Abstract
A dietary therapy for pediatric epilepsy known as the ketogenic diet has seen a revival in its clinical use in the past decade. Though the diet’s underlying mechanism remains unknown, modern scientific approaches like genetic disruption of glucose metabolism are allowing for more detailed questions to be addressed. Recent work indicates that several mechanisms may exist for the ketogenic diet including disruption of glutamatergic synaptic transmission, inhibition of glycolysis, and activation of ATP-sensitive potassium channels. Here we describe on-going work in these areas that is providing a better understanding of metabolic influences on brain excitability and epilepsy.
Keywords: Ketogenic diet, Metabolism, Neuronal Excitability, Epilepsy, seizures
The ketogenic diet treatment for epilepsy
Trends in neuroscience come and go. In the 1920’s world of clinical neurology, momentum gathered behind the idea of treating epileptic seizures not with the few and rather inadequate medications available at the time, but by a radical change in diet: elimination of all but a tiny amount of ingested carbohydrate and substitution mostly by dietary fat, a so-called “ketogenic diet” (Box 1). Then, despite many reports of the effectiveness of this diet in reducing seizures in patients with epilepsy, the diet was quick to fade from the clinical arsenal once the first really effective modern anticonvulsant drug, diphenylhydantoin, was introduced in the late 1930’s.
Box 1: The ketogenic diet.
The ketogenic diet was developed in the 1920’s to mimic the elevation in circulating ketone bodies, which were believed to be the active component of the fasting treatment for epilepsy [72, 73]. The classical ketogenic diet consists of a 4:1 ratio (by weight) of fats to carbohydrates plus proteins [3]. Patients adhere to a highly regimented diet that is specially designed by a dietician. The diet was widely used until the discovery of diphenylhydantoin (dilantin) in 1938, after which research focus was diverted towards drug development [74].
The past two decades have seen an increase in the use of the diet, especially for the roughly 30% of children for whom pharmacological treatments are unable to control their seizures [1]. The ketogenic diet is accepted as an effective treatment for drug-resistant pediatric epilepsy [2], as well as certain disorders of glucose transport [75]. Recent clinical research has also shown that modified forms of the diet such as a modified Atkins diet (MAD) [76–78] and the low glycemic index treatment (LGIT) [46, 79] can also be effective at controlling seizures. These modifications make the diet less stringent, which can make it easier for patients to stick to the diet, an important condition for the efficacy of the treatment.
Decades later and with dozens of new anticonvulsant drugs, roughly a third of epilepsy patients still fail to achieve significant relief from seizures with drug treatment [1]. Clinical interest in the ketogenic diet was renewed in the 1990’s, now specifically as a treatment for children with drug-resistant seizures. Its efficacy has been supported in retrospective and prospective studies as well as in a recent randomized trial [2], and many major centers now offer dietary therapy for epilepsy. But its utilization in epilepsy patients remains low for a variety of reasons: tolerability, compliance with the strict diet, management, and training in diet administration – as well as a general reluctance on the part of neurologists to employ dietary therapy.
Still, the diet often succeeds in control of seizures when drugs fail, indicating that the metabolic changes produced by the diet tap into anticonvulsant mechanisms that are not targeted by existing medications. Neurobiologists’ interest in dissecting – and ultimately, in reverse engineering – the nature of these mechanisms is on the rise, and here we review the latest insights from this work.
How can altered diet, and the ensuing changes in brain metabolism, affect brain excitability? Neuronal excitability is intertwined with energy metabolism in multiple ways. At the most basic level, maintenance of neuronal function incurs a substantial energy demand, and this demand must be met by very active cellular metabolism (Box 2). It is also known that certain specialized neurons and neuroendocrine cells are specially tuned to sense metabolic changes, in order to regulate hormonal secretion, energy management, and feeding behavior. But in addition to these obvious links between excitability and metabolism, many neurons whose primary function is not the sensing of metabolism can also alter their excitability in response to metabolic changes.
Box 2: Neuronal energy consumption.
The division of energy usage varies among neuronal subtypes, but the major components are consistent – housekeeping processes, maintenance of resting potentials, action potentials, and synaptic transmission. As with all cells, basic “housekeeping” and transport processes require energy usage, but these processes account for only 25–50% of neuronal energy consumption [80, 81]. The additional consumption comes from the maintenance of resting potentials and neuronal signaling. Maintaining the resting potential and reversing the changes in intracellular ionic concentrations produced by neuronal signaling primarily require ATP utilization by the Na+/K+ pump, but other pumps including the Ca2+-ATPase also consume energy [82].
This rapid energy consumption triggers energy production, some of which is thought to occur locally at sites of energy usage. For example, glycolytic enzymes have been found to be coupled to the membrane pumps, suggesting that glycolytic ATP may rapidly fuel pump activity [20, 21, 82–84]. Increased neuronal activation is also thought to increase glycolysis in astrocytes and provide neurons with lactate as a mitochondrial fuel source [85]. There is extensive evidence for neuronal usage of lactate as an energy source during activity [86–91] and studies on lactate utilization will be of importance for better understanding neuronal metabolism.
Metabolic changes associated with the ketogenic diet
The classic ketogenic diet consists of a 4:1 ratio of fats to proteins and carbohydrates [3]. This drastic decrease in carbohydrates reduces the amount of glucose utilization. Instead, fatty acids are used by the liver to produce the ketone bodies, beta-hydroxybutyrate (BHB) and acetoacetate, which fuel cellular metabolism in lieu of glucose. Much of the energy production of the body goes into fueling neurons, which have a high rate of energy expenditure (Box 2). On the ketogenic diet, ketone bodies replace glucose as the major fuel source for the brain [4].
Do ketone bodies reduce neuronal excitability?
The two major areas of focus in research on the ketogenic diet have been the ketone bodies themselves and the metabolic changes associated with decreased glucose oxidation. Early clinical studies of dietary treatment of epilepsy attributed ketosis with seizure protection and injection of ketone bodies has been described to be anticonvulsant [5–7]. In animal models, the level of ketosis has not correlated well with the degree of efficacy of the ketogenic diet [8–11]. However, in humans, there is still evidence for the importance of elevated blood ketone bodies [12, 13] and brain ketone body levels may end up correlating with seizure protection. Several recent studies have directly examined the role of ketone bodies on neuronal excitability.
Direct inhibition of vesicular glutamate loading by ketone bodies
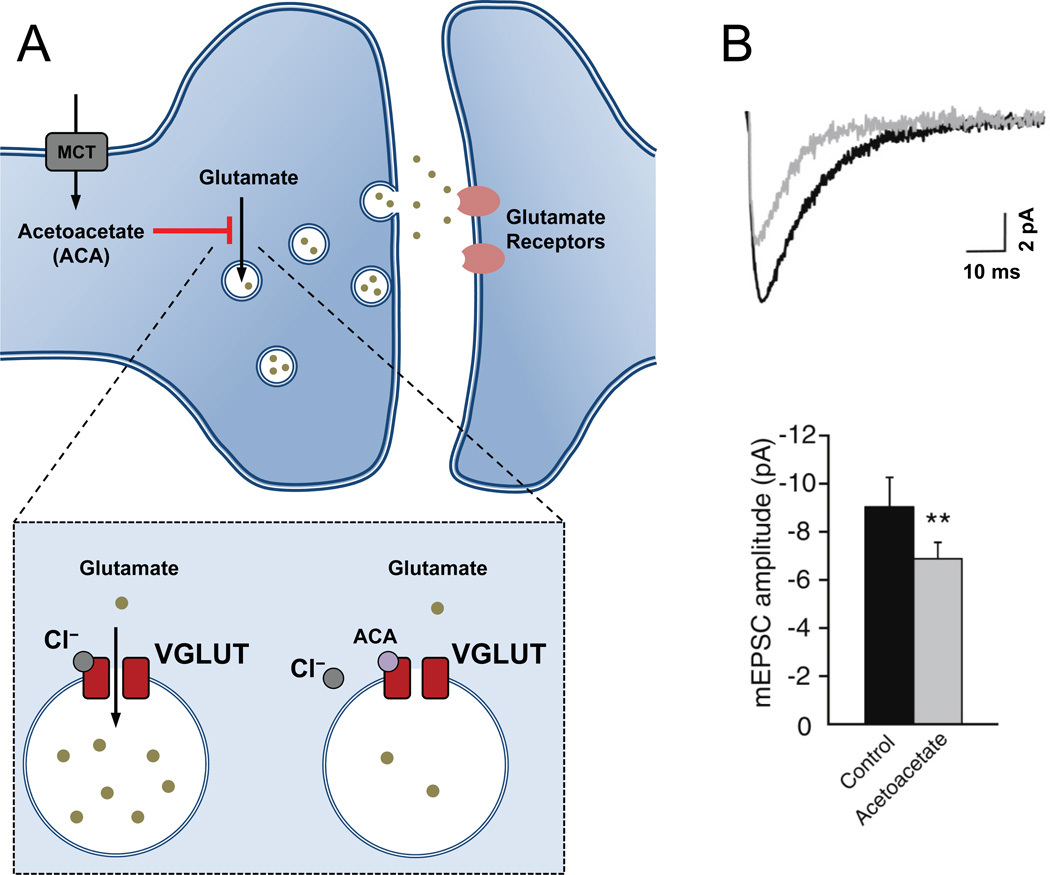
One long-standing hypothesis is that ketone bodies may act directly as pharmacological agents, but possible targets have been unclear. Recently, glutamate transport into synaptic vesicles by the vesicular glutamate transporter, VGLUT2, was found to be inhibited by the ketone body acetoacetate [14] at concentrations that are expected during the ketogenic diet. This effect can decrease glutamate release by cultured neurons exposed to acetoacetate. Furthermore, in these experiments (though not in an earlier study [15]), acetoacetate reduced excitatory glutamatergic synaptic transmission at hippocampal CA1 neurons (Figure 1). There was no effect on inhibitory synaptic input, consistent with the lack of inhibition of the vesicular GABA transporter by acetoacetate.
Figure 1. Ketone body inhibition of vesicular glutamate transport.
(A) Transport of glutamate into synaptic vesicles occurs via vesicular glutamate transporters (VGLUT). Acetoacetate (ACA), a ketone body whose level is elevated in patients on the ketogenic diet, was shown to be an inhibitor of VGLUT, competing with chloride for the site of allosteric regulation [14]. (B) When ACA was applied to hippocampal brain slices, glutamatergic synaptic transmission onto CA1 pyramidal cells was significantly reduced [14]. This reduced glutamatergic signaling may reduce brain excitability and potentially contribute to the mechanism of the ketogenic diet. Abbreviations: mEPSC, miniature excitatory postsynaptic current, **, P< 0.01. Reproduced, with permission, from [14].
Inhibition of glutamate signaling by acetoacetate would be expected to reduce neuronal excitability. Indeed, neuronal hyperexcitability induced in rats by infusion of 4-aminopyridine, a potassium channel blocker and proconvulsant, was reduced by direct infusion of acetoacetate into the brain [14]. It should be noted that, in this case, the 10 mM acetoacetate directly dialyzed into the rat brain was considerably higher than the effective concentrations required for inhibition of VGLUT2 and than expected during the ketogenic diet. Additionally, the relative seizure severity score used does not indicate the level of seizures induced (i.e. hypoactivity versus full tonic-clonic seizures), so it is unclear whether acetoacetate is effective on mild or severe seizure levels.
The reduction in glutamate release by acetoacetate is one promising candidate for how the diet might reduce seizures. However, organotypic hippocampal slice cultures chronically exposed to BHB were not protected from pharmacologically induced epileptiform activity [16]. Additionally, acetoacetate rapidly breaks down to acetone or is converted to BHB, so it remains to be shown whether acetoacetate levels in the brain during dietary therapy are actually sufficient to chronically inhibit VGLUT2.
Earlier work suggested that increased production of the inhibitory neurotransmitter GABA might result from changes in brain metabolism produced by ketogenic diet [17]. It is hypothesized that glutamate recycling via glutamine becomes more efficient when ketone bodies are available, and that this may improve GABA resynthesis for inhibitory neurotransmission even more than it affects glutamate repackaging for excitatory neurotransmission [17]. The higher GABA production would be expected to increase inhibitory signaling in the brain, though, in rodents, elevations in total brain GABA levels have not been found [18]. Such changes in GABA signaling could complement the hypothesized alteration in glutamate signaling produced by acetoacetate.
Ketone bodies can increase mitochondrial metabolism and decrease glycolysis
Because ketone bodies are directly metabolized by mitochondria, glycolysis is bypassed and even inhibited by the increase in mitochondrial metabolism [4, 19]. This metabolic shift is expected to increase mitochondrial ATP production and decrease glycolytic ATP production. Glycolytic enzymes are often found associated with membrane proteins [20–23] and may produce a compartmentation of ATP at the plasma membrane [24, 25]. Indeed, it is believed that the pumps maintaining the intracellular ionic concentrations utilize glycolytic ATP [21]. The submembrane consumption of ATP by pumps may activate nearby ATP-sensitive potassium (KATP) channels [26, 27], candidates for the link between metabolism and neuronal excitability. Intracellular ATP inhibits this channel and activation of the channel upon metabolic inhibition or ATP consumption generates a hyperpolarizing current that reduces cellular excitability [28]. Their role is best characterized in pancreatic beta cells where open KATP channels maintain the membrane potential at a negative, hyperpolarized level [29]. Increases in blood glucose levels lead to inhibition of KATP channels by ATP, which then permits membrane depolarization and triggers insulin release. Similarly, in the hypothalamus, KATP channels control the activity of glucose-sensitive neurons, which are important for regulation of energy consumption and body weight [30, 31].
KATP channels are widely expressed in the brain [32–34] and may play a role in the anticonvulsant action of the ketogenic diet, possibly via a use-dependent mechanism [35]. Increased activity of the channel has been observed with bursts of action potentials in respiratory neurons [26] and in hippocampal dentate granule neurons [27]. Inhibition of the Na+/K+ pump prevented the activation of the channels, supporting the hypothesis that ATP consumption by the pump released KATP channels from ATP inhibition. These negative feedback actions of KATP channels may be augmented by the ketogenic diet, as the presence of elevated BHB also increased the activity of KATP channels in dentate granule neurons [27], possibly via the decrease in glycolytic ATP production.
Ketone bodies reduce neuronal excitability via KATP channels
Consistent with increased KATP channel activity, ketone bodies were found to directly reduce neuronal firing rates of spontaneously active substantia nigra pars reticulata (SNr) GABAergic neurons from mouse brain slices [36]. SNr neurons are important regulators of motor output and have been considered a “seizure gate” [37]. Addition of acetoacetate or BHB produced a slowing of firing rate that was mediated by the activation of KATP channels and depended on GABAB receptors. The mechanism of activation of KATP channels may involve reductions in submembrane ATP levels or, alternatively, it may involve G-protein signaling. Consistent with this alternate method of KATP channel activation, G-protein activation of KATP channels has been linked with GABAB receptors [38] or adenosine A1 receptors [39, 40].
Brain adenosine levels affect seizure susceptibility
Adenosine signaling via A1 receptors can reduce neuronal excitability [41], and an increased level of adenosine in the brain has been hypothesized as a mechanism for the anticonvulsant effect of the ketogenic diet [42]. Recent evidence shows that the ketogenic diet can reverse seizures in mice that are produced by disruption of adenosine A1 receptor signaling. Electrographic seizure-like activity was recorded in the hippocampus of transgenic mice overexpressing adenosine kinase [43] or mice lacking the adenosine A1 receptor [44]. In the adenosine kinase transgenic mice, this hyperexcitability was reduced when they were fed a ketogenic diet [44]. The effectiveness of the ketogenic diet in reducing the seizures was reversed by injection of glucose or of DPCPX, a blocker of the adenosine A1 receptor.
These findings suggest that the ketogenic diet may increase extracellular adenosine and therefore adenosine signaling, thereby reversing the impairments producing the seizures in this model. However, it is not known whether dysfunctional adenosine signaling underlies seizures in other rodent models or in human cases. Therefore, it will be intriguing to see whether adenosine is involved in ketogenic diet seizure protection in classical rodent seizure models that do not rely directly on disruption of adenosine receptor signaling for production of seizures.
Reducing glucose usage might be more important
So far, we have examined recent ideas into how ketone bodies themselves may be important for prevention of neuronal hyperexcitability. Ketone body levels, though, have not consistently correlated with the level of seizure control. Instead, a decrease in glucose metabolism has been hypothesized to be important for the seizure protection. The anticonvulsant properties of the ketogenic diet can be rapidly reversed upon infusion of glucose [45], supporting the importance of decreased glucose usage. Additionally, new modifications to the ketogenic diet, which do not necessarily generate ketosis, are also effective in children with intractable epilepsy [46]. Thus, a reduction in glucose usage might be more important than ketone bodies. Unfortunately, dietary treatments in rodents have led to conflicting results in seizure models possibly as a result of strain differences or differences in the composition of the diet (Box 3). This has made it difficult to compare ketogenic diet results in rodents with seizure protection in humans. Recently, more direct metabolic manipulations in rodents have allowed for some new insights into how decreased glucose utilization reduces seizures.
Box 3: Seizure models and the ketogenic diet.
Newly developed tools and rodent seizure models will be important to produce future understanding of the ketogenic diet. Many of the currently used rodent seizure models were designed for the screening of anticonvulsant drugs. Results using the ketogenic diet on acute seizure models have been promising [92], but often inconsistent [93]. Future studies using rodent models with chronic, spontaneous seizures that are monitored using new methods of wireless electroencephalography (EEG) recording and automated, unsupervised behavioral analysis will allow for more realistic rodent epilepsy studies. Furthermore, using animals with genetically altered metabolism (e.g. [49]) may prevent complications created by dietary changes in rodents, which may not produce the same degree of change in fuel utilization as found in humans [94–96].
In addition to the considerations of in vivo rodent models, the results from in vitro assays used to test the role of ketone bodies on neuronal excitability may be confounded by recording conditions used. Adequate oxygenation is necessary to sustain mitochondrial respiration [91, 97], and temperature and ionic conditions can greatly affect neuronal metabolism. Additionally, using minimally invasive recording techniques to assess excitability will be important so that intracellular metabolic conditions are preserved. The use of fluorescent biosensors of metabolism [98] will also allow for real-time monitoring of metabolic changes associated with neuronal excitability.
Reduced glucose utilization confers seizure resistance
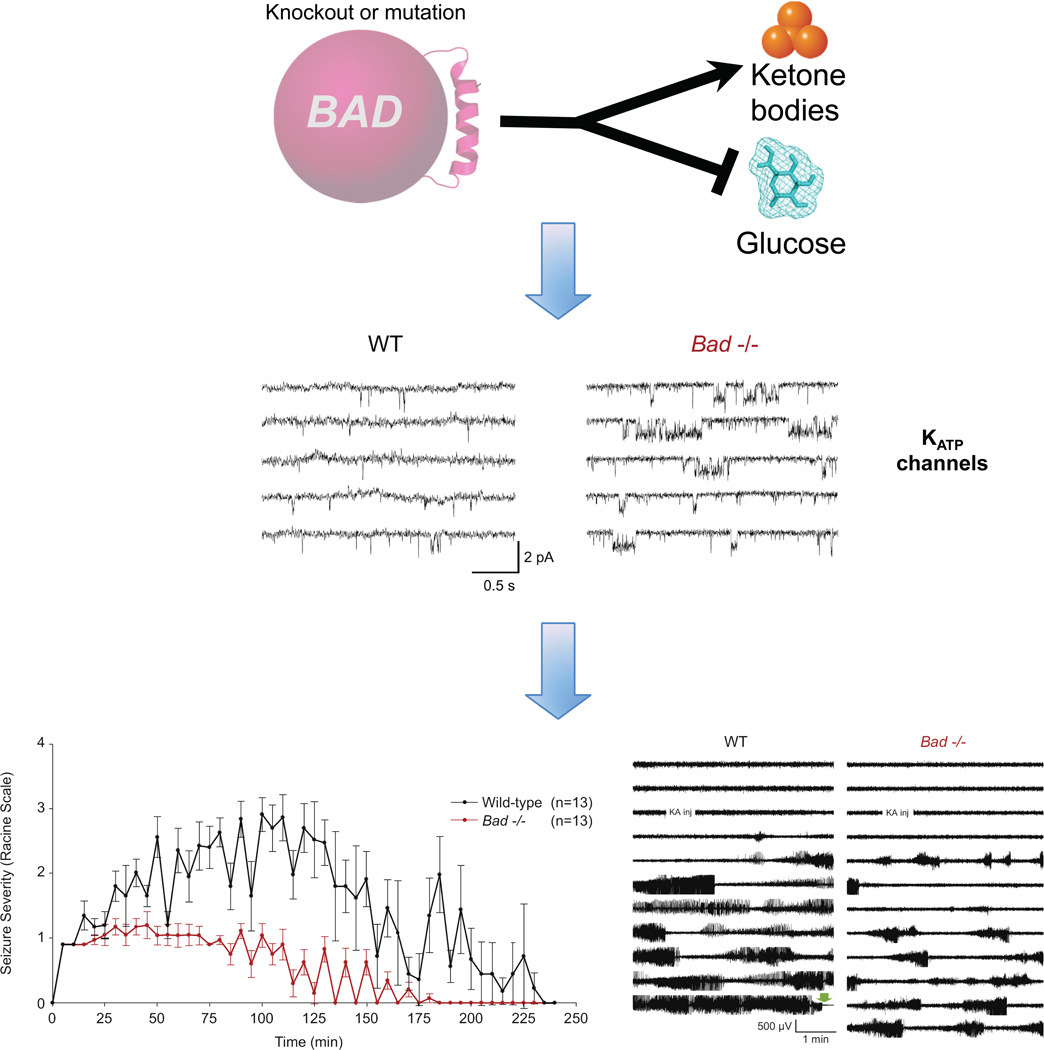
One promising approach to assess seizure susceptibility is the use of mutant mice exhibiting chronic altered metabolism. Mice lacking the protein BAD (BCL-2-associated Agonist of Cell Death) [47], have reduced cellular glucose metabolism [48]. Real-time mitochondrial oxygen consumption rates measured in cultured hippocampal neurons and astrocytes from BAD knockout mice showed reduced glucose oxidation and elevated BHB metabolism [49]. Consistent with a shift away from glucose utilization, BHB levels were elevated in brain extracts from BAD knockout animals, a result that is reminiscent of the characteristics of the ketogenic diet. Moreover, the reduced glucose metabolism in BAD knockout mice conferred resistance to acute seizures induced by kainic acid or pentylenetetrazol injection. The seizure resistance was not a result of BAD’s apoptotic role, but rather its role in glucose metabolism, as shown by parallel effects on seizures by BAD mutations with opposite effects on apoptosis.
To elucidate the link between metabolic changes and neuronal excitability, the activity of KATP channels in hippocampal brain slices from BAD mutant mice was also examined in this study [49]. KATP channels, recorded from dentate granule neurons, were significantly more active in the BAD mutant slices (Figure 2). Furthermore, whole cell KATP currents were elevated in BAD mutant neurons and increasing the intracellular ATP concentration could decrease these currents. Supporting these in vitro results, mice lacking both BAD and Kir6.2, the pore-forming subunit of the KATP channel, reversed the seizure resistance, providing genetic evidence that KATP channels were important for the seizure resistance in BAD mutant mice.
Figure 2. KATP channels mediate the seizure resistance of BAD mutant mice.
BAD regulates mitochondrial metabolism of glucose and ketone bodies. In BAD knockout or mutant animals, glucose utilization is reduced while ketone body metabolism is elevated [48]. This metabolic switch results in increased KATP channel activity, as demonstrated by cell-attached recordings of KATP channels in dentate granule neurons in brain slices from BAD knockout and mutant brains [49]. BADdeficient mice are more resistant to seizures induced by injection of kainic acid (bottom left panel) and show reduced cortical seizure activity as recorded by electroencephalogram (EEG) (bottom right panel) [49]. Reproduced, with permission, from [49] (middle and bottom panels).
The mechanism of elevated KATP channel activity in BAD mutant mice is not known. It was speculated that a down-regulation of glycolysis by the shift to ketone body oxidation might increase KATP channel activity (Figure 2), but this has not been demonstrated. In addition, it is unknown whether the elevated KATP activity recorded in dentate granule neurons occurs in other brain regions in BAD mutant mice. Dentate granule neurons are important in gating hyperexcitability from spreading beyond the dentate gyrus into other areas of the hippocampus [50–53], but it seems unlikely that changes in these cells alone would be sufficient to confer the substantial seizure resistance of BAD mutant mice. Though these questions await further investigation, BAD mutant mice provide a new tool to dissect the mechanism of metabolic reduction of seizures.
Glycolytic inhibition is anticonvulsant
The reduction in glucose levels and increase in ketone body metabolism observed during the ketogenic diet are consistent with a decrease in glycolysis. This has led to studies examining the ability of glycolytic inhibition to reduce seizures. The glucose analog, 2-deoxyglucose (2DG), inhibits glycolysis by decreasing glucose uptake [54] and competing for phosphoglucose isomerase [55]. 2DG is able to slow seizure progression in the rodent kindling seizure model [56]. This antikindling effect was proposed to result from decreased expression of brain-derived neurotrophic factor (BDNF) and the BDNF receptor, TrkB. The mechanism of the decreased expression may involve the repression of BDNF by the NADH binding protein CtBP and neuron restrictive silencing factor (NRSF). BDNF is a candidate proconvulsant and reduction of BDNF signaling via its receptor TrkB is expected to increase seizure resistance [57, 58]. A recent study supported NRSF’s role in the anticonvulsant properties of 2DG, but demonstrated that the ketogenic diet could still increase seizure resistance in mice lacking NRSF [59]. NRSF might not be required for the ketogenic diet, or multiple redundant mechanisms might exist for the diet.
Reduced oxidative stress may be involved in the seizure protection of the ketogenic diet
Metabolic changes could also improve seizure resistance by reducing reactive oxygen species (ROS). Rats injected with fructose 1,6-bisphosphate, which has been shown to shift glucose utilization to the pentose phosphate pathway [60], were more resistant to acute seizures [61]. Because the pentose phosphate pathway produces NADPH, which is used to reduce intracellular ROS, it is hypothesized that the improved antioxidant function might be important for seizure protection [62]. Several studies have presented evidence that the ketogenic diet augments mechanisms that attenuate ROS [63–66]. While reduction of ROS is known to improve cell health, it is not fully understood if this would serve only a neuroprotective role or also function to directly reduce neuronal excitability.
Neuroprotection and anti-epileptogenic effects of dietary treatment
While this review focuses on relatively direct mechanisms by which altered metabolism may reduce seizures through altered neuronal excitability, a ketogenic diet may also protect against or even reverse the chronic sequelae of seizures. Cellular metabolic stress during seizures can lead to neuronal death, and the ketogenic diet may serve a neuroprotective role, both by supplying additional cellular fuels and by reducing production of damaging reactive oxygen species [67].
The brain can also respond to seizures by “learning” to have seizures more easily, a process known as “epileptogenesis” that involves changes in intrinsic excitability, synaptic connectivity, and synaptic plasticity [68]. Dietary treatment for epilepsy may reverse these changes by blocking seizure activity itself and allowing a slow “unlearning” of seizures – many patients whose epilepsy is well-controlled for several years, either by dietary therapy or by conventional anticonvulsant medications, are able to remain seizure-free after stopping treatment. Some hypothesize that dietary treatment may also promote the “unlearning” of seizures by its effects on gene regulation [56, 58, 69, 70].
Conclusions
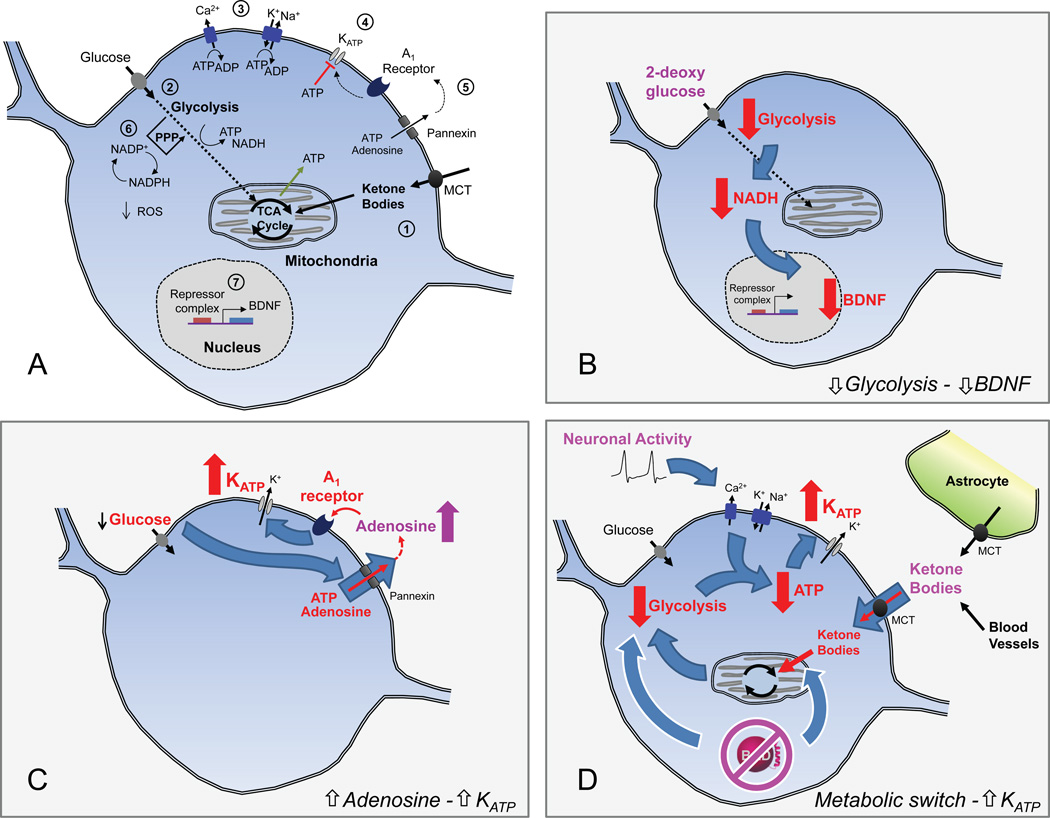
Interest in dietary treatments for pediatric epilepsy has grown in recent years, which has motivated new research studies into the mechanism of the ketogenic diet. It is evident from recent advances that multiple mechanisms are likely at play (Figure 3). Ketone bodies could be acting directly to inhibit vesicular glutamate transport, but they also produce important changes in cellular metabolism that reduce seizures. Ketone bodies alter metabolism by bypassing glycolysis and increasing mitochondrial oxidation. This metabolic change may lead to activation of KATP channels, which can reduce neuronal excitability. Recent evidence suggests that elevated KATP activity might occur with reduced glucose oxidation, as observed in BAD mutant animals, and the increased KATP activity confers seizure resistance. Inhibiting glycolysis directly using 2DG was also found to prevent seizures in rodent models through avenues involving decreased BDNF signaling. Alternatively, reducing glycolysis by shifting glucose metabolism to the pentose phosphate pathway was shown to reduce seizures, possibly through improved ROS handling.
Figure 3. Potential mechanisms of the ketogenic diet.
(A) Schematic diagram illustrating the key metabolic pathways and diet targets.
1) Ketone bodies enter neurons via the monocarboxylate transporter (MCT) and are directly metabolized by mitochondria.
2) Glucose levels are reduced during the ketogenic diet as ketone bodies become the major fuel in the brain. Reduction of glucose metabolism in BAD mutant mice, or inhibition of glycolysis using 2-deoxyglucose, results in seizure protection [49, 56].
3) Ion pumps maintain ion homeostasis and consume intracellular ATP. Neuronal function is energetically demanding [81], which allows for changes in metabolism to modulate neuronal function.
4) KATP channels hyperpolarize neurons and may link changes in metabolism to neuronal excitability [35, 36]. Decreases in cytosolic ATP, possibly via increased pump consumption of ATP, lead to increased KATP channel activity [26, 27].
5) Extracellular adenosine can signal through A1 receptors and G-proteins to activate hyperpolarizing conductances like the KATP channel [39, 40]. Release of adenine nucleotides through pannexin hemichannels or by exocytosis can modulate extracellular concentrations of adenosine.
6) Antioxidant capacity can be increased by shifts of glucose metabolism into the pentose phosphate pathway (PPP). This improves cellular handling of ROS and can act to protect neurons; such neuroprotection may be important for antiepileptogenesis [62– 65].
7) Gene expression can respond to changes in cellular metabolism, with downstream effects on excitability. 2-deoxyglucose inhibits glycolysis, which results in decreased in BDNF expression [56]. Other changes in gene expression are likely to occur during the ketogenic diet.
(B-D) Hypotheses for how metabolic changes could result in reduced hyperexcitability.
B. Reduced Glycolysis – reduced BDNF expression. Reduced glycolysis (for instance, by application of 2-deoxyglucose) leads to a decrease in cytosolic NADH, which in turn represses BDNF expression [56]. BDNF and its receptor, TrkB, have been implicated in epileptogenesis (ie. in the development of spontaneous seizures after status epilepticus) [56, 57].
C. Increased adenosine – Increased KATP. In conditions of low glucose, extracellular adenosine levels may be elevated via release of adenosine or ATP from neurons through pannexin hemichannels [40]. Activation of adenosine A1 receptors could then increase KATP channel activity and reduce neuronal excitability.
D. Metabolic switch – Increased KATP. A switch from glucose to ketone body metabolism reduces the activity of glycolysis [4]. The reduced glycolytic production of ATP, combined with pump consumption of ATP during neuronal activity, could lead to reduced ATP levels near the plasma membrane. This in turn would increase KATP channel activity and reduce the excitability of neurons. The metabolic switch can be induced either by increased availability of ketone bodies (supplied by the liver via blood vessels, when on the ketogenic diet) or by mutation of BAD (white outlines) [49]. Ketone bodies may also be produced from fatty acids by neighboring astrocytes [71].
Unraveling the complete picture of the ketogenic diet’s anticonvulsive properties will require further studies on the ways in which cellular metabolism can shift membrane excitability. Newly developed methods for monitoring chronic seizures, genetic tools for manipulation of metabolism, and improved in vitro techniques for assessing metabolism and excitability will enable future progress in answering the outstanding questions (Box 4). Diet may act through multiple simultaneous mechanisms that differ depending on cell-type and brain-region. Future work may link the individual findings from many studies of metabolic modification of neuronal activity in a unified explanation of the potent anti-seizure action of the ketogenic diet.
Box 4: Outstanding questions.
To what extent does glycolysis or oxidative phosphorylation contribute to sustaining neurons at rest and during activation? Is neuronal excitability affected by shifting metabolism towards more mitochondrial oxidation and less glycolysis?
How do different metabolic states affect the activity of specific subtypes of neurons? Is there a difference between glutamatergic and GABAergic neurons in their response to metabolic changes?
Do neurons utilize submembrane local energy production to sustain pump, channel, and receptor function? Does ATP compartmentation exist in the brain and is it important for metabolic regulation of excitability?
How do ketone-fueled neurons differ from glucose-fueled neurons? What are the acute and chronic effects of ketone body metabolism?
What in vivo and in vitro epilepsy models are best for assessing the effect and mechanisms of the ketogenic diet?
Can the therapeutic effects of dietary treatment, particularly for patients who do not respond to current drug treatment, be mimicked by new pharmacological approaches?
Acknowledgements
We thank members of the Yellen lab for helpful discussions. This work was supported by grants from the National Institutes of Health [F31 NS077633 (A.L.), R01 NS055031 and R56 NS072142 (G.Y.)].
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kwan P, Brodie M. Early identification of refractory epilepsy. N Engl J Med. 2000;342:314–319. doi: 10.1056/NEJM200002033420503. [DOI] [PubMed] [Google Scholar]
- 2.Neal E, et al. A randomized trial of classical and medium-chain triglyceride ketogenic diets in the treatment of childhood epilepsy. Epilepsia. 2009;50:1109–1126. doi: 10.1111/j.1528-1167.2008.01870.x. [DOI] [PubMed] [Google Scholar]
- 3.Peterman M. The ketogenic diet in epilepsy. J. Am. Med. Assoc. 1925;84:1979–1983. [Google Scholar]
- 4.DeVivo D, et al. Chronic ketosis and cerebral metabolism. Ann. Neurol. 1978;3:331–337. doi: 10.1002/ana.410030410. [DOI] [PubMed] [Google Scholar]
- 5.Keith H. Factors influencing experimentally produced convulsions. Arch. Neurol. Psychiatry. 1933;29:148–154. [Google Scholar]
- 6.Rho J, et al. Acetoacetate, acetone, and dibenzylamine (a contaminant in l-(+)-beta-hydroxybutyrate) exhibit direct anticonvulsant actions in vivo. Epilepsia. 2002;43:358–361. doi: 10.1046/j.1528-1157.2002.47901.x. [DOI] [PubMed] [Google Scholar]
- 7.Likhodii S, et al. Anticonvulsant properties of acetone, a brain ketone elevated by the ketogenic diet. Ann. Neurol. 2003;54:219–226. doi: 10.1002/ana.10634. [DOI] [PubMed] [Google Scholar]
- 8.Bough K, et al. Path analysis shows that increasing ketogenic ratio, but not beta-hydroxybutyrate, elevates seizure threshold in the Rat. Dev. Neurosci. 1999;21:400–406. doi: 10.1159/000017390. [DOI] [PubMed] [Google Scholar]
- 9.Bough K, et al. Higher ketogenic diet ratios confer protection from seizures without neurotoxicity. Epilepsy Res. 2000;38:15–25. doi: 10.1016/s0920-1211(99)00077-7. [DOI] [PubMed] [Google Scholar]
- 10.Likhodii S, et al. Dietary fat ketosis, and seizure resistance in rats on the ketogenic diet. Epilepsia. 2000;41:1400–1410. doi: 10.1111/j.1528-1157.2000.tb00115.x. [DOI] [PubMed] [Google Scholar]
- 11.Dell C, et al. Lipid and fatty acid profiles in rats consuming different high-fat ketogenic diets. Lipids. 2001;36:373–378. doi: 10.1007/s11745-001-0730-8. [DOI] [PubMed] [Google Scholar]
- 12.Gilbert D, et al. The ketogenic diet: seizure control correlates better with serum beta-hydroxybutyrate than with urine ketones. J. Child Neurol. 2000;15:787–790. doi: 10.1177/088307380001501203. [DOI] [PubMed] [Google Scholar]
- 13.van Delft R, et al. Blood beta-hydroxybutyrate correlates better with seizure reduction due to ketogenic diet than do ketones in the urine. Seizure. 2010;19:36–39. doi: 10.1016/j.seizure.2009.10.009. [DOI] [PubMed] [Google Scholar]
- 14.Juge N, et al. Metabolic control of vesicular glutamate transport and release. Neuron. 2010;68:99–211. doi: 10.1016/j.neuron.2010.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thio L, et al. Ketone bodies do not directly alter excitatory or inhibitory hippocampal synaptic transmission. Neurology. 2000;54:325–331. doi: 10.1212/wnl.54.2.325. [DOI] [PubMed] [Google Scholar]
- 16.Samoilova M, et al. Chronic in vitro ketosis is neuroprotective but not anticonvulsant. J. Neurochem. 2010;113:826–861. doi: 10.1111/j.1471-4159.2010.06645.x. [DOI] [PubMed] [Google Scholar]
- 17.Yudkoff M, et al. The ketogenic diet and brain metabolism of amino acids: relationship to the anticonvulsant effect. Annu. Rev. Nutr. 2007;27:415–430. doi: 10.1146/annurev.nutr.27.061406.093722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yudkoff M, et al. Brain amino acid metabolism and ketosis. J. Neurosci. Res. 2001;66:272–281. doi: 10.1002/jnr.1221. [DOI] [PubMed] [Google Scholar]
- 19.Haymond M, et al. Effects of ketosis on glucose flux in children and adults. Am. J. Physiol. 1983;245:E373–E378. doi: 10.1152/ajpendo.1983.245.4.E373. [DOI] [PubMed] [Google Scholar]
- 20.Paul R, et al. Vascular smooth muscle: aerobic glycolysis linked to sodium and potassium transport processes. Science. 1979;206:1414–1416. doi: 10.1126/science.505014. [DOI] [PubMed] [Google Scholar]
- 21.Mercer R, Dunham P. Membrane-bound ATP fuels the Na/K pump. Studies on membrane-bound glycolytic enzymes on inside-out vesicles from human red cell membranes. J. Gen. Physiol. 1981;78:547–568. doi: 10.1085/jgp.78.5.547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dubinsky W, et al. Colocalization of glycolytic enzyme activity and KATP channels in basolateral membrane of Necturus enterocytes. Am. J. Physiol. 1998;275:C1653–C1659. doi: 10.1152/ajpcell.1998.275.6.C1653. [DOI] [PubMed] [Google Scholar]
- 23.Lu M, et al. Interaction between aldolase and vacuolar H+-ATPase: evidence for direct coupling of glycolysis to the ATP-hydrolyzing proton pump. J. Biol. Chem. 2001;276:30407–30413. doi: 10.1074/jbc.M008768200. [DOI] [PubMed] [Google Scholar]
- 24.Hoffman JF, et al. On the functional use of the membrane compartmentalized pool of ATP by the Na+ and Ca++ pumps in human red blood cell ghosts. J. Gen. Physiol. 2009;134:351–361. doi: 10.1085/jgp.200910270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Proverbio F, Hoffman J. Membrane compartmentalized ATP and its preferential use by the Na,K-ATPase of human red cell ghosts. J. Gen. Physiol. 1977;69:605–632. doi: 10.1085/jgp.69.5.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Haller M, et al. Dynamic activation of KATP channels in rhythmically active neurons. J. Physiol. 2001;537:69–81. doi: 10.1111/j.1469-7793.2001.0069k.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tanner G, et al. Single KATP channel opening in response to action potential firing in mouse dentate granule neurons. J. Neurosci. 2011;31:8689–8696. doi: 10.1523/JNEUROSCI.5951-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ashcroft F, Gribble F. Correlating structure and function in ATP-sensitive K+ channels. Trends Neurosci. 1998;21:288–294. doi: 10.1016/s0166-2236(98)01225-9. [DOI] [PubMed] [Google Scholar]
- 29.Bennett K, et al. Pancreatic β-cell KATP channels: Hypoglycaemia and hyperglycaemia. Rev. Endocr. Metab. Disord. 2010;11:157–163. doi: 10.1007/s11154-010-9144-2. [DOI] [PubMed] [Google Scholar]
- 30.Parton L, et al. Glucose sensing by POMC neurons regulates glucose homeostasis and is impaired in obesity. Nature. 2007;449:228–260. doi: 10.1038/nature06098. [DOI] [PubMed] [Google Scholar]
- 31.Kong D, et al. Glucose stimulation of hypothalamic MCH neurons involves KATP channels, is modulated by UCP2, and regulates peripheral glucose homeostasis. Cell metab. 2010;12:545–597. doi: 10.1016/j.cmet.2010.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Karschin C, et al. Overlapping distribution of KATP channel-forming Kir6.2 subunit and the sulfonylurea receptor SUR1 in rodent brain. FEBS Lett. 1997;401:59–64. doi: 10.1016/s0014-5793(96)01438-x. [DOI] [PubMed] [Google Scholar]
- 33.Dunn-Meynell A, et al. Distribution and phenotype of neurons containing the ATP-sensitive K+ channel in rat brain. Brain Res. 1998;814:41–54. doi: 10.1016/s0006-8993(98)00956-1. [DOI] [PubMed] [Google Scholar]
- 34.Zawar C, et al. Cell-type specific expression of ATP-sensitive potassium channels in the rat hippocampus. J. Physiol. 1999;514(Pt 2):327–341. doi: 10.1111/j.1469-7793.1999.315ae.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yellen G. Ketone bodies, glycolysis, and KATP channels in the mechanism of the ketogenic diet. Epilepsia. 2008;49(Suppl 8):80–82. doi: 10.1111/j.1528-1167.2008.01843.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ma W, et al. Ketogenic diet metabolites reduce firing in central neurons by opening KATP channels. J. Neurosci. 2007;27:3618–3643. doi: 10.1523/JNEUROSCI.0132-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Depaulis A, et al. Endogenous control of epilepsy: the nigral inhibitory system. Prog. Neurobiol. 1994;42:33–52. doi: 10.1016/0301-0082(94)90020-5. [DOI] [PubMed] [Google Scholar]
- 38.Mironov S, Richter D. Intracellular signalling pathways modulate KATP channels in inspiratory brainstem neurones and their hypoxic activation: involvement of metabotropic receptors, G-proteins and cytoskeleton. Brain Res. 2000;853:60–67. doi: 10.1016/s0006-8993(99)02234-9. [DOI] [PubMed] [Google Scholar]
- 39.Li D-P, et al. Adenosine inhibits paraventricular pre-sympathetic neurons through ATP-dependent potassium channels. J. Neurochem. 2010;113:530–542. doi: 10.1111/j.1471-4159.2010.06618.x. [DOI] [PubMed] [Google Scholar]
- 40.Kawamura M, et al. Metabolic autocrine regulation of neurons involves cooperation among pannexin hemichannels, adenosine receptors, and KATP channels. J. Neurosci. 2010;30:3886–3981. doi: 10.1523/JNEUROSCI.0055-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Phillis J, Wu P. The role of adenosine and its nucleotides in central synaptic transmission. Prog. Neurobiol. 1981;16:187–239. doi: 10.1016/0301-0082(81)90014-9. [DOI] [PubMed] [Google Scholar]
- 42.Masino S, Geiger J. Are purines mediators of the anticonvulsant/neuroprotective effects of ketogenic diets? Trends Neurosci. 2008;31:273–281. doi: 10.1016/j.tins.2008.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fedele D, et al. Astrogliosis in epilepsy leads to overexpression of adenosine kinase, resulting in seizure aggravation. Brain. 2005;128:2383–2395. doi: 10.1093/brain/awh555. [DOI] [PubMed] [Google Scholar]
- 44.Masino S, et al. A ketogenic diet suppresses seizures in mice through adenosine A1 receptors. J. Clin. Investig. 2011;121:2679–2762. doi: 10.1172/JCI57813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Huttenlocher P. Ketonemia and seizures: metabolic and anticonvulsant effects of two ketogenic diets in childhood epilepsy. Pediatr. Res. 1976;10:536–540. doi: 10.1203/00006450-197605000-00006. [DOI] [PubMed] [Google Scholar]
- 46.Pfeifer H, Thiele E. Low-glycemic-index treatment: a liberalized ketogenic diet for treatment of intractable epilepsy. Neurology. 2005;65:1810–1812. doi: 10.1212/01.wnl.0000187071.24292.9e. [DOI] [PubMed] [Google Scholar]
- 47.Chipuk J, et al. The BCL-2 family reunion. Mol. Cell. 2010;37:299–310. doi: 10.1016/j.molcel.2010.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Danial N. BAD: undertaker by night, candyman by day. Oncogene. 2008;27(Suppl 1):S53–S70. doi: 10.1038/onc.2009.44. [DOI] [PubMed] [Google Scholar]
- 49.Giménez-Cassina A, et al. BAD-dependent regulation of fuel metabolism and KATP channel activity confers resistance to epileptic seizures. Neuron. 2012;74:719–749. doi: 10.1016/j.neuron.2012.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Brenner R, et al. BK channel beta4 subunit reduces dentate gyrus excitability and protects against temporal lobe seizures. Nat. Neurosci. 2005;8:1752–1759. doi: 10.1038/nn1573. [DOI] [PubMed] [Google Scholar]
- 51.Coulter D. Chronic epileptogenic cellular alterations in the limbic system after status epilepticus. Epilepsia. 1999;40(Suppl 1) doi: 10.1111/j.1528-1157.1999.tb00875.x. [DOI] [PubMed] [Google Scholar]
- 52.Heinemann U, et al. The dentate gyrus as a regulated gate for the propagation of epileptiform activity. Epilepsy Res. Suppl. 1992;7:273–280. [PubMed] [Google Scholar]
- 53.Hsu D. The dentate gyrus as a filter or gate: a look back and a look ahead. Prog. Brain Res. 2007;163:601–613. doi: 10.1016/S0079-6123(07)63032-5. [DOI] [PubMed] [Google Scholar]
- 54.Nakada H, Wick A. The effect of 2-deoxyglucose on the metabolism of glucose, fructose, and galactose by rat diaphragm. J. Biol. Chem. 1956;222:671–676. [PubMed] [Google Scholar]
- 55.Wick A, et al. Localization of the primary metabolic block produced by 2-deoxyglucose. J. Biol. Chem. 1957;224:963–969. [PubMed] [Google Scholar]
- 56.Garriga-Canut M, et al. 2-Deoxy-D-glucose reduces epilepsy progression by NRSF-CtBP-dependent metabolic regulation of chromatin structure. Nat. Neurosci. 2006;9:1382–1389. doi: 10.1038/nn1791. [DOI] [PubMed] [Google Scholar]
- 57.He X-P, et al. Conditional deletion of TrkB but not BDNF prevents epileptogenesis in the kindling model. Neuron. 2004;43:31–42. doi: 10.1016/j.neuron.2004.06.019. [DOI] [PubMed] [Google Scholar]
- 58.McNamara JO, Scharfman HE. Temporal lobe epilepsy and the BDNF receptor, TrkB. Epilepsia. 2010;51:46–46. [PubMed] [Google Scholar]
- 59.Hu X-L, et al. Neuron-restrictive silencer factor is not required for the antiepileptic effect of the ketogenic diet. Epilepsia. 2011;52:1609–1625. doi: 10.1111/j.1528-1167.2011.03171.x. [DOI] [PubMed] [Google Scholar]
- 60.Kelleher J, et al. Energy metabolism in hypoxic astrocytes: protective mechanism of fructose-1,6-bisphosphate. Neurochem. Res. 1995;20:785–792. doi: 10.1007/BF00969690. [DOI] [PubMed] [Google Scholar]
- 61.Lian X-Y, et al. Fructose-1,6-bisphosphate has anticonvulsant activity in models of acute seizures in adult rats. J. Neurosci. 2007;27:12007–12018. doi: 10.1523/JNEUROSCI.3163-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Stringer J, Xu K. Possible mechanisms for the anticonvulsant activity of fructose-1,6-diphosphate. Epilepsia. 2008;49(Suppl 8):101–103. doi: 10.1111/j.1528-1167.2008.01849.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kim do Y, et al. Ketone bodies are protective against oxidative stress in neocortical neurons. J. Neurochem. 2007;101:1316–1326. doi: 10.1111/j.1471-4159.2007.04483.x. [DOI] [PubMed] [Google Scholar]
- 64.Maalouf M, et al. Ketones inhibit mitochondrial production of reactive oxygen species production following glutamate excitotoxicity by increasing NADH oxidation. Neuroscience. 2007;145:256–264. doi: 10.1016/j.neuroscience.2006.11.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Jarrett S, et al. The ketogenic diet increases mitochondrial glutathione levels. J. Neurochem. 2008;106:1044–1051. doi: 10.1111/j.1471-4159.2008.05460.x. [DOI] [PubMed] [Google Scholar]
- 66.Kim do Y, et al. Ketones prevent synaptic dysfunction induced by mitochondrial respiratory complex inhibitors. J. Neurochem. 2010;114:130–141. doi: 10.1111/j.1471-4159.2010.06728.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sullivan P, et al. The ketogenic diet increases mitochondrial uncoupling protein levels and activity. Ann. Neurol. 2004;55:576–656. doi: 10.1002/ana.20062. [DOI] [PubMed] [Google Scholar]
- 68.Noebels J, et al. Jasper's Basic Mechanisms of the Epilepsies. National Center for Biotechnology Information Bookshelf, USA. (4th Edition) 2012 [PubMed] [Google Scholar]
- 69.Bough K, et al. Calorie restriction and ketogenic diet diminish neuronal excitability in rat dentate gyrus in vivo. Epilepsia. 2003;44:752–760. doi: 10.1046/j.1528-1157.2003.55502.x. [DOI] [PubMed] [Google Scholar]
- 70.McDaniel S, et al. The ketogenic diet inhibits the mammalian target of rapamycin (mTOR) pathway. Epilepsia. 2011;52:E7–E11. doi: 10.1111/j.1528-1167.2011.02981.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Guzmán M, Blázquez C. Is there an astrocyte-neuron ketone body shuttle? Trends Endocrinol Metab. 2001;12:169–173. doi: 10.1016/s1043-2760(00)00370-2. [DOI] [PubMed] [Google Scholar]
- 72.Wilder RM. The effects of ketonemia on the course of epilepsy. Mayo Clin. Proc. 1921;2:307–308. [Google Scholar]
- 73.Conklin HW. Cause and treatment of epilepsy. J. Am. Osteo. Assoc. 1922;26:11–14. [Google Scholar]
- 74.Bailey EE, et al. The use of diet in the treatment of epilepsy. Epilepsy Behav. 2005;6:4–8. doi: 10.1016/j.yebeh.2004.10.006. [DOI] [PubMed] [Google Scholar]
- 75.Pong A, et al. Glucose transporter type I deficiency syndrome: Epilepsy phenotypes and outcomes. Epilepsia. 2012;53:1503–1510. doi: 10.1111/j.1528-1167.2012.03592.x. [DOI] [PubMed] [Google Scholar]
- 76.Kossoff E, et al. Efficacy of the Atkins diet as therapy for intractable epilepsy. Neurology. 2003;61:1789–1791. doi: 10.1212/01.wnl.0000098889.35155.72. [DOI] [PubMed] [Google Scholar]
- 77.Kossoff E, et al. A modified Atkins diet is effective for the treatment of intractable pediatric epilepsy. Epilepsia. 2006;47:421–424. doi: 10.1111/j.1528-1167.2006.00438.x. [DOI] [PubMed] [Google Scholar]
- 78.Kossoff E, et al. A prospective study of the modified Atkins diet for intractable epilepsy in adults. Epilepsia. 2008;49:316–319. doi: 10.1111/j.1528-1167.2007.01256.x. [DOI] [PubMed] [Google Scholar]
- 79.Muzykewicz D, et al. Efficacy, safety, and tolerability of the low glycemic index treatment in pediatric epilepsy. Epilepsia. 2009;50:1118–1126. doi: 10.1111/j.1528-1167.2008.01959.x. [DOI] [PubMed] [Google Scholar]
- 80.Attwell D, Laughlin S. An energy budget for signaling in the grey matter of the brain. J. Cereb. Blood Flow Metab. 2001;21:1133–1178. doi: 10.1097/00004647-200110000-00001. [DOI] [PubMed] [Google Scholar]
- 81.Howarth C, et al. Updated energy budgets for neural computation in the neocortex and cerebellum. J. Cereb. Blood Flow Metab. 2012;32:1222–1232. doi: 10.1038/jcbfm.2012.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ivannikov M, et al. Calcium clearance and its energy requirements in cerebellar neurons. Cell Calcium. 2010;47:507–520. doi: 10.1016/j.ceca.2010.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Paul R, et al. Preferential support of Ca2+ uptake in smooth muscle plasma membrane vesicles by an endogenous glycolytic cascade. FASEB J. 1989;3:2298–2301. doi: 10.1096/fasebj.3.11.2528493. [DOI] [PubMed] [Google Scholar]
- 84.James J, et al. Linkage of aerobic glycolysis to sodium-potassium transport in rat skeletal muscle. Implications for increased muscle lactate production in sepsis. J. Clin. Investig. 1996;98:2388–2397. doi: 10.1172/JCI119052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Pellerin L, et al. Activity-dependent regulation of energy metabolism by astrocytes: an update. Glia. 2007;55:1251–1262. doi: 10.1002/glia.20528. [DOI] [PubMed] [Google Scholar]
- 86.Hu Y, Wilson G. A temporary local energy pool coupled to neuronal activity: fluctuations of extracellular lactate levels in rat brain monitored with rapid-response enzyme-based sensor. J. Neurochem. 1997;69:1484–1490. doi: 10.1046/j.1471-4159.1997.69041484.x. [DOI] [PubMed] [Google Scholar]
- 87.Schurr A. Lactate: the ultimate cerebral oxidative energy substrate? J. Cereb. Blood Flow Metab. 2006;26:142–152. doi: 10.1038/sj.jcbfm.9600174. [DOI] [PubMed] [Google Scholar]
- 88.Gallagher C, et al. The human brain utilizes lactate via the tricarboxylic acid cycle: a 13C-labelled microdialysis and high-resolution nuclear magnetic resonance study. Brain. 2009;132:2839–2849. doi: 10.1093/brain/awp202. [DOI] [PubMed] [Google Scholar]
- 89.van Hall G, et al. Blood lactate is an important energy source for the human brain. J. Cereb. Blood Flow Metab. 2009;29:1121–1129. doi: 10.1038/jcbfm.2009.35. [DOI] [PubMed] [Google Scholar]
- 90.Boumezbeur F, et al. The contribution of blood lactate to brain energy metabolism in humans measured by dynamic 13C nuclear magnetic resonance spectroscopy. J. Neurosci. 2010;30:13983–13991. doi: 10.1523/JNEUROSCI.2040-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ivanov A, et al. Lactate Effectively Covers Energy Demands during Neuronal Network Activity in Neonatal Hippocampal Slices. Front. Neuroenergetics. 2011;3:2. doi: 10.3389/fnene.2011.00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Bough K, et al. An anticonvulsant profile of the ketogenic diet in the rat. Epilepsy Res. 2002;50:313–325. doi: 10.1016/s0920-1211(02)00086-4. [DOI] [PubMed] [Google Scholar]
- 93.Samala R, et al. Anticonvulsant profile of a balanced ketogenic diet in acute mouse seizure models. Epilepsy Res. 2008;81:119–127. doi: 10.1016/j.eplepsyres.2008.05.001. [DOI] [PubMed] [Google Scholar]
- 94.Owen O, et al. Brain metabolism during fasting. J. Clin. Investig. 1967;46:1589–1595. doi: 10.1172/JCI105650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ruderman N, et al. Regulation of glucose and ketone-body metabolism in brain of anaesthetized rats. Biochem. J. 1974;138:1–10. doi: 10.1042/bj1380001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Zhang Y, et al. Contribution of brain glucose and ketone bodies to oxidative metabolism. Adv. Exp. Med. Biol. 2013;765:365–370. doi: 10.1007/978-1-4614-4989-8_51. [DOI] [PubMed] [Google Scholar]
- 97.Hájos N, et al. Maintaining network activity in submerged hippocampal slices: importance of oxygen supply. Eur. J. Neurosci. 2009;29:319–327. doi: 10.1111/j.1460-9568.2008.06577.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Tantama M, et al. Optogenetic reporters: Fluorescent protein-based genetically encoded indicators of signaling and metabolism in the brain. Prog. Brain Res. 2012;196:235–263. doi: 10.1016/B978-0-444-59426-6.00012-4. [DOI] [PMC free article] [PubMed] [Google Scholar]