Abstract
Objective
To examine the clinicopathologic features and clonal relationship of multifocal intraductal papillary mucinous neoplasms (IPMNs) of the pancreas.
Background
Intraductal papillary mucinous neoplasms are increasingly diagnosed cystic precursor lesions of pancreatic cancer. Intraductal papillary mucinous neoplasms can be multifocal and a potential cause of recurrence after partial pancreatectomy.
Methods
Thirty four patients with histologically documented multifocal IPMNs were collected and their clinicopathologic features catalogued. In addition, thirty multifocal IPMNs arising in 13 patients from 3 hospitals were subjected to laser microdissection followed by KRAS pyrosequencing and loss of heterozygosity (LOH) analysis on chromosomes 6q and 17p. Finally, we sought to assess the clonal relationships among multifocal IPMNs.
Results
We identified 34 patients with histologically documented multifocal IPMNs. Synchronous IPMNs were present in 29 patients (85%), whereas 5 (15%) developed clinically significant metachronous IPMNs. Six patients (18%) had a history of familial pancreatic cancer. A majority of multifocal IPMNs (86% synchronous, 100% metachronous) were composed of branch duct lesions, and typically demonstrated a gastric-foveolar subtype epithelium with low or intermediate grades of dysplasia. Three synchronous IPMNs (10%) had an associated invasive cancer. Molecular analysis of multiple IPMNs from 13 patients demonstrated nonoverlapping KRAS gene mutations in 8 patients (62%) and discordant LOH profiles in 7 patients (54%); independent genetic alterations were established in 9 of the 13 patients (69%).
Conclusions
The majority of multifocal IPMNs arise independently and exhibit a gastric-foveolar subtype, with low to intermediate dysplasia. These findings underscore the importance of life-long follow-up after resection for an IPMN.
Intraductal papillary mucinous neoplasm (IPMN) of the pancreas is a cystic precursor lesion of infiltrating pancreatic adenocarcinoma characterized by mucin production, a dilated main pancreatic duct and/or ectasia of one of its side branches, and intraductal (usually papillary) growth.1,2 Since its description nearly 3 decades ago,3 there have been numerous advances made in understanding the histopathology and natural history of solitary IPMNs.1,2,4–6 The apparent incidence of IPMNs, and in particular, asymptomatic lesions, has increased somewhat dramatically in recent years, largely paralleling the increasing use of abdominal imaging technologies.2,7,8 In contrast to the other common cystic precursor lesion of pancreatic adenocarcinoma, such as mucinous cystic neoplasms (MCNs), which are essentially always solitary in nature, a subset of IPMNs, are multifocal. The reported prevalence of multifocality in IPMNs—either as synchronously diagnosed cysts or in a metachronous setting—varies widely, from 0% to 83% (Table 1).9,10 These highly discrepant rates are largely a reflection of variations in methodologies used for assessing multifocality, particularly in the synchronous setting, with many of the reports relying solely on imaging criteria for assigning multifocality. Histopathological assessment remains critical to establishing that preoperatively suspected multifocal IPMN lesions are not extensions of a solitary IPMN or even pancreatic cysts of an entirely different histogenesis (ie, pseudocysts, retention cysts, serous cystadenomas, among others). Comparable uncertainties vis-à-vis multifocal IPMNs also extend to their natural history and optimal clinical management, a conundrum that is reflected in the absence of clearly defined recommendations in the recent guidelines from the working group of the International Association of Pancreatology on management of pancreatic cystic lesions (a.k.a., “Sendai criteria”).11–14
TABLE 1.
Published Studies on the Prevalence of Multifocal IPMNs
| Author Year Journal | Lesions | Period Treated | Criteria for Multifocality | Frequency, % (Multifocal/All Patients) |
|---|---|---|---|---|
| Cuillerier et al, 2000, Am J Gastroenterol | IPMN | 1980–1996 | Lesions separated by uninvolved pancreatic duct | 2.2 (1/45) |
| Holme et al, 2001, HPB | IPMN | 1994–1998 | Multifocal intraductal changes | 83.3 (5/6) |
| D`Angelica et al, 2004, Ann Surg | IPMN | 1983–2000 | Parenchymal margin without proliferative ducts | 1.6 (1/63) |
| Salvia et al, 2004, Ann Surg | MD-IPMN | 1990–2002 | Normal duct between lesions | 0 (0/140) |
| Kawamoto et al, 2005, Radiographics | IPMN | 2000 | Lesions located in different parts of the pancreas | 33.3 (12/36) |
| Pelaez-Luna et al, 2007, Am J Gastroenterol | BD-IPMN | 1998–2005 | > 1 BD-IPMN in distant anatomical areas of the pancreas | 37.7 (57/147)* |
| Rodriguez et al, 2007, Gastroenterology | BD-IPMN | 1990–2005 | > 1 BD-IPMN not involving the main duct | 25.5 (37/145) |
| Schmidt et al, 2007, Ann Surg | BD-IPMN | BD-IPMN: 40.8 (42/103) | ||
| MT IPMN | 1991–2006 | > 1 identifiable and distinct lesion | MT-IPMN: 65 (26/40) | |
| Niedergethmann et al, 2008, World J Surg | IPMN | 1996–2006 | ND | 2.1 (2/97) |
| Schnelldorfer et al, 2008, Arch Surg | IPMN | . | ND | IPMA: 5, IPMB: 11 IPMC; 13, IPMN invasive: 15 |
| Waters et al, 2008, J Gastrointest Surg | IPMN | 1991–2006 | > 1 identifiable and distinct lesion | 72.2 (13/18) |
| Nara et al, 2009, Pancreas | IPMN | 1984–2006 | ND | 4.1 (5/123) |
| Salvia et al, 2009, Am J Surg | multifocal BD-IPMN | 1990–2006 | > 1 cystic lesions communicating with the main duct | ND |
| Woo et al, 2009, Br J Surg | BD-IPMN | 1998–2005 | > 1 identifiable and distinct lesion (macroscopic) | 17.6 (15/85) |
Not all cases histopathologically confirmed.
BD-IPMN indicates branch-duct IPMN; MD-IPMN, main-duct IPMN; MT-IPMN, mixed-type IPMN; NA, not applicable; ND, not defined.
Another prevailing enigma regarding multifocal IPMNs pertains to the molecular pathogenesis of these lesions, for which 2 competing hypotheses have been proposed. On the one hand it has been suggested that multifocal IPMNs are clonally related neoplasms arising from a single or few progenitor cells that exhibit shared genetic alterations. The haphazard intraductal growth of a single neoplasm could mimic true multifocality. Multifocal IPMNs could also be the result of the entire ductal epithelium having an increased risk of developing a neoplasm as may be seen in the familial setting.15 In these cases, a common germline inactivating mutation is shared by all cells in the pancreas, with a “second hit” on the remaining allele likely to be observed within each independent lesion. Finally, multifocal IPMNs might also arise because of entirely unrelated independent genetic events in anatomically distinct areas of the pancreas. To date, the molecular pathogenesis of multifocal IPMNs remains poorly understood.
The goals of this study were to characterize multifocal IPMNs with respect to their clinicopathologic features and to forge a better understanding of genetic relatedness among synchronous lesions. We rigorously defined multifocal IPMNs on the basis of histological confirmation of surgically resected cysts, which allowed us to delineate a prevalence at the lower detection end with some degree of certainty. As a result of this study, we have established that the overwhelming majority of multifocal IPMNs are branch duct lesions that demonstrate gastric-foveolar type epithelium, and typically harbor low to intermediate grades of epithelial dysplasia. Not unexpectedly, a significant minority of cases in our series arise in familial pancreatic cancer kindreds, and this history needs to be elucidated in any index case presenting with multifocal cysts. Furthermore, we provide evidence of discordant molecular aberrations among noninvasive IPMNs arising in the same individual, including between synchronous cysts with no more than low or intermediate grades of dysplasia. Finally, the potential for IPMNs to be multifocal mandates that clinicians should be cognizant of the need for lifelong surveillance of the residual pancreas after partial pancreatectomy—even if histology of the primary IPMN reveals a noninvasive lesion.16
MATERIALS AND METHODS
Retrospective Review of Surgically Resected IPMNs at the Johns Hopkins Hospital
This study was approved by The Johns Hopkins University institutional review board. We retrospectively reviewed the surgical pathology database of The Johns Hopkins Hospital to identify patients who underwent resection for IPMN between January 1, 1995, and May 31, 2010. Case selection was restricted to patients who underwent resection in or after 1995 because since then all pancreatic samples were evaluated using a standardized pathologic assessment form. Patients with multifocal IPMNs were considered to have synchronous disease if they had ≥2 simultaneous IPMNs that were established to be physically isolated from one another (i.e. by interval sections without any proliferative ductal lesions). Patients were assigned to the metachronous IPMN patient group if they developed an IPMN in their pancreatic remnant after complete resection of a primary IPMN lesion. Importantly, patients showing any significant neoplastic tissue (ie, any grade of pancreatic intraepithelial neoplasia [PanIN] or IPMN) in the pancreatic resection margin after primary surgery were not included into our study, to exclude potential recurrences of incompletely resected disease versus a true metachronous lesion.
Histopathology
Histological diagnoses and classification of individual IPMN lesions were reconfirmed by 2 experts in pancreatic pathology (R.H.H. and A.M.) using the most recent World Health Organization guidelines.17 Intraductal papillary mucinous neoplasms were identified as cystic neoplasms exhibiting a tall, columnar, usually papillary epithelium, and dilatation of the main pancreatic duct and/or one of its side branches. Most IPMNs in our study measured ≥1 cm in maximum diameter. Smaller lesions were included only if they clearly matched the above mentioned diagnostic IPMN criteria and showed a characteristic finger-like papillary growth pattern and abundant extracellular mucin. The location of a cyst with respect to the ductal system (main duct IPMN [MD], branch duct IPMN [BD], or mixed type IPMN [MT]) was assessed on the basis of imaging criteria corroborated by the dissecting pathologist’s impression on the resected specimen. The neoplastic epithelium was assigned a gastric-foveolar, pancreatobiliary, intestinal, or oncocytic subtype using previously described criteria.4 All IPMNs were divided into noninvasive lesions and IPMN with an associated invasive carcinoma.17 Noninvasive IPMNs were subclassified according to the most severe degree of cytoarchitectural atypia into IPMN with low-grade dysplasia, IPMN with intermediate dysplasia, and IPMN with high-grade dysplasia.
Laser Microdissection and DNA Extraction for Molecular Analyses
Representative formalin fixed paraffin embedded (FFPE) sections of 16 noninvasive synchronous multifocal IPMNs from 6 nonfamilial cases were collected from the surgical pathology archives, Department of Pathology, The Johns Hopkins Hospital. Formalin fixed paraffin embedded tissues of an additional 14 noninvasive multifocal IPMNs from 7 nonfamilial cases were obtained through collaborating institutions (University of Verona and Sacro Cuore Hospital, Negrar, Verona, Italy, and Tohoku University, Sendai, Japan). Four of these 7 were synchronous IPMNs, whereas in 3 patients, the IPMNs occurred in a metachronous setting. For laser microdissection, 10 to 30 sections (10 µm) were placed onto PALM membrane slides (Carl Zeiss MicroImaging, Inc, Thornwood, NY) that were pretreated under ultraviolet light, followed by microdissection on a PALM Micro Beam System (see Fig 1, Supplemental Digital Content 1, available at: http://links.lww.com/SLA/A192). Large IPMN lesions in which the tumor margins could easily be defined macroscopically were microdissected using a sterile needle under a stereomicroscope (SMZ1500, Nikon, Tokyo, Japan). On average, an estimated 2000 to 4000 cells were collected from each lesion. In addition, matched nonneoplastic pancreatic tissue from the same patient was microdissected. For this purpose we chose an area of pancreatic parenchyma predominantly composed of acinar cells adjacent to the IPMN lesion that did not show any ductal proliferation. Subsequently, DNA was extracted using the QIAamp DNA Micro Kit (Qiagen Inc, Valencia, CA).
FIGURE 1.
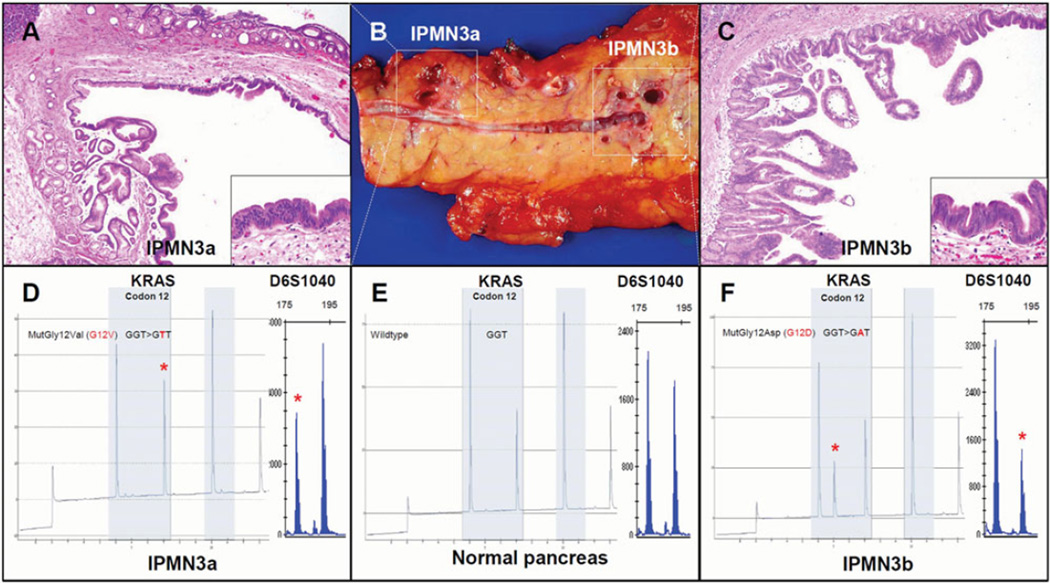
Independent molecular alterations in 2 synchronous multifocal IPMNs (patient “IPMN 3”): Case IPMN3 is illustrated in Panel B, showing one branch duct (BD)-IPMN in the pancreatic body (labeled IPMN3a) and another BD-IPMN in the pancreatic tail (IPMN3b). Photomicrographs of H + E stained sections show low-grade dysplasia in IPMN3a (Panel A, 4X and 20X magnifications) and intermediate dysplasia in IPMN3b (Panel C, 4X and 20X magnifications). Both neoplasms demonstrate a gastric-foveolar epithelial subtype characteristic of most multifocal IPMNs. The 2 synchronous lesions harbor different KRAS gene mutations and discordant LOH patterns (Panels D–F) suggesting either lack of clonal relatedness or very early clonal divergence. IPMN3a was found to have 27% G12V KRAS mutation, in contrast to the 45% G12D KRAS mutation identified in IPMN3b. At the D6S1040 locus, IPMN3a lost the longer 180 basepair allele (normalized ratio = 0.52), whereas IPM3b lost the shorter 192 basepair allele (normalized ratio = 1.88). Both the pointmutation in KRAS and the lost allele of D6S1040 are indicated by a red asterisk (*). Allelic ratios were normalized by a 4-way calculation, as described in Methods section.
KRAS Pyrosequencing
The majority of activating KRAS gene mutations in IPMNs occurs in codons 12 and 13. Pyrosequencing of both codons was performed with DNA from microdissected normal pancreas and IPMN tissues, as previously described.18 Briefly, after PCR amplification of 2 to 5 ng of DNA from laser-captured microdissectioned FFPE tissue, using the KRAS v2.0 kit (Qiagen), 10 µL of PCR product was purified using streptavidin-coated sepharose beads (GE Health care Bio-Sciences Corp., Piscataway, NJ) and QIAvac 24 Plus (Qiagen), per manufacturer’s protocol. The purified biotinylated antisense strand of PCR product was pyrosequenced on the PyroMark Q24 (Qiagen) with PyroMark Gold reagents (Qiagen) using the nucleotide dispensation order 5′-TACGACTCAGATCGTAG-3′, per the manufacturer’s protocol.
Loss of Heterozygosity Analysis
Previous studies have shown that loss of heterozygosity (LOH) in IPMNs occurs frequently on chromosomes 6q and 17p.19–21 Four polymorphic (het score range, 0.6–0.75) tetranucleotide microsatellite markers were used to assess LOH in microdissected normal pancreas and IPMN tissues, including D6S1009 (Chr6q23.3), D6S1040 (Chr6q23.1), D17S974 (Chr17p13.1), and D17S1298 (Chr17p13.2). Forward sense primers were fluorescently 5′ labeled with 6-FAM (Applied Biosystems, Foster City, CA). The primer sequences are shown in Supplemental Digital Content 2, available at: http://links.lww.com/SLA/A193. Capillary electrophoresis was performed on an ABI 3130 Genetic Analyzer and the data analyzed using GeneMapper Software Version 4.0 (Applied Biosystems). Loss of heterozygosity was assessed using a 4-way calculation of the peak height ratio of tumor DNA alleles divided by the peak height ratio of matched normal DNA alleles. Ratios between 0.59 and 1.69 were considered to be consistent with no loss of heterozygosity, whereas ratios less than 0.59 or greater than 1.69 were considered to be consistent with LOH. When the normal tissue yielded one allele size (presumably homozygous), the microsatellite was considered as noninformative for that case.
Clonality Assessment
On the basis of the pattern mentioned earlier, molecular markers we sought to assess the clonal relationships within multifocal cysts of a respective patient and assigned them to one of the 3 categories “likely clonally related,” “clonally distinct,” and “clonally indeterminate.” Overall, the below listed criteria were used:
Multifocal IPMNs That Are Clonally Related
Clonally relatedness was assumed if IPMNs arising within one pancreas demonstrated shared molecular alterations at all informative microsatellite loci and in the KRAS gene.
Multifocal IPMNs That Are Clonally Distinct
Lesions were considered clonally distinct within a single pancreas if distinct molecular alterations (either distinct KRAS mutation or differing LOH patterns) were observed among cysts. This designation was used if (a) 2 cysts had completely discordant KRAS gene mutation or LOH patterns, or if (b) the cysts shared some, but not all, molecular alterations (ie, 2 cysts harbored the same KRAS mutant clone, whereas only one of the two demonstrated a second distinct pattern of LOH).
Multifocal IPMNs That Are of Indeterminate Clonality
No conclusions about clonality could be drawn when IPMNs within one pancreas demonstrated either retention of heterozygosity or noninformative alleles at microsatellite loci, or harbored wild-type KRAS alleles.
Statistical and Clonality Analyses
Descriptive and statistical analyses were performed using SPSS version 11 (SPSS Inc., Chicago, IL).
RESULTS
Prevalence of Histologically Documented Multifocal IPMNs at a Single Institution
The histology records of patients who underwent resection for IPMN between January 1, 1995 and May 31, 2010 at the Johns Hopkins Hospital were reviewed, yielding a total of 34 cases with pathologically documented multifocal IPMN lesions. The clinicopathologic characteristics of our study population are shown in Table 2 and further details of each individual multifocal case are presented in the tables of Supplemental Digital Contents 3, available at: http://links.lww.com/SLA/A194 and 4, available at: http://links.lww.com/SLA/A196.
TABLE 2.
Clinicopathological Characteristics of Synchronous and Metachronous IPMNs in the Current Series
| Parameter | Synchronous IPMN Group |
Metachronous IPMN Group |
|---|---|---|
| Cases, all: N = 34 | 29 (85%) | 5 (15%) |
| Age, median years (range) | 68 (50–82) | 64 (47–82) |
| Gender, male : female | 11:18 | 2:3 |
| Familial pancreatic cancer history | 5 (17%) | 1 (20%) |
| Degree of dysplasia | 11 (38%) | 1 (20%) |
| IPMN, low-grade dysplasia | 12 (41%) | 1 (20%) |
| IPMN, intermediate dysplasia | 3 (10%) | 3 (60%) |
| IPMN, high-grade dysplasia | 3 (10%) | 0 (0%) |
| IPMN with invasive carcinoma | ||
| Duct location | ||
| Main | 0 (0%) | 0 (0%) |
| Branch | 25 (86%) | 5 (100%) |
| Mixed | 4 (14%) | 0 (0%) |
| Predominant epithelial subtype | ||
| Gastric-foveolar | 23 (96%) | 5 (100%) |
| Intestinal | 0 (0%) | 0 (0%) |
| Pancreatobiliary | 1 (4%) | 0 (0%) |
| Oncocytic | 0 (0%) | 0 (0%) |
| Not applied | 5 (21%) | 0 (0%) |
| Site of resected cyst | ||
| Head | 12 (41%) | 5 (100%) |
| Uncinate | 8 (27%) | 0 (0%) |
| Neck | 7 (23%) | 1 (20%) |
| Body | 14 (47%) | 4 (80%) |
| Tail | 15 (52%) | 4 (80%) |
| Number of resected IPMNs, median (range) | 2 (2–6) | 2 (2–4) |
| Maximum cyst size, cm (range) | 2 (0.5–6) | 1.2 (0.4–5.5) |
Of the 34 patients with multifocal IPMNs, 29 (85%) had synchronous IPMNs. The median age at diagnosis for this cohort was 68 years (range 50–82 years) and it included 18 female (62%) and 11 male patients (38%). Five of these 29 patients (17%) had a family history of pancreatic cancer. The resected pancreata harbored a median number of 2 synchronous IPMNs (range 2–6 lesions) with median diameter of 2 cm (range 0.5–6 cm). In 11 cases (38%), no final number of IPMNs was documented in the histology report because of diffuse involvement of the resected specimen. Pancreatic sites involved by individual IPMN lesions were as follows: pancreatic tail (N = 15; 52%), body (N = 14; 47%), head (N = 12; 41%), uncinate (N = 8; 27%), and neck (N = 7; 23%). Most patients had diagnosis of multifocal branch-duct (BD)-IPMN (N = 25; 86%) followed by mixed (MT)-IPMN (N = 4; 14%). In an overwhelming majority of the 24 available synchronous cases for histopathological review, the neoplastic epithelium was of the gastric-foveolar subtype (N = 23, 96%). One patient’s IPMNs was pancreatobiliary with respect to subtypes. The most severe histological grade present in synchronous IPMNs within any one case was most frequently either intermediate dysplasia (N = 12; 41%) or low-grade dysplasia (N = 11; 38%), without an associated invasive carcinoma. In 3 patients with high-grade dysplasia (carcinoma-in-situ, 10%), an associated invasive carcinoma was present. These included one colloid carcinoma and 2 ductal adenocarcinomas. Three patients (10%) had high-grade dysplastic epithelium in their multifocal IPMNs without invasion.
Five (15%) of the 34 patients had metachronous IPMNs. The median age at primary diagnosis for this cohort was 64 years (range 47–82 years), and 3were female (60%) and 2male (40%). The median interval between initial resection and surgery for the metachronous IPMN was 20 months (range, 15–134 months). One of the 5 patients had a known history of pancreatic cancer in the family and another patient had the Peutz-Jeghers syndrome. All but one patient had a single metachronous IPMN in their second resection, with the exception being the patient with Peutz-Jeghers syndrome that had 3 discrete cysts identified. The median cyst diameter of the primary IPMN was 1.5 cm (range, 0.5–3 cm), whereas the secondary lesions had a median maximum diameter of 1 cm (range, 0.4–5.5 cm). The initial IPMNs were most frequently found in the pancreatic head (N = 4, 80%) and all of these patients later developed disease in the pancreatic body. One patient (20%) developed metachronous disease in the pancreatic head after distal pancreatectomy for an IPMN of the pancreatic tail. Notably, all 5 of the index lesions and the subsequent metachronous IPMNs were localized to the branch ducts, and all (100%) of the cysts were primarily lined by gastric-foveolar type epithelium. In terms of histological grades of dysplasia, this did not alter in 3 cases between the index lesion and its matched metachronous IPMN (one case each had low-grade, intermediate, or high-grade dysplasia, respectively, at both time points), whereas in the remaining 2 cases, the index cyst had high-grade dysplasia whereas the metachronous lesion had intermediate dysplasia as the worst demonstrable grade (see Supplemental Digital Content 4, available at: http://links.lww.com/SLA/A196). No associated invasive carcinoma was present in the entire metachronous cohort. Of note, patients with a metachronous invasive adenocarcinoma in the absence of an associated metachronous IPMN were excluded from our analysis.
Mutational Analysis of the KRAS Gene in Multifocal IPMNs
To investigate the molecular features of multifocal IPMN lesions, we analyzed 30 lesions arising in 13 patients with no known history of familial pancreatic cancer. Somatic mutational analysis for the KRAS gene at codons 12 and 13 was performed by a quantitative pyrosequencing method.18 Sufficient DNA was available from all 30 IPMN lesions obtained from the 13 patients. The overall KRAS gene mutational rate was 80% (24 of 30 IPMNs), with 100% of the mutations being observed at codon 12, and none at codon 13. Notably, 23 of the 24 KRAS-mutant cysts (96%) were of the gastric-foveolar subtype and mutated KRAS alleles were even observed in predominantly gastric-foveolar lesions with low-grade dysplasia. This finding suggests that mutational activation of the KRAS oncogene might occur relatively early in the pathogenesis of IPMNs of this histology. An interesting corollary of this finding was that 6 IPMNs, all of the gastric-foveolar subtype, had 2 discrete KRAS mutant clones within the microdissected neoplastic epithelium, suggesting that clonal divergence (see the following text) is not uncommon in these early lesions.
The KRAS mutational profiles among multifocal IPMNs arising in the same patient were compared, and we identified 6 lesions (20%) that had a second distinct mutant clone not observed in the partner IPMN(s) (Table 3). In 5 of these cases–IPMN1, IPMN2, IPMN5, IPMN6, and IPMN12, all bearing synchronous lesions, one KRAS mutant clone was shared among the matched cysts, whereas a second mutant clone was observed in only one of the IPMNs during pyrosequencing. In 2 cases (15%), IPMN3 and IPMN5, we found evidence of distinct nonoverlapping mutant clones. In IPMN3, one of the synchronous cysts harbored an isolated G12D mutation, whereas its partner had an isolated G12V allele. Similarly, in IPMN5, a case that presented with a G12V mutation in one IPMN, there were 4 additional IPMNs all of which harbored a G12D mutation with a G12R subpopulation seen in 2 of them. In one case, IPMN4, 2 of the 3 IPMNs shared a G12V KRAS mutation, whereas the third lesion had no KRAS mutation. Another case, IPMN13, demonstrated a mutant KRAS allele in the metachronous IPMN that was not observed in the initial lesion. In this case, it is unclear whether these are genetically 2 lesions, or whether the KRAS gene mutation developed in the original neoplastic clone over time.
TABLE 3.
Molecular Analyses Reveal the Presence of Independent Genetic Events in a Subset of Multifocal Non-invasive IPMN lesions
| Patient Age, Gender |
Lesion | IPMN Diagnosis |
Subtype | KRAS | LOH-Analysis | Presence of Multiclonality |
|||
|---|---|---|---|---|---|---|---|---|---|
| D6S1040 | D6S1009 | D17S974 | D17S1298 | ||||||
| IPMN1 62f | N | Wt | Yes | ||||||
| T1 | BD-low-grade | Gastric | G12D | MSI | MSI | MSI | |||
| T2 | BD-low-grade | Gastric | G12V, G12D | ||||||
| IPMN2 71m | N | Wt | Na | Yes | |||||
| T1 | BD-intermediate | Gastric | G12D, G12V | Na | |||||
| T2 | BD-low-grade | Gastric | G12V | Na | |||||
| IPMN3 69m | N | Wt | Yes | ||||||
| T1 | BD-low-grade | Gastric | G12V | ||||||
| T2 | BD-intermediate | Gastric | G12D | ||||||
| IPMN4 54f | N | Wt | Na | Yes | |||||
| T1 | BD-low-grade | Gastric | Wt | MSI | Na | ||||
| T2 | BD-intermediate | Gastric | G12V | Na | |||||
| T3 | BD-low-grade | Gastric | G12V | Na | |||||
| IPMN5 69m | N | Wt | Yes | ||||||
| T1 | BD-low-grade | Gastric | G12D | ||||||
| T2 | BD-high-grade | Gastric | G12D | ||||||
| T3 | BD-intermediate | Gastric | G12V | ||||||
| T4 | BD-low-grade | Gastric | G12R, G12D | MSI | MSI | MSI | |||
| T5 | BD-low-grade | Gastric | G12D, G12R | ||||||
| IPMN6 66f | N | Wt | Na | Na | Yes | ||||
| T1 | BD-low-grade | Gastric | G12R | Na | Na | ||||
| T2 | BD-intermediate | Gastric | G12R, G12D | Na | Na | ||||
| IPMN7 71f | N | Wt | Na | Na | Na | Na | Indeterminate | ||
| T1 | MT-high-grade | Intestinal | Wt | Na | Na | Na | Na | ||
| T2 | MT-high-grade | Intestinal | Wt | Na | Na | Na | Na | ||
| IPMN8 68m | N | Wt | Na | Na | Na | Na | No (cysts likely related) | ||
| T1 | MT-low-grade | Gastric | G12D | Na | Na | Na | Na | ||
| T2 | MT-high-grade | Gastric/intestinal | G12D | Na | Na | Na | Na | ||
| IPMN9 66m | N | Wt | Yes | ||||||
| T1 | MT-high-grade | Oncocytic | Wt | ||||||
| T2 | MT-high-grade | Oncocytic | Wt | ||||||
| IPMN10 73f | N | Wt | No (cysts likely related) | ||||||
| T1 | BD-low-grade | Gastric | G12D | ||||||
| T2 | BD-high-grade | Oncocytic/gastric | G12D | ||||||
| IPMN11 69f | N | Wt | No (cysts likely related) | ||||||
| T1 | BD-intermediate | Gastric | G12V | ||||||
| T2 | BD-intermediate | Gastric | G12V | ||||||
| IPMN12 70m | N | Wt | Na | Na | Na | Yes | |||
| T1 | MT-high-grade | Gastric | G12D | Na | Na | Na | |||
| T2 | BD-low-grade | Gastric | G12D, G12R | Na | Na | Na | |||
| IPMN13 50m | N | Wt | Na | Na | Na | Yes | |||
| T1 | MD-high-grade | Intestinal | Wt | Na | Na | Na | |||
| T2 | MD-high-grade | Intestinal | G12V | Na | Na | Na | |||
 Both alleles present.
Both alleles present.
 Loss of longer allele.
Loss of longer allele.
 Loss of shorter allele.
Loss of shorter allele.
LOH analysis using 4 polymorphic tetranucleotide microsatellite markers was performed with allelic ratio cutoffs of <0.59 and >1.70.
MSI indicates microsatellite instability; N, matched normal tissue; Na, noninformative (single allele) or failed to amplify after multiple attempts; T, tumor tissue; Wt, wild type.
Loss of Heterozygosity
The microsatellite markers were informative in the majority of the lesions studied: 69% of cases were informative at D6S1040 and D6S1009 loci; 62% at D17S974 and D17S1298 loci (Table 3). Microsatellite shifts were detected in at least one lesion in 3 patients (23%). None of the 4 analyzed microsatellite loci could be assessed in patients IPMN7 and IPMN8 because of insufficient DNA for MSI analysis. The overall LOH rate was 27% (6 of 22 cysts) for D6S1040 (Chr6q23.1), 9% (2 of 22 cysts) for D6S1009 (Chr6q23.3), 32% (6 of 19 cysts) for D17S974 (Chr17p13.1), and 15% (3 of 20 cysts) for D17S1298 (Chr17p13.2).
Clonality Assessment
Taking results from KRAS pyrosequencing and LOH analysis into account, we sought to determine the clonal relationship within multifocal IPMNs of a respective patient. In 3 patients (23%), namely, IPMN8, IPMN10, and IPMN11, we identified identical molecular alterations within their multifocal IPMNs. Shared molecular alterations could arise through a common clonal origin, or if the IPMNs shared molecular alterations by chance. For example, the G12D mutation in KRAS is the most commonly observed alteration in IPMNs, and therefore 2 independent “hits” or events could easily produce the same G12D mutation. In one patient (8%), IPMN7, an interpretation of clonal origin was not possible. The cysts within this patient were noninformative or amplified poorly in the microsatellite analyses, and the KRAS alleles were wild-type for all lesions; thus, this case was assigned as “indeterminate” regarding clonality.
The remaining 9 patients (69%) had evidence of independent genetic alterations, suggesting clonal heterogeneity. With respect to data from KRAS and LOH analysis, multifocal IPMN lesions of patients IPMN1, IPMN2, IPMN3, IPMN4, IPMN5, and IPMN13 appeared to be clonally distinct. In patient IPMN12 one IPMN had a KRAS G12D mutation whereas the second cyst had an additional G12R clone. In IPMN9, 2 lesions had loss of opposite alleles demonstrating their clonally independent origin.
DISCUSSION
Cystic pancreatic lesions are being increasingly diagnosed using modern imaging.22 In a recent study, abdominal MR scans of 616 patients without any pancreas related symptoms revealed cystic lesions in 13.5% of the patients, of which 40% were found to be multifocal.23 Even higher incidence rates of asymptomatic pancreatic cysts can be derived from autopsy series, with incidental cysts reported in up to 25% of necropsies.24 Given that there are ever increasing numbers of abdominal CT and MR scans performed annually, the potential exists for a veritable epidemic of such asymptomatic cysts being identified. At our institution, and at other high-volume pancreatic surgery centers, IPMNs already represent the second most frequent indication for pancreatic surgery (after pancreatic ductal adenocarcinoma).25
The reported rate of multifocality of IPMNs either synchronous or metachronous varies drastically in literature (Table 1). The use of different technologies by different studies and the failure to histological document multifocality may explain some of this variability.26,27 Although modern imaging techniques are capable of detecting minimal changes in the pancreatic parenchyma, potential multifocality of IPMN lesions can best be ascertained by a meticulous histopathological analysis of the resection specimen. Lesions that appear solitary on imaging sometimes prove to be multifocal on assessment of the resection specimen. For example, in a study by Rodriguez et al, the investigators reported that histopathological examination of BD-IPMNs revealed the presence of more multifocal IPMNs than reported within preoperative radiologic evaluation (25.6% vs 14.5%). In contrast, single “grape-like” localized BD-IPMNs often mimic multifocal IPMNs, as microscopic examination of these latter lesions frequently shows an extensive solitary neoplasm with intraductal extension. Even a thorough histopathologic analysis cannot always unambiguously distinguish between a complex IPMN and multifocal independent lesions. As another example, a clear distinction between a large PanIN lesions and a small IPMN is often challenging both contributing to a misjudgment of multifocality in IPMNs. In the present study, we only included cases with histologically documented multifocality according to the final pathology report, and it is likely that the real number of multifocal IPMNs is much higher.
To characterize better the clinicopathologic correlates of multifocal IPMNs, we only included patients who had histologically documented multifocal disease. The age distribution of patients with multifocal IPMNs was similar to previous studies of solitary IPMNs.26,27 The fact that the median age of patients with synchronous IPMNs was higher than the age at primary surgery in the metachronous group (69 years vs. 64 years) may reflect greater symptomatology in patients with multifocal IPMNs, or it may be that surgeons are more likely to intervene earlier in patients with multifocal disease than they are in patients with solitary disease. The number of patients with metachronous disease was too small to draw final conclusions about onset and course of disease.
Several larger outcome studies of the recent years now allow a more evidence-based therapeutic approach for IPMNs, and there is an emerging consensus that not all pancreatic cysts require surgical resection. Criteria for the surgical versus conservative management of IPMNs were proposed at a 2004 conference organized by the International Association of Pancreatology (IAP) in Sendai, Japan, and subsequent reports have broadly validated these recommendations. On the basis of the literature that main duct IPMNs are more frequently associated with an invasive carcinoma than are branch duct-type IPMNs, it was recommended that main duct lesions should be resected. In contrast, smaller asymptomatic BD-IPMNs (ie, asymptomatic <30mm in maximum diameter without mural nodules) are mostly benign, and periodic observation without resection is considered justifiable in the majority of patients.2,10,14,26–30 For example, a retrospective study from the University of Verona and Massachusetts General Hospital assessed the histopathology of resected BD-IPMNs from 145 patients who underwent surgery.26 The rate of malignancy (defined as either invasive cancer or IPMN with high-grade dysplasia) was 22%, and strict adherence to the “Sendai criteria” would have identified cysts with high-grade dysplasia or an associated invasive carcinoma, and deemed the corresponding patients as candidates for surgery. Similarly, Salvia and colleagues31 retrospectively analyzed 109 patients with BD-IPMNs, of which 20 (~18%) underwent surgical resection on the basis of resection criteria outlined in the Sendai recommendations, whereas the remaining 89 were followed conservatively with periodic radiological examination. Five patients in the conservative observation group developed worrisome cyst features and subsequently had their lesion surgically resected, whereas the remaining 84 (~95%) patients, including 57 (64%) with multifocal lesions, remained asymptomatic without any disease-related complications for the follow up period of 32 months.
The most appropriate management of multifocal IPMNs has not been established. Some insights into the natural history of multifocal IPMNs have emerged from the previously cited study by Salvia and colleagues,31 in which 57 patients (64%) with multifocal IPMNs were followed for 32 months in the absence of surgical intervention or antecedent complications. In 2009 the same group published a retrospective study which exclusively focused on 131 patients with multifocal BD-IPMNs, 121 (92%) of whom were conservatively managed, and the entire cohort was alive after a median follow up of 40 months. Nine of the 10 patients who underwent surgical resection were alive without any signs of recurrence after a median of 56 months. The tenth patient presented with invasive carcinoma arising in association with an IPMN, and died of hepatic metastases 88 months postoperatively.32 The overall conclusion of this study was that carefully selected subsets of patients with multifocal BD-IPMNs can be followed without surgical intervention, as long as the involved cystic lesions are radiologically evaluable over time for the appearance of ominous signs. Similarly, Tajima et al33 reported the case of a 66-year-old woman who underwent pylorus-preserving pancreaticoduodenectomy for a BD-IPMN demonstrating as a grape-like multilocular cyst of 3.5 cm in the head along with numerous small BD-IPMNs in the entire rest of the pancreas. This patient is reported to be doing well without relapse 9 years after surgery.
The majority of the multifocal IPMNs included in our series were of gastric-foveolar subtype, with only low to intermediate dysplasia of the lining epithelium. Thus, on the basis of our series, and previously published data, it is reasonable to conclude that multifocality, in itself, is not a risk factor for aggressive disease, and that the “Sendai criteria” can reasonably be applied to multifocal lesions as they are to solitary IPMNs.
We also observed distinct genetic alterations among multifocal IPMNs from the same patient, suggesting that these IPMNs are genetically distinct events.
We selected KRAS gene mutations as an important determinant of clonality, because this gene has been shown to be altered early in most IPMNs.34–36 In addition, we selected 4 microsatellite loci in 2 chromosomal regions frequently lost in IPMNs.19–21 In contrast to Izawa et al,37 we restricted the analysis to true multifocal IPMNs, rather than a combination of solitary IPMNs and associated PanIN lesions. We also carefully excluded patients with a family history from the genetic component of this study, since such patients are likely to harbor a constitutional genetic predisposition in every cell in the body, including the pancreas, and may develop multifocal lesions akin to what is observed in colorectal polyposis. We observed evidence of clonal heterogeneity in as many as 69% of multifocal IPMNs studied in our series, including low grade BD-IPMN lesions. This finding helps establish that multifocal IPMNs are truly multifocal, and not simply a single lesion with multiple dominant genetically identical cysts.
Our finding of metachronous IPMNs reinforces a long-held clinical observation that the remnant pancreas in many IPMN patients remains at risk for the development of an invasive carcinoma whereas total pancreatectomy, although associated with its own risks, averts the risk of an invasive cancer arising from a metachronous IPMN.16
In conclusion, this study on multifocal IPMNs confirms that the vast majority of such lesions arise in the branch ducts, and harbor low to intermediate grades of dysplasia in the lining epithelium, which is typically of the gastric-foveolar histological subtype. Patients with multifocal IPMNs in the nonfamilial setting can be conservatively followed as long as the entire pancreatic field is amenable to careful radiological examination. The demonstration of clonal heterogeneity and distinct molecular alterations in synchronous IPMNs helps establish that multifocal IPMNs are independent lesions.
Supplementary Material
ACKNOWLEDGMENTS
The authors thank Lillian Dasko-Vincent for technical support and assistance with laser microdissection.
This work was supported by P50CA62924, the Sol Goldman Pancreatic Cancer Research Center and the Michael Rolfe Foundation for Pancreatic Cancer Research. Hanno Matthaei is supported by a grant from the Mildred-Scheel-Stiftung, Deutsche Krebshilfe, Bonn, Germany.
Footnotes
Disclosure: The authors certify that there are no commercial associations that might pose a conflict of interest in connection with the work.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s Web site (www.annalsofsurgery.com).
REFERENCES
- 1.Hruban RH, Takaori K, Klimstra DS, et al. An illustrated consensus on the classification of pancreatic intraepithelial neoplasia and intraductal papillary mucinous neoplasms. Am J Surg Pathol. 2004;28:977–987. doi: 10.1097/01.pas.0000126675.59108.80. [DOI] [PubMed] [Google Scholar]
- 2.Sohn TA, Yeo CJ, Cameron JL, Iacobuzio-Donahue CA, Hruban RH, Lillemoe KD. Intraductal papillary mucinous neoplasms of the pancreas: an increasingly recognized clinicopathologic entity. Ann Surg. 2001;234:313–321. doi: 10.1097/00000658-200109000-00005. discussion 21–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ohhashi KMY, Takekoshi T. Four cases of ‘mucin producing’ cancer of the pancreas on specific findings of the papilla of Vater. Prog Diag Endosc. 1982;20:348–351. [Google Scholar]
- 4.Longnecker DSAG, Hruban RH, Kloppel G. Intraductal papillary-mucinous neoplasms of the pancreas. In: Hamilton SRAL, editor. World Health Organization Classification of Tumours: Tumours of the Digestive System. Lyon, France: IARC Press; 2000. pp. 237–240. [Google Scholar]
- 5.Sugiyama M, Atomi Y. Intraductal papillary mucinous tumors of the pancreas: imaging studies and treatment strategies. Ann Surg. 1998;228:685–691. doi: 10.1097/00000658-199811000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kloppel GSE, Longnecker DS, et al. Histological Typing of Tumours of the Exocrine Pancreas. Berlin, Germany: Springer; 1996. World Health Organization International Classification of Tumors; pp. 11–20. [Google Scholar]
- 7.Winter JM, Cameron JL, Lillemoe KD, et al. Periampullary and pancreatic incidentaloma: a single institution’s experience with an increasingly common diagnosis. Ann Surg. 2006;243:673–680. doi: 10.1097/01.sla.0000216763.27673.97. discussion 80–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fernandez-del Castillo C, Targarona J, Thayer SP, Rattner DW, Brugge WR, Warshaw AL. Incidental pancreatic cysts: clinicopathologic characteristics and comparison with symptomatic patients. Arch Surg. 2003;138:433–434. doi: 10.1001/archsurg.138.4.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Holme JBJN, Rokkjaer M, et al. Total pancreatectomy in six patients with intraductal papillary mucinous tumour of the pancreas: the treatment of choice. HPB. 2001;3:257–262. doi: 10.1080/136518201753335539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Salvia R, Fernandez-del Castillo C, Bassi C, et al. Main-duct intraductal papillary mucinous neoplasms of the pancreas: clinical predictors of malignancy and long-term survival following resection. Ann Surg. 2004;239:678–685. doi: 10.1097/01.sla.0000124386.54496.15. discussion 85–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tang RS, Weinberg B, Dawson DW, et al. Evaluation of the guidelines for management of pancreatic branch-duct intraductal papillary mucinous neoplasm. Clin Gastroenterol Hepatol. 2008;6:815–819. doi: 10.1016/j.cgh.2008.04.005. quiz 719. [DOI] [PubMed] [Google Scholar]
- 12.Pelaez-Luna M, Chari ST, Smyrk TC, et al. Do consensus indications for resection in branch duct intraductal papillary mucinous neoplasm predict malignancy? A study of 147 patients. Am J Gastroenterol. 2007;102(8):1759–1764. doi: 10.1111/j.1572-0241.2007.01224.x. [DOI] [PubMed] [Google Scholar]
- 13.Sawhney MS, Al-Bashir S, Cury MS, et al. International consensus guidelines for surgical resection of mucinous neoplasms cannot be applied to all cystic lesions of the pancreas. Clin Gastroenterol Hepatol. 2009;7:1373–1376. doi: 10.1016/j.cgh.2009.06.026. [DOI] [PubMed] [Google Scholar]
- 14.Tanaka M, Chari S, Adsay V, et al. International consensus guidelines for management of intraductal papillary mucinous neoplasms and mucinous cystic neoplasms of the pancreas. Pancreatology. 2006;6:17–32. doi: 10.1159/000090023. [DOI] [PubMed] [Google Scholar]
- 15.Canto MI, Goggins M, Hruban RH, et al. Screening for early pancreatic neoplasia in high-risk individuals: a prospective controlled study. Clin Gastroenterol Hepatol. 2006;4:766–781. doi: 10.1016/j.cgh.2006.02.005. quiz 665. [DOI] [PubMed] [Google Scholar]
- 16.Chari ST, Yadav D, Smyrk TC, et al. Study of recurrence after surgical resection of intraductal papillary mucinous neoplasm of the pancreas. Gastroenterology. 2002;123:1500–1507. doi: 10.1053/gast.2002.36552. [DOI] [PubMed] [Google Scholar]
- 17.Bosman FCF, Hruban R, Theise N. WHO Classification of Tumours of the Digestive System IARC. Lyon, France: IARC Press; 2010. [Google Scholar]
- 18.Tsiatis AC, Norris-Kirby A, Rich RG, et al. Comparison of sanger sequencing, pyrosequencing, and melting curve analysis for the detection of KRAS mutations. Diagnostic and Clinical Implications. J Mol Diagn. 2010;12:425–432. doi: 10.2353/jmoldx.2010.090188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sato N, Rosty C, Jansen M, et al. STK11/LKB1 Peutz-Jeghers gene inactivation in intraductal papillary-mucinous neoplasms of the pancreas. Am J Pathol. 2001;159:2017–2022. doi: 10.1016/S0002-9440(10)63053-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Abe T, Fukushima N, Brune K, et al. Genome-wide allelotypes of familial pancreatic adenocarcinomas and familial and sporadic intraductal papillary mucinous neoplasms. Clin Cancer Res. 2007;13:6019–6025. doi: 10.1158/1078-0432.CCR-07-0471. [DOI] [PubMed] [Google Scholar]
- 21.Fritz S, Fernandez-del Castillo C, Mino-Kenudson M, et al. Global genomic analysis of intraductal papillary mucinous neoplasms of the pancreas reveals significant molecular differences compared to ductal adenocarcinoma. Ann Surg. 2009;249:440–447. doi: 10.1097/SLA.0b013e31819a6e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Laffan TA, Horton KM, Klein AP, et al. Prevalence of unsuspected pancreatic cysts on MDCT. AJR Am J Roentgenol. 2008;191:802–807. doi: 10.2214/AJR.07.3340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee KS, Sekhar A, Rofsky NM, Pedrosa I. Prevalence of incidental pancreatic cysts in the adult population on MR imaging. Am J Gastroenterol. 2010;105:2079–2284. doi: 10.1038/ajg.2010.122. [DOI] [PubMed] [Google Scholar]
- 24.Kimura W, Nagai H, Kuroda A, Muto T, Esaki Y. Analysis of small cystic lesions of the pancreas. Int J Pancreatol. 1995;18:197–206. doi: 10.1007/BF02784942. [DOI] [PubMed] [Google Scholar]
- 25.Bassi C, Crippa S, Salvia R. Intraductal papillary mucinous neoplasms (IPMNs): is it time to (sometimes) spare the knife? Gut. 2008;57:287–289. doi: 10.1136/gut.2007.135392. [DOI] [PubMed] [Google Scholar]
- 26.Rodriguez JR, Salvia R, Crippa S, et al. Branch-duct intraductal papillary mucinous neoplasms: observations in 145 patients who underwent resection. Gastroenterology. 2007;133:72–79. doi: 10.1053/j.gastro.2007.05.010. quiz 309–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sohn TA, Yeo CJ, Cameron JL, et al. Intraductal papillary mucinous neoplasms of the pancreas: an updated experience. Ann Surg. 2004;239:788–797. doi: 10.1097/01.sla.0000128306.90650.aa. discussion 97–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kobari M, Egawa S, Shibuya K, et al. Intraductal papillary mucinous tumors of the pancreas comprise 2 clinical subtypes: differences in clinical characteristics and surgical management. Arch Surg. 1999;134(10):1131–1136. doi: 10.1001/archsurg.134.10.1131. [DOI] [PubMed] [Google Scholar]
- 29.Terris B, Ponsot P, Paye F, et al. Intraductal papillary mucinous tumors of the pancreas confined to secondary ducts show less aggressive pathologic features as compared with those involving the main pancreatic duct. Am J Surg Pathol. 2000;24(10):1372–1377. doi: 10.1097/00000478-200010000-00006. [DOI] [PubMed] [Google Scholar]
- 30.Schmidt CM, White PB, Waters JA, et al. Intraductal papillary mucinous neoplasms: predictors of malignant and invasive pathology. Ann Surg. 2007;246:644–651. doi: 10.1097/SLA.0b013e318155a9e5. discussion 51–54. [DOI] [PubMed] [Google Scholar]
- 31.Salvia R, Crippa S, Falconi M, et al. Branch-duct intraductal papillary mucinous neoplasms of the pancreas: to operate or not to operate? Gut. 2007;56:1086–1090. doi: 10.1136/gut.2006.100628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Salvia R, Partelli S, Crippa S, et al. Intraductal papillary mucinous neoplasms of the pancreas with multifocal involvement of branch ducts. Am J Surg. 2009;198:709–714. doi: 10.1016/j.amjsurg.2008.10.022. [DOI] [PubMed] [Google Scholar]
- 33.Tajima Y, Kuroki T, Tsuneoka N, et al. Multifocal branch-duct pancreatic intraductal papillary mucinous neoplasms. Am J Surg. 2008;196:e50–e52. doi: 10.1016/j.amjsurg.2007.10.013. [DOI] [PubMed] [Google Scholar]
- 34.Schonleben F, Qiu W, Bruckman KC, et al. BRAF and KRAS gene mutations in intraductal papillary mucinous neoplasm/carcinoma (IPMN/IPMC) of the pancreas. Cancer Lett. 2007;249:242–248. doi: 10.1016/j.canlet.2006.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Licciardi M, Craparo EF, Giammona G, Armes SP, Tang Y, Lewis AL. in vitro biological evaluation of folate-functionalized block copolymer micelles for selective anti-cancer drug delivery. Macromol Biosci. 2008;8:615–626. doi: 10.1002/mabi.200800009. [DOI] [PubMed] [Google Scholar]
- 36.Stroupe KT, Stelmack JA, Tang XC, et al. Economic evaluation of blind rehabilitation for veterans with macular diseases in the Department of Veterans Affairs. Ophthalmic Epidemiol. 2008;15:84–91. doi: 10.1080/09286580802027836. [DOI] [PubMed] [Google Scholar]
- 37.Izawa T, Obara T, Tanno S, Mizukami Y, Yanagawa N, Kohgo Y. Clonality and field cancerization in intraductal papillary-mucinous tumors of the pancreas. Cancer. 2001;92:1807–1817. doi: 10.1002/1097-0142(20011001)92:7<1807::aid-cncr1697>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.