Abstract
Background
Etomidate is a sedative–hypnotic that is often given as a single intravenous bolus but rarely as an infusion because it suppresses adrenocortical function. Methoxycarbonyl etomidate and (R)-ethyl 1-(1-phenylethyl)-1H-pyrrole-2-carboxylate (carboetomidate) are etomidate analogs that do not produce significant adrenocortical suppression when given as a single bolus. However, the effects of continuous infusions on adrenocortical function are unknown. In this study, we compared the effects of continuous infusions of etomidate, methoxycarbonyl etomidate, and carboetomidate on adrenocortical function in a rat model.
Methods
A closed-loop system using the electroencephalographic burst suppression ratio as the feedback was used to administer continuous infusions of etomidate, methoxycarbonyl etomidate, or carboetomidate to Sprague–Dawley rats. Adrenocortical function was assessed during and after infusion by repetitively administering adrenocorticotropic hormone 1–24 and measuring serum corticosterone concentrations every 30 min.
Results
The sedative–hypnotic doses required to maintain a 40% burst suppression ratio in the presence of isoflurane, 1%, and the rate of burst suppression ratio recovery on infusion terminationvaried(methoxycarbonyletomidate>carboetomidate > etomidate). Serum corticosterone concentrations were reduced by 85% and 56% during 30-min infusions of etomidate and methoxycarbonyl etomidate, respectively. On infusion termination, serum corticosterone concentrations recovered within 30 min with methoxycarbonyl etomidate but persisted beyond an hour with etomidate. Carboetomidate had no effect on serum corticosterone concentrations during or after continuous infusion.
Conclusions
Our results suggest that methoxycarbonyl etomidate and carboetomidate may have clinical utility as sedative–hypnotic maintenance agents when hemodynamic stability is desirable.
Etomidate is a sedative–hypnotic commonly used in elderly and critically ill patients because it maintains hemodynamic stability.1–4 Unfortunately, etomidate also inhibits 11β-hydroxylase, causing suppression of adrenocortical steroid synthesis.5–9 This suppression is extremely potent, occurring at subhypnotic etomidate doses.10–13 It can persist for more than a day after etomidate administration has been discontinued.8,14,15 Consequently, the use of etomidate as a continuous infusion to maintain anesthesia or sedation has been almost entirely abandoned and the use of even a single dose of etomidate for anesthetic induction is controversial.16–18 We have developed two etomidate analogs that retain etomidate's potent hypnotic activity and favorable hemodynamic profile but have little or no adrenocortical effects when given as a single intravenous bolus. Methoxycarbonyl etomidate may be considered to be a “soft” analog of etomidate because it is ultrarapidly metabolized by esterases.19 After single bolus administration, recovery of adrenocortical function occurs significantly more quickly with methoxycarbonyl etomidate than etomidate.19 (R)-ethyl 1-(1-phenylethyl)-1H-pyrrole-2-carboxylate (carboetomidate) is a pyrrole analog of etomidate that was designed to bind to 11β-hydroxylase with much lower affinity than etomidate.20 Compared with etomidate, carboetomidate is three orders of magnitude less potent an inhibitor of in vitro adrenocortical cortisol synthesis and does not produce adrenocortical suppression when given to rats as a single bolus.20
Previous studies of methoxycarbonyl etomidate and carboetomidate focused exclusively on a single intravenous bolus administration. However, their greatest potential utility may be as continuously infused agents to maintain sedation or anesthesia without producing clinically significant adrenocortical suppression. Such infusions will logically result in higher total drug doses and produce longer drug exposure times than a single bolus. In the current study, we administered etomidate, methoxycarbonyl etomidate, and carboetomidate to rats by continuous infusion using a closed-loop system to achieve approximately equivalent hypnotic depths and tested the hypothesis that the sedative–hypnotic dosing requirements, recovery times, and adrenocortical inhibitory activities of the three sedative hypnotic agents vary.
Materials and Methods
Animals
All studies were conducted in accordance with the rules and regulations of the Subcommittee on Research Animal Care at Massachusetts General Hospital, Boston. Adult male Sprague–Dawley rats (300–550 g) were purchased from Charles River Laboratories (Wilmington, MA) and housed in the Massachusetts General Hospital Center for Comparative Medicine animal care facility. Drugs were administered through a lateral tail vein intravenous catheter (24 gauge, 19 mm). Blood draws were from either a tail vessel or a femoral venous catheter preimplanted by the vendor.
Drugs and Chemicals
Methoxycarbonyl etomidate and carboetomidate were synthesized (>95% purity) by Aberjona Laboratories (Beverly, MA), as previously described, and solubilized in saline (20 mg/ml) and dimethyl sulfoxide (30 mg/ml), respectively.19,20 Etomidate was obtained from Bedford Laboratories, Bedford, OH (1 mg/ml in propylene glycol, 35%, and water). Isoflurane was purchased from Baxter (Deerfield, IL), dexamethasone from American Regent (Shirley, NY), adrenocorticotropic hormone 1–24 (ACTH1–24) from Sigma–Aldrich Chemical Co (St. Louis, MO), methanol from Fisher Scientific (Fair Lawn, NJ), and bupivacaine and heparin from APP Pharmaceuticals (Schaumburg, IL).
Electroencephalographic Electrode Placement and Recording
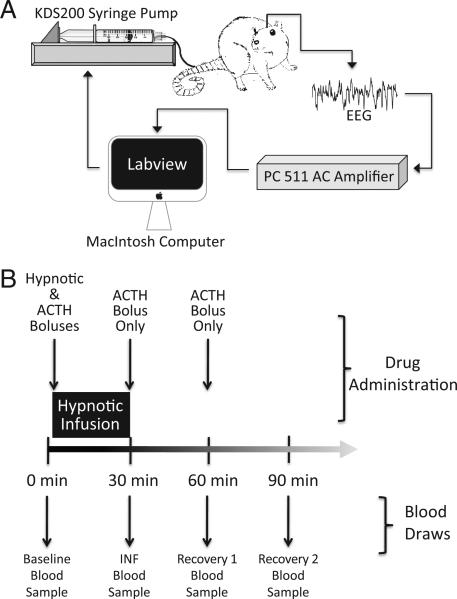
Rats were anesthetized and maintained with inhaled isoflurane, 2–3%, in 100% oxygen and placed in a stereotactic frame fitted with a nose cone. The skin was anesthetized with bupivacaine, 0.5%, containing epinephrine, 1:200,000 (approximately 0.2 ml). The skull was exposed, the periosteum was removed, and four 1.59-mm outside diameter 3.2-mm long-bone anchor screws (Stoelting, Wood Dale, IL), with attached 0.010-inch Teflon-coated stainless steel wire (A-M Systems, Sequim, WA), were inserted through the bone and reinforced with dental acrylic at the stereotactic coordinates described by Vijn and Sneyd.21 The temporal electrode with the highest signal/noise ratio was used for study. The wires were connected to an AC preamplifier (P511; Grass Technologies, West Warwick, RI). The electroencephalographic signal was amplified 5000-fold, filtered (low-frequency pass, 0.3 Hz; high-frequency pass, 0.03 kHz), and digitized at 128 Hz using a data acquisition board (USB-6009; National Instruments, Austin, TX). The burst suppression ratio (BSR) was measured in real-time with software (LabView Software, version 8.5 for Macintosh OS X; National Instruments, Austin, TX) to provide feedback for a closed-loop infusion system, as described later (fig. 1A).
Fig. 1.
(A) Schematic drawing of the closed-loop infusion system. (B) Schematic drawing of the protocol used for assessing the impact of 30-min infusions of sedatives–hypnotics and methoxycarbonyl etomidate's metabolites. A baseline preinfusion blood sample (baseline) was drawn from each rat before administering adrenocorticotropic hormone 1–24 (ACTH1–24) or intravenous sedative–hypnotic. The first dose of ACTH1–24 was given immediately before the start of the 30-min infusion (but after the bolus), and the INF blood sample was drawn at the end of the infusion. Additional doses of ACTH1–24 (but no hypnotic) were administered and blood was drawn (recovery 1 and recovery 2 blood samples) every 30 min for 1 h after the infusion was complete.
BSR Extraction and Closed-loop Infusion of Sedatives–Hypnotics
Methods described by Vijn and Sneyd21 and Rampil and Laster22 were used to continuously estimate the BSR, where the BSR is the percentage time the electroencephalographic signal spent in suppression during each 6-s epoch. Temporal differentiation (the difference between two successive data samples in the digitized electroencephalographic signal) was used to enhance BSR sensitivity.21Suppression was defined as an interval during which the time-differentiated electroencephalographic signal amplitude stays within an optimized suppression voltage window for at least 100 ms. The formula node and other virtual instruments in software (LabView 8.5 for Macintosh) were used in BSR calculations. Because of modest differences in baseline electroencephalographic signal noise, the suppression voltage window was empirically optimized for each animal before study by inducing brief electroencephalographic electrical silence with inhaled isoflurane, 4–5% (delivered in 100% oxygen at 2 l/min from a calibrated agent-specific vaporizer into a tight-fitting nose cone). The window was then reduced to the lowest value that produced a BSR measurement of more than 97% (typical value, approximately ±8μV). After optimizing the suppression voltage window, rats were equilibrated with inhaled isoflurane, 1%, by a tight-fitting nose cone for at least 45 min until the BSR stabilized before the start of any study. All studies were performed in a background of inhaled isoflurane, 1%.
An infusion pump (KDS model 200 series; KD Scientific, Holliston, MA) was used for sedative–hypnotic administration. The pump was controlled remotely via its RS 232 serial port by a Macintosh computer using a port adapter (Keyspan USB-Serial; Tripp Lite, Chicago, IL). An instrument driver (LabView 8.5) using virtual instrument software architecture protocols provided computer-to-pump communication. After 5 min of baseline BSR determination, an initial infusion rate (Iind) was set to provide an anesthetic bolus dose over 12 s predicted (based on preliminary studies) to achieve a BSR of approximately 40%.21 These boluses were 2 mg/kg for etomidate, 30 mg/kg for methoxycarbonyl etomidate, and 10 mg/kg for carboetomidate. The infusion rate was then decreased to 0.25 Iind for 1 min, after which the closed-loop continuous infusion algorithm detailed by Vijn and Sneyd21 was used to maintain the BSR near 40% for 15 min (electroencephalographic studies) or 30 min (adrenocortical studies). In this algorithm, the hypnotic infusion rate is increased (if the current BSR is <40%) or decreased (if the current BSR is >40%) every 6 s. The magnitude of the change in the infusion rate is dependent on the error (i.e., the difference between the current BSR measured in the rat and our target BSR of 40%). For the longer-acting drugs (i.e., etomidate and carboetomidate), the closed-loop infusion rate was scaled by a weighting function that reduces the infusion rate (and prevents overdosage) while hypnotic effects of the initial bolus dissipate, as described by Vijn and Sneyd.21 For all drugs, we also added a maximum infusion rate to reduce the risk of overdosage; and for methoxycarbonyl etomidate, we incorporated a minimum infusion rate and eliminated the 0.25 Iind step to accommodate its rapid pharmacokinetics.
Protocol for Assessing Adrenocortical Function on Sedative–Hypnotic Administration
To suppress baseline corticosterone production, each rat in the adrenocortical function studies was given dexamethasone (0.2 mg/kg intravenously) at the start of each experiment. During the subsequent 2–3 h, electroencephalographic electrodes were implanted, the burst suppression window was defined, and the rat was equilibrated with isoflurane, 1%, as previously described. After a second dose of dexamethasone, a blood sample (baseline blood sample in fig. 1B) was drawn to determine the baseline unstimulated serum corticosterone concentration.
A sedative–hypnotic agent was then administered as a bolus (over 12 s), followed by a 30-min continuous intravenous infusion. The rate of this infusion was controlled by the closed-loop system to maintain a BSR near 40%. Adrenocortical function (i.e., responsiveness to ACTH1–24 administration) was assessed during and after sedative–hypnotic infusion by repetitively administering ACTH1–24 (25 μg/kg intravenously) and measuring serum corticosterone concentrations 30 min later. The first dose of ACTH1–24 was given at the start of the sedative–hypnotic infusion, and the serum corticosterone concentration was measured at the end of the infusion (INF sample in fig. 1B). Thus, the corticosterone concentration in the INF sample reflects adrenocortical function during sedative–hypnotic infusion. Immediately after drawing the INF sample, a second dose of ACTH1–24 was given and the serum corticosterone concentration was measured 30 min later (recovery 1 blood sample in fig. 1B). Immediately after drawing the recovery 1 blood sample, the third and final dose of ACTH1–24 was given and the serum corticosterone concentration was measured 30 min later (recovery 2 blood sample in fig. 1B). Thus, the recovery 1 and recovery 2 blood samples reflect recovery of adrenocortical function after the sedative–hypnotic infusion has been discontinued.
The volume of each blood sample was approximately 0.3 ml. Serum corticosterone concentrations were determined as previously reported.19 Briefly, blood samples were allowed to clot at room temperature before centrifugation at 1,055g for 5 min. Serum was expressed from any resulting superficial fibrin clot using a clean pipette tip before a second centrifugation at 1,100g for 5 min. After a second centrifugation, the resultant serum layer was transferred to a fresh vial for a final high-speed centrifugation (17,000g for 5 min). The serum was promptly frozen (–20°C) pending corticosterone measurement. After thawing and heat inactivation of corticosterone-binding globulins (65°C for 20 min), serum baseline and ACTH1–24–stimulated corticosterone concentrations were quantified using an enzyme-linked immunosorbent assay (Immunodiagnostics Systems, Fountain Hills, AZ) and a 96-well plate reader (Molecular Devices, Sunnyvale, CA).
Statistical Analysis
BSR values and corticosterone concentrations were determined at each point in every rat for determination of means and SDs. For each sedative–hypnotic, the rate with which the BSR decreased on discontinuing the infusion was quantified as a time constant. This time constant was derived by fitting a plot of the BSR versus time (after infusion termination) to a single exponential equation. All data are reported as mean ± SD. Statistical analyses were performed using software: Prism v5.0 for the Macintosh (GraphPad Software, Inc., LaJolla, CA) or Igor Pro 6.1 (Wavemetrics, Lake Oswego, OR). Statistical comparisons were performed using a two-tailed t test or a repeated-measures two-way ANOVA, followed by a Bonferroni posttest (which relies on an un-paired t test with a Bonferroni correction). In the ANOVA, the two factors were time point and drug group and the posttest was used to compare differences in corticosterone concentrations among groups at each point. For all statistical analyses, P < 0.05 indicated statistical significance.
Results
Part 1: Evaluation of the Effects of Etomidate and Etomidate Analogs on Electroencephalographic BSR
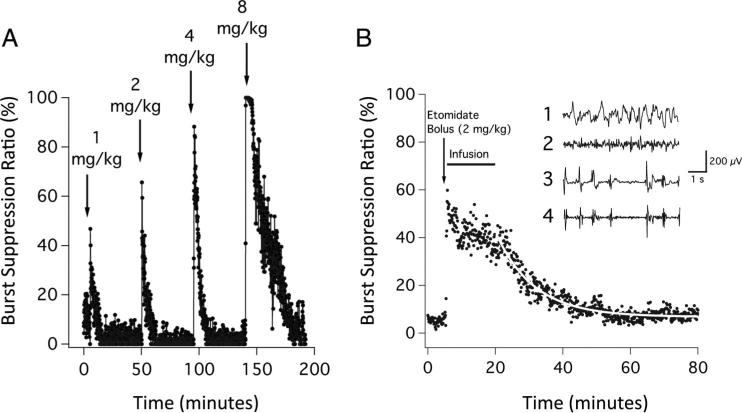
The intravenous administration of etomidate, methoxycarbonyl etomidate, or carboetomidate dose dependently increased the BSR in the presence of isoflurane, 1%. For example, figure 2A shows the change in the BSR on intravenous bolus administration of successively higher doses of etomidate to a single rat. On bolus administration, etomidate produced a rapid and reversible increase in the BSR; the peak value increased with dose. Figure 2B shows the mean BSR recorded from five rats during closed-loop etomidate infusion experiments, with a BSR target of 40%. The dose of etomidate (initial bolus plus 15-min closed-loop infusion) was 4.7 ± 1.6 mg/kg (range, 3.3–6.4 mg/kg). The BSR increased rapidly with etomidate administration, from 5.2 ± 1.3% during the 5-min preinfusion period to 41 ± 7.6% during closed-loop continuous etomidate infusion. On terminating the infusion, the BSR decreased to 7.7 ± 1.6% during the final 5 min of the experiment, with a time constant of 11.7 min (95% CI, 11.0–12.5 min).
Fig. 2.
(A) The effect of escalating bolus doses of etomidate on the burst suppression ratio in a rat. The etomidate doses represent one, two, four, and eight times the ED50 for loss of righting reflexes in rats. Each bolus was given over 6 s, and the burst suppression ratio was calculated from the time-differentiated electroencephalogram. (B) Mean burst suppression ratio during each 6 s epoch recorded from five rats on administration of a etomidate bolus (2 mg/kg), followed by a 15 min closed-loop infusion with the target burst suppression ratio of 40%. The curve is an exponential fit of the postinfusion data. Inset1: Representative baseline raw electroencephalogram obtained from an individual rat before the administration of etomidate.2 Corresponding baseline time-differentiated electroencephalogram before the administration of etomidate.3 Representative raw electroencephalogram obtained from the same rat during closed-loop infusion of etomidate.4 Corresponding time-differentiated electroencephalogram during the etomidate infusion. Each data point represents the burst suppression ratio during a 6 s epoch. All measurements were performed in the presence of inhaled isoflurane, 1%.
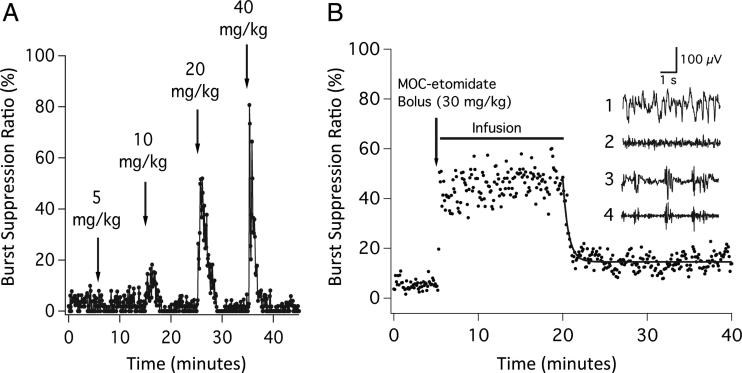
Figure 3A shows the typical changes in the BSR on intravenous bolus administration of successively higher doses of methoxycarbonyl etomidate to a rat. In common with etomidate, methoxycarbonyl etomidate produced a rapid and reversible increase in the BSR, whose peak value increased with dose. Figure 3B shows the mean BSR recorded from five rats during 15-min closed-loop methoxycarbonyl etomidate infusion experiments, with a BSR target of 40%. During the 5-min period before methoxycarbonyl etomidate administration, the BSR was 5.5 ± 2.0%. The BSR increased to 45 ± 8.3% during closed-loop continuous infusion of methoxycarbonyl etomidate. The methoxycarbonyl etomidate dose was 174 ± 27 mg/kg (range, 142–200 mg/kg). The BSR rapidly decreased on termination of the infusion, reaching 15 ± 3.1% during the final 5 min of the experiment, with a time constant of 37 s (95% CI, 31–46 s). Because the BSR was significantly higher at the end of the experiment compared with the start (15.4 ± 3.1% vs. 5.5 ± 2.0%, two-tailed Student t test), we considered the possibility that this difference was because of the presence of one or both of the metabolites formed by the hydrolysis of methoxycarbonyl etomidate (i.e., methoxycarbonyl etomidate carboxylic acid and methanol).19 To test this, we infused, over 15 min, a mixture of methoxycarbonyl etomidate carboxylic acid (appendix 1 provides a synthesis) and methanol at doses approximating those resulting from the metabolism of methoxycarbonyl etomidate during our experiments (200 and 20 mg/kg, respectively) while simultaneously measuring the BSR in rats (n = 4). The intravenous infusion of this metabolite mixture did not increase the BSR above the baseline preinfusion value (data not shown).
Fig. 3.
(A) The effect of escalating bolus doses of methoxycarbonyl (MOC) etomidate on the burst suppression ratio in a rat. The MOC etomidate doses represent one, two, four, and eight times the ED50 for loss of righting reflexes in rats. Each bolus was given over 6 s, and the burst suppression ratio was calculated from the time-differentiated electroencephalogram. (B) Mean burst suppression ratio during each 6 s epoch recorded from five rats on administration of an MOC etomidate bolus (30 mg/kg), followed by a 15 min closed-loop infusion with the target burst suppression ratio of 40%. The curve is an exponential fit of the postinfusion data. Inset1: Representative baseline raw electroencephalogram obtained from an individual rat before the administration of MOC etomidate.2 Corresponding baseline time-differentiated electroencephalogram before the administration of MOC etomidate.3 Representative raw electroencephalogram obtained from the same rat during closed-loop infusion of MOC etomidate.4 Corresponding time-differentiated electroencephalogram during the MOC etomidate infusion. Each data point represents the burst suppression ratio during a 6 s epoch. All measurements were performed in the presence of inhaled isoflurane, 1%.
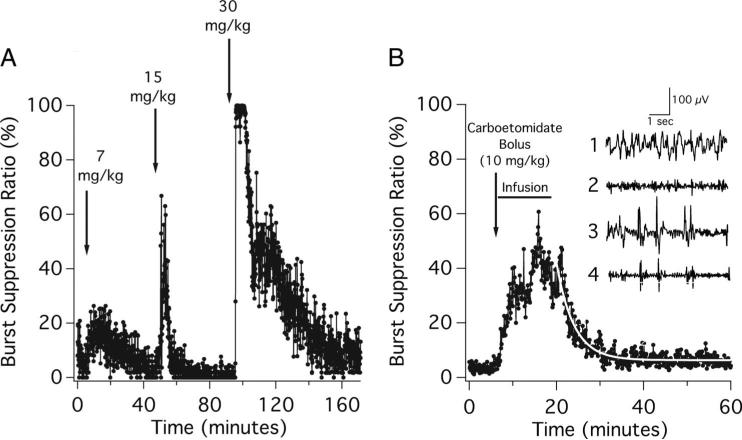
Figure 4A shows the typical changes in the BSR recorded on intravenous bolus administration of successively higher doses of carboetomidate to a rat. As with methoxycarbonyl etomidate and etomidate, successively higher doses of carboetomidate produced higher peak BSR values, although maximal burst suppression occasionally occurred more slowly. Figure 4B shows the mean BSR recorded from five rats on administration of a carboetomidate bolus (10 mg/kg), followed by a 15-min closed-loop infusion with a BSR target of 40%. The carboetomidate dose was 70 ± 16 mg/kg (range, 52–91 mg/kg). The BSR was 5.2 ± 1.2% during the 5-min preinfusion period and increased with carboetomidate administration, albeit somewhat more slowly than with etomidate and methoxycarbonyl etomidate. After this increase and during the final 10 min of the infusion, the BSR was 39 ± 7.5%. On terminating the carboetomidate infusion, the BSR decreased to 5.6 ± 1.4% during the final 5 min of the experiment, with a time constant of 3.9 min (95% CI, 3.6–4.3 min).
Fig. 4.
(A) The effect of escalating bolus doses of carboetomidate on the burst suppression ratio in a rat. The carboetomidate doses represent one, two, and four times the ED50 for loss of righting reflexes in rats. Each bolus was given over 6 s, and the burst suppression ratio was calculated from the time-differentiated electroencephalogram. (B) Mean burst suppression ratio during each 6 s epoch recorded from five rats on administration of a carboetomidate bolus (10 mg/kg), followed by a 15 min closed-loop infusion with the target burst suppression ratio of 40%. The curve is an exponential fit of the postinfusion data. Inset1: Representative baseline raw electroencephalogram obtained from an individual rat before the administration of carboetomidate.2 Corresponding baseline time-differentiated electroencephalogram before the administration of carboetomidate.3 Representative raw electroencephalogram obtained from the same rat during closed-loop infusion of carboetomidate.4 Corresponding time-differentiated electroencephalogram during the carboetomidate infusion. Each data point represents the burst suppression ratio during a 6 s epoch. All measurements were performed in the presence of inhaled isoflurane, 1%.
Part 2: Impact of Continuous Closed-loop Infusions of Etomidate and Etomidate Analogs on Adrenocortical Function
Using a separate group of rats, we assessed the impact of an intravenous bolus, followed by a 30-min closed-loop infusion (target BSR, 40%) of etomidate (n = 4 rats), methoxycarbonyl etomidate (n = 5 rats), and carboetomidate (n = 4 rats) on serum corticosterone concentrations (fig. 5). For comparison, we also studied a control group (n = 5 rats) that underwent surgical electroencephalographic electrode placement but received no intravenous sedative–hypnotic agent.
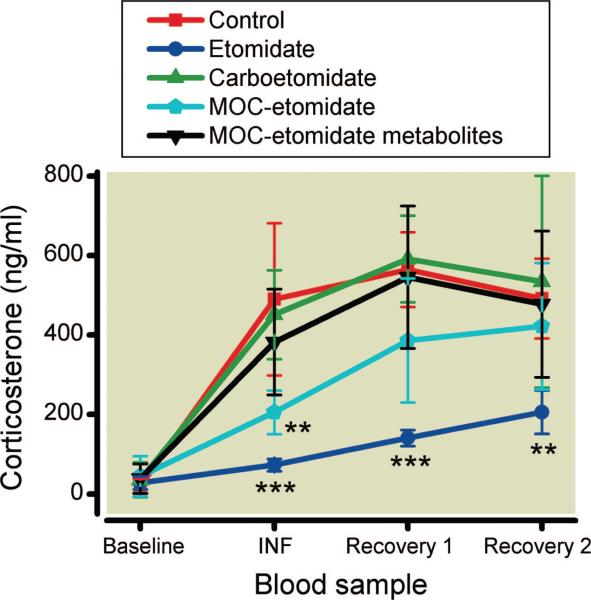
Fig. 5.
The effects of 30 min continuous infusions of etomidate (n = 4 rats), methoxycarbonyl (MOC) etomidate (n = 5 rats), MOC etomidate metabolites (n = 4 rats), carboetomidate (n = 4 rats), or nothing (control; n = 5 rats) on serum corticosterone concentrations. Dosing of etomidate, MOC etomidate, and carboetomidate was determined by closed-loop infusion. MOC etomidate metabolites were continuously infused at a rate of 400 mg/kg MOC etomidate carboxylic acid and 40 mg/kg methanol over 30 min. A baseline blood sample was drawn before the administration of adrenocorticotropic hormone 1–24, intravenous sedative–hypnotic, or metabolites. The first dose of adrenocorticotropic hormone 1–24 was given on beginning intravenous sedative–hypnotic or metabolite infusion and a blood (INF) sample was drawn 30 min later, at the end of the infusion, to assess adrenocortical function during infusion. After the infusion was complete, subsequent doses of adrenocorticotropic hormone 1–24 were administered and blood samples were drawn at 30 min intervals (recovery 1 and recovery 2 blood samples) to assess the recovery of adrenocortical function over the following hour. All studies were performed in the presence of isoflurane, 1%. **P < 0.01, ***P < 0.001 versus the corresponding control value (two-way ANOVA, followed by a Bonferroni posttest).
The serum corticosterone concentration before administration of an intravenous sedative–hypnotic or ACTH1–24 (baseline sample in fig. 5) was not statistically significantly different among the four groups of rats (control, etomidate, methoxycarbonyl etomidate, and carboetomidate), with a value of 32 ± 34 ng/ml.
The serum corticosterone concentrations of rats in the control group increased from a baseline value of 21 ± 18 ng/ml to 490 ± 192 ng/ml after administration of the first dose of ACTH1–24 (fig. 5). This concentration remained essentially unchanged over the next hour, with repeated ACTH1–24 dosing (INF, recovery 1, and recovery 2 samples: 515 ± 43 ng/ml).
Rats in the etomidate group received an average total etomidate dose of 8.3 ± 2.2 mg/kg. As with rats in the control group, their serum corticosterone concentrations increased with administration of the first dose of ACTH1–24 (fig. 5). However, this increase was an order of magnitude smaller than that observed in the control group (from 28 ± 16 to 73 ± 16 ng/ml). On terminating the etomidate infusion, serum corticosterone concentrations increased over the next hour with repeated doses of ACTH1–24. However, all of these concentrations were statistically significantly lower than the corresponding 1 s measured in the control group.
Rats in the carboetomidate group received an average total carboetomidate dose of 99 ± 20 mg/kg. Their serum corticosterone concentrations increased from a baseline value of 36 ± 44 to 450 ± 112 ng/ml with administration of the first dose of ACTH1–24 and remained essentially unchanged with repeated doses of ACTH1–24, averaging 525 ± 71 ng/ml for the three ACTH1–24–stimulated samples (fig. 5). The concentrations in these three samples (INF, recovery 1, and recovery 2 blood samples) were statistically significantly higher than the corresponding 1 s measured in the etomidate group and not statistically significantly different from those measured in the control group.
Rats in the methoxycarbonyl etomidate group received an average total methoxycarbonyl etomidate dose of 275 ± 107 mg/kg. Their serum corticosterone concentrations increased from a baseline value of 45 ± 51 to 206 ± 55 ng/ml with administration of the first dose of ACTH1–24 (fig. 5). This increase was statistically significantly lower than that measured in the control group (and not statistically significantly different from that measured in the etomidate group), implying that, during the closed-loop methoxycarbonyl etomidate infusion, adrenocortical function was suppressed. On terminating the infusion, the serum corticosterone concentrations increased with subsequent doses of ACTH1–24. These two postinfusion serum corticosterone concentrations (recovery 1 and recovery 2 blood samples) were significantly higher in the methoxycarbonyl etomidate group than in the etomidate group. Although they tended to be lower in the methoxycarbonyl etomidate group than in the control group (32% lower for the recovery 1 blood sample and 14% lower for the recovery 2 blood sample), this difference did not reach statistical significance.
We also assessed the impact of methoxycarbonyl etomidate's metabolites on adrenocortical function by continuously infusing a mixture of methoxycarbonyl etomidate carboxylic acid (400 mg/kg) and methanol (40 mg/kg) to rats over 30 min (n = 4). This metabolite mixture had no effect on serum corticosterone concentrations (fig. 5).
Discussion
In the current study, we used the electroencephalographic BSR as the feedback in a closed-loop system to continuously deliver intravenous infusions of etomidate, methoxycarbonyl etomidate, and carboetomidate, with the goal of maintaining rats at approximately equivalent hypnotic depths. In the first part of our study, we compared the sedative–hypnotic doses required to maintain a constant level of hypnosis (i.e., a BSR of 40% in the presence of isoflurane, 1%) and then measured the rate with which the BSR recovered after the infusion was discontinued. Our studies revealed that the sedative–hypnotic doses required to maintain a constant hypnotic depth and the rate of BSR recovery varied significantly among the three agents, with methoxycarbonyl etomidate > carboetomidate > etomidate. In the second part of our study, we determined the extent to which adrenocortical function was suppressed during closed-loop sedative–hypnotic infusion and assessed the rate of adrenocortical recovery after such infusions were discontinued. We found that adrenocortical function was suppressed during continuous infusions of etomidate and methoxycarbonyl-etomidate. However, on terminating the infusion, adrenocortical function recovered within 30 min with methoxycarbonyl etomidate but remained suppressed beyond an hour with etomidate. Carboetomidate had no effect on adrenocortical function either during or after continuous infusion.
We used an electroencephalogram-based closed-loop system to administer the sedatives–hypnotics because it establishes an unbiased dosing regimen to achieve approximately equivalent hypnotic depths.21,23,24 Because all three of our study drugs are thought to produce hypnosis via the same mechanism (i.e., enhancement of γ-aminobutyric acid type A receptor function in the brain), we believe that an electroencephalographic parameter, such as the BSR that varies with sedative–hypnotic dose, provides a reasonable quantitative measure of relative hypnotic depth and controls for differences in hypnotic potency and duration of action among agents when dosing.19,20,25,26 We chose a target BSR of 40% primarily because it is near the midpoint of the BSR dynamic range (0–100%) and could be maintained in our studies using reasonable quantities of methoxycarbonyl etomidate in rats. Although this represents a deep level of hypnosis, all of our experiments were performed in a background of isoflurane, 1%, which reduces the intravenous sedative–hypnotic dose required to reach this BSR. Inspection of figs. 2A, 3A, and 4A shows that, in the presence of isoflurane, 1%, a BSR of 40% can be achieved with bolus doses of our intravenous agents, which are only approximately 1.5 to 3 times their respective ED50 values for loss of righting reflexes (LORRs) in the absence of isoflurane; the ED50 values for LORR in the absence of isoflurane are 1 mg/kg etomidate, 5 mg/kg methoxycarbonyl etomidate, and 7 mg/kg carboetomidate in Sprague–Dawley rats.19,20 Because all three agents likely produce hypnosis via the same receptor mechanism (and probably by binding to the same molecular site on the γ-aminobutyric acid type A receptor), the interactions that etomidate, methoxycarbonyl etomidate, and carboetomidate make with isoflurane are strongly expected to be equivalent with respect to hypnosis.19,20 Therefore, we believe that relative dosing among the three intravenous hypnotic agents may be compared using this electroencephalographic approach in the presence of isoflurane.
With the administration of all three sedatives–hypnotics, the mean BSR measured in our rats increased and remained near our target BSR of 40% during closed-loop continuous infusion. However, the total dose delivered varied greatly among the three agents. For example, the total doses of etomidate, carboetomidate, and methoxycarbonyl etomidate required to keep the BSR at 40% for 15 min (in the presence of isoflurane, 1%) were 4.7 ± 1.6, 70 ± 16, and 174 ± 27 mg/kg, respectively. These doses correspond to ED50 multiples for LORR of 4.7, 10, and 35, respectively.19,20 The higher relative dose of methoxycarbonyl etomidate needed to maintain a BSR of 40% likely results from its faster elimination; methoxycarbonyl etomidate is rapidly hydrolyzed by esterases and has an ultrashort duration of hypnotic action when given as a single bolus.19 Consistent with that conclusion, the time constant with which the BSR decreased on discontinuing the infusion was significantly shorter with methoxycarbonyl etomidate than with carboetomidate and etomidate (37 s vs. 3.9 min and 11.7 min, respectively). However, in our experiments, the BSR did not completely return to the preinfusion baseline even 15–20 min after terminating the methoxycarbonyl etomidate infusion. This probably does not reflect the presence of accumulated metabolites because infusion of methoxycarbonyl etomidate's hydrolysis products (i.e., methoxycarbonyl etomidate carboxylic acid and methanol) did not increase the BSR higher than the low baseline value. In addition, with carboetomidate, the peak BSR tended to occur more slowly. This is broadly consistent with previous behavioral studies19 showing that LORR occurred more slowly with carboetomidate compared with etomidate (33 ± 22 vs. 4.5 ± 0.6 s).
To assess the adrenocortical effects of sedative–hypnotic infusions, we used a second group of rats and added a protocol in which ACTH1–24 was administered and blood was drawn to measure ACTH1–24–stimulated serum corticosterone every 30 min. The first ACTH1–24 dose was given at the start of the closed-loop infusion, and the first blood was drawn immediately after the infusion was complete. Thus, the corticosterone concentration in the first blood sample (INF blood sample) reflects adrenocortical responsiveness to ACTH1–24 during a continuous sedative–hypnotic infusion. Our results showed that, during infusion, both methoxycarbonyl etomidate and etomidate suppressed ACTH1–24–stimulated adrenocortical steroid synthesis. This suppression was unlikely the result of metabolite accumulation because infusion of methoxycarbonyl etomidate's hydrolysis products had no effect on serum corticosterone concentrations. Similarly, etomidate's carboxylic acid metabolite is considered to have no significant effect on steroid synthesis.27 After the infusions of etomidate and methoxycarbonyl etomidate were completed, serum corticosterone concentrations increased with additional doses of ACTH1–24, reflecting, at least in part, recovery of adrenocortical function. Because methoxycarbonyl etomidate is more rapidly metabolized to inactive metabolites than etomidate, it seems reasonable to conclude that the significantly higher serum corticosterone concentrations in the methoxycarbonyl etomidate postinfusion samples, compared with the etomidate samples, reflect methoxycarbonyl etomidate's faster rate of in vivo metabolism.19 However, we cannot exclude other possible explanations or contributions, such as the lower affinity of methoxycarbonyl etomidate for 11β-hydroxylase (which could explain why serum corticosterone concentrations tended to be higher during infusion of methoxycarbonyl etomidate vs. etomidate) or faster dissociation of methoxycarbonyl etomidate from 11β-hydroxylase (if drug dissociation is the rate-limiting step leading to recovery of adrenocortical function).28–30
In contrast to etomidate and methoxycarbonyl etomidate, carboetomidate produced no adrenocortical suppression at any point because the serum corticosterone concentrations in all blood samples drawn from rats in the carboetomidate group were not significantly different from those in the control group. Presumably, this reflects carboetomidate's low affinity for 11β-hydroxylase, the cytochrome P450 enzyme in the adrenal gland that is most sensitive to inhibition by etomidate and is necessary for corticosterone, cortisol, and aldosterone synthesis.5,12,20
Although rats are a valuable model for studying the actions of intravenous sedative–hypnotic agents, they differ from humans in important ways. First, rats are typically less sensitive than humans to the hypnotic actions of these agents. Although the anesthetic induction doses of etomidate and propofol in humans are approximately 0.2–0.3 and 2–2.5 mg/kg, respectively, these doses are insufficient to produce even LORR in rats.19 Second, in vitro and in vivo metabolism of ester-containing drugs (including etomidate) occurs much more quickly in rats than humans.31 For example, remifentanil and esmolol have in vitro metabolic half-lives of only 0.5 and 2.3 min, respectively, in rat blood compared with 37 and 27.2 min, respectively, in human blood.32,33 Remifentanil's in vivo elimination half-life is approximately 1 min in rats and longer than 10 min in humans.34–36 Because of these differences between rats and humans, we expect maintenance doses of methoxycarbonyl etomidate and carboetomidate to be 1–2 orders of magnitude lower in humans than rats on a weight-adjusted basis.
In conclusion, the sedative–hypnotic doses required to maintain a constant level of hypnosis and the rate of hypnotic recovery on infusion termination varied, with methoxycarbonyl etomidate > carboetomidate > etomidate. Serum corticosterone concentrations were reduced during continuous infusions of etomidate and methoxycarbonyl etomidate; however, on infusion termination, serum corticosterone levels recovered more quickly with methoxycarbonyl etomidate than with etomidate. Carboetomidate had no effect on serum corticosterone concentrations during or after continuous infusion. This suggests that methoxycarbonyl etomidate and carboetomidate may have clinical utility as continuously infused sedative–hypnotic maintenance agents when hemodynamic stability is desired.
What We Already Know about This Topic
Etomidate produces hypnosis without affecting hemodynamic stability but inhibits adrenocortical steroid synthesis, whereas methoxycarbonyl etomidate and carboetomidate do not significantly inhibit steroid synthesis when given as a single bolus
What This Article Tells Us That Is New
In rats, continuous infusions of etomidate and methoxycarbonyl etomidate inhibited corticosterone synthesis, but carboetomidate infusions did not
Adrenocortical function returned soon after discontinuing the methoxycarbonyl etomidate infusions but not soon after the etomidate infusions
Acknowledgments
The authors thank Sarah Whitney Raines (Wayland, Massachusetts) for her assistance with artwork and Stuart A. Forman, M.D., Ph.D., Associate Professor, Harvard Medical School, Boston, Massachusetts, Department of Anesthesia, Critical Care, and Pain Medicine, Massachusetts General Hospital, Boston, Massachusetts, for his helpful comments.
Supported by grants R01-GM087316, R21-DA029253, and K08-GM083216 from the National Institutes of Health, Bethesda, Maryland, and the Department of Anesthesia, Critical Care, and Pain Medicine, Massachusetts General Hospital. The Massachusetts General Hospital has submitted patent applications for methoxycarbonyl etomidate, carboetomidate, and related analogs (application numbers PCT/US10/41379 [July 8, 2010] and PCT/US09/38872 [March 31, 2009]). Three authors (Drs. Raines, Cotten, and Husain) and their respective laboratories, departments, and institutions could receive compensation related to the development or sale of these drugs. Dr. Raines has an equity interest in Annovation BioPharma, Inc., which has an option to license the etomidate analogs evaluated in this study.
One or more authors of this peer-reviewed article have been supported by FAER. In conjunction with the FAER 25th anniversary, articles and editorials in Anesthesiology October 2011 issue celebrate the accomplishments of FAER. For additional information visit www.FAER.org.
Appendix 1
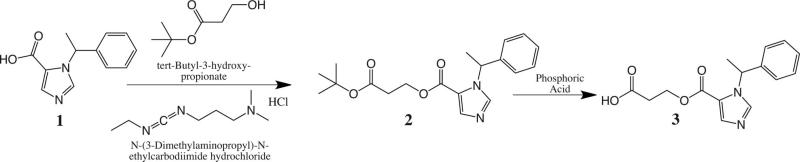
Synthesis of 3-(1-(1-phenylethyl)-1H-imidazole-5-carbonyloxy) propanoic acid (methoxycarbonyl-etomidate carboxylic acid; fig. 6).
Fig. 6.
Synthesis of methoxycarbonyl etomidate carboxylic acid.
R-1-(1-phenylethyl)-1H-imidazole-5-carboxylic acid1 was prepared from R-etomidate by alkaline hydrolysis, as previously described.32 A mixture of R-1-(1-phenylethyl)-1H-imidazole-5-carboxylic acid (3.13 g, 14.5 mmol), tert-butyl-3-hydroxypropanoate (2 g, 13.7 mmol), N-(3-dimethylaminopropyl)-N-ethylcarbodiimide hydrochloride (3 g, 15.6 mmol), and p-dimethylaminopyridine (366 mg, 3 mmol) in anhydrous dichloromethane (25 ml) was stirred at room temperature for 15 h. The reaction mixture was applied to a column of silica gel, equilibrated with dichloromethane, and eluted with ethyl acetate–dichloromethane (1:4 v/v) to yield clear viscous liquid 3-tert-3-oxopropyl 1-(1-phenylethyl)-1H-imidazole-5-carboxylate (2; 4.7 g, 93% yield). 1HNMR spectrum: (CDCl3) d 7.75 (1H, imidazole CH), 7.73 (1H, imidazole CH), 7.30 (m, 5H, phenyl), 6.33 (q, 1H, methine), 4.44 (m, 2H, methylene), 2.61 (t, 2H, methylene). 1.86 (d, 3H, methyl).
The tert-butyl ester–protecting group of 3-tert-3-oxopropyl 1-(1-phenylethyl)-1H-imidazole-5-carboxylate was selectively hydrolyzed with aqueous phosphoric acid by the procedure described by Li et al.37 For this purpose, a solution of 2 (4.6 g, 13.5 mmol) in dichloromethane (4.6 ml) was slowly added under vigorous stirring to 86.3% aqueous phosphoric acid (4.53 ml, 67.3 mmol). The mixture was vigorously stirred at room temperature for 15 h. The reaction mixture was diluted with water (22.6 ml) and cooled in ice; and the stirred mixture neutralized to pH 6.0 with sodium hydroxide, 50%. The mixture was extracted three times with 50 ml portions of dichloromethane. The combined organic layer was rotary evaporated, and the residue was purified by chromatography on a silica gel column, equilibrated with ethyl acetate–dichloromethane–acetic acid (3:7:0.25 v/v/v). The main peak obtained after elution with the equilibration solvent, followed by ethyl acetate–dichloromethane–acetic acid (7:3:0.5 v/v/v) was dried by rotary evaporation and then under high vacuum. Trituration of the product with ethyl acetate, followed by evaporation of the solvent, yielded white crystalline 3-(1-(1-phenylethyl)-1H-imidazole-5-carbonyloxy)propanoic acid (methoxycarbonyl etomidate carboxylic metabolite) (3; 2.53 g, 655). 1HNMR spectrum: (CDCl3) d 7.80 (1H, imidazole CH), 7.79 (1H, imidazole CH), 7.30 (m, 5H, phenyl), 6.35 (q, 1H, methine), 4.49 (m, 2H, methylene), 2.70 (t, 2H, methylene). 1.85 (d, 3H, methyl).
References
- 1.Criado A, Maseda J, Navarro E, Escarpa A, Avello F. Induction of anaesthesia with etomidate: Haemodynamic study of 36 patients. Br J Anaesth. 1980;52:803–6. doi: 10.1093/bja/52.8.803. [DOI] [PubMed] [Google Scholar]
- 2.Gooding JM, Weng JT, Smith RA, Berninger GT, Kirby RR. Cardiovascular and pulmonary responses following etomidate induction of anesthesia in patients with demonstrated cardiac disease. Anesth Analg. 1979;58:40–1. doi: 10.1213/00000539-197901000-00016. [DOI] [PubMed] [Google Scholar]
- 3.Ebert TJ, Muzi M, Berens R, Goff D, Kampine JP. Sympathetic responses to induction of anesthesia in humans with propofol or etomidate. Anesthesiology. 1992;76:725–33. doi: 10.1097/00000542-199205000-00010. [DOI] [PubMed] [Google Scholar]
- 4.Sarkar M, Laussen PC, Zurakowski D, Shukla A, Kussman B, Odegard KC. Hemodynamic responses to etomidate on induction of anesthesia in pediatric patients. Anesth Analg. 2005;101:645–50. doi: 10.1213/01.ane.0000166764.99863.b4. table of contents. [DOI] [PubMed] [Google Scholar]
- 5.de Jong FH, Mallios C, Jansen C, Scheck PA, Lamberts SW. Etomidate suppresses adrenocortical function by inhibition of 11 beta-hydroxylation. J Clin Endocrinol Metab. 1984;59:1143–7. doi: 10.1210/jcem-59-6-1143. [DOI] [PubMed] [Google Scholar]
- 6.Fragen RJ, Shanks CA, Molteni A, Avram MJ. Effects of etomidate on hormonal responses to surgical stress. Anesthesiology. 1984;61:652–6. doi: 10.1097/00000542-198412000-00004. [DOI] [PubMed] [Google Scholar]
- 7.Wagner RL, White PF. Etomidate inhibits adrenocortical function in surgical patients. Anesthesiology. 1984;61:647–51. doi: 10.1097/00000542-198412000-00003. [DOI] [PubMed] [Google Scholar]
- 8.Wagner RL, White PF, Kan PB, Rosenthal MH, Feldman D. Inhibition of adrenal steroidogenesis by the anesthetic etomidate. N Engl J Med. 1984;310:1415–21. doi: 10.1056/NEJM198405313102202. [DOI] [PubMed] [Google Scholar]
- 9.Lamberts SW, Bons EG, Bruining HA, de Jong FH. Differential effects of the imidazole derivatives etomidate, ketoconazole and miconazole and of metyrapone on the secretion of cortisol and its precursors by human adrenocortical cells. J Pharmacol Exp Ther. 1987;240:259–64. [PubMed] [Google Scholar]
- 10.Diago MC, Amado JA, Otero M, Lopez-Cordovilla JJ. Anti-adrenal action of a subanaesthetic dose of etomidate. Anaesthesia. 1988;43:644–5. doi: 10.1111/j.1365-2044.1988.tb04148.x. [DOI] [PubMed] [Google Scholar]
- 11.Allolio B, Schulte HM, Kaulen D, Reincke M, Jaursch-Hancke C, Winkelmann W. Nonhypnotic low-dose etomidate for rapid correction of hypercortisolaemia in Cushing's syndrome. Klin Wochenschr. 1988;66:361–4. doi: 10.1007/BF01735795. [DOI] [PubMed] [Google Scholar]
- 12.Schulte HM, Benker G, Reinwein D, Sippell WG, Allolio B. Infusion of low dose etomidate: Correction of hypercortisolemia in patients with Cushing's syndrome and dose-response relationship in normal subjects. J Clin Endocrinol Metab. 1990;70:1426–30. doi: 10.1210/jcem-70-5-1426. [DOI] [PubMed] [Google Scholar]
- 13.Drake WM, Perry LA, Hinds CJ, Lowe DG, Reznek RH, Besser GM. Emergency and prolonged use of intravenous etomidate to control hypercortisolemia in a patient with Cushing's syndrome and peritonitis. J Clin Endocrinol Metab. 1998;83:3542–4. doi: 10.1210/jcem.83.10.5156. [DOI] [PubMed] [Google Scholar]
- 14.Absalom A, Pledger D, Kong A. Adrenocortical function in critically ill patients 24 h after a single dose of etomidate. Anaesthesia. 1999;54:861–7. doi: 10.1046/j.1365-2044.1999.01003.x. [DOI] [PubMed] [Google Scholar]
- 15.Vinclair M, Broux C, Faure P, Brun J, Genty C, Jacquot C, Chabre O, Payen JF. Duration of adrenal inhibition following a single dose of etomidate in critically ill patients. Intensive Care Med. 2008;34:714–9. doi: 10.1007/s00134-007-0970-y. [DOI] [PubMed] [Google Scholar]
- 16.Jackson WL., Jr Should we use etomidate as an induction agent for endotracheal intubation in patients with septic shock? A critical appraisal. Chest. 2005;127:1031–8. doi: 10.1378/chest.127.3.1031. [DOI] [PubMed] [Google Scholar]
- 17.Annane D. ICU physicians should abandon the use of etomidate! Intensive Care Med. 2005;31:325–6. doi: 10.1007/s00134-005-2560-1. [DOI] [PubMed] [Google Scholar]
- 18.Hildreth AN, Mejia VA, Maxwell RA, Smith PW, Dart BW, Barker DE. Adrenal suppression following a single dose of etomidate for rapid sequence induction: A prospective randomized study. J Trauma. 2008;65:573–9. doi: 10.1097/TA.0b013e31818255e8. [DOI] [PubMed] [Google Scholar]
- 19.Cotten JF, Husain SS, Forman SA, Miller KW, Kelly EW, Nguyen HH, Raines DE. Methoxycarbonyl-etomidate: A novel rapidly metabolized and ultra-short-acting etomidate analogue that does not produce prolonged adrenocortical suppression. Anesthesiology. 2009;111:240–9. doi: 10.1097/ALN.0b013e3181ae63d1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cotten JF, Forman SA, Laha JK, Cuny GD, Husain SS, Miller KW, Nguyen HH, Kelly EW, Stewart D, Liu A, Raines DE. Carboetomidate: A pyrrole analog of etomidate designed not to suppress adrenocortical function. Anesthesiology. 2010;112:637–44. doi: 10.1097/ALN.0b013e3181cf40ed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vijn PC, Sneyd JR. I.V. anaesthesia and EEG burst suppression in rats: Bolus injections and closed-loop infusions. Br J Anaesth. 1998;81:415–21. doi: 10.1093/bja/81.3.415. [DOI] [PubMed] [Google Scholar]
- 22.Rampil IJ, Laster MJ. No correlation between quantitative electroencephalographic measurements and movement response to noxious stimuli during isoflurane anesthesia in rats. Anesthesiology. 1992;77:920–5. doi: 10.1097/00000542-199211000-00014. [DOI] [PubMed] [Google Scholar]
- 23.Tzabazis A, Ihmsen H, Schywalsky M, Schwilden H. EEG-controlled closed-loop dosing of propofol in rats. Br J Anaesth. 2004;92:564–9. doi: 10.1093/bja/aeh102. [DOI] [PubMed] [Google Scholar]
- 24.Struys MM, De Smet T, Versichelen LF, Van De Velde S, Van den Broecke R, Mortier EP. Comparison of closed-loop controlled administration of propofol using bispectral index as the controlled variable versus “standard practice” controlled administration. Anesthesiology. 2001;95:6–17. doi: 10.1097/00000542-200107000-00007. [DOI] [PubMed] [Google Scholar]
- 25.Jurd R, Arras M, Lambert S, Drexler B, Siegwart R, Crestani F, Zaugg M, Vogt KE, Ledermann B, Antkowiak B, Rudolph U. General anesthetic actions in vivo strongly attenuated by a point mutation in the GABA(A) receptor beta3 subunit. FASEB J. 2003;17:250–2. doi: 10.1096/fj.02-0611fje. [DOI] [PubMed] [Google Scholar]
- 26.Belelli D, Muntoni AL, Merrywest SD, Gentet LJ, Casula A, Callachan H, Madau P, Gemmell DK, Hamilton NM, Lambert JJ, Sillar KT, Peters JA. The in vitro and in vivo enantioselectivity of etomidate implicates the GABAA receptor in general anaesthesia. Neuropharmacology. 2003;45:57–71. doi: 10.1016/s0028-3908(03)00144-8. [DOI] [PubMed] [Google Scholar]
- 27.Zolle IM, Berger ML, Hammerschmidt F, Hahner S, Schirbel A, Peric-Simov B. New selective inhibitors of steroid 11beta-hydroxylation in the adrenal cortex: Synthesis and structure-activity relationship of potent etomidate analogues. J Med Chem. 2008;51:2244–53. doi: 10.1021/jm800012w. [DOI] [PubMed] [Google Scholar]
- 28.Van Hamme MJ, Ghoneim MM, Ambre JJ. Pharmacokinetics of etomidate, a new intravenous anesthetic. Anesthesiology. 1978;49:274–7. doi: 10.1097/00000542-197810000-00010. [DOI] [PubMed] [Google Scholar]
- 29.Hebron BS, Edbrooke DL, Newby DM, Mather SJ. Pharmacokinetics of etomidate associated with prolonged i.v. infusion. Br J Anaesth. 1983;55:281–7. doi: 10.1093/bja/55.4.281. [DOI] [PubMed] [Google Scholar]
- 30.Lewi PJ, Heykants JJ, Janssen PA. Intravenous pharmacokinetic profile in rats of etomidate, a short-acting hypnotic drug. Arch Int Pharmacodyn Ther. 1976;220:72–85. [PubMed] [Google Scholar]
- 31.Calvo R, Carlos R, Erill S. Etomidate and plasma esterase activity in man and experimental animals. Pharmacology. 1979;18:294–8. doi: 10.1159/000137268. [DOI] [PubMed] [Google Scholar]
- 32.Feldman PL, James MK, Brackeen MF, Bilotta JM, Schuster SV, Lahey AP, Lutz MW, Johnson MR, Leighton HJ. Design, synthesis, and pharmacological evaluation of ultrashort- to long-acting opioid analgetics. J Med Chem. 1991;34:2202–8. doi: 10.1021/jm00111a041. [DOI] [PubMed] [Google Scholar]
- 33.Quon CY, Stampfli HF. Biochemical properties of blood esmolol esterase. Drug Metab Dispos. 1985;13:420–4. [PubMed] [Google Scholar]
- 34.Haidar SH, Moreton JE, Liang Z, Hoke JF, Muir KT, Eddington ND. The pharmacokinetics and electroencephalogram response of remifentanil alone and in combination with esmolol in the rat. Pharm Res. 1997;14:1817–23. doi: 10.1023/a:1012156502624. [DOI] [PubMed] [Google Scholar]
- 35.Westmoreland CL, Hoke JF, Sebel PS, Hug CC, Jr, Muir KT. Pharmacokinetics of remifentanil (GI87084B) and its major metabolite (GI90291) in patients undergoing elective inpatient surgery. Anesthesiology. 1993;79:893–903. doi: 10.1097/00000542-199311000-00005. [DOI] [PubMed] [Google Scholar]
- 36.Glass PS, Hardman D, Kamiyama Y, Quill TJ, Marton G, Donn KH, Grosse CM, Hermann D. Preliminary pharmacokinetics and pharmacodynamics of an ultra-short-acting opioid: Remifentanil (GI87084B). Anesth Analg. 1993;77:1031–40. doi: 10.1213/00000539-199311000-00028. [DOI] [PubMed] [Google Scholar]
- 37.Li B, Berliner M, Buzon R, Chiu CK- F, Colgan ST, Kaneko T, Keene N, Kissel W, Le T, Leeman KR, Marquez B, Morris R, Newell L, Wunderwald S, Witt M, Weaver J, Zhang Z. Aqueous phosphoric acid as a mild reagent for deprotection of tert-butyl carbamates, esters, and ethers. J Org Chem. 2006;71:9045–50. doi: 10.1021/jo061377b. [DOI] [PubMed] [Google Scholar]