Abstract
Objective
Randomized trials of omega-3 polyunsaturated fatty acid (PUFA) treatment for depression have differed in outcome. Recent meta-analyses ascribe discrepancies to differential effects of eicosapentaenoic acid (EPA) vs. docosahexaenoic acid (DHA) and to diagnostic heterogeneity. This meta-analysis tests the hypothesis that EPA is the effective component in PUFA treatment of major depressive episodes.
Data Sources
PubMed was searched (1960 through June 2010) using terms “Fish Oils”[Mesh] AND (“Depressive Disorder”[Mesh] OR “Bipolar Depression”) AND “Randomized Controlled Trial”[Publication Type], for placebo-controlled trials of PUFA supplementation, a depressive episode as primary disorder, published in English, supplemented by manual bibliography review.
Study Selection
The search yielded 15 trials involving 916 participants.
Data Extraction
Sample sizes; PUFA doses; mean ages, baseline and endpoint depression ratings and standard deviations; and p values were extracted.
Data Synthesis
In a mixed-effect model, percentage of EPA in the supplements was the fixed-effect predictor, dichotomized into two groups: EPA < 60% or EPA ≥ 60% of EPA + DHA. Secondary analyses explored relevance of treatment duration, age, and EPA dose.
Results
Supplements with EPA ≥ 60% showed benefit on standardized mean depression scores (SMD, for EPA ≥ 60% = 0.558, 95% CI = (0.277, 0.838), z = 4.195, p = 0.001; for EPA < 60% = −0.026, 95% CI = (0.200, 0.148), z = −0.316, p =0.756), with negligible contribution of random effects or heteroscedasticity, and no effects of treatment duration or age. Supplements with EPA < 60% were ineffective. Exploratory analyses supported a non-linear model, with improvement determined by the dose of EPA in excess of DHA, within the range 200 to 2200 mg EPA.
Conclusions
Supplements containing EPA ≥ 60%, in dose range 200 to 2200 mg EPA in excess of DHA, were effective against primary depression. Translational studies are needed to determine mechanisms of EPA’s therapeutic benefit.
Keywords: n-3 PUFA, docosahexaenoic acid, eicosapentaenoic acid, clinical trials, depression, meta-analysis
INTRODUCTION
Low levels of omega-3 polyunsaturated fatty acids (PUFA) have been linked to depression1,2 and suicide3, as well as to cardiovascular4,5 and inflammatory disorders6, and thus may impact comorbidity of depression with diseases such as coronary heart disease7,8 and diabetes9. Previous meta-analyses disagree as to the benefit of omega-3 fatty acid supplementation for depression.10–14 However, the trials performed to date vary in important methodological aspects, including the type of placebo, diagnoses, monotherapy vs. augmentation, and doses and proportions of eicosapentaenoic acid (20:5n-3; EPA) and docosahexaenoic acid (22:6n-3; DHA) in the supplements. Two factors have recently been proposed to account for discrepancies between studies: a greater efficacy of EPA than DHA11,13, and greater effectiveness in patients with a diagnosed depressive disorder13,14. The a priori goal of this meta-analysis was to test the hypothesis that EPA is the active component of omega-3 PUFA treatment in depressive disorders. This study extends previous work by including recent clinical trials15,16 not reviewed in prior meta-analyses, and by proposing a novel model to explain the effects of EPA dosing. Determination of the most effective omega-3 PUFA supplementation regimen is important for treatment of depression and for design of future research studies.
METHOD
Literature search
Published studies eligible for this analysis were identified through a search of clinical trials in PubMed/MeSH (1960 through June 2010) using the following terms: “Fish Oils”[Mesh] AND (“Depressive Disorder”[Mesh] OR “Bipolar Depression”) AND “Randomized Controlled Trial”[Publication Type] and limited to published articles written in English. The reference lists within the resulting publications and relevant review articles were also examined to check for completeness of the assembled list of studies.
Trial selection
Trials were included if they met the following inclusion criteria: (1) prospective, randomized, double-blinded study design; (2) depressive episode as the primary complaint (with or without comorbid medical conditions); (3) administration of omega–3 PUFA supplements; (4) appropriate outcome measures to assess depressed mood; (5) a placebo comparison group, and (6) published in English.
Data extraction
Data extracted were study design, sample size, dose and percentage of EPA and DHA, subject mean ages, mean baseline and endpoint depression ratings and standard deviations (SDs) for PUFA and placebo groups, and p values. Mean and SD values not included explicitly in the published reports of Grenyer et al.17, da Silva et al.18 and Silvers et al.19 were provided electronically by the authors (Howe, P., PhD, written communication, May 28, 2010; Ferraz, A.C., PhD, written communication, June 24, 2010; Silvers, K.M., PhD, written communication, August 14, 2010, respectively). EPA was quantified as a percentage of (EPA + DHA) in the supplement, ranging from 0 (in one trial with DHA alone) to 100% (ethyl-EPA alone).
The clinical outcome of interest was standardized mean difference in the change from baseline to endpoint scores on a depression rating scale, in subjects taking PUFA supplements vs. subjects taking placebo. Trials used the Hamilton Depression Rating Scale20 for the main outcome measure except for 4 studies17,18,21,22 that used the Beck Depression Inventory23, the Montgomery–Asberg Depression Scale24, the Childhood Depression Rating Scale25, and the short form of the Depression, Anxiety, and Stress Scale26, respectively.
Primary statistical analysis
Statistical analyses were performed using R27 (R Foundation for Statistical Computing, Vienna, Austria). Where means and SDs for baseline and endpoint were available for both groups, the effect size was calculated according to the method of Hedges.28 The difference in mean baseline-endpoint change between PUFA and placebo groups was divided by an estimated SD of the change, calculated by pooling baseline and endpoint SDs in each group and multiplying by 21/2. This technique assumes that baseline and endpoint values are uncorrelated, whereas in actuality they are probably positively correlated. This conservative assumption, therefore, is likely to overestimate SD’s of the change and result in smaller estimated magnitude of effect sizes. In one study, use of the Hedges method was not possible due to limited specificity of information29, so effect size was calculated from p-values30, and standard error (SE) was imputed via a regression of SE on the reciprocal of the square root of the study size, which in this sample strongly correlated with SE (r = 0.96). The SD of the group difference was obtained by pooling SDs of the placebo and treatment groups.
A regression analysis was used to study the contribution of the EPA proportion to the effect size for omega-3 PUFA supplementation compared to placebo. The predictor variable for the fixed-effect part of the model was the percentage of EPA in the supplement, dichotomized into two groups: EPA less than 60% of DHA + EPA concentrations, or EPA greater than or equal to 60% of EPA + DHA concentrations. This cutoff was chosen based on the empirical observations that all significant positive studies used at least 60% EPA and all studies with less than 60% EPA were negative (the remaining studies used at least 60% EPA but were negative).
Two reports29,31 tested different doses of 100% ethyl-EPA; individual dose analyses within each paper were treated for purposes of meta-analysis as separate trials. This and other cases of studies by the same author may be statistically dependent, violating a basic assumption of ANOVA. To test for author effects, two classes of models were generated for the random part of the mixed model: models including ‘author’ as a random effect and those in which all studies were regarded as independent. Another concern was whether the precision of the estimated effect size might depend on study size, e.g. studies with fewer subjects might have larger variance. This and two other potential conditions of heteroscedasticity in study-wise SD were tested a priori in the mixed models: study-wise SD depended on 1) sample size or 2) EPA dichotomized at 60%, or 3) was constant. Using EPA dichotomized at 60% as the fixed-effect term and all combinations of the 2 random-effects and 3 heteroscedasticity possibilities, a family of 6 mixed-effects models was generated. One additional model was fitted, a weighted least squares regression32 with weights proportional to the reciprocal of estimated study effect size SE. The model with the smallest Bayes Information Criterion (BIC) value33 was chosen. As a further check, a Welch Two-Sample t-test was also performed, which is not based on an assumption that the SD’s in the high and low percentage EPA groups are equal.
Two sources of heterogeneity were feasible to test, given the information available in the trials included in the meta-analysis: treatment duration and mean age, included as covariates in separate regression analyses. In these analyses, the same family of 7 models was utilized, the dependent variable remained effect size, and the predictors were EPA dichotomized at 60% plus one of the covariates; interactions were also tested. Publication bias was assessed with a funnel plot.
Exploratory analysis of dose effects
Given the observed 60% threshold for significance, it was hypothesized that EPA was effective to the extent that it was in excess of DHA in the supplements. Therefore, correlations were examined between effect size and EPA dose in excess of DHA (EPA dose – DHA dose), where positive numbers represent EPA in excess, and negative numbers represent DHA in excess. A second observation was negative outcomes in 2 published studies29,34 at doses of pure ethyl EPA greater than or equal to 4,000 mg. Therefore, a non-linear relationship of EPA dose to effect size was proposed. Linear and non-linear regression models were empirically fit to the data, using the curve-estimation module from SPSS (Release 17.0.0, 2008, SPSS Inc., Chicago, IL, for Mac [Apple, Inc., Cupertino, CA]). Weighted linear and quadratic least squares regression analyses were also performed, using weights proportional to the reciprocal of estimated study effect size SE. No correction was made for multiple testing.
RESULTS
Literature search
Twenty-four reports were identified through the MeSH/PUBMED search strategy, excluding 3 studies that were not clinical trials, 4 in which the primary diagnosis was not depression, and 3 without a placebo arm. One additional article was identified through manual bibliography search, resulting in 15 double-blinded, placebo-controlled trials that fulfilled all criteria, involving 916 participants (see Table 1). Eight studies included participants with diagnosed Major Depressive Disorder16,17,21,35–39. Two studies concerned participants with a Major Depressive Episode in association with a medical illness: Parkinson’s disease18 and coronary heart disease15. One study enrolled participants with a Major Depressive Episode in context of Bipolar Disorder31. The remaining 4 studies defined the diagnostic criteria as “episode of major depression or dysthymia”40, “ongoing depression”29, “a current depressive episode”19, or “mild to moderately depressed”22. In 3 studies, depression occurred in context of pregnancy or the perinatal period35,39,40. PUFA was given as monotherapy in 6 trials 16,21,22,36,39,40, and in one trial18 as one arm of the study. The remainder gave PUFA as adjunctive to pharmacotherapy15,17,19,29,31,37,38 or psychotherapy35. All studies used an intent-to-treat analysis except one study38 that excluded 4 subjects by placebo lead-in and 6 subjects after randomization, and another study18 in which 2 patients dropped out and were not included in the efficacy analysis. The percent composition of the supplements spanned the entire range from 100% EPA to 100% DHA; doses ranged from 400–4,400 mg/day of EPA and 200–2,400 mg/day of DHA.
Table 1.
Clinical Trials of Omega-3 PUFA Supplementation Compared with Placebo in Depressive Episodes, Listed by Percentage of EPA in Supplement.
| Study | Diagnosis | Treatment Duration (Wks) | Design* | Main Depression Measure | Sample Size (ITT) | Mean Age (Yrs) | EPA (mg) | DHA (mg) | % EPA** | EPA-DHA | Results (+/−) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Nemets et al, 2002 37 | MDD | 4 | Adjunctive | HAMD | 20 | 53.4 | 2000 | 0 | 100 | 2000 | + |
| Peet & Horrobin et al, 200229 | ongoing depression | 12 | Adjunctive | HAMD | 70 | 44.7 | 1000 2000 |
0 | 100 | 1000 2000 |
+ − |
| Mischoulon et al, 200916 | MDD | 8 | Monotherapy | HAMD | 35 | 45.0 | 4000 1000 |
0 | 100 | 4000 1000 |
− − |
| Frangou et al, 2006 31 | BD | 12 | Adjunctive | HAMD | 75 | 47.0 | 1000 2000 |
0 | 100 | 1000 2000 |
+ + |
| Nemets et al, 2006 21 | MDD | 16 | Monotherapy | CDRS | 20 | 10.2 | 400 | 200 | 67 | 200 | + |
| Su et al, 2003 38 | MDD | 8 | Adjunctive | HAMD | 22*** | 38.4 | 4400 | 2200 | 67 | 2200 | + |
| Su et al, 2008 39 | MDD | 8 | Monotherapy | HAMD | 33 | 31.1 | 2200 | 1200 | 65 | 1000 | + |
| da Silva et al, 2008 18 | MDD & Parkinsons | 12 | Monotherapy Adjunctive |
MADRS | 29*** | 64.4 | 720 | 480 | 60 | 240 | + |
| Freeman et al, 2008 35 | MDD | 8 | Adjunctive | HAMD | 51 | 30.4 | 1100 | 800 | 58 | 300 | − |
| Carney et al, 2009 15 | MDD & CHD | 10 | Adjunctive | HAMD | 122 | 58.3 | 930 | 750 | 55 | 180 | − |
| Rogers et al, 2008 22 | mild-mod depression | 12 | Monotherapy | DASS | 218 | 38.1 | 630 | 850 | 43 | −220 | − |
| Grenyer et al, 2007 17 | MDD | 16 | Adjunctive | BDI | 83 | 45.3 | 600 | 2200 | 21 | −1600 | − |
| Rees et al, 2008 40 | MDD or Dysthymia | 6 | Monotherapy | HAMD | 26 | 32.9 | 414 | 1638 | 20 | −1224 | − |
| Silvers et al, 2005 19 | depressive episode | 12 | Adjunctive | HAMD | 77 | 38.8 | 600 | 2400 | 20 | −1800 | − |
| Marangell et al, 2003 36 | MDD | 6 | Monotherapy | HAMD | 35 | 47.3 | 0 | 2000 | 0 | −2000 | − |
Adjunctive to pharmacotherapy except for Freeman, et al., adjunctive to psychotherapy;
Rounded to nearest whole number;
Per protocol;
BD = Bipolar Disorder; BDI, Beck Depression Inventory; CDRS = Children’s Depression Rating Scale; CHD = Coronary Heart Disease; DASS = Depression, Anxiety and Stress Scales; HAMD = Hamilton Rating Scale for Depression; MADRS = Montgomery-Asberg Depression Rating Scale; MDD = Major Depressive Disorder; MDE=Major Depressive Episode
Data synthesis
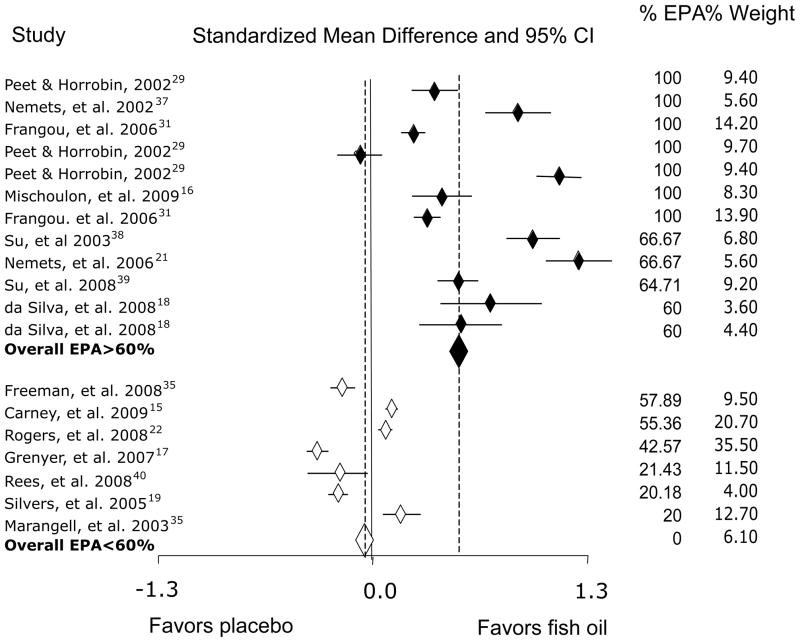
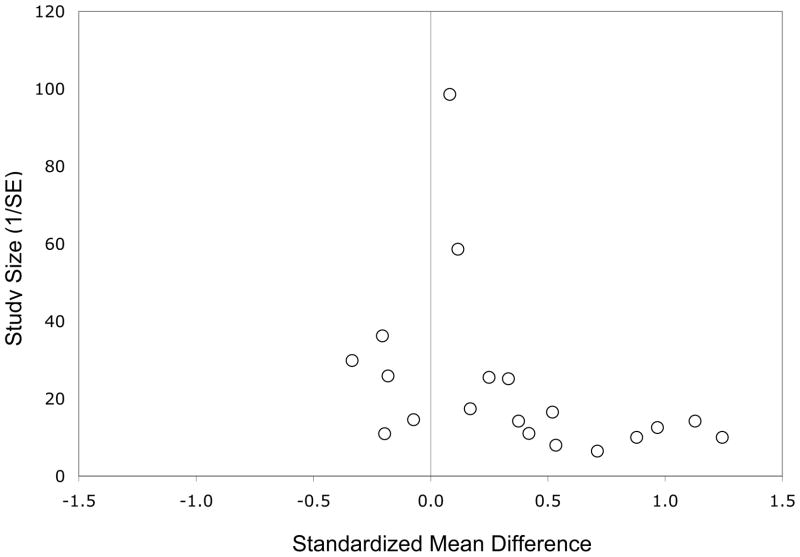
The overall effect size for 60% or greater EPA in supplements compared with placebo was 0.558 (p<0.001); for EPA at less than 60%, it was non-significant at −0.026, (see Table 2 and Figure 1). Interpretation of these findings should take into account that asymmetry of the funnel plot indicated some negative publication bias (see Figure 2).
Table 2.
Model Statistics for the Mixed-Effects Analyses of Effects of EPA, Dichotomized at 60% of Omega-3 PUFA Dose, on PUFA Supplementation Compared with Placebo.
| Effects of EPA 60%
| ||||||
|---|---|---|---|---|---|---|
| Coefficient Estimate | df | 95% CI | t-value | p | ||
|
| ||||||
| Intercept | −0.0261 | 17 | −0.2004 | 0.1482 | −0.316 | 0.7560 |
| EPA 60% | 0.5577 | 17 | 0.2772 | 0.8382 | 4.195 | 0.0006 |
|
| ||||||
| Effects of EPA 60% and Treatment Duration or Mean Age | ||||||
|
| ||||||
| df | 95% CI | t-value | p | |||
|
| ||||||
| Intercept | 0.1882 | 16 | −0.3755 | 0.7519 | 0.655 | 0.5221 |
| EPA 60% | 0.5528 | 16 | 0.2889 | 0.8168 | 4.105 | 0.0008 |
| Treatment duration | −0.0194 | 16 | −0.0681 | 0.0294 | −0.779 | 0.4474 |
|
| ||||||
| Intercept | −0.0550 | 16 | −0.4214 | 0.7979 | −0.179 | 0.8600 |
| EPA 60% | 0.5580 | 16 | 0.2673 | 0.8383 | 4.072 | 0.0009 |
| Mean age | −0.0007 | 16 | −0.0721 | 0.0334 | −0.098 | 0.9231 |
Figure 1.
Standardized Mean Differences and 95% Confidence Intervals for Studies in Depressive Episodes Comparing Antidepressant Effect Between Omega-3 Polyunsaturated Fatty Acids and Placebo, Arranged by Percentage of Eicosapentaenoic Acid (EPA) in the Supplements.
Figure 2.
Funnel Plot of Effect Sizes for Clinical Trials Included in the Meta-Analysis.
For primary and secondary analyses, the p-values of dichotomized EPA in all models were robust, ranging from 0.00046 to 0.00165. Results from models with the lowest BIC are summarized in Table 2. In the primary regression analyses, the best model was the weighted least squares regression, in which an EPA proportion of at least 60% was a significant determinant of superiority of PUFA over placebo (t = 4.19, df = 17, p-value < 0.001). A Welch Two Sample t-test confirmed the significance of the effect (t = 5.10, df = 16.83, p-value < 0.0001). In secondary covariate analyses, neither treatment duration nor age significantly predicted effect size; an EPA proportion of 60% or greater was still significant with either variable in the model. Interactions were not statistically significant.
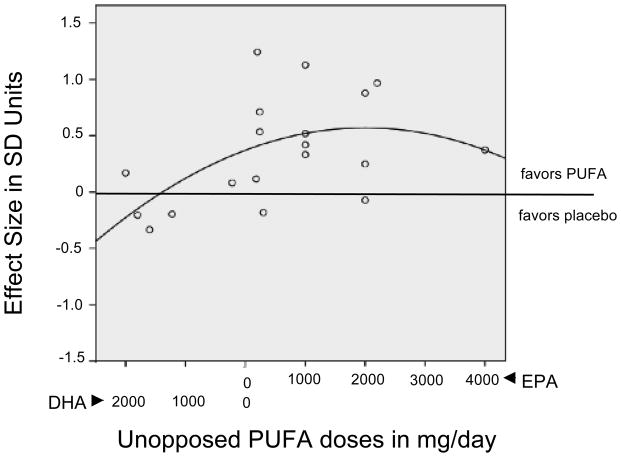
In exploratory analyses, EPA dose in excess of DHA (EPA (mg) – DHA (mg)) correlated similarly with effect size using either a linear (F=4.054, p=0.060, df1=1, df2=17) or a quadratic (F=3.399, p=0.059, df1=2, df2=16) function; neither reached significance (see Figure 3). However, weighted least squares regression analyses using weights proportional to the reciprocal of estimated study effect size SE, were significant for both linear (F=4.843, p=0.018, df1=1, df2=17) and quadratic (F=3.993, p=0.039, df1=2, df2=16) approaches.
Figure 3.
Exploratory Study of the Relationship between Unopposed PUFA Dose and Effect Size in Clinical Trials Comparing PUFA with Placebo Supplementation.
Abbreviations: SD = Standard Deviation; EPA=Eicosapentaenoic acid; DHA=Docosahexaenoic acid; PUFA=Polyunsaturated Fatty Acid. Unopposed PUFA supplement doses are defined as the absolute values of the difference between EPA (mg/day) and DHA (mg/day) and presented in a continuum, left to right, from the greatest unopposed DHA dose to the greatest unopposed EPA dose.
DISCUSSION
In agreement with Ross et al.11 and Martins13, this study identifies EPA as the effective PUFA component in treatment of depression. This finding is in contrast to the greater face validity of DHA, which is the major brain omega-3 PUFA species and is lower in brains of depressed subjects in postmortem studies2. The lack of DHA efficacy could mean that acute supplementation does not increase brain DHA concentrations. Increases in brain DHA have been reported after supplementation in piglets41 and in rats42. The effect of dietary DHA supplementation on human brain levels has not been studied; however, intravenously injected radiolabelled DHA43 resulted in an extremely low rate of DHA incorporation into brain in healthy humans: 3.8 + 1.7 mg/day, or a whole-brain half-life of 2.5 years. If this is an accurate paradigm for the fate of dietary DHA, then as noted by Umhau et al.43, effects of supplementation would not be evident in clinical trials lasting a few weeks, and the delay would be impractical for a therapeutic agent.
Possible explanations of EPA effects on depression
EPA could directly or indirectly facilitate an increase in brain DHA levels. Since EPA is a precursor of DHA, an increase in EPA might increase production of DHA44, and it has been suggested that decreased conversion of EPA to DHA could be an etiologic factor in depression45. However, supplementation with EPA has not been found to increase plasma or erythrocyte DHA levels in humans46 or brain DHA levels in rats47.
EPA could enter the brain and act directly as the effector. Given the extremely low EPA compared with DHA levels in brain (1:274 in mouse48), including in postmortem human brain2, this explanation has been considered unlikely. However, low brain levels do not necessarily indicate low uptake; they could signify rapid turnover. For example, in mouse brain, kinetic studies suggest rapid beta-oxidation of EPA upon uptake.48 Administration of ethyl-EPA increases neuronal and glial EPA content in rats49, and in differentiated PC12 cells, results in neuroprotective effects including suppression of cell death.49 This hypothesis has not been tested in vivo in humans.
EPA could have non-brain effects that cause secondary brain changes. Consistent with this model, dietary DHA and EPA exhibit differential physiologic outcomes and phospholipid partitioning.50 Following are some instances of known EPA effects, conceptualized within categories that may have relevance for depression pathophysiology: inflammation, effects on fuel supply to brain, and neuroprotection.
Inflammation
The inflammatory hypothesis of depression is based on the observations that stress precipitates both inflammatory responses and depression, inflammatory markers are increased in depression, and inflammatory cytokines can produce depressive symptoms in humans.51–53
Long-chain PUFAs and their metabolites have immunomodulatory properties.6 There is a functional opposition between omega-3 and omega-6 PUFA, in which higher relative levels of omega-3 tend to reduce the production of pro-inflammatory eicosanoids and cytokines50,52,54. Ratios of omega-6 to omega-3 PUFA are elevated in depression55–59 and in suicide risk3. These findings are in agreement with a theory proposing arachidonic acid (AA) cascade abnormalities as a cause of mood dysregulation.60–62 EPA has also been proposed29, specifically, as an important competitor with AA. For example, 1) Differences in EPA/AA ratios affect membrane fluidity and cellular responsivity50; 2) EPA competes with AA for cyclo-oxygenase, increasing production of anti-inflammatory prostaglandins29,50; and 3) lower EPA levels have been found associated with a genetic variant of phospholipase A2 (PLA2) that increased risk of interferon-induced depression63.
Effects on fuel supply to the brain
Increased PUFA oxidation could increase ketogenesis, producing ketone bodies that could bypass glucose utilization and improve energy supply to the brain.64 Increased fatty acid oxidation decreases production of triacylglycerol in rat hepatocyte cell cultures65 and increases fasting glucose concentrations in hyperlipidemic men66. Despite its low concentration in hepatocytes, EPA is a much stronger activator than DHA of peroxisome proliferator-activated receptor α (PPARα)67, an important regulator of energy homeostasis and PUFA β-oxidation68.
Neuroprotection
EPA supplementation in Bipolar Disorder has been observed to increase brain N-acetyl-aspartate69, a marker for neuronal health. EPA supplementation for 9 months also increased the ratio of cerebral phosphomonesters to phosphodiesters, an indicator of phospholipid turnover, and reversed brain atrophy, in a subject with Major Depressive Disorder.70 No comparable studies have been performed with DHA.
The role of EPA dose
The role of dose in PUFA supplements has been difficult to understand. Although EPA at ratios greater than 60% positively affected depression outcome, both successful 21,29,31,37–39 and unsuccessful 15–17,19,22,29,35,40 trials used EPA doses in the same ranges (400 to 4,000 mg/day). To address the effects of dose, we propose the following theoretical model:
There exists an approximately 1:1 competition between DHA and EPA for an unknown biological site, such that the EPA in excess of DHA exhibits a therapeutic outcome in depression. This postulate is consistent with findings of this meta-analysis, in which effects of EPA were statistically significant when the concentration of EPA in supplements rose to 10% above the DHA level. It also makes sense mechanistically, as EPA and DHA are structurally similar and might be expected to compete in approximately a 1:1 ratio for binding sites. This explanation implies a functional competition not only between omega-3 and omega-6 PUFAs61, but also within omega-3 species, with regard to depression. Thus we postulate that EPA in excess of DHA may be considered mechanistically to be unopposed EPA and the active component of PUFA supplements with regard to depression treatment.
-
There is a non-linear dose effect, such that above a certain range, unopposed doses of EPA are ineffective.
Figure 3 illustrates effect sizes as a quadratic function of unopposed PUFA dose (EPA-DHA). A cluster of positive trials was seen at 200–2,200 mg/day of unopposed EPA; the wide variance is presumably due to factors not controlled for in this analysis. The maximum dose of unopposed EPA (4,000 mg) was ineffective. The graph also shows that most studies with doses of unopposed DHA (where EPA-DHA yielded a negative number, i.e. more DHA than EPA) were less effective than placebo. This is consistent with a suggestion71 that DHA is contraindicated in depression on the basis of ex-vivo studies, in which it increased the proportion of proinflammatory markers.
The right-hand, descending portion of the quadratic curve is supported by a lone point at 4000 mg of ethyl-EPA29. However, we note the existence of another clinical trial34 in BD not included in this meta-analysis (as the sample comprised depressed and rapid-cycling patients), in which 6,000 mg of pure ethyl-EPA was not superior to placebo. It has been puzzling that these two well-designed studies were negative, as they seem to be comparable to similar, successful trials at doses of 4,400 mg of EPA in MDD38 and 6,200 mg of EPA in BD72. The problem was not the use of pure ethyl-EPA, which has been successfully used to treat depression in several clinical trials16,29,31,37. Rather, we note that in the latter, successful studies38,72, the unopposed doses of EPA were actually only 2,200 and 2,800 mg/day, respectively, consistent with our model. Thus, although the linear regression was also statistically valid, we feel that the U-shaped response curve is more likely to reflect the reality of the clinical response, although it is currently unknown why high doses of EPA may not be effective.
Effects of other factors
In a more broadly defined population, Martins13 found greater PUFA effects with shorter treatment length. In this meta-analysis, which included studies ranging from 4 to 16 weeks in duration, treatment length was not a predictor of outcome, suggesting that for patients who have a diagnosed depressive illness, effects of EPA may not be limited to the initial treatment period.
Limitations
This meta-analysis did not take into account unpublished clinical trials that would be predicted by the asymmetric funnel plot to exist. The number of potential moderators examined was limited by considerations of statistical power and inconsistent information in the source articles. Unexamined covariates that might be relevant include baseline level of depression, presence of stabilizing antioxidant in the supplement47, response by sex or ethnicity, baseline plasma PUFA levels, and dietary intakes. The selection of a diagnostic phenotype for study was limited by the relatively small number of clinical trials primarily focusing on depression, and by a lack of diagnostic clarity in some of the studies. Thus no inferences can be made about depressive episodes occurring within Major Depressive Disorder as opposed to Bipolar Disorder. The theoretical model to explain dose effects is based on a small number of studies and must be tested prospectively.
CONCLUSIONS
Recently, experts have called for more widespread use of omega-3 supplementation in patients at risk for depression10,73. However, there are no current agreed-upon guidelines concerning the optimal balance of constituents in omega-3 supplements. This meta-analysis finds no evidence that DHA is acutely effective against depression, and in fact, it may block beneficial effects of EPA at about a 1:1 dose ratio. Thus the amount of EPA unopposed by DHA may be critical for effective PUFA supplementation in depressive episodes. These findings argue against additional brief clinical trials of DHA for depression. At present, our knowledge base supports the use in acute depression of omega-3 supplements containing at least 60% EPA, with a ceiling at around 2,000 mg of EPA in excess of DHA, although the therapeutic effects of different unopposed EPA doses should be tested further in prospective studies that take into consideration diet and other potential confounds. We note that long-term efficacy and health effects of PUFA supplementation in depression have yet to be evaluated. Translational studies are also required to understand mechanisms underlying EPA effects in depression.
Acknowledgments
Sources of Financial Support: This work was partially supported by NIMH grants MH040695 and K08MH079033-03.
References
- 1.Lin PY, Huang SY, Su KP. A Meta-Analytic Review of Polyunsaturated Fatty Acid Compositions in Patients with Depression. Biol Psychiatry. 2010;68(2):140–147. doi: 10.1016/j.biopsych.2010.03.018. [DOI] [PubMed] [Google Scholar]
- 2.McNamara R, Hahn C, Jandacek R, et al. Selective Deficits in the Omega-3 Fatty Acid Docosahexaenoic Acid in the Postmortem Orbitofrontal Cortex of Patients with Major Depressive Disorder. Biol Psychiatry. 2007;62(1):17–24. doi: 10.1016/j.biopsych.2006.08.026. [DOI] [PubMed] [Google Scholar]
- 3.Sublette ME, Hibbeln JR, Galfalvy H, Oquendo MA, Mann JJ. Omega-3 polyunsaturated essential Fatty Acid status as a predictor of future suicide risk. Am J Psychiatry. 2006;163(6):1100–1102. doi: 10.1176/ajp.2006.163.6.1100. [DOI] [PubMed] [Google Scholar]
- 4.Calder PC. n-3 Fatty acids and cardiovascular disease: evidence explained and mechanisms explored. Clin Sci (Lond) 2004;107(1):1–11. doi: 10.1042/CS20040119. [DOI] [PubMed] [Google Scholar]
- 5.Gruppo Italiano per lo Studio della Sopravvivenza nell’Infarto miocardico. Dietary supplementation with n-3 polyunsaturated fatty acids and vitamin E after myocardial infarction: results of the GISSI-Prevenzione trial. Lancet. 1999;354(9177):447–455. [PubMed] [Google Scholar]
- 6.Simopoulos AP. Omega-3 fatty acids in inflammation and autoimmune diseases. J Am Coll Nutr. 2002;21(6):495–505. doi: 10.1080/07315724.2002.10719248. [DOI] [PubMed] [Google Scholar]
- 7.McNamara RK. Membrane omega-3 Fatty Acid deficiency as a preventable risk factor for comorbid coronary heart disease in major depressive disorder. Cardiovasc Psychiatry Neurol. 2009;2009:362795. doi: 10.1155/2009/362795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Parker GB, Heruc GA, Hilton TM, et al. Low levels of docosahexaenoic acid identified in acute coronary syndrome patients with depression. Psychiatry Res. 2006;141(3):279–286. doi: 10.1016/j.psychres.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 9.Holt RI, Phillips DI, Jameson KA, Cooper C, Dennison EM, Peveler RC. The relationship between depression and diabetes mellitus: findings from the Hertfordshire Cohort Study. Diabet Med. 2009;26(6):641–648. doi: 10.1111/j.1464-5491.2009.02742.x. [DOI] [PubMed] [Google Scholar]
- 10.Freeman MP, Hibbeln JR, Wisner KL, et al. Omega-3 fatty acids: evidence basis for treatment and future research in psychiatry. J Clin Psychiatry. 2006;67(12):1954–1967. doi: 10.4088/jcp.v67n1217. [DOI] [PubMed] [Google Scholar]
- 11.Ross BM, Seguin J, Sieswerda LE. Omega-3 fatty acids as treatments for mental illness: which disorder and which fatty acid? Lipids Health Dis. 2007;6:21. doi: 10.1186/1476-511X-6-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lin PY, Su KP. A meta-analytic review of double-blind, placebo-controlled trials of antidepressant efficacy of omega-3 fatty acids. J Clin Psychiatry. 2007;68(7):1056–1061. doi: 10.4088/jcp.v68n0712. [DOI] [PubMed] [Google Scholar]
- 13.Martins JG. EPA but not DHA appears to be responsible for the efficacy of omega-3 long chain polyunsaturated fatty acid supplementation in depression: evidence from a meta-analysis of randomized controlled trials. J Am Coll Nutr. 2009;28(5):525–542. doi: 10.1080/07315724.2009.10719785. [DOI] [PubMed] [Google Scholar]
- 14.Appleton KM, Rogers PJ, Ness AR. Updated systematic review and meta-analysis of the effects of n-3 long-chain polyunsaturated fatty acids on depressed mood. Am J Clin Nutr. 2010;91(3):757–770. doi: 10.3945/ajcn.2009.28313. [DOI] [PubMed] [Google Scholar]
- 15.Carney RM, Freedland KE, Rubin EH, Rich MW, Steinmeyer BC, Harris WS. Omega-3 augmentation of sertraline in treatment of depression in patients with coronary heart disease: a randomized controlled trial. JAMA. 2009;302(15):1651–1657. doi: 10.1001/jama.2009.1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mischoulon D, Papakostas GI, Dording CM, et al. A double-blind, randomized controlled trial of ethyl-eicosapentaenoate for major depressive disorder. J Clin Psychiatry. 2009 doi: 10.4088/JCP.08m04603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Grenyer B, Crowe T, Meyer B, et al. Fish oil supplementation in the treatment of major depression: A randomised double-blind placebo-controlled trial. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31(7):1393–1396. doi: 10.1016/j.pnpbp.2007.06.004. [DOI] [PubMed] [Google Scholar]
- 18.da Silva TM, Munhoz RP, Alvarez C, et al. Depression in Parkinson’s disease: a double-blind, randomized, placebo-controlled pilot study of omega-3 fatty-acid supplementation. J Affect Disord. 2008;111(2–3):351–359. doi: 10.1016/j.jad.2008.03.008. [DOI] [PubMed] [Google Scholar]
- 19.Silvers K, Woolley C, Hamilton F, Watts P, Watson R. Randomised double-blind placebo-controlled trial of fish oil in the treatment of depression. Prostaglandins, Leukotrienes and Essential Fatty Acids. 2005;72(3):211–218. doi: 10.1016/j.plefa.2004.11.004. [DOI] [PubMed] [Google Scholar]
- 20.Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nemets H, Nemets B, Apter A, Bracha Z, Belmaker RH. Omega-3 treatment of childhood depression: a controlled, double-blind pilot study. Am J Psychiatry. 2006;163(6):1098–1100. doi: 10.1176/ajp.2006.163.6.1098. [DOI] [PubMed] [Google Scholar]
- 22.Rogers PJ, Appleton KM, Kessler D, et al. No effect of n-3 long-chain polyunsaturated fatty acid (EPA and DHA) supplementation on depressed mood and cognitive function: a randomised controlled trial. Br J Nutr. 2008;99(2):421–431. doi: 10.1017/S0007114507801097. [DOI] [PubMed] [Google Scholar]
- 23.Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Arch Gen Psychiatry. 1961;4:561–571. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- 24.Montgomery SA, Asberg M. A new depression scale designed to be sensitive to change. Br J Psychiatry. 1979;134:382–389. doi: 10.1192/bjp.134.4.382. [DOI] [PubMed] [Google Scholar]
- 25.Poznanski EO, Cook SC, Carroll BJ. A depression rating scale for children. Pediatrics. 1979;64(4):442–450. [PubMed] [Google Scholar]
- 26.Lovibond PF, Lovibond SH. The structure of negative emotional states: comparison of the Depression Anxiety Stress Scales (DASS) with the Beck Depression and Anxiety Inventories. Behav Res Ther. 1995;33(3):335–343. doi: 10.1016/0005-7967(94)00075-u. [DOI] [PubMed] [Google Scholar]
- 27.R Development Core Team. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2006. [Google Scholar]
- 28.Hedges L, Olkin I. Statistical Methods for Meta-Analysis. New York: Academic Press; 1985. [Google Scholar]
- 29.Peet M, Horrobin D. A dose-ranging study of the effects of ethyl-eicosapentaenoate in patients with ongoing depression despite apparently adequate treatment with standard drugs. Arch Gen Psychiatry. 2002;59:913–919. doi: 10.1001/archpsyc.59.10.913. [DOI] [PubMed] [Google Scholar]
- 30.Rosenthal R. Parametric Measures of Effect Size. In: Cooper H, Hedges L, editors. The Handbook of Research Synthesis. New York: Russell Sage Foundation; 1994. pp. 231–244. [Google Scholar]
- 31.Frangou S, Lewis M, McCrone P. Efficacy of ethyl-eicosapentaenoic acid in bipolar depression: randomised double-blind placebo-controlled study. Br J Psychiatry. 2006;188:46–50. doi: 10.1192/bjp.188.1.46. [DOI] [PubMed] [Google Scholar]
- 32.Draper N, Smith H. Applied Regression Analysis. New York: Wiley; 1981. [Google Scholar]
- 33.Lahiri P. Model Selection. In: Lahiri P, editor. Lecture Notes-Monograph. Beachwood, Ohio: Institute of Mathematical Statistics; 2001. [Google Scholar]
- 34.Keck PE, Jr, Mintz J, McElroy SL, et al. Double-blind, randomized, placebo-controlled trials of ethyl-eicosapentanoate in the treatment of bipolar depression and rapid cycling bipolar disorder. Biol Psychiatry. 2006;60(9):1020–1022. doi: 10.1016/j.biopsych.2006.03.056. [DOI] [PubMed] [Google Scholar]
- 35.Freeman MP, Davis M, Sinha P, Wisner KL, Hibbeln JR, Gelenberg AJ. Omega-3 fatty acids and supportive psychotherapy for perinatal depression: a randomized placebo-controlled study. J Affect Disord. 2008;110(1–2):142–148. doi: 10.1016/j.jad.2007.12.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Marangell L, Martinez J, Zboyan H, Kertz B, Kim H, Puryear L. A double-blind, placebo-controlled study of the omega-3 fatty acid docosahexaenoic acid in the treatment of major depression. Am J Psychiatry. 2003;160(5):996–998. doi: 10.1176/appi.ajp.160.5.996. [DOI] [PubMed] [Google Scholar]
- 37.Nemets B, Stahl Z, Belmaker RH. Addition of omega-3 fatty acid to maintenance medication treatment for recurrent unipolar depressive disorder. The American journal of psychiatry. 2002;159(3):477–479. doi: 10.1176/appi.ajp.159.3.477. [DOI] [PubMed] [Google Scholar]
- 38.Su K, Huang S, Chiu C, Shen W. Omega-3 fatty acids in major depressive disorder. A preliminary double-blind, placebo-controlled trial. Eur Neuropsychopharmacol. 2003;13(4):267–271. doi: 10.1016/s0924-977x(03)00032-4. [DOI] [PubMed] [Google Scholar]
- 39.Su KP, Huang SY, Chiu TH, et al. Omega-3 fatty acids for major depressive disorder during pregnancy: results from a randomized, double-blind, placebo-controlled trial. J Clin Psychiatry. 2008;69(4):644–651. doi: 10.4088/jcp.v69n0418. [DOI] [PubMed] [Google Scholar]
- 40.Rees AM, Austin MP, Parker GB. Omega-3 fatty acids as a treatment for perinatal depression: randomized double-blind placebo-controlled trial. Aust N Z J Psychiatry. 2008;42(3):199–205. doi: 10.1080/00048670701827267. [DOI] [PubMed] [Google Scholar]
- 41.Huang MC, Brenna JT, Chao AC, Tschanz C, Diersen-Schade DA, Hung HC. Differential tissue dose responses of (n-3) and (n-6) PUFA in neonatal piglets fed docosahexaenoate and arachidonoate. J Nutr. 2007;137(9):2049–2055. doi: 10.1093/jn/137.9.2049. [DOI] [PubMed] [Google Scholar]
- 42.Moriguchi T, Loewke J, Garrison M, Catalan JN, Salem N., Jr Reversal of docosahexaenoic acid deficiency in the rat brain, retina, liver, and serum. J Lipid Res. 2001;42(3):419–427. [PubMed] [Google Scholar]
- 43.Umhau JC, Dauphinais KM, Patel SH, et al. The relationship between folate and docosahexaenoic acid in men. Eur J Clin Nutr. 2006;60(3):352–357. doi: 10.1038/sj.ejcn.1602321. [DOI] [PubMed] [Google Scholar]
- 44.Gao F, Kiesewetter D, Chang L, Ma K, Rapoport SI, Igarashi M. Whole-body synthesis secretion of docosahexaenoic acid from circulating eicosapentaenoic acid in unanesthetized rats. J Lipid Res. 2009;50(12):2463–2470. doi: 10.1194/jlr.M900223-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 45.Conklin SM, Runyan CA, Leonard S, Reddy RD, Muldoon MF, Yao JK. Age-related changes of n-3 and n-6 polyunsaturated fatty acids in the anterior cingulate cortex of individuals with major depressive disorder. Prostaglandins Leukot Essent Fatty Acids. 82(2–3):111–119. doi: 10.1016/j.plefa.2009.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Boston PF, Bennett A, Horrobin DF, Bennett CN. Ethyl-EPA in Alzheimer’s disease--a pilot study. Prostaglandins Leukot Essent Fatty Acids. 2004;71(5):341–346. doi: 10.1016/j.plefa.2004.07.001. [DOI] [PubMed] [Google Scholar]
- 47.Engstrom K, Saldeen AS, Yang B, Mehta JL, Saldeen T. Effect of fish oils containing different amounts of EPA, DHA, and antioxidants on plasma and brain fatty acids and brain nitric oxide synthase activity in rats. Ups J Med Sci. 2009;114(4):206–213. doi: 10.3109/03009730903268958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chen CT, Liu Z, Ouellet M, Calon F, Bazinet RP. Rapid beta-oxidation of eicosapentaenoic acid in mouse brain: an in situ study. Prostaglandins Leukot Essent Fatty Acids. 2009;80(2–3):157–163. doi: 10.1016/j.plefa.2009.01.005. [DOI] [PubMed] [Google Scholar]
- 49.Kawashima A, Harada T, Kami H, Yano T, Imada K, Mizuguchi K. Effects of eicosapentaenoic acid on synaptic plasticity, fatty acid profile and phosphoinositide 3-kinase signaling in rat hippocampus and differentiated PC12 cells. J Nutr Biochem. 2009 doi: 10.1016/j.jnutbio.2008.12.015. [DOI] [PubMed] [Google Scholar]
- 50.Smith WL. Cyclooxygenases, peroxide tone and the allure of fish oil. Curr Opin Cell Biol. 2005;17(2):174–182. doi: 10.1016/j.ceb.2005.02.005. [DOI] [PubMed] [Google Scholar]
- 51.Miller AH, Maletic V, Raison CL. Inflammation and Its Discontents: The Role of Cytokines in the Pathophysiology of Major Depression. Biol Psychiatry. 2009 doi: 10.1016/j.biopsych.2008.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Maes M, Smith RS. Fatty acids, cytokines, and major depression. Biol Psychiatry. 1998;43(5):313–314. doi: 10.1016/s0006-3223(97)00401-0. [DOI] [PubMed] [Google Scholar]
- 53.Raison CL, Capuron L, Miller AH. Cytokines sing the blues: inflammation and the pathogenesis of depression. Trends Immunol. 2006;27(1):24–31. doi: 10.1016/j.it.2005.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Calder PC. n-3 polyunsaturated fatty acids, inflammation, and inflammatory diseases. Am J Clin Nutr. 2006;83(6 Suppl):1505S–1519S. doi: 10.1093/ajcn/83.6.1505S. [DOI] [PubMed] [Google Scholar]
- 55.De Vriese SR, Christophe AB, Maes M. Lowered serum n-3 polyunsaturated fatty acid (PUFA) levels predict the occurrence of postpartum depression: further evidence that lowered n-PUFAs are related to major depression. Life Sci. 2003;73(25):3181–3187. doi: 10.1016/j.lfs.2003.02.001. [DOI] [PubMed] [Google Scholar]
- 56.Edwards R, Peet M, Shay J, Horrobin D. Omega-3 polyunsaturated fatty acid levels in the diet and in red blood cell membranes of depressed patients. J Affect Disord. 1998;48:149–155. doi: 10.1016/s0165-0327(97)00166-3. [DOI] [PubMed] [Google Scholar]
- 57.Maes M, Smith R, Christophe A, Cosyns P, Desnyder R, Meltzer H. Fatty acid composition in major depression: decreased omega 3 fractions in cholesteryl esters and increased C20: 4 omega 6/C20:5 omega 3 ratio in cholesteryl esters and phospholipids. J Affect Disord. 1996;38(1):35–46. doi: 10.1016/0165-0327(95)00092-5. [DOI] [PubMed] [Google Scholar]
- 58.Mamalakis G. Depression and adipose essential polyunsaturated fatty acids. Prostaglandins, Leukotrienes and Essential Fatty Acids. 2002;67(5):311–318. doi: 10.1054/plef.2002.0435. [DOI] [PubMed] [Google Scholar]
- 59.Peet M, Murphy B, Shay J, Horrobin D. Depletion of omega-3 fatty acid levels in red blood cell membranes of depressive patients. Biol Psychiatry. 1998;43:315–319. doi: 10.1016/s0006-3223(97)00206-0. [DOI] [PubMed] [Google Scholar]
- 60.Lee H, Rao J, Rapoport S, Bazinet R. Antimanic therapies target brain arachidonic acid signaling: Lessons learned about the regulation of brain fatty acid metabolism. Prostaglandins, Leukotrienes and Essential Fatty Acids. 2007;77(5–6):239–246. doi: 10.1016/j.plefa.2007.10.018. [DOI] [PubMed] [Google Scholar]
- 61.Rapoport SI, Basselin M, Kim HW, Rao JS. Bipolar disorder and mechanisms of action of mood stabilizers. Brain Res Rev. 2009 doi: 10.1016/j.brainresrev.2009.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sublette ME, Russ MJ, Smith GS. Evidence for a role of the arachidonic acid cascade in affective disorders: a review. Bipolar Disord. 2004;6(2):95–105. doi: 10.1046/j.1399-5618.2003.00094.x. [DOI] [PubMed] [Google Scholar]
- 63.Su KP, Huang SY, Peng CY, et al. Phospholipase A2 and cyclooxygenase 2 genes influence the risk of interferon-alpha-induced depression by regulating polyunsaturated fatty acids levels. Biol Psychiatry. 2010;67(6):550–557. doi: 10.1016/j.biopsych.2009.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Freemantle E, Vandal M, Tremblay-Mercier J, et al. Omega-3 fatty acids, energy substrates, and brain function during aging. Prostaglandins Leukot Essent Fatty Acids. 2006;75(3):213–220. doi: 10.1016/j.plefa.2006.05.011. [DOI] [PubMed] [Google Scholar]
- 65.Berge RK, Madsen L, Vaagenes H, Tronstad KJ, Gottlicher M, Rustan AC. In contrast with docosahexaenoic acid, eicosapentaenoic acid and hypolipidaemic derivatives decrease hepatic synthesis and secretion of triacylglycerol by decreased diacylglycerol acyltransferase activity and stimulation of fatty acid oxidation. Biochem J. 1999;343(Pt 1):191–197. [PMC free article] [PubMed] [Google Scholar]
- 66.Mori TA, Burke V, Puddey IB, et al. Purified eicosapentaenoic and docosahexaenoic acids have differential effects on serum lipids and lipoproteins, LDL particle size, glucose, and insulin in mildly hyperlipidemic men. Am J Clin Nutr. 2000;71(5):1085–1094. doi: 10.1093/ajcn/71.5.1085. [DOI] [PubMed] [Google Scholar]
- 67.Jump DB. N-3 polyunsaturated fatty acid regulation of hepatic gene transcription. Curr Opin Lipidol. 2008;19(3):242–247. doi: 10.1097/MOL.0b013e3282ffaf6a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.van Raalte DH, Li M, Pritchard PH, Wasan KM. Peroxisome proliferator-activated receptor (PPAR)-alpha: a pharmacological target with a promising future. Pharm Res. 2004;21(9):1531–1538. doi: 10.1023/b:pham.0000041444.06122.8d. [DOI] [PubMed] [Google Scholar]
- 69.Frangou S, Lewis M, Wollard J, Simmons A. Preliminary in vivo evidence of increased N-acetyl-aspartate following eicosapentanoic acid treatment in patients with bipolar disorder. J Psychopharmacol. 2007;21(4):435–439. doi: 10.1177/0269881106067787. [DOI] [PubMed] [Google Scholar]
- 70.Puri B, Counsell S, Hamilton G, Richardson A, Horrobin D. Eicosapentaenoic acid in treatment-resistant depression associated with symptom remission, structural brain changes and reduced neuronal phospholipid turnover. Int J Clin Pract. 2001;55(8):560–563. [PubMed] [Google Scholar]
- 71.Maes M, Mihaylova I, Kubera M, Bosmans E. Why fish oils may not always be adequate treatments for depression or other inflammatory illnesses: docosahexaenoic acid, an omega-3 polyunsaturated fatty acid, induces a Th-1-like immune response. Neuro Endocrinol Lett. 2007;28(6):875–880. [PubMed] [Google Scholar]
- 72.Stoll A, Severus E, Freeman M, et al. Omega 3 fatty acids in bipolar disorder: a preliminary double-blind, placebo-controlled trial. Arch Gen Psychiatry. 1999;56(5):407–412. doi: 10.1001/archpsyc.56.5.407. [DOI] [PubMed] [Google Scholar]
- 73.McNamara RK. Evaluation of docosahexaenoic acid deficiency as a preventable risk factor for recurrent affective disorders: current status, future directions, and dietary recommendations. Prostaglandins Leukot Essent Fatty Acids. 2009;81(2–3):223–231. doi: 10.1016/j.plefa.2009.05.017. [DOI] [PubMed] [Google Scholar]