Abstract
Dyslipidemia has been identified as an important pathogenic risk factor for diabetic neuropathy, but current animal models do not adequately reproduce the lipid profile observed in human diabetics (increased triglycerides with an elevated LDL-cholesterol and reduced HDL-cholesterol). High fat feeding of mice produces hyperlipidemia, but mice are resistant to increases in the LDL to HDL ratio, reducing the potential for peripheral lipid deposits to impact neuropathy, as is postulated to occur in human subjects. Genetic manipulations provide an alternative approach to reproducing a neuropathic plasma lipid profile. Based on findings from the atherosclerosis literature, we began with knockout of ApoE. Since knockout of ApoE alone only partially mimics the human diabetic lipid profile, we examined the impact of its combination with a well-characterized model of type 2 diabetes exhibiting neuropathy, the db/db mouse. We added further gene manipulations to increase hyperlipidemia by using mice with both ApoE and ApoB48 knockout on the ob/+ (leptin mutation) mice. In all of these models, we found that either the db/db or ob/ob genotypes had increased body weight, hyperlipidemia, hyperglycemia, and evidence of neuropathy compared with the control groups (db/+ or ob/+, respectively). We found that ApoE knockout combined with leptin receptor knockout produced a lipid profile most closely modeling human dyslipidemia that promotes neuropathy. ApoE knockout combined with additional ApoB48 and leptin knockout produced similar changes of smaller magnitude, but, notably, an increase in HDL-cholesterol. Our data suggest that the overall effects of ApoE knockout, either directly upon nerve structure and function or indirectly on lipid metabolism, are insufficient to significantly alter the course of diabetic neuropathy. Although these models ultimately do not deliver optimal lipid profiles for translational diabetic neuropathy research, they do present glycemic and lipid profile properties of value for future therapeutic investigations.
Keywords: peripheral neuropathy, dyslipidemia, diabetes, apolipoprotein E, apolipoprotein B48, lipid profile, mouse
Introduction
Dyslipidemia is now recognized as an independent risk factor for the development of neuropathy in patients with diabetes (Ansquer, et al., 2009, Vincent, et al., 2009). Lipid profiles are commonly abnormal early in the course of type 2 diabetes (high levels of plasma triglycerides, elevated very-low-density cholesterol (VLDL-C) and low-density lipoprotein cholesterol (LDL-C), and decreased high-density lipoprotein cholesterol (HDL-C)), and correlate with the onset of neuropathic symptoms (Clemens, et al., 2004). Approximately 60% of prediabetic patients with impaired glucose tolerance display painful small nerve fiber neuropathy (Singleton, et al., 2001). Furthermore, hypertriglyceridemia is epidemiologically associated with electrophysiological evidence of non-symptomatic neuropathy (Drory, et al., 1999, Kassem, et al., 2005). These findings have prompted us to establish mouse models with varying profiles of dyslipidemia and glycemia in order to confirm the role and explore the mechanisms of lipid-induced peripheral nerve injury.
Apolipoprotein E (ApoE) is a 34kDa glycoprotein constituent of lipoproteins. It facilitates the transport of lipids between the sites of synthesis or absorption to the sites of utilization or excretion (Mahley, 1988). ApoE plays a key role in the local redistribution of lipids within tissues both during normal homeostasis and during injury and repair (Donahue and Johanson, 2008, Zhang, et al., 2011). In addition to its role in lipid metabolism, ApoE is proposed to have important functions within the central and peripheral nervous systems in maintaining neuronal health and survival.
For these reasons, ApoE knockout mice have been used in dyslipidemia studies (Poirier, 2000). Commercially available ApoE knockout mice on the C57BL/6 background demonstrate decreased clearance of remnant lipoproteins that leads to hypercholesterolemia and hypertriglyceridemia (Buzello, et al., 2003). Despite the abnormalities in plasma lipid profiles, these mice exhibit normal fasting glycemic and insulin levels (Trauner, et al., 2010). In the sciatic nerves of ApoE knockout mice, there were fewer unmyelinated nerve fibers and morphological abnormalities in the axons (Fullerton, et al., 1998). These defects resulted in reduced sensitivity to noxious thermal stimuli in the feet and tail.
Evidence for peripheral nerve deficits in ApoE knockout mice led to the proposal that lack of ApoE would exacerbate the development of diabetic microvascular complications, including neuropathy, i.e., that dyslipidemia and hyperglycemia together aggravate the onset of diabetes complications. In support of this concept, ApoE knockout mice crossed with mice lacking the leptin receptor (db/db mice) have increased retinopathy compared with mice lacking either of these proteins alone (Barile, et al., 2005).
Plasma cholesterol in mice is primarily carried in ApoB48-containing lipoprotein particles (Lloyd, et al., 2008). Humans do not produce ApoB48; ApoB100 predominates, with high levels of ApoB100-containing LDL associated with atherosclerosis (Lloyd, et al., 2008). Loss of ApoB48 in mice leads to increased levels of circulating ApoB100-expressing LDLs, more closely resembling lipid profiles in humans (Lloyd, et al., 2008). With the goal of developing improved mouse models of diabetic neuropathy by developing mice with hyperglycemia, hyperlipidemia and a lipoprotein profile modeling that in humans, we examined the impact of ApoE knockout combined with leptin receptor mutation (db/db) on lipid profiles, glycemic control, and extent of neuropathy at 24 weeks of age. We added further gene manipulations to increase dyslipidemia by utilizing mice with both ApoE and ApoB48 knockout in the ob/+ (leptin mutation) mouse (Lloyd, et al., 2008). Despite dramatic changes in lipid profiles, we find small differences in neuropathy parameters between these apolipoprotein knockout models, and generally no robust effect of increasing obesity upon the degree of neuropathy.
Materials and Methods
Generation of Mouse Genotypes
ApoE knockout mice on the C57BL/6 background and heterozygous db+ mice on the C57BLKS background were obtained from Jackson Laboratories. These mice were crossed and F1 offspring carrying the ApoE+/− db/+ were bred to generate F2 offspring. These were backcrossed for another generation, and the resulting F3 ApoE−/− db/+, ApoE−/− db/db, ApoE+/+ db/+, and ApoE+/+ db/db mice were included in the study (8 mice for each genotype). Triple knockout ApoE−/− ApoB100only/ob/+ mice were generated by Dr. Murielle Veniant and colleagues (Lloyd, et al., 2008) and bred to obtain ApoE−/− ApoB100only/ob/+ and ApoE−/− ApoB100only/ob/ob littermates for this study (10 mice for each genotype). Male mice only were used in all experiments.
Mice were housed in a pathogen-free environment and cared for following the University of Michigan Committee on the Care and Use of Animals guidelines. Mice were weaned at 4 weeks of age and fed a standard rodent chow. All data were collected at 24 weeks of age.
Phenotypic Measures
Final body weight was measured using standard laboratory scales. Following a 6 hour fast, one drop of tail blood was analyzed using a standard glucometer (One Touch Profile, LIFESCAN, Inc. Milpitas, CA). Two drops of tail blood were mixed with 10 µL 50 µM EDTA for measurement of glycated hemobglobin using the Helena Laboratories Test Kit, Glyco-Tek Affinity Column Method (Helena Laboratories Corp., Beaumont, TX).
Assessment of Neuropathy
Hind paw withdrawal and tail flick latency to heat stimulus were recorded electronically per our previous studies (Lee, et al., 1990, Sullivan, et al., 2008, Vincent, et al., 2009) (6 mice per group). In the ApoE only knockout and db/+ and db/db mice, sural and sciatic nerve conduction velocities (NCV) were measured under 30/0.75 mg/kg ketamine/acepromazine IP anesthetic while maintained at 32–34°C using a heating pad (Kern, et al., 2009, Sullivan, et al., 2008) (8 mice per group). In 2010, we found that NCV measurements were significantly improved using isofluorane anesthetic (Oh, et al., 2010) and this method was used when we obtained the triple knockout mice (8 mice per group). Intraepidermal nerve fiber density (IENFD) in footpads were measured as previously described (Kern, et al., 2009, Sullivan, et al., 2008) (6 mice per group).
Terminal Metabolic Phenotyping
Mice were euthanized using an overdose of sodium pentobarbital. Blood was collected from the superior vena cava and mixed with a final concentration of 50 µM EDTA. The blood was centrifuged at 800 g for 10 min at 4°C, and plasma (supernatant) collected. A 50 µL sample from each mouse was collected for total cholesterol and triglyceride measurements using a colorimetric kit (Diagnostic Chemicals Ltd.). The remaining plasma samples from 3 mice per group were pooled and plasma lipoproteins were separated by FPLC. The levels of triglycerides and cholesterol in each fraction were measured using colorimetric kits (Diagnostic Chemicals Ltd.). Lipid measurements were performed at the Mouse Metabolomics Phenotyping Core at the University of Washington (McMillen, et al., 2005).
Data Analysis
Data analysis was performed using GraphPad Prism 5.0. When performing comparisons across more than two groups, a one-way ANOVA test was performed and Tukey’s multiple point comparison post-test p values presented. If the Bartlett’s test for equal variances was significant, a non-parametric Kruskal-Wallis test was performed and Dunn’s post-test p values are presented. If the multiple group comparison did not reach significance, the directed hypothesis that leptin/leptin receptor and ApoE knockout mice develop complications was tested using an unpaired t-test. If the f-test to compare variances was significant, Welch’s correction was performed and the t-test re-run. Due to modification of methods, nerve conduction velocity data from the triple knockout model was analyzed using the unpaired t-test, as described.
Results
Final Body Weight
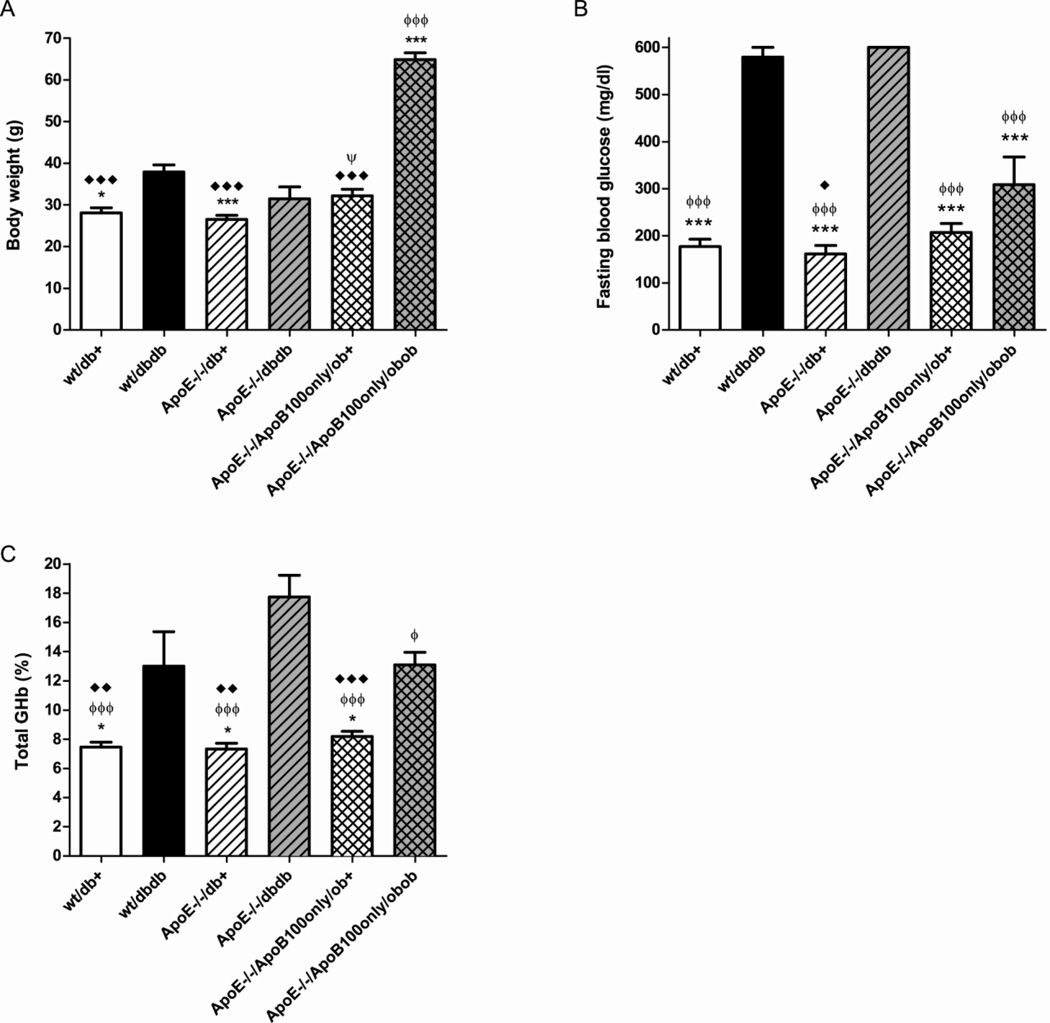
The weights of the mice are shown in Fig. 1A. In mice that were wild-type for ApoE, there was a significant increase in weight between db/+ and db/db mice (p<0.05), reflecting the hyperphagia in mice lacking leptin receptor. This weight increase resulting from loss of leptin signaling was blunted in the ApoE−/− mice. In the mice lacking both ApoE and ApoB48, the ob/+ mice showed no weight abnormality but the ob/ob littermates were significantly heavier than any of the db/db groups (p<0.001).
Figure 1. Body weight and glycemic control and at 24 weeks of age.
Body weights were recorded for all mice (A). Fasting blood samples were collected for blood glucose (B) and glycated hemoglobin (GHb) (C) determination. Data are mean ±SEM of all mice in each genotypic group. *p<0.05, ***p<0.001 vs wt/db/db; ϕ p<0.05, ϕϕϕ p<0.001 vs ApoE−/− db/db; ♦ p<0.05, ♦♦ p<0.01, ♦♦♦ p<0.001 vs ApoE−/−/ApoB100only/ob/ob; Ψ p<0.05 vs ApoE−/−/ApoB100only/ob+.
Glycemic Control
Similar to previous studies in db mouse models, fasting blood glucose was significantly elevated in db/db compared with db/+ littermates (Fig. 1B). There was no difference between mice expressing normal (wt) ApoE and ApoE knockouts in the db mice. The increase in hyperglycemia between ob/+ and ob/ob mice in the group lacking both ApoE and ApoB48 was largely eliminated and there was no significant difference between the ApoE−/−/ApoB100only/ob/+ and ApoE−/−/ApoB100only/ob/ob groups. Despite no significant difference in fasting glucose, there was a significant increase in glycated hemoglobin (GHb) between ApoE−/−/ApoB100only/ob/+ and ApoE−/−/ApoB100only/ob/ob groups (Fig. 1C) (p<0.001). Similar increases in GHb were observed between wt ApoE db/+ and wt ApoE db/db mice and between ApoE−/− db/+ and ApoE−/− db/db mice (Fig. 1C). There was a trend to increased GHb in ApoE knockout db/db mice compared to wild-type ApoE db/db mice but this was not significant, and loss of ApoE did not alter fasting glucose or GHb levels in db/+ mice.
Total plasma lipids (total triglycerides and total cholesterol)
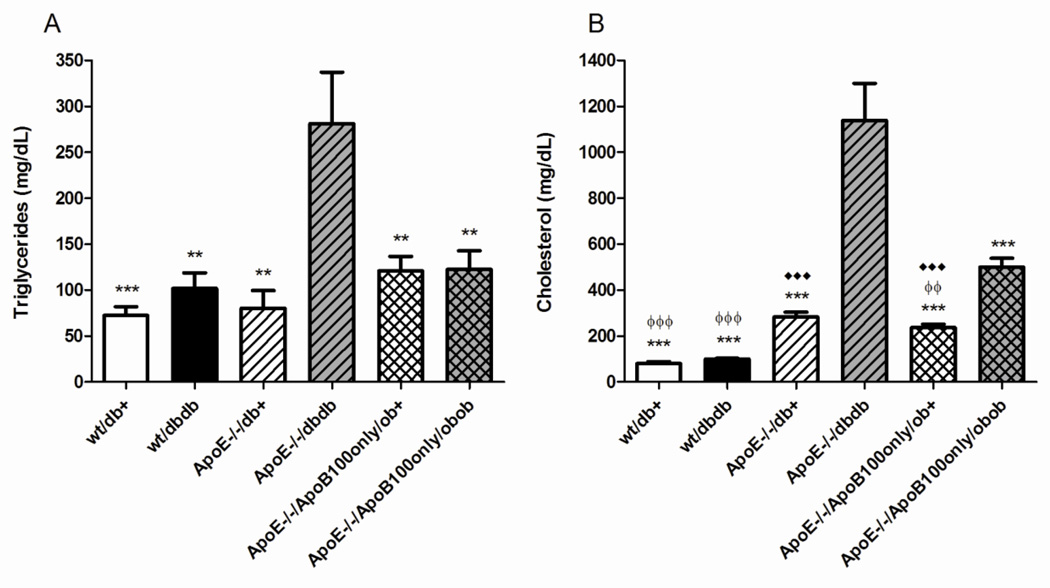
Wild-type mice display typically modest levels of triglycerides (Fig. 2A) and cholesterol (Fig. 2B) as published previously (Lloyd, et al., 2008, Vincent, et al., 2009). In mice wild-type for ApoE but with loss of leptin receptor (db/db), there was a small increase in total lipids (Fig. 2A, Fig. 2B). There was little effect of ApoE knockout alone on total plasma triglycerides (Fig. 2A). Total cholesterol was 3.5-fold increased in these ApoE knockout mice compared with wild-type db/+ mice (p<0.001) (Fig. 2B). In ApoE−/− db/db mice, there was a 3.5- and 4-fold increase in total triglycerides and cholesterol, respectively (p<0.01, p<0.001) (Fig. 2A, Fig. 2B) compared with ApoE knockout alone. In the ApoE−/−/ApoB100only/ob/+ (normoglycemic, non-obese) mice, total plasma triglycerides were not significantly different from wild-type mice, or mice with ApoE knockout alone (Fig. 2A). Cholesterol in the ApoE−/−/ApoB100only/ob/+ mice was 3-fold greater than wt/db+ (p<0.001), but not significantly different from ApoE knockout alone (Fig. 2B). In their ob/ob littermates, there was no significant difference in triglycerides, but a further doubling of total cholesterol (p<0.01) (Fig. 2A, Fig. 2B).
Figure 2. Total plasma lipids at 24 weeks of age.
Total fasting plasma triglycerides (A) and cholesterol (B) for all mice. Data are mean ±SEM. ♦♦♦ p<0.001 vs wt/db+; **p<0.01, ***p<0.001 vs ApoE−/− db/db; ϕϕ p<0.01, ϕϕϕ p<0.001 vs ApoE-/-/ApoB100only/ob/ob.
Plasma Lipid Lipoprotein Profiles
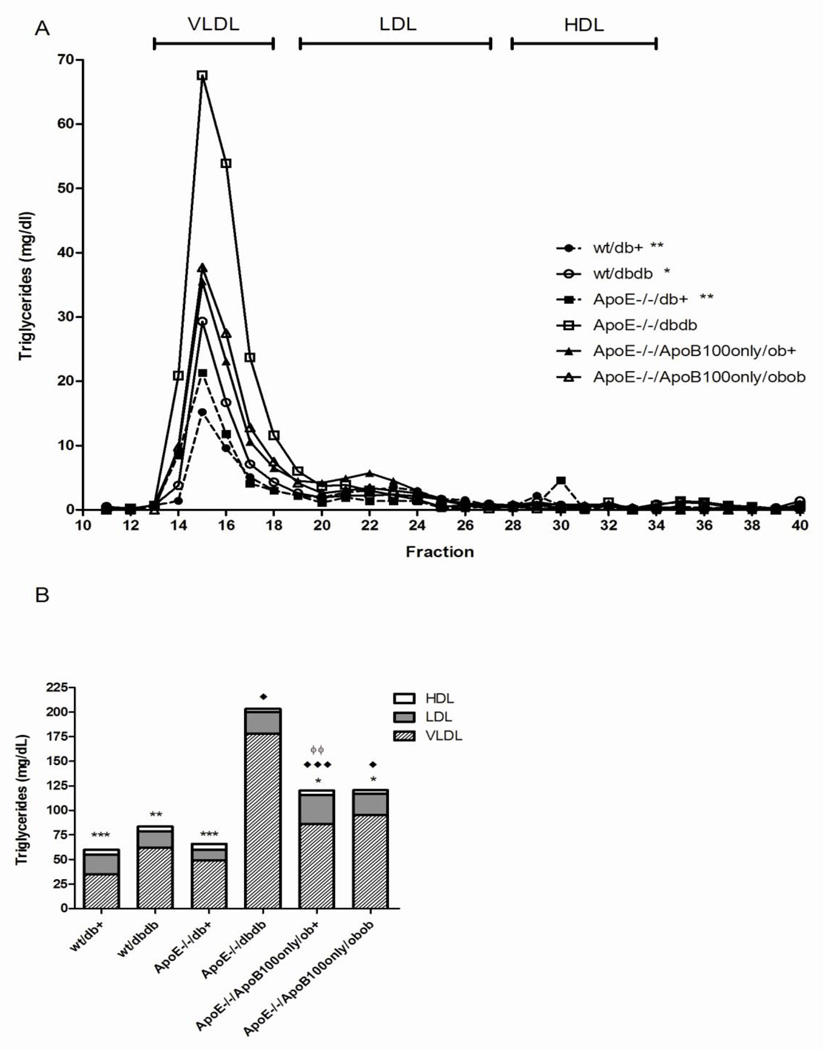
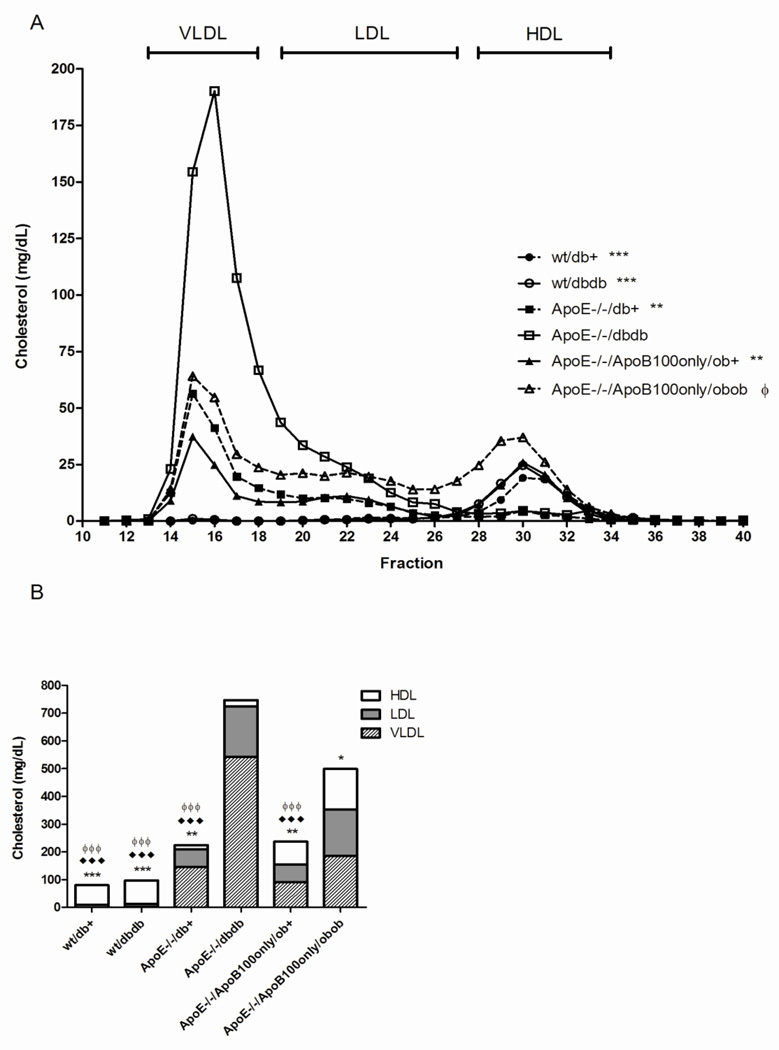
In wild-type mice, 60% of triglyceride lipoproteins were contained in the VLDL fraction and 89% of cholesterol lipoproteins in HDL (Fig. 3, Fig. 4). There was minimal effect of ApoE knockout alone on lipoprotein triglycerides, with slight redistribution from LDL-TG to VLDL-TG (35 mg/dL (59% of total lipoprotein triglycerides) in VLDL in wt/db/+; 49 mg/dL (75% of total lipoprotein triglycerides) in VLDL in ApoE−/− db/+). However, there was a substantial redistribution of cholesterol from the HDL class (decreased to 6.6% of total lipoprotein cholesterol) to the VLDL and LDL classes (increased to 64.6% and 28.8% of total lipoprotein cholesterol, respectively).
Figure 3. Plasma triglyceride profile at 24 weeks of age.
Fasting plasma samples were pooled for each group and fractionated by FPLC. Total triglycerides were measured in each fraction. In A, the concentration of triglycerides is plotted against the fraction number *p<0.05, **p<0.01, ***p<0.001 across the whole curve vs. ApoE−/−db/db. In B, the proportion of triglycerides distributed to each lipoprotein fraction is expressed for each mouse genotype. *p<0.05, **p<0.01, ***p<0.001 VLDL vs. ApoE−/− db/db; ♦ p<0.05, ♦♦♦ p<0.001 LDL vs. ApoE−/− db/+; ϕ p<0.05 LDL vs. db/db.
Figure 4. Plasma cholesterol profile at 24 weeks of age.
Fasting plasma samples were pooled for each group and fractionated by FPLC. Total cholesterol was measured in each fraction. In A, the concentration of cholesterol plotted against the fraction number **p<0.01, ***p<0.001 entire curve vs. ApoE−/− db/db; ϕ p<0.05 entire curve vs. ApoE wt db/+. In B, the proportion of cholesterol distributed to each lipoprotein fraction is expressed for each mouse genotype. *p<0.05, **p<0.01, ***p<0.001 VLDL vs. ApoE−/− db/db; ♦♦p<0.05, ♦♦♦p<0.001 LDL vs. ApoE−/− db/db; ϕ p<0.05 LDL vs. ApoE−/−/ApoB100only/ob/ob.
Compared with ApoE knockout alone, in ApoE−/− db/db mice, the low HDL lipid levels were maintained, but the majority of the lipoprotein triglycerides and cholesterol were sequestered in VLDL particles (Fig. 3, Fig. 4).
In the ApoE−/−/ApoB100only/ob/+ mice, VLDL and LDL triglycerides were elevated (86 mg/dL VLDL, 72% of total lipoprotein triglycerides; 30 mg/dL LDL, 25% of total lipoprotein triglycerides) compared with mice with ApoE knockout alone (ApoE−/− db/+). The elevated total lipoprotein cholesterol observed with ApoE knockout was maintained, with additional redistribution of VLDL-C to HDL-C in the double apolipoprotein knockout mice (91 mg/dL VLDL, 38% of total lipoprotein cholesterol; 84 mg/dL HDL, 35% of total lipoprotein). In their ob/ob littermates (ApoE−/−/ApoB100only/ob/ob), the additional loss of leptin had minimal effects on lipoprotein triglycerides, but was associated with a uniform increase in cholesterol across the lipoprotein classes (186 mg/dL VLDL; 166 mg/dL LDL; 147 mg/dL HDL; 37%, 33%, 29% of total lipoprotein cholesterol, respectively).
In only the ApoE−/−/ mice (with and without the db/db mutation) did the total cholesterol to HDL-C ratio exceed the 6.0, the threshold set for cardiovascular risk (ApoE−/−/db/+, 19.2; ApoE−/− /db/db, 48.8) (Kinosian, et al., 1994).
Behavioral Neuropathy Phenotyping
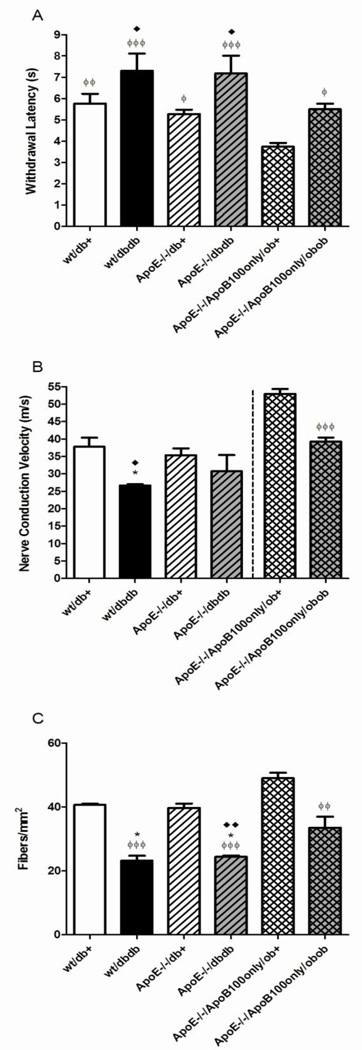
We determined the latency of a tail flick response to a heat stimulus (Fig. 5A). In both wild-type and ApoE−/− models, tail flick latency was increased in db/db compared to db/+ mice (reaching significance for the ApoE−/− model, p<0.05). Comparison of wt/db/+ with ApoE−/−/db/+ or wt/db/db with ApoE−/−/db/db shows loss of ApoE itself did not result in differences in latency. Basal tail flick latency was significantly lower in ApoE−/−/ApoB100only/ob/+ mice compared with all other groups. The relative difference between ApoE−/−/ApoB100only/ob/+ and ApoE−/− /ApoB100only/ob/ob mice was similar to that observed for the db/+ and db/db mice with the ApoE deletion (p<0.05).
Figure 5. Neuropathy phenotyping at 24 weeks of age.
The figure displays the tail flick response to heat stimulus (A), sciatic motor nerve conduction velocity (B), and intraepidermal nerve fiber density (C) for all groups. Data are mean ±SEM. ♦p<0.05, ♦♦p<0.01 vs ApoE−/−/db+; ϕ p<0.05, ϕϕ p<0.01, ϕϕϕ p<0.001 vs ApoE−/−/ApoB100only/ob+; *p<0.05 vs wt/db/+.
Electrophysiological Neuropathy Phenotyping
Sural sensory (not shown) and sciatic motor (Fig. 5B) NCV measurements were performed. Sciatic motor NCV measures presented show the same trends as sural NCV. Between developing the db/db models (prior to 2010) and obtaining the ob/ob model, we changed our methodology for NCV measures due to improved understanding of the effects of anesthesia (Oh, et al., 2010). However, while the absolute measures tend to be lower in the older data in ketamine-anesthetized mice, the statistical differences between groups obtained at the same time are comparable between experiments (Oh, et al., 2010). There was a significant decrease in sciatic motor NCV between db/db mice and their db/+ littermates (p<0.05). Addition of the ApoE deletion to the leptin receptor mutation (db/db) did not significantly affect sciatic motor NCV compared with either ApoE deletion or db/db mutation alone. There was a significant (p<0.001) decrease in sciatic motor NCV between ApoE−/−/ApoB100only/ob/+ mice and their ApoE−/−/ApoB100only/ob/ob littermates.
Morphological Neuropathy Phenotyping
IENFD was calculated as an index of small fiber neuropathy. There was a similar significant decrease in IENFD between db/+ and db/db littermates in mice wild-type for ApoE (41 ± 0.3 fibers/mm2 to 23 ± 2 fibers/mm2, p<0.05) and with ApoE knockout (40 ± 1 fibers/mm2 to 24 ± 0.4 fibers/mm2, p<0.01) (Fig. 5C). In the triple knockout model, there was a significant decrease in IENFD from 49 ± 2 fibers/mm2 in ApoE−/−/ApoB100only/ob/+ to 34 ± 4 fibers/mm2 in ApoE−/−/ApoB100only/ob/ob mice.
Discussion
Attempts to develop a hyperlipidemic mouse that display parallel lipid profiles to human patients with dyslipidemia have had limited success to date (Lloyd, et al., 2008). Unlike humans, mice do not markedly increase atherogenic LDL particles, and instead sequester extra plasma cholesterol in the more protective HDL particles. With this in mind, we explored the metabolic and neuropathy profiles of mice with progressive genetic alterations in an effort to produce a model of diabetic neuropathy with a human-like dyslipidemia, using knockout of the ApoE and ApoB proteins combined with genetic interference of leptin signaling.
Knockout of ApoE alone did not produce a marked increase in body weight, which is not surprising as it is only predicted to shift the plasma lipid profile. However, adding the loss of the leptin receptor to ApoE knockout mice only moderately increased body weight. This was unexpected because mice lacking leptin receptor become obese, secondary to hyperphagia (Sullivan, et al., 2007). Similarly, knockout of ApoE and ApoB48 together did not increase body weight, however, adding loss of leptin (ob/ob) to these mice led to a marked weight gain. On a standard diet, it takes the added genetic deletion of leptin for ApoE knockout mice to develop obesity-associated diabetes. These data support clinical studies examining the relationship of plasma lipids to diet and calorie intake. Patients on a low-fat diet tend to be able to sustain an appropriate calorie intake and maintain a healthy body weight. However, dyslipidemia such as low HDL and high LDL/VLDL caused by endogenous hypertriglyceridemia does not normalize following weight loss (Gonen, et al., 1985). Therefore, dyslipidemia related to genetic determinants has little impact upon, or response to, feeding behavior.
ApoE −/− produced modest hypercholesterolemia, but combined deletion of ApoE and the leptin receptor (db/db) produced severe hypertriglyceridemia and hypercholesterolemia, characterized by huge expansion of VLDL-triglycerides, and a substantial increase in the total cholesterol to HDL-C ratio. This ratio is the strongest lipid ratio index of vascular risk (Millan, et al., 2009) and improved ratios are associated with improved NCV deficits in genetically diabetic mice (Inaba, et al., 1986). In only the ApoE−/−/ mice (with and without the db/db mutation) did the total cholesterol to HDL-C ratio exceed the 6.0 threshold set for cardiovascular risk (ApoE−/−/db/+, 19.2; ApoE−/−/db/db, 48.8) (Kinosian, et al., 1994). Although LDL-C elevation was modest, notably, it was small LDL (fractions 19–23) that was elevated, a factor that is independently correlated with neuropathy in diabetic patients (Isomaa, et al., 2001). We had predicted that the loss of ApoE in combination with ApoB48 would produce more profound dyslipidemia, with increased LDL but not HDL. However, this double apolipoprotein knockout model proved disappointing in its effect on the lipid profiles, even with additional leptin deletion.
Three measures of neuropathy development were utilized. The behavioral tail flick response to a heat stimulus, and electrophysiological assessment of sciatic NCV were employed as measures of sensory and motor neuropathy. Small fiber neuropathy was evaluated by counting IENFD (Cheng, et al., 2011, Smith, et al., 2006, Sullivan, et al., 2008). Analysis of these data showed that mutation of the leptin receptor increases latency to tail flick, and decreases NCV and IENFD, with no additional effects of ApoE deletion. A similar relationship was observed for the triple knockout model, with the leptin deficiency increasing latency to tail flick, and decreasing NCV and IENFD compared with their ob+ littermates.
In contrast to the current study, Fullerton et al. (2008) reported impaired hind paw and tail flick measures of unmyelinated, small fiber function in ApoE knockout mice. The mice used by Fullerton and colleagues were younger (8–14 weeks) and on a pure C57BL/6 background, which may have contributed to the different outcome. Functional deficits observed in the Fullerton study could have been related to weight (Massaro and Massaro, 2008) and in addition, ApoE−/− mice on the mixed C57BL/6/C57BKS background in this study did not exhibit the hypertriglyceridemia found on the C57BL/6 background (Buzello, et al., 2003, Trauner, et al., 2010). As patients with high triglycerides displayed the greatest propensity to develop neuropathy in our clinical study (Wiggin, et al., 2009), the lack of ApoE knockout-associated nerve function deficits on the mixed C57BLKS/C57BL/6 background, is likely related to triglyceride status. Indeed, elevation of plasma triglycerides upon additional mutation of the leptin receptor was associated with impaired tail flick responses in the present study.
We conclude that loss of ApoE does not alter the course of the development of neuropathy in mice, despite previous data that loss of ApoE may promote brain neuron injury (Lane and Farlow, 2005) and retinopathy (Barile, et al., 2005). However, astrocytes and retinal Muller cells are highly immunoreactive for ApoE, while ApoE levels are modest in the PNS (Boyles, et al., 1985), suggesting that loss of ApoE may not severely impact peripheral nerve function. In summary, knockout of ApoE, and additional deletion of ApoB48 are poor strategies to produce a mouse model with the lipid profile changes observed in human diabetics with neuropathy. ApoE knockout combined with leptin receptor knockout produced hyperglycemia, hypertriglyceridemia, hypercholesterolemia, and a lipid profile most closely modeling human dyslipidemia that promotes neuropathy, i.e. increased triglycerides with an expansion of LDL-cholesterol and decrease in HDL-cholesterol. ApoE knockout combined with additional ApoB48 and leptin knockout produced similar changes of smaller magnitude, but, importantly, an increase in HDL-cholesterol.
Conclusion
Our data suggest that the overall effects of ApoE and ApoB48 knockout, either directly upon nerve structure and function or indirectly on lipid metabolism, are not sufficient to significantly alter the course of diabetic neuropathy. Although the combination of ApoE knockout and leptin receptor deficiency (db/db) did not exacerbate the neuropathic phenotype, of the models tested it produces neuropathy and a lipid profile most closely modeling that of human patients with diabetic neuropathy. It is possible that because the hyperlipidemia in the ApoE−/−/db/db mice reflects a genetic defect, rather than the acquired defect observed in patients with diabetes, the lack of an exacerbated phenotype is not unexpected. This is further supported by the fact that individuals with inherited dyslipidemia either do not develop a neuropathy (Gotoda, et al., 2012) or develop a mild neuropathy later in life (McManis, et al., 1994). Although glycemia and specific lipid metabolic defects are known to be associated with neuropathic deficits, these apolipoprotein knockout models ultimately do not deliver optimal lipid profiles for translational diabetic neuropathy research, i.e. increased triglycerides with dramatic expansion of LDL-cholesterol. However, these mice represent models with glycemic and lipid profile features that may be useful in testing therapies targeted for the human condition.
Highlights.
We employed genetic manipulations to produce improved models of diabetic neuropathy.
db/db and ob/ob genotypes had hyperglycemia, dyslipidemia and evidence of neuropathy.
Apolipoprotein knockout mice had dyslipidemia, but normoglycemia and no neuropathy.
Apolipoprotein knockout combined with db/db or ob/ob exacerbated dyslipidemia.
Combined apolipoprotein and leptin signaling mutations did not exacerbate neuropathy.
Acknowledgments
This work was supported by NIH 1 R24 DK082841-01 (Feldman and Pennathur), NIH 1 UO1 DK076160 (Feldman), NIH 1RC 1NS068182 (Feldman), The A. Alfred Taubman Institute and The Program for Neurology Research and Discovery. This work utilized the Michigan Diabetes Research and Training Center at the University of Michigan, Ann Arbor, Michigan for GHb measurements, and the Mouse Metabolic Phenotyping Center Core at the University of Washington, Seattle, Washington (U24 DK076126) for plasma lipid measurements. We thank Dr. A. M. Heacock and Dr. C. Sims-Robinson for critical review of the manuscript.
Abbreviations
- DN
diabetic neuropathy
- IENFD
intraepidermal nerve fiber density
- NCV
nerve conduction velocity
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ansquer JC, Foucher C, Aubonnet P, Le Malicot K. Fibrates and microvascular complications in diabetes--insight from the FIELD study. Curr Pharm Des. 2009;15:537–552. doi: 10.2174/138161209787315701. [DOI] [PubMed] [Google Scholar]
- 2.Barile GR, Pachydaki SI, Tari SR, Lee SE, Donmoyer CM, Ma W, Rong LL, Buciarelli LG, Wendt T, Horig H, Hudson BI, Qu W, Weinberg AD, Yan SF, Schmidt AM. The RAGE axis in early diabetic retinopathy. Invest Ophthalmol Vis Sci. 2005;46:2916–2924. doi: 10.1167/iovs.04-1409. [DOI] [PubMed] [Google Scholar]
- 3.Boyles JK, Pitas RE, Wilson E, Mahley RW, Taylor JM. Apolipoprotein E associated with astrocytic glia of the central nervous system and with nonmyelinating glia of the peripheral nervous system. J Clin Invest. 1985;76:1501–1513. doi: 10.1172/JCI112130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Buzello M, Tornig J, Faulhaber J, Ehmke H, Ritz E, Amann K. The apolipoprotein e knockout mouse: a model documenting accelerated atherogenesis in uremia. J Am Soc Nephrol. 2003;14:311–316. doi: 10.1097/01.asn.0000045048.71975.fc. [DOI] [PubMed] [Google Scholar]
- 5.Cheng HT, Dauch JR, Hayes JM, Yanik BM, Feldman EL. Nerve growth factor/p38 signaling increases intraepidermal nerve fiber densities in painful neuropathy of type 2 diabetes. Neurobiol Dis. 2011 doi: 10.1016/j.nbd.2011.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clemens A, Siegel E, Gallwitz B. Global risk management in type 2 diabetes: blood glucose, blood pressure, and lipids--update on the background of the current guidelines. Exp Clin Endocrinol Diabetes. 2004;112:493–503. doi: 10.1055/s-2004-821306. [DOI] [PubMed] [Google Scholar]
- 7.Donahue JE, Johanson CE. Apolipoprotein E, amyloid-beta, and blood-brain barrier permeability in Alzheimer disease. J Neuropathol Exp Neurol. 2008;67:261–270. doi: 10.1097/NEN.0b013e31816a0dc8. [DOI] [PubMed] [Google Scholar]
- 8.Drory VE, Groozman GB, Rubinstein A, Korczyn AD. Hypertriglyceridemia may cause a subclinical peripheral neuropathy. Electromyogr Clin Neurophysiol. 1999;39:39–41. [PubMed] [Google Scholar]
- 9.Fullerton SM, Strittmatter WJ, Matthew WD. Peripheral sensory nerve defects in apolipoprotein E knockout mice. Exp Neurol. 1998;153:156–163. doi: 10.1006/exnr.1998.6872. [DOI] [PubMed] [Google Scholar]
- 10.Gonen B, Patsch W, Kuisk I, Goldberg A, Phair R, Schonfeld G. Altered HDL subclasses in endogenous hypertriglyceridemia are not affected by weight reduction. Metabolism. 1985;34:494–501. doi: 10.1016/0026-0495(85)90217-3. [DOI] [PubMed] [Google Scholar]
- 11.Gotoda T, Shirai K, Ohta T, Kobayashi J, Yokoyama S, Oikawa S, Bujo H, Ishibashi S, Arai H, Yamashita S, Harada-Shiba M, Eto M, Hayashi T, Sone H, Suzuki H, Yamada N. Diagnosis and management of type I and type V hyperlipoproteinemia. J Atheroscler Thromb. 2012;19:1–12. doi: 10.5551/jat.10702. [DOI] [PubMed] [Google Scholar]
- 12.Inaba H, Kato K, Yano T. [Effect of trapidil on neuropathy in diabetic mice] Nihon Yakurigaku Zasshi. 1986;88:95–99. doi: 10.1254/fpj.88.95. [DOI] [PubMed] [Google Scholar]
- 13.Isomaa B, Henricsson M, Almgren P, Tuomi T, Taskinen MR, Groop L. The metabolic syndrome influences the risk of chronic complications in patients with type II diabetes. Diabetologia. 2001;44:1148–1154. doi: 10.1007/s001250100615. [DOI] [PubMed] [Google Scholar]
- 14.Kassem HS, Azar ST, Zantout MS, Sawaya RA. Hypertriglyceridemia and peripheral neuropathy in neurologically asymptomatic patients. Neuro Endocrinol Lett. 2005;26:775–779. [PubMed] [Google Scholar]
- 15.Kern TS, Berkowitz BA, Feldman EL. National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) meeting summary: Advances toward measuring diabetic retinopathy and neuropathy: from the bench to the clinic and back again (April 4–5, 2007, Baltimore, Maryland) J Diabetes Complications. 2009;23:219–223. doi: 10.1016/j.jdiacomp.2007.11.001. [DOI] [PubMed] [Google Scholar]
- 16.Kinosian B, Glick H, Garland G. Cholesterol and coronary heart disease: predicting risks by levels and ratios. Ann Intern Med. 1994;121:641–647. doi: 10.7326/0003-4819-121-9-199411010-00002. [DOI] [PubMed] [Google Scholar]
- 17.Lane RM, Farlow MR. Lipid homeostasis and apolipoprotein E in the development and progression of Alzheimer's disease. J Lipid Res. 2005;46:949–968. doi: 10.1194/jlr.M400486-JLR200. [DOI] [PubMed] [Google Scholar]
- 18.Lee JH, Cox DJ, Mook DG, McCarty RC. Effect of hyperglycemia on pain threshold in alloxan-diabetic rats. Pain. 1990;40:105–107. doi: 10.1016/0304-3959(90)91057-P. [DOI] [PubMed] [Google Scholar]
- 19.Lloyd DJ, McCormick J, Helmering J, Kim KW, Wang M, Fordstrom P, Kaufman SA, Lindberg RA, Veniant MM. Generation and characterization of two novel mouse models exhibiting the phenotypes of the metabolic syndrome: Apob48-/-Lepob/ob mice devoid of ApoE or Ldlr. Am J Physiol Endocrinol Metab. 2008;294:E496–E505. doi: 10.1152/ajpendo.00509.2007. [DOI] [PubMed] [Google Scholar]
- 20.Mahley RW. Apolipoprotein E: cholesterol transport protein with expanding role in cell biology. Science. 1988;240:622–630. doi: 10.1126/science.3283935. [DOI] [PubMed] [Google Scholar]
- 21.Massaro D, Massaro GD. Apoetm1Unc mice have impaired alveologenesis, low lung function, and rapid loss of lung function. Am J Physiol Lung Cell Mol Physiol. 2008;294:L991–L997. doi: 10.1152/ajplung.00013.2008. [DOI] [PubMed] [Google Scholar]
- 22.McManis PG, Windebank AJ, Kiziltan M. Neuropathy associated with hyperlipidemia. Neurology. 1994;44:2185–2186. doi: 10.1212/wnl.44.11.2185. [DOI] [PubMed] [Google Scholar]
- 23.McMillen TS, Heinecke JW, LeBoeuf RC. Expression of human myeloperoxidase by macrophages promotes atherosclerosis in mice. Circulation. 2005;111:2798–2804. doi: 10.1161/CIRCULATIONAHA.104.516278. [DOI] [PubMed] [Google Scholar]
- 24.Millan J, Pinto X, Munoz A, Zuniga M, Rubies-Prat J, Pallardo LF, Masana L, Mangas A, Hernandez-Mijares A, Gonzalez-Santos P, Ascaso JF, Pedro-Botet J. Lipoprotein ratios: Physiological significance and clinical usefulness in cardiovascular prevention. Vasc Health Risk Manag. 2009;5:757–765. [PMC free article] [PubMed] [Google Scholar]
- 25.Oh SS, Hayes JM, Sims-Robinson C, Sullivan KA, Feldman EL. The effects of anesthesia on measures of nerve conduction velocity in male C57Bl6/J mice. Neurosci Lett. 2010;483:127–131. doi: 10.1016/j.neulet.2010.07.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Poirier J. Apolipoprotein E and Alzheimer's disease. A role in amyloid catabolism. Ann N Y Acad Sci. 2000;924:81–90. doi: 10.1111/j.1749-6632.2000.tb05564.x. [DOI] [PubMed] [Google Scholar]
- 27.Singleton JR, Smith AG, Bromberg MB. Painful sensory polyneuropathy associated with impaired glucose tolerance. Muscle Nerve. 2001;24:1225–1228. doi: 10.1002/mus.1136. [DOI] [PubMed] [Google Scholar]
- 28.Smith AG, Russell J, Feldman EL, Goldstein J, Peltier A, Smith S, Hamwi J, Pollari D, Bixby B, Howard J, Singleton JR. Lifestyle intervention for pre-diabetic neuropathy. Diabetes Care. 2006;29:1294–1299. doi: 10.2337/dc06-0224. [DOI] [PubMed] [Google Scholar]
- 29.Sullivan KA, Hayes JM, Wiggin TD, Backus C, Su OhS, Lentz SI, Brosius F, 3rd, Feldman EL. Mouse models of diabetic neuropathy. Neurobiol Dis. 2007;28:276–285. doi: 10.1016/j.nbd.2007.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sullivan KA, Lentz SI, Roberts JL, Jr, Feldman EL. Criteria for creating and assessing mouse models of diabetic neuropathy. Curr Drug Targets. 2008;9:3–13. doi: 10.2174/138945008783431763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Trauner M, Claudel T, Fickert P, Moustafa T, Wagner M. Bile acids as regulators of hepatic lipid and glucose metabolism. Dig Dis. 2010;28:220–224. doi: 10.1159/000282091. [DOI] [PubMed] [Google Scholar]
- 32.Vincent AM, Hayes JM, McLean LL, Vivekanandan-Giri A, Pennathur S, Feldman EL. Dyslipidemia-induced neuropathy in mice: the role of oxLDL/LOX-1. Diabetes. 2009;58:2376–2385. doi: 10.2337/db09-0047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vincent AM, Hinder LM, Pop-Busui R, Feldman EL. Hyperlipidemia: a new therapeutic target for diabetic neuropathy. J Peripher Nerv Syst. 2009;14:257–267. doi: 10.1111/j.1529-8027.2009.00237.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wiggin TD, Sullivan KA, Pop-Busui R, Amato A, Sima AA, Feldman EL. Elevated triglycerides correlate with progression of diabetic neuropathy. Diabetes. 2009;58:1634–1640. doi: 10.2337/db08-1771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang H, Wu LM, Wu J. Cross-talk between apolipoprotein E and cytokines. Mediators Inflamm. 2011:949072. doi: 10.1155/2011/949072. [DOI] [PMC free article] [PubMed] [Google Scholar]