Abstract
While dietary wholegrain Flaxseed (FS) has potent anti-inflammatory, anti-fibrotic and antioxidant properties in murine models of acute and chronic lung injury, the main bioactive ingredient that contributes to these protective effects remains unknown. This study evaluated the lignan complex of FS (FLC) enriched in secoisolariciresinol diglucoside with respect to lung radioprotective and tumor radiosensitizing efficacy using a mouse model of thoracic radiation-induced pneumonopathy. C57/Bl6 mice were fed 0% FS, 10% FS, 10% FLC or 20% FLC for 3 weeks, then irradiated with a single fraction (13.5 Gy) of X-ray radiation treatment (XRT). Mouse survival was monitored for 4 months after irradiation and inflammatory lung parameters were evaluated in bronchoalveolar lavage (BAL) fluid. Gene and protein levels of protective antioxidant and phase II enzymes were evaluated in lung tissue using qPCR and protein levels were verified by immunoblotting. Prolonged administration of the FLC diet was well tolerated and was not associated with any toxicity. Importantly, comparable to the whole grain 10% FS diet, irradiated mice fed 10% and 20% FLC diets displayed improved survival. Improved hemodynamic measurements were also recorded in irradiated mice fed 10% FS or 10% FLC diet compared to irradiated 0% FS fed mice. Flaxseed lignan complex diet also attenuated polymorphonuclear infiltration and overall lung inflammation to levels comparable to those in nonirradiated mice. Flaxseed lignan complex, similarly to FS, up-regulated gene expression as well as protein levels of protective antioxidant enzymes such as heme oxygenase-1 (HO-1) and NAD(P)H quinone oxidoreductase 1 (NQO1). Dietary FLC induced radiosensitizing effects in our murine model of metastatic lung cancer. Importantly, protection of normal tissue does not thwart tumor cell death by radiation treatment. The dietary lignan complex of FS, mainly consisting of the phenolic secoisolariciresinol, is protective against radiation pneumonopathy in vivo while not hindering the tumoricidal effects of radiotherapy.
INTRODUCTION
The efficacy of radiotherapy against thoracic malignancy is significantly limited by radiation pneumonopathy that occurs on a spectrum of toxicities from acute radiation pneumonitis to chronic pulmonary fibrosis (1, 2). Acute radiation pneumonitis may manifest as cough, dyspnea on exertion and hypoxemia occurring within weeks to months after X-ray radiation therapy (XRT) resolving with or without active treatment. Pulmonary fibrosis occurs months to years after radiation therapy and can lead to permanent impairment in gas transfer and ultimately to decreased lung function. Recent evidence has suggested that radiation-induced effects are caused partly by chronic inflammation and oxidative stress (3). Volatile compounds known as reactive oxygen species (ROS) and reactive nitrogen species (RNS) are generated in large quantities by radiation therapy and have been implicated in this form of lung injury (4). Increased ROS production has been shown in vivo and in vitro to accelerate lipid peroxidation, oxidation of DNA and cellular proteins, as well as activation of proinflammatory cytokines.
There is currently no effective pharmacologic therapy for the prevention of acute or chronic radiation pneumonopathy. To date, the only way to avoid life-threatening radiation pneumonopathy is to modify the irradiation technique to minimize the volume of normal lung receiving a significant radiation dose. Thus, a safe and effective biological radioprotector that inhibits or alters the mechanisms that result in pulmonary damage would be extremely useful. Preclinical data suggest that antioxidant molecules and/or enzymes might offer protection for the lung (5, 6).
Clinical interest has grown for flaxseed (FS) because of its potential health benefits. For the past few years, our group has studied the role of wholegrain dietary FS in radiation-induced injuries. We have shown that FS decreased radiation-induced inflammation and oxidative stress in mice in different clinically related scenarios (7, 8). Importantly, dietary FS mitigated thoracic radiation-induced pneumonitis and fibrosis in experimental rodent models (9). It has also been reported that the protective effects of FS against various types of cancer such as breast (10), prostate (11) and colon cancer (12) are attributed to the presence of plant lignans. Although it is not the main focus of this study, the tumoricidal activity of the flaxseed lignan complex is being evaluated in the context of radiosensitization of tumor cells in lung.
Flaxseed is the richest known source of the mammalian lignan precursor, secoisolariciresinol diglucoside (SDG). As a plant phenolic, SDG was shown in vitro to have direct hydroxyl radical scavenging properties and to inhibit lipid peroxidation (13–15). Flaxseed lignan SDG has been reported to provide potential health benefits in several disease conditions [for review see ref (16)] related to hypercholesterolemia (17), diabetes (18), postmenopausal symptoms (19, 20), cardiovascular health (21), metabolic syndrome and bone health (22) and other diseases. Our group has performed extensive research in characterizing the beneficial effects of this plant phenolic in experimental models of acute respiratory distress syndrome (ARDS), ischemia-reperfusion injury (IRI), radiation pneumonopathy and hyperoxia (7, 8, 23, 24). However, this is the first study focusing on the lung protective effects of the lignan complex of FS, enriched in SDG.
Normal lung parenchyma has an exceptionally low tolerance of ionizing radiation thus clearly limits maximal radiotherapy. Much research is dedicated to finding a radioprotector that would permit higher tumoricidal doses. In this study, we explored the use of the dietary FS lignan complex (FLC) enriched in the phenolic SDG as a potential safeguard against radiation-induced lung injury in our murine model of thoracic XRT. We evaluated the effects of dietary FLC on acute pneumonitis and late radiation fibrosis. To determine whether this therapy is likely to increase the therapeutic index of thoracic XRT, we have also evaluated the impact of FLC on the radiosensitivity of lung cancer cells both in vitro and using an orthotopic lung cancer model. This study will establish if FLC, enriched in SDG, is the main bioactive ingredient of FS that is responsible for radioprotective properties in our murine lung radiation model.
MATERIALS AND METHODS
Animal Protocol
Our studies used 8-week-old female C57/Bl6 mice, a strain that is well characterized in our studies of radiation exposure (9, 25–27). Mice were obtained from Charles River (Wilmington, MA) and housed under animal protocols approved by the Institutional Animal Care and Use Committee (IACUC) of the University of Pennsylvania. Animals were housed in conventional cages under standardized conditions with controlled temperature and humidity and a 12 h light/dark cycle. Animals had free access to water and formulated study diets. For this study, we used n = 15 for each of the nonirradiated control (no XRT) and n =25 for each of the irradiated mouse cohorts (XRT, 13.5 Gy) unless otherwise stated. The data shown here represent combined data from two separate in vivo studies.
Diet Composition and Dietary Treatments
Four diets were used in this study, all of which were based on a semi-purified AIN-93G diet that was modified to contain the test ingredient. Importantly, control (no test ingredient added) and experimental diets were isocaloric, isonitrogenous, and contained equal amounts of dietary lipid and carbohydrate. A detailed dietary composition and ingredient list is provided in Table 1.
TABLE 1.
Composition of Study Dietsa
| Nutrient profile | Diet
|
|||
|---|---|---|---|---|
| 0% FS | 10% FS | 10% FLCb | 20% FLCc | |
| g/kg diet | ||||
| Corn starch | 322.87 | 312.70 | 319.50 | 316.13 |
| Casein - vitamin free | 221.50 | 200.00 | 221.50 | 221.50 |
| Dextrin | 132.00 | 132.00 | 132.00 | 132.00 |
| Sucrose | 100.00 | 100.00 | 100.00 | 100.00 |
| Corn oil (tocopherol stripped)d | 94.35 | 54.70 | 94.35 | 94.35 |
| Powdered cellulose | 78.68 | 50.00 | 78.68 | 78.68 |
| AIN-93G mineral mix | 35.00 | 35.00 | 35.00 | 35.00 |
| AIN-93 vitamin mixe | 10.00 | 10.00 | 10.00 | 10.00 |
| L-Cystine | 3.00 | 3.00 | 3.00 | 3.00 |
| Choline bitartrate | 2.50 | 2.50 | 2.50 | 2.50 |
| Flaxseed | 0.00 | 100.00 | 0.00 | 0.00 |
| Flaxseed-lignan complex | 0.00 | 0.00 | 3.37 | 6.74 |
| SDGf | 0.00 | 1.18 | 1.18 | 2.36 |
| % Energyg | ||||
| Protein | 20.50 | 20.60 | 20.60 | 20.70 |
| Fat | 21.80 | 22.00 | 21.90 | 22.00 |
| Carbohydrate | 57.70 | 57.40 | 57.50 | 57.40 |
| Physiological fuel value (kJ/g) | 21.30 | 21.34 | 21.32 | 21.36 |
Notes. Study diets were formulated to contain (wt/wt) 0% flaxseed (0% FS), 10% flaxseed (10% FS), 10% flaxseed lignan complex (10% FLC) matching the SDG lignan content in 10% FS diet, and 20% flaxseed lignan complex (20% FLC) matching the SDG lignan content in 20% FS diet. Study diets were formulated to be isocaloric, isonitrogenous, and to contain the same amount of carbohydrate and dietary fat.
The basal diet against which flaxseed and FLC diets were compared was based on a semi-purified AIN-93G formula containing corn oil.
Ten percent FLC diet contains flaxseed lignan SDG (contained in the added FLC powder) comparable to that in a 10% flaxseed diet.
Twenty percent FLC diet contains flaxseed lignan SDG (contained in the added FLC powder) comparable to that in a 20% flaxseed diet.
Dietary corn oil was tocopherol-stripped to eliminate variability in antioxidant contribution.
Vitamin mix contains 75 IU/Kg vitamin E.
Flaxseed lignan secoisolariciresinol diglucoside (SDG).
Dietary ingredients were adjusted to formulate isocaloric study diets.
Diets contained either 10% (wt/wt) whole grain FS, a dose selected based on our previous studies (7, 8), or flaxseed lignan complex added to 2 different concentrations (10 and 20%). These concentrations reflected amounts of the FS lignan SDG comparable to those in 10 and 20% wholegrain FS diets. Analytical evaluation of the SDG content in wholegrain was performed at the School of Food Systems, University of South Dakota.
Specifically, for formulating the FLC diets, the semi-purified AIN-93G diet was supplemented with 0.337% and 0.674% (wt/wt) FLC powder. The FLC enriched in the lignan SDG (35% SDG content) was kindly provided by Archer Daniels Midland Inc., (ADM, IL). Flaxseed lignan complex was analyzed prior to incorporation in the diets and found to contain negligible amounts of vitamin E. Specifically, it contained <0.003 mg total tocopherol/g of FLC and <0.000024 mg alpha-tocopherol/g of FLC. The study diets used corn oil that was tocopherol-stripped to ensure that all diets contained an adequate but equal amount of vitamin E (75 IU/kg).
Mice were maintained on control (0% FS) or treatment (10% FS, 10 and 20% FLC) diets given ad libitum for three weeks prior to irradiation and for the entire duration of the experiment, unless otherwise noted in the text. This timeframe was determined by mass-spectroscopic evaluation of murine plasma to be optimal for circulating levels of FS lignan metabolites (28).
Analytical Evaluation of FS Lignan Metabolite Levels in Murine Plasma Samples
To ensure consumption of formulated test diets, analytical evaluation of plasma lignan levels (enterolactone and enterodiol) was performed as previously described (7–9) using LC/MS/MS.
Irradiation Procedure
Mice were anesthetized and irradiated, as previously described (29). Briefly, using an immobilization jig that allowed bilateral exposure of the lungs of up to 8 mice simultaneously while lead shielding (3 mm) the head, abdomen and extremities, 13.5 Gy or 15 Gy (tumor experiments) was delivered to the midplane using a 250 kVp orthovoltage machine (Philips RT 250). Thoracic irradiation was administered at a dose rate of 1.7 Gy/min and a source-to-skin distance of 33 cm through a 0.2 mm copper filter and a tube current of 13 mA. For quality assurance, thermoluminescent dosimeters were placed over selected mice to verify correct dose administration.
Evaluation of Cardiopulmonary Function Parameters
Prior to sacrifice, at 16 weeks after irradiation, pulse oximetry was performed on conscious mice (n = 5/group) using a MouseOx noninvasive vital signs monitor (STARR Life Sciences Corp., Oakmont, PA). A mouse collar sensor was used to obtain measurements for arterial oxygen saturation, pulse distension, breath rate and heart rate. To minimize stress and maintain body temperature, mice were placed on a heating pad. True measurements were recorded for a minimum of 3 min and values were reported as mean ± SEM for the total recording. Pulse distention, an additional hemodynamic measurement obtained by the pulse oximetry software, is a measure of change in path length of light passing through the carotids, and it has true linear distance units of micrometer.
Bronchoalveolar Lavage Fluid Analysis
Mice were sacrificed using an overdose of ketamine (100 mg/ml) and xylazine (20 mg/ml) at 16 weeks after irradiation. Bronchoalveolar lavage (BAL) was performed as described previously (9, 27, 29). Briefly, BAL fluid was obtained using a 20-gauge angiocatheter (BD Pharmingen, San Diego, CA), with intra-tracheal instillation of 1 ml of phosphate-buffered saline (PBS) containing an antiprotease cocktail (Sigma) and 5 mM EDTA that was given in 0.5 ml increments (8, 9, 27). An aliquot was immediately separated to measure total leukocyte cell counts (cells/ml BAL fluid) using a hemocytometer. The remaining BAL fluid was centrifuged at 1,200g for 10 min and the cell-free supernatant was frozen at −80°C for cytokine and protein analysis. The amount of total protein in the BAL fluid was assayed using the BCA Protein Assay Kit (Pierce, Rockford, IL) according to manufacturer’s instructions. Absorbance was read at 560 nm (MRX Microplate Reader, Dynatech Laboratories, Chantilly, VA) and protein levels in mg/ml of BAL fluid were calculated.
Tissue Harvesting and Evaluation of Pulmonary Fibrosis and Oxidative Tissue Damage
Radiation experiments were terminated at 16 weeks after irradiation, corresponding to a time point when radiation-induced pulmonary fibrosis is readily detectable in our model (9, 27, 29) using both biochemical assays and histopathological evaluation. Semiquantitative evaluation of pulmonary fibrosis using the fibrotic index (FI) scale was prepared histologically by scoring trichrome and H&E stained murine lung sections as described previously (9). Collagen content of murine lung tissue was evaluated quantitatively by determining the hydroxyproline content using acid hydrolysis according to Woessner et al. (30). Data is expressed as micrograms hydroxyproline/whole lung.
Gene Expression Analysis by Real-Time qPCR
Quantitative real-time Polymerase Chain Reaction (qPCR) was performed using TaqMan® Probe-Based Gene Expression Assays supplied by Applied Biosystems, Life Technologies (Carlsbad, CA). Individual TaqMan gene expression assays were selected for nuclear factor erythroid-derived 2 (Nrf2), heme oxygenase-1 (HO-1) and NAD(P)H quinone oxidoreductase 1 (NQO1). Briefly, total RNA was isolated from lung tissue of mice fed 0% FS, 10% FS, 10% FLC or 20% FLC for 3 weeks using a commercially available kit: RNeasy Plus Mini Kit, supplied by Qiagen (Valencia, CA). Total RNA was quantified using a NanoDrop 2000 (ThermoFisher Scientific, Waltham, MA). Reverse transcription of RNA to cDNA was then performed on a Veriti® Thermal Cycler using the high capacity RNA to cDNA kit supplied by Applied Biosystems, Life Technologies. qPCR was performed using 25 ng of cDNA per reaction well on a StepOnePlus™ Real-Time PCR System (Applied Biosystems). Gene expression data was normalized to 18S ribosomal RNA and was calibrated to untreated control samples according to the ΔΔCT method.
Immunoblotting
Mice were fed 0% FS, 10% or 20% FLC for 3 weeks, at which point murine lung tissue was harvested. Immunoblot analysis was performed on whole lung homogenates, as previously described (8), by using primary antibodies against heme oxygenase-1 (HO-1) (Stressgen, San Diego, CA) and NAD(P)H quinone oxidoreductase 1 (NQO-1) (Novus Biologicals, Littleton, CO). Densitometry of Western blots with β-actin normalization of expression was performed using Gel-Pro Analyzer software (Version 6.0) (MediaCybernetics, Silver Spring, MD).
Tumor Morphometry
Mice were pre-fed either a 0% FS, 10% FS or 10% FLC diet for 10 days. Mouse cohorts (n = 5/group) were then intravenously injected with 2 million murine Lewis Lung carcinoma cells (LLC cells), as previously described. Multiple metastatic lung tumors were established within 14 days after injection as judged by parallel animals that were sacrificed and evaluated histologically. At this time, a single dose of radiation to the thorax (15 Gy) was administered. Lungs were evaluated for tumor burden 7 days after irradiation. Lung weight was also recorded. Quantitative morphometric analysis was performed on 5 μm serial lung sections stained with H&E. Image analysis was performed using the Aperio ScanScope SC (Aperio Technologies, Vista, CA), Aperio ImageScope and Aperio Genie Histology Pattern Recognition Software. The H&E glass slides with the lung sections were first scanned and whole slide images were created at 12.5× magnification. Using the Genie software, a unique algorithm was created based on pattern recognition to separate: (1) tumor nodules, (2) benign lung tissue and (3) glass background. The slides were visualized with Aperio ImageScope for quality control and subsequently by employing the algorithm the digital images were analyzed and a quantitative measurement of the tumor area was obtained. Data was provided as percentage of tumor area to total lung. Sections from all lung lobes were evaluated from each animal (n = 5 mice per experimental cohort). For each lung specimen analyzed, three sections, 25 microns apart were used for tumor morphometry evaluation per lung lobe. Data reflected the average from all 3 sectioning levels.
Statistical Analysis
Results are expressed as mean ± SEM of two independent experiments. Statistical differences among groups were determined using one-way analysis of variance (ANOVA). When statistically significant differences were found (P < 0.05), individual comparisons were made using the Bonferoni/Dunn test (StatView 4.0). The survivor function was calculated using the Kaplan-Meier estimation method. Subsequently, Kaplan-Meier survival curves were generated using Stata data analysis and statistical software (Release 12, StataCorp, College Station, TX). Overall log-rank test for equality of survivor functions among mouse cohorts was performed as well as subsequent post-hoc analysis between individual treatments.
RESULTS
Dietary FLC was Well Tolerated over Prolonged Ingestion Achieving Stable Systemic Levels In Vivo
Mice tolerated FS supplementation very well throughout the duration of the study. Basal control diet (purified AIN-93G) was compared with 10% FS, and 10–20% FLC diets to confirm the physiological fuel values of treatment diets. Table 1 showed that the physiological fuel value of the diets remained similar.
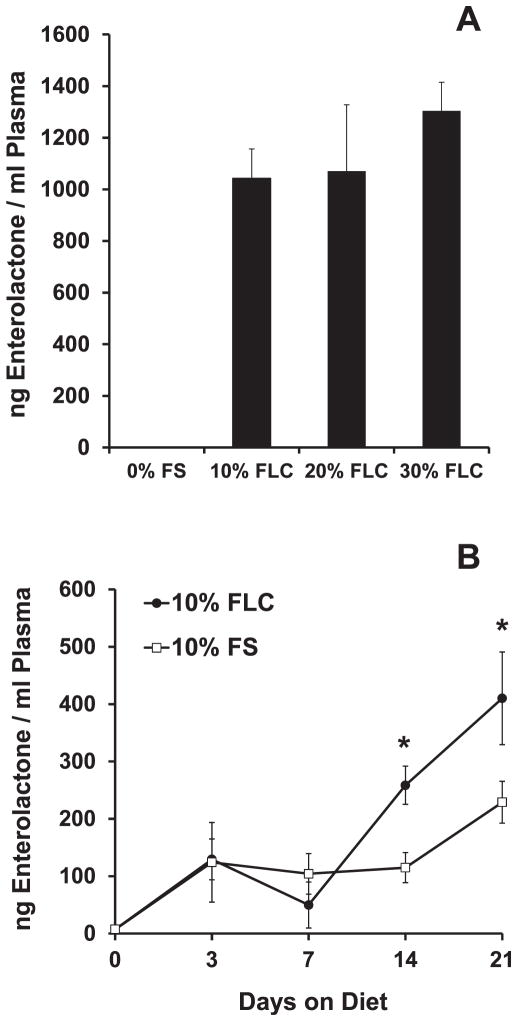
Circulating levels of the FS metabolite enterolactone (EL) were quantified from the plasma of mice fed 0%, 10%, 20% and 30% FLC diets over 3 weeks. As expected, EL levels were notably higher in all groups fed FLC compared to mice fed 0% FS diets (Fig. 1A). Furthermore, a separate comparative analysis was done to detect the difference in enterolactone levels in the plasma of mice fed 10% FS vs. 10% FLC diets. Enterolactone was detectable in both groups, but lignan levels were notably higher in mice fed 10% FLC diet compared to mice fed 10% FS diet (Fig. 1B). After 3 weeks, enterolactone levels in mice fed 10% FLC averaged 410.2 ng/ml, while enterolactone levels in mice fed 10% FS averaged 229.0 ng/ml. All study diets were well tolerated over the course of the study (4 months). As a measure of diet palatability, no differences in feed intake were noted. This was evidenced by no differences in mouse weights among nonirradiated control groups fed 10% FS and 10% FLC diets (Fig. 2B).
FIG. 1.
Detection of flaxseed lignan metabolite enterolactone (EL) in plasma of mice fed FS and FLC diets. Circulating mammalian lignan enterolactone levels in plasma of mice were determined using GC/MS/MS. Panel A: Mice were fed 0% FS, 10% FLC, 20% FLC or 30% FLC diet for 3 weeks. At 3 weeks, plasma was collected and levels of enterolactone were determined. Panel B: Mice were fed 10% FS or 10% FLC for 0, 3, 7, 14 and 21 days. At each time point, plasma was collected and levels of enterolactone were determined. Data is represented as mean ± SEM (n = 5 mice/group). *P < 0.05 for 10% FS vs. 10% FLC.
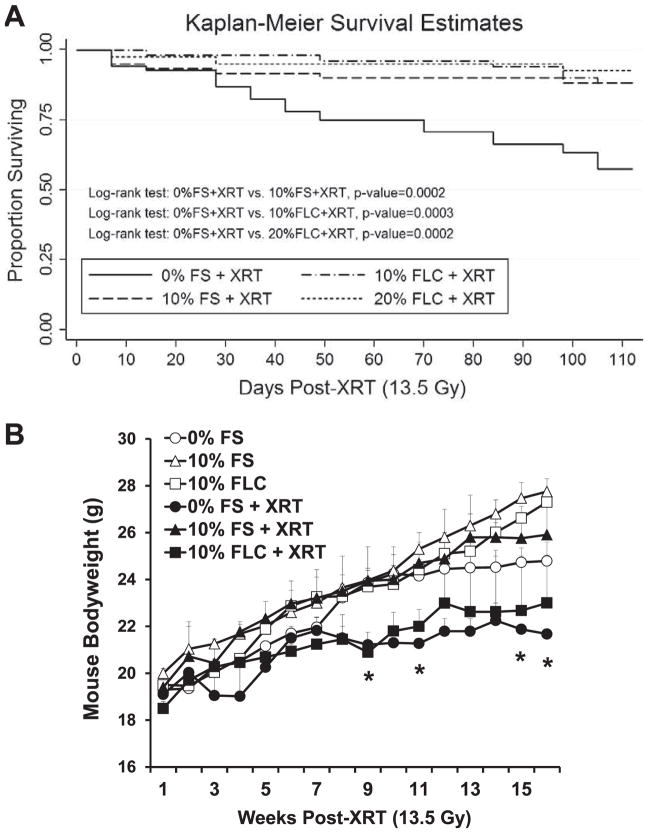
FIG. 2.
Effect of flaxseed lignan diet (FLC) on the survival and body weight of mice after radiation therapy (XRT). Panel A: Kaplan-Meier curves for overall survival. Mice were fed diets containing 0% FS, 10% FS, 10% FLC or 20% FLC for 3 weeks prior to thoracic irradiation and survival was observed up to 16 weeks post-XRT. No mice were lost in the nonirradiated cohorts (data not shown). Log-rank P values (shown in figure) were calculated by log-rank testing between irradiated mouse cohorts. Panel B: Mouse weight (n = 10/group) changes observed over 16 weeks post-XRT. Mice were pre-fed (3 weeks) diets containing 0% FS, 10% FS or 10% FLC prior to thoracic irradiation. Data is represented as mean ± SEM. Significant weight loss is observed only with irradiated 0% FS cohorts (*P < 0.05).
Dietary FLC Improved Survival and Prevented Weight Loss after Thoracic Radiation Therapy
We evaluated the radioprotective effect of FLC diet on XRT-induced mortality in mice. The treatment diets (FS and FLC) were started 3 weeks prior to radiation exposure. Nonirradiated mouse cohorts fed experimental diets (0% FS, 10% FS and 10% FLC) displayed robust survival throughout the course of the study as no mice were lost in these cohorts. Within the first 2 weeks of exposure, all groups of mice began exhibiting signs of XRT-induced morbidity and mortality except those mice on FLC diets (Fig. 2A). As expected, the group fed the control diet and irradiated with 13.5 Gy showed a progressive pattern of XRT-induced mortality. After 4 months, only 57% of irradiated mice fed 0% FS survived compared to their nonirradiated counterparts. Our results showed that percentage survival was significantly improved when mice were fed treatment (10% FS or 10–20% FLC) diet compared to the control diet (no FS or FLC) prior to irradiation (Fig. 2A).
As anticipated, feeding mice 10% FS diet led to a significant (P = 0.0002) improvement in survival (86%) compared to controls (57%) irradiated after basal diet. Feeding mice 10% FLC diet delayed onset of radiation-induced mortality as first death was observed 2 weeks after treatment in this group. The 10% and 20% FLC diet significantly (P ≤ 0.0003) enhanced the survival of irradiated animals by 33% and 36%, respectively. The Kaplan-Meier survival curve illustrated that the time to death is significantly longer for animals fed with either of the experimental diets (10–20% FLC) compared to the irradiated mice fed the control diet and the survival of FLC fed animals is similar to the survival of FS fed animals.
Figure 2B compares mouse body weights over 16 week among the different diets with or without irradiation. Most notably, the irradiated cohort on the control diet had the lowest body weights overall. Their weights differed significantly from nonirradiated mice fed the control diet. Statistical significance was noted first at week 9 and ended significantly lower. Importantly, irradiated mice fed 10% FLC had body weights not significantly different from their nonirradiated counterparts fed 10% FLC.
Dietary FLC Protected Lung Inflammation Induced by a Single Fraction of Thoracic Radiation Therapy
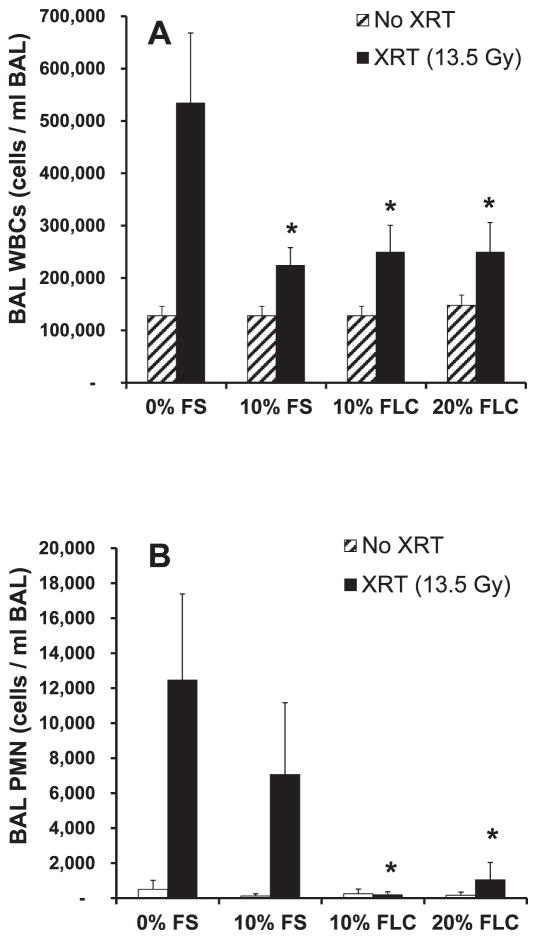
High levels of inflammatory cells within the lung characterize radiation pneumonopathy. To test if dietary FLC is as effective as FS as shown in our previous studies (8, 9), we measured total white blood cells (WBC) and neutrophils (PMN) cells/ml of BAL in irradiated mice that were fed the test diets. The cells/ml BAL were significantly increased (P ≤ 0.01) in irradiated mice fed control (0% FS) diet compared to nonirradiated mice fed control diet. Notably, mice pre-fed 10% FS and 10–20% FLC diets showed decreased inflammation (P < 0.05) after irradiation with reduced WBC cells/ml BAL (Fig. 3A). PMN cells/ml BAL were notably higher in irradiated animals (0% FS fed) compared to their nonirradiated control counterparts. Importantly, PMNs were significantly reduced (P < 0.01) when mice were fed the 10% or 20% FLC diet prior to XRT (Fig. 3B). It is important to note that Fig. 3B illustrates the baseline PMNs in the lungs prior to an exposure were very low, alluding to the role PMNs may play in inflammation and lung injury.
FIG. 3.
Evaluation of lung inflammation in mice at 16 weeks after radiation therapy (XRT). Mice were fed diets containing 0% FS, 10% FS, 10% FC, or 20% FLC for 3 weeks prior to thoracic irradiation. Data is represented as mean ± SEM of two independent experiments (n = 20 and n = 30 mice for nonirradiated and irradiated groups, respectively). Panel A: Total WBC counts in bronchoalveolar lavage (BAL) fluid of mice at 16 weeks post-XRT. *P < 0.05 for irradiated 0% FS vs. irradiated 10% FS, 10% FLC and 20% FLC. Panel B: Total PMN in bronchoalveolar lavage (BAL) fluid of mice at 16 weeks post-XRT. *P < 0.01 for irradiated 0% FS vs. irradiated 10% FLC and 20% FLC.
Dietary FLC Prevented Cardiopulmonary Deterioration after a Single Fraction of Thoracic Radiation Therapy
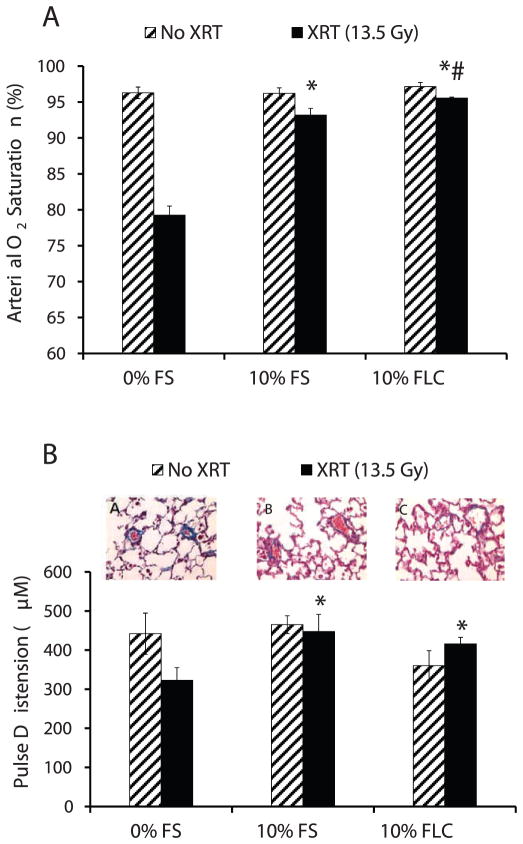
Pulse oximetry measured via a noninvasive sensor collar clip attached to each mouse yielded multiple cardiopulmonary measurements, including arterial oxygen saturation and pulse distention (Fig. 4). Arterial oxygen saturation of irradiated mice pre-fed 3 weeks of 0% FS, 10% FS or 10% FLC diet was compared to nonirradiated mice pre-fed the same diets at 16 weeks after radiation therapy (Fig. 4A). Irradiated mice pre-fed FLC had higher saturations with activity at 16 weeks after radiation therapy (P ≤ 0.01). Irradiated mice pre-fed control diets had much lower average oxygen saturations in the high 70% range compared to FLC pre-fed mice that maintained average saturations in the mid-to-high 90% range. Moreover, irradiated mice fed 10% FLC had higher saturations than the irradiated mice fed 10% FS (P < 0.05), perhaps pointing to FLC as the primary ingredient in flaxseed that yields its radioprotector effects.
FIG. 4.
Evaluation of pulmonary function parameters in mice at 16 weeks after radiation therapy (XRT). Mice were fed diets containing 0% FS, 10% FS or 10% FLC for 3 weeks prior to 13.5 Gy thoracic irradiation. At 16 weeks post-XRT, pulmonary function parameters were evaluated by pulse oximetry analysis (n =5 mice/group). Data is represented as mean ± SEM. Panel A: Arterial O2 saturation; *P < 0.001 for irradiated 0% FS vs. irradiated 10% FS and 10% FLC. #P < 0.05 for irradiated 10% FS vs. irradiated 10% FLC. Panel B: Pulse oximeter measurements of pulse distension. Micrographs A, B, and C display perivascular fibrosis at 16 weeks post-XRT in representative irradiated mice fed 0% FS, 10% FS and 10% FLC, respectively. *P ≤ 0.05 for irradiated 0% FS vs. irradiated 10% FS and 10% FLC.
At 16 weeks after radiation therapy, mice pre-fed 10% FLC diet prior to irradiation showed higher pulse distention compared to irradiated mice on the control diet (Fig. 4B). Qualitative histopathological evaluations of blood vessels within the irradiated lung sections from mice on the different diets revealed an association of less perivascular fibrosis with both FLC and FS [Fig. 4B: micrographs showing post-XRT perivascular fibrosis with comparisons between (A) 0% FS, (B) 10% FS and (C) 10% FLC].
Dietary FLC Protected against Fibrosis after a Single Fraction of Thoracic Radiation Therapy
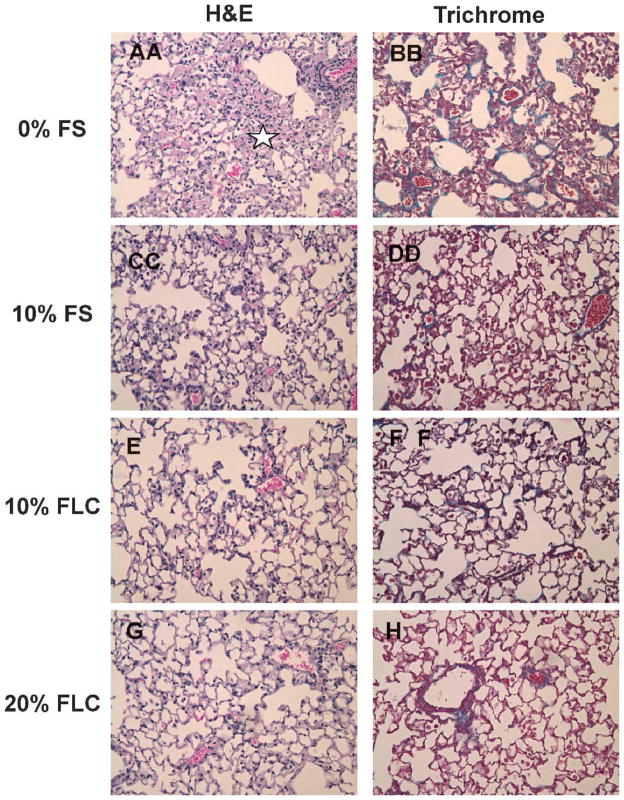
Late effects in the lung from radiation exposure include collagen deposition and fibrosis. We therefore evaluated both histopathologically and quantitatively the role of FLC in preventing XRT-induced fibrosis. Semiquantitative analyses using an established scale of fibrosis/inflammation, as previously described in Lee et al. (9), on trichrome-stained sections was performed to assess the extent of radiation fibrosis within murine lung tissue. Representative samples of lungs from mice fed test diets are shown in Fig. 5, where trichrome staining revealed higher collagen deposition in irradiated murine lungs on control diet compared to any FS or FLC diets. This was supported by decreased alveolar congestion and edema (stars indicate pink exudate in alveolar space). Additionally, the fibrotic index was significantly higher (P < 0.05) in irradiated mice fed 0% FS diet (2.83 ± 0.17) compared to their nonirradiated counterparts (0.70 ± 0.20). This elevated fibrotic index score of 2.83 ± 0.17 observed in lung sections from irradiated mice fed 0% FS was less in irradiated mice fed 10% FS (2.00 ± 0.71), 10% FLC (2.43 ± 0.35) and 20% FLC (2.07 ± 0.73).
FIG. 5.
Histological evaluation of murine lungs at 16 weeks after radiation therapy (XRT). Mice were fed diets containing 0% FS, 10% FS, 10% FLC or 20% FLC for 3 weeks prior to thoracic irradiation. Lungs were harvested at 16 weeks post-XRT and were processed for histology (H&E) and trichrome staining to evaluate collagen deposition and fibrosis. Panels A, C, E, G represent H&E (stars indicate pink exudate in alveolar space) and panels B, D, F, H represent trichrome staining of representative lung sections (200× magnification).
Results of histopathological staining for collagen were further reinforced with quantification of the hydroxyproline content in the lung. Murine lung tissue from two in vivo experiments was analyzed for hydroxyproline content. In both analyses, a significant induction in lung hydroxyproline content was found in irradiated mice fed control 0% FS diet (n = 5 mice/group/analysis). When irradiated, in both repeat experiments lung hydroxyproline levels increased significantly in mice fed control 0% FS diet. Levels increased from baseline 98.02 ± 12.42 and 68.58 ±5.31 to 171.35 ± 11.38 and 147.95 ± 14.47 μg per lung, respectively. As anticipated from our previous studies, in both experiments lung tissue of irradiated mice fed 10% FS displayed significantly (P < 0.05) decreased fibrosis compared to the control diet, with 117.51 ± 7.29 and 111.33 ± 3.33 μg/lung hydroxyproline content, which reflected a 31.4% and 24.7% decrease in fibrosis, respectively. While combined data from both experiments showed only a trend in decreased lung fibrosis with 10% and 20% FLC, in each separate experiment either 10% FLC or 20% FLC showed significant (P < 0.05) reduction in hydroxyproline content compared to irradiated mice fed 0% FS. Specifically, in two experiments there was a 12.5% and 47.9% reduction with 10% FLC and a 24.8% reduction with 20% FLC. Along with hydroxyproline analysis, histopathological scoring confirmed our hypothesis that pre-feeding mice any of the treatment diets (10% FS, 10% FLC or 20% FLC) significantly decreased the extent of pulmonary fibrosis after a single fraction of 13.5 Gy.
Dietary FLC Did Not Impede the Tumoricidal Effect of a Single Fraction of Thoracic Radiation Therapy
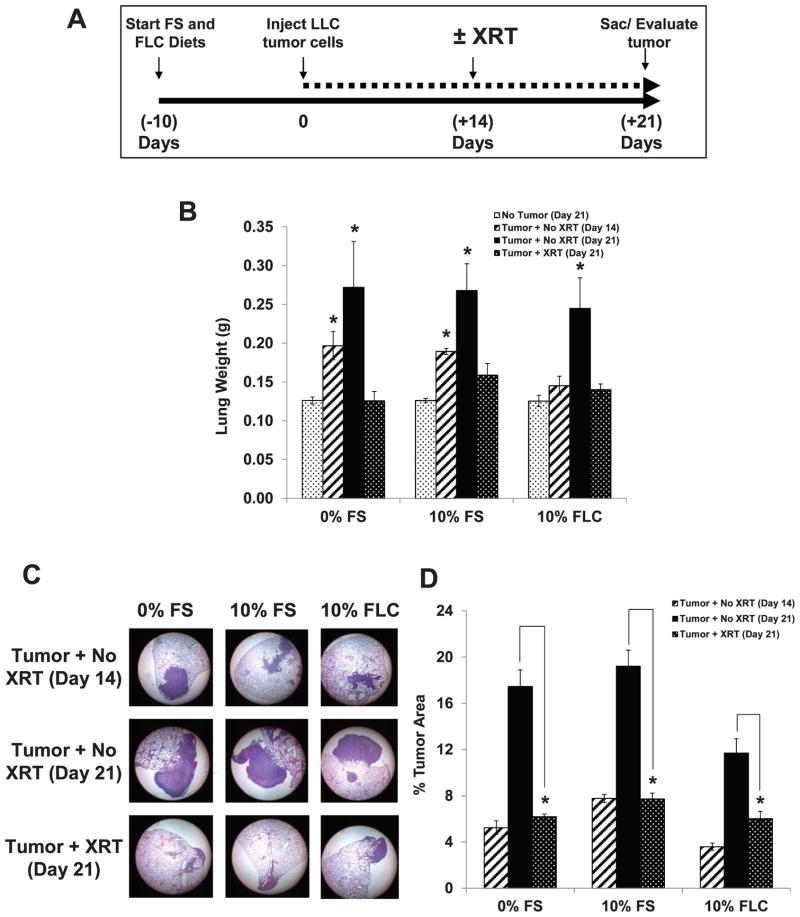
Since FLC is enriched in SDG, which is a potent phenolic antioxidant, the question remained as to whether FLC would similarly radioprotect tumor cells to the same degree as normal lung tissue, and thus have no net impact on the therapeutic index of thoracic XRT. We have shown that FS is able to protect normal tissue against XRT damage (9). While the dose of radiation used in these studies is sufficient to induce fibrosis (9), it would be subtherapeutic for this model of lung cancer. Mice were fed 0% FS or 10% FLC diet for 10 days (24 days prior to planned XRT). Mouse cohorts (n =5/group) received tail vein injections of 2 × 106 LLC at 14 days prior to planned XRT, observed for 14 days, irradiated once with 15 Gy and after 7 days were sacrificed for lung tumor burden evaluation (Fig. 6A).
FIG. 6.
Effect of FS lignan diet (FLC) on murine lung tumor burden post-XRT. Mice were pre-fed either 0% FS, 10% FS or a 10% FLC diet for 10 days. Mouse cohorts (n =5/group) were then intravenously injected with 2 × 106 LLC cells. Once tumors were established in the lungs at day 14 (confirmed by sacrificing and evaluating the lungs for a mouse cohort on day 14), lungs of mice were irradiated (single fraction 15 Gy to the thorax). All remaining mouse cohorts were then sacrificed at day 21 (7 days post-XRT). Tumor burden was quantified using image analysis from H&E stained histological sections. Panel A: Schematic presentation of experimental plan. Panel B: Lung tissue weights (n = 5/group). Data is represented as mean ± SEM. *P < 0.05 for comparisons to no tumor (day 21) within each respective cohort. Panel C: Histological profiles of lung sections stained with H&E in each cohort (magnification 12.5×). Panel D: Tumor morphometry analysis (n =5 mice/group) using the Aperio system. Data reflects the average from all 3 sectioning levels represented as mean ± SEM. *P < 0.01 for irradiated vs. nonirradiated mouse cohorts.
As seen in Fig. 6B, significant increases (P < 0.05) in lung tissue weights were apparent at 14 days after LLC injection for mice fed 0% and 10% FS. At 21 days post-injection, lung tissue weights were significantly increased in mice injected with LLC tumor cells, while receiving no XRT (P < 0.05). Administration of 15 Gy XRT to the thorax reduced tumor burden to levels comparable to untreated mice. Tumor morphometry was analyzed via the Aperio system, a digital histopathological slide analyzer. Tumor burden in terms of percent tumor occupied area in the lung was compared in irradiated and nonirradiated lungs of mice fed either 0% FS, 10% FS or 10% FLC. Similar to lung tissue weights, LLC tumor cell injection increased tumor burden as measured histopathologically. Thoracic irradiation of 15 Gy reduced tumor burden across all study diets (Fig. 6D) showing that under conditions where pulmonary radioprotection is observed, FLC did not protect the tumor. Figure 6C shows histopathological profiles of lung sections stained with H&E in each cohort. Low magnification was selected to better illustrate the lung area covered by tumor rather than the details within the tumor. It is evidenced by both histological analysis and morphometry as well as lung weight (tumor burden) that radiation dose was sufficient to decrease tumor area, and that none of our test diets prevented tumoricidal dose of radiation.
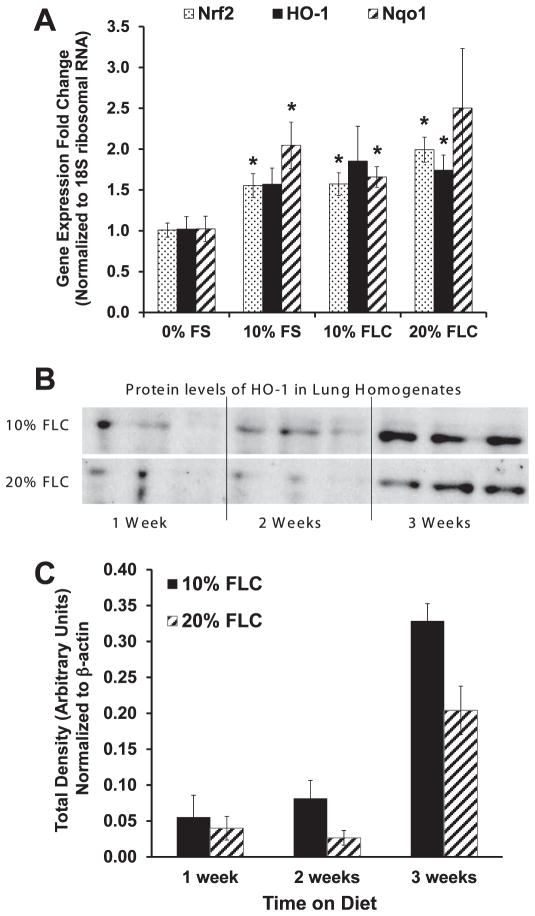
Dietary FLC Augmented Expression of Protective Antioxidant Enzymes in Lung Tissues
We have shown in prior studies that FS supplementation increased antioxidant enzyme expression in lung tissue that was exposed to varying stressors (7, 8). Here, we evaluated the effect of dietary FLC in modulating gene expression changes irrespective of any stressor on the expression of selected representative antioxidant enzymes NQO1 and HO-1, both under Nrf2 regulation. Mice were fed 0% FS, 10% FS, 10% FLC or 20% FLC diet for 3 weeks. All treatment diets increased gene expression levels of antioxidant enzymes significantly (Fig. 7A). Mice fed 10% FS had a notable 1.5-fold increase in Nrf2 and HO-1 mRNA expression compared to mice fed the control diet. In mice fed the 10% FS diet, a twofold increase in NQO1 levels was observed compared to mice fed the control diet. FLC supplementation also increased levels in a dose-dependent trend of Nrf2, HO-1 and NQO1 gene expression compared to mice fed the control diet. A significant (P < 0.05) increase in HO-1 gene expression and ultimately in protein expression was observed in mice fed FLC diet. Importantly, gene expression changes were confirmed by protein level analysis of selected genes. Figure 7B shows the time dependent increase in lung HO-1 protein levels with dietary administration of 10% and 20% FLC. Densitometric analysis of HO-1 protein levels that normalized to β-actin were also compiled (Fig. 7C).
FIG. 7.
Effect of FS lignan diet (FLC) on the expression of antioxidant enzymes in murine lung tissue. Panel A: Mice were fed diets composed of 0% FS, 10% FS, 10% FLC and 20% FLC for 3 weeks. Lung tissue was harvested and evaluated by quantitative real-time PCR analysis for Nrf2, HO-1, and NQO1 gene expression (n =3/group). Analysis was performed in duplicate and gene expression normalized to 18S ribosomal RNA. Data is represented as mean ± SEM. *P < 0.05 for 0% FS vs. 10% FS, 10% FLC and 20% FLC. Panel C: Mice were fed diets composed of 0% FS, 10% FLC and 20% FLC for 1, 2 and 3 weeks. Lung tissue was harvested and evaluated by Western blotting for HO-1 protein expression. Panel B: Densitometric analysis of band intensity was normalized to β-actin.
DISCUSSION
Radiotherapy for lung carcinoma is frequently limited secondary to damage on a spectrum from pneumonitis to fibrosis that is inflicted upon surrounding normal tissues, and this progressive damage ultimately results in worsened pulmonary function. The search for an ideal radioprotector led us to identify the potent antioxidant, anti-fibrotic, anti-inflammatory properties of wholegrain flaxseed (8, 9) in our mouse model of lung injury from thoracic radiation therapy. The current study identified for the first time the FLC as the bioactive ingredient of this grain responsible for the protective effects. We demonstrate here that dietary FLC enriched in SDG, a non-toxic phenolic compound with numerous beneficial properties is easily tolerated for 16 weeks with no side effects and appropriate maintenance of weight. Moreover, biologically active levels of FLC metabolites could be detected in mice fed the treatment diets. Mice fed the FLC-supplemented diet showed dramatically reduced levels of pulmonary inflammation and fibrosis with the prevention of deterioration of pulse oximetry several months after a single fraction of XRT. Finally, in our perhaps most clinically applicable data, we showed that despite the radioprotection afforded by FLC in normal pulmonary tissue, it did not abrogate the tumoricidal effect of radiation.
Our group has had an interest in exploring the use of natural dietary phenolic compounds as a means of ameliorating acute and chronic lung diseases caused by the oxidative stress of thoracic radiation. One such dietary supplement, flaxseed that contains phenolics, is nontoxic with anti-inflammatory, anti-fibrotic, and antioxidant properties due to its high concentrations of omega-3 fatty acids and plant lignans (31), our particular focus in this study. Flaxseed’s bioactive metabolites have been extensively studied in other organ systems and have proven to be beneficial, mostly with respect to cancer therapies (32–35). Our group was the first to show that dietary FS could reduce inflammation and lipid peroxidation in murine models of acid aspiration and hyperoxia (7) and more recently, in ischemia/reperfusion injury associated with lung transplantation (8). For the first time, we demonstrate here that the lignan complex (FLC) possesses these same antioxidant, anti-inflammatory, anti-fibrotic, and lung radioprotecting properties of the wholegrain.
FLC could be ingested over a prolonged period and could achieve biologically significant levels in vivo. This was a lengthy study and prolonged feeding (>16 weeks) did not cause any adverse health effects evidenced by weight monitoring. Additionally, circulating levels of the FLC metabolite enterolactone could be quantified reaching biologically comparable (if not superior to) levels achieved in our previous studies (7–9) that featured wholegrain flaxseed. Dietary FLC led to improved survival in mice irradiated with a single fraction of 13.5 Gy XRT. Time to radiation-induced mortality (first death from XRT) was delayed by 2 weeks in mice pre-fed with FLC prior to irradiation. Survival curves showed irradiated mice fed 10% FLC were alive longer than mice fed 10% FS and alive far longer than mice fed a diet without flaxseed supplementation. Giving FLC may permit administration of higher and physiologically more effective doses of radiation to achieve higher tumoricidal effects while avoiding immediate morbidity and mortality. More investigation is needed in this aspect of the role of FLC in radiation-induced lung injury.
We discovered that FLC reduced the influx of inflammatory cells into the airways. Neutrophils are the first responders during the acute phase of radiation-induced lung injury. Mice irradiated after being pre-fed FLC had reduced numbers of white blood cells (with reduced numbers of neutrophils) in their BAL fluid compared to irradiated mice fed diets that were not supplemented with flaxseed. Recent studies by Lee at al. (8) showed that inflammatory cells isolated from lung lavage of FS-fed animals had diminished respiratory bursts and reduced oxidative enzyme release when stimulated. Importantly, in our recent work on the genomic profiling of dietary FS in lung tissues, we showed the immunomodulatory properties of FS mediated by down-regulation of gene expression levels of key cytokine receptors that were involved in the inflammatory cascade (36). This may help explain the anti-inflammatory protective effects of FLC in radiation injury.
The current study compiled the first clinically relevant cardiopulmonary measurements through use of noninvasive sensor collars that were attached to each mouse. Most notably, pulse oximetry data showed that mice fed a diet of FLC had higher oxygen saturations with activity 16 weeks after a single dose of radiotherapy compared to mice irradiated after being fed a control diet. Before and after irradiation, FLC-fed mice had oxygen saturations well within physiologically acceptable ranges (≥95%) compared to the control diet fed mice that struggled to maintain saturations in the low 80% range. Oxygen saturation at 80% in the clinic patient could manifest itself as cyanosis, breathlessness and fatigue as a patient struggles to interact with the physician; while a patient with a saturation of 95% could have a conversation, breathe comfortably and carry on with a normal life. This highly favorable physiologic parameter dovetailed with our hydroxyproline assays and trichrome-stained lung section evaluations confirming reduced fibrosis in the lung tissue of FLC-fed mice.
Another intriguing and novel piece of data came in the form of pulse distention values. These values could translate to physiologic estimates of flow in a cardiopulmonary circuit (37). X-ray radiation treatment (just as it does in pulmonary parenchyma) alters the dynamic equilibrium of tissue types within blood vessel walls (37, 38). It also stimulates inflammatory pathways that lead to scars in areas of wall injury (37–39). The wall then becomes less compliant, less distensible due to a change in prevailing tissue type from highly dynamic muscle cells to rigid less distensible collagen scars (37–39). Pulse distention could serve as a noninvasive surrogate for flow and derivation of resistance within the cardiopulmonary and possibly systemic circulations. X-ray radiation treatment as we have shown is tumoricidal, but inflames surrounding normal tissue enacting a repair cascade that ultimately ends in fibrosis. Mice pre-fed FLC demonstrated higher pulse distention. Although not causative, this intriguing finding of an association between increased pulse distention and protection from vascular fibrosis needs to be pursued further. Importantly, the possibility that this may be due to destruction of endothelial cells by oxygen radicals, hence reduction in production of vasodilators and/or unopposed action of circulating vasoconstrictors, must be further investigated.
Our study showed significant up-regulation of antioxidant enzyme gene expression (HO-1, NQO1) in lung tissue of mice fed FLC for 3 weeks, correlating with elevated plasma lignan levels. We also reported significant increases in Nrf2 gene expression that, along with HO-1 and NQO1 is regulated by the Nrf2 transcription factor. The ability of both 10% FLC and 20% FLC diets to up-regulate baseline levels of phase II antioxidant enzyme gene expression suggests FS lignan interaction with the Nrf2-Keap1 signaling pathway, which serves as the intracellular sensor of oxidative stress. Along with direct ROS scavenging (9, 15), activation of this pathway and subsequent Nrf2 up-regulation of antioxidant gene expression in unchallenged lung tissues of FLC-fed mice may explain the radioprotective properties of FLC. The ability of FLC diets to up-regulate antioxidant and phase II enzymes is further supported by immunoblot analysis of lung tissue harvested from FLC-fed mice in which HO-1 protein expression was elevated after 3 weeks of feeding.
In summary, we have evaluated a nontoxic and widely available dietary phenolic compound that yielded long-term protective benefits after thoracic radiotherapy. We have studied the beneficial properties of the wholegrain in the past, but herein for the first time have demonstrated that the lignan complex surpassed the wholegrain in its antioxidant, anti-inflammatory and anti-fibrotic properties. Our long-term goal is to permit greater doses of radiation to improve clinical responses and cures for thoracic malignancies, while providing adequate radioprotection against the side effects in normal lung parenchyma.
CONCLUSION
We demonstrated for the first time the role of Flaxseed Lignan Complex (FLC) in protecting against radiation-induced lung injury in our murine model. Mice tolerated pre-feeding with FLC, achieving biologically significant systemic levels, demonstrating reduced lung inflammation, fibrosis and improved survival after exposure to thoracic irradiation. Most importantly, FLC did not inhibit tumoricidal doses of radiation. Therefore, the current study suggests that dietary FLC supplementation potentially protects normal lung parenchyma against radiation injury and may also be clinically useful in narrowing the limitations of radiation therapy in thoracic malignancies.
Acknowledgments
Funded in part by: NIH-R01 CA133470-04 (MCS), NIH-RC1AI081251-01 (MCS), the American Institute for Cancer Research no. AICR-03B024 (MCS), the University of Pennsylvania Research Foundation (MCS), and by pilot project support from 1P30 ES013508-02 awarded to MCS (its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIEHS, NIH).
References
- 1.Ghafoori P, Marks LB, Vujaskovic Z, Kelsey CR. Radiation-induced lung injury. Assessment, management, and prevention. Oncology. 2008;22(1):37–47. [PubMed] [Google Scholar]
- 2.Graves PR, Siddiqui F, Anscher MS, Movsas B. Radiation pulmonary toxicity: from mechanisms to management. Semin Radiat Oncol. 2010;20(3):201–7. doi: 10.1016/j.semradonc.2010.01.010. [DOI] [PubMed] [Google Scholar]
- 3.Zhao W, Robbins ME. Inflammation and chronic oxidative stress in radiation-induced late normal tissue injury: therapeutic implications. Curr Med Chem. 2009;16(2):130–43. doi: 10.2174/092986709787002790. [DOI] [PubMed] [Google Scholar]
- 4.Riley PA. Free radicals in biology: oxidative stress and the effects of ionizing radiation. Int J Radiat Biol. 1994;65(1):27–33. doi: 10.1080/09553009414550041. [DOI] [PubMed] [Google Scholar]
- 5.Greenberger JS. Radioprotection. In Vivo. 2009;23(2):323–36. [PMC free article] [PubMed] [Google Scholar]
- 6.Greenberger JS, Epperly MW. Review. Antioxidant gene therapeutic approaches to normal tissue radioprotection and tumor radiosensitization. In Vivo. 2007;21(2):141–6. [PubMed] [Google Scholar]
- 7.Kinniry P, Amrani Y, Vachani A, Solomides CC, Arguiri E, Workman A, et al. Dietary flaxseed supplementation ameliorates inflammation and oxidative tissue damage in experimental models of acute lung injury in mice. J Nutr. 2006;136(6):1545–51. doi: 10.1093/jn/136.6.1545. [DOI] [PubMed] [Google Scholar]
- 8.Lee JC, Bhora F, Sun J, Cheng G, Arguiri E, Solomides CC, et al. Dietary flaxseed enhances antioxidant defenses and is protective in a mouse model of lung ischemia-reperfusion injury. Am J Physiol Lung Cell Mol Physiol. 2008;294(2):L255–65. doi: 10.1152/ajplung.00138.2007. [DOI] [PubMed] [Google Scholar]
- 9.Lee JC, Krochak R, Blouin A, Kanterakis S, Chatterjee S, Arguiri E, et al. Dietary flaxseed prevents radiation-induced oxidative lung damage, inflammation and fibrosis in a mouse model of thoracic radiation injury. Cancer Biol Ther. 2009;8(1):47–53. doi: 10.4161/cbt.8.1.7092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen H, Sun B, Pan S, Jiang H, Sun X. Dihydroartemisinin inhibits growth of pancreatic cancer cells in vitro and in vivo. Anticancer Drugs. 2009;20(2):131–40. doi: 10.1097/CAD.0b013e3283212ade. [DOI] [PubMed] [Google Scholar]
- 11.Demark-Wahnefried W, Polascik TJ, George SL, Switzer BR, Madden JF, Ruffin MT, et al. Flaxseed supplementation (not dietary fat restriction) reduces prostate cancer proliferation rates in men presurgery. Cancer Epidemiol Biomark Prev. 2008;17(12):3577–87. doi: 10.1158/1055-9965.EPI-08-0008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jenab M, Thompson LU. The influence of flaxseed and lignans on colon carcinogenesis and beta-glucuronidase activity. Carcinogenesis. 1996;17(6):1343–8. doi: 10.1093/carcin/17.6.1343. [DOI] [PubMed] [Google Scholar]
- 13.Prasad K. Hydroxyl radical-scavenging property of secoisolariciresinol diglucoside (SDG) isolated from flax-seed. Mol Cell Biochem. 1997;168(1–2):117–23. doi: 10.1023/a:1006847310741. [DOI] [PubMed] [Google Scholar]
- 14.Prasad K. Antioxidant Activity of secoisolariciresinol diglucoside-derived metabolites, secoisolariciresinol, enterodiol, and enterolactone. Int J Angiol. 2000;9(4):220–5. doi: 10.1007/BF01623898. [DOI] [PubMed] [Google Scholar]
- 15.Kitts DD, Yuan YV, Wijewickreme AN, Thompson LU. Antioxidant activity of the flaxseed lignan secoisolariciresinol diglycoside and its mammalian lignan metabolites enterodiol and enterolactone. Mol Cell Biochem. 1999;202(1–2):91–100. doi: 10.1023/a:1007022329660. [DOI] [PubMed] [Google Scholar]
- 16.Adolphe JL, Whiting SJ, Juurlink BH, Thorpe LU, Alcorn J. Health effects with consumption of the flax lignan secoisolariciresinol diglucoside. Br J Nutr. 2010;103(7):929–38. doi: 10.1017/S0007114509992753. [DOI] [PubMed] [Google Scholar]
- 17.Fukumitsu S, Aida K, Shimizu H, Toyoda K. Flaxseed lignan lowers blood cholesterol and decreases liver disease risk factors in moderately hypercholesterolemic men. Nutr Res. 2010;30(7):441–6. doi: 10.1016/j.nutres.2010.06.004. [DOI] [PubMed] [Google Scholar]
- 18.Pan A, Demark-Wahnefried W, Ye X, Yu Z, Li H, Qi Q, et al. Effects of a flaxseed-derived lignan supplement on C-reactive protein, IL-6 and retinol-binding protein 4 in type 2 diabetic patients. Br J Nutr. 2009;101(8):1145–9. doi: 10.1017/S0007114508061527. [DOI] [PubMed] [Google Scholar]
- 19.Hallund J, Tetens I, Bugel S, Tholstrup T, Bruun JM. The effect of a lignan complex isolated from flaxseed on inflammation markers in healthy postmenopausal women. Nutr Metab Cardiovasc Dis. 2008;18(7):497–502. doi: 10.1016/j.numecd.2007.05.007. [DOI] [PubMed] [Google Scholar]
- 20.Hallund J, Tetens I, Bugel S, Tholstrup T, Ferrari M, Teerlink T, et al. Daily consumption for six weeks of a lignan complex isolated from flaxseed does not affect endothelial function in healthy postmenopausal women. J Nutr. 2006;136(9):2314–8. doi: 10.1093/jn/136.9.2314. [DOI] [PubMed] [Google Scholar]
- 21.Jenkins DJ, Kendall CW, Vidgen E, Agarwal S, Rao AV, Rosenberg RS, et al. Health aspects of partially defatted flaxseed, including effects on serum lipids, oxidative measures, and ex vivo androgen and progestin activity: a controlled crossover trial. Am J Clin Nutr. 1999;69(3):395–402. doi: 10.1093/ajcn/69.3.395. [DOI] [PubMed] [Google Scholar]
- 22.Cornish SM, Chilibeck PD, Paus-Jennsen L, Biem HJ, Khozani T, et al. A randomized controlled trial of the effects of flaxseed lignan complex on metabolic syndrome composite score and bone mineral in older adults. Appl Physiol Nutr Metab. 2009;34(2):89–98. doi: 10.1139/H08-142. [DOI] [PubMed] [Google Scholar]
- 23.Metnitz PG, Bartens C, Fischer M, Fridrich P, Steltzer H, Druml W. Antioxidant status in patients with acute respiratory distress syndrome. Intens Care Med. 1999;25(2):180–5. doi: 10.1007/s001340050813. [DOI] [PubMed] [Google Scholar]
- 24.Prasad K. Dietary flax seed in prevention of hypercholesterolemic atherosclerosis. Atherosclerosis. 1997;132(1):69–76. doi: 10.1016/s0021-9150(97)06110-8. [DOI] [PubMed] [Google Scholar]
- 25.Christofidou-Solomidou M, McDonough J, Scherpereel A, Wiewrodt R, Argyris E, Solomides CC, et al. Immunoconjugates of catalase attenuate radiation-induced pulmonary fibrosis in C57bl mice. Int J Radiat Oncol Biol Phys; Proc ASTRO..2001. [Google Scholar]
- 26.Christofidou-Solomidou M, Scherpereel A, Solomides CC, Muzykantov VR, Machtay M, Albelda SM, et al. Changes in plasma gelsolin concentration during acute oxidant lung injury in mice. Lung. 2002;180(2):91–104. doi: 10.1007/s004080000084. [DOI] [PubMed] [Google Scholar]
- 27.Lee JC, Kinniry PA, Arguiri E, Serota M, Kanterakis S, Chatterjee S, Solomides CC, et al. Dietary curcumin increases antioxidant defenses in lung, ameliorates radiation-induced pulmonary fibrosis, and improves survival in mice. Radiat Res. 2010;173(5):590–601. doi: 10.1667/RR1522.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kinniry P, Pick J, Stephens S, Jain D, Solomides CC, Niven R, et al. KL(4)-surfactant prevents hyperoxic and LPS-induced lung injury in mice. Pediatr Pulmonol. 2006;41(10):916–28. doi: 10.1002/ppul.20468. [DOI] [PubMed] [Google Scholar]
- 29.Machtay M, Scherpereel A, Santiago J, Lee J, McDonough J, Kinniry P, et al. Systemic polyethylene glycol-modified (PEGylated) superoxide dismutase and catalase mixture attenuates radiation pulmonary fibrosis in the C57/bl6 mouse. Radiother Oncol. 2006;81(2):196–205. doi: 10.1016/j.radonc.2006.09.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Woessner JF., Jr The determination of hydroxyproline in tissue and protein samples containing small proportions of this imino acid. Arch Biochem Biophys. 1961;93:440–7. doi: 10.1016/0003-9861(61)90291-0. [DOI] [PubMed] [Google Scholar]
- 31.Thompson L. Flaxseed in Human Nutrition. 2. Champaign: AOCS Press; 2003. [Google Scholar]
- 32.Bergman Jungestrom M, Thompson LU, Dabrosin C. Flaxseed and its lignans inhibit estradiol-induced growth, angiogenesis, and secretion of vascular endothelial growth factor in human breast cancer xenografts in vivo. Clin Cancer Res. 2007;13(3):1061–7. doi: 10.1158/1078-0432.CCR-06-1651. [DOI] [PubMed] [Google Scholar]
- 33.Bommareddy A, Arasada BL, Mathees DP, Dwivedi C. Chemo-preventive effects of dietary flaxseed on colon tumor development. Nutr Cancer. 2006;54(2):216–22. doi: 10.1207/s15327914nc5402_8. [DOI] [PubMed] [Google Scholar]
- 34.Dwivedi C, Natarajan K, Matthees DP. Chemopreventive effects of dietary flaxseed oil on colon tumor development. Nutr Cancer. 2005;51(1):52–8. doi: 10.1207/s15327914nc5101_8. [DOI] [PubMed] [Google Scholar]
- 35.Oikarinen S, Heinonen SM, Nurmi T, Adlercreutz H, Mutanen M. No effect on adenoma formation in Min mice after moderate amount of flaxseed. Eur J Nutr. 2005;44(5):273–80. doi: 10.1007/s00394-004-0521-z. [DOI] [PubMed] [Google Scholar]
- 36.Dukes F, Kanterakis S, Lee J, Pietrofesa R, Andersen ES, Arguiri E, et al. Gene expression profiling of flaxseed in mouse lung tissues-modulation of toxicologically relevant genes. BMC Complem Altern Med. 2012;12(1):47. doi: 10.1186/1472-6882-12-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Russell NS, Hoving S, Heeneman S, Hage JJ, Woerdeman LA, de Bree R, et al. Novel insights into pathological changes in muscular arteries of radiotherapy patients. Radiother Oncol. 2009;92(3):477–83. doi: 10.1016/j.radonc.2009.05.021. [DOI] [PubMed] [Google Scholar]
- 38.Basavaraju SR, Easterly CE. Pathophysiological effects of radiation on atherosclerosis development and progression, and the incidence of cardiovascular complications. Med Phys. 2002;29(10):2391–403. doi: 10.1118/1.1509442. [DOI] [PubMed] [Google Scholar]
- 39.Virmani R, Farb A, Carter AJ, Jones RM. Comparative pathology: radiation-induced coronary artery disease in man and animals. Semin Interv Cardiol. 1998;3(3–4):163–72. [PubMed] [Google Scholar]