Abstract
The effect of the (R,S)-ketamine metabolites (R,S)-norketamine, (R,S)-dehydronorketamine, (2S,6S)-hydroxynorketamine and (2R,6R)- hydroxynorketamine on the activity of α7 and α3β4 neuronal nicotinic acetylcholine receptors was investigated using patch-clamp techniques. The data indicated that (R,S)-dehydronorketamine inhibited acetylcholine-evoked currents in α7-nicotinic acetylcholine receptor, IC50 = 55 ± 6 nM, and that (2S,6S)-hydroxynorketamine, (2R,6R)-hydroxynorketamine and (R,S)-norketamine also inhibited α7-nicotinic acetylcholine receptor function at concentrations ≤1μM, while (R,S)-ketamine was inactive at these concentrations. The inhibitory effect of (R,S)-dehydronorketamine was voltage-independent and the compound did not competitively displace selective α7-nicotinic acetylcholine receptor ligands [125I]-α-bungarotoxin and [3H]-epibatidine indicating that (R,S)-dehydronorketamine is a negative allosteric modulator of the α7-nicotinic acetylcholine receptor. (R,S)-Ketamine and (R,S)-norketamine inhibited (S)-nicotine-induced whole-cell currents in cells expressing α3β4-nicotinic acetylcholine receptor, IC50 3.1 and 9.1μM, respectively, while (R,S)-dehydronorketamine, (2S,6S)-hydroxynorketamine and (2R,6R)-hydroxynorketamine were weak inhibitors, IC50 >100μM. The binding affinities of (R,S)-dehydronorketamine, (2S,6S)-hydroxynorketamine and (2R,6R)-hydroxynorketamine at the NMDA receptor were also determined using rat brain membranes and the selective NMDA receptor antagonist [3H]-MK-801. The calculated Ki values were 38.95 μM for (S)-dehydronorketamine, 21.19 μM for (2S,6S)-hydroxynorketamine and > 100 μM for (2R,6R)-hydroxynorketamine. The results suggest that the inhibitory activity of ketamine metabolites at the α7-nicotinic acetylcholine receptor may contribute to the clinical effect of the drug.
Keywords: Dehydronorketamine, hydroxynorketamine, norketamine, depression, pain, negative allosteric modifiers, neuronal nicotinic acetylcholine receptors
1. Introduction
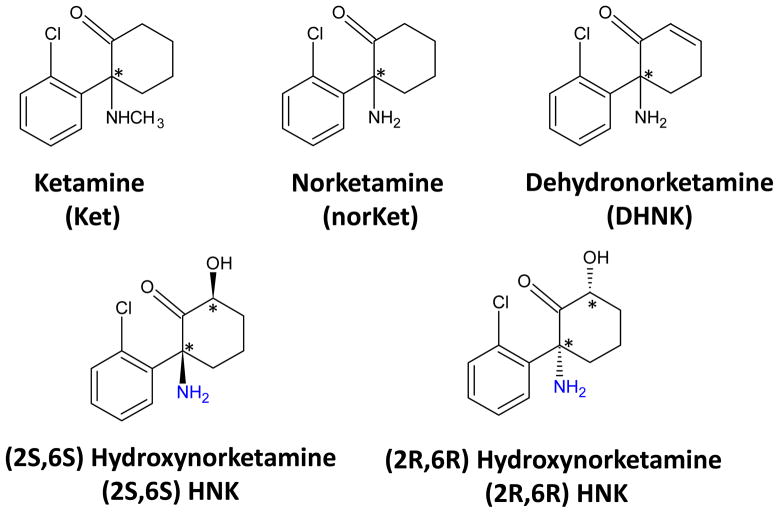
(R,S)-Ketamine (Fig. 1) is a chiral phencyclidine derivative that was developed as an anesthetic agent (Hirota and Lambert, 2011). (R,S)-Ketamine is extensively transformed by microsomal enzymes into a number of metabolites including (R,S)-norketamine, (R,S)-dehydronorketamine and a series of hydroxynorketamines including (2S,6S)-hydroxynorketamine and (2R,6R)-hydroxynorketamine (Fig. 1) (Trevor, et al., 1983), and the P450 isoforms associated with these transformation have been recently identified (Desta, et al., 2012). Initial studies utilizing (R,S)-ketamine, (R,S)-norketamine and (2S,6S;2R,6R)-hydroxynorketamine determined that the CNS activities associated with general anesthesia and recovery were produced by (R,S)-ketamine and (R,S)-norketamine but not (2S,6S;2R,6R)-hydroxynorketamine (Leung and Baillie, 1986). Thus, (R,S)-ketamine was identified as an anesthetic agent, (R,S)-norketamine as an “active” metabolite and (2S,6S;2R,6R)- hydroxynorketamine was considered inactive. When subsequent studies indicated that (R,S)-ketamine and (R,S)-norketamine inhibited the NMDA receptor, this activity became the accepted explanation of the pharmacological effects of (R,S)-ketamine and (R,S)-norketamine (Hirota and Lambert, 2011).
Figure 1.
The structures of the compounds used in this study
Since the operating hypothesis was centered upon the activity of (R,S)-ketamine and (R,S)-norketamine, most metabolic, pharmacokinetic, pharmacological and clinical studies have concentrated on these compounds. However, while this approach may be valid for anesthetic dosing of (R,S)-ketamine, it does not appear to be applicable to sub-anesthetic dosing of the compound, which is currently being investigated for treatment of neuropathic and acute pain and depression (Hirota and Lambert, 2011). For example, a population-pharmacokinetic analysis of patients receiving low-dose (R,S)-ketamine for the treatment of bipolar depression demonstrated that following the distribution of (R,S)-ketamine and (R,S)-norketamine may not accurately reflect the pharmacodynamics and that (2S,6S)- hydroxynorketamine, (2R,6R)- hydroxynorketamine and (R,S)-dehydronorketamine were major plasma components (Zhao, et al., 2012). In addition, a study of clinical response in 67 patients treated with low-dose (R,S)-ketamine for major depressive disorder and bipolar depression indicated plasma concentrations of (R,S)-dehydronorketamine, (2S,5S;2R,5R)-hydroxynorketamine, and (2S,5R;2R,5S)- hydroxynorketamine were associated with lower psychotomimetic or dissociative side effects (Zarate, et al., 2012). Thus, the observed clinical responses produced by low dose (R,S)-ketamine may be due to unexplored pharmacological activities of (R,S)-ketamine metabolites.
Recent studies have demonstrated that mecamylamine has therapeutic efficacy in the treatment of depression and that this effect may be related to its activity as an open-channel non-competitive inhibitor of neuronal nicotinic acetylcholine receptors (Philip, et al., 2010). (R,S)-Ketamine is also an open-channel non-competitive inhibitor of homomeric (α7) and heteromeric (α4β2, α3β4) nicotinic acetylcholine receptor subtypes (Yamakura, et al., 2000; Coates, et al., 2001), but little is known about the activity of (R,S)-ketamine metabolites at the nicotinic acetylcholine receptor. This study was designed to assess the effect of (R,S)-norketamine, (2S,6S)-hydroxynorketamine, (2R,6R)- hydroxynorketamine and (R,S)-dehydronorketamine on agonist-induced whole-cell current in cells expressing α3β4-nicotinic acetylcholine receptor and α7-nicotinic acetylcholine receptor and their binding affinities to the NMDA receptor. In addition, the binding affinities of (R)- and (S)-dehydronorketamine at the α7-nicotinic acetylcholine receptor were determined.
2. Material and methods
2.1. Materials
(R,S)-Ketamine, (R)-ketamine, (S)-ketamine, (R,S)-norketamine, (R)-norketamine, (S)-norketamine, (R,S)-dehydronorketamine, (R)-dehydronorketamine, (S)-dehydronorketamine, (2S,6S)-hydroxynorketamine and (2R,6R)-hydroxynorketamine were prepared as previously described (Moaddel, et al., 2010). Minimum Essential Medium with Earles Salts and L-glutamine (MEM), fetal bovine serum (FBS), penicillin, streptomycin, geneticin were purchased from Gibco (Carlsbad, CA, USA). (±)-Epibatidine dihydrochloride, (S)-nicotine tartrate, acetylcholine chloride, and the other chemicals used in the study were purchased from Sigma (St. Louis, MO, USA). Mecamylamine was purchased from Ascent Scientific (Princeton, NJ, USA), and [125I]-α-bungarotoxin, [3H]-MK-801 and [3H]-epibatidine dihydrochloride were purchased from PerkinElmer Life Sciences (Boston, MA, USA). Methyllycaconitine was generously provided by Research Triangle Institute through the National Institute on Drug Abuse.
2.2. Cell lines and culture
The KXα3β4R2 (expressing rat α3β4-nicotinic acetylcholine receptor) and KXα7R1 (expressing rat α7-nicotinic acetylcholine receptor) cell lines have been previously described (Xaio, et al., 1998; Xaio, et al., 2009). The cell lines were maintained in MEM supplemented with 10% FBS, 100 units/ml penicillin G, 100 μg/ml streptomycin and 0.7 mg/ml geneticin at 37°C with 5% CO2 in a humidified incubator.
2.3. Whole-cell current recording in KXα7R1 cells
The KXα7R1 cell line was studied in the whole-cell configuration of the patch-clamp technique using an Axopatch 200B amplifier (Molecular Devices, Sunnyvale, CA, USA). The borosilicate glass capillary electrodes (Sutter Instrument Company, Novato, CA, USA), had a resistance of 2.5–3.5 MΩ when filled with 110mM trisphosphate dibasic, 28mM Tris base, 11mM EGTA, 2mM MgCl2, 0.1mM CaCl2 and 4mM Mg-ATP (pH 7.3). In some cells ~85% of electrode resistance was compensated electronically, so that the effective series resistance in the whole-cell configuration was accepted when less than 20 MΩ. Generation of voltage-clamp protocols and acquisition of the data were carried out using pCLAMP 9.0 software (Molecular Devices). Sampling frequency was 5 kHz and current signals were filtered at 5 or 10 kHz before digitization and storage. All experiments were performed at room temperature (22–25°C).
KXα7R1 cells were studied 2–3 days after plating on 15-mm round plastic cover slips (Thermanox, Nalge Nunc, Napierville, IL, USA) in an experimental chamber mounted on the stage of an Olympus IX50 inverted microscope (Olympus Corporation, Tokyo, Japan). The experimental chamber was constantly perfused (1–2 ml/min) with 140mM NaCl, 3mM KCl, 2mM MgCl2, 25mM D-glucose, 10mM HEPES and 2mM CaCl2 (pH 7.4). The high speed solution exchange system, HSSE-2 (ALA Scientific Instruments, Westbury, NY, USA) was used to deliver control and test solutions. The delay in switching between solutions was ~10ms. Data presented herein were obtained through subtraction from the leak current.
The cells expressing α7-nicotinic acetylcholine receptors were initially exposed to 280μM acetylcholine (~EC50), followed by pre-exposure (1–3 min) to test compounds at various concentrations prior to co-application with 280μM acetylcholine. The acetylcholine-evoked currents in the presence of the test compounds were measured at −80 mV and normalized to the amplitude of the current elicited by acetylcholine alone. The IC50 and nH values for (R,S)-dehydronorketamine were evaluated with the Origin 5.0 program (Microcal, North Hampton, MA, USA). The acetylcholine-evoked currents in the presence of (R,S)-dehydronorketamine were measured at −80 mV and normalized to the amplitude of the current elicited by acetylcholine alone. Values were plotted against the concentrations of (R,S)-dehydronorketamine on a logarithm scale and fitted with the equation:
Where: I is the current amplitude at the antagonist concentration [antagonist]; Imax is the maximum current; nH is the Hill coefficient. Results are presented as the mean ± S.E.M for the number of cells (n), where n = 4 per concentration.
2.4. Whole-Cell current recording α3β4-nicotinic acetylcholine receptor
Whole cell current was measured using EPC 10 HEKA Instruments amplifier. All drugs were applied by fast perfusion system (DynaFlow, Celletricon AB, Mölndal, Sweden) with 16 channels chip. Cells were clamped at -60mV holding potential. KXα3β4R2 cells were collected at 70% confluence and suspended in HEPES [10mM, pH 7.4] containing 120mM NaCl, 3.1mM KCl, 2mM CaCl2, 5mM glucose at room temperature. The glass pipettes with 5–7M resistance were filled with HEPES [10 mM, pH 7.2], containing 145mM CsCl, 1mM MgCl2, 2mM ATP, 1mM EGTA. All experiments were performed under visual control using an Olympus CKX 41 inverted microscope (Olympus Corporation). Test compounds were applied at concentrations of 0.1nM, 1nM, 10nM, 100nM, 1μM, 10μM and 100μM in the absence of (S)-nicotine for stimulation or in the presence of 100μM (S)-nicotine for inhibition measurements. For inhibition studies, the decrease in current relative to 100μM (S)-nicotine (100%) was determined. Raw data was collected using PatchMaster Version 2.32 software (Celletricon AB) and analysed using GraphPad Prism Version 5.0 software (GraphPad Software, Inc., La Jolla, CA, USA).
2.5 KXα7R1 membrane binding studies
Binding of [125I]-α-bungarotoxin and of [3H]-epibatidine to membranes prepared from the KXα7R1 cell line was measured as described previously (Xaio, et al., 2009). Briefly, membrane preparations were incubated with ~ 1.7 nM [125I]-α-bungarotoxin or ~ 2.1 nM [3H]-epibatidine in Tris.HCl [50 mM, pH7.4] containing 0.2 mg/ml BSA for 2h at 24°C in a final volume of 0.1 ml. Total binding and nonspecific binding were determined in the absence and presence of 1,000 μM (S)-nicotine. Ten concentrations of each test compound were incubated to afford a binding inhibition curve. Bound and free ligands were separated by vacuum filtration through Whatman GF/C filters (Thermo Fisher Scientific, Waltham, MA, USA) treated with 0.5% polyethylenimine and 0.5 mg/ml BSA. The filter-retained radioactivity was measured by liquid scintillation counting. Specific binding was defined as the difference between total binding and nonspecific binding. Data from competition binding assays were analyzed using GraphPad Prism version 5.0 (GraphPad Software).
2.6 NMDA receptor binding studies
The binding affinities of the test compounds to the NMDA receptor were determined using rat brain membranes and the high affinity and selective antagonist [3H]MK-801 using previously described procedures (Wong, et al., 1986). In brief, membranes were prepared from previously frozen rat brains by homogenization in Tris buffer [5 mM, pH 7.7], centrifugation, re-homogenization and a second centrifugation. The final membrane pellet was suspended in 5 mM Tris buffer at a concentration of 1.0 g original wet weight per 50 ml. Binding was conducted in a final volume of 1.0 ml, using 1.65 nM [3H]MK-801 as the radioligand and unlabeled MK-801 (10 μM) to define non-specific binding. The reaction was incubated for 1 h at 25°C and filtered over glass fiber filters soaked in 0.05% polyethyleneimine (PEI, EMD Millipore Corp, Belleria, MA, USA).
3. Results
3.1 The effect on α7-nicotinic acetylcholine receptor activity
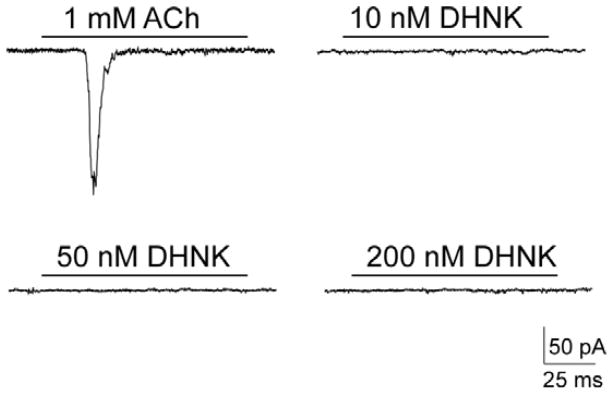
The effect of the test compounds on the function of α7-nicotinic acetylcholine receptors was evaluated using patch-clamp technique in the whole-cell configuration. Initial studies examined their ability to induce current activation at concentrations equal to 10, 50 and 200 nM. No measurable effect was observed at any tested concentration (n = 4), as illustrated for (R,S)-dehydronorketamine (Fig. 2).
Figure 2.
The effect of 10, 50 and 200nM (R,S)-dehydronorketamine {(R,S)-DHNK} on the current traces recorded using KXα7R1 cells in the whole-cell configuration of patch-clamp technique in the presence of 1 mM ACh with a holding potential of −80mV.
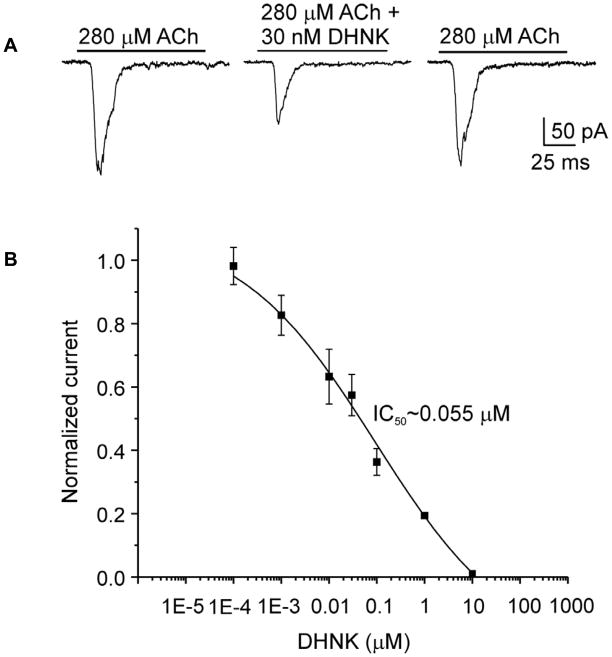
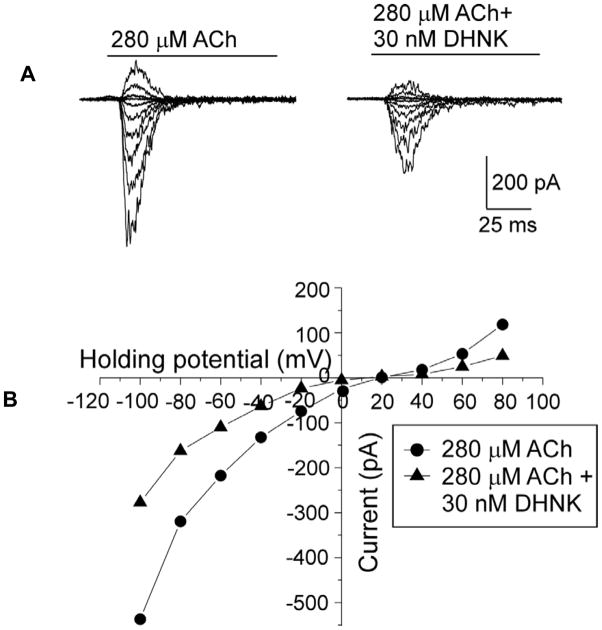
The ability of the test compounds to affect acetylcholine-induced current was assessed using 280 μM acetylcholine, corresponding to previously determined EC50 concentration for rat α7-nicotinic acetylcholine receptor (Xiao, et al., 2009) and 100 nM or 1 μM of the test compounds. (R,S)-Ketamine had no observable effect, data not shown, which is consistent with previous finding that (R,S)-ketamine is a non-competitive inhibitor of α7-nicotinic acetylcholine receptor with an IC50 ~20μM with no significant inhibitory activity at concentrations ≤1 μM (Coates and Flood, 2001). At 100 nM concentrations, (R,S)-ketamine metabolites inhibited acetylcholine-induced current in a range from ~60%, (R,S)-dehydronorketamine (Fig. 3A), to ~45%, (R,S)-norketamine (Table 1). Acetylcholine-activated current recovered upon washout of (R,S)-dehydronorketamine (Fig. 3A). The inhibitory potency of (R,S)-dehydronorketamine was assessed at a range of concentrations from 0.0001 to 10 μM, and the IC50 was 55 ± 6 nM (nH = 0.66 ± 0.05; n = 3–5 for each tested concentration) (Fig. 3B). The effect of voltage on the (R,S)-dehydronorketamine mediated inhibition of acetylcholine-induced current was examined using a 30 nM concentration of (R,S)-dehydronorketamine and by varying the holding potential between −100 and + 80 mV, (Fig. 4A,B). The inhibitory effect of (R,S)-dehydronorketamine on α7-nicotinic acetylcholine receptors was voltage-independent (n=3) (Fig. 4B), suggesting that (R,S)-dehydronorketamine does not act as an ion channel blocker on this nicotinic acetylcholine receptor subtype.
Figure 3.
A. The effect of 30 nM (R,S)-DHNK on the ACh-induced current in KXα7R1 cells illustrates a reversible pronounced (45%) inhibition of the whole-cell current. Holding potential was −80 mV.
B. Dose-dependent effect of (R,S)-DHNK on the ACh-induced current in KXα7R1 cells. The curve was fitted to Hill equation. Symbols and bars represent the mean ± S.E.M.
Table 1.
The effect of (R,S)-norketamine, (R,S)-dehydronorketamine, (2S,6S)-hydroxynorketamine and (2R,6R)-hydroxynorketamine on the currents evoked by 280 μM acetylcholine in the KXα7R1 cell line.
| Compound | Concentration | Mean | SE |
|---|---|---|---|
| (R,S)-norKetamine | 100 nM | 0.548 | 0.099 |
| 1000 nM | 0.287 | 0.075 | |
| (R,S)-dehydronorketamine | 100 nM | 0.407 | 0.053 |
| 1000 nM | 0.205 | 0.013 | |
| (2S,6S)-hydroxynorketamine | 100 nM | 0.461 | 0.06 |
| 1000 nM | 0.361 | 0.07 | |
| (2R,6R)-hydroxynorketamine | 100 nM | 0.489 | 0.077 |
| 1000 nM | 0.354 | 0.084 |
The data was obtained at −80 mV and normalized to the amplitude of the current elicited by acetylcholine alone, n = 4.
Figure 4.
A. Superimposed traces of the ACh-induced currents in the absence and presence of DHNK in KXα7R1 cells.
B. Voltage-dependence of inhibitory effect of (R,S)-DHNK on the ACh-induced current in KXα7R1 evoked at various holding potentials from −100 to +80 mV, quantified at its maximal amplitude in the absence (circles) and presence (triangles) of 30 nM DHNK and plotted versus the corresponding holding potential.
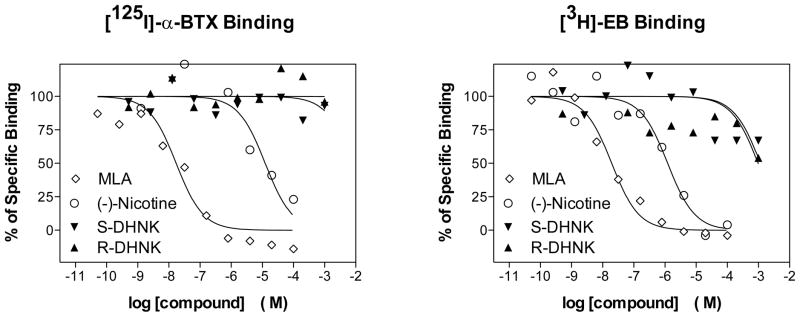
In order to determine whether the inhibitory effect of (R,S)-dehydronorketamine in α7-nicotinic acetylcholine receptor is a function of competitive binding to the agonist binding site, the binding affinities, Ki values, of (R)-dehydronorketamine and (S)-dehydronorketamine were determined using cellular membranes from the KXα7R1 cell line. Competitive displacement studies were carried out using the selective α7-nicotinic acetylcholine receptor ligands [125I]-α-bungarotoxin and [3H]-epibatidine with (S)-nicotine and methyllycaconitine as positive controls. In previous studies utilizing KXα7R1 cellular membranes (Xiao, et al., 2009), [125I]- α-bungarotoxin, Kd = 0.38 nM, was displaced by methyllycaconitine, Ki = 9.1 nM, and by (S)-nicotine, Ki = 570 nM. Under the same experimental conditions, (S)-dehydronorketamine and (R)-dehydronorketamine did not displace [125I]-α-bungarotoxin at concentrations up to 1,000 μM (Fig. 5A). These results were confirmed using [3H]-epibatidine, Kd = 1.8 nM (Xiao, et al., 2009), which was fully displaced by methyllycaconitine and (S)-nicotine, but was not displaced by (S)-dehydronorketamine or (R)-dehydronorketamine at concentrations up to 1,000 μM (Fig 5B). While non-specific binding at the higher concentrations of epibatidine produced a large scatter in the data, the results demonstrate that (S)-dehydronorketamine and (R)-dehydronorketamine have low binding affinities for the binding site probed by epibatidine and are consistent with the results from the binding studies using [125I]-α-bungarotoxin.
Figure 5.
Competitive displacement of [125I]-α-BTX and [3H]-EB from cellular membranes obtained from the KXα7R1 cell line by (R)-DHNK, (S)-DHNK, MLA and (S)-nicotine.
3.2 The effect on α3β4-nicotinic acetylcholine receptor activity
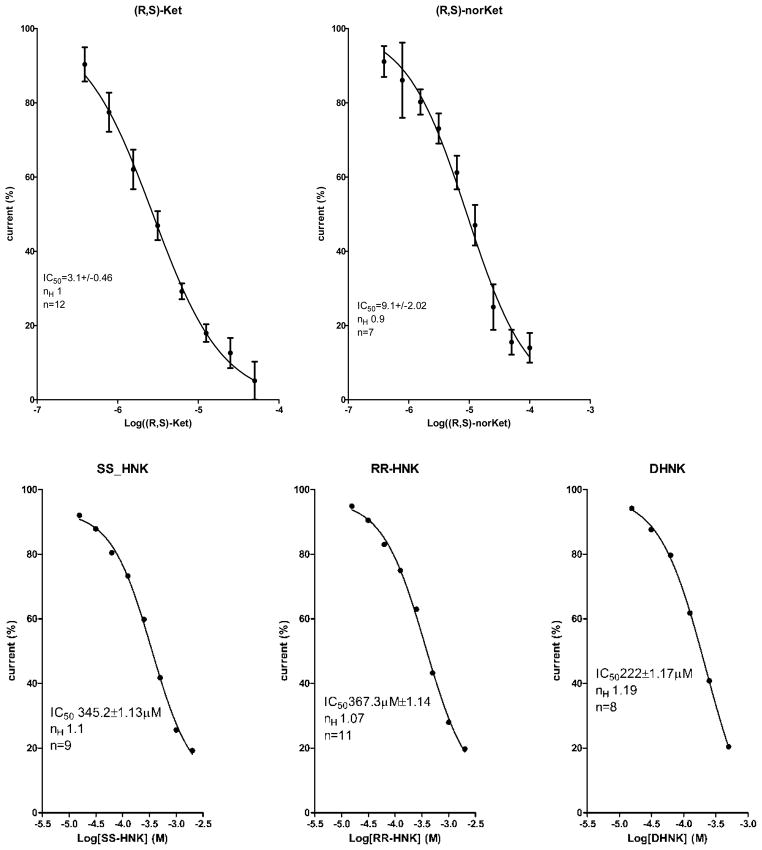
The effect of the test compounds on the function of the α3β4-nicotinic acetylcholine receptors was evaluated using patch-clamp technique in the whole-cell configuration. The compounds were first examined for their ability to induce current activation at the range of concentrations 0.1 nM-100 μM. No measurable response was observed at any tested concentration, data not shown. The ability of the test compounds to affect (S)-nicotine-induced current was assessed using 100 μM (S)-nicotine and appropriate concentration range of (R,S)-ketamine and metabolites (Fig. 6). (R,S)-Ketamine and (R,S)-norketamine produced a significant suppression of the currents with IC50 values of 3.1μM and 9.1μM, respectively. (R,S)-Ketamine has been previously reported as a non-competitive inhibitor of the α3β4 nicotinic acetylcholine receptor, IC50 = 9.5μM (Yamakura, et al., 2000). The application of (R,S)-dehydronorketamine, (2S,6S)-hydroxynorketamine and (2R,6R)-hydroxynorketamine had no significant effect on the induced current, IC50 > than 200μM (Fig 5) indicating that these compounds had little effect on α3β4-nicotinic acetylcholine receptor activity.
Figure 6.
The effect of the concentration of (R,S)-ketamine {(R,S)-Ket}, (R,S)-norketamine {(R,S)-norKet}, (2S,6S)-hydroxynorketamine {SS-HNK}, (2R,6R)-hydroxynorketamine {RR-HNK} and (R,S)-dehydronorketamine {(R,S)-DHNK} on the (S)-nicotine-induced current in KXα3β4R2 cells determined using whole-cell configuration of the patch-clamp technique. The calculated IC50 values are included in the figure.
3.3 Binding affinities to the NMDA receptor
The binding affinities of the compounds used in this study to the NMDA receptor were investigated using displacement binding studies (Table 2). The marker ligand was MK-801, a non-competitive inhibitor of the NMDA receptor, which binds to phencyclidine binding sites within the ion pore of the receptor.
Table 2.
The binding affinities (Ki values) of (R)- and (S)-ketamine, (R)- and (S)-norketamine, (R)- and (S)-dehydronorketamine and (2S,6S)- and (2R,6R)-hydroxynorketamine to the NMDA receptor determined using displacement binding studies with [3H]-MK801as the marker ligand, where n = 4 for MK801 and 2 for all other measurements.
| Compound | Ki (μM) |
|---|---|
| MK801 | 0.0047 ± 0.0004 |
| (R,S)-Ketamine | 1.06 ± 0.14 |
| (S)-Ketamine | 0.69 ± 0.09 |
| (R)-Ketamine | 2.57 ± 0.28 |
| (S)-norKetamine | 2.25 ± 0.22 |
| (R)-norKetamine | 26.46 |
| (S)-Dehydronorketamine | 38.95 |
| (R)-Dedhydronorketamine | 74.55 |
| (2S,6S)-Hydroxynorketamine | 21.19 |
| (2R,6R)-Hydroxynorketamine | >100 |
The calculated Ki values for (S)-ketamine (0.69 μM), (R)-ketamine (2.57 μM), (S)-norketamine (2.25 μM) and (R)-norketamine (24.46 μM) were consistent with previously reported data as were the enantioselectivities with the (S)-enantiomer having a greater relative binding affinity the (R)-enantiomer (Ebert, et al., 1997). The binding affinities of (S)-dehydronorketamine (38.95 μM) and (2S,6S)-hydroxynorketamine (21.19 μM) were weaker than the corresponding (S)-enantiomers of ketamine and norketamine, but stronger than the corresponding (R)-dehydronorketamine (74.55 μM) and (2R,6R)-hydroxynorketamine (> 100 μM) enantiomers (Table 2).
4. Discussion
Numerous in vitro studies have determined that (R,S)-ketamine is extensively metabolized by microsomal enzymes producing (R,S)-norketamine (Trevor et al., 1983; Kharasch and Labroo, 1992; Desta, et al., 2012), (R,S)- dehydronorketamine (Bolze and Boulieu, 1998; Desta, et al., 2012) and a series of diastereomeric hydroxynorketamine metabolites (Adams et al., 1981; Trevor et al., 1983; Woolf and Adams, 1987). The in vitro data were confirmed in studies in healthy volunteers (Turfus, et al., 2009) and patients receiving the drug in the treatment of bipolar and major depression (Zhao, et al., 2012; Zarate, et al., 2012) and complex regional pain syndrome (Moaddel, et al., 2010). However, while the extensive metabolism of (R,S)-ketamine has been recognized, little is known about the pharmacological activity of its metabolites other than (R,S)-norketamine. This study reports the initial examination of the pharmacological activity of (2S,6S)-hydroxynorketamine, (2R,6R)-hydroxynorketamine, (R)-dehydronorketamine and (S)-dehydronorketamine at the α7 nicotinic acetylcholine receptor, α3β4-nicotinic acetylcholine receptor and NMDA receptor.
In this study, patch-clamp techniques were utilized to determine the pharmacological effect of (R,S)-dehydronorketamine, (2S,6S)-hydroxynorketamine and (2R,6R)-hydroxynorketamine on the activity of the α7 nicotinic acetylcholine receptor and α3β4-nicotinic acetylcholine receptor. The data from the patch-clamp studies utilizing KXα7R1 cells indicate that 100 nM concentrations of (R,S)-norKetamine, (R,S)-dehydronorketamine, (2S,6S)-hydroxynorketamine and (2R,6R)-hydroxynorketamine inhibited acetylcholine-induced current, while (R,S)-ketamine had no activity at this concentration (Table 1). (R,S)-dehydronorketamine appeared to be the most potent inhibitor of the tested metabolites, IC50 = 55 ± 6 nM, acting as a negative allosteric modulator of the α7-nicotinic acetylcholine receptor. The allosteric modulation of nicotinic acetylcholine receptor by (R,S)-dehydronorketamine is consistent with recent studies that have characterized allosteric binding sites at the protein lipid interface of the nicotinic acetylcholine receptor, to which general anesthetics bind and potentially modulate different transitions of the receptor (Nury, et al., 2010). The magnitude of the inhibitory effects of (R,S)-norketamine, (2S,6S)-hydroxynorketamine and (2R,6R)-hydroxynorketamine were similar (Table 1) indicating that inhibition of the α7-nicotinic acetylcholine receptor by these metabolites may play a role in the therapeutic effects of (R,S)-ketamine.
The effect of (R,S)-ketamine, (R,S)-norketamine, (R,S)-dehydronorketamine, (2S,6S)-hydroxynorketamine and (2R,6R)-hydroxynorketamine on the α3β4-nicotinic acetylcholine receptor was also investigated. The data indicate that both (R,S)-ketamine and (R,S)-norketamine effectively inhibited (S)-nicotine-induced current in KXα3β4R2 cells with IC50 values of 3.1 μM and 9.1 μM, respectively (Fig. 6). Under the same conditions (R,S)-dehydronorketamine, (2S,6S)-hydroxynorketamine and (2S,6R)-hydroxynorketamine were essentially inactive with IC50 values >200 μM.
(R,S)-Ketamine and (R,S)-norketamine have been previously characterized as NMDA receptor antagonists and the clinical effects of (R,S)-ketamine are attributed to this pharmacological effect (Hirota and Lambert, 2011). Therefore, we determined the ability of (2S,6S)-hydroxynorketamine, (2R,6R)-hydroxynorketamine, (R)-dehydronorketamine and (S)-dehydronorketamine to displace the NMDA receptor marker ligand [3H]-MK801 in rat brain tissue preparations. The results indicate that the metabolites interact weakly with the phencyclidine-binding site of the NMDA receptor as the calculated Ki values ranged from 21 μM (2S,6S)-hydroxynorketamine to >100 μM (2R,6R)-hydroxynorketamine (Table 2). The observed affinities were lower than those obtained using (S)-ketamine (0.69 μM), (R)-ketamine (2.57 μM) and (S)-norketamine (2.25 μM) as the displacers, while the Ki of (2S,6S)-hydroxynorketamine was equivalent to that of (R)-norketamine (26.46 μM) (Table 2). These results are consistent with the data from an earlier study of (R,S)-ketamine and (R,S)-norketamine at the NMDA receptor in which (R,S)-ketamine had the highest binding affinity to the receptor (Ki = 0.53 μM) (Ebert, et al., 1997).
The relative binding affinities of the tested compounds demonstrated that an S-configuration at the 2-position of the phencyclidine ring was associated with a higher affinity than the corresponding compounds with an R-configuration at that site. These results are consistent with previous NMDA receptor binding data obtained with ketamine and norketamine stereoisomers (Ebert, et al., 1997), and with the observations that (R,S)-ketamine is a more potent anesthetic agent than (R,S)-norketamine and that (S)-ketamine and (S)-norketamine are more potent than the corresponding (R)-enantiomers (Hirota and Lambert, 2011). It is of interest to consider that the large difference in the Ki values between the enantiomeric (2S,6S)-hydroxynorketamine and (2R,6R)-hydroxynorketamine may explain the initial observation that the hydroxynorketamine metabolite is pharmacologically inactive since the (2S,6S;2R,6R)-hydroxynorketamine racemate was used in these studies (Leung and Baillie, 1986). However, even though the calculated Ki values for (2S,6S)-hydroxynorketamine and (S)-dehydronorketamine suggest that these compounds may act as inhibitors of the NMDA receptor, the clinical effect should be weak and on the order of the inhibition produced by (R)-norketamine.
The results of this study indicate that (R,S)-dehydronorketamine is a potent and selective inhibitor of the α7-nicotinic acetylcholine receptor with little effect at the α3β4-nicotinic acetylcholine receptor or NMDA receptor. Preliminary studies have also indicated that this compound had no effect on acetylcholine-evoked currents in α4β2-nicotinic acetylcholine receptor (data not shown). The data also demonstrate that (2S,6S)-hydroxynorketamine and (2R,6R)-hydroxynorketamine are inhibitors of the α7-nicotinic acetylcholine receptor with little effect at the α3β4-nicotinic acetylcholine receptor and NMDA receptor.
The potential clinical relevance of the inhibition of α7-nicotinic acetylcholine receptor activity by (R,S)-ketamine metabolites is suggested by the plasma concentrations of (R,S)-dehydronorketamine in patients with bipolar depression treated with a 40-min infusion of 0.5 mg/kg (R,S)-ketamine. In these patients the average plasma concentrations of (R,S)- dehydronorketamine were above the IC50 value for the inhibition of α7-nicotinic acetylcholine receptor activity at each measured time point, including 24 h after the completion of the infusion (Zarate, et al., 2012). It is important to note that 41% of the bipolar depression patients treated with (R,S)-ketamine reported a positive response 24 h after treatment and the response rate was 30% at 72 h, and that the plasma concentrations of (R,S)-dehydronorketamine, (2S,5S;2R,5R)-hydroxynorketamine, and (2S,5R;2R,5S)-hydroxynorketamine were associated with lower psychotomimetic or dissociative side effects (Zarate, et al., 2012). The contribution of an α7-nicotinic acetylcholine receptor antagonist such as (R,S)-dehydronorketamine to a positive response in the treatment of depression is consistent with the emerging clinical use of these agents (Mineur and Picciotto, 2010; Philip, et al., 2010). This is exemplified by the use of mecamylamine in the treatment of depression, which like ketamine is an open-channel non-competitive inhibitor of the α7-nicotinic acetylcholine receptor and α3β4-nicotinic acetylcholine receptor (Papke, et al., 2001).
The results of this study indicate that a further examination of the pharmacological effects of (R,S)-dehydronorketamine, (2S,6S)-hydroxynorketamine and (2R,6R)-hydroxynorketamine is warranted and studies involving cellular and animal models have been initiated. The results will be presented elsewhere.
Acknowledgments
Research Programs, National Institute of Mental Health, National Institutes of Health, Bethesda, MD
This work was supported by the Intramural Research Programs of the National Institute of Aging (IWW) and the National Institute of Mental Health (CAZ), the Brain & Behavior Research Foundation Bipolar Disorders Award (CAZ), NIA contract number N01AG-3-1009 (GA,LT,LJ), the Foundation for Polish Science (FOCUS and TEAM Programmes) (KJ).
Footnotes
Disclosure
Dr. Zarate (CAZ) is listed as a co-inventor on a patent application for the use of ketamine and its metabolites in major depression. CAZ has assigned his rights to the US government but will share a percentage of any royalties that may be received by the government. CAZ, IWW, and RM have submitted a patent, assigned to the US government, for use of ketamine metabolites in treatment of bipolar disorder and major depression.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adams JD, Jr, Baillie TA, Trevor AJ, Castagnoli N. Studies on the biotransformation of ketamine 1-Identification of metabolites produced in vitro from rat liver microsomal preparations. Biomed Mass Spec. 1981;8:527–538. doi: 10.1002/bms.1200081103. [DOI] [PubMed] [Google Scholar]
- Bolze S, Boulieu R. HPLC determination of ketamine, norketamine and dehydronorketamine in plasma with a high-purity reversed-phase sorbent. Clin Chem. 1998;44:560–564. [PubMed] [Google Scholar]
- Coates KM, Flood P. Ketamine and its preservative benzethonium chloride, both inhibit human recombinant α7 and α4β2 neuronal nicotinic acetylcholine receptors in Xenopus oocytes. Br J Pharmacol. 2001;134:871–879. doi: 10.1038/sj.bjp.0704315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desta Z, Moaddel R, Ogburn ET, Xu C, Ramamoorthy A, Venkata SLV, Sanghvi M, Goldberg ME, Torjman MC, Wainer IW. Stereoselective and regiospecific hydroxylation of ketamine and norketamine. Xenobiotica. 2012;42:1076–1087. doi: 10.3109/00498254.2012.685777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebert B, Mikkelsen S, Thorkildsen C, Borgbjerg FM. Norketamine, the main metabolite of ketamine, is a non-competitive NMDA receptor antagonist in the rat cortex and spinal cord. Eur J Pharmacol. 1997;333:99–104. doi: 10.1016/s0014-2999(97)01116-3. [DOI] [PubMed] [Google Scholar]
- Hirota K, Lambert DG. Ketamine: new uses for an old drug? Br J Anesth. 2011;107:123–126. doi: 10.1093/bja/aer221. [DOI] [PubMed] [Google Scholar]
- Kharasch ED, Labroo R. Metabolism of ketamine stereoisomers by human liver microsomes. Anesthesiology. 1992;77:1201–1207. doi: 10.1097/00000542-199212000-00022. [DOI] [PubMed] [Google Scholar]
- Leung LY, Baillie TA. Comparative pharmacology in the rat of ketamine and its two principal metabolites, norketamine and (Z)-6-hydroxynorketamine. J Med Chem. 1986;29:2396–2399. doi: 10.1021/jm00161a043. [DOI] [PubMed] [Google Scholar]
- Mineur YS, Picciotto MR. Nicotine receptors and depression: revisiting the cholinergic hypothesis. Trends Pharmacol Sci. 2010;31:580–586. doi: 10.1016/j.tips.2010.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moaddel R, Jozwiak K, Yamaguchi R, Wainer IW. Direct chromatographic determination of dissociation rate constants of ligand-receptor complexes: assessment of the interaction of noncompetitive inhibitors with an immobilized nicotinic acetylcholine receptor-based liquid chromatography stationary phase. Anal Chem. 2005;77:5421–5426. doi: 10.1021/ac0504464. [DOI] [PubMed] [Google Scholar]
- Moaddel R, Venkata SLV, Tanga MJ, Bupp JE, Green CE, LaIyer L, Furimsky A, Goldberg ME, Torjman MC, Wainer IW. A parallel chiral-achiral liquid chromatographic method for the determination of the stereoisomers of ketamine and ketamine metabolites in the plasma and urine of patients with complex regional pain syndrome. Talanta. 2010;82:1892–1904. doi: 10.1016/j.talanta.2010.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nury H, Van Renterghem C, Weng Y, Tran A, Baaden M, Dufresne V, Changeux JP, Sonner JM, Delarue M, Corringer JP. X-ray structures of general anaesthetics bound to a pentameric ligand-gated ion channel. Nature. 2010;469:428–431. doi: 10.1038/nature09647. [DOI] [PubMed] [Google Scholar]
- Philip NS, Carpenter LL, Tyrka AR, Price LH. Nicotinic acetylcholine receptors and depression: a review of the preclinical and clinical literature. Psychopharmacology. 2010;212:1–12. doi: 10.1007/s00213-010-1932-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trevor AJ, Woolf TF, Baillie TA, Adams JD, Castagnoli N. Stereoselective metabolism of ketamine enantiomers. In: Kamenka JM, Domino EF, Geneste P, editors. Phencyclidine and Related Arylcyclohexylamines, Present and Future Applications. Ann Arbor, MI: NPP Books; 1983. pp. 279–289. [Google Scholar]
- Turfus SC, Parkin MC, Cowan DA, Halketamine JM, Smith NW, Braithwaite RA, Elliot SP, Steventon GB, Kicman AT. Use of human microsomes and deuterated substrates; an alternative approach for the identification of novel metabolites of ketamine by mass spectrometry. Drug Metab Dispos. 2009;37:1769–1778. doi: 10.1124/dmd.108.026328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong EH, Kemp JA, Priestley T, Knight AR, Woodruff GN, Iversen LL. The anticonvulsant MK-801 is a potent N-methyl-D-aspartate antagonist. Proc Natl Acad Sci USA. 1986;83:7104–7108. doi: 10.1073/pnas.83.18.7104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolf TF, Adams JD. Biotransformation of ketamine, (Z)-6-hydroxyketamine, and (E)-6-hydroxyketamine by rat, rabbit, and human liver microsomal preparations. Xenobiotica. 1987;17:839–847. doi: 10.3109/00498258709043993. [DOI] [PubMed] [Google Scholar]
- Xiao Y, Meyer EL, Thompson JM, Surin A, Wroblewski J, Kellar KJ. Rat alpha3/beta4 subtype of neuronal nicotinic acetylcholine receptor stably expressed in a transfected cell line: pharmacology of ligand binding and function. Molec Pharmacol. 1998;54:322–333. doi: 10.1124/mol.54.2.322. [DOI] [PubMed] [Google Scholar]
- Xiao Y, Abipolar depressionrakhmanova GR, Baydyuk M, Hernandez S, Kellar KJ. Rat neuronal nicotinic acetylcholine receptors containing alpha7 subunit: pharmacological properties of ligand binding and function. Acta Pharmacol Sin. 2009;30:842–850. doi: 10.1038/aps.2009.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamakura T, Chavez-Noriega LE, Harris RA. Subunit-dependent inhibition of human neuronal nicotinic acetylcholine receptors and other ligand-gated ion channels by dissociative anesthetics ketamine and dizocilpine. Anesthesiology. 2000;92:1144–1155. doi: 10.1097/00000542-200004000-00033. [DOI] [PubMed] [Google Scholar]
- Zarate CA, Jr, Brutsche N, Laje G, Luckenbaugh DA, Ramamoorthy A, Moaddel R, Wainer IW. Relationship of Ketamine’s Plasma Metabolites with Response and Diagnosis, and Side Effects in Major Depression. Biol Psychiatry. 2012;72:331–338. doi: 10.1016/j.biopsych.2012.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao X, Venkata SLV, Moaddel R, Luckenbaugh DA, Brutsche NE, Ibrahim L, Zarate CA, Jr, Mager DE, Wainer IW. Simultaneous Population Pharmacokinetic Modeling of Ketamine and Three Major Metabolites in Patients with Treatment-Resistant Bipolar Depression. Br J Clin Pharmacol. 2012;74:304–314. doi: 10.1111/j.1365-2125.2012.04198.x. [DOI] [PMC free article] [PubMed] [Google Scholar]