Abstract
Purpose
The primary objective of this study was to examine whether children with low-grade glioma (LGG) or craniopharyngioma had impaired learning and memory after conformal radiation therapy (CRT). A secondary objective was to determine whether children who received chemotherapy before CRT, a treatment often used to delay radiation therapy in younger children with LGG, received any protective benefit with respect to learning.
Methods and Materials
Learning and memory in 57 children with LGG and 44 children with craniopharyngioma were assessed with the California Verbal Learning Test–Children’s Version and the Visual-Auditory Learning tests. Learning measures were administered before CRT, 6 months later, and then yearly for a total of 5 years.
Results
No decline in learning scores after CRT was observed when patients were grouped by diagnosis. For children with LGG, chemotherapy before CRT did not provide a protective effect on learning. Multiple regression analyses, which accounted for age and tumor volume and location, found that children treated with chemotherapy before CRT were at greater risk of decline on learning measures than those treated with CRT alone. Variables predictive of learning and memory decline included hydrocephalus, shunt insertion, younger age at time of treatment, female gender, and pre-CRT chemotherapy.
Conclusions
This study did not reveal any impairment or decline in learning after CRT in over-all aggregate learning scores. However, several important variables were found to have a significant effect on neurocognitive outcome. Specifically, chemotherapy before CRT was predictive of worse outcome on verbal learning in LGG patients. In addition, hydrocephalus and shunt insertion in craniopharyngioma were found to be predictive of worse neurocognitive outcome, suggesting a more aggressive natural history for those patients.
Introduction
Children with low-grade glioma (LGG) and craniopharyngioma are typically treated less intensively and have relatively better survival rates than those with more aggressive tumors (1). Treatment of LGG and craniopharyngioma often requires irradiation. Therefore, more research is necessary to determine whether focal irradiation can produce neurocognitive side effects outside the domain of intellectual function (2, 3). Evidence of subtle neurocognitive sequelae in children with LGG and craniopharyngioma years after treatment has been documented (4–7).
Identification of clinical and treatment factors that contribute to long-term neurocognitive sequelae may help with treatment planning (eg, when to use irradiation instead of chemotherapy for LGG or surgery instead of irradiation for craniopharyngioma) (6, 8, 9). LGG and craniopharyngioma share some similarities in clinical presentation and management based primarily on centralized tumor location. These similarities include hydrocephalus, tumor-related morbidity, limitations in surgical access, potential for complications, radiotherapeutic target volumes and doses, and the potential for late effects due to irradiation of normal brain tissue.
Tumor location plays a major role in determining the use of irradiation. Irradiation is often used to treat diencephalic and optic pathway tumors. Craniopharyngioma is generally limited to the suprasellar region and other central brain structures. Complications associated with surgical removal of craniopharyngioma often make this tumor a good candidate for irradiation.
It is well known that younger children are more vulnerable to the adverse side effects of radiation therapy. Chemotherapy is often chosen as the first-line treatment for young children with LGG to reduce these risks, and surgery may be considered in lieu of irradiation for young children with craniopharyngioma (3). Newer radiation therapy methods have the potential to reduce the radiation dose to normal tissues and subsequently decrease late effects (2, 6, 10). However, data to support this hypothesis are limited, particularly in regard to neurocognitive late effects outside the domain of global intelligence. Declines in intelligence quotient may actually be secondary to other more primary neurocognitive deficits such as learning and memory, attention, and speed of processing (4, 5).
The primary goal of this study was to examine whether children with LGG and craniopharyngioma treated with conformal radiation therapy (CRT) experienced a decline in verbal and visual-auditory learning several years after completion of CRT. The governing hypothesis for the study was that irradiated treatment volumes could be reduced without altering treatment efficacy i.e., change in failure rate or pattern), thereby minimizing exposure of healthy brain tissue to radiation and diminishing the risk of adverse effects on learning and memory. Our secondary goal was to examine the influence of clinical and treatment factors on verbal and visual-auditory learning, with a particular interest in whether the use of chemotherapy before CRT conferred any protective benefit with respect to learning.
Methods and Materials
Patients
Children with LGG (n=57) and craniopharyngioma (n=44) were treated with CRT between July 1997 and August 2007, and prospective longitudinal cognitive testing was conducted. Patients included in this study had a diagnosis of localized LGG or craniopharyngioma, were between 1 and 21 years old at the time of irradiation and had no prior irradiation or ongoing chemotherapy and an Eastern Cooperative Oncology Group (ECOG) performance grade 0–2 (11). For participants with LGG, median age at the start of CRT was 8.0 years, and median length of last follow-up was 59 months (range = 0.0–66.6 months). For participants with craniopharyngioma, median age at the start of CRT was 8.2 years, and median length of last follow-up was 48 months (range = 0.0–63.7 months). Five-year event-free survival rates for children with craniopharyngioma and low-grade glioma were 86% ± 7% and 84% ± 5%, respectively.
Clinical and demographic characteristics are presented in Table 1. All patients received CRT or intensity modulated radiation therapy to 54 Gy, using fractionation of 1.8 Gy per day. Target volume definitions included a 10- or 5-mm clinical target volume margin, depending on treatment era, and have been reviewed in previous studies (12, 13).
Table 1.
Patient demographic and clinical characteristics
| Variable | No. of patients with craniopharyngioma (n=44) (%) |
No. of patients with low-grade glioma (n=57) (%) |
|---|---|---|
| Sex | ||
| Female | 25 (56.8) | 30 (52.6) |
| Male | 19 (43.2) | 27 (47.4) |
| Tumor location | ||
| Infratentorial | 0 | 13 (22.8) |
| Supratentorial | 44 (100) | 44 (77.2) |
| Hydrocephalus | ||
| Yes | 24 (54.5) | 20 (35.1) |
| No | 20 (45.5) | 37 (64.9) |
| CSF shunting | ||
| Yes | 19 (43.2) | 20 (35.1) |
| No | 25 (56.8) | 37 (64.9) |
| Pre-CRT chemotherapy | ||
| Yes | 2 (4.5) | 16 (28.1) |
| No | 42 (95.5) | 41 (71.9) |
| Extent of resection | ||
| Bx or NBx | 14 (31.8) | 34 (59.6) |
| GTR, NTR, STR | 30 (68.2) | 23 (40.4) |
| Number of pre-CRT surgeries | ||
| 0–1 | 31 (70.5) | 44 (77.2) |
| 2–3 | 13 (29.5) | 13 (22.8) |
| Tumor laterality | ||
| Midline | 44 (100) | 39 (68.4) |
| Left | 0 | 7 (12.3) |
| Right | 0 | 11 (19.3) |
| Mean ± SD age at CRT (y) | 9.0 ± 3.6 | 8.9 ± 3.2 |
| Mean ± SD time from diagnosis to CRT (y) | 0.7 ± 1.0 | 1.9 ± 2.4 |
Abbreviations: CRT = conformal radiation therapy; CSF = cerebrospinal fluid; GTR = gross total resection; NTR = near-total resection; STR = subtotal resection; Bx = biopsy; NBx = no biopsy.
Preirradiation chemotherapy
Sixteen (28.1%) of 57 children with LGG and 2 (4.5%) of 44 children with craniopharyngioma received chemotherapy before irradiation. Patients with LGG typically received carboplatin/ vincristine. The 2 patients with craniopharyngioma who received chemotherapy were treated using interferon.
Measures of verbal and visual auditory learning
Neurocognitive testing was performed before treatment (baseline), at 6 months, and then yearly after CRT for a total of 5 years. Verbal learning was assessed with the California Verbal Learning Test–Children’s Version (CVLT-C) (14). This task presents a word list over 5 trials; the total recall score for trials 1 to 5 was used. Age-standardized scores were derived with a mean of 50 and standard deviation (SD) of 10. Higher scores indicate better performance. Visual learning was assessed with the Visual- Auditory Learning (VAL) test from Woodcock-Johnson Tests of Cognitive Ability: Revised (15). The VAL test is an associative learning task of word-symbol pairings. Age-standardized scores are derived with a mean of 100 and SD of 15. Higher scores indicate better performance. The treatment protocol allowed enrollment of children ages 1–21 years. Patients under the age of 4 years at the time of irradiation did not undergo baseline or subsequent CVLT-C testing until they reached the appropriate age. This group included 1 patient with craniopharyngioma and 2 with low-grade glioma. No participants had motor, vision, or hearing impediments that interfered with testing. Patients did not undergo testing after disease progression.
Statistical analyses
All variables in Table 1 were included in univariate and multivariate analyses, with the exception of tumor laterality because most participants had midline tumors. Pre-CRT chemotherapy was not analyzed in the craniopharyngioma group because only 2 of the 44 craniopharyngioma patients received chemotherapy before CRT. Infratentorial vs supratentorial tumor location was not included in analyses for craniopharyngioma as all tumors were supratentorial. Tumor volumes at the time of irradiation were included in a detailed analysis involving the LGG group to examine the association between tumor volume, age, and use of pre-CRT chemotherapy and their effects on longitudinal change of CVLT-C score (see Supplementary Material Appendix E1). Average baseline scores, mean rates of longitudinal change on CVLT-C and VAL scores, and average scores 5 years after CRT were estimated using linear mixed-effects models with random coefficients (16). A linear relationship was found and tested in the analysis. Analyses were completed separately for the craniopharyngioma group (Table 2) and the LGG group (Table 3), with only significant findings included.
Table 2.
Significant predictors of learning in children with craniopharyngioma (n=44)
| Baseline |
Change per month |
Estimated 5-y score |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Variable | Subgroup | n | Estimate | SE | P1 | Estimate | SE | P2 | Estimate | SE | P3 |
| Verbal learning (CVLT-C) | |||||||||||
| Hydrocephalus | No | 20 | 51.48 | 2.33 | .01* | .102 | .065 | .12 | 57.58 | 3.40 | .16 |
| Yes | 24 | 43.43 | 2.28 | .129 | .058 | .02* | 51.16 | 2.91 | |||
| Shunt | No | 25 | 49.81 | 2.12 | .07 | .139 | .057 | .01* | 58.15 | 2.90 | .04* |
| Yes | 19 | 43.45 | 2.79 | .093 | .072 | .20 | 49.03 | 3.32 | |||
| Visual auditory learning | |||||||||||
| Age at CRT | <8 y | 21 | 90.25 | 4.66 | .03* | .091 | .066 | .17 | 95.72 | 4.93 | .22 |
| ≥8 y | 23 | 104.40 | 4.37 | .000 | .071 | .99 | 104.47 | 5.04 | |||
| Sex | Female | 25 | 90.55 | 4.22 | .01* | .030 | .067 | .65 | 92.37 | 4.26 | <.01* |
| Male | 19 | 106.80 | 4.67 | .084 | .072 | .25 | 111.77 | 4.95 | |||
| Hydrocephalus | No | 20 | 105.90 | 4.51 | .02* | .108 | .075 | .16 | 112.41 | 4.94 | <.01* |
| Yes | 24 | 90.49 | 4.31 | .015 | .069 | .83 | 91.38 | 4.33 | |||
| Shunt | No | 25 | 107.90 | 3.58 | <.01* | .060 | .061 | .33 | 111.50 | 4.01 | <.01* |
| Yes | 19 | 84.35 | 4.55 | .029 | .086 | .74 | 86.09 | 4.72 | |||
Abbreviations CRT = conformal radiation therapy; P1 = difference at baseline; P2 = change over time; P3 = difference 5 y after CRT; SE = standard error.
P≤.05, statistically significant.
Table 3.
Predictors of learning in children with low-grade glioma (n=57)
| Baseline |
Change per month |
Estimated 5-y score |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Variable | Subgroup | n | Estimate | SE | P1 | Estimate | SE | P2 | Estimate | SE | P3 |
| Verbal learning (CVLT-C) | |||||||||||
| Shunt | No | 37 | 49.48 | 2.10 | .10 | .041 | .046 | .37 | 51.95 | 2.55 | .02* |
| Yes | 20 | 43.66 | 2.86 | −.080 | .061 | .19 | 38.83 | 3.33 | |||
| Pre-CRT Chemotherapy | No | 41 | 46.79 | 2.07 | .64 | .051 | .040 | .21 | 49.84 | 2.49 | .04* |
| Yes | 16 | 48.57 | 3.24 | −.139 | .062 | .03* | 40.21 | 3.96 | |||
| Visual auditory learning | |||||||||||
| Shunt | No | 37 | 94.76 | 3.24 | .01* | .173 | .057 | <.01* | 105.15 | 3.74 | .55 |
| Yes | 20 | 80.58 | 4.43 | .347 | .075 | <.01* | 101.41 | 4.92 | |||
Abbreviations CRT = conformal radiation therapy; P1 = difference at baseline; P2 = change over time; P3 = difference 5 y after CRT; SE = standard error.
P≤.05, statistically significant.
Multiple regression analyses used a backward selection method whereby all variables with a P value of ≤.05 for the slope were retained as both an intercept and a slope term. Variables were also retained if their removal significantly reduced overall model fit (P≤.05). Continuous covariates were split at their median value for inclusion in models (ie, age at CRT, time in months from diagnosis to CRT). Correlations between all variables were run to screen for multicolinearity. One-way ANOVA revealed no significant differences in verbal and visual-auditory outcome scores between those tested more frequently (>4 evaluations) and those tested less frequently (≤4 evaluations) for either the LGG group (CVLT-C, P=.43; VAL, P=.46) or the craniopharyngioma group (CVLT-C, P=.29; VAL, P=.80), providing no preliminary evidence of practice effects.
Results
Overall learning and memory outcomes
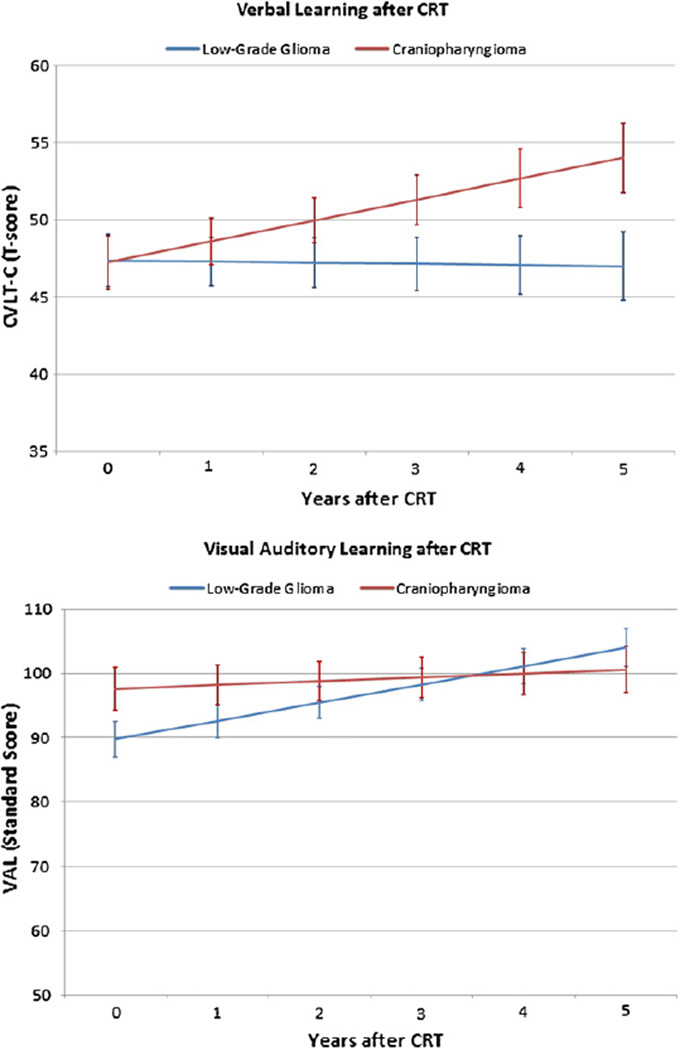
For children with LGG, mean scores (± SD) on the CVLT-C test (47.39 ± 1.69) and VAL test (89.80 ± 2.77) were in the average range at the start of irradiation. CVLT-C scores remained stable over time (−.01 ± .036 points/month; P=.86), and there was a statistically significant increase in VAL scores (.24 ± .046 points/month; P<.001) during the first 5 years after treatment.
For children with craniopharyngioma, mean ± SD scores on the CVLT-C test (47.26 ± 1.72) and VAL test (91.58 ± 3.31) were in the average range at the start of irradiation. After treatment, there was a statistically significant increase in CVLT-C scores (.11 ± .044 points/month; P=.01), and scores on the VAL remained stable over time (.05 ± .048 points/month; P=.31). Figure 1 plots global longitudinal trends in learning for both tumor groups.
Fig. 1.
Longitudinal change in verbal (top) and visual-auditory (bottom) learning scores after CRT in children with LGG and craniopharyngioma. Scores (mean ± SD) ranging from 40–60 (50 ± 10) and 85–115 (100 ± 15) represent the average ranges for CVLT-C test scores and VAL test standard scores, respectively. Standard error bars are included.
Demographic and clinical predictors of Learning–univariate analysis
Craniopharyngioma
In the craniopharyngioma group, hydrocephalus predicted lower scores at baseline for verbal learning but not 5 years after CRT (Table 2). Shunt insertion predicted worse performance for verbal learning 5 years after CRT but not at baseline. In the domain of visual-auditory learning, female sex, hydrocephalus, shunt insertion, and younger age all predicted lower scores at baseline. These same variables, with the exception of younger age, also predicted lower scores in the domain of visual-auditory learning 5 years after CRT.
Low-grade glioma
In the LGG group, there were no variables that predicted significant differences at baseline for verbal learning (Table 3). However, shunt insertion and pre-CRT chemotherapy did predict worse performance for verbal learning 5 years after CRT. In the domain of visual auditory learning, shunt insertion did predict lower scores at baseline in the LGG group but not 5 years after CRT.
Preirradiation chemotherapy and learning outcome
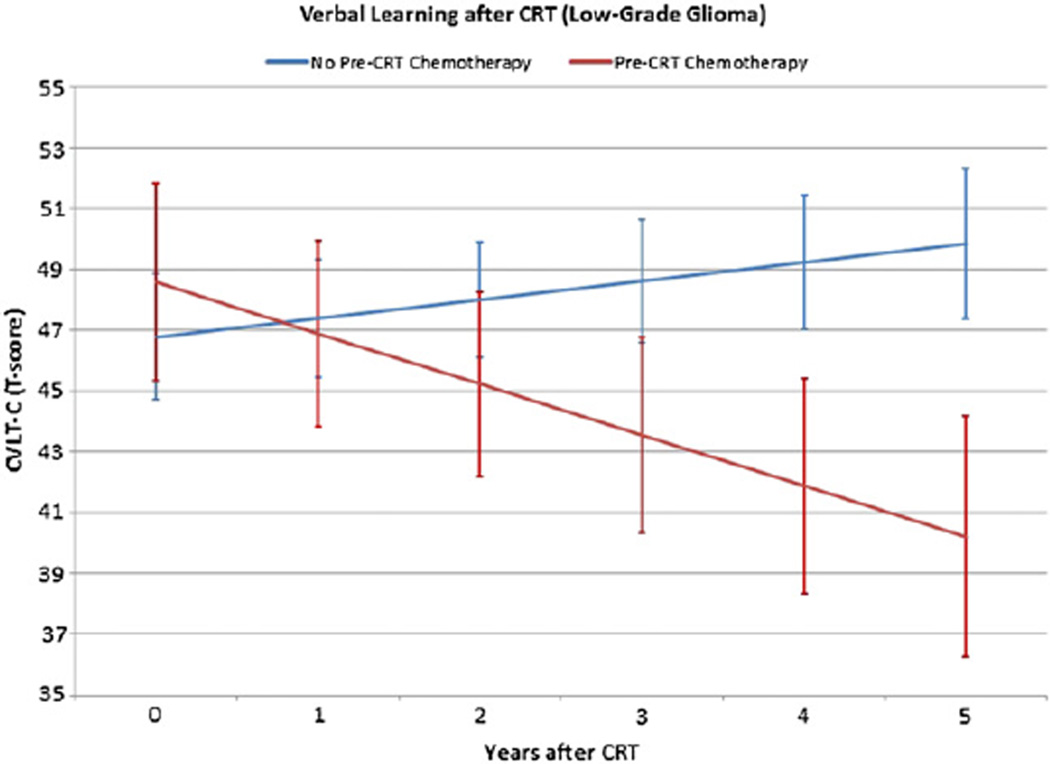
Those patients in the LGG group who received preirradiation chemotherapy had lower verbal learning (CVLT-C) scores 5 years after CRT (Fig. 2). The length of time from diagnosis to start of irradiation was significantly longer in children who received chemotherapy than in those who did not (P<.0001). There were no other significant differences between variables across subgroups, including tumor volumes, at the time of CRT, except for age at diagnosis. Those patients who received pre-CRT chemotherapy (n=16) were significantly younger at diagnosis than those who did not receive chemotherapy (n=41). Table 4 displays a comparison of clinical and demographic characteristics between LGG patients who received chemotherapy and those who did not.
Fig. 2.
The effect of chemotherapy prior to CRT (pre-CRT chemotherapy) on verbal learning in children with LGG assessed with CVLT-C test scores. Scores ranging from 85–115 (100 ± 15) represent the average range. Standard error bars are included.
Table 4.
Patient characteristics in low-grade glioma (chemotherapy vs no chemotherapy)
| Variable | No. of patients who had pre-CRT chemotherapy (n=16) (%) |
No. of patients who did not have chemotherapy (n=41) (%) |
P value |
|---|---|---|---|
| Tumor location | |||
| Infratentorial | 2 (12.5) | 11 (26.8) | .31 |
| Supratentorial | 14 (87.5) | 30 (73.2) | |
| Hydrocephalus | |||
| Yes | 5 (31.3) | 15 (36.6) | .70 |
| No | 11 (68.8) | 26 (63.4) | |
| CSF shunting | |||
| No | 9 (56.3) | 28 (68.3) | .39 |
| Yes | 7 (43.8) | 13 (31.7) | |
| Pre-CRT progression | |||
| No | 2 (12.5) | 14 (34.1) | .19 |
| Yes | 14 (87.5) | 27 (65.9) | |
| Extent of resection | |||
| Bx or NBx | 10 (62.5) | 24 (58.5) | .78 |
| GTR, NTR, STR | 6 (37.5) | 17 (41.5) | |
| No. of pre-CRT surgeries | |||
| 0–1 | 12 (75.0) | 32 (78.0) | 1.0 |
| 2–3 | 4 (25.0) | 9 (22.0) | |
| Mean±SD age at diagnosis (y) | 3.6±2.9 | 8.3±3.6 | <.0001†‡* |
| Mean±SD age at CRT (y) | 7.5±2.6 | 9.5±3.3 | .037†‡* |
| Mean±SD time from diagnosis to CRT (y) | 3.9±2.2 | 1.2±2.0 | <.0001†‡* |
Abbreviations CRT = conformal radiation therapy; CSF = cerebrospinal fluid; GTR = gross total resection; NTR = near-total resection; STR = subtotal resection; Bx = biopsy; NBx = no biopsy.
† P values derived either by chi-square or Fisher’s exact test* or by t test‡.
Multiple regression
Multiple regression analyses were used to account for age at diagnosis, as well as all other clinical and demographic characteristics listed in Table 1. Significant correlations were found between hydrocephalus and shunt insertion for management of hydrocephalus, suggesting an expected shared variance and multicolinearity between these variables. No other highly correlated variable pairs were found. Multiple regression analyses, which exclude variables with shared variance, helped control for multicolinearity. For the craniopharyngioma group, hydrocephalus predicted significantly lower baseline scores on the CVLT-C (−7.52; P<.01) test but had no effect on the rate of learning (change per month in learning scores) after irradiation. Among children with craniopharyngioma, shunt insertion predicted significantly lower scores at baseline on the VAL (−21.89; P<.001) test, as did female sex (−13.33; P<.01). Furthermore, multiple regression analyses revealed that these variables had no effect on the rate of learning (change per month) after CRT, indicating that these lower baseline scores persisted throughout the 5-year period after irradiation. For children with LGG, shunt insertion predicted significantly lower baseline scores on the CVLT-C (−8.21; P<.01) test but had no effect on the rate of learning over time. For children with LGG, pre-CRT chemotherapy had no significant effect on baseline CVLT-C scores but was associated with a reduced rate of learning after irradiation. Specifically, preirradiation chemotherapy predicted a decline in CVLT-C scores over time (−0.14 ± .06 points/month; P=.03). In the LGG group, shunt insertion predicted lower scores at baseline (−10.06; P<.05) on the VAL test but not on the rate of learning after treatment. Children with LGG who underwent tumor resection that was more extensive than a biopsy had no significant differences in VAL scores at baseline but did have a significant increase in VAL scores over time (0.43 ± .06 points/month; P=.001).
After we adjusted for age at diagnosis for both the intercept and slope, the pre-CRT chemotherapy subgroup still showed a decline in CVLT-C scores over time (−.10 ± .078 points/month) vs those of the subgroup that did not receive pre-CRT chemotherapy, whose CVLT-C scores improved over time (.10 ± .093 points/month). The slope of the pre-CRT chemotherapy subgroup was significantly different (lower) than that of the non-pre-CRT chemotherapy subgroup (P=.01). The finding of lower CVLT-C scores in the pre-CRT chemotherapy LGG subgroup remained even after we accounted for tumor volume in conjunction with age at time of diagnosis following more comprehensive multiple regression analyses (see Supplementary material Appendix E-1).
Discussion
This study revealed that learning was not impaired in children with LGG and craniopharyngioma who receive CRT. These findings support our primary hypothesis that reducing the volume of irradiation in an effort to spare normal tissues may reduce the risk of a significant longitudinal decline in learning. This study also found several other factors besides irradiation that contributed to cognitive change. For children with LGG, those who received pre-CRT chemotherapy had a decline in verbal learning after irradiation. These findings were still present even after accounting for age of diagnosis, which was the only significant difference between these subgroups. Further investigation is required to determine why children with LGG treated with chemotherapy appear to be at greater risk for neurocognitive side effects. The direct effect of chemotherapy on the developing brain, earlier age at diagnosis, morbidity associated with a protracted course of treatment, and the effect of delaying treatment of growth hormone deficiency should be considered.
However, these findings should be interpreted cautiously, as children who received pre-CRT chemotherapy were on average 3.6 years old at time of diagnosis compared to children who received irradiation only who were significantly older (8.3 years) at time of diagnosis. If this younger subgroup had received irradiation at 3.6 years of age for instance, the effects of irradiation could have been more deleterious and resulted in similar longitudinal trends. Nonetheless, in another study examining a younger group of patients with ependymoma, where the ages between those who received preirradiation chemotherapy vs those who did not were less discrepant, preirradiation chemotherapy was still found to be a significant risk factor for adverse neurocognitive outcome (2).
Research has shown that children treated with chemotherapy alone are also at risk for neurocognitive side effects (17). The biological mechanisms of chemotherapy-induced neurotoxicity are still under investigation, but proposed mechanisms include (1) direct damage to oligodendrocytes resulting in demyelination, (2) secondary inflammatory responses, and (3) microvascular injury resulting in demyelination, with deep white matter tracts most vulnerable to disruption (17). Most research concerning chemotherapy-related neurocognitive effects in pediatric oncology is focused on children treated for acute lymphoblastic leukemia or medulloblastoma. Those children are exposed to agents and irradiation regimens that differ considerably from those used for children with LGG. The only valid comparison to determine the effect of chemotherapy would be to compare our results with the neurocognitive outcome of LGG children treated with chemotherapy alone. Such data do not exist at our institution, and we are unaware of any such studies in the peer-reviewed literature.
It is important to note the effect of chemotherapy cannot be extrapolated to the general LGG population because our study sample included only those patients who received CRT. Our results are exploratory and not confirmatory, suggesting further investigation. A confirmatory study would require a statistical design involving a randomized control study with chemotherapy.
Delaying irradiation is common for young children with LGG. Five-year progression-free survival rates for children treated with chemotherapy range from 35%–48%. Thus, although most patients may be able to delay irradiation for a few years, further investigation is necessary to determine whether this delay reduces the risk of neurocognitive impairment. Deferring irradiation may pose its own set of unique risk factors (eg, the negative sequelae associated with disease progression and combined effects of chemotherapy and irradiation). Furthermore, infection, adverse events associated with chemotherapy, prolonged school absences, and additional factors might further contribute to the increased risk for neurocognitive effects (18).
The current study revealed that hydrocephalus and permanent cerebrospinal fluid shunting had a broad effect on neurocognitive outcomes for both tumor types relative to those with other treatment variables. Hydrocephalus in craniopharyngioma may represent a more complicated disease course characterized by faster disease progression (19). A study examining morbidity in a large sample of children with craniopharyngioma found hydrocephalus was a significant predictor of poor disease control and medical complications (19). In the current study, the presence of hydrocephalus and shunt insertion in craniopharyngioma had an adverse effect on learning at baseline and 5 years after CRT. The location of craniopharyngioma in the suprasellar brain region puts it near limbic structures that are important to learning and memory formation, particularly in regard to the transfer of information from short- to long-term memory (20). Ventriculomegaly, which may occur despite shunt insertion, may result in more chronic interruption to the encoding process beyond that associated with acute surgical changes (20). The risk for herniation and damage may be greater in children with craniopharyngioma experiencing hydrocephalus-associated complications than in children with posterior fossa tumors who show more recovery over time.
Conclusions
The aggregate learning scores in patients with LGG and craniopharyngioma do not suggest significant impairment in learning after conformal irradiation, even in younger children. Results of this study show the importance of numerous factors that may affect neurocognitive outcomes in children with these tumors, including preirradiation chemotherapy, hydrocephalus, and shunt insertion. These findings may help parents and caregivers make more informed decisions about treatment and facilitate the development of interventions to mitigate untoward side effects.
Supplementary Material
Summary.
A prospective trial showed that conformal radiation therapy spared memory and learning in children with low-grade glioma and craniopharyngioma. After correcting for age and tumor location, it was found that children treated with chemotherapy prior to irradiation were at greater risk of a decline in learning measures. These findings suggest that it is important to individualize decisions about the sequencing of chemotherapy and surgery even for the youngest patients
Acknowledgments
This work was supported in part by Cancer Center support grant CA21765 from the National Cancer Institute and Research Project Grant RPG-99-252-01-CCE from the American Cancer Society and by the American Lebanese Syrian Associated Charities.
Footnotes
Conflict of interest: none.
Supplementary material for this article can be found at www.redjournal.org.
References
- 1.Packer RJ, MacDonald T, Vezina G. Central nervous system tumors. Pediatr Clin N Am. 2008;55:121–145. doi: 10.1016/j.pcl.2007.10.010. [DOI] [PubMed] [Google Scholar]
- 2.Di Pinto M, Conklin H, Chenghong L, et al. Investigating verbal and visual auditory learning after conformal radiation therapy for childhood ependymoma. Int J Radiat Oncol Biol Phys. 2010;77:1002–1008. doi: 10.1016/j.ijrobp.2009.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nathan PC, Patel SK, Dilley K, et al. Guidelines for identification of, advocacy for, intervention in neurocognitive problems in survivors of childhood cancer: a report from the Children’s Oncology Group. Arch Pediatr Adolesc Med. 2007;161:798–806. doi: 10.1001/archpedi.161.8.798. [DOI] [PubMed] [Google Scholar]
- 4.Ris D, Beebe DW. Neurodevelopmental outcomes of children with low-grade gliomas. Dev Dis. 2008;14:196–202. doi: 10.1002/ddrr.27. [DOI] [PubMed] [Google Scholar]
- 5.Turner C, Chordas C, Liptak C, et al. Medical, psychological, cognitive and educational late effects in pediatric low-grade glioma survivors treated with surgery only. Pediatr Blood Cancer. 2009;53:417–423. doi: 10.1002/pbc.22081. [DOI] [PubMed] [Google Scholar]
- 6.Merchant M, Conklin H, Wu S, et al. Late effects with conformal radiation therapy for pediatric patients with low-grade glioma: prospective evaluation of cognitive, endocrine, and hearing deficits. J Clin Oncol. 2009;27:3691–3697. doi: 10.1200/JCO.2008.21.2738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bawden H, Salisbury S, Eskes G, et al. Neuropsychological functioning following craniopharyngioma removal. J Clin Exp Neuropsychol. 2009;31:140–144. doi: 10.1080/13803390802064599. [DOI] [PubMed] [Google Scholar]
- 8.Mabbott DJ, Spiegler BJ, Greenberg ML, et al. Serial evaluation of academic and behavioral outcome after treatment with cranial radiation in childhood. J Clin Oncol. 2005;23:2256–2263. doi: 10.1200/JCO.2005.01.158. [DOI] [PubMed] [Google Scholar]
- 9.Turner C, Chordas C, Liptak C, et al. Medical, psychological, cognitive and educational late effects in pediatric low-grade glioma survivors treated with surgery only. Pediatr Blood Cancer. 2009;53:417–423. doi: 10.1002/pbc.22081. [DOI] [PubMed] [Google Scholar]
- 10.Conklin H, Chenghong L, Xiaoping X, et al. Predicting change in academic abilities after conformal radiation therapy for localized ependymoma. J Clin Oncol. 2008;26:3965–3970. doi: 10.1200/JCO.2007.15.9970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Oken MM, Creech RH, Tormey DC, et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol. 1982;5:649–655. [PubMed] [Google Scholar]
- 12.Merchant TE, Kiehna EN, Kun LE, et al. Phase II trial of conformal radiation therapy for pediatric patients with craniopharyngioma and correlation of surgical factors and radiation dosimetry with change in cognitive function. J Neurosurg. 2006;104(suppl 2):S94–S102. doi: 10.3171/ped.2006.104.2.5. [DOI] [PubMed] [Google Scholar]
- 13.Merchant TE, Kun LE, Wu S, et al. Phase II trial of conformal radiation therapy for pediatric low-grade glioma. J Clin Oncol. 2009;27:3598–3604. doi: 10.1200/JCO.2008.20.9494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Delis DC, Kramer JH, Kaplan E, et al. California Verbal Learning Test: Children’s Version. New York: Harcourt, Brace, Jovanovich; 1994. [Google Scholar]
- 15.Woodcock R, Johnson M. The Woodcock-Johnson Tests of Cognitive Ability Revised. New York: Riverside Publishing; 1989. [Google Scholar]
- 16.Littell RC, Milliken GA, Stroup WW, et al. SAS for Mixed Models. 2nd ed. Cary, NC: SAS Institute Inc; 2006. [Google Scholar]
- 17.Saykin AJ, Ahles TA, McDonald BC. Mechanisms of chemotherapyinduced cognitive disorders: neuropsychological, pathophysiological, and neuroimaging perspectives. Semin Clin Neuropsychiatry. 2003;8:201–206. [PubMed] [Google Scholar]
- 18.Puga González B, Ferrández Longás A, Oyarzábal M, et al. for the Grupo Colaborativo Español. The effects of growth hormone deficiency and growth hormone replacement therapy on intellectual ability, personality and adjustment in children. Pediatr Endocrinol Rev. 2010;7:328–338. [PubMed] [Google Scholar]
- 19.De Vile CJ, Grant DB, Kendall BE, et al. Management of childhood craniopharyngioma: can the morbidity of radical surgery be predicted? J Neurosurg. 1996;85:73–81. doi: 10.3171/jns.1996.85.1.0073. [DOI] [PubMed] [Google Scholar]
- 20.Carpentieri SC, Waber DP, Scott MR, et al. Memory deficits among children with craniopharyngiomas. Neurosurgery. 2001;49:1053–1058. doi: 10.1097/00006123-200111000-00005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.