Abstract
The C-terminal 69 residues of the J-protein Zuo1 are sufficient to activate Pdr1, a transcription factor involved in both pleiotropic drug resistance (PDR) and growth control. Little is understood about the pathway of activation by this primarily ribosome-associated Hsp40 co-chaperone. Here we report that only the C-terminal 13 residues of Zuo1 are required for activation of Pdr1, with hydrophobic residues being critical for activity. Two-hybrid interaction experiments suggest that the interaction between this 13-residue Zuo1 peptide and Pdr1 is direct, analogous to the activation of Pdr1 by xenobiotics. However, simply dissociation of Zuo1 from the ribosome is not sufficient for induction of Pdr1 transcriptional activity, as the C-terminal 86 residues of Zuo1 fold into an autoinhibitory left-handed four-helix bundle. Hydrophobic residues critical for interaction with Pdr1 are sequestered within the structure of this C-terminal domain (CTD), necessitating unfolding for activation. Thus, although expression of the CTD does not result in activation, alterations that destabilize the structure cause induction of PDR. These destabilizing alterations also result in dissociation of the full-length protein from the ribosome. Thus, our results are consistent with an activation pathway in which unfolding of Zuo1’s C-terminal helical bundle domain results in ribosome dissociation followed by activation of Pdr1 via a direct interaction.
Keywords: molecular chaperone, Hsp40, PDR, pleiotropic drug resistance, ribosome
Introduction
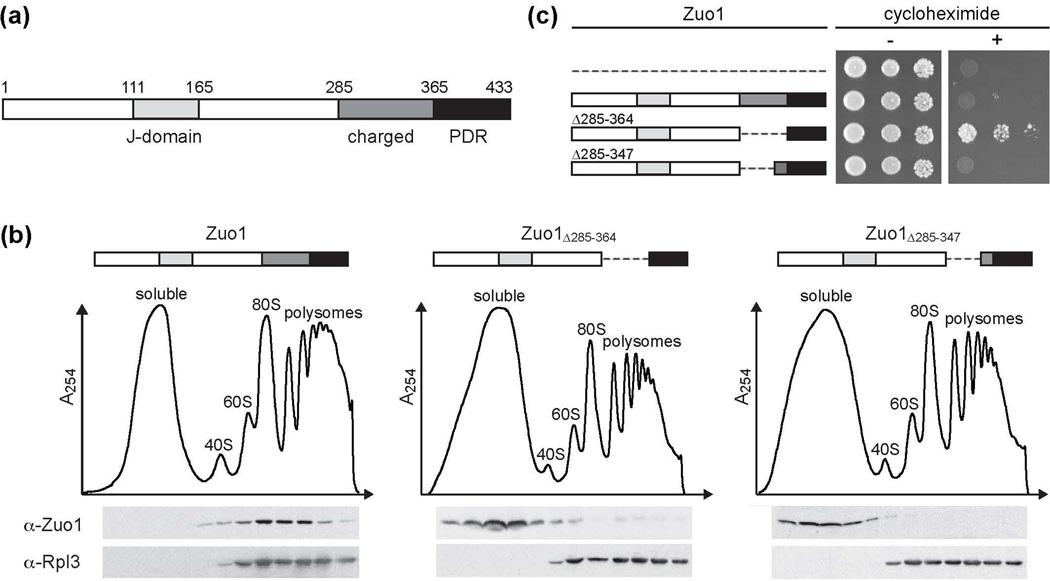
The eukaryote-specific J-protein Zuo1 is an Hsp70 co-chaperone that is primarily associated with ribosomes and widely accepted to play an important role in the folding of nascent polypeptides1–3. However, evidence from several organisms indicates that Zuo1 also has a direct role in transcriptional regulation4,5. In Saccharomyces cerevisiae, Zuo1 has been identified as an activator of the zinc cluster transcription factor (TF) Pdr16,7. Zuo1 is a 433 amino acid protein, however, the 69 residues at Zuo1’s C-terminus (Zuo1365–433) are sufficient to activate Pdr1-dependent transcription6,7 (Fig. 1a). The remainder of the protein contains regions, such as the J-domain, known to be important for its ribosome-associated role in protein folding2,3,8. Particularly relevant to this report, the segment adjacent to the Pdr1-activating region is a positively charged RNA-binding region necessary for association with the ribosome.
Figure 1.
Dissociation of Zuo1 from the ribosome is not sufficient for Pdr1 activation. (a) Domain architecture of the J-protein Zuo1. (b) Δzuo1 cells were transformed with vector containing DNA encoding Zuo1 or variants of Zuo1 lacking portions of the charged region under control of the endogenous ZUO1 promoter. Lysates of cells harboring the indicated plasmids were separated on 5–50% sucrose gradients and fractions were collected and analyzed by immunoblotting for the presence of Zuo1 and Rpl3. The migration of ribosomal subunits was monitored by absorbance at 254 nm and plotted versus the time course of fraction collection. (c) Serial dilutions of wild-type (wt) cells harboring either empty vector (dotted line) or vector containing DNA encoding the indicated Zuo1 variants were spotted onto medium without (−) or with (+) cycloheximide.
Pdr1, like many TFs, has a C-terminal activation domain that has minimal activity until activated by a specific signal9,10. Activation by either Zuo1 or a variety of xenobiotics initiates a highly specific transcriptional response, upregulating a set of genes belonging to the pleiotropic drug resistance (PDR) regulon6,11,12. Pdr1 target genes include ATP-binding cassette (ABC) transporters, such as Pdr5 and Snq2. These transporters extrude xenobiotics from cells, rendering them resistant to a variety of toxic compounds13,14. Evidence indicates that the PDR pathway also functions in growth regulation, perhaps by extruding small molecules sensed by neighboring cells6,15.
Pdr1 is constitutively bound to DNA and not known to shuttle between the nucleus and cytosol16–18. The C-terminus of Zuo1 interacts with Pdr1 in yeast two-hybrid assays, suggesting that Zuo1 activates Pdr1 directly6. Since Zuo1 is primarily associated with the ribosome, this localization raises the question of the pathway of activation. Here, we report that the C-terminal domain (CTD) of Zuo1 forms an autoinhibitory four-helix bundle sequestering residues critical for Pdr1 activation. Unfolding of the CTD causes both dissociation of Zuo1 from the ribosome and release of autoinhibition necessary for PDR activation.
Results
Dissociation of Zuo1 from the ribosome is not sufficient for Pdr1 activation
We previously reported that a Zuo1 variant lacking the ribosome-binding region (Zuo1Δ285–364) was competent to activate Pdr1, but full-length Zuo1 was not, even when overexpressed7. This observation raised the question as to whether dissociation from the ribosome is sufficient for activation. Thus, we made constructs encoding variants having smaller deletions within the ribosome-binding region to test both ribosome association using sucrose gradient centrifugation and ability to induce PDR by plating on medium containing the drug cycloheximide. One variant, Zuo1Δ285–347, which has the same N-terminal deletion boundary as Zuo1Δ285–364, but retains 17 more residues at the C-terminal boundary, was not ribosome associated (Fig 1b). However, cells expressing Zuo1Δ285–347 did not grow on drug-containing plates, while Zuo1Δ285–364-expressing cells grew as expected (Fig. 1c). We conclude that dissociation of Zuo1 from the ribosome is not sufficient to activate PDR.
Residues 348 to 364 inhibit the ability of Zuo1’s C-terminus to activate Pdr1
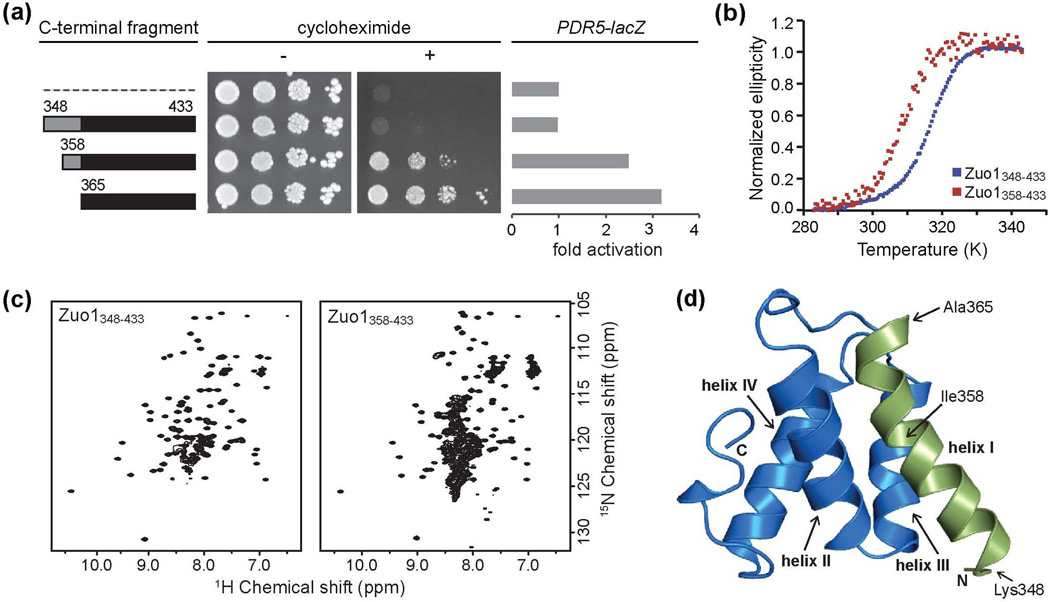
The only difference between the inactive Zuo1Δ285–347 and active Zuo1Δ285–364 is the presence of 17 residues (348-364) in the former. Zuo1365–433, the region immediately C-terminal to these 17 residues, is sufficient to activate Pdr1. Therefore, we reasoned that the inactivity of Zuo1Δ285–347 might be due to an inhibitory effect of these 17 residues. To test this idea, we created a construct encoding a tandem affinity purification (TAP)-tagged fusion analogous to TAPZuo1365-433, which we previously reported to be competent to induce PDR, that included these 17 residues (TAP-Zuo1348–433). Unlike cells expressing TAP-Zuo1365–433, cells expressing the longer TAP-Zuo1348–433 fusion showed no observable drug resistance (Fig. 2a, Supplementary Fig. 1a). We also assessed the ability of the TAP-Zuo1 fusions to activate the promoter of a Pdr1 target gene, PDR5, using a PDR5-lacZ fusion. Results were consistent with the drug resistance test. Cells expressing TAP-Zuo1365–433 had 3.2-fold higher levels of β-galactosidase than control cells expressing only the TAP tag, but the TAP-Zuo1348–433 fusion showed no activity above this background control level (Fig. 2a). Since our results were consistent with an inhibitory role for residues 348-364, we also made an intermediate construct that encoded TAP-Zuo1358–433. TAPZuo1358-433-expressing cells grew in the presence of cycloheximide, but more slowly than those expressing TAP-Zuo1365–433 (Fig. 2a, Supplementary Fig. 1a), suggesting partial inhibition of activity. Expression from the PDR5 promoter was also intermediate, with TAP-Zuo1358–433-expressing cells showing 2.5-fold activation compared to the 3.2-fold activation observed with the shorter fragment. These data indicate that residues 348-364 prevent activation of Pdr1 by the C-terminus of Zuo1. Thus sequences sufficient for both transcriptional activation and inhibition of activity are contained within the last 86 residues of Zuo1.
Figure 2.
The C-terminal 86 residues of Zuo1 form an autoinhibitory four-helix bundle. (a) Residues 348-364 inhibit C-terminal activity. Wt cells were transformed with vector (pRS415-GPD) containing DNA encoding a tandem affinity purification (TAP) tag alone (dotted line) or TAP fused to the indicated fragments of the C-terminus of Zuo1. Serial dilutions of cells harboring the indicated plasmids were spotted onto medium without (−) or with (+) cycloheximide. β-galactosidase activity was measured for cells containing an integrated PDR5-lacZ reporter and transformed with the indicated plasmids. The average activity of 3 transformants of each was quantified and reported as fold activation over cells expressing TAP alone. (b) Circular dichroism thermal melting curves of the inactive Zuo1348–433 fragment (blue) and the partially active Zuo1358–433 fragment (red) collected at 222 nm. (c) Two-dimensional 15N–1H heteronuclear single quantum coherence (HSQC) spectra of Zuo1348–433 and Zuo1358–433. (d) Ribbon diagram of the solution structure of the four-helix bundle formed by the C-terminal 86 residues of Zuo1 (348-433), referred to as the C-terminal domain (CTD). Residues 348-364, which inhibit C-terminal activity, are shown in green.
Inactive and active C-terminal fragments differ in both stability and fold
To better understand the autoinhibitory effect of residues 348-364, we initiated a biochemical characterization of the Zuo1 C-terminal fragments. We were unable to obtain sufficient quantities of Zuo1365–433. Therefore, we compared the partially active Zuo1358–433 and inactive Zuo1348–433 fragments. The melting temperatures of Zuo1348–433 and Zuo1358–433, determined using circular dichroism, were substantially different, 43.5 °C and 35.5 °C, respectively (Fig. 2b). Thus removal of N-terminal residues, which resulted in partial activity, also resulted in a decrease in thermal stability. Analysis of the 15N-1H heteronuclear single quantum coherence (HSQC) NMR spectra of Zuo1348–433 revealed chemical shift dispersion and uniform peak intensity consistent with a single folded domain (Fig 2c). The 15N-1H HSQC of the Zuo1358–433 sample, on the other hand, contained approximately twice the number of expected peaks, suggesting the presence of multiple structural populations. These data indicate that C-terminal fragments of Zuo1 that differ in their in vivo activity also differ in both stability and fold, suggesting the possibility that a structural transition in Zuo1’s C-terminus is responsible for activation of the protein’s transcriptional activity.
Autoinhibited C-terminus is a four-helix bundle
To understand the structural basis for this autoinhibition, we determined the solution structure of Zuo1348–433 using an automated procedure for iterative NOE assignment (Table 1). Zuo1348–433 folds into a left-handed four-helix bundle (Fig. 2d, Supplementary Fig. 1b). To assure that sequences immediately N-terminal do not substantially affect the structure of the helical bundle, we generated two longer constructs extended by 13 or 32 residues. An overlay of the 15N-1H HSQC spectra of Zuo1335–433 and Zuo1306–433 with that of Zuo1348–433 revealed the addition of only random coil peaks and showed no significant chemical shift perturbations between these fragments (Supplementary Fig. 1c), suggesting that the four-helix bundle formed by Zuo1348–433, which we refer to as the C-terminal domain (CTD), forms whether or not adjacent N-terminal residues are present.
Table 1.
NMR and refinement statistics for the 20 Zuo1348-433 conformers
| Experimental constraints | ||
| Distance constraints | ||
| Long [|i − j| > 5] | 318 | |
| Medium [1 < |i − j| ≤ 5] | 463 | |
| Sequential [|i − j| = 1] | 272 | |
| Intraresidue [i = j] | 418 | |
| Total | 1471 | |
| Dihedral angle constraints (ϕ and ψ) | 148 | |
| Average atomic RMSD to the mean structure (Å) | ||
| (Residues 349-433) | ||
| Backbone (Cα, C′, N) | 0.44 ± 0.08 | |
| Heavy atoms | 0.87 ± 0.06 | |
| Deviations from idealized covalent geometry | ||
| Bond lengths (Å) | 0.014 | |
| Torsion angles (°) | 1.2 | |
| Constraint violations | ||
| NOE distance | Number > 0.3 Å | 0.00 ± 0.00 |
| NOE distance | RMSD (Å) | 0.021 ± 0.001 |
| Torsion-angle violations | Number > 5 ° | 0.00 ± 0.00 |
| Torsion-angle violations | RMSD (°) | 0.456 ± 0.062 |
| WHATCHECK quality indicators | ||
| Z-score | 2.90 ± 0.14 | |
| RMS Z-score | ||
| Bond lengths | 0.62 ± 0.02 | |
| Bond angles | 0.58 ± 0.03 | |
| Bumps | 0.00 ± 0.00 | |
| Lennard-Jones energy (kJ mol−1) | −1,694 ± 72 | |
| Ramachandran statistics (% of all residues) | ||
| Most favored | 91.9 ± 1.5 | |
| Additionally allowed | 6.6 ± 1.5 | |
| Generously allowed | 0 ± 0 | |
| Disallowed | 1.5 ± 0.6 | |
Residues 348-364, which we identified as being inhibitory to Zuo1’s transcriptional activation activity, form the first helix of the bundle (Fig. 2d, green). Ile358, the N-terminal residue of the partially active Zuo1358–433 fragment, is located in the center of helix I and buried within the structure. Comparison of the 15N-1H HSQC spectra of Zuo1358–433 with that of the inactive CTD revealed a very similar pattern of dispersed peaks (Fig. 2c, Supplementary Fig. 1d), suggesting that the structured protein present in the partially active Zuo1358–433 sample is similar in conformation to that of the inactive CTD. The additional peaks observed in the Zuo1358–433 sample appear to be predominantly clustered around the random coil chemical shift value of ~8.2 ppm, suggesting that a significant population of unfolded protein is present in the sample. These data are consistent with the idea that helix I is critical for stability of the CTD and that truncation of the N-terminal half of the helix results in partial destabilization.
Unfolding of Zuo1’s C-terminal domain releases autoinhibition
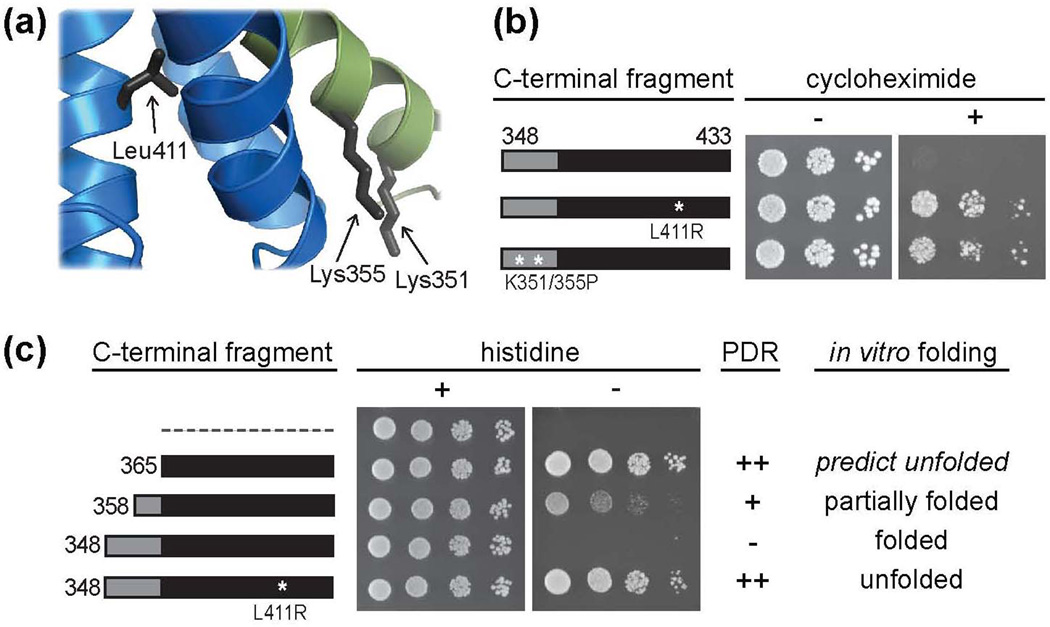
Our in vivo data indicate that Zuo1 is only fully active to induce PDR in the absence of helix I, which is integral to the CTD fold. Thus, we hypothesized that the C-terminus of Zuo1 activates Pdr1 in an unfolded conformation and that C-terminal sequences required to form the helical bundle structure cause autoinhibition. To test this idea, we designed two amino acid alterations aimed at destabilizing the fold of the domain: (1) the buried hydrophobic residue, Leu411, was replaced with the charged residue Arg and (2) Lys351 and Lys355 were replaced with prolines with the goal of preventing helix I from folding properly (Fig. 3a). We analyzed the 15N-1H HSQC spectra of these variants. The spectra of both Zuo1348–433 L411R and Zuo1348–433 K351/355P showed poor peak dispersion with the majority of peaks clustered around the random coil chemical shift value (Supplementary Fig. 2a), indicating that these amino acid alterations were sufficient to prevent folding of the CTD.
Figure 3.
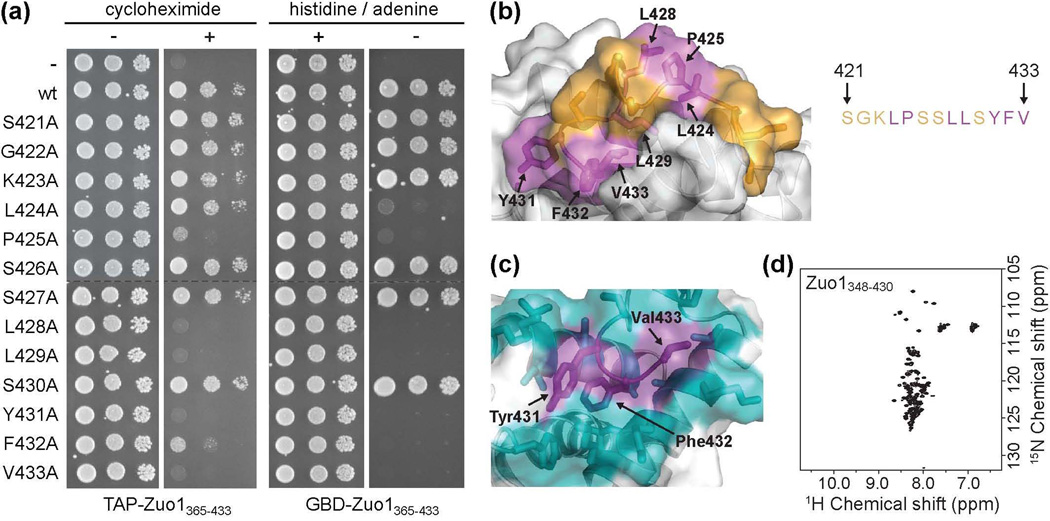
Unfolding of Zuo1’s C-terminal helical bundle releases autoinhibition. (a) Ribbon diagram of a portion of the CTD with side chains of residues predicted to disrupt domain structure upon substitution indicated. (b) Wt cells were transformed with DNA encoding TAP tag fusions of the CTD with or without a destabilizing L411R or K351/355P alteration. Serial dilutions of cells containing the indicated plasmids were spotted onto medium without (−) or with (+) cycloheximide. (c) Modified yeast two-hybrid and correlation between protein fold and PDR induction. Cells containing an integrated Gal1-HIS3 reporter were transformed with DNA encoding the Gal4 DNA binding domain (GBD) alone (dotted line) or GBD fused to fragments of the C-terminus of Zuo1, with the N-terminal residue of each fragment indicated. Transformants were spotted in serial dilutions onto medium with (+) or without (−) histidine. Table includes summarized PDR induction data from Fig. 2a and Fig. 3b with (++) representing a high level of induction and (−) representing no induction and summarized in vitro folding data from Fig. 2c and Supplementary Fig. 2a. Zuo1365–433 is predicted to be unfolded (see text).
To test in vivo activity, we assessed the ability of TAP-Zuo1348–433 L411R and TAP-Zuo1348-433 K351/355P to induce PDR. Both were competent to render cells resistant to cycloheximide (Fig. 3b, Supplementary Fig. 2b). As a second in vivo test, we used a modified yeast two-hybrid assay that we described previously6. In this assay, Zuo1365–433 was tethered to GAL1 promoters by expressing it as a fusion to the DNA binding domain of Gal4 (GBD). This fusion auto-activated transcription in a Pdr1-dependent manner, presumably by recruiting Pdr1, which in turn recruits RNA polymerase. Activity was assessed by growth on medium lacking histidine in a test strain containing a fusion between the GAL1 promoter and the HIS3 gene. We generated a GBD fusion construct analogous to GBD-Zuo1365–433 but containing the entire CTD, either having or lacking the L411R mutation that prevents folding. Cells expressing GBD-Zuo1348–433 did not form colonies (Fig. 3c), though expressed at expected levels (Supplementary Fig. 2c). However, cells expressing GBD-Zuo1348–433 L411R grew as well as GBD-Zuo1365–433-expressing cells. We also tested a GBD fusion of Zuo1 residues 358-433, the fragment that had a mixture of folded and unfolded conformations in vitro. Cells expressing GBD-Zuo1358–433 formed colonies, but grew more poorly than cells expressing the shorter GBD-Zuo1365–433 fusion (Fig. 3c, Supplementary Fig. 2c). Together our data strongly support the idea that Zuo1’s C-terminus activates Pdr1 in an unfolded conformation.
The C-terminal 13 residues of Zuo1 are necessary and sufficient for Pdr1 activation
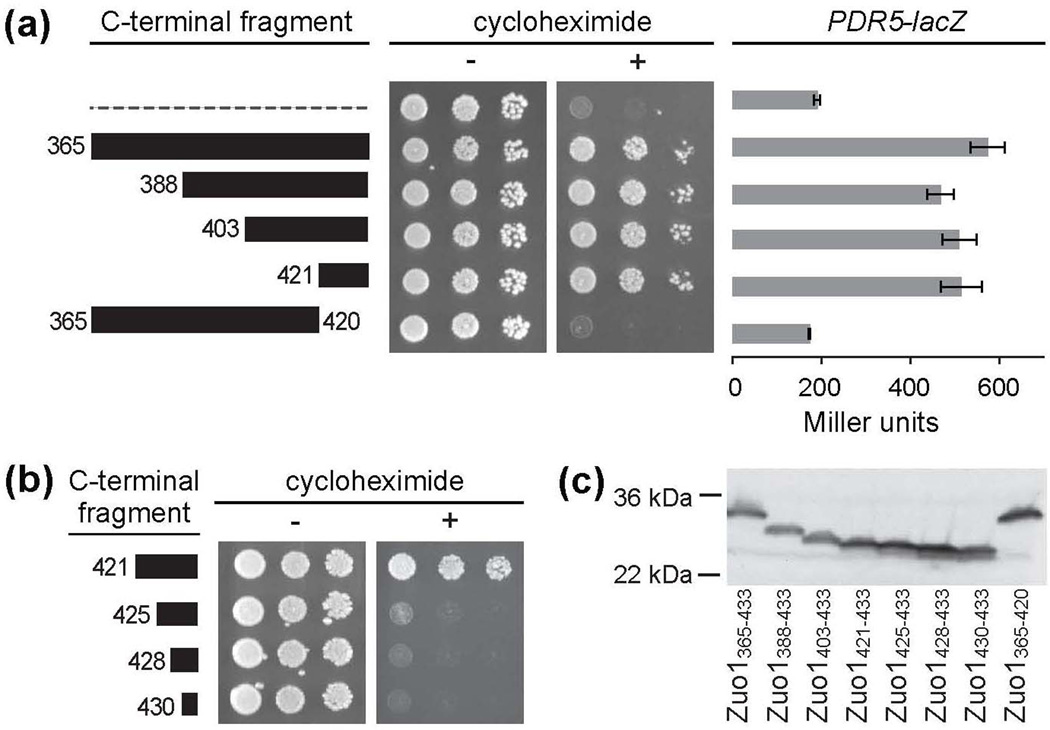
While our results indicate that unfolding of Zuo1’s CTD is required for activation of Pdr1 and that the C-terminal 69 residues (365-433) are sufficient for this activity, the residues necessary for activity were not known. Thus, we constructed a series of truncations to generate TAP-tag fusions beginning at residues 388, 403 and 421, but all ending at C-terminal residue 433, to test whether a smaller segment is sufficient. All shorter C-terminal fragments tested enabled cells to grow similarly to those expressing TAP-Zuo1365–433 on cycloheximide-containing plates (Fig. 4a). Also, β-galactosidase activities in cells expressing each of the shorter C-terminal truncations were statistically indistinguishable from the activity observed in TAP-Zuo1365–433-expressing cells, indicating an equivalent ability of the fusions to activate the PDR5 promoter. These results indicate that the C-terminal 13 residues of Zuo1 (421-433) are sufficient to activate PDR.
Figure 4.
The C-terminal 13 residues are necessary and sufficient for Pdr1 activation. Cells were transformed with vector containing DNA encoding TAP (dotted line) or TAP fused to fragments of the C-terminus of Zuo1, with the N-terminal residue of each fragment indicated. (a) Serial dilutions of wt cells harboring the indicated plasmids were spotted onto medium without (−) or with (+) cycloheximide. β-galactosidase activity was measured for cells containing an integrated PDR5-lacZ reporter and transformed with the indicated plasmids. The average activity of 3 transformants of each was quantified and reported in Miller units with error bars indicating standard error. (b) Serial dilutions of wt cells harboring the indicted plasmids were performed as described in a. (c) Cell extracts were prepared from cultures used for serial dilutions in (a) and (b) and subjected to immunoblot analysis using rabbit IgG to detect the TAP-tagged Zuo1 fusions.
We carried out two additional experiments to determine the necessity of these 13 residues for activation. First, to confirm that these residues are necessary for activation in the context of the larger Zuo1365–433 fragment, we generated a construct that lacks the codons for these 13 residues, TAP-Zuo1365–420. Cells expressing TAP-Zuo1365–420 did not form colonies on cycloheximide-containing plates and had β-galactosidase activity similar to the basal level found in cells expressing only the TAP tag (Fig. 4a). Second, we constructed three additional TAP fusions having even smaller segments of the extreme C-terminus of Zuo1, generating TAPZuo1425-433, TAP-Zuo1428-433 and TAP-Zuo1430-433. None of these shorter C-terminal fusions were able to support growth on drug-containing plates (Fig. 4b,c). Thus, we conclude that the 13 extreme C-terminal residues (421-433) of Zuo1 are both necessary and sufficient for Pdr1 activation.
Residues critical for Pdr1 activation are buried in the C-terminal helical bundle
To identify individual residues required for Pdr1 activation within the 13-residue segment identified as sufficient, we performed alanine scanning mutagenesis. Cells expressing either wt TAP-Zuo1365–433 or TAP-Zuo1365–433 containing one of the 13 alanine substitutions were plated on medium containing or lacking cycloheximide. Cells expressing the Leu428, Leu429, Tyr431 or Val433 substitution variants did not grow on cycloheximide-containing plates (Fig. 5a, Supplementary Fig. 3a). Those expressing the Pro425 or Phe432 substitutions grew much more slowly than those expressing the wt fusion. Alteration of only one hydrophobic residue, Leu424, was tolerated, while alteration of the 6 other residues had no obvious affect on Pdr1 activation.
Figure 5.
Residues critical for Pdr1 activation are buried in the C-terminal helical bundle. (a) Alanine scanning mutagenesis of residues 421-433. (left) Wt cells were transformed with vector containing DNA encoding TAP (-) or TAP fused to wt Zuo1365–433 (wt) or Zuo1365–433 containing one of the 13 alanine point mutations and plated onto medium without (−) or with (+) cycloheximide. (right) PJ6933 cells were transformed with vector containing DNA encoding GBD (−) or GBD fused to wt Zuo1365–433 (wt) or Zuo1365–433 containing one of the 13 alanine point mutations and plated onto medium with (+) or without (−) histidine and adenine to detect auto-activation of Gal1-HIS3 and Gal2-ADE2 reporters. All serial dilutions on the same medium were plated on the same plate. Dotted lines indicate where the columns were aligned for display purposes. (b) Residues important for Pdr1 activation, as determined by loss of activity upon alteration to alanine as described in (a), are indicated in magenta. Residues in which substitution to alanine had no effect on activity in either assay are shown in orange. (c) The three most C-terminal residues, Tyr431, Phe432, and Val433 (purple), are well constrained in the hydrophobic core of the domain, as indicated by the large number of NOEs observed between these and surrounding residues, shown in cyan. (d) 15N–1H HSQC spectrum of the C-terminal domain lacking the three most C-terminal residues (Zuo1348-430).
We also performed an analogous alanine scan using the modified two-hybrid assay. The pattern of effects of alterations in the GBD-Zuo1365–433 fusions was very similar to that found with the TAP-Zuo1365–433 fusions. Cells expressing GBD-Zuo1365–433 in which any of the 7 hydrophobic residues in the extreme C-terminus (Leu424, Pro425, Leu428, Leu429, Tyr431, Phe432, or Val433) were altered showed no growth on selective medium, indicating a failure to activate the Gal4-inducible reporters (Fig. 5a, Supplementary Fig. 3b). Alteration of any of the polar or charged residues, on the other hand, had no effect on activity. Thus, we conclude that hydrophobic residues within the extreme C-terminus play critical roles in PDR induction.
Examination of the position of these critical hydrophobic residues within the helical bundle structure revealed, as expected, that these residues are predominantly buried within the hydrophobic core of the domain (Fig. 5b). Thus, we hypothesized that residues required for PDR activation also play important roles in domain structure. To test this idea, we generated a construct to express the C-terminal domain of Zuo1 lacking the 3 most C-terminal residues (Tyr431, Phe432, and Val433), as these residues are not only important for activity, but also deeply buried and particularly well constrained within the hydrophobic core of the domain (Fig. 5c). The 15N-1H HSQC of this variant, Zuo1348-430, showed poor peak dispersion with the majority of peaks centered around the random coil chemical shift value, consistent with the protein being in an unfolded conformation (Fig. 5d), confirming the importance of these residues in maintaining domain structure.
Specificity of Zuo1’s hydrophobic C-terminus in Pdr1-dependent transcriptional activation
The sufficiency of such a short segment of Zuo1 for PDR activation, coupled with the importance of hydrophobic residues, raised the question as to the specificity of activation, as short hydrophobic peptides have been found to act as general transcriptional activators when tethered to DNA. Thus, we compared the transcription activation potential of the Zuo1 peptide with two other hydrophobic peptides, referred to as P201 (YLLPTCIP) and P223 (YLLPFLPY), which were originally selected for their ability to activate transcription from the GAL1 promoter when fused to GBD19. Unlike cells expressing only GBD, cells expressing any one of the three peptide fusions, GBD-Zuo1421-433, GBD-P201 and GBD-P233, grew on plates lacking histidine, indicating activation of the Gal1-HIS3 reporter (Fig. 6, left). We next tested the ability of the fusions to activate transcription in the absence of Pdr1. As expected, Δpdr1 cells expressing GBD-Zuo1421-433 did not grow in the absence of histidine. GBD-P201 and GBD-P223, on the other hand, activated Gal1-HIS3 even in the absence of Pdr1.
Figure 6.
Specificity of Zuo1’s hydrophobic C-terminus in Pdr1-dependent transcriptional activation. (left) Wt or Δpdr1 cells containing an integrated Gal1-HIS3 reporter were transformed with DNA encoding GBD (-) or GBD fused to one of three peptides: Zuo1421-433, P201, or P223. Cells were spotted in serial dilutions onto medium with (+) or without (−) histidine. (right) Wt or Δpdr1 cells were transformed with DNA encoding TAP (−) or TAP fused to the indicated peptides and spotted in serial dilutions onto medium without (−) or with (+) cycloheximide.
As a second test of specificity, we asked whether cells expressing TAP tag fusions of peptides P201 and P223 could, like Zuo1421-433, activate PDR when not tethered to DNA. Unlike cells expressing TAP-Zuo1421-433, no detectable drug resistance was observed for cells expressing TAP-P201 or TAP-P223, whether or not Pdr1 was present (Fig. 6, right, Supplementary Fig. 4). Together these data indicate that, although activation of Pdr1 by Zuo1 requires a short, hydrophobic peptide, the observed transcriptional activation is distinct from the general transcriptional properties observed previously for hydrophobic peptides tethered to DNA.
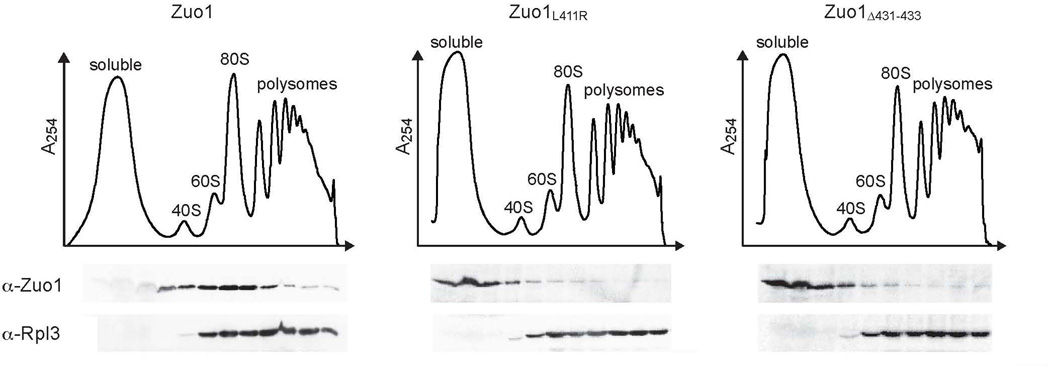
C-terminal unfolding results in dissociation of Zuo1 from the ribosome
The results discussed above indicate that both dissociation of Zuo1 from the ribosome and unfolding of the C-terminal domain are necessary for the specific activation of Pdr1. To assess the effect of unfolding of the CTD on ribosome association, we tested the migration in sucrose gradients of two Zuo1 variants having amino acid alterations that cause unfolding of the CTD, Zuo1L411R and Zuo1Δ431–433. Unlike wt Zuo1, neither variant co-migrated with ribosomes during centrifugation (Fig. 7). Rather both remained at the top of the gradient. The lack of ribosome association of these variants is consistent with a folded CTD being required for association of Zuo1 with the ribosome.
Figure 7.
Unfolding of the CTD results in dissociation of Zuo1 from the ribosome. Δzuo1 cells were transformed with vector containing DNA encoding Zuo1 or variants of Zuo1 with alterations that result in unfolding of the CTD under control of the endogenous ZUO1 promoter. Lysates of cells harboring the indicated plasmids were separated on 5–50% sucrose gradients and fractions were collected and analyzed by immunoblotting for the presence of Zuo1 and Rpl3. The migration of ribosomal subunits was monitored by absorbance at 254 nm and plotted versus the time course of fraction collection.
Discussion
The data presented here indicate that unfolding of the extreme C-terminal domain of Zuo1 results in both its dissociation from the ribosome and release of autoinhibition unleashing its ability to specifically activate the Pdr1 transcription factor. This dual effect of unfolding of the CTD suggests a pathway of communication between the cytosolic translational apparatus and the nuclear transcriptional machinery.
The extreme C-terminal 13 residues of Zuo1 are necessary and sufficient for activation of Pdr1, with hydrophobic residues playing critical roles in this activation. Although short, hydrophobic peptides have been identified as recruiters of the general transcription machinery20,21, Zuo1’s C-terminus activates transcription specifically through Pdr1. This specificity is consistent with previously reported microarray data demonstrating that the PDR regulon is the major class of genes upregulated by the C-terminus of Zuo16. Positive two-hybrid interaction analyses indicate that the interaction between Zuo1 and Pdr1 is direct6, pointing to a model in which Zuo1’s C-terminal hydrophobic peptide interacts directly with Pdr1, leading to mobilization of Pdr1’s activation domain which is thought to be inhibited by its central regulatory region10,22. Such a mode of activation is analogous to the direct binding of xenobiotics observed for both yeast and mammalian transcription factors in pleiotropic drug resistance9,23,24. It should be noted, however, that Pdr1 activation may be more complex, as Zuo1 forms a very stable heterodimer with the atypical Hsp70 Ssz125, often called the ribosome associated complex (RAC). Ssz1, when not ribosome associated, also activates Pdr126. Thus, it is likely that Zuo1 and Ssz1 act in concert as a complex in the natural environment to activate Pdr1.
For Zuo1 to activate Pdr1 its C-terminus must be unfolded, as hydrophobic residues in the extreme C-terminus critical for activation of Pdr1 are sequestered within a four-helix bundle formed by the 86 C-terminal residues and are thus inaccessible for intermolecular interactions in the folded conformation. The positive correlation between unfolding and transcriptional activation we observed points to a scenario in which Zuo1’s activity in Pdr1-dependent transcriptional activation is regulated by autoinhibition conferred by the CTD structure, with activity being induced upon a folding:unfolding transition of its helical bundle domain. Although to our knowledge there are few, if any, examples of unfolding as a requirement for a protein to activate a transcription factor, unfolding as a mode of positive regulation certainly has precedents. Perhaps best understood is N-WASP, which, in its structured form, sequesters key hydrophobic residues in its C-terminal VCA region required for actin polymerization27. Cdc42 binding releases this autoinhibition by disrupting the folded structure of a helical GTPase-binding domain. Unfolding of this domain releases the cofilin homology motif and allows the VCA region to activate the Arp2/3 complex.
Previous work identified an 80-residue charged region in Zuo1 (285-364) required for ribosome binding3,28. The structural analysis reported here demonstrates that residues at the C-terminal boundary of this ribosome-binding region are necessary for formation of the C-terminal helical bundle. More experiments will be required to understand the molecular interactions responsible for Zuo1’s association with the ribosome, both in terms of protein:RNA and protein:protein contacts, and the intermolecular effects of unfolding of the CTD. However, even without this information in hand, a plausible hypothesis is that the two requisite steps for Pdr1 activation, ribosome dissociation and unfolding of the CTD to expose critical hydrophobic residues, are coupled, as alterations that promote unfolding of Zuo1’s CTD also result in dissociation of Zuo1 from the ribosome. Thus, a shift in the equilibrium of the CTD to an unfolded conformation likely increases the amount of soluble Zuo1 free to activate Pdr1.
The idea that Zuo1’s CTD is active in an unfolded conformation raises the question of how the autoinhibited structure gains access to the unfolded state that results in both ribosome dissociation and exposure of residues required for Pdr1 activation. Depending on the free energy of folding (ΔGfold), a significant population of unfolded CTD may exist in equilibrium with the folded structure, as observed by 2D NMR for the Zuo1358–433 construct (Fig. 2c). Based on the NMR spectra and CD measurements of thermal unfolding, we estimate that the Zuo1348–433 and Zuo1358–433 C-terminal fragments fold with relatively small margins of stability, with ΔGfold ~ –2 and –1 kcal/mol respectively. These estimates suggest that in the absence of other stabilizing or destabilizing interactions, roughly 4% of the CTD would be unfolded, a percentage consistent with observations that the vast majority of Zuo1 is ribosome-associated under typical laboratory conditions. When Zuo1 is bound to the ribosome and the majority of the CTD is folded, we predict that the Pdr1-activating epitope would be exposed at low levels by a basal level of equilibrium unfolding. Such a low amount of the unfolded, ribosome-free form is likely insufficient to induce Pdr1 activation. However, a binding partner that recognizes a distinct segment of the unfolded CTD would shift the equilibrium toward the unfolded state, with the net effect of destabilizing the autoinhibitory conformation and promoting Pdr1 activation.
In a broader physiological context, the relationship between Zuo1 (and Ssz1), the ribosome, and cellular signaling is intriguing. The translational apparatus has been repeatedly linked to growth control and cell cycle regulation29,30. Indeed, activation of Pdr1 leads to premature growth arrest as cells transition from preferred to non-preferred carbon sources, presumably due to sensing by neighboring cells of metabolites exported by induced transporters. It is worth noting that transcriptional regulatory functions of ribosome-associated chaperones may extend beyond Zuo1:Ssz1. Components of the nascent chain associated complex (NAC) have been reported to function as transcriptional co-activators31,32. The work presented here is a start in unraveling the complex regulatory pathway for RAC components. Further work will be necessary to understand the dynamics of the interaction of these chaperones with the ribosome, including the identification of factors that affect the folding:unfolding equilibrium of Zuo1’s CTD, and the balance between their function in protein folding at the ribosome and their regulatory roles in extra-ribosomal transcriptional activation.
Materials and Methods
Yeast strains and plasmids
Yeast strains used were isogenic with DS10 and contain the following mutations: his3-11,15 leu2-3,112 lys1 lys2 trp1Δ ura3-52. Δzuo1::HIS31 and Δpdr1::TRP16 have been described previously. A strain containing an integrated PDR5-lacZ reporter was created by digesting the pTH120 plasmid containing PDR5-lacZ::HIS326 with StuI and transforming the resulting fragment into DS10 wt or Δpdr1::TRP1 to direct integration at the PDR5 locus. TAP and TAPZuo1365-433 plasmids were described previously6. Additional plasmids created for this study are described in Supplementary Table 1.
Assays for PDR induction
To assay drug resistance, approximately equal numbers of cells were subjected to 10-fold serial dilutions and spotted on selective minimal glucose medium containing 0, 0.7 or 1 μg/ml cycloheximide. Plates were incubated at 30°C for 2–3 days before photographing. β-galactosidase assays were performed as previously described7 using strains containing an integrated PDR5-lacZ reporter. A minimum of three independent transformants was tested and the average activity determined.
Analysis of ribosome association
Δzuo1 cells containing the indicated plasmids were grown to an OD600 between 0.5 and 0.8 in selective minimal medium, treated with 100 μg/ml cycloheximide, and harvested by centrifugation at 4 °C. Cells were resuspended in 20 mM Tris-HCl pH 7.5, 50 mM KCl, 5 mM MgCl2 buffer containing 0.1 mM phenylmethylsulfonyl fluoride and 1.5 mM Pepstatin and treated with RNasin (Promega) at a dilution of 1:1,000. Yeast lysates were prepared by bead beating for 5 min and clarified by centrifugation at 14,000 rpm for 10 min. To fractionate polysomes, approximately 10 OD260 units of lysate were applied to the top of a 4-ml 5–50% sucrose gradient in the resuspension buffer and centrifuged for 80 min at 45,000 rpm at 4 °C in a SW50.1 Ti rotor (Beckman). Gradients were monitored for absorbance at 254 nm to detect monosomes and polysomes. Fractions were precipitated with 10% TCA, separated by SDS-PAGE, and subjected to immunoblotting.
Modified yeast two-hybrid
Strains PJ69 and PJ69 Δpdr1::TRP1, the GBD and GBD-Zuo1365–433 plasmids, and yeast two-hybrid methods were described previously6,33. Additional plasmids generated for this study are described in Supplementary Table 1. Modified yeast two-hybrid was carried out by detecting auto-activation of Gal1–HIS3 and/or Gal2-ADE2 reporters of PJ69 wt or Δpdr1 cells by monitoring growth on minimal medium lacking uracil for plasmid selection and either histidine and adenine or histidine and containing 2 mM 3-aminotriazole, as described previously6. Plates were incubated for 2–3 days at 30°C before photographing.
Protein expression and purification
8xHis-tagged Zuo1 C-terminal fragments were expressed in E. coli BL21[pREP4] cells from pQE308HT34-based plasmids described in Supplementary Table 1. Cells were grown at 37 °C to an OD600 ≈ 0.8 in LB medium containing 150 μg/ml ampicillin and 50 μg/ml kanamycin, expression was induced by the addition of isopropyl β-D-thiogalactopyranoside to a final concentration of 1 mM, and cells were grown for an additional 3 hours at 15°C following induction. Isotopically labeled proteins were prepared for NMR by growing cultures in M9 medium containing 15N-ammonium chloride and/or 13C-glucose as the sole nitrogen and carbon sources, respectively. Cells harvested from a 1-L culture were resuspended in 50 mM sodium phosphate pH 7.4, 300 mM NaCl, 40 mM imidazole, 0.1% (w/v) 2-mercaptoethanol buffer containing an EDTA-free Complete Protease Inhibitor Cocktail tablet (Roche). Cells were lysed using a French pressure cell and protein was purified at 4 °C by immobilized metal-ion affinity chromatography using Ni-sepharose 6 Fast Flow resin (GE Healthcare) according to a previously published protocol35. Following purification, the protein solutions were dialyzed 2× into 2 L of 20 mM sodium phosphate pH 6.5, 50 mM NaCl, 1 mM dithiothreitol. Dialyzed protein was concentrated to approximately 500μl for analysis by NMR and the purity and identity was verified by SDS-PAGE and/or mass spectrometry.
Circular dichroism spectroscopy
Samples of Zuo1348–433 and Zuo1358–433 were prepared at a concentration of 20 μM in buffer containing 20 mM sodium phosphate pH 6.5 and 50 mM NaCl. Thermal denaturation experiments were performed in a 1 mm cuvette and the ellipticity was monitored at 222 nm over a temperature range of 10–70 °C. Thermal denaturation curves were analyzed by nonlinear least-squares fitting as previously described to determine the melting temperature36.
NMR spectroscopy
NMR samples were prepared in buffer containing 20 mM sodium phosphate pH 6.5, 50 mM sodium chloride, 1 mM dithiothreitol, and 5–10% 2H2O. All 2D 15N-1H HSQC spectra were acquired at 20 or 25 °C on a Bruker 500 or 600 MHz spectrometer equipped with a triple-resonance CryoProbe™ and processed with NMRPipe software37. The Zuo1348–433 sample used for structure determination was prepared in the identical buffer at a concentration of 1.2 mM. All structural data were acquired at 10 °C using a field strength of 600 MHz. Backbone 1H, 15N, and 13C chemical shift assignments for Zuo1348–433 were obtained automatically as previously described using peak lists from 15N-1H HSQC, HNCO, HN(CO)CA, HN(CO)CACB, HNCA, HNCACB, HN(CA)CO, and CC(CO)NH38. Sidechain assignments were completed manually from 3D HBHACONH, HCCONH, HCCH total correlation spectroscopy, and 13C(aromatic)-edited NOESY-HSQC spectra. Chemical shift assignments were >99% complete for Zuo1348–433. Heteronuclear NOE values were measured from an interleaved pair of 2D 15N-1H sensitivity enhanced correlation spectra recorded with and without a 5s proton saturation period.
Structure calculation and analysis
The Zuo1348–433 structure was calculated using distance constraints obtained from 3D 15N-edited NOESY-HSQC and 13C-edited NOESY-HSQC (τmix = 80 ms). Backbone ϕ and ψ dihedral angle constraints were generated from secondary shifts of the 1H, 13Cα, 13Cβ, 13C’ and 15N nuclei using the program TALOS39. Structure calculations were performed using the torsion angle dynamics program CYANA40 followed by iterative rounds of manual refinement to eliminate constraint violations. Of the 100 CYANA structures calculated, the 20 conformers with the lowest target function were subjected to a molecular dynamics protocol in explicit solvent41 using XPLOR-NIH42. PyMOL (Schrödinger LLC) was used to generate all structure images.
Accession numbers
Coordinates and related data have been deposited at the RSCB PDB (2LWX) and NMR data at BMRB (17685).
Supplementary Material
Acknowledgements
We thank Scott Moye-Rowley (University of Iowa, Iowa City) for sharing plasmids, Peter Kuhn for helpful discussions as well as assistance with data collection, Justin Hines for helpful advice, and Davin Jenson for assistance with data collection. This work was supported by National Institutes of Health grants GM31107 (E.A.C.) and AI063325 (B.F.V.).
Abbreviations used
- PDR
pleiotropic drug resistance
- TF
transcription factor
- CTD
C-terminal domain
- ABC
ATP-binding cassette
- TAP
tandem affinity purification
- HSQC
heteronuclear single quantum coherence
- NOE
nuclear Overhauser effect
- GBD
Gal4 DNA binding domain
References
- 1.Hundley H, Eisenman H, Walter W, Evans T, Hotokezaka Y, Wiedmann M, Craig E. The in vivo function of the ribosome-associated Hsp70, Ssz1, does not require its putative peptide-binding domain. Proc Natl Acad Sci U S A. 2002;99:4203–4208. doi: 10.1073/pnas.062048399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gautschi M, Mun A, Ross S, Rospert S. A functional chaperone triad on the yeast ribosome. Proc Natl Acad Sci U S A. 2002;99:4209–4214. doi: 10.1073/pnas.062048599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yan W, Schilke B, Pfund C, Walter W, Kim S, Craig EA. Zuotin, a ribosome-associated DnaJ molecular chaperone. EMBO J. 1998;17:4809–4817. doi: 10.1093/emboj/17.16.4809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Richly H, Rocha-Viegas L, Ribeiro JD, Demajo S, Gundem G, Lopez-Bigas N, Nakagawa T, Rospert S, Ito T, Di Croce L. Transcriptional activation of polycomb-repressed genes by ZRF1. Nature. 2010;468:1124–1128. doi: 10.1038/nature09574. [DOI] [PubMed] [Google Scholar]
- 5.Inoue T, Shoji W, Obinata M. MIDA1, an Id-associating protein, has two distinct DNA binding activities that are converted by the association with Id1: a novel function of Id protein. Biochem Biophys Res Commun. 1999;266:147–151. doi: 10.1006/bbrc.1999.1779. [DOI] [PubMed] [Google Scholar]
- 6.Prunuske AJ, Waltner JK, Kuhn P, Gu B, Craig EA. Role for the molecular chaperones Zuo1 and Ssz1 in quorum sensing via activation of the transcription factor Pdr1. Proc Natl Acad Sci U S A. 2012;109:472–477. doi: 10.1073/pnas.1119184109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Eisenman HC, Craig EA. Activation of pleiotropic drug resistance by the Jprotein and Hsp70-related proteins, Zuo1 and Ssz1. Mol Microbiol. 2004;53:335–344. doi: 10.1111/j.1365-2958.2004.04134.x. [DOI] [PubMed] [Google Scholar]
- 8.Fiaux J, Horst J, Scior A, Preissler S, Koplin A, Bukau B, Deuerling E. Structural analysis of the ribosome-associated complex (RAC) reveals an unusual Hsp70/Hsp40 interaction. J Biol Chem. 2010;285:3227–3234. doi: 10.1074/jbc.M109.075804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thakur JK, Arthanari H, Yang F, Pan SJ, Fan X, Breger J, Frueh DP, Gulshan K, Li DK, Mylonakis E, Struhl K, Moye-Rowley WS, Cormack BP, Wagner G, Naar AM. A nuclear receptor-like pathway regulating multidrug resistance in fungi. Nature. 2008;452:604–609. doi: 10.1038/nature06836. [DOI] [PubMed] [Google Scholar]
- 10.Kolaczkowska A, Kolaczkowski M, Delahodde A, Goffeau A. Functional dissection of Pdr1p, a regulator of multidrug resistance in Saccharomyces cerevisiae. Mol Genet Genomics. 2002;267:96–106. doi: 10.1007/s00438-002-0642-0. [DOI] [PubMed] [Google Scholar]
- 11.Devaux F, Marc P, Bouchoux C, Delaveau T, Hikkel I, Potier MC, Jacq C. An artificial transcription activator mimics the genome-wide properties of the yeast Pdr1 transcription factor. EMBO Rep. 2001;2:493–498. doi: 10.1093/embo-reports/kve114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.DeRisi J, van den Hazel B, Marc P, Balzi E, Brown P, Jacq C, Goffeau A. Genome microarray analysis of transcriptional activation in multidrug resistance yeast mutants. FEBS Lett. 2000;470:156–160. doi: 10.1016/s0014-5793(00)01294-1. [DOI] [PubMed] [Google Scholar]
- 13.Jungwirth H, Kuchler K. Yeast ABC transporters-- a tale of sex, stress, drugs and aging. FEBS Lett. 2006;580:1131–1138. doi: 10.1016/j.febslet.2005.12.050. [DOI] [PubMed] [Google Scholar]
- 14.Kolaczkowski M, Kolaczowska A, Luczynski J, Witek S, Goffeau A. In vivo characterization of the drug resistance profile of the major ABC transporters and other components of the yeast pleiotropic drug resistance network. Microb Drug Resist. 1998;4:143–158. doi: 10.1089/mdr.1998.4.143. [DOI] [PubMed] [Google Scholar]
- 15.Hlavacek O, Kucerova H, Harant K, Palkova Z, Vachova L. Putative role for ABC multidrug exporters in yeast quorum sensing. FEBS Lett. 2009;583:1107–1113. doi: 10.1016/j.febslet.2009.02.030. [DOI] [PubMed] [Google Scholar]
- 16.Fardeau V, Lelandais G, Oldfield A, Salin H, Lemoine S, Garcia M, Tanty V, Le Crom S, Jacq C, Devaux F. The central role of PDR1 in the foundation of yeast drug resistance. J Biol Chem. 2007;282:5063–5074. doi: 10.1074/jbc.M610197200. [DOI] [PubMed] [Google Scholar]
- 17.Delahodde A, Pandjaitan R, Corral-Debrinski M, Jacq C. Pse1/Kap121- dependent nuclear localization of the major yeast multidrug resistance (MDR) transcription factor Pdr1. Mol Microbiol. 2001;39:304–312. doi: 10.1046/j.1365-2958.2001.02182.x. [DOI] [PubMed] [Google Scholar]
- 18.Hallstrom TC, Moye-Rowley WS. Hyperactive forms of the Pdr1p transcription factor fail to respond to positive regulation by the hsp70 protein Pdr13p. Mol Microbiol. 2000;36:402–413. doi: 10.1046/j.1365-2958.2000.01858.x. [DOI] [PubMed] [Google Scholar]
- 19.Lu X, Ansari AZ, Ptashne M. An artificial transcriptional activating region with unusual properties. Proc Natl Acad Sci U S A. 2000;97:1988–1992. doi: 10.1073/pnas.040573197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wu Z, Belanger G, Brennan BB, Lum JK, Minter AR, Rowe SP, Plachetka A, Majmudar CY, Mapp AK. Targeting the transcriptional machinery with unique artificial transcriptional activators. J Am Chem Soc. 2003;125:12390–12391. doi: 10.1021/ja036685v. [DOI] [PubMed] [Google Scholar]
- 21.Lu Z, Ansari AZ, Lu X, Ogirala A, Ptashne M. A target essential for the activity of a nonacidic yeast transcriptional activator. Proc Natl Acad Sci U S A. 2002;99:8591–8596. doi: 10.1073/pnas.092263499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.MacPherson S, Larochelle M, Turcotte B. A fungal family of transcriptional regulators: the zinc cluster proteins. Microbiol Mol Biol Rev. 2006;70:583–604. doi: 10.1128/MMBR.00015-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Willson TM, Kliewer SA. PXR, CAR and drug metabolism. Nat Rev Drug Discov. 2002;1:259–266. doi: 10.1038/nrd753. [DOI] [PubMed] [Google Scholar]
- 24.Kliewer SA, Goodwin B, Willson TM. The nuclear pregnane X receptor: a key regulator of xenobiotic metabolism. Endocr Rev. 2002;23:687–702. doi: 10.1210/er.2001-0038. [DOI] [PubMed] [Google Scholar]
- 25.Gautschi M, Lilie H, Funfschilling U, Mun A, Ross S, Lithgow T, Rucknagel P, Rospert S. RAC, a stable ribosome-associated complex in yeast formed by the DnaK- DnaJ homologs Ssz1p and zuotin. Proc Natl Acad Sci U S A. 2001;98:3762–3767. doi: 10.1073/pnas.071057198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hallstrom TC, Katzmann DJ, Torres RJ, Sharp WJ, Moye-Rowley WS. Regulation of transcription factor Pdr1p function by an Hsp70 protein in Saccharomyces cerevisiae. Mol Cell Biol. 1998;18:1147–1155. doi: 10.1128/mcb.18.3.1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Panchal SC, Kaiser DA, Torres E, Pollard TD, Rosen MK. A conserved amphipathic helix in WASP/Scar proteins is essential for activation of Arp2/3 complex. Nat Struct Biol. 2003;10:591–598. doi: 10.1038/nsb952. [DOI] [PubMed] [Google Scholar]
- 28.Peisker K, Braun D, Wolfle T, Hentschel J, Funfschilling U, Fischer G, Sickmann A, Rospert S. Ribosome-associated complex binds to ribosomes in close proximity of Rpl31 at the exit of the polypeptide tunnel in yeast. Mol Biol Cell. 2008;19:5279–5288. doi: 10.1091/mbc.E08-06-0661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Spriggs KA, Bushell M, Willis AE. Translational regulation of gene expression during conditions of cell stress. Mol Cell. 2010;40:228–237. doi: 10.1016/j.molcel.2010.09.028. [DOI] [PubMed] [Google Scholar]
- 30.Van Der Kelen K, Beyaert R, Inze D, De Veylder L. Translational control of eukaryotic gene expression. Crit Rev Biochem Mol Biol. 2009;44:143–168. doi: 10.1080/10409230902882090. [DOI] [PubMed] [Google Scholar]
- 31.Meury T, Akhouayri O, Jafarov T, Mandic V, St-Arnaud R. Nuclear alpha NAC influences bone matrix mineralization and osteoblast maturation in vivo. Mol Cell Biol. 2010;30:43–53. doi: 10.1128/MCB.00378-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yotov WV, Moreau A, St-Arnaud R. The alpha chain of the nascent polypeptide-associated complex functions as a transcriptional coactivator. Mol Cell Biol. 1998;18:1303–1311. doi: 10.1128/mcb.18.3.1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.James P, Halladay J, Craig EA. Genomic libraries and a host strain designed for highly efficient two-hybrid selection in yeast. Genetics. 1996;144:1425–1436. doi: 10.1093/genetics/144.4.1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Waltner JK, Peterson FC, Lytle BL, Volkman BF. Structure of the B3 domain from Arabidopsis thaliana protein At1g16640. Protein Sci. 2005;14:2478–2483. doi: 10.1110/ps.051606305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lytle BL, Peterson FC, Qiu SH, Luo M, Zhao Q, Markley JL, Volkman BF. Solution structure of a ubiquitin-like domain from tubulin-binding cofactor B. J Biol Chem. 2004;279:46787–46793. doi: 10.1074/jbc.M409422200. [DOI] [PubMed] [Google Scholar]
- 36.Allen DL, Pielak GJ. Baseline length and automated fitting of denaturation data. Protein science : a publication of the Protein Society. 1998;7:1262–1263. doi: 10.1002/pro.5560070524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Delaglio F, Grzesiek S, Vuister GW, Zhu G, Pfeifer J, Bax A. NMRPipe: a multidimensional spectral processing system based on UNIX pipes. J. Biomol. NMR. 1995;6:277–293. doi: 10.1007/BF00197809. [DOI] [PubMed] [Google Scholar]
- 38.Markley JL, Bahrami A, Eghbalnia HR, Peterson FC, Ulrich EL, Westler WM, Volkman BF. Gu J, Bourne PE. Structural Bioinformatics. 2nd edit. John Wiley; 2009. Macromolecular Structure Determination by NMR Spectroscopy; pp. 93–142. [Google Scholar]
- 39.Cornilescu G, Delaglio F, Bax A. Protein backbone angle restraints from searching a database for chemical shift and sequence homology. J Biomol NMR. 1999;13:289–302. doi: 10.1023/a:1008392405740. [DOI] [PubMed] [Google Scholar]
- 40.Guntert P. Automated NMR structure calculation with CYANA. Methods Mol Biol. 2004;278:353–378. doi: 10.1385/1-59259-809-9:353. [DOI] [PubMed] [Google Scholar]
- 41.Linge JP, Williams MA, Spronk CA, Bonvin AM, Nilges M. Refinement of protein structures in explicit solvent. Proteins. 2003;50:496–506. doi: 10.1002/prot.10299. [DOI] [PubMed] [Google Scholar]
- 42.Schwieters CD, Kuszewski JJ, Tjandra N, Marius Clore G. The Xplor- NIH NMR molecular structure determination package. J Magn Reson. 2003;160:65–73. doi: 10.1016/s1090-7807(02)00014-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.