Abstract
Background
The U.S. National HIV/AIDS Strategy targets for 2015 include increasing access to care and improving health outcomes for persons living with HIV in the United States (PLWH-US).
Objective
To demonstrate the utility of the NA-ACCORD (North American AIDS Cohort Collaboration on Research and Design) for monitoring trends in the HIV epidemic in the United States and to present trends in HIV treatment and related health outcomes.
Design
Trends from annual cross-sectional analyses comparing patients from pooled, multicenter, prospective, clinical HIV cohort studies with PLWH-US, as reported to national surveillance systems in 40 states.
Setting
U.S. HIV outpatient clinics.
Patients
HIV-infected adults with 1 or more HIV RNA plasma viral load (HIV VL) or CD4 T-lymphocyte (CD4) cell count measured in any calendar year from 1 January 2000 to 31 December 2008.
Measurements
Annual rates of antiretroviral therapy use, HIV VL, and CD4 cell count at death.
Results
45 529 HIV-infected persons received care in an NA-ACCORD–participating U.S. clinical cohort from 2000 to 2008. In 2008, the 26 030 NA-ACCORD participants in care and the 655 966 PLWH-US had qualitatively similar demographic characteristics. From 2000 to 2008, the proportion of participants prescribed highly active antiretroviral therapy increased by 9 percentage points to 83% (P < 0.001), whereas the proportion with suppressed HIV VL (≤2.7 log10 copies/mL) increased by 26 percentage points to 72% (P < 0.001). Median CD4 cell count at death more than tripled to 0.209 × 109 cells/L (P < 0.001).
Limitation
The usual limitations of observational data apply.
Conclusion
The NA-ACCORD is the largest cohort of HIV-infected adults in clinical care in the United States that is demographically similar to PLWH-US in 2008. From 2000 to 2008, increases were observed in the percentage of prescribed HAART, the percentage who achieved a suppressed HIV VL, and the median CD4 cell count at death.
Primary Funding Source
National Institutes of Health, Centers for Disease Control and Prevention, Canadian Institutes of Health Research, Canadian HIV Trials Network, and the government of British Columbia, Canada.
In the 30 years since the HIV epidemic was recognized in the United States, remarkable advances in treatment have turned a rapidly fatal disease into a chronic illness for persons who are aware of their infection and can access effective care (1, 2). The Centers for Disease Control and Prevention (CDC) estimates that 1.2 million persons live with HIV in the United States (3). The estimated annual rate of new HIV infections between 2006 and 2009 ranged from 19.0 to 22.5 per 100 000 population (approximately 47 800 to 56 000 new infections per year), with most occurring among men who have sex with men (MSM) and African Americans (4). A central component of the National HIV/AIDS Strategy (5) is to monitor the health of the growing number of Americans living with HIV infection who receive HIV treatment. Although seemingly simple, such monitoring is actually a substantial epidemiologic challenge because of the complexity of the U.S. health care system.
Many of the studies that have reported trends in the clinical outcomes of persons receiving HIV care (6–12) have been limited to discrete populations, and their findings have not been generalizable to all HIV-infected Americans. Two projects have been specifically designed to be nationally representative. The HCSUS (HIV Cost and Services Utilization Study) (13, 14) enrolled a national probability sample of HIV-infected adults receiving care from 1996 to 1998. Although useful a decade ago, data from this population no longer reflect the substantial improvements in HIV care in the past 14 years. The Medicapl Monitoring Project is an ongoing CDC-sponsored, multisite, supplemental national surveillance project designed to capture contemporary data about behaviors, medical care, and health status of HIV-infected adults in the United States through annual cross-sectional surveys (15). Data are compiled from the medical records of persons in care, who are selected through a 3-stage probability sampling scheme designed to produce a representative sample. Participants in the Medical Monitoring Project are not followed longitudinally, which limits its capacity to evaluate such associations as those between treatment and clinical outcomes, including survival. Conversely, even very large longitudinal cohort studies are not perfectly representative. Neither longitudinal cohort studies nor cross-sectional probability surveys alone can provide the most complete and accurate picture of HIV-infected persons in care; however, by addressing limitations of the other, together they provide highly useful, complementary information.
The NA-ACCORD (North American AIDS Cohort Collaboration on Research and Design) is the continent’s largest collaboration of longitudinal HIV cohort studies and has compiled clinical data from more than 100 clinical sites in the United States and Canada since 2005 (16). In this analysis, we assessed the extent to which attributes of persons receiving HIV care in an NA-ACCORD–participating U.S. clinical cohort are similar to those of persons living with HIV in the United States (PLWH-US) who are reported to the CDC’s HARS (Center’s for Disease Control’s HIV/AIDS Reporting System). We then examined the following illustrative and linked health outcomes among HIV-infected persons in care: trends in prescribed antiretroviral therapy (ART), HIV RNA plasma viral load (HIV VL), and CD4 T-lymphocyte (CD4) cell count at death. Our objective was to investigate the utility of NA-ACCORD for monitoring the U.S. HIV epidemic and for informing the progress made toward achieving the targets of the National HIV/AIDS Strategy.
Methods
Study Design and Population
The NA-ACCORD is a multisite collaboration of interval- and clinic-based cohort studies of HIV-infected persons receiving care in the United States and Canada (17). It is one of the regional cohort studies sponsored by the International Epidemiologic Databases to Evaluate AIDS consortium of the National Institutes of Health. Details on the NA-ACCORD collaboration and participating cohort studies have been published elsewhere (16). Each contributing cohort has developed standardized, cohort-specific methods of data collection. At scheduled intervals, these cohorts submit data about enrolled participants’ demographic characteristics; dates of prescribed antiretroviral medications; dates and results of laboratory tests, including HIV VL and CD4 cell count; dates of clinical diagnoses; and vital status. These data are securely transferred to the NA-ACCORD’s central data management core, where they undergo quality control for completeness and accuracy before they are combined into harmonized data files. Quality control includes measures to reduce the probability that a person was participating in more than 1 clinical cohort. The human subjects activities of the NA-ACCORD and of each of the participating cohort studies have been reviewed and approved by their respective institutional review boards.
We compared the characteristics of NA-ACCORD participants with those of PLWH-US reported to HARS from 2000 to 2008, the latest year for which complete data for NA-ACCORD participants and PLWH-US were available (18). Estimates for PLWH-US were based on data reported to the CDC from a subset of 40 states where confidential, name-based HIV surveillance systems were well-established; these estimates were adjusted for reporting delays in diagnoses and deaths as well as for missing risk factors. The remaining 10 states (California, Delaware, Hawaii, Maryland, Massachusetts, Montana, Oregon, Rhode Island, Vermont, and Washington) and the District of Columbia have surveillance systems that report HIV infections to the CDC but had not been reporting long enough to calculate stable adjusted estimates. Surveillance data were de-identified by the states before transmission to the CDC. Persons reported to surveillance systems were not necessarily receiving clinical care.
Inclusion and Exclusion Criteria
Data were combined from contributing U.S. clinic-based cohort studies of HIV-infected adults (aged ≥18 years) who received care from 1 January 2000 to 31 December 2008. Persons with 1 or more HIV VL or CD4 cell count measurements in any calendar year during this time were defined as “in care” for that year and included in our analysis. Interval cohort studies and Canadian cohort studies that participate in NA-ACCORD were excluded from this analysis because of our focus on trends in HIV care in the United States.
Outcomes
Our outcomes of interest were ART use, log10 HIV VL, and CD4 cell count at death. Antiretroviral regimens were summarized and categorized at the month level for each patient during each calendar year in which his or her CD4 cell count or HIV VL was measured. A regimen was defined as highly active antiretroviral therapy (HAART) if it contained at least 3 drugs, including a protease inhibitor (PI); a nonnucleoside reverse transcriptase inhibitor (NNRTI); an entry inhibitor or integrase inhibitor (new agents); or 3 nucleoside reverse-transcriptase inhibitors, including abacavir or tenofovir. Any other combination of antiretroviral medications was defined as ART. We defined persons as treatment-naive if they had no documented history of being prescribed HAART or ART. We defined persons as “off HAART” or “off ART” if they had not been prescribed either therapy during an entire calendar year but had previously been prescribed it. To ensure that we included only persons who successfully initiated HAART, “prescribed HAART” was defined as this therapy having been prescribed 2 or more months. “Prescribed ART” was defined as this therapy (and no HAART) having been prescribed 1 or more months during a calendar year to reduce misclassifying treatment-exposed persons into the treatment-naive group. The HAART regimen for a calendar year was defined as the regimen that was prescribed for the largest proportion of the year.
We selected the HIV VL measured closest to 30 June to calculate the mean and median HIV VL at the midpoint of each calendar year. For our analyses, we defined virologic suppression as an HIV VL of 2.7 log10 copies/mL or less (≤500 copies/mL) this limit was uniformly imputed for all HIV VL measurements at or below this limit. Results of undetectable HIV VL reported by an assay with a lower limit of detection greater than 2.7 log10 copies/mL were discarded; these constituted fewer than 1% of all HIV VL measurements during the study period. A participant was considered to have a missing HIV VL measurement if no such measurement was recorded in a calendar year between the first measurement and the last measurement, the participant’s death, or 31 December 2008, whichever occurred first.
The CD4 cell count measurement closest to death in the preceding 18 months was efined as the CD4 cell count at death. These counts are reported as × 109 cells/L (to convert to cells/mm3, multiply by 1000). Contributing cohorts use standardized methods to ascertain deaths through national and regional U.S. death registries.
Covariates
Age was calculated using year of birth. Race and ethnicity was categorized as non-Hispanic black, non-Hispanic white, Hispanic, and other or unknown. Risk group for HIV transmission was categorized as MSM, injection drug users (IDUs), heterosexual contact, and other or unknown. Participants who were both MSM and IDUs were categorized as IDUs.
Statistical Analysis
To compare demographic characteristics of PLWH-US with those of NA-ACCORD participants, we used adjusted data reported to HARS from the 40 states with stable, confidential, name-based reporting of HIV infection. Differences in the characteristics of PLWH-US and NA-ACCORD participants were determined by using the chi-square test.
To estimate the percentage of PLWH-US who were NA-ACCORD participants, we divided the number of NA-ACCORD participants who were alive as of 31 December 2008 in the U.S. clinical cohorts by the unadjusted number of adults and adolescents in all 50 states and the District of Columbia who were reported to HARS as living with HIV infection at the end of 2008 (adjusted data for PLWH-US were available for only 40 states, whereas unadjusted data were available for all 50 states and the District of Columbia). This calculation slightly underestimated the proportion of PLWH-US who were NA-ACCORD participants, because the denominator for each state included persons aged 13 to 17 years. Two clinical cohort studies participating in this analysis did not report the participants’ state of residence; the state in which the participating clinic of these participants was located was used as an approximation. Another clinical cohort study included in this analysis, the VACS (Veterans Aging Cohort Study), did not report the participants’ state of residence or the clinic location and thus was excluded from this part of the analysis.
Among NA-ACCORD participants, the following measures were calculated annually from 2000 to 2008: proportion prescribed antiretroviral medications, proportion with a suppressed HIV VL (≤2.7 log10 copies/mL), mean and median log10 HIV VL, and median CD4 cell count at death. These annual calculations provided a serial cross-sectional evaluation of the trends over time. For all analyses not involving initial regimens among treatment-naive HAART initiators or death, a person could contribute data to more than 1 calendar year. Statistical comparisons across calendar years were made by using generalized linear models with generalized estimating equations that included an independent working correlation matrix to take repeated measures from individuals into account (an identity link with normal variance was specified for trends in continuous variables and a log link with binomial variance was specified for trends in categorical variables). Because many participants had an HIV VL of 2.7 log10 copies/mL or less, statistical comparisons across calendar years were made using random-effects models with left-censoring (19, 20) that included a random intercept for each person to take repeated measures into account. For analyses of trends in the proportions of initial regimens among treatment-naive HAART initiators and in median CD4 cell count at death, a person could contribute data to only 1 calendar year during the study period; the Cochran Armitage and Cuzick tests of trends were used for proportions and medians, respectively. A P value less than 0.05 was used to guide statistical interpretation. Analyses were performed using SAS, version 9.2 (SAS Institute, Cary, North Carolina).
Role of the Funding Source
Funding was provided by the National Institutes of Health; the CDC; the Canadian Institutes of Health Research; the Canadian HIV Trials Network; and the government of British Columbia, Canada. The funding sources had no role in the design or conduct of the study; collection, management, analysis, and interpretation of the data; or preparation or review of the manuscript. This manuscript was reviewed and approved by the CDC before submission for peer review; the findings and conclusions in this report are those of the authors and do not necessarily represent the views of the CDC. Participating investigators and contributors are CDC employees. Employees of the CDC conducted the surveillance data analyses, and the report was reviewed and approved by the CDC.
Results
In the U.S. clinical cohort studies participating in the NA-ACCORD, 45 529 persons had at least 1 CD4 cell count or HIV VL measurement between 1 January 2000 and 31 December 2008 and were therefore included in our analysis; 14 014 were treatment-naive HAART initiators. The Table shows the demographic characteristics of the 26 030 NA-ACCORD participants who were alive and in care as of 31 December 2008, compared with the 655 966 PLWH-US reported to HARS from the 40 states with stable, confidential HIV surveillance systems as of 31 December 2008. The NA-ACCORD population of HIV-infected persons was qualitatively similar to PLWH-US; proportions of demographic characteristics differed by 7 or fewer percentage points, except for the HIV transmission risk category (Table). The NA-ACCORD had fewer IDUs than PLWH-US (16% vs. 24%); however, risk factors for HIV transmission were difficult to compare because substantially more NA-ACCORD participants than PLWH-US were classified as belonging to the “other or unknown” risk group (12% vs. 1%).
Table 1.
Table Demographic Characteristics of NA-ACCORD Participants in U.S. Clinical Cohorts Compared With PLWH-US as of 31 December 2008*
| Characteristic | PLWH-US, n (%)† | NA-ACCORD, n (%) |
|---|---|---|
| Total participants | 655 966 | 26 030 |
| Age | ||
|
| ||
| 18–19 y | 3764 (1) | 38 (0) |
|
| ||
| 20–24 y | 21 197 (3) | 468 (2) |
|
| ||
| 25–29 y | 39 603 (6) | 1164 (4) |
|
| ||
| 30–34 y | 54 895 (8) | 1863 (7) |
|
| ||
| 35–39 y | 83 935 (13) | 3128 (12) |
|
| ||
| 40–44 y | 121 465 (19) | 4765 (18) |
|
| ||
| 45–49 y | 128 546 (20) | 5455 (21) |
|
| ||
| 50–54 y | 94 957 (14) | 4236 (16) |
|
| ||
| 55–59 y | 57 359 (9) | 2658 (10) |
|
| ||
| 60–64 y | 28 141 (4) | 1345 (5) |
|
| ||
| ≥65 y | 22 103 (3) | 910 (3) |
|
| ||
| Sex | ||
| Female | 175 392 (27) | 5472 (21) |
|
| ||
| Male | 480 570 (73) | 20 558 (79) |
|
| ||
| Race/ethnicity | ||
| White, not Hispanic | 214 895 (33) | 10 541 (40) |
|
| ||
| Black, not Hispanic | 310 622 (47) | 10 429 (40) |
|
| ||
| Hispanic | 113 944 (17) | 3481 (13) |
|
| ||
| Other/unknown | 16 506 (3) | 1579 (6) |
|
| ||
| HIV transmission risk | ||
| Male-to-male sexual contact | 306 613 (47) | 11 231 (43) |
|
| ||
| Injection drug use‡ | 157 286 (24) | 4194 (16) |
|
| ||
| Heterosexual contact | 184 266 (28) | 7485 (29) |
|
| ||
| Other/unknown | 7801 (1) | 3120 (12) |
CDC = Centers for Disease Control and Prevention; NA-ACCORD = North American AIDS Cohort Collaboration on Research and Design; PLWH-US = persons living with HIV in the United States.
P values were calculated by using the chi-square test. For comparisons by population (PLWH-US vs. NA-ACCORD), P < 0.001 for all characteristics. Percentages may not add to 100% because of rounding.
These CDC surveillance data are from 40 states with stable, confidential, name-based HIV surveillance systems as of 2008 (Alabama, Alaska, Arizona, Arkansas, Colorado, Connecticut, Florida, Georgia, Idaho, Illinois, Indiana, Iowa, Kansas, Kentucky, Louisiana, Maine, Michigan, Minnesota, Mississippi, Missouri, Nebraska, New Hampshire, New Jersey, New Mexico, New York, Nevada, North Carolina, North Dakota, Ohio, Oklahoma, Pennsylvania, South Carolina, South Dakota, Tennessee, Texas, Utah, Virginia, West Virginia, Wisconsin, and Wyoming). Data have been adjusted for reporting delays and missing risk factors.
Includes participants who reported male-to-male sexual contact in addition to injection drug use.
We estimated that 3.1% of NA-ACCORD participants (26 030 of 831 578) who were alive as of 31 December 2008 and in care in 2008 were among the unadjusted estimate of PLWH-US in all 50 states and the District of Columbia at the end of 2008. Figure 1 shows the estimated percentages by state, excluding data from the multicenter VACS cohort (2146 participants), which reported neither the participants’ state of residency nor their clinical care location. The remaining 23 884 NA-ACCORD participants (91.8%) who were alive and in care as of 31 December 2008 represented 2.8% of the 831 578 PLWH-US in all 50 states and the District of Columbia at the end of 2008. No NA-ACCORD participants reported residing or receiving clinical care in 14 states (Arkansas, Connecticut, Idaho, Indiana, Kansas, Maine, Nebraska, New Hampshire, New Mexico, North Dakota, Rhode Island, South Dakota, Vermont, and Wisconsin).
Figure 1.
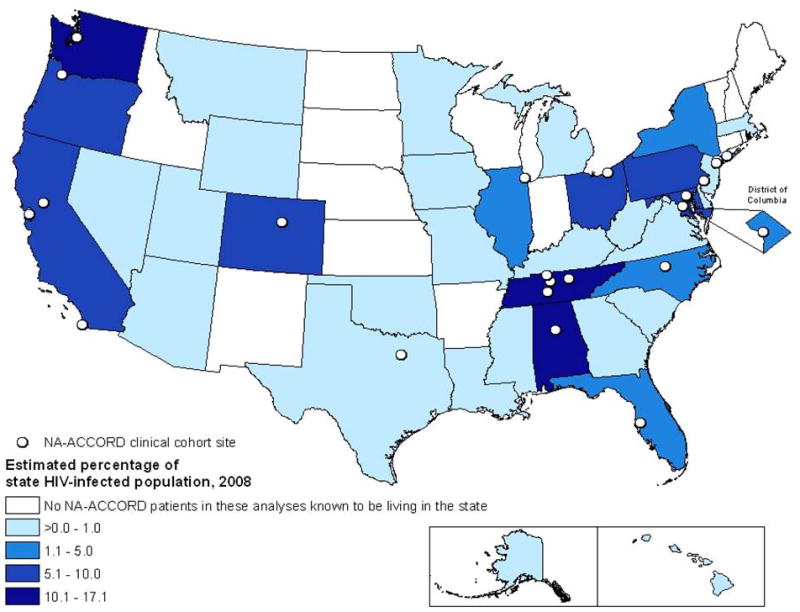
Unadjusted percentages of persons living with HIV infection in the 50 states and the District of Columbia who were alive and in care among U.S. clinical cohorts participating in NA-ACCORD (n = 23 884), by state, year-end 2008. Data from the multicenter Veteran’s Aging Cohort Study are excluded because residency information was not available for participants or for their site of clinical care. Two additional cohorts, the HIV Research Network and the HIV Outpatient Study, report residency by the location of clinical care. As of 2008, stable, confidential, name-based systems for reporting persons living with HIV infection to the CDC were used in Alabama, Alaska, Arizona, Arkansas, Colorado, Connecticut, Florida, Georgia, Idaho, Illinois, Indiana, Iowa, Kansas, Kentucky, Louisiana, Maine, Michigan, Minnesota, Mississippi, Missouri, Nebraska, New Hampshire, New Jersey, New Mexico, New York, Nevada, North Carolina, North Dakota, Ohio, Oklahoma, Pennsylvania, South Carolina, South Dakota, Tennessee, Texas, Utah, Virginia, West Virginia, Wisconsin, and Wyoming. Estimated totals from these states were adjusted for reporting delays and missing risk factors. The remaining 10 states (California, Delaware, Hawaii, Maryland, Massachusetts, Montana, Oregon, Rhode Island, Vermont, and Washington) and the District of Columbia reported only unadjusted estimates for year-end 2008. NA-ACCORD = North American AIDS Cohort Collaboration on Research and Design. * No NA-ACCORD participants eligible for this analysis were known to be living in the state.
The median age of the study population increased from 41 years in 2000 to 46 years in 2008 (P < 0.001). The proportion of participants aged 50 years or older increased by an average of 2 percentage points each year (19% to 36%; P < 0.001). From 2000 to 2008, the annual proportion of NA-ACCORD participants who were female remained stable at 21% to 22% (P = 0.62). Annual racial and ethnic distributions were also essentially stable from 2000 to 2008, with only small fluctuations (non-Hispanic white remained stable at 40% to 41% [P = 0.42]; non-Hispanic black decreased from 43% to 40% [P < 0.001]; Hispanic remained stable at 13% to 14% [P < 0.001]; and other or unknown, increased from 4% to 6% [P < 0.001]). The annual percentage of IDUs in care decreased by 7 percentage points (23% to 16%; P < 0.001). After the 2146 VACS participants were excluded in a sensitivity analysis (because more VACS participants were male and older than in other cohort studies), the trends by sex, race and ethnicity, HIV transmission risk, and age remained similar to our overall results.
The annual proportion of participants prescribed HAART increased from 74% in 2000 to 83% in 2008 (P < 0.001), with concomitant decreases in the percentages of treatment-naive participants (from 15% to 10%; P < 0.001) and of participants prescribed ART or who were classified as off ART or off HAART (from 11% to 7%; P < 0.001) (Figure 2, top). Between 2000 and 2008, most participants were prescribed PI-based HAART regimens (42% in 2008), followed by NNRTI-based regimens (31% in 2008), with little change in this pattern over time (Figure 2, middle). However, in 2000 nearly equal percentages of treatment-naïve participants initiating HAART were prescribed PI-based and NNRTI-based regimens (41% vs. 47%, P = 0.001); by 2008 the percentage of initiators prescribed an NNRTI-based regimen increased to 57% and the percentage initiating PI-based HAART decreased to 37% (P < 0.001, Figure 2, bottom).
Figure 2.
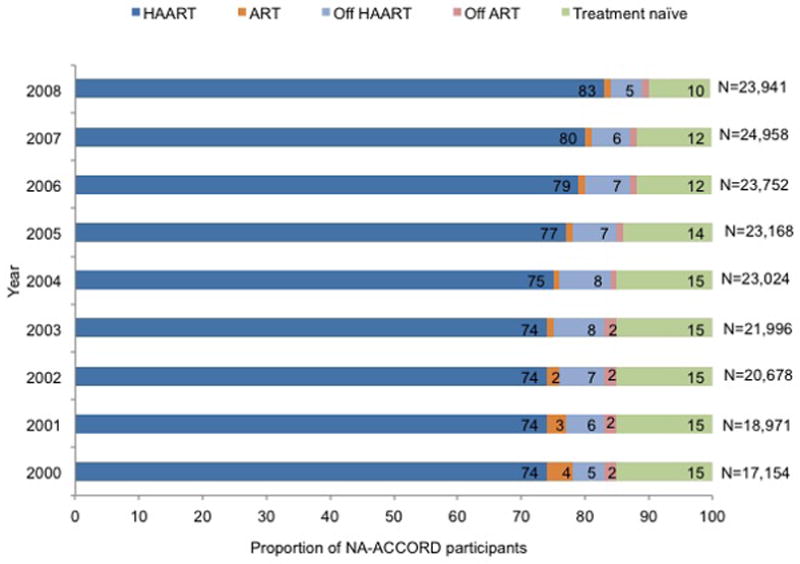
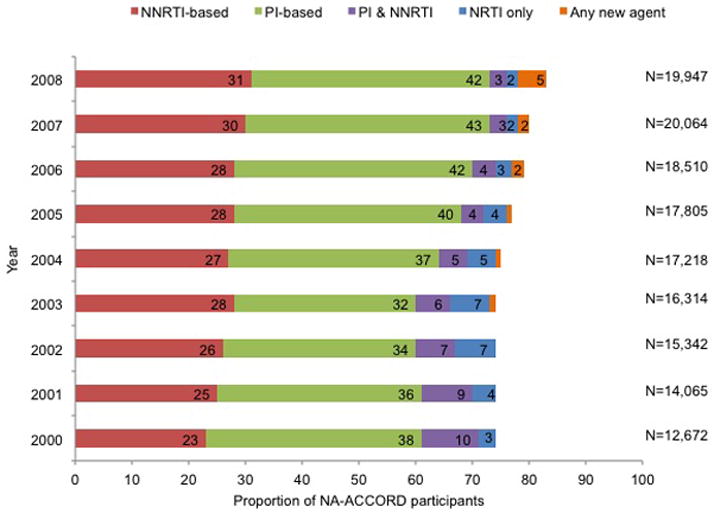
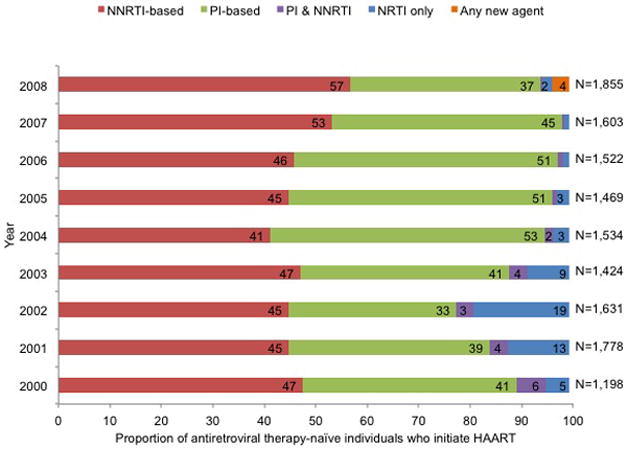
Trends in prescribed ART for HIV infection among participants in U.S. clinical cohort studies in NA-ACCORD, 2000 to 2008. Proportions are noted in the horizontal bars if >1%. P values for the top and middle panels were calculated by using general linear models with generalized estimating equations for repeated measures; for values in the bottom panel, the Cochran Armitage test of trend was used. ART = antiretroviral therapy; HAART = highly active antiretroviral therapy; NA-ACCORD = North American AIDS Cohort Collaboration on Research and Design; NNRTI = nonnucleoside reverse transcriptase inhibitor; NRTI = nucleoside reverse transcriptase inhibitor; PI = protease inhibitor. Top. Antiretroviral treatment status among all participants. (P for trend < 0.001 for HAART, ART, off ART, and treatment-naive; P for trend = 0.137 for off HAART.) Middle. Prescribed HAART, by drug class. (P for trend < 0.001 for all groups.) Bottom. Prescribed therapy, by drug class, among treatment-naive participants initiating HAART. (P for trend < 0.001 for all groups.)
As more participants were prescribed HAART, the percentage of these participants with a suppressed HIV VL increased (from 54% in 2000 to 81% in 2008; P < 0.001), as did the percentage of all participants regardless of treatment status (from 46% in 2000 to 72% in 2008; P < 0.001) (Figure 3). Mean HIV VL also decreased among participants prescribed HAART (from 3.4 to 3.0 log10 copies/mL; P < 0.001) and among all participants regardless of treatment status (from 3.5 to 3.1 log10 copies/mL; P < 0.001). The median HIV VL remained at 2.7 log10 copies/mL or less from 2000 to 2008 among participants prescribed HAART and from 2004 to 2008 among all participants regardless of treatment status. However, from 2000 to 2008 mean HIV VL remained essentially unchanged without trend among participants receiving ART (range, 3.6 to 3.7 log10 copies/mL) and those who were treatment-naive or had stopped receiving HAART or ART (range, 3.7 to 4.1 log10 copies/mL).
Figure 3.
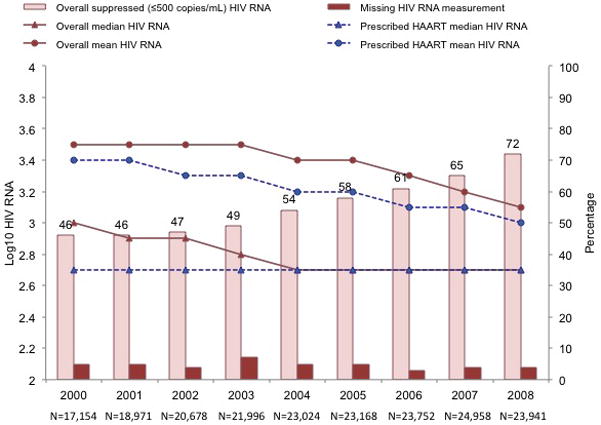
Trends in the proportion of participants with suppressed plasma HIV viral load (≤2.7 log10 copies/mL [≤500 copies/mL]) and midyear plasma HIV VL by ART status among participants in U.S. clinical cohort studies in the NA-ACCORD from 2000 to 2008. For midyear HIV VL, we used the measurement obtained closest to 30 June. (P for trend < 0.001 for overall suppressed HIV VL, missing HIV VL, and mean HIV VL [overall and for HAART recipients]. P values for trends in median HIV VL are not reported.) ART = antiretroviral therapy; HAART = highly active antiretroviral therapy; NA-ACCORD = North American AIDS Cohort Collaboration on Research and Design; VL = viral load.
Of the 5144 participants who died from 2000 to 2008, 4417 (86%) had a CD4 cell count measured within 18 months before death (median time from last CD4 cell count to death, 85 days [interquartile range, 39 to 180 days]). From 2000 to 2008, median CD4 cell count at death increased from 0.060 to 0.209 × 109 cells/L (P < 0.001) (Figure 4).
Figure 4.
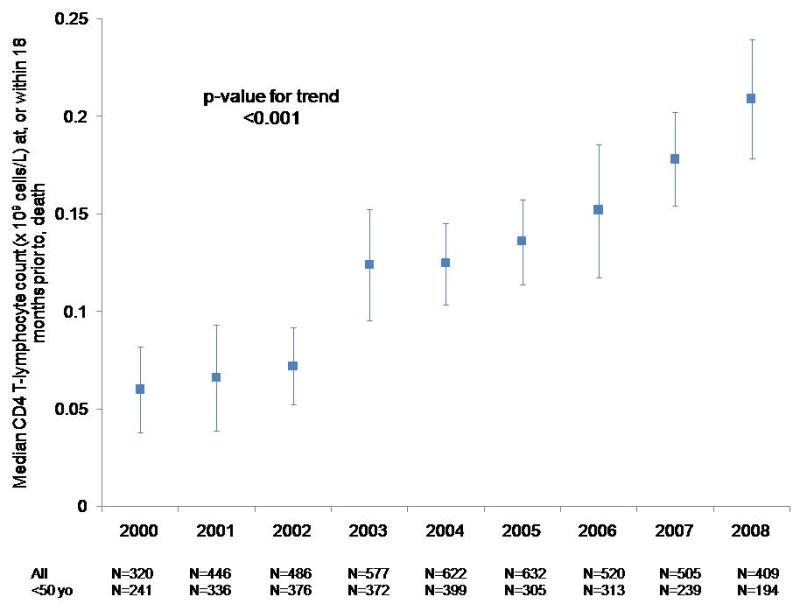
Median CD4 cell count within 18 months before death among NA-ACCORD participants in U.S. clinical cohort studies from 2000 to 2008. Among the 5144 decedents, 4417 had a CD4 cell count measured at or within 18 months before death. NA-ACCORD = North American AIDS Cohort Collaboration on Research and Design.
Discussion
The NA-ACCORD is the largest collaborative study of cohorts of HIV-infected persons receiving clinical care in the United States. We estimate that approximately 3% of PLWH-US are enrolled in clinical cohort studies that participate in the NA-ACCORD and that these NA-ACCORD participants are demographically similar to PLWH-US as reported to the CDC from 40 states with stable HIV infection reporting systems as of 2008. Describing longitudinal trends in HIV treatment and related health outcomes in a population similar to that of PLWH-US demonstrates that use of NA-ACCORD data to fill gaps in existing knowledge, together with national surveillance and cross-sectional surveys, can inform progress toward national HIV goals.
Distributions of age structure, percentage of women, and race and ethnicity were qualitatively similar to PLWH-US; because of the large numbers, even small differences were likely to be statistically significant. However, IDUs are slightly underrepresented in the NA-ACCORD compared with PLWH-US (a difference of 8 percentage points). Twelve percent of NA-ACCORD participants could not be classified into an HIV transmission risk group; some of these individuals were likely to be IDUs Trends in HIV VL could be affected by this underrepresentation because IDUs tend to have poorer clinical response to treatment (21–23). The CD4 cell count at death could also be affected because of the increased risk for death at higher CD4 cell counts among IDUs than among other HIV transmission risk groups (24). Another potential difference to note is geographical coverage of the NA-ACCORD and adjusted CDC surveillance data. NA-ACCORD participants do not live in all 50 states. Adjusted CDC surveillance data for PLWH-US are missing for many states, some of which (such as California) contain large fractions of PLWH-US.
According to the cumulative estimated number of AIDS diagnoses, an estimate for which complete and stable national reporting exists, as of 2009 the 40 states for which adjusted data were available represent approximately 75% of AIDS diagnoses in the 50 states and the District of Columbia (18). The CDC will report adjusted data on PLWH-US for all states in its 2011 HIV surveillance report, and our comparison can then be updated accordingly. However, these additional national surveillance data on HIV infection should not meaningfully alter our comparison.
We acknowledge that clinical cohorts in the NA-ACCORD do not include HIV-infected adults who are not receiving HIV clinical care. An estimated 75% of PLWH-US successfully link into HIV care within a year of receiving their HIV diagnosis, and 90% link into HIV care within 3 to 5 years after diagnosis (25). Although only 45% are estimated to be engaged in regular care (≥2 visits annually, ≥3 months apart) (26), many patients reengage in care sporadically (25). Thus, we believe that by virtue of its size and demographic characteristics, the NA-ACCORD provides and will continue to provide the most generalizable cohort data available about the clinical epidemiology of Americans in care for HIV infection. In addition, the NA-ACCORD is especially well-positioned to monitor longitudinal trends in the use of and response to HIV therapy, as well as the adoption of HIV-related guidelines and other quality-of-care standards.
We note that our estimate of persons receiving any ART in 2007 was 81%, whereas the self-reported estimate was 85% from the 3643 persons included in the 2007 cycle of the MMP (27). Although NNRTI-based regimens were consistently the initial therapies of choice from 2000 to 2008, most patients were prescribed PI-based regimens in each of these years. To our knowledge, our findings provide the first publicly available data on national trends in use of ART. These data, in conjunction with those on health outcomes from NA-ACCORD, can be used for clinical and cost-effectiveness modeling to inform improvements in HIV treatment, both now and when future antiretroviral agents become available.
Population-level ecological analyses from San Francisco (28) and British Columbia (29) have reported that decreases in new HIV infections paralleled increases in the fractions of HIV-infected patients in these jurisdictions who were treated and who achieved virologic suppression. Our analysis of clinical cohort data demonstrates at a national level that increasing use of HAART is accompanied by decreasing HIV VL in the observed population; such decreases have been shown definitively to reduce the amount of virus in a patient’s plasma and genital secretions (30) and substantially reduce individual transmission events and new infections (31). Data from the NA-ACCORD on HIV viral suppression can inform models of transmission dynamics.
The CD4 cell count is an established and useful barometer of immune status in HIV-infected adults. Many recent reports, including previous ones from the NA-ACCORD, have described trends in CD4 cell counts among persons in care in the United States. They usually focus on CD4 cell counts at the time of HIV diagnosis (32) or at presentation to care (7, 33–36) or while the patient is receiving treatment (37–40). Although change in mortality is the gold standard for assessing the effects of morbidity and quality of care, we are not aware of any surveillance reports or reports from larger U.S. cohort studies that have looked at CD4 cell count at death. At a national level, we have found a steady increase in immune system preservation at death. We can infer from this finding that immune suppression and associated conditions (opportunistic illnesses) are probably contributing less to mortality, as has been observed in smaller U.S. cohort studies (41–45) that have reported a shift in mortality from AIDS-related to non–AIDS-related causes. When we compared deaths in 2000 or 2001 with those in 2007 or 2008 among our study participants, 60% and 53%, respectively, had information about the cause of death. From these limited data, the proportion of deaths with a primary or underlying cause that was not associated with an AIDS-defining illness increased from 52% to 58% (P = 0.001). Additional data are being collected for a more comprehensive examination of causes of death in the NA-ACCORD.
Our analysis has limitations. First, the NA-ACCORD is limited to adults with HIV infection—adolescent and pediatric information is not available. Second, as a longitudinal cohort study, our observations are obtained from a convenience sample; however, we believe our sample is sufficiently similar in its demographic diversity to the population of PLWH-US and is sufficiently large to adequately represent for monitoring trends. Third, participants were not classified into the multiple transmission risk category of MSM and IDU. All NA-ACCORD participating cohorts contribute a primary single HIV transmission risk category; some cohorts provide additional information on multiple risks for HIV transmission. Participants who are both MSM and IDUs may have been at higher risk for negative health outcomes than those with either risk alone.
Finally, the 3-fold increase in CD4 cell count at death may have been due to a cohort effect. The clinical cohorts contributing to our analyses are dynamic (participants entered and left clinical care during the study period), which reduces the influence of a cohort effect compared with a closed cohort. We observed higher mortality rates among participants with a CD4 cell count less than 0.200 × 109 cells/L, regardless of age; however, differences in mortality rates by CD4 count stratum increased with age (data not shown) (Appendix, available at www.annals.org). Thus, the increase in CD4 cell count at death that we observed was probably not due to participants with lower CD4 cell counts dying at younger ages and thereby enriching the remaining cohort with survivors who had higher counts. The Appendix presents additional data.
Our study’s strengths include the low cost of monitoring trends in HIV treatment and related outcomes in the NA-ACCORD. Participation in this cohort requires no new data collection (only standardized collation of existing data), which lowers the operating cost of the study, speeds dissemination of summary data to within 1 to 4 years after collection of the primary data, and reduces barriers to participation by other cohorts. Cohorts of HIV-infected adults can join the NA-ACCORD at any time, and membership continues to grow. Having more participants will probably increase the generalizability of our collaborative findings. The characteristics of NA-ACCORD participants and PLWH-US will continue to be monitored, and methods will be employed as needed to improve the generalizability of findings to specified target populations (46). Although cross-sectional probability surveys are designed to produce a representative sample, differential nonparticipation requires statistical correction and greater operating expense and longitudinal data are not available to monitor trends. We believe that longitudinal cohort studies and cross-sectional probability surveys provide complementary information and that both are necessary to fully characterize the U.S. HIV epidemic and inform public health action to optimize the morbidity and mortality of Americans living with HIV infection.
In summary, the NA-ACCORD is uniquely positioned to provide timely longitudinal data on the clinical epidemiology and health of adults living with HIV infection in the United States. To our knowledge, our analysis is the first to provide data about national trends in antiretroviral prescription. We show that from 2000 to 2008, the percentage of U.S. participants in clinical care who were prescribed HAART increased, as did the percentage of all patients who achieved a suppressed HIV VL. Simultaneously, the median CD4 cell count at death increased by 0.149 × 109 cells/L to greater than 0.200 × 109 cells/L. New cohort studies continue to join the NA-ACCORD. We expect their addition to increase the similarity of NA-ACCORD participants to a national probability sample of persons living with HIV infection, and that this collaboration will remain an important means of monitoring trends that document efforts to improve health outcomes for these persons, as articulated in the National HIV/AIDS Strategy (5).
Supplementary Material
Acknowledgments
Grant Support: By grants U01-AI069918, U10-AA13566, U01-AI31834, U01-AI34989, U01-AI34993, U01-AI34994, U01-AI35004, U01-AI35039, U01-AI35040, U01-AI35041, U01-AI35042, U01-AI35043, U01-AI37613, U01-AI37984, U01-AI38855, U01-AI38858, U01-AI42590, U01-AI68634, U01-AI68636, U01-HD32632, U10-EY08057, U10-EY08052, U10-EY08067, UL1-RR024131, UL1-RR024131, M01-RR-00052, M01-RR00071, M01-RR00079, M01-RR00083, M01-RR00722, M01-RR025747, P30-AI27757, P30-AI27767, P30-AI27763, P30-AI50410, P30-AI54999, R01-DA04334, R01-DA12568, R01-DA11602, R01-AA16893, R24-AI067039, Z01-CP010176, AHQ290-01-0012, N02-CP55504, AI-69432, AI-69434, K01-AI071725, K23-AI610320, K23-EY013707, K24-DA00432, K01-AI093197 (Dr. Althoff) and F31-DA30254 (D. Hanna) from the National Institutes of Health; contract CDC200-2006-18797 from the CDC; grants TGF-96118, HCP-97105, CBR-86906, CBR-94036, KRS-86251, and 169621 from the Canadian Institutes of Health Research; the Canadian HIV Trials Network, project 24; and the Government of British Columbia. The CDC funds all U.S. states and the District of Columbia to conduct HIV surveillance and provides technical assistance to all funded areas. Participating investigators and contributors are CDC employees. Employees of the CDC conducted the surveillance data analyses, and the report was reviewed and approved by the CDC.
Appendix 1
We have reported median CD4 cell counts at death among participants who had at least 1 CD4 cell count or HIV VL measurement from 2000 to 2008 in U.S. clinical cohorts that participate in the NA-ACCORD; therefore, the extent to which the median CD4 cell count at death was a function of the aging cohort (a cohort effect) must be addressed. A cohort effect is more prominent in a closed cohort. The clinical cohorts contributing to our analyses are dynamic (participants entered and left clinical care during the study period), which reduces the influence of a cohort effect compared with a closed cohort. The Appendix Table describes the dynamics of the cohort.
Appendix Table.
Study Population Dynamics, by Year
| Variable | 2000 | 2001 | 2002 | 2003 | 2004 | 2005 | 2006 | 2007 | 2008 |
|---|---|---|---|---|---|---|---|---|---|
| Total Participants, n | 45 852 | 45 667 | 45 271 | 44 863 | 44 281 | 43 536 | 42 851 | 42 215 | 41 397 |
| Not yet enrolled, n | 26 039 | 22 269 | 18 616 | 14 880 | 11 362 | 8070 | 4883 | 1581 | 0 |
| In care, n* | 16 831 | 18 654 | 20 328 | 21 589 | 22 598 | 22 823 | 23 400 | 24 615 | 23 665 |
| Out of care, n† | 2336 | 3822 | 5275 | 7106 | 8909 | 11 309 | 13 270 | 14 695 | 16 720 |
| Died, n | 323 | 461 | 526 | 644 | 706 | 667 | 649 | 662 | 506 |
HIV VL = HIV RNA plasma viral load
Defined as having a CD4 cell count or HIV VL measurement in the calendar year.
Defined as previously enrolled, not dead, but having no CD4 cell count or HIV VL measurement in the calendar year.
Although some participants entered and left care during the study period, many who enter are retained in care for many years, increasing the overall age of the cohort (Appendix Figure 1). Cohort effects may therefore have affected our analysis of CD4 cell count at death (47). Because the risk for death is higher at lower CD4 cell counts regardless of age, persons with lower counts may have had a higher risk for exiting the cohort by dying, thus enriching the remaining cohort under observation with survivors who had higher counts and resulting in higher counts at death even if the age- and CD4 cell count–specific mortality rates remained unchanged. To investigate this, we examined trends in mortality rates by CD4 cell count, stratified by age group (Appendix Figure 2).
Appendix Figure 1.
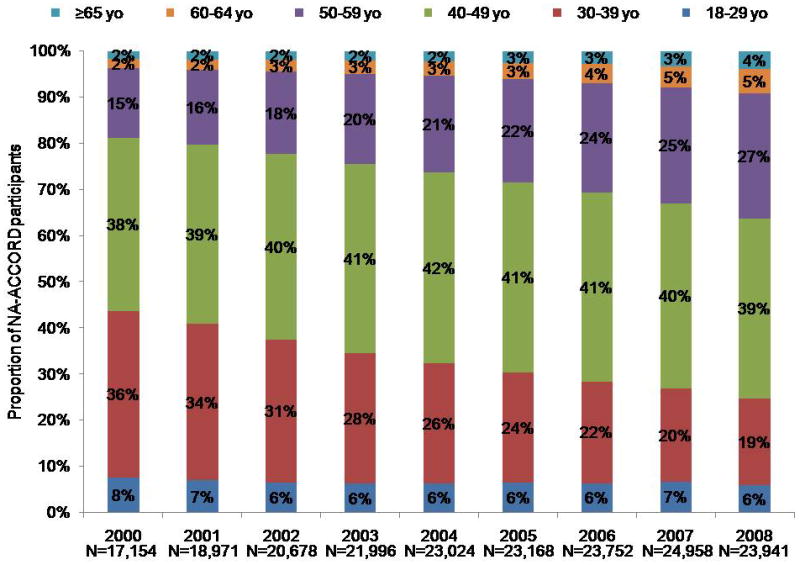
Age distribution of study population, by year.
Appendix Figure 2.
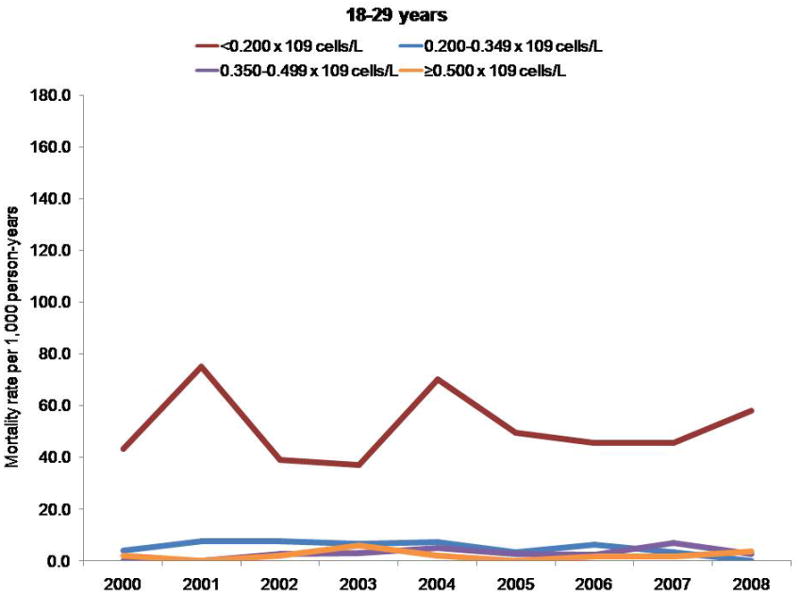
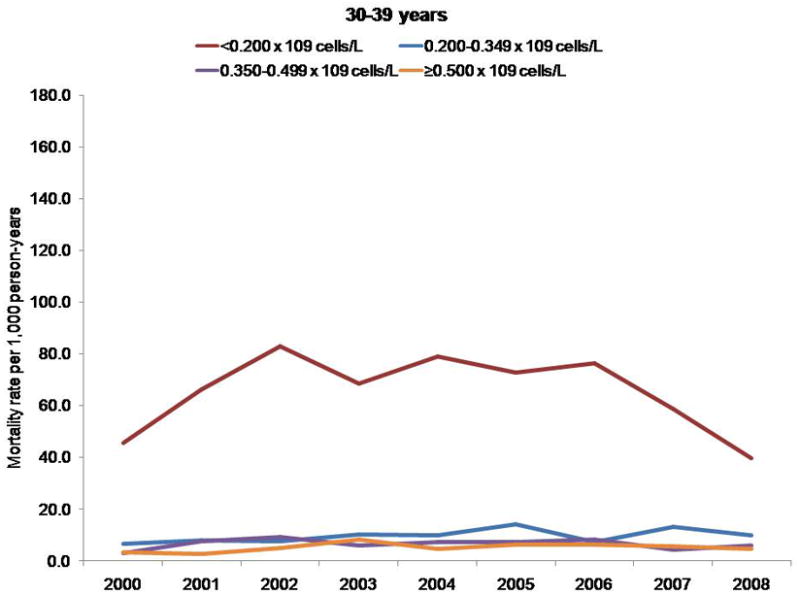
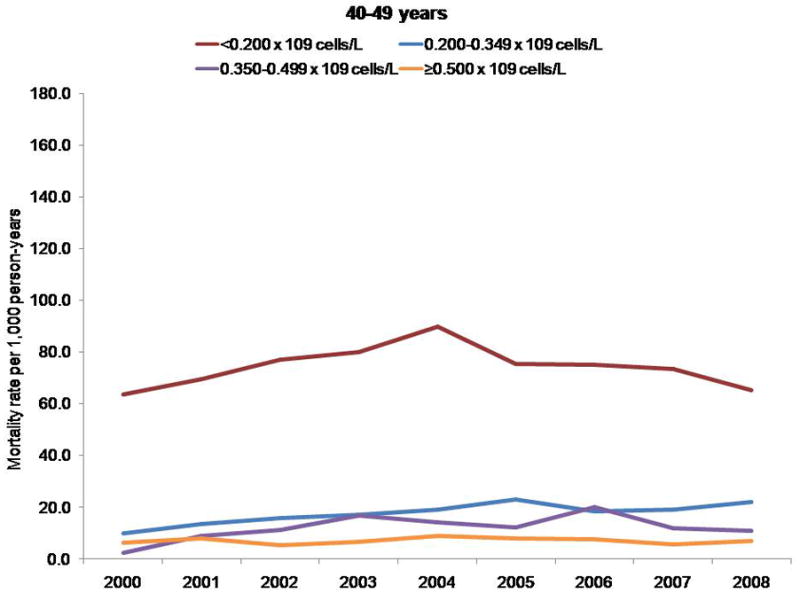
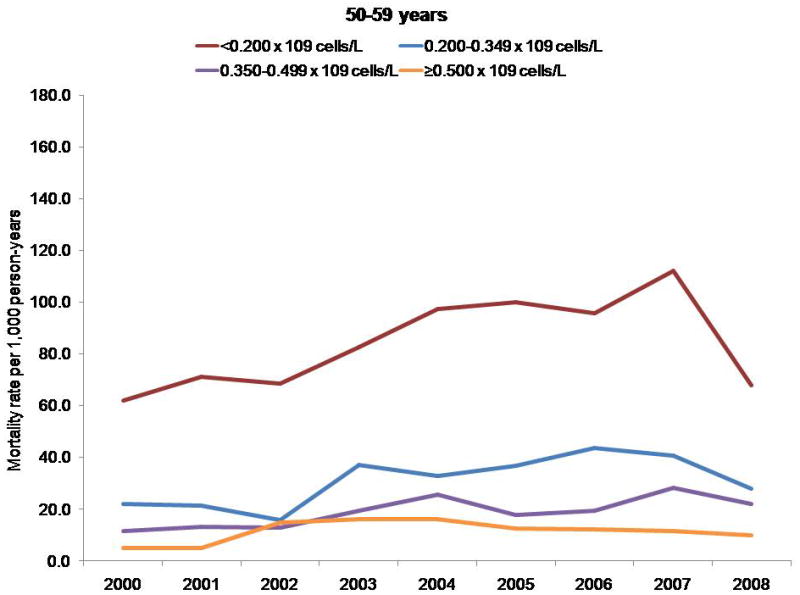
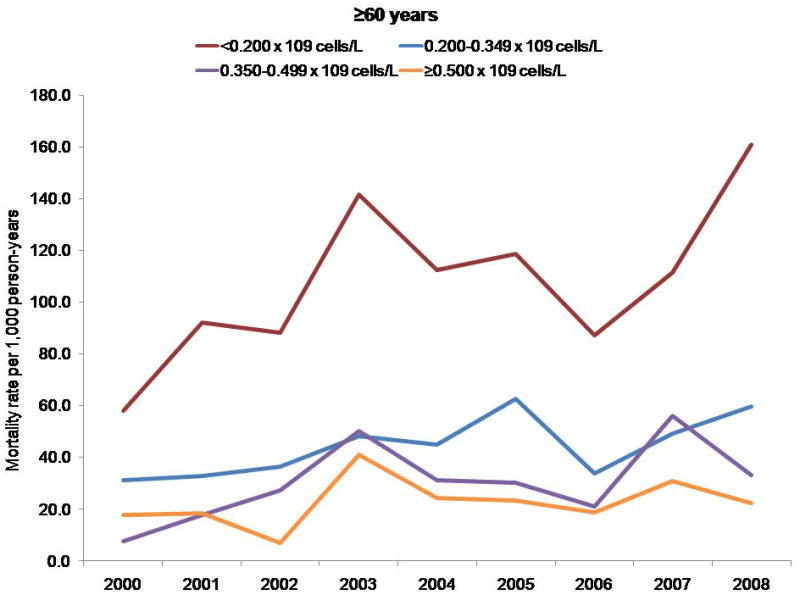
Mortality rates per 1000 person-years, by CD4 cell count and age group.
Persons with a CD4 cell count less than 0.200 × 109 cells/L had a higher mortality rate, regardless of age; however, differences in mortality rates by CD4 cell count stratum increased with age. The NA-ACCORD has shown that older persons enter into care in NA-ACCORD clinics with lower CD4 cell counts than younger persons (33). Thus, the observed increase in CD4 cell count at death was probably not due to persons with lower CD4 cell counts dying at younger ages and thereby leaving only survivors with higher CD4 counts under observation.
Appendix 2: Cohorts and Representatives of the NA-ACCORD
AIDS Link to the IntraVenous Experience: Gregory D. Kirk.
Adult AIDS Clinical Trials Group Longitudinal Linked Randomized Trials: Constance A. Benson, Ronald J. Bosch, and Ann C. Collier.
Fenway Health HIV Cohort: Stephen Boswell, Chris Grasso, and Ken Mayer.
HAART Observational Medical Evaluation and Research: Robert S. Hogg, Richard Harrigan, Julio Montaner, and Angela Cescon.
HIV Outpatient Study: John T. Brooks and Kate Buchacz.
HIV Research Network: Kelly A. Gebo.
Johns Hopkins HIV Clinical Cohort: Richard D. Moore.
John T. Carey Special Immunology Unit Patient Care and Research Database, Case Western Reserve University: Benigno Rodriguez.
Kaiser Permanente Northern California: Michael A. Horberg and Michael J. Silverberg.
Longitudinal Study of Ocular Complications of AIDS: Jennifer E. Thorne.
Multicenter Hemophilia Cohort Study–II: James J. Goedert.
Multicenter AIDS Cohort Study: Lisa P. Jacobson.
Montreal Chest Institute Immunodeficiency Service Cohort: Marina B. Klein.
Ontario HIV Treatment Network Cohort Study: Sean B. Rourke, Ann Burchell, and Anita R. Rachlis.
Southern Alberta Clinic Cohort: M. John Gill.
Studies of the Consequences of the Protease Inhibitor Era: Steven G. Deeks and Jeffery N. Martin.
University of Alabama at Birmingham 1917 Clinic Cohort: Michael S. Saag, Michael Mugavero, and James Willig.
University of North Carolina, Chapel Hill, HIV Clinic Cohort: Joseph J. Eron and Sonia Napravnik.
University of Washington HIV Cohort: Mari M. Kitahata and Heidi M. Crane.
Vetrans Aging Cohort Study: Amy C. Justice, Robert Dubrow, and David Fiellin.
Vanderbilt-Meharry Centers for AIDS Research Cohort: Timothy R. Sterling, David Haas, and Sam Stinnette.
Women’s Interagency HIV Study: Stephen J. Gange and Kathryn Anastos.
Executive Committee: Richard D. Moore, Michael S. Saag, Stephen J. Gange, Mari M. Kitahata, Rosemary G. McKaig, and Aimee M. Freeman.
Epidemiology and Biostatistics Core: Stephen J. Gange, Alison G. Abraham, Bryan Lau, Keri N. Althoff, Jinbing Zhang, Jerry Jing, Elizabeth Golub, Shari Modur, David Hanna, Peter Rebeiro, Adell Mendes, and Aaron Platt.
Data Management Core: Mari M. Kitahata, Stephen E. Van Rompaey, Heidi M. Crane, Eric Webster, Liz Morton, and Brenda Simon.
Footnotes
Disclaimer: Dr. Althoff and Mr. Zhang had full access to all of the NA-ACCORD data in the study; they take responsibility for the integrity of the data and the accuracy of the data analysis. Drs. Buchacz and Hall had full access to the HARS data in the study; they take responsibility for the integrity of the data and the accuracy of the data analysis. The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the CDC.
Potential Conflicts of Interest: Disclosures can be viewed at www.acponline.org/authors/icmje/ConflictOfInterestForms.do?msNum=M12-0355.
Reproducible Research Statement: Study protocol: For more information about the NA-ACCORD, please go to http://statepiaps.jhsph.edu/naaccord/. Statistical code and data set: Available from Dr. Althoff (e-mail, kalthoff@jhsph.edu).
References
- 1.Palella FJ, Jr, Delaney KM, Moorman AC, Loveless MO, Fuhrer J, Satten GA, et al. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. HIV Outpatient Study Investigators. N Engl J Med. 1998;338:853–60. doi: 10.1056/NEJM199803263381301. [DOI] [PubMed] [Google Scholar]
- 2.Antiretroviral Therapy Cohort Collaboration. Life expectancy of individuals on combination antiretroviral therapy in high-income countries: a collaborative analysis of 14 cohort studies. Lancet. 2008;372:293–9. doi: 10.1016/S0140-6736(08)61113-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention (CDC) HIV surveillance---United States, 1981–2008. MMWR Morb Mortal Wkly Rep. 2011;60:689–93. [PubMed] [Google Scholar]
- 4.Prejean J, Song R, Hernandez A, Ziebell R, Green T, Walker F, et al. HIV Incidence Surveillance Group. Estimated HIV incidence in the United States, 2006–2009. PLoS One. 2011;6:e17502. doi: 10.1371/journal.pone.0017502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.White House Office of National AIDS Policy. National HIV/AIDS Strategy for the United States. Washington, DC: White House Office of National AIDS Policy; 2010. [Accessed at on 3 July 2012.]. www.aids.gov/federal-resources/policies/national-hiv-aids-strategy/nhas.pdf. [Google Scholar]
- 6.McGowan CC, Weinstein DD, Samenow CP, Stinnette SE, Barkanic G, Rebeiro PF, et al. Drug use and receipt of highly active antiretroviral therapy among HIV-infected persons in two U.S. clinic cohorts. PLoS One. 2011;6:e18462. doi: 10.1371/journal.pone.0018462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Seal PS, Jackson DA, Chamot E, Willig JH, Nevin CR, Allison JJ, et al. Temporal trends in presentation for outpatient HIV medical care 2000–2010: implications for short-term mortality. J Gen Intern Med. 2011;26:745–50. doi: 10.1007/s11606-011-1693-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Howe CJ, Cole SR, Napravnik S, Eron JJ. Enrollment, retention, and visit attendance in the University of North Carolina Center for AIDS Research HIV clinical cohort, 2001–2007. AIDS Res Hum Retroviruses. 2010;26:875–81. doi: 10.1089/aid.2009.0282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Belperio PS, Mole LA, Boothroyd DB, Backus LI. Trends in uptake of recently approved antiretrovirals within a national healthcare system. HIV Med. 2010;11:209–15. doi: 10.1111/j.1468-1293.2009.00764.x. [DOI] [PubMed] [Google Scholar]
- 10.Losina E, Schackman BR, Sadownik SN, Gebo KA, Walensky RP, Chiosi JJ, et al. Racial and sex disparities in life expectancy losses among HIV-infected persons in the united states: impact of risk behavior, late initiation, and early discontinuation of antiretroviral therapy. Clin Infect Dis. 2009;49:1570–8. doi: 10.1086/644772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Silverberg MJ, Leyden W, Quesenberry CP, Jr, Horberg MA. Race/ethnicity and risk of AIDS and death among HIV-infected patients with access to care. J Gen Intern Med. 2009;24:1065–72. doi: 10.1007/s11606-009-1049-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Scott JD, Wald A, Kitahata M, Krantz E, Drolette L, Corey L, et al. Hepatitis C virus is infrequently evaluated and treated in an urban HIV clinic population. AIDS Patient Care STDS. 2009;23:925–9. doi: 10.1089/apc.2009.0099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bozzette SA, Berry SH, Duan N, Frankel MR, Leibowitz AA, Lefkowitz D, et al. The care of HIV-infected adults in the United States. HIV Cost and Services Utilization Study Consortium. N Engl J Med. 1998;339:1897–904. doi: 10.1056/NEJM199812243392606. [DOI] [PubMed] [Google Scholar]
- 14.Shapiro MF, Morton SC, McCaffrey DF, Senterfitt JW, Fleishman JA, Perlman JF, et al. Variations in the care of HIV-infected adults in the United States: results from the HIV Cost and Services Utilization Study. JAMA. 1999;281:2305–15. doi: 10.1001/jama.281.24.2305. [DOI] [PubMed] [Google Scholar]
- 15.McNaghten AD, Wolfe MI, Onorato I, Nakashima AK, Valdiserri RO, Mokotoff E, et al. Improving the representativeness of behavioral and clinical surveillance for persons with HIV in the United States: the rationale for developing a population-based approach. PLoS One. 2007;2:e550. doi: 10.1371/journal.pone.0000550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gange SJ, Kitahata MM, Saag MS, Bangsberg DR, Bosch RJ, Brooks JT, et al. Cohort profile: the North American AIDS Cohort Collaboration on Research and Design (NA-ACCORD) Int J Epidemiol. 2007;36:294–301. doi: 10.1093/ije/dyl286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lau B, Gange SJ, Moore RD. Interval and clinical cohort studies: epidemiological issues. AIDS Res Hum Retroviruses. 2007;23:769–76. doi: 10.1089/aid.2006.0171. [DOI] [PubMed] [Google Scholar]
- 18.Centers for Disease Control and Prevention. HIV Surveillance Report, 2009. Vol. 21. Atlanta: Centers for Disease Control and Prevention; 2011. [Google Scholar]
- 19.Thiébaut R, Jacqmin-Gadda H. Mixed models for longitudinal left-censored repeated measures. Comput Methods Programs Biomed. 2004;74:255–60. doi: 10.1016/j.cmpb.2003.08.004. [DOI] [PubMed] [Google Scholar]
- 20.Hughes JP. Mixed effects models with censored data with application to HIV RNA levels. Biometrics. 1999;55:625–9. doi: 10.1111/j.0006-341x.1999.00625.x. [DOI] [PubMed] [Google Scholar]
- 21.Henrich TJ, Lauder N, Desai MM, Sofair AN. Association of alcohol abuse and injection drug use with immunologic and virologic responses to HAART in HIV-positive patients from urban community health clinics. J Community Health. 2008;33:69–77. doi: 10.1007/s10900-007-9069-1. [DOI] [PubMed] [Google Scholar]
- 22.Mehta SH, Lucas G, Astemborski J, Kirk GD, Vlahov D, Galai N. Early immunologic and virologic responses to highly active antiretroviral therapy and subsequent disease progression among HIV-infected injection drug users. AIDS Care. 2007;19:637–45. doi: 10.1080/09540120701235644. [DOI] [PubMed] [Google Scholar]
- 23.Wood E, Montaner JS, Yip B, Tyndall MW, Schechter MT, O’Shaughnessy MV, et al. Adherence and plasma HIV RNA responses to highly active antiretroviral therapy among HIV-1 infected injection drug users. CMAJ. 2003;169:656–61. [PMC free article] [PubMed] [Google Scholar]
- 24.Lau B, Gange SJ, Moore RD. Risk of non-AIDS-related mortality may exceed risk of AIDS-related mortality among individuals enrolling into care with CD4+ counts greater than 200 cells/mm3. J Acquir Immune Defic Syndr. 2007;44:179–87. doi: 10.1097/01.qai.0000247229.68246.c5. [DOI] [PubMed] [Google Scholar]
- 25.Gardner EM, McLees MP, Steiner JF, Del Rio C, Burman WJ. The spectrum of engagement in HIV care and its relevance to test-and-treat strategies for prevention of HIV infection. Clin Infect Dis. 2011;52:793–800. doi: 10.1093/cid/ciq243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hall HI, Gray KM, Tang T, Li J, Shouse L, Mermin J. Retention in care of adults and adolescents living with HIV in 13 U.S. areas. J Acquir Immune Defic Syndr. 2012;60:77–82. doi: 10.1097/QAI.0b013e318249fe90. [DOI] [PubMed] [Google Scholar]
- 27.Blair JM, McNaghten AD, Frazier EL, Skarbinski J, Huang P, Heffelfinger JD. Clinical and behavioral characteristics of adults receiving medical care for HIV infection ---- Medical Monitoring Project, United States, 2007. MMWR Surveill Summ. 2011;60(11):1–20. [PubMed] [Google Scholar]
- 28.Das M, Chu PL, Santos GM, Scheer S, Vittinghoff E, McFarland W, et al. Decreases in community viral load are accompanied by reductions in new HIV infections in San Francisco. PLoS One. 2010;5:e11068. doi: 10.1371/journal.pone.0011068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Montaner JS, Lima VD, Barrios R, Yip B, Wood E, Kerr T, et al. Association of highly active antiretroviral therapy coverage, population viral load, and yearly new HIV diagnoses in British Columbia, Canada: a population-based study. Lancet. 2010;376:532–9. doi: 10.1016/S0140-6736(10)60936-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Baeten JM, Kahle E, Lingappa JR, Coombs RW, Delany-Moretlwe S, Nakku-Joloba E, et al. Partners in Prevention HSV/HIV Transmission Study Team. Genital HIV-1 RNA predicts risk of heterosexual HIV-1 transmission. Sci Transl Med. 2011;3:77ra29. doi: 10.1126/scitranslmed.3001888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cohen MS, Chen YQ, McCauley M, Gamble T, Hosseinipour MC, Kumarasamy N, et al. HPTN 052 Study Team. Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med. 2011;365:493–505. doi: 10.1056/NEJMoa1105243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Centers for Disease Control and Prevention. Reported CD4+ T-lymphocyte results for adults and adolescents with HIV/AIDS—33 states, 2005. HIV/AIDS Surveill Suppl Rep. 2005;11:1–31. [Google Scholar]
- 33.Althoff KN, Gange SJ, Klein MB, Brooks JT, Hogg RS, Bosch RJ, et al. Late presentation for human immunodeficiency virus care in the United States and Canada. Clin Infect Dis. 2010;50:1512–20. doi: 10.1086/652650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gandhi NR, Skanderson M, Gordon KS, Concato J, Justice AC. Delayed presentation for human immunodeficiency virus (HIV) care among veterans: a problem of access or screening? Med Care. 2007;45:1105–9. doi: 10.1097/MLR.0b013e3181271476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Keruly JC, Moore RD. Immune status at presentation to care did not improve among antiretroviral-naive persons from 1990 to 2006. Clin Infect Dis. 2007;45:1369–74. doi: 10.1086/522759. [DOI] [PubMed] [Google Scholar]
- 36.Gay CL, Napravnik S, Eron JJ., Jr Advanced immunosuppression at entry to HIV care in the southeastern United States and associated risk factors. AIDS. 2006;20:775–8. doi: 10.1097/01.aids.0000216380.30055.4a. [DOI] [PubMed] [Google Scholar]
- 37.Lifson AR, Krantz EM, Eberly LE, Dolan MJ, Marconi VC, Weintrob AC, et al. Infectious Disease Clinical Research Program (IDCRP) HIV Working Group. Long-term CD4+ lymphocyte response following HAART initiation in a U.S. Military prospective cohort. AIDS Res Ther. 2011;8:2. doi: 10.1186/1742-6405-8-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lok JJ, Bosch RJ, Benson CA, Collier AC, Robbins GK, Shafer RW, et al. ALLRT team. Long-term increase in CD4+ T-cell counts during combination antiretroviral therapy for HIV-1 infection. AIDS. 2010;24:1867–76. doi: 10.1097/QAD.0b013e32833adbcf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kelley CF, Kitchen CM, Hunt PW, Rodriguez B, Hecht FM, Kitahata M, et al. Incomplete peripheral CD4+ cell count restoration in HIV-infected patients receiving long-term antiretroviral treatment. Clin Infect Dis. 2009;48:787–94. doi: 10.1086/597093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Moore RD, Keruly JC. CD4+ cell count 6 years after commencement of highly active antiretroviral therapy in persons with sustained virologic suppression. Clin Infect Dis. 2007;44:441–6. doi: 10.1086/510746. [DOI] [PubMed] [Google Scholar]
- 41.Smith C, Sabin CA, Lundgren JD, Thiebaut R, Weber R, Law M, et al. Data Collection on Adverse Events of Anti-HIV drugs (D:A:D) Study Group. Factors associated with specific causes of death amongst HIV-positive individuals in the D:A:D Study. AIDS. 2010;24:1537–48. doi: 10.1097/QAD.0b013e32833a0918. [DOI] [PubMed] [Google Scholar]
- 42.Leone S, Gregis G, Quinzan G, Velenti D, Cologni G, Soavi L, et al. Causes of death and risk factors among HIV-infected persons in the HAART era: analysis of a large urban cohort. Infection. 2011;39:13–20. doi: 10.1007/s15010-010-0079-z. [DOI] [PubMed] [Google Scholar]
- 43.Marin B, Thiébaut R, Bucher HC, Rondeau V, Costagliola D, Dorrucci M, et al. Non-AIDS-defining deaths and immunodeficiency in the era of combination antiretroviral therapy. AIDS. 2009;23:1743–53. doi: 10.1097/QAD.0b013e32832e9b78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pacheco AG, Tuboi SH, May SB, Moreira LF, Ramadas L, Nunes EP, et al. Temporal changes in causes of death among HIV-infected patients in the HAART era in Rio de Janeiro, Brazil. J Acquir Immune Defic Syndr. 2009;51:624–30. doi: 10.1097/QAI.0b013e3181a4ecf5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sackoff JE, Hanna DB, Pfeiffer MR, Torian LV. Causes of death among persons with AIDS in the era of highly active antiretroviral therapy: New York City. Ann Intern Med. 2006;145:397–406. doi: 10.7326/0003-4819-145-6-200609190-00003. [DOI] [PubMed] [Google Scholar]
- 46.Cole SR, Stuart EA. Generalizing evidence from randomized clinical trials to target populations: The ACTG 320 trial. Am J Epidemiol. 2010;172:107–15. doi: 10.1093/aje/kwq084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Holford TR. Understanding the effects of age, period, and cohort on incidence and mortality rates. Annu Rev Public Health. 1991;12:425–57. doi: 10.1146/annurev.pu.12.050191.002233. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


