Abstract
OBJECTIVES
The etiology of bacterial vaginosis (BV) remains unknown. We sought to describe longitudinal changes in vaginal microbiota.
METHODS
Thirty-nine women self-collected vaginal specimens twice-weekly for 16 weeks as part of a vaginal douching cessation study. In an analysis where each woman serves as her own control, we used Conditional Logistic Regression to evaluate daily, time-varying factors associated with a woman’s incident BV episode(s) as compared to her persistently BV-negative sample(s). BV was defined by Nugent’s Gram stain score ≥7.
RESULTS
The mean age was 36.8 years; 56.4% were African-American. 46.2% of participants had BV in the first four weeks of observation. Rapid fluctuation of vaginal microbiota was observed in 226 transitions to BV or spontaneous remission. Duration of BV was often short: 51% of the episodes lasted only one sample interval (three days). Among women who had at least one BV episode, the median number of episodes per woman was 8.7 (SD=7.4, range: 1–22). Lubricant use one day prior to specimen collection (adjusted odds ratio (aOR): 11.75, 95% confidence interval (CI): 1.96–70.27) and rectal sex two days prior (aOR: 4.48, 95% CI: 2.79–7.17) were associated with BV onset.
CONCLUSION
Rapid fluctuation of the vaginal microbiota was observed. Longitudinal studies with long intervals between sampling are likely to miss episodes of BV. Recent report of lubricant use and rectal sex were associated with incident BV.
Keywords: bacterial vaginosis, Nugent score, Gram stain, vaginal microbiota, epidemiology
INTRODUCTION
The vaginal microbiota play an important protective role in maintaining the health of women. Disruption of the mutualistic relationship that exists between microbial communities in the vagina and their hosts can lead to bacterial vaginosis (BV), a condition traditionally characterized by a shift in the composition and structure of vaginal microbial communities that results in decreased numbers of lactic acid producing bacteria and elevated vaginal pH. (1) BV has been shown to be an independent risk factor for adverse outcomes including preterm delivery and low infant birth weight, acquisition of sexually transmitted infections (STIs), and development of pelvic inflammatory disease. (2–5) Nationally representative surveys in the U.S. indicate the prevalence of BV is 29% (6), and despite considerable effort, the etiology of BV remains unknown. Moreover, recurrence after antibiotic treatment is common. (7)
In reproductive-age woman, the vaginal microbiota have been shown by cultivation methods to be a dynamic ecosystem subject to shifts over the time course of the menstrual cycle. (8) Vaginal microbial communities may also experience various kinds of chronic and acute disturbances caused by exogenous and endogenous factors such as the use of antibiotics, hormonal contraceptives, sexual intercourse, vaginal lubricants, vaginal douching, aging, phase of the menstrual cycle, pregnancy, and stress. (9–16)
Prospective longitudinal sampling is required to evaluate the factors which may lead to a disturbed microbiota. However, the majority of existing epidemiologic studies of BV were designed such that samples were collected cross-sectionally (at the time BV was diagnosed), monthly or less frequently, and therefore are not adequate for addressing the short-term structural and compositional variations of vaginal microbial communities. There are a few prospective studies with high frequency sampling, and each has suggested a role for several important time-varying behaviors. (12;13;17;18)
We sought to conduct an epidemiologic analysis of daily, time-varying factors and their relation to disturbed vaginal microbiota in a study of vaginal specimens which were self-collected twice-weekly for 16 weeks. As there is a great deal of literature on population-level risk factors for BV (6) (studies which compare risk factors between women), we use a case-crossover analysis in which each participant acts as her own control during prospective BV and non-BV intervals. The strength of this approach is that we do not need to account for all the variation (and confounding) that occurs between women and we are able to decipher the factors which are associated with an individual woman’s risk for incident BV.
MATERIALS AND METHODS
Cohort design
Thirty-nine non-pregnant women who reported the use of vaginal douche products in the two months prior to screening were enrolled in the Parent study, a vaginal douching cessation pilot study. (19) Participants were asked to continue their usual practice and frequency (same hygiene products as they normally used) of vaginal douching during a 4-week observation (phase I) and then cease using all feminine hygiene products for the following 12-weeks (phase II). Thirty-three women successfully completed the longitudinal study (85%). Participants were screened for pregnancy (urine hCG), Chlamydia trachomatis and Neisseria gonorrhoeae (nucleic acid amplification, Becton Dickinson, Sparks, Maryland, BD ProbeTec ET) at enrollment and final visits.
Self-collected vaginal smears were obtained twice-weekly during the 16-week study. Behavioral diaries included a daily yes/no check-off list to report menstrual bleeding, vaginal douching, sexual activity (vaginal intercourse, receptive oral sex, digital penetration, rectal sex, sex toys, condoms, spermicides, lubricants), thong undergarment, medications, use of a diaphragm, sanitary napkin and tampons. These daily, time-varying variables are the focus of the current analysis.
Participants sent the vaginal smears and diaries by mail to the laboratory weekly and one microbiologist batched and evaluated the slides in random order. Vaginal smears were Gram stained, and a microscopy score of 0 to 10 was assigned by using the standardized method described by Nugent. (20) Nugent’s scores reflect the range of vaginal microbiota disruption-- A score of 0–3 is considered normal, 4–6 is designated intermediate disruption and 7–10 is considered BV. Gram stains were evaluated microscopically for polymorphonuclear leukocytes quantified on the basis of the number present per field (X1000). Vaginal samples were positive for neutrophils if there was one or more per field. In this analysis, BV was defined by Nugent’s score ≥ 7. With a sensitivity of 89% and specificity of 83% (21) compared to Amsel’s clinical criteria (22), the Nugent score is a useful research tool, (6) and it can be performed on self-collected vaginal smears, (23) facilitating longitudinal field-based studies (12;19).
The protocol was approved by the Institutional Review Board of the Johns Hopkins University School of Medicine and the University of Maryland School of Medicine. All participants provided written informed consent.
Case crossover analysis
In this longitudinal analysis of vaginal smears, participants served as their own control. A woman had to have experienced both BV onset and persistently-negative samples to be included in the model (n=22). The outcome was observation of incident BV, defined by a BV-positive sample which was immediately preceded by a BV-negative sample. The incident BV sample was compared to a woman’s own persistently negative samples defined by the second BV-negative sample in a series. Sample intervals were approximately 3 days (twice-weekly). Seventy-four incident BV intervals and 340 persistently normal microbiota intervals were available for modeling.
Conditional logistic regression was used to evaluate time-varying factors associated with a woman’s incident BV compared to her persistently normal intervals. Because each woman serves as her own control, this approach eliminates problems of confounding between on time-invariant factors (24) such as risk history and demographics. The model is also able to account for within subject correlations.
Time-varying factors collected from daily diaries that had been identified on the basis of previous literature, biologic plausibility and preliminary univariable analyses were evaluated as possible confounders. In addition, the regression analyses were performed with backward and forward selection procedures to verify the significant predictors of incident BV. Model fit was also evaluated using Akaike’s Information Criteria. Variables that were felt to be biologically relevant, even if they did not achieve statistical significance, were retained in the final model. Data were analyzed using STATA/SE 10.0 for Windows (Stata Corporation, College Station, Texas).
RESULTS
Parent study cohort (n=39)
The mean age of the study participants was 36.8 years (range: 22–53); and 56.4% were African-American, while the remainder were white (35.9%) and other ethnicities (7.7%). During the study observation period, participants reported engaging in douching (95%), vaginal lubricant use (26%) and vaginal intercourse (82%). Nine women (23%) reported rectal sex, however, six women reported the act on just one day and the other three women reported on four, seven and 15 days. One woman was prescribed metronidazole for symptomatic BV and 4 women recorded the use of vaginal antimycotics (topical, over the counter). Among women who completed the 16-week study (n=33), the average number of longitudinally-collected specimens per woman was 32.3 (SD: 1.2, range: 29–34) and behavioral dairies were submitted from 99% of enrollment weeks. (19)
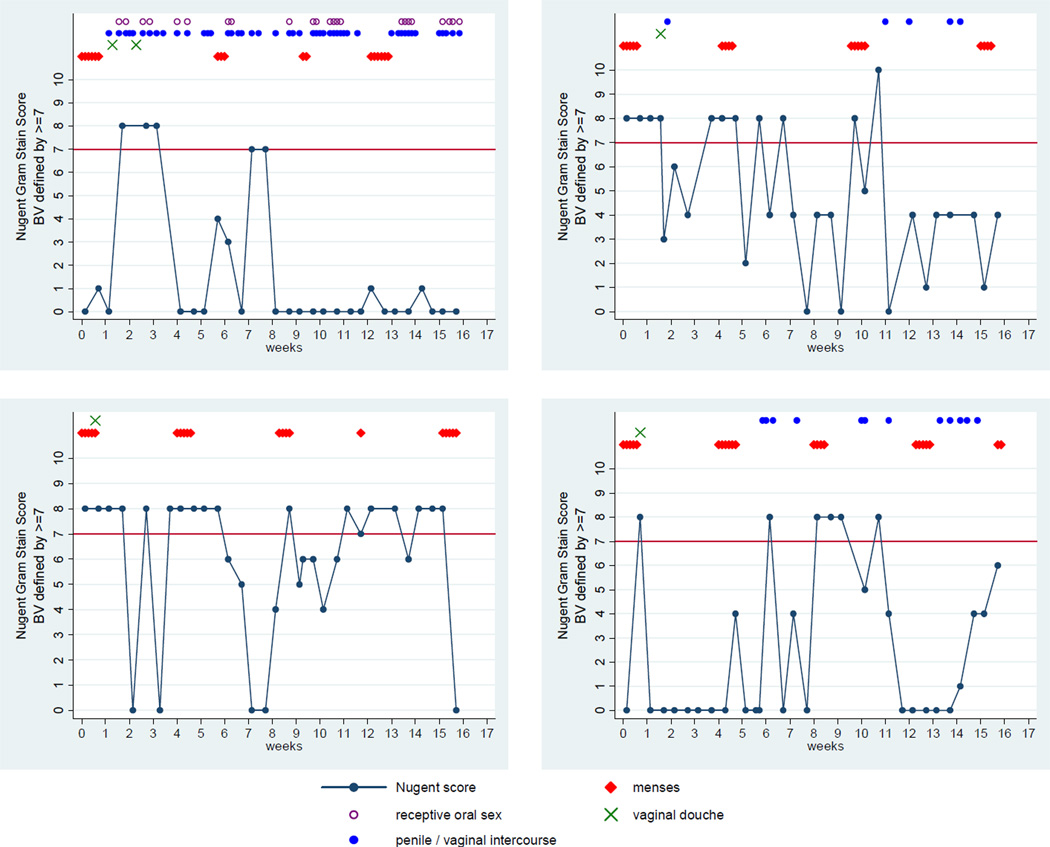
Rapid fluctuation in BV status was often observed. A total of 226 transitions between normal, intermediate and BV were documented (Figure 1). Of the 113 observed remissions, all but one were spontaneous (not the result of antibiotic treatment). Of the 33 women who completed the study, nine women (27%) were observed to have persistently normal vaginal microbiota, while the status of 16 women (49%) fluctuated, as defined by two or more fluctuations between normal and BV. (Table 1) Condom use (ever) was the only significant factor which differed between BV fluctuation groups (Table 2).
Figure 1.
Nugent Gram stain score fluctuation in four participants over the course of the 16-week study.
Table 1.
Number of women with fluctuation of vaginal microbiota over 16 weeks as defined by Nugent's Gram stain score (n=33)*
| N | % | |
|---|---|---|
| single BV episode | 3 | 9 |
| 2 or more BV episodes | 16 | 48 |
| normal to intermediate fluctuation | 5 | 15 |
| persistently normal | 9 | 27 |
Reflected in this table are only the 33 women who completed the 16-week study so that we may have complete observation periods in order to properly classify them by fluctuation status. However, the full analysis includes longitudinal data from 39 women.
TABLE 2.
Descriptive characteristics of women by fluctuation status of Nugent's Gram's stain score* during 16 weeks of twice-weekly specimen collection, Baltimore, MD, 2006–2007, n=33†
| Normal persister |
Intermediate fluctuation |
Fluctuation | p- value |
||||
|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | ||
| Age (years): | 0.23 | ||||||
| 18–29 | 2 | 22.2 | 1 | 20.0 | 3 | 15.8 | |
| 30–39 | 4 | 44.4 | 0 | 0.0 | 10 | 52.6 | |
| 40+ | 3 | 33.3 | 4 | 80.0 | 6 | 31.6 | |
| Ethnicity: | 0.22 | ||||||
| Black | 2 | 22.2 | 2 | 40.0 | 12 | 63.2 | |
| White | 6 | 66.7 | 2 | 40.0 | 6 | 31.6 | |
| Other | 1 | 11.1 | 1 | 20.0 | 1 | 5.3 | |
| Educational attainment: | 0.64 | ||||||
| Less than high school | 2 | 22.2 | 1 | 20.0 | 4 | 21.1 | |
| High school graduate (or GED)‡ | 2 | 22.2 | 1 | 20.0 | 9 | 47.4 | |
| More than high school | 5 | 55.6 | 3 | 60.0 | 6 | 31.6 | |
| Self-reported behavioral factors: | |||||||
| Ever smoked | 4 | 44.4 | 3 | 60.0 | 9 | 47.4 | 1.00 |
| Sexually transmitted infection in 12 months prior to study entry | 1 | 11.1 | 1 | 20.0 | 1 | 5.3 | |
| Lifetime number of sexual partners:§ | 0.08 | ||||||
| 1 | 1 | 11.1 | 1 | 20.0 | 1 | 5.6 | |
| 2–5 | 1 | 11.1 | 3 | 60.0 | 8 | 44.4 | |
| 6–10 | 2 | 22.2 | 1 | 20.0 | 7 | 38.9 | |
| 11+ | 5 | 55.6 | 0 | 0.0 | 2 | 11.1 | |
| Primary contraceptive method reported at baseline | 0.57 | ||||||
| Tubal ligation | 4 | 44.4 | 3 | 60.0 | 8 | 42.1 | |
| Oral contraceptive | 1 | 11.1 | 0 | 0.0 | 4 | 21.1 | |
| Condom | 4 | 44.4 | 1 | 20.0 | 2 | 10.5 | |
| Intrauterine device | 0 | 0.0 | 0 | 0.0 | 1 | 5.3 | |
| None reported | 0 | 0.0 | 1 | 20.0 | 4 | 21.1 | |
| Report of at least one day for each time-varying exposure during 16 weeks of observation | |||||||
| Condom use | 6 | 66.7 | 0 | 0.0 | 8 | 40.0 | 0.05 |
| Digital penetration | 5 | 55.6 | 1 | 20.0 | 10 | 50.0 | 0.50 |
| Insertive sex toy | 2 | 22.2 | 2 | 40.0 | 9 | 45.0 | 0.55 |
| Menses | 9 | 100.0 | 5 | 100.0 | 20 | 100.0 | - |
| Receptive oral sex | 5 | 55.6 | 3 | 60.0 | 12 | 60.0 | 1.00 |
| Rectal sex | 2 | 22.2 | 1 | 20.0 | 5 | 25.0 | 1.00 |
| Thong undergarment | 4 | 44.4 | 2 | 40.0 | 5 | 25.0 | 0.51 |
| Vaginal douching | 9 | 100.0 | 5 | 100.0 | 20 | 100.0 | - |
| Vaginal intercourse | 8 | 88.9 | 4 | 80.0 | 17 | 85.0 | 1.00 |
| Vaginal lubricant | 1 | 11.1 | 2 | 40.0 | 7 | 35.0 | 0.49 |
Women were categorized to fluctuation status based on Nugent's Gram's stain score of twice-weekly collected specimens over 16 weeks of study observation. Nugent’s scores reflect the range of vaginal microbiota disruption. A score of 0–3 is considered normal, 4–6 is designated intermediate disruption and 7–10 is disrupted. In the table, fluctuation is defined by at least 1 incident transitions from normal microbiota to disrupted. Intermediate fluctuation is an individual who fluctuated from normal to intermediate but did not reach full disruption. Normal persistor is an indiviudal who persisted with normal Nugent scores for the duration of the study. The women in the Fluctuation column are those that were included in the crossover analysis.
Although 39 women intiated the study, 33 women completed the full 16 weeks of twice-weekly sampling. Reflected in this table are only the 33 women who completed the study so that we may have complete observation periods in order to properly classify participants by vaginal microbiota fluctuation status.
GED, general education degree
One woman refused report of lifetime number of sexual partners.
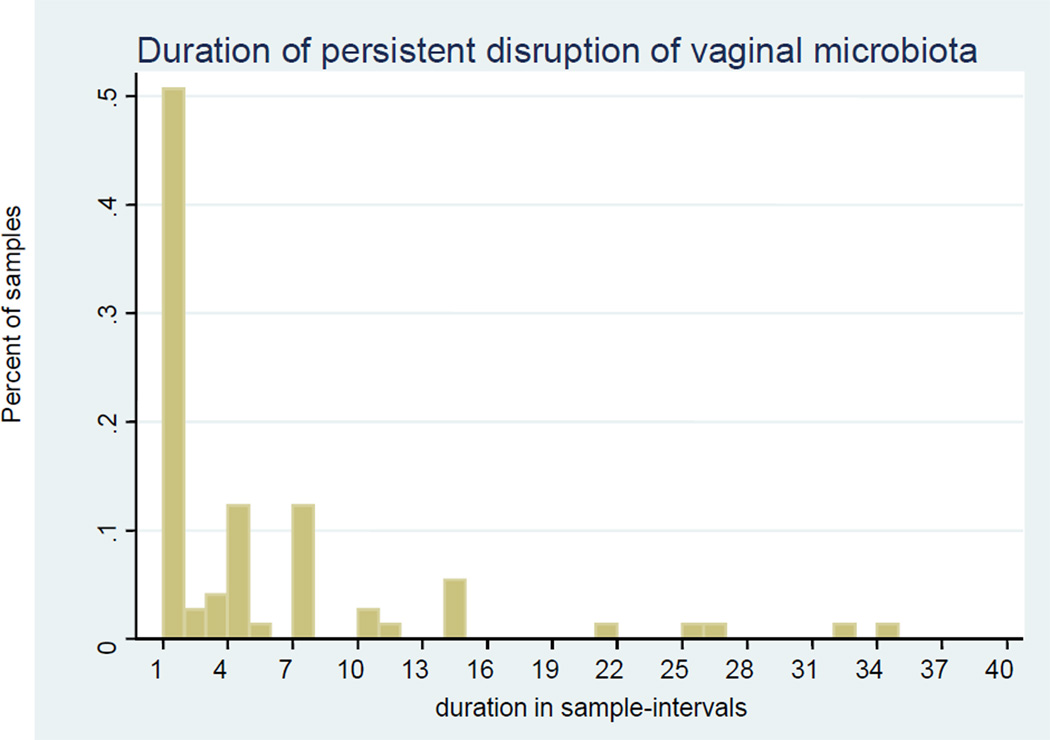
The duration of BV varied widely, but often (51%) was very short, one sample interval lasting less than three days (Figure 2). Among women who had episodes of BV, the mean number of episodes observed was 8.7 (SD: 7.4, range: 1–22, median: 6), with an average of approximately two episodes per month. The estimated average number of sample-days observed with BV was 5.1 (SD: 7.2, range: 1–35, median: 1).
Figure 2.
Duration of disruption of microbiota based on Nugent’s Gram stain scores of vaginal swabs obtained twice weekly. Sample intervals were approximately three days.
Case crossover analysis
Demographics for the sub-sample included in the case crossover analysis (women with at least one BV episode and one persistently normal interval, n=22) are shown in Table 2 (Fluctuation column). The mean age of the sub-sample was 36.5 years (range 22–48).
To determine time-varying factors associated with BV onset, we contrasted factors that preceded BV onset with those reported during observation intervals of normal microbiota. In multivariable modeling, menses in the prior 10 days (aOR: 2.28, 95% CI: 1.11–4.70), vaginal lubricant use reported the day prior (aOR: 11.75, 95% CI: 1.96–70.27) and rectal sex within two days prior (aOR: 4.48, 95% CI: 2.79–7.17) were all associated with a woman’s BV onset as compared to her persistently normal samples (Table 3). The adjusted point estimate for vaginal douching practiced the day prior suggested a trend that douching is associated with BV onset, however it was not statistically significant (aOR: 3.71, 95% CI: 0.79–17.36).
Table 3.
Case crossover analysis for risk of incident bacterial vaginosis compared to persistently normal samples by exposures occurring in the 1–3 days prior to specimen sampling* (n=20)
| Number (%) of intervals with the factor |
OR† | p- value |
95% CI | aOR‡ | p- value |
95% CI | |||
|---|---|---|---|---|---|---|---|---|---|
| Menses | |||||||||
| 1 day prior | 82 (19.90) | 1.45 | 0.34 | 0.68 | 3.10 | ||||
| 3 days prior | 120 (29.13) | 1.34 | 0.39 | 0.69 | 2.61 | ||||
| 10 days prior | 215 (52.18) | 1.94 | 0.08 | 0.92 | 4.11 | 2.28 | 0.03 | 1.11 | 4.70 |
| Menstrual hygiene | |||||||||
| not menstruating | 358 (87.10) | - | - | - | - | ||||
| Tampon | 13 (3.16) | 2.75 | 0.20 | 0.58 | 13.09 | ||||
| Pad | 36 (8.76) | 0.91 | 0.84 | 0.37 | 2.25 | ||||
| Tampon and pad | 4 (0.97) | 3.60 | 0.13 | 0.68 | 18.96 | ||||
| Rectal sex | |||||||||
| 1 day prior | 2 (0.49) | - | - | - | - | ||||
| 2 day prior | 3 (0.73) | 4.14 | 0.00 | 2.15 | 7.96 | 4.48 | 0.00 | 2.79 | 7.17 |
| 3 days prior | 5 (1.22) | 4.06 | 0.00 | 1.60 | 10.34 | ||||
| Vaginal douching | |||||||||
| 1 day prior | 15 (3.65) | 3.91 | 0.10 | 0.78 | 19.52 | 3.71 | 0.10 | 0.79 | 17.36 |
| 2 days prior | 23 (5.60) | 1.57 | 0.51 | 0.41 | 5.96 | ||||
| 3 days prior | 31 (7.56) | 1.25 | 0.74 | 0.33 | 4.72 | ||||
| Vaginal lubricant | |||||||||
| 1 day prior | 12 (2.92) | 7.51 | 0.13 | 0.56 | 100.09 | 11.75 | 0.01 | 1.96 | 70.27 |
| 2 days prior | 23 (5.60) | 2.83 | 0.16 | 0.65 | 12.20 | ||||
| 3 days prior | 31 (7.56) | 1.41 | 0.42 | 0.61 | 3.21 | ||||
| Condom use | |||||||||
| 1 day prior | 21 (5.11) | 1.52 | 0.66 | 0.24 | 9.66 | ||||
| 2 days prior | 33 (8.03) | 1.10 | 0.86 | 0.39 | 3.13 | ||||
| 3 days prior | 51 (12.44) | 1.09 | 0.90 | 0.30 | 3.89 | ||||
| Digital penetration | |||||||||
| 1 day prior | 33 (8.03) | 1.17 | 0.82 | 0.30 | 4.62 | ||||
| 2 days prior | 45 (10.95) | 0.80 | 0.72 | 0.22 | 2.82 | ||||
| 3 days prior | 66 (16.10) | 0.68 | 0.62 | 0.15 | 3.06 | ||||
| Insertive sex toy | |||||||||
| 1 day prior | 9 (2.19) | 0.65 | 0.67 | 0.09 | 4.53 | ||||
| 2 days prior | 15 (3.65) | 0.96 | 0.96 | 0.16 | 5.88 | ||||
| 3 days prior | 22 (5.37) | 1.48 | 0.46 | 0.52 | 4.17 | ||||
| Receptive oral sex | |||||||||
| 1 day prior | 34 (8.27) | 1.43 | 0.67 | 0.28 | 7.17 | ||||
| 2 days prior | 51 (12.41) | 1.40 | 0.54 | 0.48 | 4.12 | ||||
| 3 days prior | 80 (19.51) | 1.25 | 0.56 | 0.59 | 2.61 | ||||
| Thong undergarment | |||||||||
| 1 day prior | 32 (7.79) | 0.79 | 0.88 | 0.04 | 16.27 | ||||
| 2 days prior | 37 (9.00) | 1.20 | 0.91 | 0.05 | 27.54 | ||||
| 3 days prior | 42 (10.24) | 0.93 | 0.96 | 0.05 | 18.83 | ||||
| Vaginal intercourse | |||||||||
| 1 day prior | 81 (19.71) | 1.34 | 0.42 | 0.66 | 2.72 | ||||
| 2 days prior | 131 (31.87) | 0.82 | 0.48 | 0.48 | 1.42 | ||||
| 3 days prior | 180 (43.90) | 0.75 | 0.34 | 0.42 | 1.35 | ||||
Incident bacterial vaginosis defined as new onset of vaginal smear with Nugent's Gram stain score ≥ 7. Persistently negative defined as the second consecutive smear which had a Nugent's Gram stain score ≤ 6. Exposures do not include the sampling day. A woman had to have experienced both BV onset and persistently-negative samples to be included in the model (n=22 women with 74 incident BV episodes).
OR, unadjusted odds ratio
aOR, adjusted odds ratio. Adjusted for days since last antibiotic treatment (1 individual was treated with metronidazole for 3 sample days), days since vaginal antimycotic use (4 individuals for 6 sample days), and other variables listed in the table.
As a proxy for vaginal inflammation, we adjusted for white blood cell presence and the point estimates were not affected. The analysis also demonstrated that sexual activities (vaginal intercourse 1 day prior), receptive oral sex (1–3 days prior), digital penetration (1 day prior), insertive sex toy use (3 days prior) resulted in positive point estimates in their univariable association with BV onset but were not statistically significant (Table 3).
When disruption of vaginal microbiota was defined by Nugent score ≥=4 (representing intermediate disruption), the results were similar except that menses and rectal sex were no longer statistically significant in multivariable modeling (data not shown). Duration of menstruation (1–3, 4–6, >7 days) and vaginal intercourse immediately after menstruation (1–3, 4–7, 8–11, >12 days) were also not significantly associated with incident BV (data not shown).
DISCUSSION
As defined by Nugent’s Gram stain score (20), our study confirms that women may have short episodes of disruption in vaginal microbiota that spontaneously resolve. It was surprising that half of enrolled women had rapid fluctuation in their vaginal microbial communities. This observation reveals a weakness of traditional cross-sectional or long-interval sampling studies, as BV episodes will be missed if samples are collected on a single observation or are collected weekly, monthly, or less frequently. Such studies would underestimate the incidence of BV and the number of recurrences. Our data also indicate that physical disturbances may be associated with BV onset. It is, however, not clear why disruption of the microbiota is more prevalent in some women after such activities (or menses) but not in all women. We hypothesize that vaginal microbial community types vary between women and that a number of community types are more susceptible to disturbance and subsequent risk for BV.
Poor sampling technique or observation error cannot account entirely for our observed fluctuations in Nugent scores. Several longitudinal studies with daily sampling have documented rapid fluctuation in Gram stain vaginal smears. (13;17;18;25)
Lubricant use was a strong predictor of BV onset in this study. Lubricants contain a wide variety of ingredients, including glycerin and chlorhexidine. Chlorhexidine is a broad spectrum microbicide that may cause significant toxic effects to mucosal surfaces. Glycerin may increase local osmolarity and its effect on protective lactic acid producing bacteria is not known.
We also found that rectal sex was associated with BV onset. This association was inferred from only 5 women, but the point estimate was significant with a reasonably tight 95% confidence interval. Transfer of microorganisms from the rectum to the vagina may disrupt the vaginal equilibrium or induce local inflammatory responses that may lead to an increased susceptibility to vaginal microbiota disruption and BV. Despite the frequent report of this sexual practice by women (25% of participants in the Parent study), there are few and conflicting data on the association between rectal sex and BV. (26–28)
In the current analysis, douching the day prior was marginally associated with BV onset. Because samples were collected every 3 days, the study design was not be sufficient to determine if the observed association was causal or due to women douching in response to BV symptoms.
A strength and unique aspect of our study is the analytical method which allows each woman to serve as her own control. Therefore, we do not need to control for time-independent factors which vary between women (such as age or medical history). However, a limitation of this case-crossover approach is that only women who have discordant pairs (women with fluctuating BV status) contribute to the analytic model. Our results, however, are consistent with standard between-woman evaluations of time-varying risk factors. (12)
There are several limitations to our study. Time-varying exposures noted as associated with BV onset are hypothesis-generating and therefore were not corrected for multiple comparisons. Our findings were data driven. It is not known what time frame, whether one or three days prior to sampling, should be used to evaluate different intravaginal exposures. In addition, daily information on vaginal symptoms was not collected. As the Parent study was a longitudinal field-based study with self-sampling, the study design did not include evaluation for BV by Amsel’s clinical criteria. (22) The study population may also not be generalizable. The study recruited women who reported use of vaginal douche products, and this population may represent a group of women who have increased variability in their vaginal microbiota. However, the reasons for douching were varied (19) and as demonstrated by 27% of women persisting with normal Nugent scores throughout the 16 weeks of observation, women with both disturbed and undisturbed vaginal microbiota were sampled. African American women were over-represented in the cohort and they are at higher risk for BV (6) and potentially recurrent BV. Fifty-seven percent of this cohort had at least one episode of BV, while data from a nationally representative- survey found a prevalence of BV of approximately 38% among U.S. women who reported recent douching. (6) Finally, risk for BV may be modulated by contraceptive method (15) and 45% of participants reported a history of tubal ligation.
Both the etiology of BV and the vaginal microbiome remain poorly understood. It is not known how the transient fluctuations observed on Gram stain reflect a clinician’s diagnosis of BV. The fluctuations may represent temporary states of limited clinical significance. However, future research on the implications of longitudinal changes in vaginal microbiota is warranted. If a healthy microbiota is a protective state and a disrupted microbiota is viewed as a non-protective state (guards down) in part because of the bactericidal and virucidal actions of lactic acid, fluctuations of vaginal microbial communities may result in intervals of increased susceptibility to STIs and HIV. (3;4;29–31) Use of new molecular technologies will facilitate a more comprehensive understanding of the changes in vaginal microbial populations that characterize BV and the differences in microbial species composition and abundance that occur between healthy and BV-prone microbiota.
Together with prior studies (13;17;18;25), we have highlighted the dynamic nature of the vaginal microbiota and demonstrated the need for frequent prospective sampling in order to significantly advance our understanding of the dynamics of vaginal microbiota structure and function, and the microbiological, biochemical, molecular, and behavioral contributions to onset and remission of BV.
Key messages.
Rapid fluctuations in vaginal bacterial communities are common.
Women may have short episodes of bacterial vaginosis that spontaneously resolve without antibiotic therapy.
Longitudinal studies may miss bacterial vaginosis episodes if samples are collected infrequently.
Recent report of lubricant use and rectal sex were associated with incident bacterial vaginosis as defined by Gram stain.
Acknowledgements
Funding
This study was supported by NIH grants: K12-RR023250 (to Brotman), UH2-AI083264 (to Ravel), R03-AI061131 (to Zenilman) and K24-AI001633 (to Zenilman).
Footnotes
Competing Interest: None declared.
Parts of this study were presented at the British Association for Sexual Health and HIV – American Sexually Transmitted Diseases Association 3rd Joint Conference, May 7–10, 2008, Brooklyn, NY, Oral presentation # O-20 and The International Society for Sexually Transmitted Disease Research, 18th Biennial Congress, June 28–July 1, 2009, London, Poster # P4.137
Contributors:
RMB participated in the design and conduct of the Parent study, performed the secondary data analysis and wrote the initial manuscript draft. JR participated in the discussion of findings and contributed to the writing of the manuscript. RAC provided guidance, and participated in the interpretation of results and writing of the manuscript. JMZ was involved with designing the Parent study, monitoring of study procedures, interpretation of findings and writing of the manuscript.
Statement on license:
The Corresponding Author has the right to grant on behalf of all authors and does grant on behalf of all authors, an exclusive licence (or non exclusive for government employees) on a worldwide basis to the BMJ Publishing Group Ltd to permit this article (if accepted) to be published in STI and any other BMJPGL products and sub-licences such use and exploit all subsidiary rights, as set out in our licence http://group.bmj.com/products/journals/instructions-for-authors/licence-forms".
Reference List
- 1.Hillier SL, Holmes KK, Marrazzo JM. Sexually Transmitted Diseases. 4th ed. New York: McGraw-Hill, Health Professions Division; 2008. Bacterial Vaginosis; pp. 737–768. [Google Scholar]
- 2.Hillier SL, Nugent RP, Eschenbach DA, Krohn MA, Gibbs RS, Martin DH, et al. Association between bacterial vaginosis and preterm delivery of a low-birth-weight infant. The Vaginal Infections and Prematurity Study Group. N Engl J Med. 1995;333(26):1737–1742. doi: 10.1056/NEJM199512283332604. [DOI] [PubMed] [Google Scholar]
- 3.Schwebke JR, Desmond R. A randomized trial of metronidazole in asymptomatic bacterial vaginosis to prevent the acquisition of sexually transmitted diseases. American Journal of Obstetrics and Gynecology. 2007;196(6):517. doi: 10.1016/j.ajog.2007.02.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Taha TE, Hoover DR, Dallabetta GA, Kumwenda NI, Mtimavalye LA, Yang LP, et al. Bacterial vaginosis and disturbances of vaginal flora: association with increased acquisition of HIV. AIDS. 1998;12(13):1699–1706. doi: 10.1097/00002030-199813000-00019. [DOI] [PubMed] [Google Scholar]
- 5.Ness RB, Kip KE, Hillier SL, Soper DE, Stamm CA, Sweet RL, et al. A cluster analysis of bacterial vaginosis-associated microflora and pelvic inflammatory disease. American Journal of Epidemiology. 2005;162(6):585–590. doi: 10.1093/aje/kwi243. [DOI] [PubMed] [Google Scholar]
- 6.Koumans EH, Sternberg M, Bruce C, McQuillan G, Kendrick J, Sutton M, et al. The prevalence of bacterial vaginosis in the United States, 2001–2004; associations with symptoms, sexual behaviors, and reproductive health. Sex Transm Dis. 2007 Nov;34(11):864–869. doi: 10.1097/OLQ.0b013e318074e565. [DOI] [PubMed] [Google Scholar]
- 7.Bradshaw CS, Morton AN, Hocking J, Garland SM, Morris MB, Moss LM, et al. High recurrence rates of bacterial vaginosis over the course of 12 months after oral metronidazole therapy and factors associated with recurrence. J Infect Dis. 2006;193(11):1478–1486. doi: 10.1086/503780. [DOI] [PubMed] [Google Scholar]
- 8.Bartlett JG, Onderdonk AB, Drude E, Goldstein C, Anderka M, Alpert S, et al. Quantitative bacteriology of the vaginal flora. The Journal of infectious diseases. 1977;136(2):271–277. doi: 10.1093/infdis/136.2.271. [DOI] [PubMed] [Google Scholar]
- 9.Nansel TR, Riggs MA, Yu KF, Andrews WW, Schwebke JR, Klebanoff MA. The association of psychosocial stress and bacterial vaginosis in a longitudinal cohort. Am J Obstet Gynecol. 2006 Feb;194(2):381–386. doi: 10.1016/j.ajog.2005.07.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eschenbach DA, Thwin SS, Patton DL, Hooton TM, Stapleton AE, Agnew K, et al. Influence of the normal menstrual cycle on vaginal tissue, discharge, and microflora. Clin Infect Dis. 2000;30(6):901–907. doi: 10.1086/313818. [DOI] [PubMed] [Google Scholar]
- 11.Guise JM, Mahon SM, Aickin M, Helfand M, Peipert JF, Westhoff C. Screening for bacterial vaginosis in pregnancy. Am J Prev Med. 2001 Apr;20(3 Suppl):62–72. doi: 10.1016/s0749-3797(01)00256-2. [DOI] [PubMed] [Google Scholar]
- 12.Schwebke JR, Richey CM, Weiss HL. Correlation of behaviors with microbiological changes in vaginal flora. J Infect Dis. 1999;180(5):1632–1636. doi: 10.1086/315065. [DOI] [PubMed] [Google Scholar]
- 13.Hay PE, Ugwumadu A, Chowns J. Sex, thrush and bacterial vaginosis. Int J STD AIDS. 1997;8(10):603–608. doi: 10.1258/0956462971918850. [DOI] [PubMed] [Google Scholar]
- 14.Brotman RM, Klebanoff MA, Nansel TR, Andrews WW, Schwebke JR, Zhang J, et al. A longitudinal study of vaginal douching and bacterial vaginosis--a marginal structural modeling analysis. Am J Epidemiol. 2008 Jul 15;168(2):188–196. doi: 10.1093/aje/kwn103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rifkin SB, Smith MR, Brotman RM, Gindi RM, Erbelding EJ. Hormonal contraception and risk of bacterial vaginosis diagnosis in an observational study of women attending STD clinics in Baltimore, MD. Contraception. 2009 Jul;80(1):63–67. doi: 10.1016/j.contraception.2009.01.008. [DOI] [PubMed] [Google Scholar]
- 16.Wilson JD, Lee RA, Balen AH, Rutherford AJ. Bacterial vaginal flora in relation to changing oestrogen levels. International Journal of STD & AIDS. 2007;18(5):308–311. doi: 10.1258/095646207780749583. [DOI] [PubMed] [Google Scholar]
- 17.Priestley CJ, Jones BM, Dhar J, Goodwin L. What is normal vaginal flora? Genitourinary medicine. 1997;73(1):23–28. doi: 10.1136/sti.73.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Keane FE, Ison CA, Taylor-Robinson D. A longitudinal study of the vaginal flora over a menstrual cycle. International Journal of STD & AIDS. 1997;8(8):489–494. doi: 10.1258/0956462971920631. [DOI] [PubMed] [Google Scholar]
- 19.Brotman RM, Ghanem KG, Klebanoff MA, Taha TE, Scharfstein DO, Zenilman JM. The effect of vaginal douching cessation on bacterial vaginosis: a pilot study. Am J Obstet Gynecol. 2008 Jun;198(6):628–627. doi: 10.1016/j.ajog.2007.11.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nugent RP, Krohn MA, Hillier SL. Reliability of diagnosing bacterial vaginosis is improved by a standardized method of gram stain interpretation. J Clin Microbiol. 1991;29(2):297–301. doi: 10.1128/jcm.29.2.297-301.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schwebke JR, Hillier SL, Sobel JD, McGregor JA, Sweet RL. Validity of the vaginal gram stain for the diagnosis of bacterial vaginosis. Obstet Gynecol. 1996;88(4 Pt 1):573–576. doi: 10.1016/0029-7844(96)00233-5. [DOI] [PubMed] [Google Scholar]
- 22.Amsel R, Totten PA, Spiegel CA, Chen KC, Eschenbach D, Holmes KK. Nonspecific vaginitis. Diagnostic criteria and microbial and epidemiologic associations. Am J Med. 1983;74(1):14–22. doi: 10.1016/0002-9343(83)91112-9. [DOI] [PubMed] [Google Scholar]
- 23.Morgan DJ, Aboud CJ, McCaffrey IM, Bhide SA, Lamont RF, Taylor-Robinson D. Comparison of Gram-stained smears prepared from blind vaginal swabs with those obtained at speculum examination for the assessment of vaginal flora. Br J Obstet Gynaecol. 1996;103(11):1105–1108. doi: 10.1111/j.1471-0528.1996.tb09591.x. [DOI] [PubMed] [Google Scholar]
- 24.Maclure M. The case-crossover design: a method for studying transient effects on the risk of acute events. American Journal of Epidemiology. 1991;133(2):144–153. doi: 10.1093/oxfordjournals.aje.a115853. [DOI] [PubMed] [Google Scholar]
- 25.Schwebke JR, Morgan SC, Weiss HL. The use of sequential self-obtained vaginal smears for detecting changes in the vaginal flora. Sex Transm Dis. 1997;24(4):236–239. doi: 10.1097/00007435-199704000-00009. [DOI] [PubMed] [Google Scholar]
- 26.Marrazzo JM, Koutsky LA, Eschenbach DA, Agnew K, Stine K, Hillier SL. Characterization of vaginal flora and bacterial vaginosis in women who have sex with women. The Journal of infectious diseases. 2002;185(9):1307–1313. doi: 10.1086/339884. [DOI] [PubMed] [Google Scholar]
- 27.Cherpes TL, Hillier SL, Meyn LA, Busch JL, Krohn MA. A delicate balance: risk factors for acquisition of bacterial vaginosis include sexual activity, absence of hydrogen peroxide-producing lactobacilli, black race, and positive herpes simplex virus type 2 serology. Sex Transm Dis. 2008 Jan;35(1):78–83. doi: 10.1097/OLQ.0b013e318156a5d0. [DOI] [PubMed] [Google Scholar]
- 28.Antonio MA, Rabe LK, Hillier SL. Colonization of the rectum by Lactobacillus species and decreased risk of bacterial vaginosis. J Infect Dis. 2005;192(3):394–398. doi: 10.1086/430926. [DOI] [PubMed] [Google Scholar]
- 29.Cherpes TL, Meyn LA, Krohn MA, Lurie JG, Hillier SL. Association between acquisition of herpes simplex virus type 2 in women and bacterial vaginosis. Clin Infect Dis. 2003;37(3):319–325. doi: 10.1086/375819. [DOI] [PubMed] [Google Scholar]
- 30.Cohen MS. Sexually transmitted diseases enhance HIV transmission: no longer a hypothesis. Lancet. 1998;351(Suppl 3):5–7. doi: 10.1016/s0140-6736(98)90002-2. [DOI] [PubMed] [Google Scholar]
- 31.Watts DH, Fazzari M, Minkoff H, Hillier SL, Sha B, Glesby M, et al. Effects of bacterial vaginosis and other genital infections on the natural history of human papillomavirus infection in HIV-1-infected and high-risk HIV-1-uninfected women. J Infect Dis. 2005 Apr 1;191(7):1129–1139. doi: 10.1086/427777. [DOI] [PubMed] [Google Scholar]