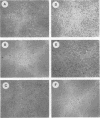
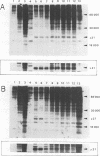
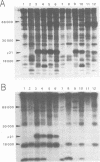
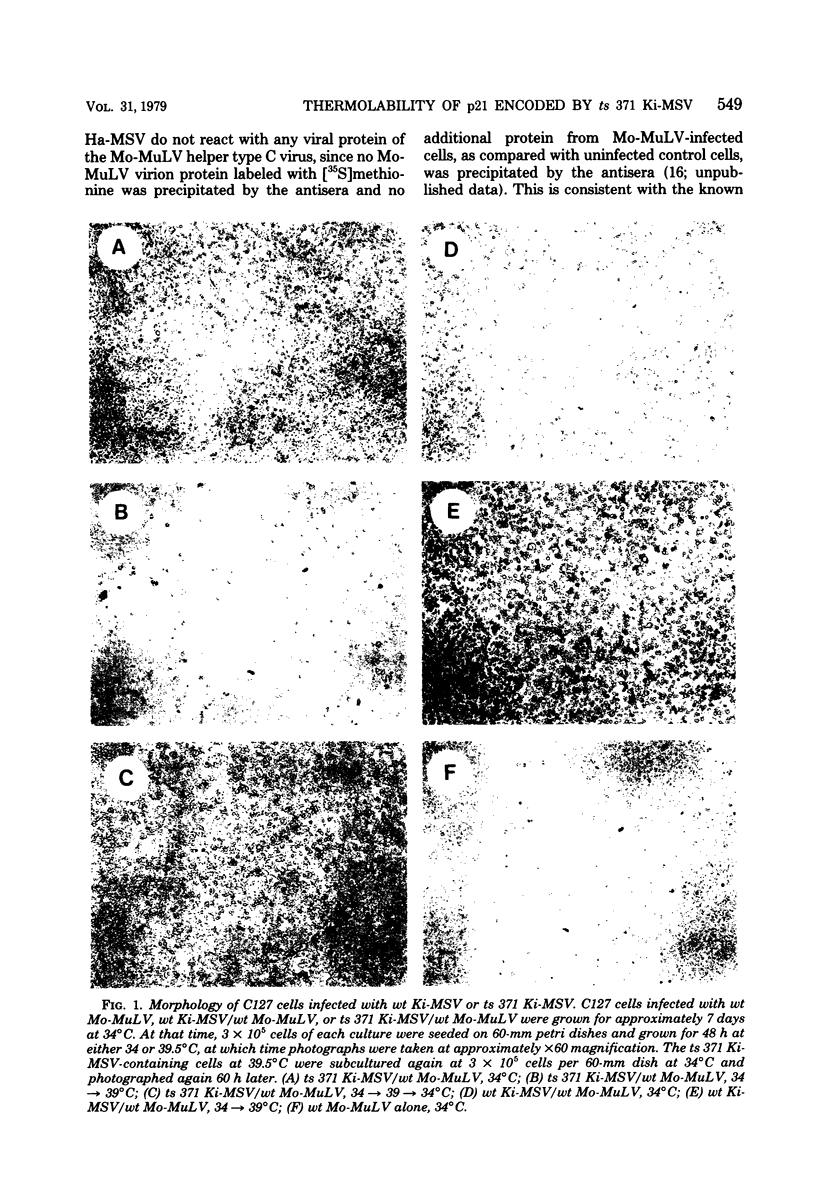
Abstract
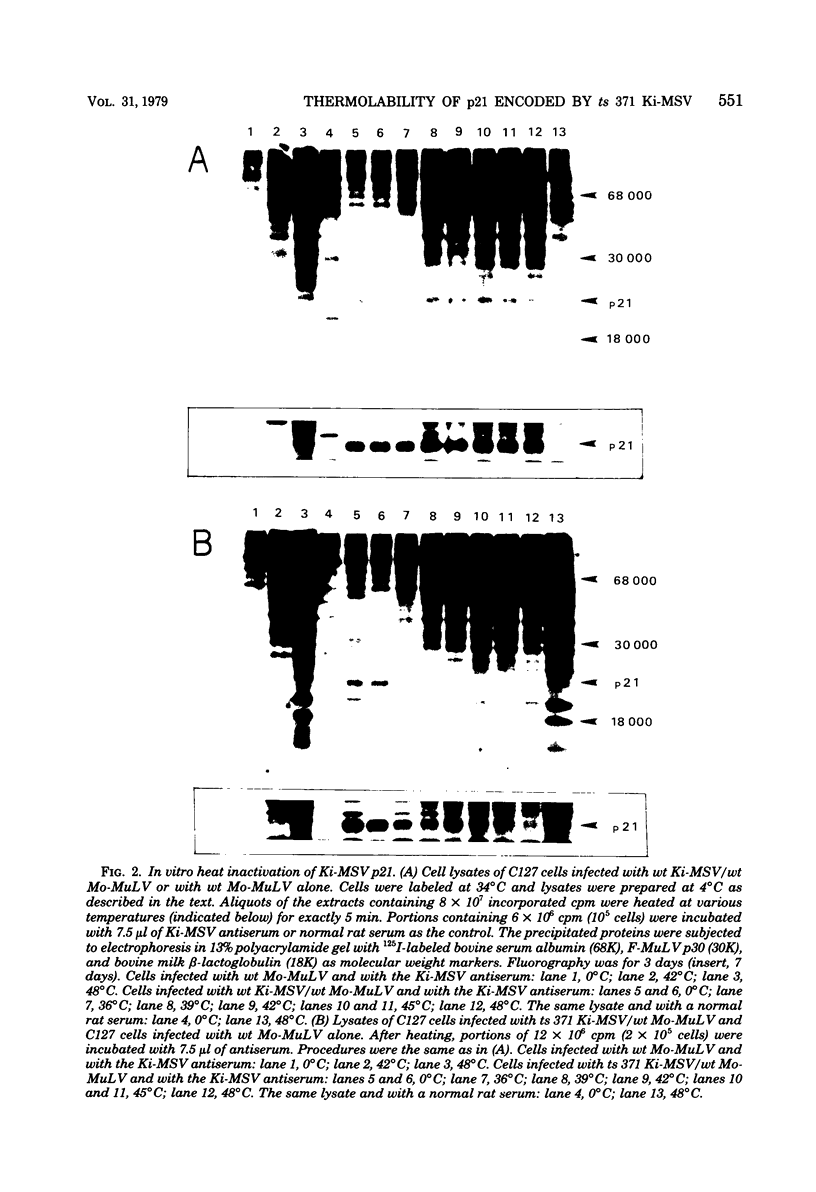
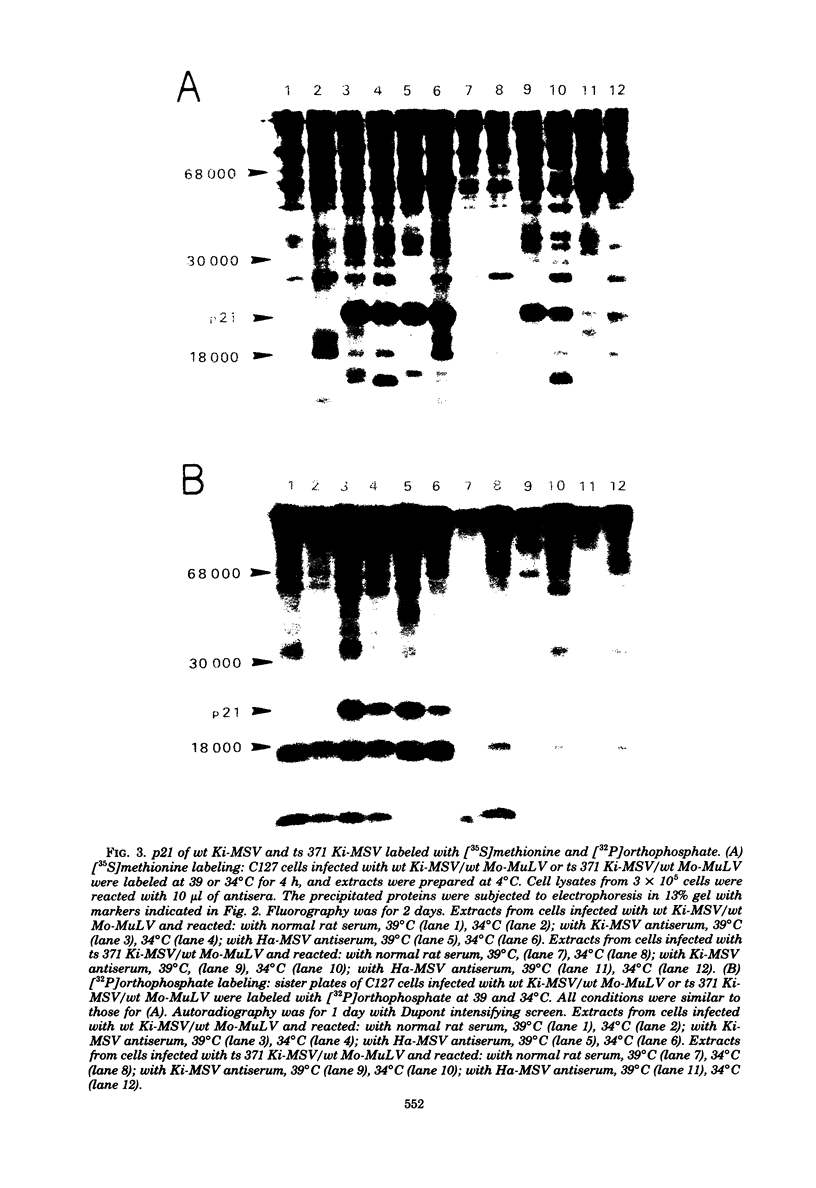
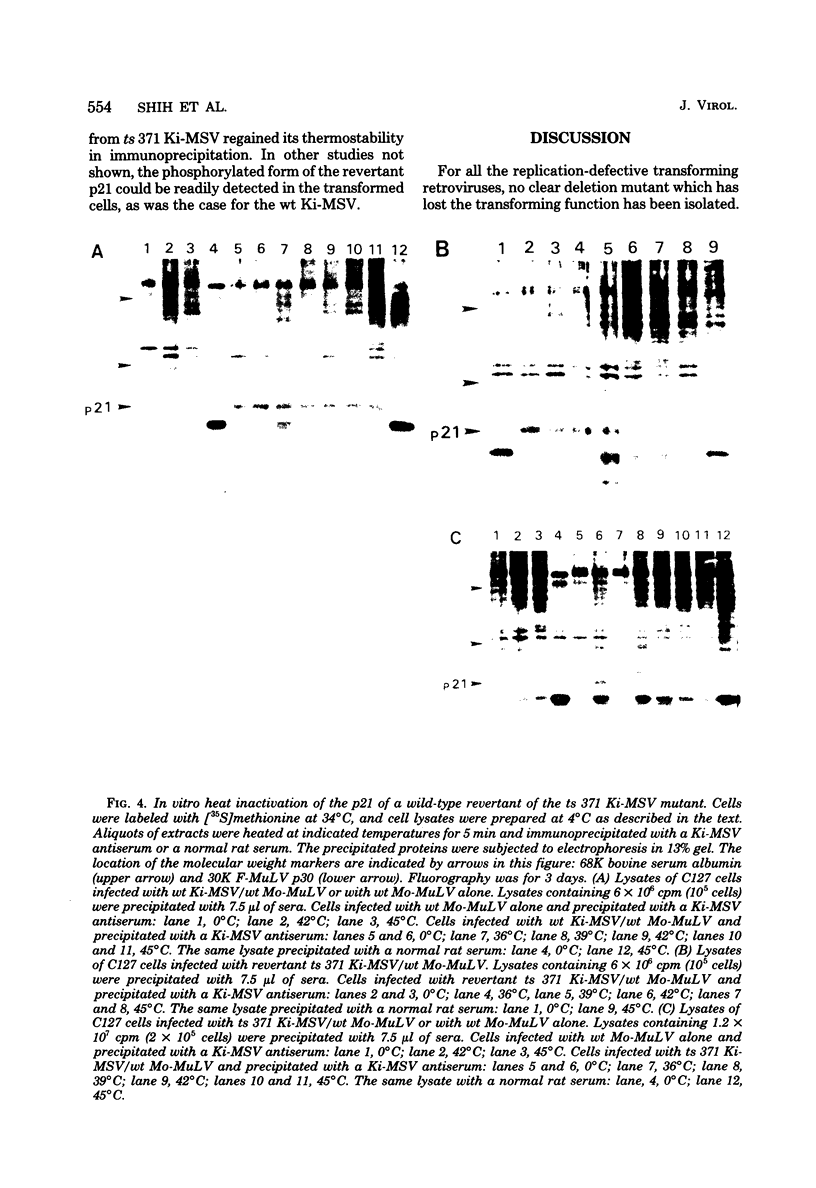
We have recently described an intracellular protein, p21, in nonproducer cells transformed by either the Kirsten (Ki-MSV) or Harvey (Ha-MSV) strain of murine sarcoma virus (Shih et al., Virology, in press). The p21 is phosphorylated and has been shown to be coded for by either Ki-MSV or Ha-MSV. In this report, we compare the thermal stability of the newly synthesized [35S]methionine-labeled p21 in cells transformed by the wild-type Ki-MSV or by a mutant of Ki-MSV (ts 371) which is temperature sensitive in a viral function required for the maintenance of several properties of the transformed phenotype. The immunoprecipitability of the p21 coded for by the ts 371 Ki-MSV was markedly more thermolabile than the p21 of the wild-type Ki-MSV when the cell extracts are heated in vitro. The present finding suggests that the p21 is required for the maintenance of transformation induced by Ki-MSV.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bassin R. H., Tuttle N., Fischinger P. J. Isolation of murine sarcoma virus-transformed mouse cells which are negative for leukemia virus from agar suspension cultures. Int J Cancer. 1970 Jul 15;6(1):95–107. doi: 10.1002/ijc.2910060114. [DOI] [PubMed] [Google Scholar]
- Benveniste R. E., Scolnick E. M. RNA in mammalian sarcoma virus transformed nonproducer cells homologous to murine leukemia virus RNA. Virology. 1973 Feb;51(2):370–382. doi: 10.1016/0042-6822(73)90436-4. [DOI] [PubMed] [Google Scholar]
- Brugge J. S., Steinbaugh P. J., Erikson R. L. Characterization of the avian sarcoma virus protein p60src. Virology. 1978 Nov;91(1):130–140. doi: 10.1016/0042-6822(78)90361-6. [DOI] [PubMed] [Google Scholar]
- Collett M. S., Erikson E., Erikson R. L. Structural analysis of the avian sarcoma virus transforming protein: sites of phosphorylation. J Virol. 1979 Feb;29(2):770–781. doi: 10.1128/jvi.29.2.770-781.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graf T., Ade N., Beug H. Temperature-sensitive mutant of avian erythroblastosis virus suggests a block of differentiation as mechanism of leukaemogenesis. Nature. 1978 Oct 12;275(5680):496–501. doi: 10.1038/275496a0. [DOI] [PubMed] [Google Scholar]
- Hartley J. W., Rowe W. P. Production of altered cell foci in tissue culture by defective Moloney sarcoma virus particles. Proc Natl Acad Sci U S A. 1966 Apr;55(4):780–786. doi: 10.1073/pnas.55.4.780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huu Duc-Nguyen, Rosenblum E. N., Zeigel R. F. Persistent infection of a rat kidney cell line with Rauscher murine leukemia virus. J Bacteriol. 1966 Oct;92(4):1133–1140. doi: 10.1128/jb.92.4.1133-1140.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jainchill J. L., Aaronson S. A., Todaro G. J. Murine sarcoma and leukemia viruses: assay using clonal lines of contact-inhibited mouse cells. J Virol. 1969 Nov;4(5):549–553. doi: 10.1128/jvi.4.5.549-553.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler S. W. Rapid isolation of antigens from cells with a staphylococcal protein A-antibody adsorbent: parameters of the interaction of antibody-antigen complexes with protein A. J Immunol. 1975 Dec;115(6):1617–1624. [PubMed] [Google Scholar]
- Levinson A. D., Oppermann H., Levintow L., Varmus H. E., Bishop J. M. Evidence that the transforming gene of avian sarcoma virus encodes a protein kinase associated with a phosphoprotein. Cell. 1978 Oct;15(2):561–572. doi: 10.1016/0092-8674(78)90024-7. [DOI] [PubMed] [Google Scholar]
- Rowe W. P., Pugh W. E., Hartley J. W. Plaque assay techniques for murine leukemia viruses. Virology. 1970 Dec;42(4):1136–1139. doi: 10.1016/0042-6822(70)90362-4. [DOI] [PubMed] [Google Scholar]
- Scolnick E. M., Parks W. P. Harvey sarcoma virus: a second murine type C sarcoma virus with rat genetic information. J Virol. 1974 Jun;13(6):1211–1219. doi: 10.1128/jvi.13.6.1211-1219.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scolnick E. M., Rands E., Williams D., Parks W. P. Studies on the nucleic acid sequences of Kirsten sarcoma virus: a model for formation of a mammalian RNA-containing sarcoma virus. J Virol. 1973 Sep;12(3):458–463. doi: 10.1128/jvi.12.3.458-463.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scolnick E. M., Stephenson J. R., Aaronson S. A. Isolation of temperature-sensitive mutants of murine sarcoma virus. J Virol. 1972 Oct;10(4):653–657. doi: 10.1128/jvi.10.4.653-657.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shih T. Y., Young H. A., Coffin J. M., Scolnick E. M. Physical map of the Kirsten sarcoma virus genome as determined by fingerprinting RNase T1-resistant oligonucleotides. J Virol. 1978 Jan;25(1):238–252. doi: 10.1128/jvi.25.1.238-252.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somers K., Kit S. Temperature-dependent expression of transformation by cold-sensitive mutant of murine sarcoma virus. Proc Natl Acad Sci U S A. 1973 Aug;70(8):2206–2210. doi: 10.1073/pnas.70.8.2206. [DOI] [PMC free article] [PubMed] [Google Scholar]