Abstract
Purpose
In this study we examine the association of blood pressure (BP), retinal thickness (RT), and vessel caliber in patients with type 2 diabetes and high HbA1c (elevated long-term blood glucose), either with or without mild or moderate non-proliferative diabetic retinopathy (NPDR).
Methods
Forty-three type 2 diabetes patients with high HbA1c measures (23 without NPDR and 20 with mild to moderate NPDR) and 22 age-matched, non-diabetic controls participated. BP, RT (Stratus OCT3), fundus photography, and HbA1c were measured. Correlations between BP, HbA1c, vessel caliber, and RT were evaluated.
Results
1) Diastolic BP is positively and significantly associated with RT in patients with NPDR (p <0.02). BP was not associated with retinal thickness in patients without NPDR (p = 0.83). 2) There is an association between higher HbA1c and higher diastolic BP within the NPDR group (p<0.02). Furthermore, HbA1c modifies the slope of the relationship between diastolic BP and RT in NPDR patients. 3) Greater venule diameters and a loss of the correlation between decreased arteriole size and increased systolic blood pressure, seen in controls, were observed in patients with and without NPDR.
Conclusions
The results of this study show that HbA1c and BP together have an impact on the retinal thickness measures of patients with diabetic retinopathy. These measures should be considered when evaluating retinal thickness in patients with diabetic retinopathy, both clinically and in future OCT studies on this population.
Keywords: diabetes, diabetic retinopathy, retinal thickness, blood pressure, retinal vessels
Diabetes is the leading cause of blindness in people aged 21–74 in the United States, and is an increasing problem around the world today.1 Macular edema, the leaking of fluid from vessels into the retinal tissue, is one of the leading causes of vision loss in these patients.2–4 Preventing macular edema in at-risk individuals would help to prevent vision loss.
Currently, most treatments for macular edema are aimed at slowing or reducing edema revealed through fundus examination, fluorescein angiography, or optical coherence tomography (OCT). Reports suggest that the combination of pharmaceutical and laser treatments are effective in treating macular edema in diabetes.5 However, earlier diagnosis of edema even at a sub-clinical level could be helpful in reducing the impact on vision, and lessen the need for these invasive treatments.6, 7 Finding modifiable factors that influence retinal thickness and vessel permeability would be especially helpful in this process.
As macular edema is caused by a release of fluid and proteins from the retinal vessels, a co-existing hypertension is of particular importance. Hypertension puts additional strain on the small venules and arterioles of the body, which are already affected by hyperglycemia in diabetes. In subjects without diabetes, the retinal arterioles are affected by hypertension alone, tending to become smaller in caliber.8 It has also been shown that the retina has a protective mechanism from hypertension in healthy individuals, a myogenic constriction of the arterioles, and that this effect can be impaired by hyperglycemia in patients with type 1 diabetes.9
Other studies have found links between diabetic retinal changes and increased blood pressure. In subjects with non-proliferative diabetic retinopathy (NPDR), an elevated systolic blood pressure has been shown to be predictive of an increase in retinopathy over a four year period.10 Furthermore, associations between higher systolic or diastolic blood pressure and retinopathy progression over time in type 1 diabetes patients have been noted.11, 12 Several studies have shown a relationship between blood pressure and macular edema, with high systolic blood pressure shown to be correlated with diffuse macular edema 13 and improved blood pressure control reducing the risk of macular edema.14
This study evaluates the association between blood pressure and retinal structure, both overall retinal thickness as well as blood vessel calibers, in patients with type 2 diabetes and poor blood glucose control with and without NPDR and no edema. We hypothesize that in these patients with poor control of their diabetes, that higher blood pressure could be associated with increased retinal thickness.
METHODS
Subjects
Forty-three subjects with type 2 diabetes (23 with no retinopathy and 20 with moderate or mild non-proliferative diabetic retinopathy) and 22 healthy non-diabetic controls were included. None of the controls were taking hypertensive medications, but all of the included subjects with diabetes were taking both diabetes and hypertensive medications, prescribed by their primary care doctors. In summary, there were 13 patients taking diuretics, 5 patients taking beta blockers, 5 taking angiotensin II receptor blockers, 2 taking ACE inhibitors, and 2 taking calcium channel blockers. Sixteen of the patients reported taking a hypertension medication but did not know the name of the medication. In addition, 20 of the patients reported taking aspirin, 3 reported taking insulin, and all 43 diabetic patients were taking an oral diabetes medication. No interventions were performed to change blood pressure or blood glucose levels during the course of the study. One eye of each subject was randomly chosen for inclusion. All subjects were Caucasian and Latino or Non-Latino, and subject demographic data is shown in Table 1.
Table 1.
Characteristics of Subject Groups.
| Group | Non-Diabetic Controls |
No Retinopathy | Moderate or Mild Non-proliferative Retinopathy |
|---|---|---|---|
| Number of subjects | 22 | 23 | 20 |
| Age (Years) | 51.0 ±9.2 | 52.5 ± 9.5 | 58.2 ± 6.9 |
| Gender | 11M:11F | 12M:11F | 14M:6F |
| Blood Pressure (mm Hg) | 117.3/73.8 ± (16.2/8.0) | 121.2/75.4 ± (13.8/8.0) | 125.2/75.1 ± (15.7/11.0) |
| Retinal thickness (microns) | 245.2 ± 13.6 | 247.3 ± 12.2 | 244.9 ± 21.0 |
| Duration of Diabetes (years) | N/A | 8.2 ± 2.5 | 10.7 ± 5.9 |
| HbA1c (%) | N/A | 8.8 ± 1.6 | 9.0 ± 1.8 |
Values are means ± SD.
Blood pressure (LAS on Automatic cuff, Omron Model HEM-773, Bannockburn, IL), retinal thickness (Stratus OCT3, Carl Zeiss Meditec, Dublin, CA), and fundus photography covering the central 50 degrees (Carl Zeiss Meditec, Dublin, CA) were measured on all subjects. HbA1c (FlexSite Diagnostics, Palm City, FL) was also measured on subjects with diabetes. All subjects provided written informed consent and procedures adhered to the tenets of the Declaration of Helsinki and the UC Committee for the Protection of Human Subjects.
All subjects had a best-corrected visual acuity of 20/25 or better and a spherical equivalent refractive error between −6D and +4D. The pupils were dilated with 1% tropicamide and 2.5% phenylephrine to at least 7mm to insure high quality OCT and fundus photograph clarity. Fundus photographs were graded for level of retinopathy by a retinal specialist, and subjects with patches of edema in the central 50 degrees were not included in the study.
OCT
Two different OCT scanning procedures were used. First a standard fast macular scan was done which takes six 6mm scans of the central macula at one time. Second, a 12 radial scan protocol was employed to give better resolution. The 12 radial scans were taken sequentially with each scan comprised of 512 axial samples. This 12 scan technique has been fully described by Neuville et al.15 in our laboratory and the same protocol and retinal groupings were used in this study. The retinal thickness was taken from the vitreo-retinal surface to the RPE/outer segment interface. The two scan modalities gave the same average retinal thickness. Scans with a signal strength under 7 were not used. The 12 scan protocol was used to provide greater resolution of the thickness of 37 separate retinal regions, as there are more data points captured with the 12 scan protocol. The 12 scans were interpolated to identify the thickness in 37 different macular locations which were averaged together. The number of A-scans within each hexagon ranged from 63 at a peripheral location to 707 at the center hexagon.
Blood Vessel Analysis
Fundus photographs of the optic discs of all subjects were used for the analysis of retinal blood vessels using the IVAN software (University of Wisconsin, Madison, WI). The software measures and summarizes the caliber of retinal venules and arterioles within a 0.5 to 1 disk diameter ring around the outside of the optic nerve. A centered photo of the optic nerve taken on the same camera by a qualified photographer was used for this analysis. We followed the standard protocol previously described in detail.16–18
Data Analysis
Univariate and multivariate linear regression were used to determine the significance of the associations. The means of the subject groups were compared using Student t-tests. The significance levels of linear regressions performed for multiple groups were corrected for multiple comparisons with a Bonferroni correction. The resulting significant P-value is stated in each section as needed.
RESULTS
Comparisons of Subject Groups
The subject groups were selected to be very similar with respect to average blood pressure (p =0.46) and average HbA1c (p= 0.69). They also had similar durations of diabetes (p = 0.09), but the duration of hypertension was unknown for these subjects. Perhaps surprisingly, the mean retinal thicknesses (p = 0.66) of the three groups were also not significantly different (Table 1). The HbA1c of the groups with diabetes were elevated and none of the patients fell within the normal range (patient range of 6.7–13.1%), indicating generally poor blood glucose control for these subjects. Blood pressures, on the other hand, were fairly well controlled in the majority of individuals in our diabetes population, and fell within the normal range for most subjects. Only 4 patients (3 with NPDR and 1 without) had a high diastolic blood pressure (DBP) above 90mm Hg and 12 subjects (7 with NPDR and 5 without) had an elevated systolic blood pressure (SBP), above 130 mmHg.
Blood Pressure and Retinal Thickness
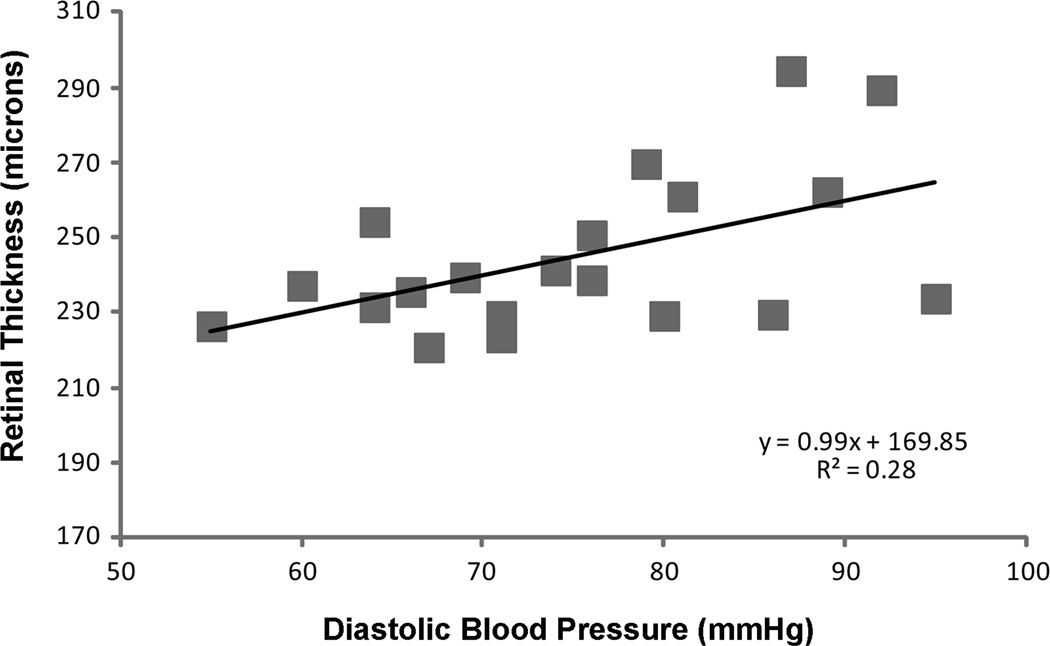
A positive correlation was found between mean retinal thickness and diastolic blood pressure in the NPDR group (r2= 0.27, p < 0.02; Figure 1). Systolic blood pressure displayed a similar trend (r2=0.18, p < 0.06) (data not shown). Over a diastolic blood pressure range of 50 − 90 mm Hg the macular thickness increased on average by 15 %. There was no correlation between blood pressure and retinal thickness in the control group (r2= 0.048, p=0.26 systolic; r2 =0.002, p=0.78 diastolic) or the patients without NPDR (r2 = 0.011, p=0.42 systolic; r2=0.0016, p=0.83 diastolic).
Figure 1.
The relationship between diastolic blood pressure and retinal thickness in patients with controlled hypertension and nonproliferative diabetic retinopathy.
Multivariate Regression of Blood Pressure, HbA1c, and Retinal Thickness
A positive correlation was found between increased diastolic blood pressure (DBP) and increased HbA1c in the patients with retinopathy (p<0.02). However, there was no relationship between HbA1c and DBP in the patients without retinopathy (p = 0.33) and there was no relationship between HbA1c and retinal thickness for either group (p=0.35; p = 0.25). HbA1c data was not gathered for the control group. To establish the most accurate relationship between the three factors and determine if HbA1c modifies the relationship between DBP and retinal thickness, a multivariate regression of diastolic blood pressure, HbA1c, and retinal thickness was performed for the patients with retinopathy.
The multivariate regression confirmed that there is no direct relationship between HbA1c and retinal thickness. However, HbA1c does act as a modifier of the relationship between retinal thickness and DBP, changing the linear slope (Table 2). Therefore, it should also be taken into account when considering retinal thickness measures in this population. Since this is a cross-sectional study, no conclusions can be drawn about causal relationships between the factors, but it is clear given the model that there is a complex relationship between HbA1c and DBP in patients with retinopathy, and that DBP is associated with retinal thickness in these patients.
Table 2.
Coefficients and P values of univariate and multivariate regression.
| Regression | Coefficient | P-value |
|---|---|---|
| RT vs DBP | 1.00 | 0.02 |
| DBP vs HbA1c | 4.11 | 0.001 |
| RT vs HbA1c | 3.15 | 0.253 |
| RT vs DBP | 1.18 | 0.04 |
| And HbA1c | 1.72 | 0.601 |
The table highlights that the coefficient for the relationship of retinal thickness (RT) and diastolic blood pressure (DBP) changes by almost 20% with the addition of HbA1c (bolded). This indicates that HbA1c is a confounder, modifying the relationship. The final model is the last one presented in italics.
Vessel Caliber Analysis
As vessels are included in the retinal thickness measures, and known to be altered in diabetes, the caliber of the retinal blood vessels was also examined for all subjects in this study. First, the vessel calibers were compared between the three groups. Second, associations between vessel calibers and blood pressure were assessed.
Comparison of Vessel Calibers Between Subject Groups
The subjects with retinopathy had significantly larger venules than controls (p < 0.03). There were no differences between the three groups with respect to arteriole size (p=0.22). Since the arteriole size was relatively unchanged and the venules became larger in diabetic retinopathy, the arteriole to venule ratio (AVR) was also smaller in the subjects with NPDR than the other groups (p < 0.03) (Table 3).
Table 3.
Vessel Caliber size measurements.
| Group | Non-Diabetic Controls | No Retinopathy | Moderate or Mild Non-proliferative Retinopathy |
|---|---|---|---|
| Arteriole Size (microns) | 185.1 ± 14.4 | 185.0 ± 21.8 | 179.3 ± 22.8 |
| Venule Size (microns) | 223.1 ± 27.2 *. | 226.7 ± 31.8 | 243.4 ± 31.2 * |
| AVR | 0.831 ± 0.091 | 0.823 ± 0.090 | .768 ± 0.090 † |
Values are mean ± SD. Bolded values are significantly different.
Venules were larger in patients with retinopathy compared to controls. (p< 0.03)
Patients with retinopathy have significantly smaller AVR than the other two groups. (p<0.03)
Associations between Retinal Thickness, Blood Pressure and Vessel Caliber
None of the subject groups demonstrated correlations between retinal thickness and any of the vessel measures. There were also no associations or trends between systolic or diastolic blood pressure and any vessel caliber measures for the subjects with diabetes, either with or without NPDR (p = 0.87). As expected, a negative correlation was found between arteriole caliber and systolic blood pressure in the control group, with a similar trend for diastolic blood pressure (r2 =0.33, p < 0.03 systolic; r2= 0.29, p < 0.049 diastolic; where p <0.03 is significant, data not shown).
DISCUSSION
In this study, the association between blood pressure, vessel caliber, and retinal thickness was examined in patients with type 2 diabetes and controlled hypertension, with or without retinopathy, and with poor blood glucose control. We found that within this selected group, diastolic blood pressure is positively and linearly associated with retinal thickness in subjects with mild to moderate NPDR but no edema, even within the normal blood pressure range. The slope of this linear relationship is modified by long-term blood glucose control. For the highest blood pressures in these subjects, the level of retinal thickening is consistent with sub-clinical edema. This suggests that blood pressure and blood glucose together could play an important role in the development of edema in these NPDR subjects. This correlation was not found in patients without retinopathy, who were also hypertensive and in poor diabetic health with long durations of diabetes and high HbA1c measures. This indicates that the presence of retinopathy is an important risk factor for increased retinal thickness in diabetics with poor health. This is in agreement with other studies that have also found a combination of these factors to be associated with diabetic edema and retinopathy progression. 14, 19–21
We included a group of patients with fairly well controlled blood pressure but poor control of their diabetes. No interventions for blood pressure or blood glucose control were performed in this study and none of the diabetic patients in either the no retinopathy or the NPDR group had a HbA1c in the normal range. This is a unique group of subjects who are likely at a higher risk of damage to their retina, and retinal edema than a group of well controlled diabetics.22 This difference could account for some of the differences in the data here and other OCT and vessel caliber studies in diabetes with wider ranges of blood glucose control. Those studies found OCT readings that are thinner in the central fovea and wider arterioles in moderate to severe retinopathy.23, 24 Patients with well-controlled diabetes were not examined here and it is not clear whether results from this study could translate to diabetics with retinopathy in better health with lower HbA1c measures. It would be interesting to examine groups of well-controlled diabetics, with and without retinopathy, to learn more about the role blood glucose control over time plays in these measures and associations as a logical follow up to this work. A recent study by Moon et al.25 found a change in HbA1c in diabetic patients was related to a change in macular thickness, but blood pressure was not included in that comparison. It would also be useful to gather information about the duration that the patients have had hypertension to see if that plays a role, which was not done here. Previous studies have shown a relationship between higher blood pressure and higher HbA1c (poorer diabetic health),26, 27 as well as a relationship between these factors and the presence of both retinopathy and macular edema.28–30 However, to our knowledge, there is only one other study that examined the relationship between blood pressure and retinal thickness measured by OCT in subjects with diabetes. Asefzadeh et al.31 found no correlation between the measures in patients with either mild NPDR or no retinopathy. We also found no correlation in subjects without retinopathy. However, a moderate NPDR group was not evaluated by Asefzadeh et al., and most of our retinopathy group had moderate NPDR, with only 15% of the subjects here having mild central NPDR.
As with other studies which noted increased venular caliber in diabetes,32–33 we noted that the venule size is greater in subjects with diabetic retinopathy. Some studies have also noted an increase in arteriole size in diabetes,24,34 but we did not. This may be because, by dividing the subjects into groups by retinopathy status, we did not have a large enough sample in each group to have adequate statistical power for this measure. Moreover, we noted that in patients with diabetes, both with and without retinopathy, there was no relationship between increased blood pressure and decreased arteriole diameter as there was for control patients. This indicates that alterations to blood vessel regulation in diabetic patients happen before clinical signs of retinopathy develop, when there is poor blood glucose control. This is in agreement with many studies that have also found a decrease in auto-regulation in the diabetic retina.35–37 It is important to note that while the blood vessel measures were obtained from the larger vessels around the optic disc, and the retinal thickness measures encompass areas of the macula with small capillaries, we have no reason to believe that an effect which is absent in the larger blood vessels would be selectively present in the smaller vessels with less and weaker muscles.
The relationship between blood pressure and retinal thickness we observed is consistent with the breakdown of the blood-retinal barrier in diabetic retinopathy causing leakage of fluid into the retinal layers. This leakage is exacerbated by increased blood pressure. While no fluid is clearly visible on the fundus photograph or in the OCT, a sub-clinical diffuse edematous event could be present. This is also consistent with the correlation between blood pressure and retinal thickness being weakest in the central fovea and strongest in the areas with greater numbers of intraretinal vessels.
If the results seen in our study are a manifestation of a sub-clinical edema caused by pushing of fluid out of the retinal vessels by a higher blood pressure, then proper control of blood pressure early in the diabetes process could be essential to reducing the risk of sight threatening clinically significant macular edema. Future studies are needed using higher definition OCT and examining both blood pressure interventions and changes in retinal thickness with changes in blood pressure. These follow up studies should also consider a larger sample size of patients with varying levels of blood glucose control and retinopathy.
In conclusion, the blood vessels within the eye are altered early in diabetes patients with high HbA1c values. Even in patients with no retinopathy, the correlation between arteriole size and blood pressure (present in controls) is absent. Furthermore, higher blood pressures are associated with greater retinal thickness in subjects with moderate or mild non-proliferative diabetic retinopathy. This occurs even when blood pressures are largely within the normal range, and the slope of the relationship is modified by the HbA1c. This indicates that blood pressure plays an important role in the mechanisms leading to increased retinal thickness and possibly retinal edema in patients with diabetes and poor control of their blood glucose.
ACKNOWLEDGMENTS
This study was funded by NIH NEI EY007043 to AJA. Part of this work was completed while WWH was the recipient of an Ezell Fellowship from the American Optometric Foundation. The authors thank Drs. Jason Ng, Kevin Bronson-Castain, Kavita Dhamdhere, Brian Wolff, Glen Ozawa, and Jessica Neuville, as well as Mr. Royce Lam and Ms. Melissa Au-Yeung, for their assistance with this project.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Centers for Disease Control and Prevention. National Diabetes Fact Sheet: General Information and National Estimates on Diabetes in the United States, 2007. Atlanta, GA: U.S. Department of Health and Human Services; 2008. [Accessed September 24, 2012]. Available at: National Diabetes Fact Sheet: General Information and National Estimates on Diabetes in the United States, 2007. [Google Scholar]
- 2.Girach A, Lund-Andersen H. Diabetic macular oedema: a clinical overview. Int J Clin Pract. 2007;61:88–97. doi: 10.1111/j.1742-1241.2006.01211.x. [DOI] [PubMed] [Google Scholar]
- 3.Klein R, Klein BE, Moss SE, Cruickshanks KJ. The Wisconsin Epidemiologic Study of Diabetic Retinopathy. XV. The long-term incidence of macular edema. Ophthalmology. 1995;102:7–16. doi: 10.1016/s0161-6420(95)31052-4. [DOI] [PubMed] [Google Scholar]
- 4.Goebel W, Kretzchmar-Gross T. Retinal thickness in diabetic retinopathy: a study using optical coherence tomography (OCT) Retina. 2002;22:759–767. doi: 10.1097/00006982-200212000-00012. [DOI] [PubMed] [Google Scholar]
- 5.Elman MJ, Aiello LP, Beck RW, Bressler NM, Bressler SB, Edwards AR, Ferris FL, 3rd, Friedman SM, Glassman AR, Miller KM, Scott IU, Stockdale CR, Sun JK. Randomized trial evaluating ranibizumab plus prompt or deferred laser or triamcinolone plus prompt laser for diabetic macular edema. Ophthalmology. 2010;117:1064–1077. e35. doi: 10.1016/j.ophtha.2010.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Browning DJ, Fraser CM, Clark S. The relationship of macular thickness to clinically graded diabetic retinopathy severity in eyes without clinically detected diabetic macular edema. Ophthalmology. 2008;115:533–539. doi: 10.1016/j.ophtha.2007.06.042. [DOI] [PubMed] [Google Scholar]
- 7.Brown JC, Solomon SD, Bressler SB, Schachat AP, DiBernardo C, Bressler NM. Detection of diabetic foveal edema: contact lens biomicroscopy compared with optical coherence tomography. Arch Ophthalmol. 2004;122:330–335. doi: 10.1001/archopht.122.3.330. [DOI] [PubMed] [Google Scholar]
- 8.Ikram MK, Witteman JC, Vingerling JR, Breteler MM, Hofman A, de Jong PT. Retinal vessel diameters and risk of hypertension: the Rotterdam Study. Hypertension. 2006;47:189–194. doi: 10.1161/01.HYP.0000199104.61945.33. [DOI] [PubMed] [Google Scholar]
- 9.Blum M, Kloos C, Gunther S, Hunger-Dathe W, Muller UA. Improved metabolic control results in better myogenic response of retinal arterioles in patients with diabetes mellitus type 1. Ophthalmologica. 2008;222:373–377. doi: 10.1159/000151278. [DOI] [PubMed] [Google Scholar]
- 10.Manaviat MR, Rashidi M, Afkhami-Ardekani M. Four years incidence of diabetic retinopathy and effective factors on its progression in type II diabetes. Eur J Ophthalmol. 2008;18:572–577. doi: 10.1177/112067210801800412. [DOI] [PubMed] [Google Scholar]
- 11.Klein BE, Klein R, Moss SE, Palta M. A cohort study of the relationship of diabetic retinopathy to blood pressure. Arch Ophthalmol. 1995;113:601–606. doi: 10.1001/archopht.1995.01100050069033. [DOI] [PubMed] [Google Scholar]
- 12.Chase HP, Garg SK, Jackson WE, Thomas MA, Harris S, Marshall G, Crews MJ. Blood pressure and retinopathy in type I diabetes. Ophthalmology. 1990;97:155–159. doi: 10.1016/s0161-6420(90)32611-8. [DOI] [PubMed] [Google Scholar]
- 13.Lopes de Faria JM, Jalkh AE, Trempe CL, McMeel JW. Diabetic macular edema: risk factors and concomitants. Acta Ophthalmol Scand. 1999;77:170–175. doi: 10.1034/j.1600-0420.1999.770211.x. [DOI] [PubMed] [Google Scholar]
- 14.Beulens JW, Patel A, Vingerling JR, Cruickshank JK, Hughes AD, Stanton A, Lu J, Mc GTSA, Grobbee DE, Stolk RP. Effects of blood pressure lowering and intensive glucose control on the incidence and progression of retinopathy in patients with type 2 diabetes mellitus: a randomised controlled trial. Diabetologia. 2009;52:2027–2036. doi: 10.1007/s00125-009-1457-x. [DOI] [PubMed] [Google Scholar]
- 15.Neuville JM, Bronson-Castain K, Bearse MA, Jr, Ng JS, Harrison WW, Schneck ME, Adams AJ. OCT reveals regional differences in macular thickness with age. Optom Vis Sci. 2009;86:810–816. doi: 10.1097/OPX.0b013e3181adff59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hubbard LD, Brothers RJ, King WN, Clegg LX, Klein R, Cooper LS, Sharrett AR, Davis MD, Cai J. Methods for evaluation of retinal microvascular abnormalities associated with hypertension/sclerosis in the Atherosclerosis Risk in Communities Study. Ophthalmology. 1999;106:2269–2280. doi: 10.1016/s0161-6420(99)90525-0. [DOI] [PubMed] [Google Scholar]
- 17.Wong TY, Klein R, Klein BE, Meuer SM, Hubbard LD. Retinal vessel diameters and their associations with age and blood pressure. Invest Ophthalmol Vis Sci. 2003;44:4644–4650. doi: 10.1167/iovs.03-0079. [DOI] [PubMed] [Google Scholar]
- 18.Knudtson MD, Lee KE, Hubbard LD, Wong TY, Klein R, Klein BE. Revised formulas for summarizing retinal vessel diameters. Curr Eye Res. 2003;27:143–149. doi: 10.1076/ceyr.27.3.143.16049. [DOI] [PubMed] [Google Scholar]
- 19.Jeganathan VS, Cheung N, Tay WT, Wang JJ, Mitchell P, Wong TY. Prevalence and risk factors of retinopathy in an Asian population without diabetes: the Singapore Malay Eye Study. Arch Ophthalmol. 2010;128:40–45. doi: 10.1001/archophthalmol.2009.330. [DOI] [PubMed] [Google Scholar]
- 20.Wong TY, Mwamburi M, Klein R, Larsen M, Flynn H, Hernandez-Medina M, Ranganathan G, Wirostko B, Pleil A, Mitchell P. Rates of progression in diabetic retinopathy during different time periods: a systematic review and meta-analysis. Diabetes Care. 2009;32:2307–2313. doi: 10.2337/dc09-0615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sultan MB, Starita C, Huang K. Epidemiology, risk factors and management of paediatric diabetic retinopathy. Br J Ophthalmol. 2012;96:312–317. doi: 10.1136/bjophthalmol-2011-300169. [DOI] [PubMed] [Google Scholar]
- 22.Chou TH, Wu PC, Kuo JZ, Lai CH, Kuo CN. Relationship of diabetic macular oedema with glycosylated haemoglobin. Eye (Lond) 2009;23:1360–1363. doi: 10.1038/eye.2008.279. [DOI] [PubMed] [Google Scholar]
- 23.Bressler NM, Edwards AR, Antoszyk AN, Beck RW, Browning DJ, Ciardella AP, Danis RP, Elman MJ, Friedman SM, Glassman AR, Gross JG, Li HK, Murtha TJ, Stone TW, Sun JK. Retinal thickness on Stratus optical coherence tomography in people with diabetes and minimal or no diabetic retinopathy. Am J Ophthalmol. 2008;145:894–901. doi: 10.1016/j.ajo.2007.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kifley A, Wang JJ, Cugati S, Wong TY, Mitchell P. Retinal vascular caliber, diabetes, and retinopathy. Am J Ophthalmol. 2007;143:1024–1026. doi: 10.1016/j.ajo.2007.01.034. [DOI] [PubMed] [Google Scholar]
- 25.Moon SW, Kim HY, Kim SW, Oh J, Huh K, Oh IK. The change of macular thickness measured by optical coherence tomography in relation to glycemic control in diabetic patients. Graefes Arch Clin Exp Ophthalmol. 2011;249:839–848. doi: 10.1007/s00417-010-1562-z. [DOI] [PubMed] [Google Scholar]
- 26.Voorham J, Haaijer-Ruskamp FM, Wolffenbuttel BH, de Zeeuw D, Stolk RP, Denig P. Differential effects of comorbidity on antihypertensive and glucose-regulating treatment in diabetes mellitus--a cohort study. PLoS One. 2012;7:e38707. doi: 10.1371/journal.pone.0038707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Torchinsky MY, Gomez R, Rao J, Vargas A, Mercante DE, Chalew SA. Poor glycemic control is associated with increased diastolic blood pressure and heart rate in children with Type 1 diabetes. J Diabetes Complications. 2004;18:220–223. doi: 10.1016/S1056-8727(03)00031-X. [DOI] [PubMed] [Google Scholar]
- 28.UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33) Lancet. 1998;352:837–853. [PubMed] [Google Scholar]
- 29.Hypertension in Diabetes Study (HDS): I. Prevalence of hypertension in newly presenting type 2 diabetic patients and the association with risk factors for cardiovascular and diabetic complications. J Hypertens. 1993;11:309–317. doi: 10.1097/00004872-199303000-00012. [DOI] [PubMed] [Google Scholar]
- 30.UK Prospective Diabetes Study Group. Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38. BMJ. 1998;317:703–713. [PMC free article] [PubMed] [Google Scholar]
- 31.Asefzadeh B, Fisch BM, Parenteau CE, Cavallerano AA. Macular thickness and systemic markers for diabetes in individuals with no or mild diabetic retinopathy. Clin Experiment Ophthalmol. 2008;36:455–463. doi: 10.1111/j.1442-9071.2008.01769.x. [DOI] [PubMed] [Google Scholar]
- 32.Bronson-Castain KW, Bearse MA, Jr, Neuville J, Jonasdottir S, King-Hooper B, Barez S, Schneck ME, Adams AJ. Adolescents with Type 2 diabetes: early indications of focal retinal neuropathy, retinal thinning, and venular dilation. Retina. 2009;29:618–626. doi: 10.1097/IAE.0b013e31819a988b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sun C, Wang JJ, Mackey DA, Wong TY. Retinal vascular caliber: systemic, environmental, and genetic associations. Surv Ophthalmol. 2009;54:74–95. doi: 10.1016/j.survophthal.2008.10.003. [DOI] [PubMed] [Google Scholar]
- 34.Tikellis G, Wang JJ, Tapp R, Simpson R, Mitchell P, Zimmet PZ, Shaw J, Wong TY. The relationship of retinal vascular calibre to diabetes and retinopathy: the Australian Diabetes, Obesity and Lifestyle (AusDiab) study. Diabetologia. 2007;50:2263–2271. doi: 10.1007/s00125-007-0822-x. [DOI] [PubMed] [Google Scholar]
- 35.Frederiksen CA, Jeppesen P, Knudsen ST, Poulsen PL, Mogensen CE, Bek T. The blood pressure-induced diameter response of retinal arterioles decreases with increasing diabetic maculopathy. Graefes Arch Clin Exp Ophthalmol. 2006;244:1255–1261. doi: 10.1007/s00417-006-0262-1. [DOI] [PubMed] [Google Scholar]
- 36.Jeppesen P, Bek T. Impaired retinal autoregulation in small retinal arterioles before and after focal laser treatment for diabetic maculopathy. Br J Ophthalmol. 2006;90:198–201. doi: 10.1136/bjo.2005.078808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shibata M, Oku H, Sugiyama T, Kobayashi T, Tsujimoto M, Okuno T, Ikeda T. Disruption of gap junctions may be involved in impairment of autoregulation in optic nerve head blood flow of diabetic rabbits. Invest Ophthalmol Vis Sci. 2011;52:2153–2159. doi: 10.1167/iovs.10-6605. [DOI] [PubMed] [Google Scholar]