Abstract
Object
The authors performed a study to determine if lesion expansion occurs in humans during the early hours after spinal cord injury (SCI), as has been established in rodent models of SCI, and to identify factors that might predict lesion expansion.
Methods
The authors studied 42 patients with acute cervical SCI and admission American Spinal Injury Association Impairment Scale Grades A (35 patients) and B (7 patients) in whom 2 consecutive MRI scans were obtained 3-134 hours after trauma. They recorded demographic data, clinical information, Injury Severity Score (ISS), admission MRI-documented spinal canal and cord characteristics, and management strategies.
Results
The characteristics of the cohort were as follows: male/female ratio 37:5; mean age, 34.6 years; and cause of injury, motor vehicle collision, falls, and sport injuries in 40 of 42 cases. The first MRI study was performed 6.8 ± 2.7 hours (mean ± SD) after injury, and the second was performed 54.5 ± 32.3 hours after injury. The rostrocaudal intramedullary length of the lesion on the first MRI scan was 59.2 ± 16.1 mm, whereas its length on the second was 88.5 ± 31.9 mm. The principal factors associated with lesion length on the first MRI study were the time between injury and imaging (p = 0.05) and the time to decompression (p = 0.03). The lesion’s rate of rostrocaudal intramedullary expansion in the interval between the first and second MRI was 0.9 ± 0.8 mm/hour. The principal factors associated with the rate of expansion were the maximum spinal cord compression (p = 0.03) and the mechanism of injury (p = 0.05).
Conclusions
Spinal cord injury in humans is characterized by lesion expansion during the hours following trauma. Lesion expansion has a positive relationship with spinal cord compression and may be mitigated by early surgical decompression. Lesion expansion may be a novel surrogate measure by which to assess therapeutic effects in surgical or drug trials.
Keywords: trauma, spinal cord injury, magnetic resonance imaging, American Spinal Injury Association Impairment Scale, lesion expansion
Spinal cord injury remains one of the foremost unsolved challenges in medicine. Worldwide, the incidence of SCI ranges from 10 to 83 per million people per year, with half of these individuals suffering a complete injury and one-third becoming tetraplegic.55 At present, little can be done to undo or repair the initial damage to spinal cord tissues, but great hope lies in stopping the chain of molecular events triggered by primary injury that are believed to complicate the damage and worsen outcome.
Preclinical studies in rodent models of SCI have revealed a mechanism of secondary injury that is unique to the CNS. During the hours following injury, a dynamic process ensues wherein a hemorrhagic contusion enlarges progressively, resulting in the autodestruction of spinal cord tissues.27,50,52 Individual, discrete petechial hemorrhages appear, first around the site of injury, then in more distant areas.33 As petechial hemorrhages continue to form and coalesce, the lesion gradually expands, exhibiting a characteristic region of hemorrhage that “caps” the advancing front of the lesion. A small hemorrhagic lesion that initially involves primarily the capillary-rich gray matter enlarges several-fold during a period of 3–24 hours after injury.6,32 The advancing hemorrhage results from delayed progressive catastrophic failure of the structural integrity of capillaries, a phenomenon termed “progressive hemorrhagic necrosis.”50,51
Although well established in experimental SCI, the concept of lesion expansion in humans with SCI has not been validated. Histopathological studies have shown findings consistent with lesion enlargement that occurs primarily in the first 24 hours after trauma.22 Magnetic resonance imaging has shown that patients with complete SCI have significantly longer intramedullary lesions, greater MCC, and greater MSCC than patients with incomplete injuries.40 However, lesion expansion in humans remains poorly characterized. To our knowledge, no MRI study involving human SCI has examined serial images obtained in individual patients, which would be required to characterize the temporal evolution of a dynamic pathological process.
We hypothesized that, in patients with severe cervical SCI, the intramedullary lesion observed on MRI would expand at a definable rate. We sought to define the rate of expansion of intramedullary lesions in patients with cervical motor-complete SCI and to assess factors associated with lesion expansion. Our data confirm that lesion expansion occurs in humans, suggesting that, if expansion could be halted, final lesion sizes could be reduced.
Methods
Table 1 lists all the nonstandard acronyms used in this paper.
TABLE 1. Summary of nonstandard abbreviations.
| Abbreviation | Definition |
|---|---|
| Di | midsagittal diameter of the spinal canal at injury site |
| Da | midsagittal diameter of the spinal canal above injury site |
| Db | midsagittal diameter of the spinal canal below injury site |
| di | anteroposterior diameter of the spinal cord at injury site |
| da | anteroposterior diameter of the spinal cord above injury site |
| db | anteroposterior diameter of the spinal cord below injury site |
| Interval-I | time btwn trauma & 1st MRI study |
| Interval-II | time btwn trauma & 2nd MRI study |
| LL1 | rostrocaudal intramedullary lesion length measured on the 1st MRI study |
| LL2 | rostrocaudal intramedullary lesion length measured on the 2nd MRI study |
| MCC | maximal canal compromise |
| MSCC | maximal spinal cord compression |
Study Population
This study was approved by the Institutional Review Board of the University of Maryland School of Medicine. From March 1, 2004 through July 30, 2010, 101 patients with motor complete SCI were admitted to the R Adams Cowley Shock Trauma Center of the University of Maryland. Of these, 42 patients were eligible for the present study. Patients were included if they had the following: 1) motor complete SCI, with ASIA Impairment Scale Grades A and B; 2) known exact scene, injury, transfer, imaging studies, traction, and surgical intervention timing; 3) known ASIA impairment and ASIA motor examinations at several time points following injury; 4) 2 consecutive MRI studies within 6 days of trauma; and 5) a well-defined intramedullary lesion on T2-weighted or STIR MRI studies. Patients with penetrating cervical spinal cord trauma or impairment reflected by ASIA grades of C, D, or E were excluded.
Transfer of the patients to the Shock Trauma Center occurred directly from the scene of accident in 33 cases, with a mean scene time of 1 hour in 26 patients. Prehospital, trauma resuscitation unit, critical care, surgical, and rehabilitation management were performed according to the Guidelines for Advanced Trauma Life Support, the Brain Trauma Foundation, and Guidelines for the Management of Acute Cervical Spine and Spinal Cord Injuries.3,28 Computed tomography studies of the cervical spine were obtained in all patients at a mean of 3.6 ± 2.7 hours (range 1.2-11 hours) after trauma. Short-term methylprednisolone therapy was administered in 34 patients.8 Since the beginning of debate about methylprednisolone treatment of acute SCI,29-31 the University of Maryland has slowly adopted as “an option” the use of steroids for acute SCI. At present, steroid agent use for cervical SCI is only at the level of an “option” and only for younger patients with severe injury to the spinal cord. Thirty-four of 42 patients underwent circumferential (ACDF or corpectomy) decompression, alignment, and internal fixation of the cervical spine. Seven patients underwent ACDF or corpectomy and 1 patient underwent only laminectomy and fusion. The mean interval between injury and surgical intervention was 16.2 ± 6.7 hours (range 7.2–44 hours). Decompression was conducted within 12 hours of injury in 13 patients and between 12.5 and 44 hours in 29 patients (Table 2).
TABLE 2. Characteristics of 42 patients with motor complete subaxial cervical SCIs*.
| Variable | Value |
|---|---|
| demographics | |
| male sex | 37 (88) |
| mean age (yrs) | 34.6 ± 15 |
| mechanism of injury | |
| motor vehicle crash | 24 (57.1) |
| fall | 9 (21.4) |
| sport | 7 (16.7) |
| other | 2 (4.8) |
| clinical characteristics | |
| admission ASIA motor score | 12.7 ± 11.2 |
| admission ASIA impairment grade | |
| A | 35 |
| B | 7 |
| time btwn injury & surgery (hrs) | 16.2 ± 6.7 |
| surgical decompression | |
| circumferential† | 34 |
| ACDF or corpectomy + fusion | 7 |
| laminectomy & fusion | 1 |
| injury severity | |
| ISS | 41.3 ± 18 |
| midsagittal diameter of spinal canal at injury site (mm) | 6.9 ± 2.2 |
| MCC (%) | 47.1 ± 15.3 |
| MSCC (%) | 7.9 ± 27 |
| Interval-I (hrs) | 6.8 ± 2.7 |
| intramedullary lesion length (mm) on 1st MRI (LL1) | 59.2 ± 16.1 |
| interval-II (hrs) | 54.5 ± 32.3 |
| intramedullary lesion length (mm) on 2nd MRI (LL2) | 88.5 ± 31.9 |
| Interval-II − Interval-I (hours) | 47.7 ± 32.4 |
| LL2 − LL1 (mm) | 30.3 ± 22.8 |
| rate of expansion btwn Interval-I & -II (mm/hr) | 0.9 ± 0.8 |
| timing of surgical decompression | |
| decompression w/in 12 hrs | 13 |
| lesion length (mm) | 46.7 |
| rate of lesion expansion (mm/hour) | 0.86 |
| decompression btwn 12.5 & 44 hrs | 29 |
| lesion length (mm) | 64.8 |
| rate of lesion expansion (mm/hr) | 0.89 |
| administration of methylprednisolone | |
| patients who received steroid protocol | 34 |
| lesion length (mm) | 57.6 |
| rate of lesion expansion (mm/hr) | 0.79 |
| patients who did not receive steroid protocol | 8 |
| lesion length (mm) | 65.8 |
| rate of lesion expansion (mm/hr) | 1.26 |
| follow-up (mos) | 7.2 ± 7 |
Mean values are presented ± SD. The remaining values represent the number of patients and, parenthetically, the percentage. Abbreviation: ISS = Injury Severity Score.
A circumferential decompression is defined as a discectomy and/or corpectomy plus laminectomy plus fusion.
Study Parameters
The baseline MRI was performed at a mean of 6.8 ± 2.7 hours (range 3–15 hours) after traumatic injury; this period is called Interval-I. The second MRI was performed at 54.5 ± 32.3 hours (range 15–134 hours) after traumatic injury; this period is called Interval-II. The mean duration between the first and second MRI studies was 47.7 ± 32.4 hours (range 12–126 hours).
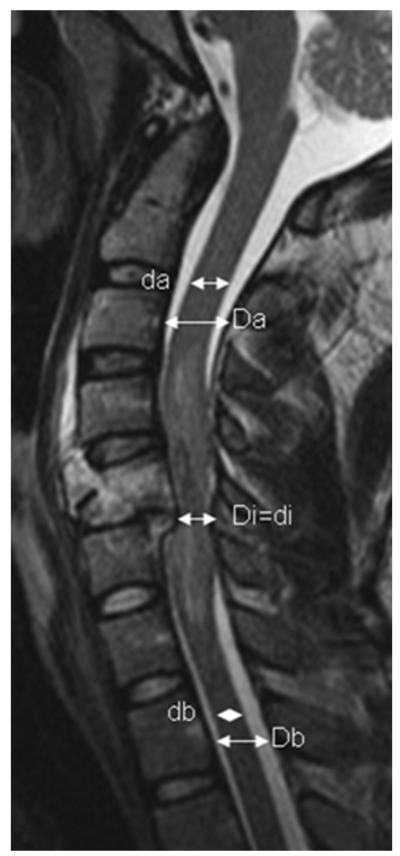
Admission MRI scans were used to determine several parameters (Figs. 1 and 2 [see Table 1 for abbreviation key]): 1) the rostrocaudal intramedullary lesion length at the end of Interval-I (LL1, in mm); 2) the midsagittal diameter of the spinal canal at the site of skeletal injury (Di, in mm); 3) the MCC at the injury site (in percentage); 4) the MSCC at the injury site (in percentage). Both the MCC and MSCC were calculated using previously validated formulas:17,23
where Di is defined as above, Da is the diameter of the spinal canal above the level of spinal injury, and Db is the diameter of the spinal canal below the level of spinal injury. We calculated MSCC using the formula:
where da is the diameter of the spinal cord at a normal segment above the level of SCI, db is the diameter of the spinal cord one segment below the level of SCI, and di is the diameter of the spinal cord at the level of SCI.
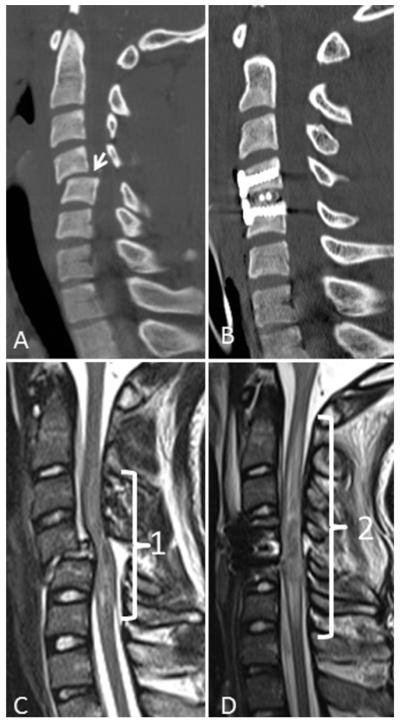
Fig. 1.
Reformatted CT and MRI scans obtained in a 17-year-old boy who sustained a cervical spine injury during a motor vehicle collision and was left tetraplegic. His admission motor function was ASIA Score 8 and impairment severity was ASIA Grade A. A and B: Images showing distractive flexion injury at the C4-5 motion segment. C: Initial T2-weighted MRI study, acquired 5.5 hours after the accident, demonstrating the mixed-intensity signal of an intramedullary lesion 69.2 mm in length. D: Second MRI study, obtained 42.5 hours after injury, revealing expansion of the intramedullary lesion to a length of 102.8 mm. In this case the expansion rate of the intramedullary lesion was 0.91 mm/hour.
Fig. 2.
Reformatted MRI scan of cervical spine acquired in a 24-year-old man who sustained a flexion teardrop fracture of C-5 vertebral body in an accidental fall. Admission scores were as follows: Injury Severity Score 75, ASIA motor Score 10, and ASIA impairment Grade A. The midsagittal diameters of the spinal canal and spinal cord (Da and da) above the site of injury were 14.2 mm and 8.6 mm, respectively. The midsagittal diameter of the spinal canal, which was equal to the midsagittal diameter of the spinal cord at the injury epicenter, was 6.8 mm (Di and di). The diameters of the spinal canal and spinal cord below the level of injury were 12.3 and 6.5 mm, respectively (Db and db). Calculations indicated that the MCC was 49.6% and the MSCC was 10.5%.
The second MRI scan was used to measure the rostrocaudal intramedullary lesion length at the end of Interval-II (LL2, in millimeters).
The rate of intramedullary lesion expansion was calculated as:
Final values of LL1, LL2, Di, Da, Db, di, da, and db used in the calculations were determined as the mean of 3 values obtained by two trauma neuroradiologists (S.E.M. and K.S.) and a trauma neurosurgeon (B.A.) working independently in a blinded fashion using electronic calipers.
Statistical Analysis
Statistical calculations were performed using SAS (Version 9.1, SAS Institute, Inc.). Univariate intergroup comparisons, with regard to the continuous outcome measures of lesion length and rate of lesion spread, were evaluated using the Student t-test for binary variables and ANOVA for variables having 3 or more categories. Correlation coefficients were computed to determine the univariate relationship between continuous factors and measured outcome. Redundant variables were then ascertained by examination of cross-tabulations and correlation calculations between possible independent variables. For the outcome measure of LL1, multiple linear regression models were constructed to examine the effect of time to MRI before and after controlling for specific descriptive factors and impairment scales. Linear regression techniques were also applied to determine risk factors for rate of spread of the intramedullary lesion. For each regression model, p values and adjusted regression coefficients, along with their 95% CIs, were reported. A p value < 0.05, corresponding to a 95% CI that does not include 1.00, was taken as indicating statistical significance for all tests.
Results
Clinical Characteristics of the Cohort
The clinical characteristics of the 42 patients are listed in Table 2. Most patients were injured in motor vehicle accidents, falls, and sports accidents; in 2 patients, the injury was from an altercation or a tree branch. Injury Severity Scores ranged from 10 to 75.43,46 In addition to spine injuries and SCIs, evidence of multiple trauma was present in 36 patients in the following places: thorax in 20 patients, neck in 18, traumatic brain injury and facial injuries each in 16, lower extremities in 15, upper extremities in 10, and intraabdominal visceral in 8. The cervical spine level of injury was at C-5 in 29, C-6 in 17, C-4 in 16, C-7 in 14, C-3 in 6, and T-1 in 3 patients. In 20 patients CT angiography revealed evidence of vertebral artery injury.20,21
Magnetic Resonance Imaging Characteristics of the Cohort
Magnetic resonance imaging studies with T2-weighted and STIR sequences indicated hematomyelia, myelomalacia and spinal cord edema in all patients.23,37,40,48,49 The first MRI study was performed at 6.8 ± 2.7 hours (mean ± SD) after injury; the second was performed at 54.5 ± 32.3 hours.
The MRI characteristics of the 42 patients are listed in Table 2. The midsagittal diameter of the spinal canal at the point of fracture dislocation (Di) ranged from 3.5 to 13.8 mm (mean 6.9 ± 2.2 mm), MCC ranged from 6.1% to 73.2% (mean 47.1% ± 15.3%), MSCC ranged from −43.2% (mainly swelling) to 55.4% (mainly compression) (mean 7.9% ± 27%).
The rostrocaudal intramedullary lesion length seen on the first MRI scan was 59.2 ± 16.1 mm, whereas the lesion length on the second scan was 88.5 ± 31.9 mm. The rate of rostrocaudal intramedullary lesion expansion during the interval between the first and second MRI was 0.9 ± 0.8 mm/hour.
Factors Associated With the Intramedullary Lesion Length
Univariate analysis indicated that use of methylprednisolone, long-term ASIA impairment grade, and worsening neurological status had no relationship with lesion length, whereas decompression within 12 hours of injury significantly reduced lesion length (p < 0.001). Analysis following construction of 8 linear regression models, controlling for 5 other descriptive and impairment scale variables, indicated that the time between injury and the first MRI (Interval-I; p = 0.05) and time to decompression (p = 0.03) were significantly associated with the rostrocaudal intramedullary lesion length (LL1) (Tables 3 and 4).
TABLE 3. Differences in lesion length for 5 new factors*.
| Variable | No. of Cases |
Mean Lesion Length (mm) |
p Value |
|---|---|---|---|
| steroids | |||
| yes | 34 | 57.6 ± 17.1 | 0.20 |
| no | 8 | 65.9 ± 9.3 | |
| motor outcome | |||
| improved | 18 | 58.4 ± 13.7 | 0.89 |
| same | 10 | 60.5 ± 20.8 | |
| worsened | 8 | 61.2 ± 12.2 | |
| ASIA grade | |||
| A | 35 | 60.6 ± 16.2 | 0.21 |
| B, C, or D | 7 | 52.2 ± 14.5 | |
| time to decompression | |||
| ≤12 hrs | 13 | 46.7 ± 15.0 | <0.001 |
| >12 hrs | 29 | 64.8 ± 13.3 |
Mean values are presented ± SD.
TABLE 4. Linear regression analysis of lesion length compared with time to MRI adjusted for covariate regression coefficients and corresponding 95% CIs*.
| Variable | No. of Cases |
Coefficient | 95% CI | p Value |
|---|---|---|---|---|
| age | 42 | −0.2 | −0.5 to 0.1 | 0.28 |
| ISS | ||||
| 10–30 | 16 | 0.0 | referent | |
| 31–50 | 17 | 3.6 | −6.6 to 13.9 | 0.47 |
| 51–75 | 9 | 8.8 | −3.7 to 21.3 | 0.16 |
| ASIA motor score | ||||
| 0–10 | 23 | 0.0 | referent | |
| 11–20 | 9 | 10.2 | −1.9 to 22.4 | 0.10 |
| 21–40 | 10 | −8.1 | −19.1 to 2.8 | 0.14 |
| ASIA impairment grade | ||||
| A | 35 | 0.0 | referent | |
| B | 7 | −7.8 | −19.5 to 3.8 | 0.18 |
| MCC (%)† | ||||
| 6–40 | 12 | 0.0 | referent | |
| >40 | 30 | 8.9 | −0.2 to 18.1 | 0.06 |
| time to decompression | ||||
| >12 hrs | 29 | 0.0 | referent | |
| ≤12 hrs | 13 | −12.1 | −22.9 to −1.3 | 0.03 |
| time to MRI† | 42 | 2.0 | 0.0 to 4.0 | 0.05 |
Time to decompression (≤ 12 hours vs > 12 hours) was added to the model.
Expect lesion length to increase by 2.0 for a unit increase in time to MRI, while holding the other variables constant.
Factors Associated With the Rate of Expansion of the Intramedullary Lesion
Univariate analysis did not indicate a relationship between expansion rate and use of steroid agents, ASIA impairment grade at follow-up, worsening neurological status, and the timing of surgical decompression. Analysis following construction of 7 regression models, controlling for 6 other descriptive and impairment scale variables, indicated that the MSCC computed from the first MRI study (p = 0.03) and sport injuries (p = 0.05) were significantly associated with the rate of expansion of the intramedullary lesion (Tables 3 and 5).
TABLE 5. Significant risk factors for rate of spread of intramedullary lesion adjusted by other covariates in a multiple linear regression model*.
| Variable | No. of Cases |
Coefficient | 95% CI | p Value |
|---|---|---|---|---|
| age | 40 | −0.001 | −0.02 to 0.2 | 0.95 |
| mechanism | ||||
| sport/other | 9 | 0.0 | referent | |
| fall | 9 | −0.8 | −1.5 to −0.01 | 0.05 |
| motor vehicle crash | 22 | −0.7 | −1.4 to −0.01 | 0.05 |
| ASIA motor score | ||||
| 0–10 | 22 | 0.0 | referent | |
| 11–20 | 9 | 0.3 | −0.3 to 1.0 | 0.35 |
| 21–40 | 9 | −0.1 | −0.7 to 0.6 | 0.85 |
| ASIA impairment grade | ||||
| A | 33 | 0.0 | referent | |
| B | 7 | −0.2 | −0.8 to 0.5 | 0.64 |
| MSCC (%)† | ||||
| <0 | 15 | 0.0 | referent | |
| ≤0 | 25 | 0.7 | 0.1 to 1.2 | 0.03 |
| time to decompression | ||||
| >12 hrs | 29 | 0.0 | referent | |
| ≤12 hrs | 13 | 0.2 | −0.4 to 0.8 | 0.54 |
Time to decompression (≤ 12 hours vs > 12 hours) added to model.
Expect rate of spread to increase by 0.7 for a patient with MSCC greater than 0 relative to a patient with an MSCC less than 0, while holding the other variables constant.
Illustrative Case
This 17-year-old boy was involved in a motor vehicle collision and was transferred to the Trauma Resuscitation Unit with tetraplegia (ASIA impairment Grade A). His ASIA motor score was 8, and a cervical CT study, which was performed 2 hours after the accident, demonstrated a C4–5 distractive flexion injury, Stage 2 of the classification reported by Allen et al.2 (Fig. 1A). His admission MRI scan, obtained 5.5 hours after injury, revealed a hemorrhagic intramedullary lesion (Fig. 1C). The midsagittal canal diameter was 4.8 mm, the MCC was 61.6%, and the MSCC was 9.2%. The length of the intramedullary lesion was 69.2 mm (Fig. 1C). The patient was taken to the operating room 11.5 hours postadmission for realignment and internal fixation (Fig. 1B). Following his surgery, the patient had his second cervical MRI study (Fig. 1D), obtained 42.5 hours after injury, which revealed an intramedullary lesion length of 102.8 mm. Thus, during the 37 hours between MRI scans, the lesion expanded 33.6 mm, a mean rate of 0.91 mm per hour.
Discussion
The major finding of the present study is that lesion expansion occurs in humans during the early hours after cervical SCI, as has been established in rodent models of SCI. In addition, the present study suggests that the initial length of the intramedullary lesion and its rate of subsequent expansion may be predicted by specific independent variables. The timing of acquisition of the first MRI after SCI predicted the intramedullary lesion length (p = 0.05). Also, lesion length was not greater in patients who suffered multiple traumas, as indicated by higher injury severity scores. The most important independent variable associated with the rate of intramedullary lesion expansion was the extent of spinal cord compression at the time of initial imaging (p = 0.03). To our knowledge, factors predicting lesion length and lesion expansion after cervical SCI have not been defined previously.
In SCI, the translation of kinetic energy into parenchymal damage at the moment of trauma, which is termed the “primary injury,” is instantaneous. The primary injury is followed by secondary injury, which continues as a dynamic process for hours to days.45,50 It is the combination of “primary” and “secondary” injuries that is visually evident on MR images. Imaging studies in humans are snapshots that are usually obtained several hours after trauma,4,7,19,26,36,39,48,49 with the time of inception of specific MRI signal changes being impossible to determine. In a rat model of cervical SCI, MRI revealed that the earliest changes in signal characteristics of damaged spinal cord were evident as early as 36 minutes after the trauma.41 In a unique tetraplegic patient with traumatic cervical disc herniation reported by Aoyama and colleagues,5 an MRI study performed 120 minutes after trauma did not show any signal change, although by 8 hours, signal changes were evident on T2-weighted images. In our study, signal changes were clearly evident in patients in whom imaging studies were performed as early as 3 hours after trauma.
During the past 20 years, investigators have tried to define intramedullary signal changes using qualitative and quantitative methodologies. Signal changes on T2-weighted images have been characterized as high, mixed and low intensity, with high-intensity signals indicating edema, low-intensity signals indicating intramedullary hematoma, and mixed-intensity signals indicating contusion.4,7,19,36,48,49 The length of the signal change has been reported using metamers (the number of motion segments covered by the intramedullary lesion) or millimeters.4,48,49 There is a sharp distinction between complete and incomplete SCI applying the aforementioned visual characteristics. Intramedullary low signal changes at the point of translation of kinetic energy usually are associated with complete injury, whereas pure high signal changes typically denote incomplete SCI.40 Approximately 34% of 155 patients reported in 4 series had low-intensity signal change on T2-weighted imaging, characteristic of a complete SCI.4,7,48,49,54 In a study of 100 patients with SCI, Miyanji et al.40 reported that patients with complete injuries had longer lesions and more prominent MCC and MSCC; lesion length in complete SCI was 40 mm whereas in incomplete SCI it was 20 mm. Similarly Aarabi et al.1 reported a lesion length of 29.4 mm in 42 patients with acute traumatic central cord syndrome. In the present study, lesion length measured on the first MRI study was 59.2 ± 16.1 mm whereas on the second it was 88.5 ± 31.9 mm.
Knowing the dynamic characteristics of lesion expansion following SCI and its response to pharmacotherapy or surgical decompression may have important clinical implications. Preclinical studies with rat models indicate that MRI-defined lesion expansion can be reduced by pharmacotherapy.14,41 In their rat model of cervical SCI, Nout et al.41 showed that hypertonic saline attenuated spinal cord swelling and decreased the volume of the hypointense core and the edema of the injured spinal cord. In a rat model of T-10 SCI, Chou et al.14 showed that S-nitrosoglutathione reduced the size of the MRI-defined intramedullary edema and hematoma. In the present investigation, lesion length was significantly shorter in patients in whom decompression was performed within 12 hours of injury. While there is a biological rationale for early spinal cord decompression in SCI, clinical evidence for better motor or functional outcome in such a setting is less strong.10-12,15,24,25,34,42,44 The recently published results of STASCIS (Surgical Treatment for Acute Spinal Cord Injury Study) indicate that interruption of the continually compressed spinal cord following SCI within the first 24 hours of injury might contribute to a better long-term functional outcome.18 It is conceivable that at least part of the treatment effect of early spinal cord decompression may be through attenuation of secondary injury that contributes to lesion expansion.9,13,16,35,38,47,53 If the findings of the present study are validated, investigators could use the rate of lesion expansion of the secondary injury on MRI as a surrogate outcome measure for future therapeutic trials.
Conclusions
The present study demonstrates that acute SCI in humans is a dynamic process. Rostrocaudal expansion of the intramedullary lesion continues for many hours after trauma. It is our inclination to suggest that not only the magnitude of the initial impact but also the severity of the spinal cord compression and earlier decompression may significantly affect lesion length on MRI. Lesion expansion may be a novel surrogate measure to assess the therapeutic effects of surgical or drug trials.
Acknowledgments
Dr. Schwartzbauer reports receiving a Neurosurgery Research and Education Foundation grant from the AANS/CNS.
Abbreviations used in this paper
- ACDF
anterior cervical discectomy and fusion
- ASIA
American Spinal Injury Association
- MCC
maximum canal compromise
- MSCC
maximum spinal cord compression
- SCI
spinal cord injury
Footnotes
Disclosure
The authors report no conflict of interest concerning the materials or methods used in this study or the findings specified in this paper.
Author contributions to the study and manuscript preparation include the following. Conception and design: Aarabi. Acquisition of data: Aarabi, Mirvis, Shanmuganathan. Analysis and interpretation of data: Aarabi, Simard, Kufera, Alexander. Drafting the article: Aarabi. Critically revising the article: all authors. Reviewed submitted version of manuscript: all authors. Approved the final version of the manuscript on behalf of all authors: Aarabi. Statistical analysis: Kufera, Alexander, Mirvis. Administrative/technical/material support: Aarabi. Study supervision: Aarabi.
This study was presented at the 2011 Annual Scientific Meetings of the AANS and CNS.
References
- 1.Aarabi B, Alexander M, Mirvis SE, Shanmuganathan K, Chesler D, Maulucci C, et al. Predictors of outcome in acute traumatic central cord syndrome due to spinal stenosis. Clinical article. J Neurosurg Spine. 2011;14:122–130. doi: 10.3171/2010.9.SPINE09922. [DOI] [PubMed] [Google Scholar]
- 2.Allen BL, Jr, Ferguson RL, Lehmann TR, O’Brien RP. A mechanistic classification of closed, indirect fractures and dislocations of the lower cervical spine. Spine (Phila Pa 1976) 1982;7:1–27. doi: 10.1097/00007632-198200710-00001. [DOI] [PubMed] [Google Scholar]
- 3.American College of Surgeons: Advanced Trauma Life Support (ATLS) ed 7 American College of Surgeons; Chicago: 2004. [Google Scholar]
- 4.Andreoli C, Colaiacomo MC, Rojas Beccaglia M, Di Biasi C, Casciani E, Gualdi G. MRI in the acute phase of spinal cord traumatic lesions: relationship between MRI findings and neurological outcome. Radiol Med (Torino) 2005;110:636–645. [PubMed] [Google Scholar]
- 5.Aoyama T, Hida K, Akino M, Yano S, Iwasaki YS, Saito H. Ultra-early MRI showing no abnormality in a fall victim presenting with tetraparesis. Spinal Cord. 2007;45:695–699. doi: 10.1038/sj.sc.3102014. [DOI] [PubMed] [Google Scholar]
- 6.Balentine JD. Pathology of experimental spinal cord trauma. I. The necrotic lesion as a function of vascular injury. Lab Invest. 1978;39:236–253. [PubMed] [Google Scholar]
- 7.Bondurant FJ, Cotler HB, Kulkarni MV, McArdle CB, Harris JH., Jr Acute spinal cord injury. A study using physical examination and magnetic resonance imaging. Spine (Phila Pa 1976) 1990;15:161–168. [PubMed] [Google Scholar]
- 8.Bracken MB, Shepard MJ, Collins WF, Holford TR, Young W, Baskin DS, et al. A randomized, controlled trial of methylprednisolone or naloxone in the treatment of acute spinal-cord injury. Results of the Second National Acute Spinal Cord Injury Study. N Engl J Med. 1990;322:1405–1411. doi: 10.1056/NEJM199005173222001. [DOI] [PubMed] [Google Scholar]
- 9.Campagnolo DI, Esquieres RE, Kopacz KJ. Effect of timing of stabilization on length of stay and medical complications following spinal cord injury. J Spinal Cord Med. 1997;20:331–334. doi: 10.1080/10790268.1997.11719484. [DOI] [PubMed] [Google Scholar]
- 10.Carlson GD, Gorden CD, Oliff HS, Pillai JJ, LaManna JC. Sustained spinal cord compression: part I: time-dependent effect on long-term pathophysiology. J Bone Joint Surg Am. 2003;85-A:86–94. [PubMed] [Google Scholar]
- 11.Carlson GD, Minato Y, Okada A, Gorden CD, Warden KE, Barbeau JM, et al. Early time-dependent decompression for spinal cord injury: vascular mechanisms of recovery. J Neurotrauma. 1997;14:951–962. doi: 10.1089/neu.1997.14.951. [DOI] [PubMed] [Google Scholar]
- 12.Carlson GD, Warden KE, Barbeau JM, Bahniuk E, Kutina-Nelson KL, Biro CL, et al. Viscoelastic relaxation and regional blood flow response to spinal cord compression and decompression. Spine (Phila Pa 1976) 1997;22:1285–1291. doi: 10.1097/00007632-199706150-00002. [DOI] [PubMed] [Google Scholar]
- 13.Chipman JG, Deuser WE, Beilman GJ. Early surgery for thoracolumbar spine injuries decreases complications. J Trauma. 2004;56:52–57. doi: 10.1097/01.TA.0000108630.34225.85. [DOI] [PubMed] [Google Scholar]
- 14.Chou PC, Shunmugavel A, El Sayed H, Desouki MM, Nguyen SA, Khan M, et al. Preclinical use of longitudinal MRI for screening the efficacy of S-nitrosoglutathione in treating spinal cord injury. J Magn Reson Imaging. 2011;33:1301–1311. doi: 10.1002/jmri.22574. [DOI] [PubMed] [Google Scholar]
- 15.Dolan EJ, Tator CH, Endrenyi L. The value of decompression for acute experimental spinal cord compression injury. J Neurosurg. 1980;53:749–755. doi: 10.3171/jns.1980.53.6.0749. [DOI] [PubMed] [Google Scholar]
- 16.Duh MS, Shepard MJ, Wilberger JE, Bracken MB. The effectiveness of surgery on the treatment of acute spinal cord injury and its relation to pharmacological treatment. Neurosurgery. 1994;35:240–249. doi: 10.1227/00006123-199408000-00009. [DOI] [PubMed] [Google Scholar]
- 17.Fehlings MG, Rao SC, Tator CH, Skaf G, Arnold P, Benzel E, et al. The optimal radiologic method for assessing spinal canal compromise and cord compression in patients with cervical spinal cord injury. Part II: Results of a multicenter study. Spine (Phila Pa 1976) 1999;24:605–613. doi: 10.1097/00007632-199903150-00023. [DOI] [PubMed] [Google Scholar]
- 18.Fehlings MG, Vaccaro A, Wilson JR, Singh AW, Cadotte D, Harrop JS, et al. Early versus delayed decompression for traumatic cervical spinal cord injury: results of the Surgical Timing in Acute Spinal Cord Injury Study (STASCIS) PLoS ONE. 2012;7:e32037. doi: 10.1371/journal.pone.0032037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Flanders AE, Schaefer DM, Doan HT, Mishkin MM, Gonzalez CF, Northrup BE. Acute cervical spine trauma: correlation of MR imaging findings with degree of neurologic deficit. Radiology. 1990;177:25–33. doi: 10.1148/radiology.177.1.2399326. [DOI] [PubMed] [Google Scholar]
- 20.Fleck SK, Langner S, Baldauf J, Kirsch M, Kohlmann T, Schroeder HW. Incidence of blunt craniocervical artery injuries: use of whole body CT trauma imaging with adapted computed tomography angiography. Neurosurgery. 2011;69:615–624. doi: 10.1227/NEU.0b013e31821a8701. [DOI] [PubMed] [Google Scholar]
- 21.Fleck SK, Langner S, Baldauf J, Kirsch M, Rosenstengel C, Schroeder HW. Blunt craniocervical artery injury in cervical spine lesions: the value of CT angiography. Acta Neurochir (Wien) 2010;152:1679–1686. doi: 10.1007/s00701-010-0685-7. [DOI] [PubMed] [Google Scholar]
- 22.Fleming JC, Norenberg MD, Ramsay DA, Dekaban GA, Marcillo AE, Saenz AD, et al. The cellular inflammatory response in human spinal cords after injury. Brain. 2006;129:3249–3269. doi: 10.1093/brain/awl296. [DOI] [PubMed] [Google Scholar]
- 23.Furlan JC, Kailaya-Vasan A, Aarabi B, Fehlings M. A novel approach to quantitatively assess posttraumatic cervical spinal canal compromise and spinal cord compression: a multicenter responsiveness study. Spine (Phila Pa 1976) 2011;36:784–793. doi: 10.1097/BRS.0b013e3181e7be3a. [DOI] [PubMed] [Google Scholar]
- 24.Furlan JC, Noonan V, Cadotte DW, Fehlings MG. Timing of decompressive surgery of spinal cord after traumatic spinal cord injury: an evidence-based examination of pre-clinical and clinical studies. J Neurotrauma. 2011;28:1371–1399. doi: 10.1089/neu.2009.1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Guha A, Tator CH, Endrenyi L, Piper I. Decompression of the spinal cord improves recovery after acute experimental spinal cord compression injury. Paraplegia. 1987;25:324–339. doi: 10.1038/sc.1987.61. [DOI] [PubMed] [Google Scholar]
- 26.Gupta SK, Rajeev K, Khosla VK, Sharma BS, Paramjit, Mathuriya SN, et al. Spinal cord injury without radiographic abnormality in adults. Spinal Cord. 1999;37:726–729. doi: 10.1038/sj.sc.3100900. [DOI] [PubMed] [Google Scholar]
- 27.Guth L, Zhang Z, Steward O. The unique histopathological responses of the injured spinal cord. Implications for neuro-protective therapy. Ann N Y Acad Sci. 1999;890:366–384. doi: 10.1111/j.1749-6632.1999.tb08017.x. [DOI] [PubMed] [Google Scholar]
- 28.Hadley MN, Walters BC, Grabb PA, Oyesiku NM, Prezybylski GJ, Resnick DK, et al. Guidelines for the management of acute cervical spine and spinal cord injuries. Neurosurgery. 2002;50:S1–S199. doi: 10.1097/00006123-200203001-00003. [DOI] [PubMed] [Google Scholar]
- 29.Hugenholtz H. Methylprednisolone for acute spinal cord injury: not a standard of care. CMAJ. 2003;168:1145–1146. [PMC free article] [PubMed] [Google Scholar]
- 30.Hurlbert RJ. The role of steroids in acute spinal cord injury: an evidence-based analysis. Spine (Phila Pa 1976) 2001;26(24 Suppl):S39–S46. doi: 10.1097/00007632-200112151-00009. [DOI] [PubMed] [Google Scholar]
- 31.Hurlbert RJ, Moulton R. Why do you prescribe methylprednisolone for acute spinal cord injury? A Canadian perspective and a position statement. Can J Neurol Sci. 2002;29:236–239. doi: 10.1017/s0317167100002006. [DOI] [PubMed] [Google Scholar]
- 32.Iizuka H, Yamamoto H, Iwasaki Y, Yamamoto T, Konno H. Evolution of tissue damage in compressive spinal cord injury in rats. J Neurosurg. 1987;66:595–603. doi: 10.3171/jns.1987.66.4.0595. [DOI] [PubMed] [Google Scholar]
- 33.Kawata K, Morimoto T, Ohashi T, Tsujimoto S, Hoshida T, Tsunoda S, et al. Experimental study of acute spinal cord injury: a histopathological study. No Shinkei Geka. 1993;21:45–51. Jpn. [PubMed] [Google Scholar]
- 34.Kobrine AI, Evans DE, Rizzoli HV. Experimental acute balloon compression of the spinal cord. Factors affecting disappearance and return of the spinal evoked response. J Neurosurg. 1979;51:841–845. doi: 10.3171/jns.1979.51.6.0841. [DOI] [PubMed] [Google Scholar]
- 35.Krengel WF, III, Anderson PA, Henley MB. Early stabilization and decompression for incomplete paraplegia due to a thoracic-level spinal cord injury. Spine (Phila Pa 1976) 1993;18:2080–2087. doi: 10.1097/00007632-199310001-00027. [DOI] [PubMed] [Google Scholar]
- 36.Mahmood NS, Kadavigere R, Avinash KR, Rao VR. Magnetic resonance imaging in acute cervical spinal cord injury: a correlative study on spinal cord changes and 1 month motor recovery. Spinal Cord. 2008;46:791–797. doi: 10.1038/sc.2008.55. [DOI] [PubMed] [Google Scholar]
- 37.Martin D, Schoenen J, Lenelle J, Reznik M, Moonen G. MRI-pathological correlations in acute traumatic central cord syndrome: case report. Neuroradiology. 1992;34:262–266. doi: 10.1007/BF00588177. [DOI] [PubMed] [Google Scholar]
- 38.McLain RF, Benson DR. Urgent surgical stabilization of spinal fractures in polytrauma patients. Spine (Phila Pa 1976) 1999;24:1646–1654. doi: 10.1097/00007632-199908150-00005. [DOI] [PubMed] [Google Scholar]
- 39.Miranda P, Gomez P, Alday R, Kaen A, Ramos A. Brown-Sequard syndrome after blunt cervical spine trauma: clinical and radiological correlations. Eur Spine J. 2007;16:1165–1170. doi: 10.1007/s00586-007-0345-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Miyanji F, Furlan JC, Aarabi B, Arnold PM, Fehlings MG. Acute cervical traumatic spinal cord injury: MR imaging findings correlated with neurologic outcome—prospective study with 100 consecutive patients. Radiology. 2007;243:820–827. doi: 10.1148/radiol.2433060583. [DOI] [PubMed] [Google Scholar]
- 41.Nout YS, Mihai G, Tovar CA, Schmalbrock P, Bresnahan JC, Beattie MS. Hypertonic saline attenuates cord swelling and edema in experimental spinal cord injury: a study utilizing magnetic resonance imaging. Crit Care Med. 2009;37:2160–2166. doi: 10.1097/CCM.0b013e3181a05d41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nyström B, Berglund JE. Spinal cord restitution following compression injuries in rats. Acta Neurol Scand. 1988;78:467–472. doi: 10.1111/j.1600-0404.1988.tb03689.x. [DOI] [PubMed] [Google Scholar]
- 43.Osler T, Rutledge R, Deis J, Bedrick E. ICISS: an international classification of disease-9 based injury severity score. J Trauma. 1996;41:380–388. doi: 10.1097/00005373-199609000-00002. [DOI] [PubMed] [Google Scholar]
- 44.Rabinowitz RS, Eck JC, Harper CM, Jr, Larson DR, Jimenez MA, Parisi JE, et al. Urgent surgical decompression compared to methylprednisolone for the treatment of acute spinal cord injury: a randomized prospective study in beagle dogs. Spine (Phila Pa 1976) 2008;33:2260–2268. doi: 10.1097/BRS.0b013e31818786db. [DOI] [PubMed] [Google Scholar]
- 45.Rowland JW, Hawryluk GW, Kwon B, Fehlings MG. Current status of acute spinal cord injury pathophysiology and emerging therapies: promise on the horizon. Neurosurg Focus. 2008;25(5):E2. doi: 10.3171/FOC.2008.25.11.E2. [DOI] [PubMed] [Google Scholar]
- 46.Rutledge R, Osler T, Kromhout-Schiro S. Illness severity adjustment for outcomes analysis: validation of the ICISS methodology in all 821,455 patients hospitalized in North Carolina in 1996. Surgery. 1998;124:187–196. [PubMed] [Google Scholar]
- 47.Sapkas GS, Papadakis SA. Neurological outcome following early versus delayed lower cervical spine surgery. J Orthop Surg (Hong Kong) 2007;15:183–186. doi: 10.1177/230949900701500212. [DOI] [PubMed] [Google Scholar]
- 48.Schaefer DM, Flanders A, Northrup BE, Doan HT, Osterholm JL. Magnetic resonance imaging of acute cervical spine trauma. Correlation with severity of neurologic injury. Spine (Phila Pa 1976) 1989;14:1090–1095. doi: 10.1097/00007632-198910000-00011. [DOI] [PubMed] [Google Scholar]
- 49.Schaefer DM, Flanders AE, Osterholm JL, Northrup BE. Prognostic significance of magnetic resonance imaging in the acute phase of cervical spine injury. J Neurosurg. 1992;76:218–223. doi: 10.3171/jns.1992.76.2.0218. [DOI] [PubMed] [Google Scholar]
- 50.Simard JM, Tsymbalyuk O, Ivanov A, Ivanova S, Bhatta S, Geng Z, et al. Endothelial sulfonylurea receptor 1-regulated NC Ca-ATP channels mediate progressive hemorrhagic necrosis following spinal cord injury. J Clin Invest. 2007;117:2105–2113. doi: 10.1172/JCI32041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Steward O, Schauwecker PE, Guth L, Zhang Z, Fujiki M, Inman D, et al. Genetic approaches to neurotrauma research: opportunities and potential pitfalls of murine models. Exp Neurol. 1999;157:19–42. doi: 10.1006/exnr.1999.7040. [DOI] [PubMed] [Google Scholar]
- 52.Stiller K, Simionato R, Rice K, Hall B. The effect of intermittent positive pressure breathing on lung volumes in acute quadriparesis. Paraplegia. 1992;30:121–126. doi: 10.1038/sc.1992.39. [DOI] [PubMed] [Google Scholar]
- 53.Vaccaro AR, Daugherty RJ, Sheehan TP, Dante SJ, Cotler JM, Balderston RA, et al. Neurologic outcome of early versus late surgery for cervical spinal cord injury. Spine (Phila Pa 1976) 1997;22:2609–2613. doi: 10.1097/00007632-199711150-00006. [DOI] [PubMed] [Google Scholar]
- 54.Wang M, Dai Y, Han Y, Haacke EM, Dai J, Shi D. Susceptibility weighted imaging in detecting hemorrhage in acute cervical spinal cord injury. Magn Reson Imaging. 2011;29:365–373. doi: 10.1016/j.mri.2010.10.016. [DOI] [PubMed] [Google Scholar]
- 55.Wyndaele M, Wyndaele JJ. Incidence, prevalence and epidemiology of spinal cord injury: what learns a worldwide literature survey? Spinal Cord. 2006;44:523–529. doi: 10.1038/sj.sc.3101893. [DOI] [PubMed] [Google Scholar]