cell Stem Cell. 2007;1:635–645. doi: 10.1016/j.stem.2007.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mrozek K, Heerema NA, Bloomfield CD. Cytogenetics in acute leukemia. Blood Rev. 2004;18:115–136. doi: 10.1016/S0268-960X(03)00040-7. [DOI] [PubMed] [Google Scholar]
- Ni Chonghaile T, Sarosiek KA, Vo TT, Ryan JA, Tammareddi A, Moore Vdel G, Deng J, Anderson KC, Richardson P, Tai YT, et al. Pretreatment mitochondrial priming correlates with clinical response to cytotoxic chemotherapy. Science. 2011;334:1129–1133. doi: 10.1126/science.1206727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nitiss JL. Targeting DNA topoisomerase II in cancer chemotherapy. Nat Rev Cancer. 2009;9:338–350. doi: 10.1038/nrc2607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oltersdorf T, Elmore SW, Shoemaker AR, Armstrong RC, Augeri DJ, Belli BA, Bruncko M, Deckwerth TL, Dinges J, Hajduk PJ, et al. An inhibitor of Bcl-2 family proteins induces regression of solid tumours. Nature. 2005;435:677–681. doi: 10.1038/nature03579. [DOI] [PubMed] [Google Scholar]
- Opferman JT, Iwasaki H, Ong CC, Suh H, Mizuno S, Akashi K, Korsmeyer SJ. Obligate role of anti-apoptotic MCL-1 in the survival of hematopoietic stem cells. Science. 2005;307:1101–1104. doi: 10.1126/science.1106114. [DOI] [PubMed] [Google Scholar]
- Opferman JT, Letai A, Beard C, Sorcinelli MD, Ong CC, Korsmeyer SJ. Development and maintenance of B and T lymphocytes requires antiapoptotic MCL-1. Nature. 2003;426:671–676. doi: 10.1038/nature02067. [DOI] [PubMed] [Google Scholar]
- Roberts AW, Seymour JF, Brown JR, Wierda WG, Kipps TJ, Khaw SL, Carney DA, He SZ, Huang DC, Xiong H, et al. Substantial susceptibility of chronic lymphocytic leukemia to BCL2 inhibition: results of a phase I study of navitoclax in patients with relapsed or refractory disease. J Clin Oncol. 2012;30:488–496. doi: 10.1200/JCO.2011.34.7898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan JA, Brunelle JK, Letai A. Heightened mitochondrial priming is the basis for apoptotic hypersensitivity of CD4+ CD8+ thymocytes. Proc Natl Acad Sci U S A. 2010;107:12895–12900. doi: 10.1073/pnas.0914878107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlenk RF, Dohner K, Krauter J, Frohling S, Corbacioglu A, Bullinger L, Habdank M, Spath D, Morgan M, Benner A, et al. Mutations and treatment outcome in cytogenetically normal acute myeloid leukemia. N Engl J Med. 2008;358:1909–1918. doi: 10.1056/NEJMoa074306. [DOI] [PubMed] [Google Scholar]
- Sekeres MA, Stone R. Older adults with acute myeloid leukemia. Curr Oncol Rep. 2002;4:403–409. doi: 10.1007/s11912-002-0034-y. [DOI] [PubMed] [Google Scholar]
- Tse C, Shoemaker AR, Adickes J, Anderson MG, Chen J, Jin S, Johnson EF, Marsh KC, Mitten MJ, Nimmer P, et al. ABT-263: a potent and orally bioavailable Bcl-2 family inhibitor. Cancer Res. 2008;68:3421–3428. doi: 10.1158/0008-5472.CAN-07-5836. [DOI] [PubMed] [Google Scholar]
- van Delft MF, Wei AH, Mason KD, Vandenberg CJ, Chen L, Czabotar PE, Willis SN, Scott CL, Day CL, Cory S, et al. The BH3 mimetic ABT-737 targets selective Bcl-2 proteins and efficiently induces apoptosis via Bak/Bax if Mcl-1 is neutralized. Cancer Cell. 2006;10:389–399. doi: 10.1016/j.ccr.2006.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei MC, Lindsten T, Mootha VK, Weiler S, Gross A, Ashiya M, Thompson CB, Korsmeyer SJ. tBID, a membrane-targeted death ligand, oligomerizes BAK to release cytochrome c. Genes Dev. 2000;14:2060–2071. [PMC free article] [PubMed] [Google Scholar]
- Wei MC, Zong WX, Cheng EH, Lindsten T, Panoutsakopoulou V, Ross AJ, Roth KA, MacGregor GR, Thompson CB, Korsmeyer SJ. Proapoptotic BAX and BAK: a requisite gateway to mitochondrial dysfunction and death. Science. 2001;292:727–730. doi: 10.1126/science.1059108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willis SN, Chen L, Dewson G, Wei A, Naik E, Fletcher JI, Adams JM, Huang DC. Proapoptotic Bak is sequestered by Mcl-1 and Bcl-xL, but not Bcl-2, until displaced by BH3-only proteins. Genes Dev. 2005;19:1294–1305. doi: 10.1101/gad.1304105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson WH, O'Connor OA, Czuczman MS, LaCasce AS, Gerecitano JF, Leonard JP, Tulpule A, Dunleavy K, Xiong H, Chiu YL, et al. Navitoclax, a targeted high-affinity inhibitor of BCL-2, in lymphoid malignancies: a phase 1 dose-escalation study of safety, pharmacokinetics, pharmacodynamics, and antitumour activity. Lancet Oncol. 2010;11:1149–1159. doi: 10.1016/S1470-2045(10)70261-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou H, Li Y, Liu X, Wang X. An APAF-1.cytochrome c multimeric complex is a functional apoptosome that activates procaspase-9. J Biol Chem. 1999;274:11549–11556. doi: 10.1074/jbc.274.17.11549. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.