Abstract
Purpose
Conformal and intensity modulated radiation therapies have the potential to preserve cognitive outcomes in children with ependymoma; however, functional behavior remains uninvestigated. This longitudinal investigation prospectively examined intelligence quotient (IQ) and adaptive functioning during the first 5 years after irradiation in children diagnosed with ependymoma.
Methods and Materials
The study cohort consisted of 123 children with intracranial ependymoma. Mean age at irradiation was 4.60 years (95% confidence interval [CI], 3.85–5.35). Serial neurocognitive evaluations, including an age-appropriate IQ measure and the Vineland Adaptive Behavior Scales (VABS), were completed before irradiation, 6 months after treatment, and annually for 5 years. A total of 579 neurocognitive evaluations were included in these analyses.
Results
Baseline IQ and VABS were below normative means (P<.05), although within the average range. Linear mixed models revealed stable IQ and VABS across the follow-up period, except for the VABS Communication Index, which declined significantly (P=.015). Annual change in IQ (−.04 points) did not correlate with annual change in VABS (−.90 to +.44 points). Clinical factors associated with poorer baseline performance (P<.05) included preirradiation chemotherapy, cerebrospinal fluid shunt placement, number and extent of surgical resections, and younger age at treatment. No clinical factors significantly affected the rate of change in scores.
Conclusions
Conformal and intensity modulated radiation therapies provided relative sparing of functional outcomes including IQ and adaptive behaviors, even in very young children. Communication skills remained vulnerable and should be the target of preventive and rehabilitative interventions.
Introduction
Ependymoma accounts for approximately 5%–7% of all pediatric brain tumors and is diagnosed most frequently in children 4 years of age or younger (1). Optimal treatment outcomes generally result from gross total resection and subsequent irradiation (2), with resulting 3-year disease-free survival rates approaching 75% (3). The use of postoperative conformal and intensity modulated radiation therapy has become the standard of care for ependymoma because the prescription dose can be precisely shaped to the targeted volume, reducing the dose to normal, uninvolved tissue. This treatment approach is not without functional risks: children who receive treatment for ependymoma and other posterior fossa tumors are at risk for parenchymal and vascular damage, endocrinopathy, and cognitive deficits (2). Given the high survival rates associated with ependymoma and the generally young age at diagnosis, it is important to understand the functional outcomes these children can expect in order to prepare families appropriately and design interventions to ameliorate deficits.
Children who receive treatment for brain tumor including radiation therapy, chemotherapy and surgery are at risk for cognitive late effects, such as global declines in intellectual function and academic achievement (4–8), with resulting concerns about overall quality of life and the ability to function independently at older ages. Radiation dose to normal brain tissue has been identified as a direct predictor of postirradiation intelligence quotient (IQ) in ependymoma (9). Furthermore, ependymoma survivors exhibit greater stability in IQ scores after treatment with focally administered conformal and intensity modulated irradiation than children with similarly located tumors, including medulloblastoma, who are treated with craniospinal irradiation (8). Additional evidence of spared verbal learning ability (10) and academic skills including math and spelling (6) suggests progress toward reducing late effects; however, this progress is not global. Continued declines are noted in reading ability, with younger age at treatment conferring additional risk (6). Measures of academic achievement offer a glimpse into real-world performance, yet further investigation of functional outcomes following newer methods of irradiation is needed.
Adaptive functioning, or the ability to perform the tasks of daily living at an age-appropriate level, has not been thoroughly examined in this population. In typically developing individuals, correlations between adaptive and intellectual functioning are small to moderate (11), suggesting that these measures identify related but not identical constructs. Few studies have examined adaptive functioning in children with brain tumors. In heterogeneous samples of children with brain tumors, declines in adaptive functioning have been found in children undergoing surgery only (12) and in those receiving conventional radiation therapy and chemotherapy (13, 14). Children without brain tumors who receive low-dose whole-brain irradiation and subsequent bone marrow transplantation also experience a decline in global adaptive function (15). Hydrocephalus, a common complication of ependymoma, confers independent risk for adaptive deficits, and children with congenital hydrocephalus perform below age-level expectations on daily living skills and communication skills as adults (16). Despite these risks, no studies to our knowledge have explicitly examined adaptive functioning after newer methods of irradiation in this population.
Children with ependymoma are at risk for a host of cognitive and functional sequelae as a result of disease- and treatment-related factors, given the young age at diagnosis, the need for aggressive resection, and potential risks associated with hydrocephalus. Based on emerging literature suggesting relative sparing of some cognitive skills in this population with advanced treatment techniques, the need to examine outcomes in daily functioning is paramount. This study capitalized on the availability of a large sample of children whose disease was homogenously diagnosed and treated, permitting greater reliability and generalizability of findings. Aims of the study were to examine the trajectory of adaptive behavior scores in children treated with conformal irradiation for localized ependymoma; to compare the rate of change in IQ and adaptive behavior scores; and to identify clinical, demographic, and treatment-related variables that influence the change in scores over time. We hypothesized that this cohort would experience a decline in adaptive functioning over time and that the change in adaptive behaviors would correspond with a change in IQ scores.
Methods and Materials
Participants
This study enrolled 123 children in a single-institution phase II trial of conformal radiation therapy for localized ependymoma between July 1997 and January 2008 (3). Study entry criteria for the phase II trial included age between 1 and 25 years at time of treatment, histologic confirmation of ependymoma, no evidence of disseminated disease, no ongoing chemotherapy, no previous irradiation, and adequate performance status (ie, according to Eastern Cooperative Oncology Group Grade 0–2 criteria) (17). Additionally, participants must have completed at least 2 serial neurocognitive assessments, which required English as the primary language, and have no sensory or motor impairment that prohibited neurocognitive testing. Parents provided consent for this investigation, which was approved by the institutional review board.
Medical treatment and clinical factors
All patients underwent surgical resection before irradiation, with additional surgery performed as needed to maximize extent of resection before treatment. Children who received chemotherapy before irradiation typically received cyclophosphamide, cisplatin, or carboplatin, etoposide, and vincristine. Hydrocephalus was identified by neuroimaging at diagnosis. Radiation treatment parameters have been described previously (6, 9, 18). All participants received conformal (n = 115) or intensity modulated radiation therapy (n = 8) at St. Jude Children’s Research Hospital, using conventional fractionation (1.8 Gy per day) with a prescribed dose of 59.4 Gy. The dose was attenuated to 54.0 Gy for children younger than 18 months of age after gross total resection. The irradiated clinical target volume included a 10-mm margin surrounding the tumor and/or tumor bed to control microscopic disease and an additional 3- to 5-mm margin expansion in 3 dimensions to form the planning target volume and account for uncertainty in patient positioning and image registration.
Neurocognitive assessment
Participants underwent serial neurocognitive assessment at pre-irradiation baseline 6 months after treatment and annually thereafter for 5 years. Intellectual function was assessed using the Bayley Scales of Infant Development, second edition (children < 4 years of age) (19), and the Block Design, Similarities, and Information subtests from the age-appropriate Wechsler scale (children 4 years and older) (20–22). Abbreviated Wechsler IQ scores were derived from a formula provided by Sattler (23), which yields an estimated IQ (EIQ) that correlates highly (r=0.93) with IQ scores obtained from full administration. All measures yield an age-normed standard score with a mean of 100 and a standard deviation of 15.
Adaptive functioning was assessed with the Vineland Adaptive Behavior Scales (VABS) (11), which is a psychometrically validated parent interview administered by a trained psychological examiner that assesses adaptive behaviors at developmental levels from birth through adulthood. Several domains are assessed, yielding index scores for Communication, Daily Living Skills, Socialization, and Motor Skills (for children up through age 5). An overall Adaptive Behavior Composite is obtained. All index scores have an age-referenced mean of 100 and a standard deviation of 15, where higher scores reflect better skills. A reduced number of children received Motor Skills Index scores at each time point due to the age constraints for the scale; therefore, it was not included in analyses.
Analyses
Descriptive analyses were conducted to characterize the clinical, demographic, and neurocognitive features of the study group at baseline. Frequencies were calculated to determine proportions of the sample with EIQ and VABS standard scores below average (<85) at each time point. Longitudinal changes in EIQ and adaptive functioning were examined using linear mixed models. The intercept served as the standard score at baseline, and the slope represented the mean change in score per month. Pearson correlations were then used to investigate the relationships between changes (ie, slope values) in EIQ and VABS index scores over the 5-year follow-up period. Finally, univariate linear mixed models were used to examine the effects of demographic, clinical, and treatment-related variables on changes in adaptive functioning.
Results
Demographic, clinical, and treatment-related characteristics of the study cohort are presented in Table 1. Mean age at irradiation was 4.60 years (95% confidence interval [CI], 3.85–5.35), and the group was balanced with respect to sex. Most participants underwent near total or gross total resection, and 37% required more than one surgery. In 80%, tumors were located within the posterior fossa. Approximately 24% received preirradiation chemotherapy, and nearly 65% experienced hydrocephalus as a complication of their disease. There was a strong association between age at diagnosis (P=.001) or age at the time of irradiation (P=.0240) and the use of preirradiation chemotherapy. The patients treated by pre-CRT chemotherapy were younger than those not treated by pre-CRT chemotherapy.
Table 1.
Baseline demographic and clinical characteristics (n=123)
| Variable | Mean no. of patients (95% CI) |
Range |
|---|---|---|
| Age at RT | 4.60 (3.85–5.35) | 1.02–17.64 |
| Sex | ||
| Male | 61 | 50 |
| Female | 62 | 50 |
| Race | ||
| African-American | 10 | 8 |
| Caucasian | 107 | 87 |
| Other | 6 | 5 |
| Tumor location | ||
| Infratentorial | 98 | 80 |
| Supratentorial | 25 | 20 |
| Number of surgeries | ||
| 1 | 78 | 63 |
| 2 | 36 | 29 |
| 3 or more | 9 | 7 |
| Extent of pre-RT surgery | ||
| Biopsy only | 0 | 0 |
| STR | 13 | 11 |
| NTR | 9 | 7 |
| GTR | 101 | 82 |
| Pre-RT chemotherapy | 29 | 24 |
| Hydrocephalus | 80 | 65 |
| Shunt placement | 46 | 37 |
Abbreviations: CI = confidence interval; GTR = gross total resection (macroscopic complete); NTR = near total resection (≤5-mm residual disease); RT = conformal or intensity modulated radiation therapy; SD = standard deviation; STR = subtotal resection (>5-mm residual disease).
Percentages may not total 100% due to rounding procedures.
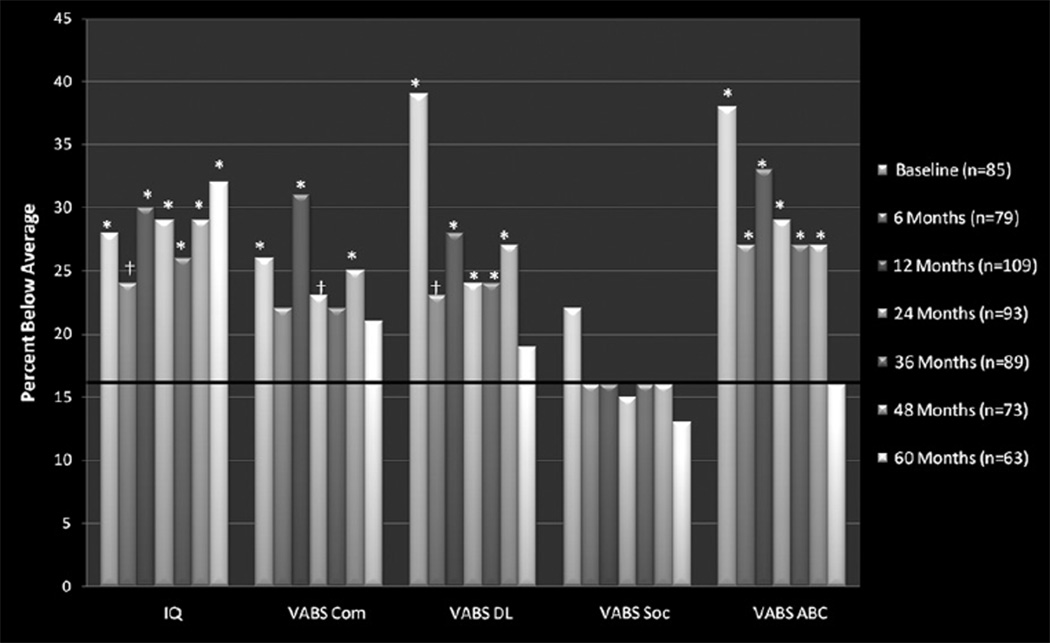
Participants completed a total of 579 neurocognitive evaluations. All 123 participants completed at least 2 VABS; 117 participants completed at least two EIQ measures. Incomplete evaluations resulted from patient illness/fatigue, parental refusal, treatment/travel scheduling conflicts, and failure to attend evaluation appointments. Baseline EIQ and VABS scores are presented in Table 2. Group means were below normative means (P<.05) for EIQ and all VABS indices; however, none was outside of the average range (85–115). The proportion of the sample with EIQ and adaptive behavior scores falling below the average range at each time point was calculated. Based on the normal distribution of these scores in the general population, it was anticipated that 16% of the sample would score less than 85 on any given index. Significantly more (P<.05) than 16% of the sample scored below average on EIQ and VABS Communication, Daily Living Skills, and Adaptive Behavior Composite indices across nearly all time points during the first 4 years. Proportions of VABS indices falling below average returned to expected levels at Year 5. In contrast, the proportion scoring below average on the VABS Socialization Index never exceeded population expectations. Results for all indices across time points are shown in Fig.
Table 2.
Baseline and longitudinal neurocognitive scores
| Variable | Baseline (n = 85) mean (95% CI) |
P* | Correlation with baseline IQ Pearson r (P)† |
Annual change | P‡ | Correlation with IQ change Pearson r (P)§ |
|---|---|---|---|---|---|---|
| EIQ | 95.75 (91.52–99.98)∥ | .023 | - | −0.04 | .898 | - |
| VABS Com | 96.93 (93.72–100.14)∥ | .026 | .39 (<.0001)∥ | −0.90∥ | .015 | .04 (.6715) |
| VABS DL | 92.62 (89.25–95.99)∥ | <.001 | .46 (<.0001)∥ | +0.44 | .265 | .14 (.1336) |
| VABS Soc | 97.54 (94.71–100.37)∥ | .043 | .41 (<.0001)∥ | +0.39 | .322 | −.10(.2604) |
| VABS ABC | 92.73 (89.17–96.29∥ | <.001 | .52 (<.0001)∥ | +0.30 | .468 | .04 (.7006) |
Abbreviations: ABC = Adaptive Behavior Composite; CI = confidence interval; Com = Communication Index; DL = Daily Living Skills Index; EIQ = estimated IQ; Soc = Socialization Index; VABS = Vineland Adaptive Behavior Scales.
Models are valid for up to 5 years after irradiation.
Compared with normative mean of 100 (±15 SD).
Pearson correlation between baseline IQ score (first row) and VABS indices.
Significant decline over time.
Pearson correlation between change in IQ (slope) and change in VABS indices.
Significant at a P level of <.05.
Fig.
Percentage of IQ and adaptive behavior scores falling below the average range (<85) at each time point. Solid line at 16% denotes the expected proportion of below-average scores based on a normally distributed population estimate. ABC = Adaptive Behavior Composite; Com = Communication Index; DL = Daily Living Skills index; Soc = Socialization index; VABS = Vineland Adaptive Behavior Scale. *Significantly greater than 16% is indicated at P<.05; †nonsignificant trend at P<.10.
Linear mixed models revealed the trajectory of change in EIQ and adaptive scores over the 5-year follow-up period. Only the VABS Communication Index declined significantly at a loss of nearly 1 standard score point per year (P=.015). Pearson correlations performed on the slopes for each index score revealed no significant (P>.05) correlations between change in EIQ and change in VABS indices over time.
Univariate linear mixed models were used to examine the effects of clinical, demographic, and treatment-related factors on change in adaptive behaviors over time. Several variables exerted significant impact on baseline EIQ and adaptive behavior scores (Table 3). Younger age at irradiation, chemotherapy prior to irradiation, and cerebrospinal fluid shunt placement resulted in lower baseline scores across nearly all indices (P<.05). Extent of preirradiation surgical resection affected baseline VABS Daily Living Skills Index (P=.046); near total or gross total resection was associated with higher baseline scores. A trend was noted for the effect of sex on daily living skills, where girls had higher baseline scores than boys (P=.057). No variables significantly affected the rate of change in EIQ or any VABS indices.
Table 3.
Clinical and demographic variables affecting baseline performance
| EIQ |
VABS Com |
|||
|---|---|---|---|---|
| Variable | Intercept (95% CI) | P | Intercept (95% CI) | P |
| Age at RT* | 87.14 (82.22–92.06) | <.001‡ | 96.86 (92.90–100.82) | .986 |
| No. of surgeries† | 104.44 (95.80–113.08) | .036‡ | 108.35 (102.45–114.25) | <.001‡ |
| Sex | .495 | .118 | ||
| Male | 94.52 (89.50–99.54) | 94.83 (91.11–98.55) | ||
| Female | 97.05 (91.86–102.24) | 99.09 (95.31–102.87) | ||
| Extent of surgery | .999 | .628 | ||
| STR | 95.59 (84.30–106.88) | 93.81 (85.62–102.00) | ||
| NTR | 95.74 (82.06–109.42) | 94.57 (83.83–105.31) | ||
| GTR | 95.76 (91.76–99.76) | 97.59 (94.65–100.53) | ||
| Pre-RT chemotherapy | .020‡ | .006‡ | ||
| Yes | 87.84 (80.41–95.30) | 90.34 (85.09–95.59) | ||
| No | 98.04 (94.02–102.06) | 99.03 (96.05–102.01) | ||
| Shunt placement | <.001‡ | <.001‡ | ||
| Yes | 86.46 (80.58–92.34) | 90.17 (85.94–94.40) | ||
| No | 100.67 (96.51–104.83) | 100.82 (97.68–103.96) | ||
| VABS DL |
VABS Soc |
VABS ABC |
|||
|---|---|---|---|---|---|
| Intercept (95% CI) | P | Intercept (95% CI) | P | Intercept (95% CI) | P |
| 86.95 (83.11–90.79) | <.001‡ | 93.82 (90.49–97.15) | .003‡ | 88.09 (83.90–92.28) | .004‡ |
| 102.48 (96.07–108.89) | .001‡ | 106.09 (100.70–111.48) | .001‡ | 105.76 (99.21–112.31) | <.001‡ |
| .057§ | .480 | .222 | |||
| 89.94 (86.04–93.84) | 96.69 (93.38–100.00) | 90.89 (86.73–95.05) | |||
| 95.38 (91.42–99.34) | 98.40 (95.03–101.77) | 94.60 (90.39–98.81) | |||
| .046‡ | .463 | .195 | |||
| 82.79 (74.44–91.14) | - | 93.69 (86.48–100.90) | 84.94 (75.94–93.94) | ||
| 96.92 (85.87–107.97) | .048 | 95.11 (85.39–104.83) | 91.78 (79.98–103.58) | ||
| 93.59 (90.59–96.59) | .019 | 98.16 (95.55–100.77) | 93.79 (84.79–102.79) | ||
| .008‡ | <.001‡ | <.001‡ | |||
| 85.96 (80.43–91.49) | 89.94 (85.45–94.43) | 83.16 (77.48–88.64) | |||
| 94.77 (91.63–97.91) | 100.00 (97.43–102.57) | 95.80 (92.57–99.03) | |||
| <.001‡ | <.001‡ | <.001‡ | |||
| 83.84 (79.51–88.17) | 91.17 (87.41–94.93) | 82.73 (78.24–87.22) | |||
| 97.53 (94.34–100.72) | 101.07 (98.33–103.81) | 98.32 (95.01–101.63) | |||
Abbreviations: ABC = Adaptive Behavior Composite; CI = confidence interval; Com = Communication Index; DL = Daily Living Skills Index; EIQ = estimated IQ; GTR = gross total resection macroscopic complete); NTR = near total resection (≤5-mm residual disease); RT = conformal and intensity modulated radiation therapy; SEM = standard error of the mean; Soc = Socialization Index; STR = subtotal resection (>5-mm residual disease); VABS = Vineland Adaptive Behavior Scales.
Clinical and demographic variables are included in this table if their relationship with IQ and VABS scores was significant or trended toward significance in univariate models.
Younger age at RT was associated with lower scores, such that scores increased significantly with each additional year of age at the time of RT for EIQ, VABS DL, VABS Soc, and VABS ABC.
More than one surgery was associated with significantly lower scores across EIQ and all VABS indices, where scores worsened with each additional surgery.
Significant at a P value of <.05.
Nonsignificant trend at a P value of <.10.
Discussion
Contrary to predictions, children treated with conformal and intensity-modulated radiation therapy for localized ependymoma experienced relative stability in their adaptive functioning over the 5-year follow-up period. These results provide novel and clinically meaningful information about the ability of these patients to perform developmentally appropriate tasks of daily living and add to the existing literature that suggests relative stability in IQ (8), verbal learning (10), and academic skills including math and spelling (6). These ependymoma survivors demonstrated less pronounced cognitive and functional effects up to 5 years after treatment relative to those of an older cohort of medulloblastoma survivors who received craniospinal irradiation (24). Prior reports have suggested that the use of craniospinal irradiation to treat medulloblastoma is the primary risk factor differentiating these 2 groups (24), indicating that the use of conformal radiation therapy may play a large role in sparing healthy brain tissue, resulting in better functional performance.
In this cohort, baseline scores were below population means, suggesting deleterious effects of preirradiation factors on developmental progress that must be considered in addition to the effects of radiation therapy. Indeed, young age at treatment, the need for a shunt to manage hydrocephalus, preirradiation chemotherapy, and multiple surgical resections required to obtain minimal residual disease before treatment were related to lower baseline performance on nearly all IQ and adaptive behavior indices. It should be noted that young age and preirradiation chemotherapy are highly related, given that chemotherapy is often administered in order to delay irradiation for very young children. Tumor growth alone is likely to disrupt functional outcome; however, additional clinical factors prior to irradiation must be considered. Despite the significant impact on baseline scores, these factors were not found to significantly affect the trajectory of change over time. Children who begin the treatment course with lower scores may remain at lower performance levels but are not predicted to experience any more significant decline than children who performed at higher levels before treatment.
The trajectory of change in adaptive behaviors was not associated with the rate of change in IQ scores, suggesting that adaptive functioning is a unique outcome that warrants continued assessment. Measures of adaptive behavior and IQ are modestly correlated in typically developing individuals and those diagnosed with intellectual impairment (11); however, acquired brain injuries and other neurologically based disorders (eg, attention-deficit/hyperactivity disorder) have a less predictable effect on this relationship (25). Physical factors such as motor impairment, decreased balance, and sensory deficits may all play a moderating role in adaptive outcomes following treatment for ependymoma and warrant further investigation. Likewise, psychosocial factors such as exposure to developmentally appropriate tasks and parental expectations of performance, which are known to be altered in childhood cancer survivors (26), may also affect adaptive functioning in this population.
While this sample exhibited general stability in their adaptive performance across time, the VABS Communication Index declined significantly over the 5-year study period. It is important to note that the Communication Index encompasses skills that may be uniquely affected by tumors in the posterior fossa. For example, speech production in general can be impacted by post-operative posterior fossa syndrome, and the effects of this syndrome on language production and organization can linger indefinitely, even if productive, intelligible speech improves (27). At older ages, items comprising the Communication Index include writing and advanced reading skills. These have been shown to be diminished in ependymoma survivors (6), and thus their emergence as weaknesses in more functional settings is not surprising.
It is notable that, while group mean scores remained within the average range across indices, a larger-than-expected proportion of children exhibited scores below the average range. Based on the normal distribution of IQ and adaptive scores in the general population, approximately 16% of the population can be expected to score below 85; yet, scores for a greater proportion of this sample fell below the average range on most scores at nearly all time points. The proportion of children with scores below average appeared to diminish at the 5-year point for all VABS indices. In contrast, the proportion of the cohort with below-average EIQ scores remained high across time. This dissociation between intellectual and adaptive functioning warrants further scrutiny to determine which factors promote buffering of adaptive functions. Interestingly, the proportion of children with below-average scores on the VABS Socialization Index never exceeded population expectations. This may be due to the ongoing social exposure that is inherent in receiving cancer treatment at a children’s hospital, suggesting less disruption to these developmental skills. Continued longitudinal follow-up is needed to determine whether these trends continue.
Young age at treatment has been identified as a prominent risk factor for more significant cognitive late effects, possibly due to disrupted exposure to developmentally appropriate material during critical periods (eg, learning to read) or to more pronounced liabilities in attention and executive function, making new learning more difficult across domains. These deficits are suspected to be heavily related to disrupted neural development of white matter in early childhood. Following central nervous system-directed therapies, reduced normal-appearing white matter volumes are overwhelmingly correlated with performance on measures of attention, impulsivity, and processing speed (28). However, much of what is known about these late effects stems from research into the use of craniospinal irradiation for treatment of medulloblastoma (29). In this sample of children with ependymoma, younger age at treatment was associated with lower baseline scores but not with the rate of change in IQ or adaptive scores. This finding is consistent with those of other reports of young ependymoma survivors that suggest stable intellectual (18), memory (10), and math and spelling skills (6), lending support for the early treatment of very young children with focal irradiation as a conservative yet effective measure of disease control.
Although these findings are promising with regard to functional outcomes following focused irradiation, they are not without some important limitations. The VABS is a widely used measure of adaptive functioning, but its reliance on parent report is subject to bias. Scores obtained using this measure, although reliable and generally stable, rely on a child’s opportunity to demonstrate skills at an age-appropriate level, and this is sometimes affected by factors unrelated to treatment (eg, parental expectations, socioeconomic limitations). Clinician observation of tasks of daily living might provide a less biased assessment of skills but would certainly add a burden to research that might prohibit large-scale investigations. Results related to IQ change across time may be affected by changes in IQ instruments at different age levels. When changing from a measure of infant IQ to one for preschoolers or older children, variations in scoring criteria and normative samples may eclipse true IQ findings or add variability that is a result of the psychometric properties of the test. This may be addressed with more consistent measures across the age range in future studies. Finally, these results are based on group performance across time, thereby limiting the predictive power of results for individual patients. Future research might approach the development of predictive algorithms that could provide specific individual risks for patients based on their clinical and demographic histories.
Conclusions
In summary, these findings suggest relative stability of IQ and adaptive behaviors following treatment for ependymoma with conformal radiation therapy methods. Behaviors most likely to decline include communication skills, which may be affected by tumor location in the posterior fossa and cannot be easily separated from academic skills such as reading and writing. Future directions include the development of patient-specific risk models to help inform parents about appropriate expectations, supports, and behavioral demands. Given the lack of a strong relationship with the trajectory of change in IQ, these results highlight a need to continue evaluating adaptive functioning as a separate yet important functional outcome for survivors of ependymoma. Further follow-up 5 to 10 years postirradiation will be needed to examine whether functional performance remains stable. These findings suggest that baseline performance and preirradiation factors may prove to be the strongest predictors of functional outcome.
Summary.
A prospective trial showed that conformal radiation therapy spared adaptive behavior in children with ependymoma. The study cohort included a vulnerable population including children as young as 12 months of age at the time of irradiation. Although immediate postoperative radiation therapy has been adopted as a standard of care for these patients, these findings secure the ability of advanced methods of irradiation and target volume reduction to reduce or eliminate cognitive effects in children with brain tumors.
Acknowledgments
Supported in part by National Cancer Institute Cancer Center grant CA21765 and American Cancer Society research project grant RPG-99-252-01-CCE and the American Lebanese Syrian and Associated Charities.
Footnotes
Conflict of interest: none.
References
- 1.Central Brain Tumor Registry of the United States. CBTRUS Statistical Report: Primary Brain and Central Nervous System Tumors Diagnosed in the United States in 2004–2006. Hinsdale, IL: CBTRUS; 2010. [Google Scholar]
- 2.Merchant TE, Fouladi M. Ependymoma: new therapeutic approaches including radiation and chemotherapy. J Neurooncol. 2005;75:287–299. doi: 10.1007/s11060-005-6753-9. [DOI] [PubMed] [Google Scholar]
- 3.Merchant TE, Li C, Xiong X, et al. Conformal radiotherapy after surgery for paediatric ependymoma: a prospective study. Lancet Oncol. 2009;10:258–266. doi: 10.1016/S1470-2045(08)70342-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kun LE, Mulhern RK, Crisco JJ. Quality of life in children treated for brain tumors. Intellectual, emotional, and academic function. J Neurosurg. 1983;58:1–6. doi: 10.3171/jns.1983.58.1.0001. [DOI] [PubMed] [Google Scholar]
- 5.Butler RW, Haser JK. Neurocognitive effects of treatment for childhood cancer. Ment Retard Dev Disabil Res Rev. 2006;12:184–191. doi: 10.1002/mrdd.20110. [DOI] [PubMed] [Google Scholar]
- 6.Conklin HM, Li C, Xiong X, et al. Predicting change in academic abilities after conformal radiation therapy for localized ependymoma. J Clin Oncol. 2008;26:3965–3970. doi: 10.1200/JCO.2007.15.9970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Merchant TE, Conklin HM, Wu S, et al. Late effects of conformal radiation therapy for pediatric patients with low-grade glioma: prospective evaluation of cognitive, endocrine, and hearing deficits. J Clin Oncol. 2009;27:3691–3697. doi: 10.1200/JCO.2008.21.2738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mulhern RK, Merchant TE, Gajjar A, et al. Late neurocognitive sequelae in survivors of brain tumours in childhood. Lancet Oncol. 2004;5:399–408. doi: 10.1016/S1470-2045(04)01507-4. [DOI] [PubMed] [Google Scholar]
- 9.Merchant TE, Kiehna EN, Li C, et al. Radiation dosimetry predicts IQ after conformal radiation therapy in pediatric patients with localized ependymoma. Int J Radiat Oncol Biol Phys. 2005;63:1546–1554. doi: 10.1016/j.ijrobp.2005.05.028. [DOI] [PubMed] [Google Scholar]
- 10.Di Pinto M, Conklin HM, Li C, et al. Investigating verbal and visual auditory learning after conformal radiation therapy for childhood ependymoma. Int J Radiat Oncol Biol Phys. 2009;77(4):1002–1008. doi: 10.1016/j.ijrobp.2009.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sparrow SS, Balla DA, Cicchetti DV. Vineland Adaptive Behavior Scales. Circle Pines, MN: American Guidance Service, Inc; 1984. [Google Scholar]
- 12.Ris MD, Beebe DW, Armstrong FD, et al. Cognitive and adaptive outcome in extracerebellar low-grade brain tumors in children: a report from the Children’s Oncology Group. J Clin Oncol. 2008;26:4765–4770. doi: 10.1200/JCO.2008.17.1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stargatt R, Rosenfeld JV, Anderson V, et al. Intelligence and adaptive function in children diagnosed with brain tumour during infancy. J Neurooncol. 2006;80:295–303. doi: 10.1007/s11060-006-9187-0. [DOI] [PubMed] [Google Scholar]
- 14.Mulhern RK, Horowitz ME, Kovnar EH, et al. Neurodevelopmental status of infants and young children treated for brain tumors with preirradiation chemotherapy. J Clin Oncol. 1989;7:1660–1666. doi: 10.1200/JCO.1989.7.11.1660. [DOI] [PubMed] [Google Scholar]
- 15.Kramer JH, Crittenden MR, DeSantes K, et al. Cognitive and adaptive behavior 1 and 3 years following bone marrow transplantation. Bone Marrow Transplant. 1997;19:607–613. doi: 10.1038/sj.bmt.1700699. [DOI] [PubMed] [Google Scholar]
- 16.Hommet C, Billard C, Gillet P, et al. Neuropsychologic and adaptive functioning in adolescents and young adults shunted for congenital hydrocephalus. J Child Neurol. 1999;14:144–150. doi: 10.1177/088307389901400302. [DOI] [PubMed] [Google Scholar]
- 17.Oken MM, Creech RH, Tormey DC, et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol. 1982;5:649–655. [PubMed] [Google Scholar]
- 18.Merchant TE, Mulhern RK, Krasin MJ, et al. Preliminary results from a phase II trial of conformal radiation therapy and evaluation of radiation-related CNS effects for pediatric patients with localized ependymoma. J Clin Oncol. 2004;22:3156–3162. doi: 10.1200/JCO.2004.11.142. [DOI] [PubMed] [Google Scholar]
- 19.Bayley N. Bayley Scales of Infant Development. 2nd ed. New York: The Psychological Corporation; 1993. [Google Scholar]
- 20.Wechsler D. Wechsler Preschool and Primary Scale of Intelligence. Revised. San Antonio, TX: The Psychological Corporation; 1989. [Google Scholar]
Supplementary Material
- 21.Wechsler D. Wechsler Intelligence Scale for Children. 3rd ed. New York: Psychological Corporation; 1991. [Google Scholar]
- 22.Wechsler D. Wechsler Adult Intelligence Scale. 3rd ed. New York: Psychological Corporation; 1997. [Google Scholar]
- 23.Sattler JM. Assessment of Children. 3rd ed. San Diego, CA: Jerome M. Sattler Publisher, Inc.; 1992. [Google Scholar]
- 24.Hoppe-Hirsch E, Brunet L, Laroussinie F, et al. Intellectual outcome in children with malignant tumors of the posterior fossa: influence of the field of irradiation and quality of surgery. Childs Nerv Syst. 1995;11:340–346. doi: 10.1007/BF00301666. [DOI] [PubMed] [Google Scholar]
- 25.Stein MA, Szumowski E, Blondis TA, et al. Adaptive skills dysfunction in ADD and ADHD children. J Child Psychol Psychiatry. 1995;36:663–670. doi: 10.1111/j.1469-7610.1995.tb02320.x. [DOI] [PubMed] [Google Scholar]
- 26.Eiser C, Richard Eiser J, Greco V. Parenting a child with cancer: promotion and prevention-focused parenting. Pediatr Rehabil. 2002;5:215–221. doi: 10.1080/1363849031000078610. [DOI] [PubMed] [Google Scholar]
- 27.Levisohn L, Cronin-Golomb A, Schmahmann JD. Neuropsychological consequences of cerebellar tumour resection in children: cerebellar cognitive affective syndrome in a paediatric population. Brain. 2000;123(Pt 5):1041–1050. doi: 10.1093/brain/123.5.1041. [DOI] [PubMed] [Google Scholar]
- 28.Mulhern RK, White HA, Glass JO, et al. Attentional functioning and white matter integrity among survivors of malignant brain tumors of childhood. J Int Neuropsychol Soc. 2004;10:180–189. doi: 10.1017/S135561770410204X. [DOI] [PubMed] [Google Scholar]
- 29.Reddick WE, Glass JO, Palmer SL, et al. Atypical white matter volume development in children following craniospinal irradiation. Neuro Oncol. 2005;7:12–19. doi: 10.1215/S1152851704000079. [DOI] [PMC free article] [PubMed] [Google Scholar]