Abstract
Rats repeatedly exposed to variable prenatal stress (PNS) exhibit behavioral features often observed in neuropsychiatric disorders including elevated sensitivity to stimulants and impairments of attention, inhibitory control and memory-related task performance. However, to date there have been relatively few studies designed to assess the effects of PNS on anxiety, stress and fear responses, or the function of the hypothalamic-pituitary-adrenal (HPA) axis (a system clearly linked to stress and fear-related responses as well as neuropsychiatric disorders). In the current study, rats exposed to variable PNS were evaluated for anxiety-related behaviors in open field, elevated plus maze, and light/dark preference tasks. Innate fear responses were assessed using a predatory odor task and learned fear and extinction were assessed with a contextual fear conditioning task. As an indicator of HPA axis function, serum corticosterone levels were determined by enzyme immunoassay at various time points. The results indicated that PNS resulted in several behavioral anomalies including decreased innate fear responses to predator odor, impaired fear extinction, increased locomotor activity and stereotypic-like behaviors. Baseline levels of corticosterone in PNS subjects were similar to non-stressed controls; however, when exposed to acute stress, they exhibited an increase in corticosterone that was greater in magnitude. PNS was not associated with increased anxiety-like behaviors or deficits in learning or retention during contextual fear conditioning. Collectivity, these data support the argument that variable PNS in rats is a valid model system for studying some behavioral components of neuropsychiatric disorders as well as the influence of stress hormones.
Keywords: maternal stress, corticosterone, cognition, memory, schizophrenia, stereotypy
1. Introduction
During the prenatal period in mammalian species, the rapid growth of the central nervous system makes the fetus particularly vulnerable to insults [30]. This phenomenon is evident from the results of several independent (and prospective) human studies which indicate that maternal stress during pregnancy is associated with adverse neurodevelopmental outcomes in the child later in life, including attention-deficit/hyperactivity disorder (ADHD), autism, schizophrenia, and anxiety disorders [2,12,20,25,27,43,49,51]. However, there any many aspects of this relationship (i.e., prenatal stress to neuropsychiatric disorders) that are unclear and the development of appropriate animal models for the purpose of elucidating this relationship as well as for evaluating novel therapeutic interventions is greatly needed [29,55]. It has been suggested that repeated variable prenatal stress in rodents (henceforth referred to as PNS) might be an etiologically appropriate neurodevelopmental model for some components of schizophrenia [22]. Exposure to variable PNS was previously found to result in social withdrawal, elevated amphetamine-induced locomotor activity, and deficits in sensory-motor gating; behavioral characteristics commonly associated with a schizophrenia-related phenotype [22,24]. Further, PNS subjects were also found to exhibit impairments in spatial and recognition memory-related tasks, attention, and inhibitory control [31,52].
While several domains of cognition have been evaluated in the variable PNS model, relatively little is known about its effects on anxiety, stress, and fear-related responses. Anxiety, maladaptive fear responses, and impaired fear extinction are primary symptoms of a number of neuropsychiatric disorders including post traumatic stress disorder (PTSD) and schizophrenia. An additional question relates how PNS might affect the function of the hypothalamic-pituitary-adrenal (HPA) axis, a system that is intimately linked to stress, and fear-related responses. It is relatively well established that dysfunction of the HPA axis is a common feature in adults with neuropsychiatric disorders (e.g., schizophrenia, anxiety disorders) [14,28,54], however, it is currently unclear how PNS might alter HPA axis regulation and affect the susceptibility to psychopathology later in life. [9,15,19,32]. Interestingly, Koenig and colleagues previously reported that rats exposed to variable PNS have significantly higher plasma levels of the stress hormone corticosterone following exposure to a mild restraint stress than control animals suggesting an altered response of the HPA axis to acute stress [21]. Importantly, corticosterone (in rodents) is known to play a crucial role in anxiety-related behaviors as well cognitive processes including conditioned fear and extinction learning [13,34,45].
There were, therefore, four objectives of the current study: 1) to determine if PNS in rats is associated with anxiety like behaviors in open field, elevated plus maze (EPM), and light/dark preference tasks, 2) to determine if PNS produces impairments in innate fear responses (using cat odor), 3) to determine if PNS is associated with learning, memory, and extinction deficits in a fear conditioning task (CFC), and 4) to determine how PNS affects the function of the HPA (via measurements of serum corticosterone) in the adult.
2. Material and methods
2.1. Animals
Timed pregnant Sprague-Dawley female rats (Harlan Sprague-Dawley, Inc., Indianapolis, IN, USA) arriving on day five of gestation were housed individually in a temperature-controlled (25°C) and light-controlled (12-h light/dark cycle) facility. Pregnant animals had free access to food (Teklad Rodent Diet 8604 pellets, Harlan, Madison, WI, USA) and water following their arrival. All procedure employed during this study were reviewed and approved by the Georgia Health Sciences University Institutional Animal Care and Use Committee and are consistent with the AAALAC guidelines. Measures were taken to minimize pain or discomfort in accordance with the National Institutes of Health Guide for Care and Use of Laboratory Animals. Animals were handled for one week (beginning on postnatal day, PND 56) prior to all testing and training to reduce human contact-related stress and anxiety-related behaviors. Study subjects were transferred (in their home cages) to the behavioral testing rooms each morning approximately 30 min prior to training and testing. Both male and female rats were tested in each behavioral task and testing began on PND 63.
2.2. Stress paradigm
The repeated variable prenatal stress paradigm used in this study was adapted from Koenig and colleagues [21,22]. Pregnant rats were exposed to the paradigm beginning on day 14 of gestation until delivery of pups on gestational day 22 or 23. The stress paradigm consisted of: (1) restraint in Broome style rodent restrainers (PLAS Labs, Inc) (1 h); (2) exposure to a cold environment (4 ± 1°C, 6 h); (3) overnight food deprivation; (4) forced swim in room temperature water (15 min); (5) reversal of the light–dark cycle; and (6) social stress induced by overcrowded housing during the dark phase of the cycle. Stressors were applied in a randomized manner to prevent accommodation with one to three stress sessions per day. Pregnant control rats were exposed to normal animal care and maintenance procedures during this period. Following birth, all dams and pups were left undisturbed until weaning on postnatal day 22. Offspring were double housed with same sex littermate, food and water was allowed ad libitum. Exposure to the prenatal stress procedures did not result in changes to the number of live born pups or the latency to parturition. Further, there were no differences in the number of pups per litter or the ratio of male to female pups between the control groups or those exposed to the paradigm (data not shown)
2.3. Serum corticosterone levels
2.3.1. Serum sample collection
Blood sample collection was performed on both pregnant rats exposed to the variable prenatal stress paradigm and pregnant control rats. Samples were collected on gestational day 13 (baseline levels), day 16 (3rd day of stress paradigm), and day 19 (6th day of stress paradigm). Samples were taken at a specific time point for each animal on all days which correlated with the completion of stress in day 16 and day 19. To minimize additional stress associated with the blood sampling, dams were anesthetized by delivering isoflourane via a Vetroson small animal anesthesia machine (Summit Hill Laboratories, Wavesink, NJ). Animals were under approximately 90s, during which blood was collected from the tail. Blood sample collection was also performed on both control and prenatally stressed (PNS) pups (i.e., both male and female) on PND 75 with minimal stress and PND 76 following a foot shock (1 mA AC, 1000 ms) 30 minutes prior to collection. Rats were placed on heating pad (to promote vasodilation) and briefly anesthetized (< 90 s) using isofluorane. Blood was taken by using a razor to produce a small tail nick and collected in BD Microtainer® tubes (approx. 100 ul) with serum separator (Becton, Dickinson and Company, Franklin Lakes, NJ). Collected blood was incubated at room temperature for 30 min to allow clotting, followed by centrifugation at 6,000 × g for 90 sec. Serum samples were stored at −20°C.
2.3.2. Corticosterone measurements
Serum corticosterone levels were determined using a corticosterone enzyme immunoassay (EIA) kit (Abnova, Taipei City, Taiwan). The EIA was carried out according to the manufacturer’s instructions. Absorbance was measured at 450 nm and concentrations were calculated from the measured absorbance values using Gen5 data analysis software (BioTek, Winooski, VT). The intra-assay and inter-assay coefficients of variation ranged between 4.9% and 7.4%.
2.4. Locomotor activity
Rat open field activity monitors (43.2 × 43.2 cm, Med Associates, St. Albans, VT, USA) were used to analyze locomotor activity of control and PNS rats in a novel environment. Animals were placed in the middle of the test arena facing the back wall. The following parameters were recorded for the entire 30 min test duration: horizontal activity (horizontal photobeam breaks or counts), number of stereotypical movements (repeated photobeam breaks), and vertical activity (vertical photobeam breaks). Thus, spontaneous locomotor activity, exploratory activity (rearing and sniffing movements) and stereotypical movements were assessed. Time spent in the center and peripheral zone was also recorded.
2.5. Elevated plus maze
To assess the effects of prenatal stress on anxiety, animals were evaluated using an elevated plus maze (EPM) consisting of a plus shaped maze made of opaque Plexiglas with two opposite open arms (50 × 10 cm) and two closed arms (50 × 10 cm with 40 cm walls) under low lighting (50 lux, lumen/m2). The task was initiated by placing the test subject into the center of the maze facing an open arm. Activity was monitored via a mounted overhead camera and video tracking system (Noldus EthoVision® Pro 3.1) for a 10 min period. The total distance traveled, entries into each arm, and the time spent in open arms, closed arms, and center of maze were evaluated. The frequency of head dips (the rat dipping its head into the space below the open arm and observing the environment) was also scored manually from the video files).
2.6. Light/dark preference test
To further assess the effects of prenatal stress on anxiety levels, a light/dark preference test (also referred to as light/dark exploration or emergence neophobia test) was conducted. For these experiments, Med Associates Inc. (St. Albans, VT) rat open field activity monitors (43.2 × 43.2 cm) fitted with dark box inserts (which are opaque to visible light) to cover half the open field area, thus separating the apparatus into two zones of equal area (i.e., a brightly lit zone and a darkened zone) were utilized. Lamps were located above the activity monitors to provide an illumination level of approximately 1000 lux in the brightly lit zone, whereas the illumination level in the darkened zone was approximately 5 lux. The task was initiated by placing the test subject into the lighted zone of the activity chamber. Time spent in the light and dark zones of the apparatus was monitored and recorded continuously for 5 min. Latency to entry into dark zone was also recorded.
2.7. Predatory odor avoidance task
A predatory odor avoidance task was used to determine if prenatal stress results in alterations of innate defensive behaviors towards predatory odors [38]. Cat hair (compressed into a ball, 10 cm in diameter) was obtained from a domestic male cat prior to the day of testing and kept in airtight (Zip-Lock) plastic bag until use. Similar textured “fake” hair (polyester fiber filling) was used as a control stimulus. Testing was conducted in a 35 × 26 × 50 cm box under low lighting (70 lux). The arena was divided into four equal quadrants. Animals were recorded via an overhead camera. The box was cleaned with 10% alcohol solution between each subject. On day 1, fake hair was placed in the center of a quadrant. Rats were then placed in the opposite quadrant facing the wall and allowed to freely explore for 5 min. The following day, fake hair was replaced with cat hair and experimental animals were once again placed into the opposite quadrant and allowed to freely explore for 5 min. Contact (animal directing its nose to the hair ball at a distance of less than 2 cm and/or by the animal touching the object), freezing (no movement except for respiration) and avoidance (time spent in the quadrant furthest from the cat hair or fake hair) was scored for both sessions. The number of rearings was counted as a measure of locomotor activity.
2.8. Odor discrimination
As the predatory odor avoidance task relies heavily on intact olfactory function, a simple odor task was used to determine if exposure to prenatal stress resulted in impaired olfactory discrimination. Animals were tested in an apparatus consisting of open field box (65.5 × 41.0 × 36.5 cm) with normal rodent bedding material (Sani-Chips®, Harlan Teklad Madison, WI) covering the floor. Two identical perforated polyethylene tubes (2.5 × 13 cm) were filled with either food pellets (45 mg chow pellet, BioServ, Frenchtown, NJ, USA) or “fake” food pellets (zinc coated 4.5 mm BB pellets). The tubes were placed near two adjacent corners of the box and the rat was placed into the box equidistant from each tube during the test session. Animals were allowed to explore freely for 3 min. The box was cleaned between each trial and all sessions were recorded via mounted overhead cameras. The total amount of time exploring (animal directing its nose at object at a distance of less than 2 cm and/or by the animal touching the object with its nose, mouth or paws) each tube was recorded. The proportion of the total exploration time that the animal spent investigating the tube with food pellets is suggestive of olfactory discrimination and this preference index was calculated as: TA/(TA+TB) * 100, where TA is time spent exploring food tube (food pellets) and TB is time spent exploring fake food tube (BB pellets).
2.9. Contextual fear conditioning task
The contextual fear conditioning (CFC) task, used to test PNS and control animals, was modified from Nalloor and colleagues [38]. Test subjects were moved to an isolated room with minimal noise levels at least 1 hr prior to the start of task, food and water were allowed ad libitum. CFC was conducted in a 50 × 10 × 19 cm box with dim lighting (100 lux). Foot shocks were administered via stainless steel floor plates electrified by a constant current shock generator. Animals were recorded via an overhead camera.
2.9.1. CFC training
Experimental animals were placed in the test box apparatus and allowed to freely explore for a 3 min habituation period. At the end of the habituation period, two foot shocks (0.7 mA AC, 1000 ms, 1 min apart) were administered. Animals were removed from test box 1 min following the completion of the second foot shock and returned to their home cage. Time spent freezing (no movement except for respiration), horizontal activity (number of crossings from one end of the box to the other), and vertical activity (number of rearings) were scored throughout habituation and training. A training criterion of freezing >15% of the post-shock epoch was used to ensure reliable fear conditioning. Fear conditioning retention testing was measured 48 hrs after training. Animals were placed back into testing apparatus for a 5 min period (no foot shock administered) and freezing was recorded.
2.9.2. CFC extinction
Fear extinction to the CFC context was performed by reintroducing the animal into the CFC apparatus for 5 min per day for 4 consecutive days following fear conditioning retention testing, without foot shocks. Freezing was scored. The Extinction Index was a measure of magnitude of extinction and was calculated as: 100–100*(EDx/ED1), where ED1 and EDx are the percent time spent freezing on extinction days 1 and 2, 3, 4, or 5 respectively.
2.10. Y maze CFC task
Due to the lack of freezing of female rats in both the control and PNS groups during the previously described contextual fear conditioning task, female rats were evaluated using a Y maze version of contextual fear condition previously described [52]. Test subjects were moved to an isolated room with minimal noise levels at least 1 hr prior to the start of task, food and water were allowed ad libitum. The Y maze consisted of three arms, separated by 120°, each 50 × 10 × 19 cm, however each arm was uniquely different in color and texture. The arms were covered with translucent Plexiglas lids. Sessions were conducted under dim lighting (100 lux) conditions and recorded via an overhead camera. Foot shocks were administered via stainless steel floor plates electrified by a constant current shock generator.
2.10.1. CFC training
Animals were habituated by allowing the rats to freely explore the Y maze for 8 min each on two consecutive days. Time spent freezing (no movement except for respiration), time spent per arm, and entries per arm were recorded for each habituation session. On the day of training, rats were placed in one arm (shock arm) that was blocked off from the rest of the maze. After 2 minutes, rats received the first of two foot shocks (0.7 mA AC, 1000 ms) delivered at 1-min intervals. One minute after the last footshock, the rats were returned to their home cages. Time spent freezing, horizontal activity (number of crossings from one end of the box to the other) and vertical activity (number of rearings) were scored during training. Fear conditioning retention testing was measured 48 hrs after training. Animals were placed back into the Y maze in an arm where they did not receive a foot shock and allowed to freely explore all arms for a 5 min period (no foot shock administered). Time spent freezing, time spent per arm, entries per arm, and latency to first entry into shock arm were recorded.
2.10.2. CFC extinction
Fear extinction to the CFC context was performed by reintroducing the animal into the Y maze for 5 min per day for 4 consecutive days following fear conditioning retention testing, without foot shocks. Freezing was recorded for each session. The Extinction Index was a measure of magnitude of extinction and was calculated as: 100–100*(EDx/ED1), where ED1 and EDx are the percent time spent freezing on extinction days 1 and 2, 3, 4, or 5 respectively.
2.11. Hot plate nociception
A HotScan™ incrementing temperature hot plate (AccuScan Instruments, Inc., Columbus, OH) was used to analyze supraspinal nociception of control and PNS animals. The apparatus consisted of a Plexiglas enclosure resting on a digitally regulated hotplate which was manually triggered on and off. The surface temperature of the plate was maintained at 40°C at the start of a trial increasing by 3°C/min to a maximum of 49°C, at which point the temperature automatically reset to 40°C. The characteristic behavior of licking a hindpaw was used as the criterion for nociception, at which point the plate temperature was immediately reversed to the original setting and the trial was terminated. The animal was removed from the chamber and the temperature recorded (none of the animals remained on the plate long enough to reach the cutoff temperature of 49°C).
2.12. Tail flick nociception
A tail-flick apparatus (San Diego Instruments Inc., San Diego, California) was used to analyze spinal nociception of control and PNS animals. Rats was placed on the platform of the tail-flick apparatus and held in place while the distal regions of their tails were placed flat against the radiant heat source. The amount of time taken for the animal to move (flick) its tail away from the heat is recorded. Each test subject was given two consecutive trials with a maximum latency of 10 sec for each trial.
2.13. Statistics
Results are expressed as means ± standard error of mean of the individual values of rats from each group. Data set were tested for normality (Shapiro-Wilk test). Comparisons for single factors between two groups were performed with an unpaired two-tailed t-test. Statistical comparisons of groups with two-factors were conducted using a two way repeated measures ANOVA followed by post hoc analysis using the Student–Newman–Keuls multiple comparison method (SigmaStat 2.03, SPSS Inc., Chicago, IL, USA). Statistical significance was assessed using an alpha level of 0.05. Trends toward significance were considered at the p < 0.10. When gender differences were not statistically significant, analyses were collapse over gender.
3. Results
3.1. Corticosterone levels
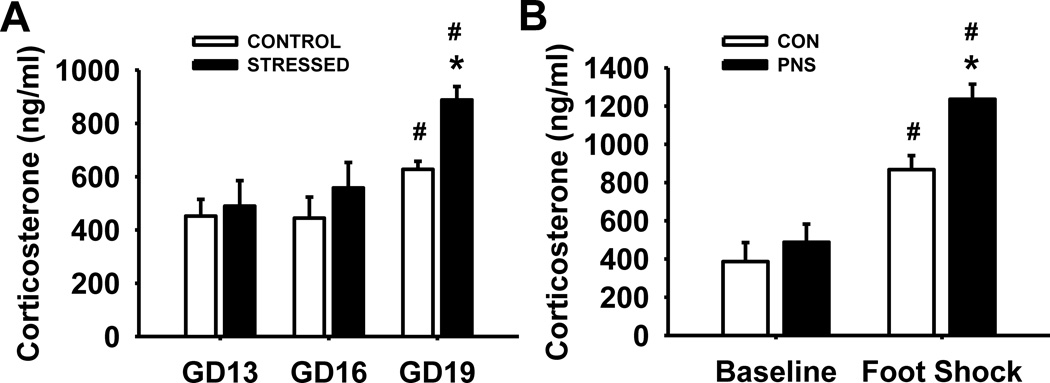
Serum samples were evaluated on day 13, 16, and 19 of gestation (GD) to determine the effects of the variable prenatal stress paradigm on corticosterone levels in pregnant rats (n=4 per group) (Fig.1A). Statistical analysis revealed the following results, main effect of group F(1,12) = 2.36, p=0.17; day, F(2,23) = 25.18, p<0.001; group by day interaction F(2,23) = 3.26, p=0.07. Post hoc analyses indicated that both control pregnant dams and pregnant dams exposed to the prenatal stress paradigm had elevated corticosterone levels on GD 19 compared to those on GD 13 and 16 (p<0.001). However, corticosterone levels were elevated to a higher degree in pregnant dams exposed to the variable prenatal stress paradigm than those exposed to normal animal maintenance (controls) on GD 19 (p=0.03).
Fig. 1.
Effects of exposure to variable prenatal stress on corticosterone levels. (A) Corticosterone levels of dams at baseline on gestational day (GD) 13 and following exposure to prenatal stress on GD 16 and 19. (B) Corticosterone levels of pups at baseline on postnatal day (PND) 75 and following a foot shock on PND 76. Each bar represents the mean ± SEM for each test group.* represents a significant difference (p<0.05) in corticosterone levels between rats exposed to the prenatal stress paradigm and control rats. # represents a significant difference compared to baseline levels. PNS = prenatally stressed; CON = non-stressed controls. Dams N = 4, Pups N = 6.
Serum corticosterone levels in pups were also evaluated to determine the long term effects of the prenatal stress paradigm on baseline levels of corticosterone as well as following exposure to acute stress (n=6 per group) (Fig. 1B). Statistical analysis revealed the following results, main effect of group F(1,10) = 5.27, p<0.05; day, F(1,23) = 78.70, p<0.001; group by day interaction F(1,23) = 3.71, p=0.08. Post hoc analyses indicated that both PNS and control rats had a significant increase in corticosterone levels following exposure to acute stress (foot shock) compared to baseline levels (p<0.001). In addition, prenatally stressed (PNS) rats had significantly higher corticosterone levels following acute stress compared to control rats (p=0.008), although there was no significant difference between PNS and control rats at baseline levels.
3.2. Locomotor activity
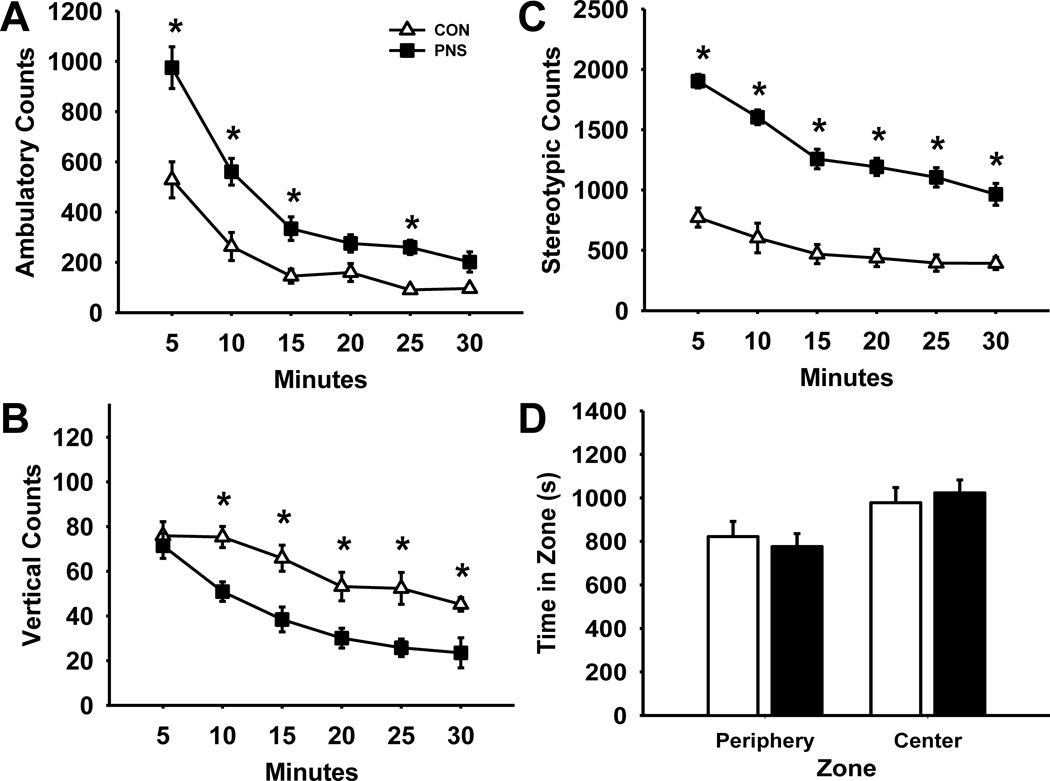
Fig. 2 illustrates the effects of prenatal stress on locomotor activity over a 30 min time period during an open field task (n=12 per group). Statistical analysis of ambulatory counts (Fig. 2A) revealed a significant main effect of group F(1,110) = 29.78, p<0.001, time F(5,143) = 63.60, p<0.001, and group by time interaction F(5,143) = 5.15, p<0.001. Post hoc analysis indicated a significant increase in ambulatory counts of PNS rats compared to controls at 5, 10, 15, and 25 min time intervals; however during the last five min of the session there was no longer a significant difference between PNS and control rats. The number of ambulatory counts of both groups significantly decreased with time revealing that both groups habituated to the new environment over time. Statistical analysis of vertical counts (Fig. 2B) also revealed a significant main effect of group F(1,110) = 18.28, p<0.001, and time F(5,143) = 22.32, p<0.001; without a significant group by time interaction F(5,143) = 1.72, p=0.14. Post hoc analysis indicated a significant decrease in the number of vertical counts in PNS rats compared to controls except during the first five min of the task. Statistical analysis of stereotypic counts (Fig. 2C) revealed a significant main effect of group F(1,110) = 110.6, p<0.001, time F(5,143) = 46.06, p<0.001, and group by time interaction F(5,143) = 5.64, p<0.001. Post hoc analysis indicated a significant increase in stereotypic counts of PNS rats during the entire 30 min session compared to controls. Further, statistical analysis of time rats spent in either center zone or periphery zone (Fig. 2D) revealed a significant main effect of zone F(1,22) = 4.66, p<0.05 without a main effect of group F(1,47) = 1.00, p=0.33, or group by zone interaction F(1,47) = 0.17, p=0.68. Post hoc analysis suggested that rats from either condition spent significantly more time in the center zone than in the peripheral zone.
Fig. 2.
Effects of prenatal stress on locomotor activity. (A) Ambulatory counts, (B) vertical counts, or (C) stereotypic counts over a thirty minute period. (D) Time spent (s) in peripheral and center zones. Each point or bar represents the mean ± SEM for each test group.* represents a significant difference (p<0.05) in activity between PNS and control rats. PNS = prenatally stressed; CON = non-stressed controls. N = 12.
3.3. Elevated plus maze
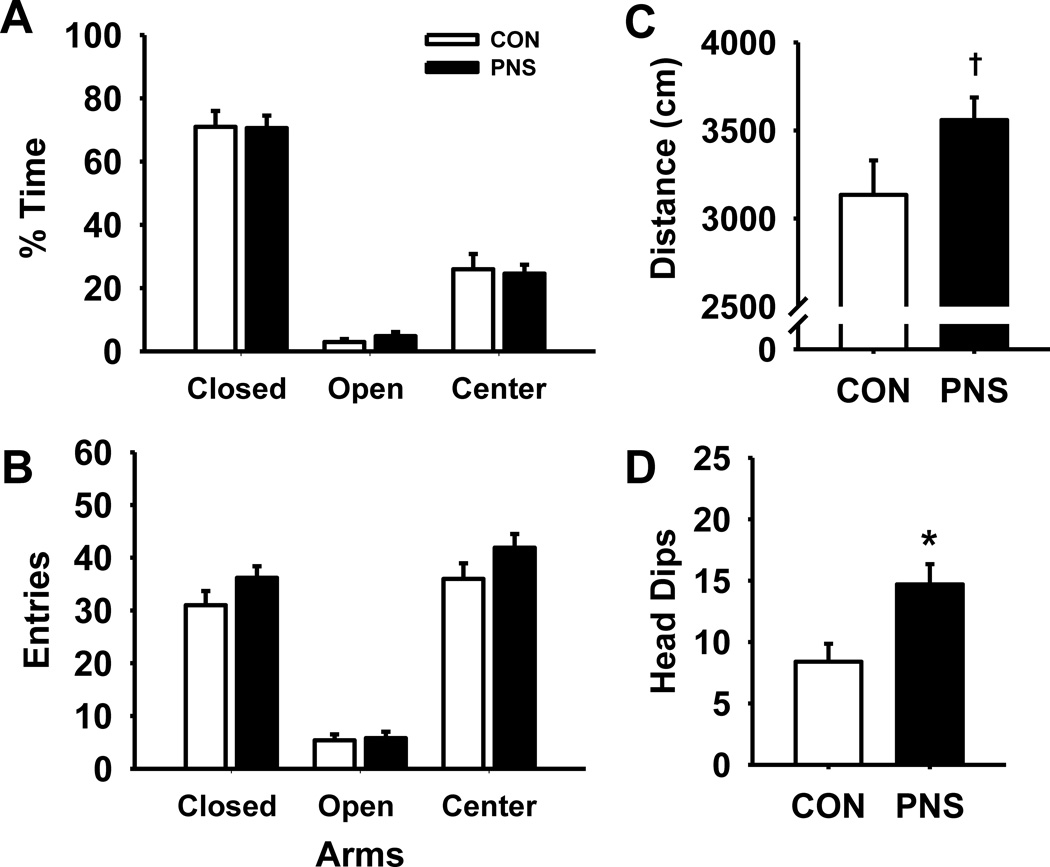
Fig. 3 illustrates the effects of prenatal stress on performance of an EPM task (n=12 per group). While statistical analysis on the percentage of time rats spent in each of the zones (Fig. 3A) revealed a significant main effect of zone F(2,59) = 126.42, p<0.001, the main effect of group F(1,36) = 0.98, p=0.37 and group by zone interaction F(2,59) = 0.07, p=0.93 were not significant. As expected, post hoc analysis indicated that both PNS and control rats spent a significant portion of time in the closed arms compared to the open arms or center zone. Similarly, in the analysis of the number of entries into each of the zones (Fig. 3B) there was a significant effect of zone F(2,59) = 266.50, p<0.001, but not a main effect of group F(1,36) = 2.16, p=0.16 or group by zone interaction F(2,59) = 1.86, p=0.17. Post hoc analysis indicated that there were significantly fewer entries into the open arms compared to the closed arms and center zone by both groups. There was also a trend toward significance differences (t = 1.82, df = 18, p=0.08) in the total distance traveled (Fig. 3C) between PNS and control rats. Additionally, PNS rats had a significant increase in the number of head dips (t = −2.87, df = 18, p<0.05) compared to controls (Fig. 3D).
Fig. 3.
Effects of prenatal stress on anxiety during an elevated plus maze task. (A) % time spent in closed arms, open arms, and center zone, (B) entries into closed arms, open arms, and center zone, (C) total distance traveled, or (D) number of head dips. Each bar represents the mean ± SEM for each test group.* represents a significant difference (p<0.05) in performance between PNS and control rats. † represents a trend toward a significant difference (p < 0.10). PNS = prenatally stressed; CON = non-stressed controls. N = 12.
3.4. Light/dark preference
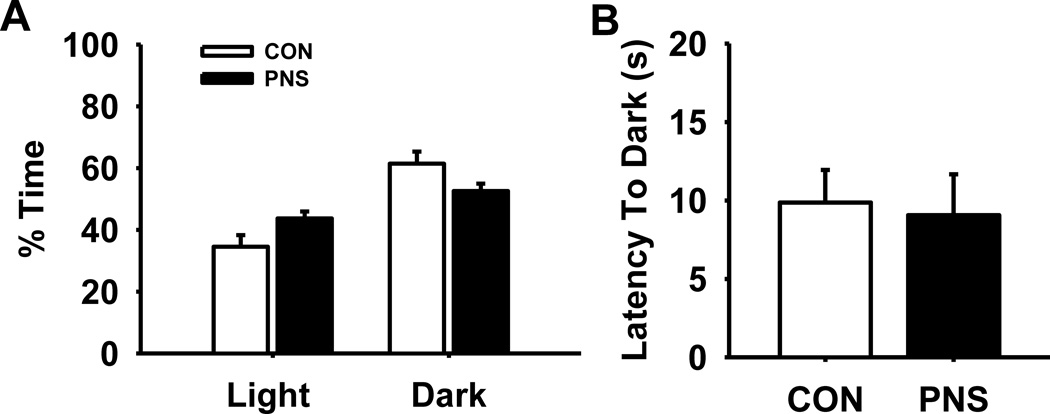
Fig. 4 illustrates the effects of prenatal stress on performance of a light/dark preference task (n=12 per group). Statistical analysis on the % time rats spent in each of the zones (Fig. 4A) indicated a significant effect of zone F(1,47) = 15.91, p<0.001, however the main effect of group F(1,22) = 0.18, p=0.68 and the group by zone interaction F(1,47) = 4.64 were not significant, (p=0.06). As expected, both PNS and control rats spent a modest (but significant) portion of time in the dark zone compared to the light zone. There was no significant difference in latency into the dark zone between PNS and control rats (Fig. 4B).
Fig. 4.
Effects of prenatal stress on anxiety during a light/dark preference task. (A) % time spent in light and dark zones, or (B) latency (s) into the dark zone. Each bar represents the mean ± SEM for each test group. PNS = prenatally stressed; CON = non-stressed controls. N = 12.
3.5. Predatory odor avoidance
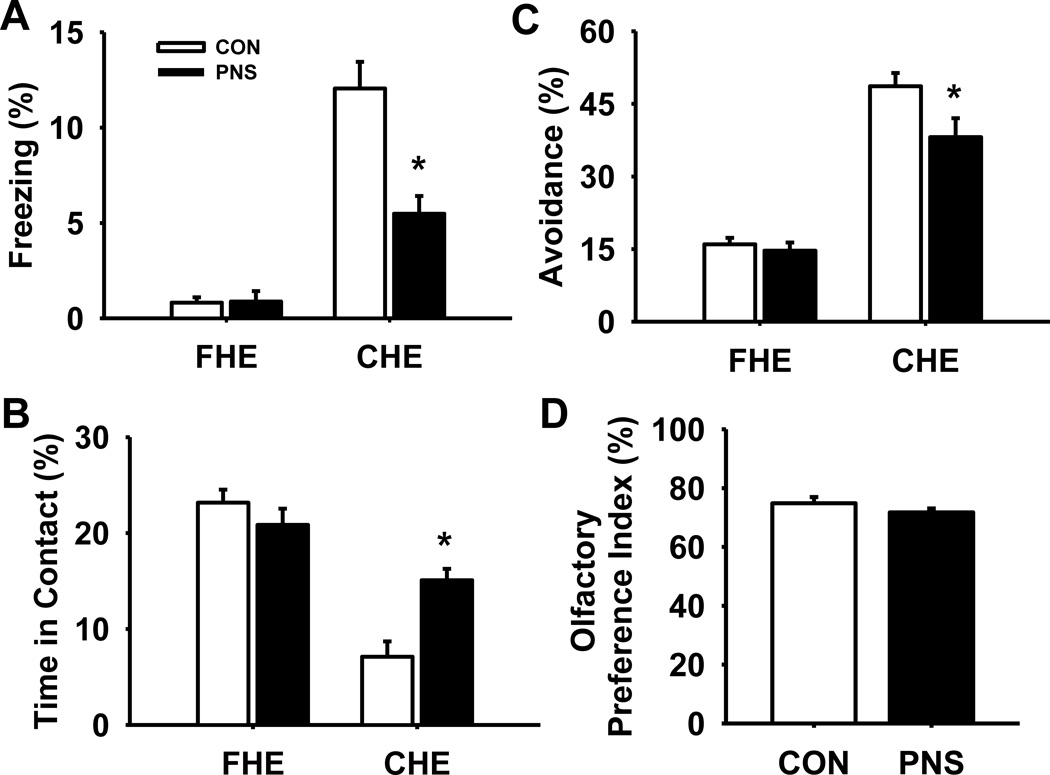
Fig. 5 illustrates the effects of prenatal stress on performance of a predatory odor avoidance task (n=12 per group). Statistical analysis of the percentage of time animals spent freezing (Fig. 5A) revealed a significant main effect of group F(1,22) = 11.53, p<0.05, exposure F(1,47) = 93.44, p<0.001, and group by exposure interaction F(1,47) = 16.36, p<0.001. Post hoc analysis indicated that while there was no significant difference in freezing between PNS and control rats during fake hair exposure, freezing was significantly lower in PNS rats compared to controls during cat hair exposure (p<0.001). Nonetheless, there was a significant increase in freezing of both PNS and control rats during cat hair exposure compared to fake hair exposure. Further, analysis of the % time animals spent in contact with the cat hair or fake hair (Fig. 5B) also indicated a significant main effect of group F(1,22) = 5.66, p<0.05, and exposure F(1,47) = 105.03, p<0.001, without a group by exposure interaction F(1,47) = 2.83, p=0.11. Post hoc analysis indicated that PNS spent significantly more time in contact with the cat hair compared to controls (p=0.007), yet there was no significant difference in time in contact between PNS and control rats during fake hair exposure. Further, there was a significant decrease in time spent in contact with cat hair compared to fake hair in both test groups. Statistical analysis of the percentage of time rats spent avoiding the cat hair or fake hair altogether (i.e., time spent in a quadrant not containing animal hair) (Fig. 5C) revealed the following results, main effect of group F(1,22) = 3.53, p=0.07; exposure, F(1,47) = 57.54, p<0.001; group by exposure interaction F(1,47) = 12.80, p<0.05. Post hoc analysis indicated that PNS rats spent significantly less time avoiding the cat hair compared to control rats (p<0.001), although there was no significant difference in % avoidance between PNS and control rats during fake hair exposure. In addition, there was a significant increase in the time animals spent avoiding the cat hair in both groups compared to % avoidance of fake hair.
Fig. 5.
Effects of prenatal stress on fear response during a predatory odor avoidance task. (A) % time freezing, (B) % time in contact with cat or fake hair, or (C) % avoidance time from hair (i.e., time in opposite quadrant). (D) Olfactory preference index during an odor discrimination task. Each bar represents the mean ± SEM for each test group.* represents a significant difference (p<0.05) in performance between PNS and control rats. PNS = prenatally stressed; CON = non-stressed controls; FHE = faked hair exposure; CHE = cat hair exposure. N = 12.
3.6. Odor discrimination
An odor discrimination task was conducted to ensure that prenatal stress did not result in deficits in olfactory discrimination that might have confounded the predatory odor avoidance task analysis (Fig. 5D). There were no significant differences in olfactory preference index between PNS and control rats, showing the PNS rats had olfactory function similar to controls.
3.7. Contextual fear conditioning
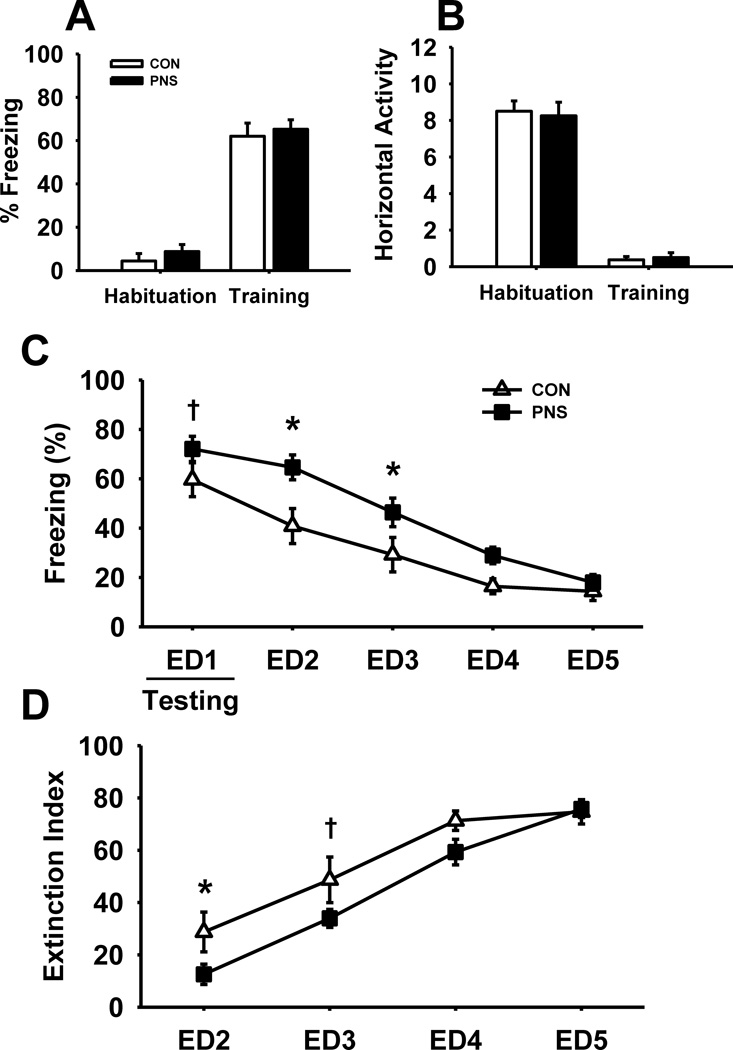
3.7.1. Male rats
Fig. 6 illustrates the effects of prenatal stress on performance of male rats during a contextual fear conditioning task (n=8 per group). As expected, after exposure to foot shocks (i.e., training results) there were significant increases in freezing and decreases in horizontal activity in both test groups. However, there were no differences in performance between the test groups. In addition, there was also no significant difference between PNS or control male rats in vertical activity in either habituation or training (data not shown).
Fig. 6.
Effects of prenatal stress on fear response and extinction during a contextual fear conditioning task in male rats. (A) % time freezing, or (B) horizontal activity during habituation and training of the task. (C) % time freezing throughout extinction, or (D) extinction index for extinction days 2–5. Each bar or point represents the mean ± SEM for each test group.* represents a significant difference (p<0.05) in performance between PNS and control rats. † represents a trend toward a significant difference (p < 0.10). PNS = prenatally stressed; CON = non-stressed controls; ED = extinction day. N = 8.
Statistical analysis of freezing throughout the extinction trials (Fig. 6C) however, did reveal significant group-related differences, main effect of group F(1,56) = 5.56, p<0.05, day F(4,79) = 71.36, p<0.001, with a trend toward a group by day interaction F(4,79) = 2.33, p=0.07. Post hoc analysis indicated significantly higher levels of freezing of PNS male rats on both extinction day (ED) 2 and 3 compared to controls, with a trend toward significance on ED1 (p=0.06). As expected, there was a significant decrease in % freezing of both PNS and control male rats throughout the extinction period. Further, statistical analysis of the extinction index (Fig. 6D) also revealed a significant main effect of group F(1,42) = 4.72, p<0.05, and day F(3,63) = 55.65, p<0.001, without a group by day interaction F(3, 63) = 1.78, p=0.17. Post hoc analysis indicated a significant decrease in the extinction index of PNS male rats compared to controls on ED2, as well as a trend toward significance on ED3 (p=0.06).
In addition to the freezing outcome measure in the extinction experiments, the time spent per arm, entries per arm, and latency to enter shock arm were also assessed (data not shown). As opposed the freezing outcome measure, there was quite a bit of variance in the aforementioned exploratory measures and no statistically significant group × day related interactions were detected.
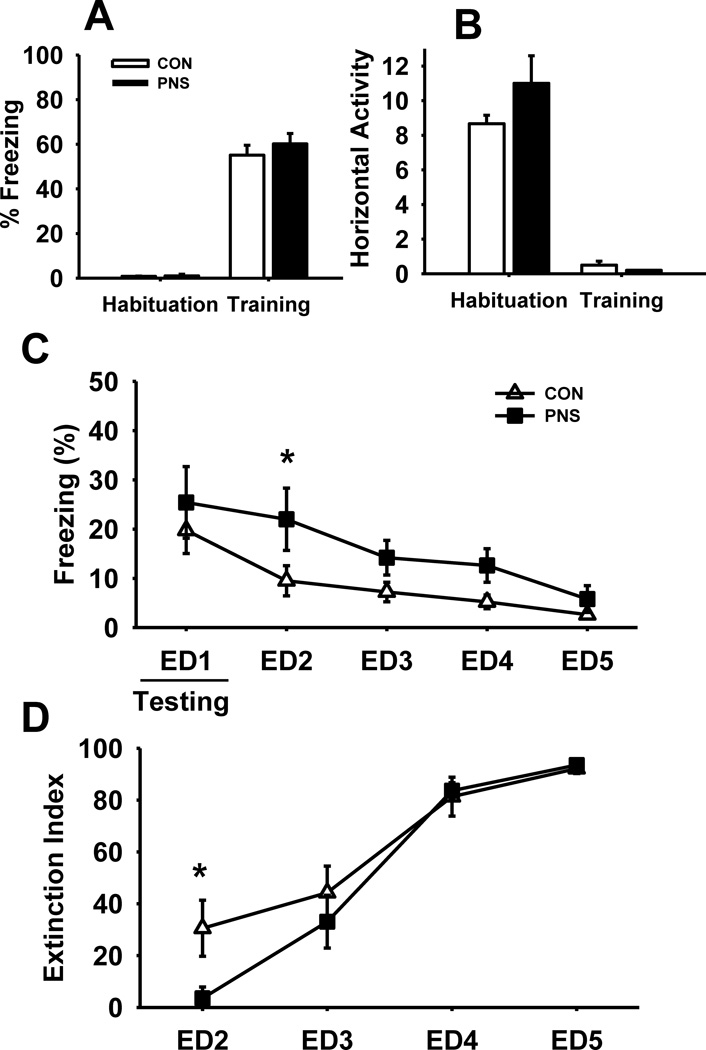
3.7.2. Female rats
Fig. 7 illustrates the effects of prenatal stress on performance of female rats during a Y-maze contextual fear conditioning task. Similar to what was observed in male rats, there was no significant difference in either freezing (Fig. 7A) or horizontal activity (Fig. 7B) between PNS and control female rats during habituation and training.
Fig. 7.
Effects of prenatal stress on fear response and extinction during a contextual fear conditioning task in female rats. (A) % time freezing, or (B) horizontal activity during habituation and training of the task. (C) % time freezing and (D) extinction index throughout extinction. Each bar or point represents the mean ± SEM for each test group.* represents a significant difference (p<0.05) in performance between PNS and control rats. † represents a trend toward a significant difference (p < 0.10). PNS = prenatally stressed; CON = non-stressed controls; ED = extinction day. N = 8.
Similar to PNS male rats, exposure to PNS resulted in impaired extinction in female rats. (Fig. 7C) main effect of group F(1,56) = 5.40, p<0.05, day F(4,79) = 7.05 p<0.001, without a group by day interaction F(4,79) = 0.47, p=0.76. Post hoc analysis indicated a significant increase in freezing of PNS female rats compared to control female rats on ED2. Predictably, there was a significant decrease in freezing of both PNS and control female rats throughout the extinction period. Statistical analysis of the extinction indexes (Fig. 7D) revealed similar results, main effect of group F(1,42) = 0.66, p=0.44; day F(3,63) = 31.41, p<0.001; group by day interaction F(3,63) = 1.73, p=0.18. Post hoc analysis indicated a significant (p<0.05) decrease in the extinction index of PNS female rats on ED2 compared to control females.
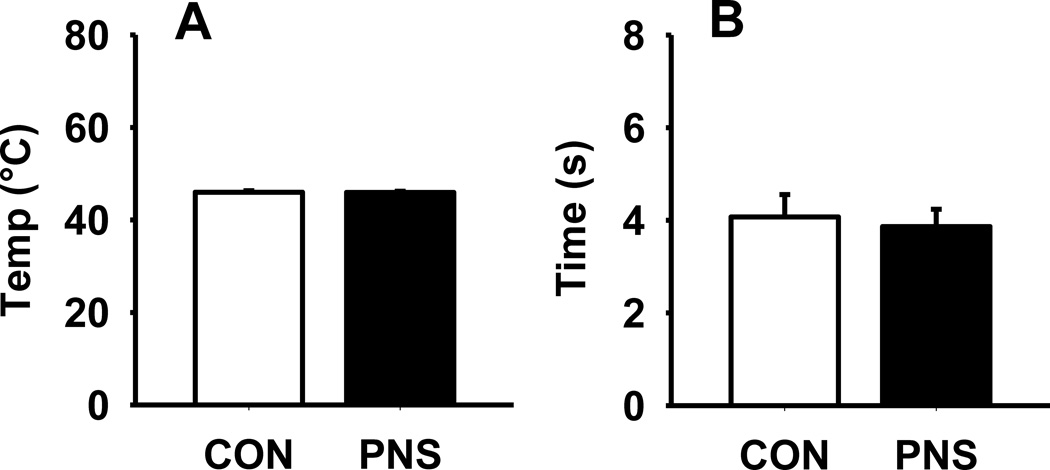
3.8. Nociception
The two nociception task, hot plate and tail flick, were conducted to ensure that prenatal stress did not result in deficits in nociception that might have confounded the contextual fear conditioning task results. During the hot plate task, there was no significant difference in the temperature (°C) at which the animals would lick their hind paws between the PNS and control rats (Fig. 8A). Likewise, during the tail flick task, there was no significant difference between PNS or control rats in the time (s) it took the animals to move their tails (Fig. 8B).
Fig. 8.
Effects of prenatal stress on nociception during a hot plate and tail flick task. (A) Temperature (°C) at which animals licked their hind paws, or (B) time (s) for the animals to remove (flick) their tail. Each bar represents the mean ± SEM for each test group. PNS = prenatally stressed; CON = non-stressed controls. N = 12.
4. Discussion
The results of this study can be summarized as follows: 1) pregnant dams exposed to a variable stress procedure exhibited elevations in corticosterone levels (relative to non-stressed control pregnant dams) late during gestation, 2) the offspring of stressed dams had similar baseline levels of corticosterone to non-stressed controls, however, when exposed to acute stress, they exhibited an increase in corticosterone that was greater in magnitude, 3) the offspring of stressed dams exhibited several behavioral anomalies including decreased innate fear responses to predator odor, impaired fear extinction, increased locomotor activity, and increased stereotypic-like behaviors, 4) there were no indications that exposure to PNS resulted in increased anxiety-like behaviors or deficits in learning or retention during contextual fear conditioning.
The analyses of corticosterone levels in this study were important for several reasons. The perception of physical and psychological challenges prompts a stress response characterized by the activation of the hypothalamic-pituitary-adrenal (HPA) axis [10]. Circulating levels of glucocorticoids (e.g., corticosterone and cortisol) are relatively well-established as markers of HPA axis activation and dysregulation [16]. Further, maternal prenatal glucocorticoids cross the placenta and can influence other aspects of the prenatal environment. Elevated glucocorticoid levels have been associated with several negative outcomes including delayed fetal growth and development, attention and temperament problems, and psychopathology in adulthood [8]. For example, HPA axis dysregulation is a consistent component of several affective disorders including depression, PTSD and other anxiety disorders which are often considered to be stress-related/ stress-initiated [13]. Further, corticosterone acting via glucocorticoid and mineralocorticoid receptors is known influence anxiety-related behaviors, fear learning and memory, as well as extinction [1,3,11,23], i.e., important factors for this study.
As noted above, the initial results of this study indicated that a few stress exposures is not sufficient to elevate corticosterone levels in pregnant dams, as corticosterone levels on GD 16 following stress were not different than baseline levels on GD 13. However, since plasma samples were collected from the pregnant dams following completion of stress (i.e., later in the day), it is possible that the normal diurnal variation in corticosterone levels may have contributed to our results, though were collected at the same time of the day for each animal across time and controls were time-matched [41]. Nonetheless, repeated stress exposures significantly increased corticosterone levels in pregnant dams in response to a subsequent (acute) stressor, as dams exposed to the stress paradigm had significantly higher levels of corticosteroids on GD 19 compared to controls. Importantly, these results support the argument that repeated exposures to variable stressors elicit more intense HPA axis responses in pregnant rats which may affect the developing fetus [50]. Prior to exposure to the variable prenatal stress paradigm, there were no differences in baseline corticosterone levels between either dams exposed to stress or controls. We further observed that on GD 19 (sixth day of stress), both control dams and those exposed to the variable prenatal stress paradigm had a significant increase in plasma corticosterone levels, most likely due to the natural elevation of corticosterone levels that occur during the last days of gestation [18].
Exposure to PNS also resulted in elevated stress responses in the offspring. While offspring of dams exposed to the variable prenatal stress paradigm had similar baseline levels of corticosterone to control offspring, PNS rats had significantly elevated corticosterone levels following exposure to an intense acute stress. As expected, there was a significant increase in corticosterone levels in both groups following stress, however PNS rats had significantly higher levels 30 min post stress compared to controls. These data are consistent with previous findings by Koenig and colleagues as well as studies of rats prenatal exposed to lipopolysaccharide [21,22,26]. These observations further support that idea that exposure to prenatal stress reprograms the HPA axis, resulting in greater elevation of corticosterone following stress [18,33].
Contextual fear conditioning (CFC) is a commonly used model to study fear memory processes of acquisition, consolidation, retrieval and extinction in rodents [47]. Exposure to variable PNS did not result in impaired learning and memory during CFC in male rats, as both groups had similar increases in freezing and reduction in locomotor activity during training and retention of the task. However, exposure to variable PNS resulted in impaired extinction, as PNS male rats maintained higher levels of freezing on ED2 and ED3 (compared to non-stressed controls), as well as a significant decrease in extinction index on ED2. These results support previous studies that reported delayed fear extinction, albeit extinction of cue-conditioned fear, in male rats exposed to prenatal stress [13, 31]. Interestingly, Koenig and colleagues found no evidence of impaired contextual fear extinction in either male or female rats, although this may be due to variations in the behavioral test protocol (e.g., combination of cued and context testing vs. context only) [31]. Extinction of fear is a learned inhibition of previous fear response in lieu of a new set of rules, and thus is thought to be a form of cognitive flexibility [17,53]. Consistent with this interpretation are results from one of our previous studies where variable PNS was associated with impaired cognitive flexibility (i.e., elevated timeout responses) in a 5-choice serial reaction time task [56].
For female rats, a Y-maze version of contextual fear conditioning was used due to low levels of freezing in both the control and PNS groups during the previously described contextual fear conditioning task (data not shown). These results are in accordance with previous literature indicating that female rats do not show conditioned freezing in response to the classical contextual fear conditioning, but displayed increased escape behavior compared to males, suggesting an active response by females as opposed to freezing shown by males [42]. Interestingly, performance of female rats in the Y-maze version of the task was similar to those seen in males during the standard version of the task (although females had higher variability). While exposure to variable PNS did not result in impaired learning and memory in the fear conditioning task, PNS females had impaired extinction, with increased freezing on ED2, as well as a significant decrease in extinction index. As we did not control for the female rats estrous cycle, it is possible that some of the variability observed in female rats may be due to high or low levels of estrogen. Importantly, Milad and colleagues previously observed that depending on the phase of the estrous cycle, there were significant differences in fear extinction [35]. The results in the contextual fear conditioning studies did not appear to be confounded by alterations of nociception, as there was no differences between PNS males or females and non-stressed controls in either the hot plate task (supraspinal nociception) or tail flick task (spinal nociception).
Exposure to predator odor (cat or fox odor), a natural unconditioned stimulus, results in a range of fear responses in prey animals including freezing and avoidance [4,7,46]. As expected, exposure to cat hair produced significant fear responses in both PNS and non-stressed control groups with increased freezing and avoidance as well as decreased time in contact compared to exposure to fake hair. However, rats exposed to variable PNS showed a significant reduction in freezing and avoidance compared to controls. Additionally, PNS rats spent significantly more time in contact with the cat hair than controls, suggesting that exposure to variable PNS decreases innate fear responses to predator odor. Similar results in olfactory preference indices of both groups suggest that alterations in fear response are not due to PNS-induced impairments in odor discrimination. It is important to note however, that a previous study by Dielenberg and colleagues found strong and selective activation of the posteroventral medial amygdala in rats exposed to cat odor, raising the possibility that cat odor is processed as a pheromone-like stimulus instead of as a conventional odor stimulus [5]. Further, these results do not seem to be due to the inability to freeze, a possible result of elevated locomotor activity, as exposure to a foot shock produced similar freezing in both groups. However, it has been previously found that conditioned freezing to cat hair context was 4 times less than conditioned freezing to foot shock context, suggesting that exposure to cat hair is a mild stressor compared to foot shock [38]. Interestingly, it has been found that noradrenergic transmission is critical for the expression of innate fear responses to predator odor [6]. In a previous study we suggested (based on positive effects of the norepinephrine reuptake inhibitor, atomoxetine, on sustained attention) that exposure to variable PNS may result in altered noradrenergic neurotransmission [56]. Thus, the decrease in innate fear responses observed in PNS rats in the current study may be due to changes in noradrenergic transmission.
The open field task is a popular model for assessing spontaneous locomotor activity, stereotypic activity, exploratory behavior, and anxiety-like behaviors [40]. During the open field task, exposure to PNS significantly increased spontaneous locomotor activity relative to controls; however PNS rats did not exhibit altered habituation to the novel environment. In addition, exposure to PNS also resulted in a significant increase in stereotypic-like behaviors. This is important as hyperactivity as well as increases in stereotypic-like behaviors are symptoms of several neuropsychiatric disorders (e.g., schizophrenia, ADHD, autism) [12,36,39]. Surprisingly, PNS rats exhibited significantly lower vertical exploratory activity (vertical counts) compared to controls. These results are very similar to those seen in rats prenatally exposed to valproic acid, an animal model of autism [48]. It may be possible though, that the decrease in vertical exploratory behavior is simply a consequence of increased non goal-directed locomotor activity and increased stereotypic activity [44].
The deficits in innate fear and fear extinction in PNS rats (noted above) were not paralleled by significant alterations in anxiety levels. For example, there was no evidence in either the PNS or control rats of thigmotaxis (innate behavioral response in rodents to stay close to the walls [40]) during the open field task as both groups spent significantly more time in the center zone than in the periphery. In the elevated plus maze, exposure to variable PNS did not result in any significant differences in entries or time spent in open arms compared to controls. Interestingly, PNS rats had a significant increase in the number of head dips (ethological measurement of “risk assessment”) compared to controls, thus suggesting that variable PNS may increase risk assessment behaviors. However, these results may be confounded by the increase in locomotor activity of PNS rats, as seen previously (open field) and further supported by the trend toward a significant increase in total distance traveled during the EPM task. Additionally, there was no indication of increased anxiety due to exposure of PNS during light/dark preference task, as there were no significant differences in the latencies into the dark zone or time spent in the light/dark compartments between control and PNS rats. The lack of an increase in anxiety-like behavior in PNS rats (in the presence of elevated corticosterone) would appear to contrast with studies which show that prenatal exposure to the synthetic glucocorticoid dexamethasone, increases anxiety-like behaviors in rats. Further our studies would also appear to contrast with observations of rats prenatally exposed to lipopolysaccharide which resulted in elevations of anxiety-like behaviors and increases in stress-induced corticosterone responses in adult rats. [26, 37]. However, direct comparisons between these studies is difficult and can be misleading, as there are at least a couple of substantial differences produced by the experimental models that are relevant to the interpretation of anxiety behaviors. First, prenatal LPS infusions did not alter general locomotion in an open field of a size comparable to that used in our study (45×45 vs. 43.5×43.5 cm in our study), while we observed increased locomotion in prenatally stressed rats (figure 2). An impaired ability to suppress locomotion could lead to a false negative result in the center measure of the open field and in the EPM. Secondly, in the study of dexamethasone, animals were placed in the dark zone of the test apparatus (in the current study they were placed in the lit zone). In previous preliminary studies done in our lab, we found that placing experimental rats in the dark zone resulted in the reduced likelihood of the animal emerging and exploring the lit zone which could lead to misinterpretation of the results.
In conclusion, the results of this study suggest that exposure to variable PNS alters stress and fear responses in rats, effects that may be due in part to altered HPA axis function. In addition, PNS was associated with impaired fear extinction (evidence of deficits in cognitive flexibility), increased locomotor activity, and increased stereotypic-like behaviors. These observations along with previous reports of impairments in attention, inhibitory control, object recognition memory and spatial reference memory [22,31,52] suggest that phenomenological similarities exist between prenatally stressed rodents and humans afflicted with neuropsychiatric disorders (e.g., schizophrenia). Such observations further support the face validity of this model system for studying several aspects of these conditions.
Research Highlights.
Prenatal stress leads to decreased innate fear responses to predator odor
Prenatal stress leads impaired fear extinction
Prenatal stress leads to increased locomotor activity and stereotypy
Prenatal stress was not associated with increased anxiety-like behaviors
Acknowledgements
The experiments described in this manuscript were supported in part by grants from the National Institute on Drug Abuse (DA029127), the National Institute of Environmental Health Sciences (ES012241), and the National Institute on Aging (AG029617).
Abbreviations
- PNS
prenatal stress
- ADHD
attention deficit/hyperactivity disorder
- PTSD
post traumatic stress disorder
- HPA
hypothalamic-pituitary-adrenal
- EPM
elevated plus maze
- CFC
contextual fear conditioning
- GD
gestational day
- PND
postnatal day
- ED
extinction day
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Brinks V, Berger S, Gass P, de Kloet ER, Oitzl MS. Mineralocorticoid receptors in control of emotional arousal and fear memory. Horm Behav. 2009;56(2):232–238. doi: 10.1016/j.yhbeh.2009.05.003. [DOI] [PubMed] [Google Scholar]
- 2.Davis EP, Sandman CA. Prenatal psychobiological predictors of anxiety risk in preadolescent children. Psychoneuroendocrinology. 2012;37(8):1224–1233. doi: 10.1016/j.psyneuen.2011.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.de Kloet ER, Oitzl MS, Joëls M. Stress and cognition: are corticosteroids good or bad guys? Trends Neurosci. 1999;22(10):422–426. doi: 10.1016/s0166-2236(99)01438-1. [DOI] [PubMed] [Google Scholar]
- 4.Dielenberg RA, McGregor IS. Defensive behavior in rats towards predatory odors: a review. Neurosci Biobehav Rev. 2001;25(7–8):597–609. doi: 10.1016/s0149-7634(01)00044-6. [DOI] [PubMed] [Google Scholar]
- 5.Dielenberg RA, Hunt GE, McGregor IS. "When a rat smells a cat": the distribution of Fos immunoreactivity in rat brain following exposure to a predatory odor. Neuroscience. 2001;104(4):1085–1097. doi: 10.1016/s0306-4522(01)00150-6. [DOI] [PubMed] [Google Scholar]
- 6.Do Monte FH, Canteras NS, Fernandes D, Assreuy J, Carobrez AP. New perspectives on beta-adrenergic mediation of innate and learned fear responses to predator odor. J Neurosci. 2008;28(49):13296–13302. doi: 10.1523/JNEUROSCI.2843-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dringenberg HC, Oliveira D, Habib D. Predator (cat hair)-induced enhancement of hippocampal long-term potentiation in rats: involvement of acetylcholine. Learn Mem. 2008;15:112–116. doi: 10.1101/lm.778108. [DOI] [PubMed] [Google Scholar]
- 8.Field T, Diego M. Cortisol: the culprit prenatal stress variable. Int J Neurosci. 2008;118(8):1181–1205. doi: 10.1080/00207450701820944. [DOI] [PubMed] [Google Scholar]
- 9.Fossati P, Amar G, Raoux N, Ergis AM, Allilaire JF. Executive functioning and verbal memory in young patients with unipolar depression and schizophrenia. Psychiatry Res. 1999;89:171–187. doi: 10.1016/s0165-1781(99)00110-9. [DOI] [PubMed] [Google Scholar]
- 10.Glover V, O'Connor TG, O'Donnell K. Prenatal stress and the programming of the HPA axis. Neurosci Biobehav Rev. 2010;35(1):17–22. doi: 10.1016/j.neubiorev.2009.11.008. [DOI] [PubMed] [Google Scholar]
- 11.Gourley SL, Kedves AT, Olausson P, Taylor JR. A history of corticosterone exposure regulates fear extinction and cortical NR2B, GluR2/3, and BDNF. Neuropsychopharmacology. 2009;34(3):707–716. doi: 10.1038/npp.2008.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grizenko N, Shayan YR, Polotskaia A, Ter-Stepanian M, Joober R. Relation of maternal stress during pregnancy to symptom severity and response to treatment in children with ADHD. Rev Psychiatr Neurosci. 2008;33(1):10–16. [PMC free article] [PubMed] [Google Scholar]
- 13.Green MK, Rani CS, Joshi A, Soto-Piña AE, Martinez PA, Frazer A, Strong R, Morilak DA. Prenatal stress induces long term stress vulnerability, compromising stress response systems in the brain and impairing extinction of conditioned fear after adult stress. Neuroscience. 2011;192:438–451. doi: 10.1016/j.neuroscience.2011.06.041. [DOI] [PubMed] [Google Scholar]
- 14.Guest PC, Martins-de-Souza D, Vanattou-Saifoudine N, Harris LW, Bahn S. Abnormalities in metabolism and hypothalamic-pituitary-adrenal axis function in schizophrenia. Int Rev Neurobiol. 2011;101:145–168. doi: 10.1016/B978-0-12-387718-5.00006-7. [DOI] [PubMed] [Google Scholar]
- 15.Holt DJ, Lebron-Milad K, Milad MR, Rauch SL, Pitman RK, Orr SP, Cassidy BS, Walsh JP, Goff DC. Extinction memory is impaired in schizophrenia. Biol Psychiatry. 2009;65(6):455–463. doi: 10.1016/j.biopsych.2008.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Joëls M. Impact of glucocorticoids on brain function: relevance for mood disorders. Psychoneuroendocrinology. 2011;36(3):406–414. doi: 10.1016/j.psyneuen.2010.03.004. [DOI] [PubMed] [Google Scholar]
- 17.Kaczorowski CC, Davis SJ, Moyer JR., Jr Aging redistributes medial prefrontal neuronal excitability and impedes extinction of trace fear conditioning. Neurobiol Aging. 2012;33(8):1744–1757. doi: 10.1016/j.neurobiolaging.2011.03.020. [DOI] [PubMed] [Google Scholar]
- 18.Kapoor A, Dunn E, Kostaki A, Andrews MH, Matthews SG. Fetal programming of hypothalamo-pituitary-adrenal function: prenatal stress and glucocorticoids. J Physiol. 2006;572(1):31–44. doi: 10.1113/jphysiol.2006.105254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Koenen KC, Driver KL, Oscar-Berman M, Wolfe J, Folsom S, Huang MT, Schlesinger L. Measures of prefrontal system dysfunction in posttraumatic stress disorder. Brain Cogn. 2001;45:64–78. doi: 10.1006/brcg.2000.1256. [DOI] [PubMed] [Google Scholar]
- 20.Kinney DK, Munir KM, Crowley DJ, Miller AM. Prenatal stress and risk for autism. Neurosci Biobehav Rev. 2008;32(8):1519–1532. doi: 10.1016/j.neubiorev.2008.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kinnunen AK, Koenig JI, Bilbe G. Repeated variable prenatal stress alters pre- and postsynaptic gene expression in the rat frontal pole. J Neurochem. 2003;86:736–748. doi: 10.1046/j.1471-4159.2003.01873.x. [DOI] [PubMed] [Google Scholar]
- 22.Koenig JI, Elmer GI, Shepard PD, Lee PR, Mayo C, Joy B, Hercher E, Brady DL. Prenatal exposure to a repeated variable stress paradigm elicits behavioral and neuroendocrinological changes in the adult offspring: potential relevance to schizophrenia. Behav Brain Res. 2005;156:251–261. doi: 10.1016/j.bbr.2004.05.030. [DOI] [PubMed] [Google Scholar]
- 23.Korte SM. Corticosteroids in relation to fear, anxiety and psychopathology. Neurosci Biobehav Rev. 2001;25(2):117–142. doi: 10.1016/s0149-7634(01)00002-1. [DOI] [PubMed] [Google Scholar]
- 24.Lee PR, Brady D, Shapiro RA, Dorsa DM, Koenig JI. Prenatal stress generates deficits in rat social behavior: reversal by oxytocin. Brain Res. 2007;1156:152–167. doi: 10.1016/j.brainres.2007.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lewis DA, Levitt P. Schizophrenia as a disorder of neurodevelopment. Annu Rev Neurosci. 2002;25:409–432. doi: 10.1146/annurev.neuro.25.112701.142754. [DOI] [PubMed] [Google Scholar]
- 26.Lin YL, Lin SY, Wang S. Prenatal lipopolysaccharide exposure increases anxiety-like behaviors and enhances stress-induced corticosterone responses in adult rats. Brain Behav Immun. 2012;26(3):459–468. doi: 10.1016/j.bbi.2011.12.003. [DOI] [PubMed] [Google Scholar]
- 27.Linnet KM, Dalsgaard S, Obel C, Wisborg K, Henriksen TB, Rodriguez A, Kotimaa A, Moilanen I, Thomsen PH, Olsen J, Jarvelin MR. Maternal lifestyle factors in pregnancy risk of attention deficit hyperactivity disorder and associated behaviors: review of the current evidence. Am J Psychiatry. 2003;160:1028–1040. doi: 10.1176/appi.ajp.160.6.1028. [DOI] [PubMed] [Google Scholar]
- 28.Ma L, Chen YH, Chen H, Liu YY, Wang YX. The function of hypothalamus-pituitaryadrenal axis in children with ADHD. Brain Res. 2011;1368:159–162. doi: 10.1016/j.brainres.2010.10.045. [DOI] [PubMed] [Google Scholar]
- 29.Marco EM, Macrì S, Laviola G. Critical age windows for neurodevelopmental psychiatric disorders: evidence from animal models. Neurotox Res. 2011;19(2):286–307. doi: 10.1007/s12640-010-9205-z. [DOI] [PubMed] [Google Scholar]
- 30.Markham JA, Koenig JI. Prenatal stress: Role in psychotic and depressive diseases. Psychopharmacology. 2011;214(1):89–106. doi: 10.1007/s00213-010-2035-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Markham JA, Taylor AR, Taylor SB, Bell DB, Koenig JI. Characterization of the cognitive impairments induced by prenatal exposure to stress in the rat. Front Behav Neurosci. 2012;4:173. doi: 10.3389/fnbeh.2010.00173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Martínez KG, Quirk GJ. Extending fear extinction beyond anxiety disorders. Biol Psychiatry. 2009;65(6):453–454. doi: 10.1016/j.biopsych.2008.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McCormick CM, Smythe JW, Sharma S, Meaney MJ. Sex-specific effects of prenatal stress on hypothalamic–pituitary–adrenal responses to stress and brain glucocorticoid receptor density in adult rats. Brain Res Dev Brain Res. 1995;84:55–61. doi: 10.1016/0165-3806(94)00153-q. [DOI] [PubMed] [Google Scholar]
- 34.McEown K, Treit D. Mineralocorticoid receptors in the medial prefrontal cortex and hippocampus mediate rats' unconditioned fear behaviour. Horm Behav. 2011;60(5):581–588. doi: 10.1016/j.yhbeh.2011.08.007. [DOI] [PubMed] [Google Scholar]
- 35.Milad MR, Igoe SA, Lebron-Milad K, Novales JE. Estrous cycle phase and gonadal hormones influence conditioned fear extinction. Neuroscience. 2009;164(3):887–895. doi: 10.1016/j.neuroscience.2009.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Morrens M, Hulstijn W, Lewi PJ, De Hert M, Sabbe BG. Stereotypy in schizophrenia. Schizophr Res. 2006;84(2–3):397–404. doi: 10.1016/j.schres.2006.01.024. [DOI] [PubMed] [Google Scholar]
- 37.Nagano M, Ozawa H, Suzuki H. Prenatal dexamethasone exposure affects anxiety-like behaviour and neuroendocrine systems in an age-dependent manner. Neurosci Res. 2008;60(4):364–371. doi: 10.1016/j.neures.2007.12.005. [DOI] [PubMed] [Google Scholar]
- 38.Nalloor R, Bunting K, Vazdarjanova A. Predicting impaired extinction of traumatic memory and elevated startle. PLoS One. 2011;6(5):e19760. doi: 10.1371/journal.pone.0019760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pierce K, Courchesne E. Evidence for a cerebellar role in reduced exploration and stereotyped behavior in autism. Biol Psychiatry. 2001;49:655–664. doi: 10.1016/s0006-3223(00)01008-8. [DOI] [PubMed] [Google Scholar]
- 40.Prut L, Belzung C. The open field as a paradigm to measure the effects of drugs on anxiety-like behaviors: a review. Eur J Pharmacol. 2003;463(1–3):3–33. doi: 10.1016/s0014-2999(03)01272-x. [DOI] [PubMed] [Google Scholar]
- 41.Qian X, Droste SK, Lightman SL, Reul JM, Linthorst AC. Circadian and Ultradian Rhythms of Free Glucocorticoid Hormone Are Highly Synchronized between the Blood, the Subcutaneous Tissue, and the Brain. Endocrinology. 2012;153(9):4346–4353. doi: 10.1210/en.2012-1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ribeiro AM, Barbosa FF, Godinho MR, Fernandes VS, Munguba H, Melo TG, Barbosa MT, Eufrasio RA, Cabral A, Izídio GS, Silva RH. Sex differences in aversive memory in rats: possible role of extinction and reactive emotional factors. Brain Cogn. 2010;74(2):145–151. doi: 10.1016/j.bandc.2010.07.012. [DOI] [PubMed] [Google Scholar]
- 43.Rice F, Jones I, Thapar A. The impact of gestational stress and prenatal growth on emotional problems in offspring: a review. Acta Psychiatr Scand. 2007;115:171–183. doi: 10.1111/j.1600-0447.2006.00895.x. [DOI] [PubMed] [Google Scholar]
- 44.Roth KA, Katz RJ. Stress, behavioral arousal, and open field activity--a reexamination of emotionality in the rat. Neurosci Biobehav Rev. 1979;3(4):247–263. doi: 10.1016/0149-7634(79)90012-5. [DOI] [PubMed] [Google Scholar]
- 45.Roozendaal B. Stress and memory: opposing effects of glucocorticoids on memory consolidation and memory retrieval. Neurobiol Learn Mem. 2002;78:578–595. doi: 10.1006/nlme.2002.4080. [DOI] [PubMed] [Google Scholar]
- 46.Rosen JB, Pagani JH, Rolla KL, Davis C. Analysis of behavioral constraints and the neuroanatomy of fear to the predator odor trimethylthiazoline: a model for animal phobias. Neurosci Biobehav Rev. 2008;32(7):1267–1276. doi: 10.1016/j.neubiorev.2008.05.006. [DOI] [PubMed] [Google Scholar]
- 47.Rudy JW, Huff NC, Matus-Amat P. Understanding contextual fear conditioning: insights from a two-process model. Neurosci Biobehav Rev. 2004;28(7):675–685. doi: 10.1016/j.neubiorev.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 48.Schneider T, Przewłocki R. Behavioral alterations in rats prenatally exposed to valproic acid: animal model of autism. Neuropsychopharmacology. 2005;30(1):80–89. doi: 10.1038/sj.npp.1300518. [DOI] [PubMed] [Google Scholar]
- 49.Spauwen J, Krabbendam L, Lieb R, Wittchen HU, van Os J. Early maternal stress and health behaviours and offspring expression of psychosis in adolescence. Acta Psychiatr Scand. 2004;110:356–364. doi: 10.1111/j.1600-0447.2004.00429.x. [DOI] [PubMed] [Google Scholar]
- 50.Takahashi LK, Turner JG, Kalin NH. Prolonged stress-induced elevation in plasma corticosterone during pregnancy in the rat: implications for prenatal stress studies. Psychoneuroendocrinology. 1998;23:571–581. doi: 10.1016/s0306-4530(98)00024-9. [DOI] [PubMed] [Google Scholar]
- 51.van Os J, Selten J. Prenatal exposure to maternal stress and subsequent schizophrenia. The May 1940 invasion of The Netherlands. Br J Psychiatry. 1998;172:324–326. doi: 10.1192/bjp.172.4.324. [DOI] [PubMed] [Google Scholar]
- 52.Vazdarjanova A, McGaugh JL. Basolateral amygdala is not critical for cognitive memory of contextual fear conditioning. Proc Natl Acad Sci U S A. 1998;95(25):15003–15007. doi: 10.1073/pnas.95.25.15003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Vazdarjanova A, Bunting K, Muthusamy N, Bergson C. Calcyon upregulation in adolescence impairs response inhibition and working memory in adulthood. Mol Psychiatry. 2011;16(6):672–684. doi: 10.1038/mp.2011.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wingenfeld K, Wolf OT. HPA axis alterations in mental disorders: impact on memory and its relevance for therapeutic interventions. CNS Neurosci Ther. 2011;17(6):714–722. doi: 10.1111/j.1755-5949.2010.00207.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wilson C, Terry AV., Jr Neurodevelopmental Animal Models of Schizophrenia: Role in Novel Drug Discovery and Development. Clinical Schizophrenia & Related Psychosis. 2010;4(2):124–137. doi: 10.3371/CSRP.4.2.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wilson C, Schade R, Terry AV., Jr Variable prenatal stress results in impairments of sustained attention and inhibitory response control in a 5-choice serial reaction time task in rats. Neuroscience. 2012;218:126–137. doi: 10.1016/j.neuroscience.2012.05.040. [DOI] [PMC free article] [PubMed] [Google Scholar]