Abstract
Traumatic brain injury (TBI) is a global problem reaching near epidemic numbers that manifests clinically with cognitive problems that decades later may result in dementias like Alzheimer’s disease (AD). Presently, little can be done to prevent ensuing neurological dysfunctions by pharmacological means. Recently, it has become apparent that several CNS diseases share common terminal features of neuronal cell death. The effects of exendin-4 (Ex-4), a neuroprotective agent delivered via a subcutaneous micro-osmotic pump, were examined in the setting of mild TBI (mTBI). Utilizing a model of mTBI, where cognitive disturbances occur over time, animals were subjected to four treatments: sham; Ex-4; mTBI and Ex-4/mTBI. mTBI mice displayed deficits in novel object recognition, while Ex-4/mTBI mice performed similar to sham. Hippocampal gene expression, assessed by gene array methods, showed significant differences with little overlap in co-regulated genes between groups. Importantly, changes in gene expression induced by mTBI, including genes associated with AD were largely prevented by Ex-4. These data suggest a strong beneficial action of Ex-4 in managing secondary events induced by a traumatic brain injury.
Keywords: mild traumatic brain injury (mTBI), glucagon-like peptide-1, excendin-4, gene expression, novel object recognition, Alzheimer’s disease
Introduction
Head trauma is a global problem caused by household injuries, automobile accidents, sports related events and warfare (Faul et al. 2010; DeKosky et al. 2010; Hoge et al. 2008; Mendez et al. 2005). The incidence of traumatic brain injury (TBI) in the United States alone, based on figures from a Centers for Disease Control and Prevention report published in 2009, are in the order of ~1.7 million cases per year (Faul et al. 2010). Gender and age analysis indicated that there is a greater occurrence of injuries in males than in females and that young (infants to 19 years old) and older individuals (65 years old and older) were more prone to these types of injuries. Adults of 75 years or more presented the highest rates of head injury related to hospitalization and death. The majority of head trauma cases were found to require a visit to an emergency department, after which the individuals were sent home. Head trauma victim survival from primary events has improved due to better surgical treatments initiated shortly after the occurrence of the trauma. More often than not, depending on the nature of the TBI, patients who survive the initial insult develop serious secondary emotional, memory and cognitive impairments that have significant impacts upon the patient, the patient’s family members and health care providers. Currently, there are no clear pharmaceutical-based therapies of benefit to manage the secondary pathological events associated with TBI patients; moreover, data relating to the cellular and molecular mechanisms underlying the transition from acute to chronic pathology and behavioral deficits after TBI have yet to be fully identified. Secondary TBI effects manifest over time from weeks to months or even over longer periods of time after the event, often decades later. Of particular relevance to the long-term implications of TBI, there is a growing body of evidence that illustrates a strong association between head trauma and the development of neurodegenerative disorders, particularly dementia related syndromes exemplified by Alzheimer’s disease (AD), (Costanza et al. 2011; Johnson et al. 2010; Kiraly and Kiraly 2007; Uryu et al. 2003).
Different forms of TBI have been modeled in animal studies, with the majority of human TBIs described as mild in nature (De Kruijck et al. 2001). Consequently we have used a mild traumatic brain injury (mTBI) model where no overt tissue damage occurs at the time of injury, yet cognitive impairments manifest over time post injury (Pan et al. 2003, Zohar et al. 2003, Milman et al. 2005). We have shown time-dependent disturbances in animal behavior and cognition and, at early time points, changes in neuronal apoptosis have been reported (Tashlykov et al. 2007, 2009; Tweedie et al. 2007). Several studies suggest a strong commonality between the biochemical cascades resulting in neuronal cell death from TBI and neurodegenerative disease. This commonality may well provide clinicians an opportunity to exploit existing treatments for neurodegenerative conditions that could possibly provide cross-over therapeutic potential to TBI.
We have recently demonstrated the beneficial utility of the glucagon-like peptide-1 (GLP-1) receptor signaling pathway in preclinical models of several CNS related neurological disorders such as AD, Parkinson’s disease (PD), stroke, amyotrophic lateral sclerosis (ALS) and Huntington’s disease (HD), (Li et al. 2009, 2010, 2012; Martin et al. 2009; Salcedo et al. 2012). Due to the apparent benefits of GLP-1 receptor signaling in quite diverse neurodegenerative conditions, we examined potential benefits from the peripheral administration of a GLP-1 receptor agonist, exendin-4 (Ex-4) which readily crosses the blood-brain barrier (Kastin and Akerstrom, 2003), in the setting of mild brain trauma. The focus of this study was three fold: one, to investigate the impact of GLP-1 receptor activation on cognitive deficits induced by mTBI; two, to identify potential gene expression changes induced by mTBI that may underlie the observed cognitive changes and three, to identify genes regulated by treatment with Ex-4 prior to mTBI. In the present study, we have demonstrated GLP-1 receptor mediated benefits in behavior and identified many mouse hippocampal genes regulated by: Ex-4 (drug no trauma); mTBI (vehicle with mTBI) and Ex-4/mTBI (drug prior to mTBI). The identities of these genes may shed insight on the development of novel therapeutic approaches that limit neurological dysfunction observed in animals and that may translate to humans. This report describes selected Gene Ontologies and gene set pathways relevant to neuronal and neurodegenerative conditions, specifically those related to AD.
Material and method
Animal studies
Male ICR mice weighing 30–40 g were kept five per cage under a constant 12-h light/dark cycle, at room temperature (23°C). Food (Purina rodent chow) and water were available ad libitum. Each mouse was used for one experiment and for one time point only. The Ethics Committee of the Sackler Faculty of Medicine approved the experimental protocol (M-09-055), in compliance with the guidelines for animal experimentation of the National Institutes of Health (DHEW publication 85–23, revised, 1995). A minimal number of mice were used for the study and all efforts were made to minimize suffering. All experimental manipulations were conducted during the light phase of the cycle.
Closed head mTBI injury and implantation of exendin-4 mini pumps
Experimental mTBI was induced using the concussive head trauma device described previously (Milman et al. 2005; Zohar et al. 2003). Briefly, mice were lightly anesthetized (Isoflurane) and placed under the weight-drop concussive head trauma instrument. The device consists of a metal tube (inner diameter 13 mm), placed vertically over the mouse head. A metal weight (30 g) was dropped from the top of the tube (80 cm) and struck the skull at the temporal right side between the corner of the eye and the ear. A sponge supported the head, allowing some antero-posterior motion without any rotational head movement at the moment of the impact. Immediately after the injury, mice were placed back in their cages for recovery. The effect of the injury upon behavior and cognition was studied from 7 days following the trauma (Figure 1A). Sham treated mouse groups were treated identically only the weight was not dropped.
Fig. 1.
A: Experimental time course, D-2 refers to 2 days prior to injury and mini-pump implantation; D0 refers day 0 i.e. the induction of mTBI; D7 refers to day 7 after induction of mTBI and the initiation of behavior testing; D14 refers to day 14 after the induction of mTBI and the time of euthanasia. B: mTBI (30 g) induces a deficit in novel object recognition memory in mice determined from 7 days after the induction of trauma, ** P<0.01.
The peptide Ex-4 was obtained from Bachem (Torrance, CA). In order to obtain a constant steady-state concentration of Ex-4 in mice prior to injury; Ex-4 was delivered by use of micro-osmotic pumps (7 day infusion duration), 2 days prior to the induction of mTBI. For the Ex-4 treated animals the peptide was dissolved in an equal volume of saline and dimethyl sulfoxide (DMSO) mixture and delivered from a subcutaneously implanted ALZET Micro-osmotic pump (Model 1007D, Alzet, Cupertino, CA) at a rate of 3.5 pM/kg/min. This dose of Ex-4 was utilized based upon prior studies in which it provided significant benefits to neurological markers in mouse models of neurodegeneration (Li et al., 2010; 2012). In drug treatment control groups, saline and DMSO pumps were implanted in each animal following the same surgical procedure as used for the Ex-4 treatment animals. In all animals, pumps were placed, posterior to the scapulae. Pump implantation was performed under anesthesia (ketamine/xylazine) utilizing sterile procedures. Mini pumps were implanted 48 hours prior to the weight drop injury. The behavioral study treatment groups were as follows, sham, n = 12; mTBI, n = 12; Ex-4, n = 17; Ex-4/ mTBI, n = 18.
In a parallel series of animals (sham, n = 3; Ex-4, n = 3), similar osmotic minipumps containing either vehicle alone or Ex-4 (3.5 pM/kg/min) were subcutaneously implanted and core body temperature was recorded rectally with a pediatric digital thermometer prior to mini pump implantation (0 hour) and at 12 and 36 hours afterwards. To assess for potential Ex-4-induced changes in core body temperature over time a repeated measures analysis of variance was performed. To assess for any differences in animal core body temperature between sham and Ex-4 treated animals an un-paired t test was performed, with significance determined at p<0.05, (GraphPad InStat version 3.05 for Windows 95, GraphPad Software, San Diego California USA).
Behavioral measurement
Mouse cognition was assessed using the novel object recognition paradigm. For a diagram of the experimental time course see Fig. 1 A. The experimental room was sound-insulated and had a constant level of illumination.
Novel object recognition paradigm
The Novel object recognition task was used to evaluate recognition memory; methodologies used have been described elsewhere in detail (Messier. 1997; Baratz et al. 2011; Edut et al. 2011). Briefly, this task is based on an inherent tendency of rodents to explore unfamiliar objects found in their local environment. The use of this natural property allows observers to make assessments as to whether the rodent, in this case the mouse, is able to discriminate between a familiar and a novel object. A discrimination preference index was calculated by calculating the mean time spent exploring each object.
Mouse novel object recognition data analysis
All results are given as mean ± SEM and were analyzed using SPSS 17 software (Genius Systems, Petah Tikva, Israel). One-way ANOVA was used to analyze the novel object recognition data, p values of post hoc tests were adjusted using the Fisher LSD or Tukey's HSD tests and a nominal significance level of 0.05 was used. When a comparison was made between familiar and novel objects within a specific group to show the level of recall within the group, a two tailed t-test was used.
Hippocampus RNA extraction and cDNA microarray hybridizations
After the completion of the novel object behavioral assessment, animals were euthanized and the hippocampus dissected for total RNA isolation. Total RNA was prepared using the Qiagen RNeasy Mini Kit (Qiagen, Inc. Valencia CA) following the manufacturer's specifications. Quantity and quality of the RNA was assessed using the Agilent 2100 Bioanalyzer with RNA 6000 Nano Chips. The total RNA (500 ng) was used to generate biotin-labeled cRNA using the Illumina TotalPrep RNA Amplification Kit (Ambion; Austin, TX, cat # IL1791). In short, 500 ng of total RNA was first converted into single-stranded cDNA with reverse transcriptase using an oligo-dT primer containing the T7 RNA polymerase promoter site and then copied to produce double-stranded cDNA molecules. The double stranded cDNA was cleaned and concentrated with the supplied columns and used in an overnight in vitro transcription reaction where single-stranded RNA (cRNA) was generated and labeled by incorporation of biotin-16-UTP. This cRNA was used in the hybridization reaction. Briefly, a total of 750 ng of biotin-labeled cRNA was hybridized at 58°C for 16 hours to Illumina's SentrixMouse Ref-8, v2 Expression BeadChips (Illumina, San Diego, CA). Each BeadChip has ~24,000 well-annotated RefSeq transcripts with an approximately 30-fold redundancy. The arrays were washed, blocked and the biotin labeled cRNA was detected by staining with streptavidin-Cy3. Arrays were scanned at a resolution of 0.8 µm using the Beadstation 500 X from Illumina, data was extracted from the image using Illumina BeadStudio software, V3. Mouse treatment groups utilized in the gene expression study were as follows: sham n= 5; Ex-4 n= 5; mTBI n= 4; Ex-4/mTBI n= 4. The tissues chosen to be used for gene expression studies were randomly selected from the larger set of samples generated by the behavioral experiments.
Bioinformatic array data analysis
Raw array chip hybridization image signals were filtered and processed to generate normalized data that was then transformed to create Z-scores for each gene. The Z-score transformed data was then utilized to generate a Z-ratio measurement, which allowed for the statistical analysis of the gene expression data sets. Only genes that displayed consistent significant expression changes in all samples from a given treatment group were considered for further statistical analysis. Mouse treatment groups utilized in the study are indicated in the above section. Hippocampus gene expression profile comparisons were made between the following mouse data sets: Ex-4 vs. sham mice; mTBI vs. sham mice; sham vs. Ex-4/mTBI mice and lastly Ex-4/mTBI vs. mTBI mice. Data sets underwent Parametric Analysis of Gene set Enrichment analysis (PAGE) for Gene Ontology and pathways which enabled the identification of treatment effects on Gene Ontology listings, Cellular Component, Biological Process and Molecular Function, and pathway mapping. When Z-score and Z-ratio data are compared for individual genes or gene sets (i.e. ontology or pathways) the measured values obtained for Ex-4/mTBI compared to mTBI illustrated in graphical format represent an overall change in gene/gene set expression between treatment groups, and are not represented as geometric averaged values. The original data file and the filtered, normalized results are available online in the Gene Expression Omnibus (Accession number to be provided by authors).
Array validation by quantitative (Q)-RT-PCR
In an effort to validate our cDNA array data, four genes were selected for characterization by Q-RT-PCR; gene expression comparisons were performed between Ex-4 vs. sham and mTBI vs. sham and the Genes of interest (GOI) were Mal, HexB, Junb and Apoe. Total RNA was extracted from mouse hippocampal tissue as described in the above subsection. Total RNA was reverse transcribed into complementary deoxyribonucleic acid (cDNA) using SuperScript II reverse transcriptase with random hexamers (Invitrogen, Carlsbad, CA). Quantitative PCR was performed on two occasions once in duplicate and the other in single well format, using the iTaq Sybr Green supermix with ROX (Bio-Rad, Hercules, CA). The final reaction volume of 20 µl consisted of 10 ul of the pre-made reaction mix, 1 µL primer pair and 9 µl cDNA (20 ng) in water. The reaction conditions for qPCR for the Step 1 plus qPCR System (Applied Biosystems, Foster City, CA) were as follows: 95°C for 10 min and 45 cycles of 95°C for 30 sec (denaturation step) and 60°C for 45 sec (combined annealing and extension steps). A melting curve analysis was performed by denaturation at 95°C for 15 sec, annealing at 60°C for 1 min and at a melting rate of 0.3°C/sec to 95°C. Primers for amplification were: Mal forward 5’- TTTGTGAGTTTGTCTTTGGAGGC-3’ and reverse 5’-CCGCCATGAGTACCAATTATGT-3’ (Primer Bank 6857808a1, Gene Bank accession no. NM_010762.4, 157 bp); Hexb forward 5’- TGATCCCGATGGAACAATGAGT -3’ and reverse 5’- GAGATGCCGTTTCAGTTGTCT -3’ (Primer Bank 667906a3, Gene Bank accession no. NM_008697.2, 112 bp); Junb forward 5’-GACGACCTGCACAAGATGAA-3’ and reverse 5’- TGCTGAGGTTGGTGTAGACG-3’ (Gene Bank accession no. NM_008416.1, 130 bp); ApoE forward 5’-CTGACAGGATGCCTAGCCG-3’ and reverse 5’- CGCAGGTAATCCCAGAAGC-3’ (Primer Bank 6753102a1, Gene Bank accession no. NM_009696.2, 107 bp), tubulin forward 5’-TAGCAGAGATCACCAATGCC-3’ and reverse 5’-GGCAGCAAGCCATGTATTTA-3’ (Gene Bank accession no. NM_011654.2, 87 bp).
The relative levels of transcript were quantified by standard curve methodology. Levels of mRNA expression are reported as a geometrical average of the expression of the GIO relative to the housekeeper gene mRNA for tubulin (i.e. Mal/tubulin). The geometrical average from both assessments was utilized to generate the fold change (mean ± SEM) values as shown in Fig. 7. The geometrical average data were: (1) utilized to represent the overall treatment-induced change and: (2) used for statistical analysis between the appropriate groups (Unpaired students t-test). The fold change in expression level determined by Q-RT-PCR was compared to that obtained from the array.
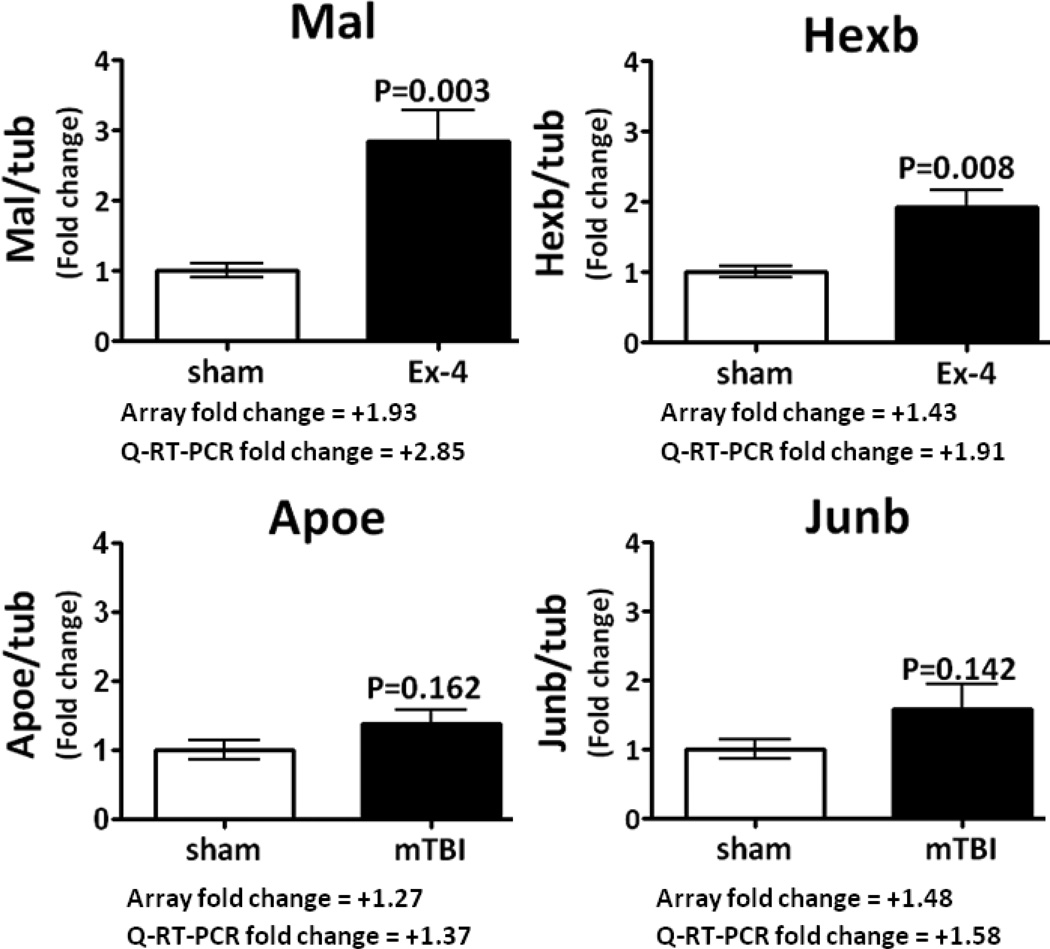
Fig. 7.
Q-RT-PCR data obtained for four hippocampal genes are presented; the relative levels of gene transcripts are expressed as a geometric average of data normalized to the house keeper gene tubulin (see Material and Methods, subsection Array validation by quantitative (Q)-RT-PCR). The genes Mal; Hexb, Apoe and Junb illustrate changes in transcript levels compared to the sham samples with near identical fold changes observed with both methods of gene expression analysis. Data are expressed as mean ± SEM, of n, observations; sham n=5, Ex-4 n=5, and mTBI n=4. The appropriate statistical P values are presented in Fig. 7.
Results
Novel object recognition behavioral measurement
The novel object recognition test was used in order to examine the visual recognition memory of the mice. mTBI mice demonstrated a reduced tendency to explore the novel object when compared to sham animals. Ex-4/mTBI mice demonstrated a tendency to explore the novel object to the same degree as was observed with the sham animals. One-way ANOVA revealed a significant effect of group [F(3, 55) = 4.93, p=0.004]. Fisher LSD post hoc analysis revealed that the preference index of the mTBI mice was significantly lower when compared with all the other groups. When the visual memory of the mice was assessed across groups, all animals apart from mTBI mice showed a significant preference for the novel object over the familiar object (Fig. 1B, t-test **p<0.01). Treatment with Ex-4 by itself did not change animal behavior, nor did Ex-4 treatment alter body core temperature (Table 1). The deficit in novel object recognition implicates abnormal neurological function in visual memory processes that were prevented by pretreatment with Ex-4. Due to the significance of the hippocampus in learning and memory and its vulnerability to mTBI (Tweedie et al. 2007), we then utilized a cDNA array screen approach to identify which genes and pathways were altered in the hippocampus by Ex-4; mTBI and in the setting of Ex-4/mTBI.
Table 1.
Mean core body temperature in sham control and Ex-4 treated mice prior and post mini-pump implantation.
| Treatment | 0 hours (pre-pump implantation) |
12 hours (post-pump implantation |
12 hours (post-pump implantation |
|---|---|---|---|
| Sham (n = 3) | *36.3 ± 0.2 | 36.6 ± 0.5 | 36.9 ± 0.4 |
| Ex-4 (n = 3) | 36.6 ± 0.3 | 36.9 ± 0.2 | 36.9 ± 0.3 |
Values represent mean ± S.E.M.
There were no statistically significant differences between pre- vs. post-treatment core temperatures, or between sham and Ex-4 values.
cDNA microarray analysis of hippocampus genes obtained from sham; Ex-4; mTBI and Ex-4/mTBI mice
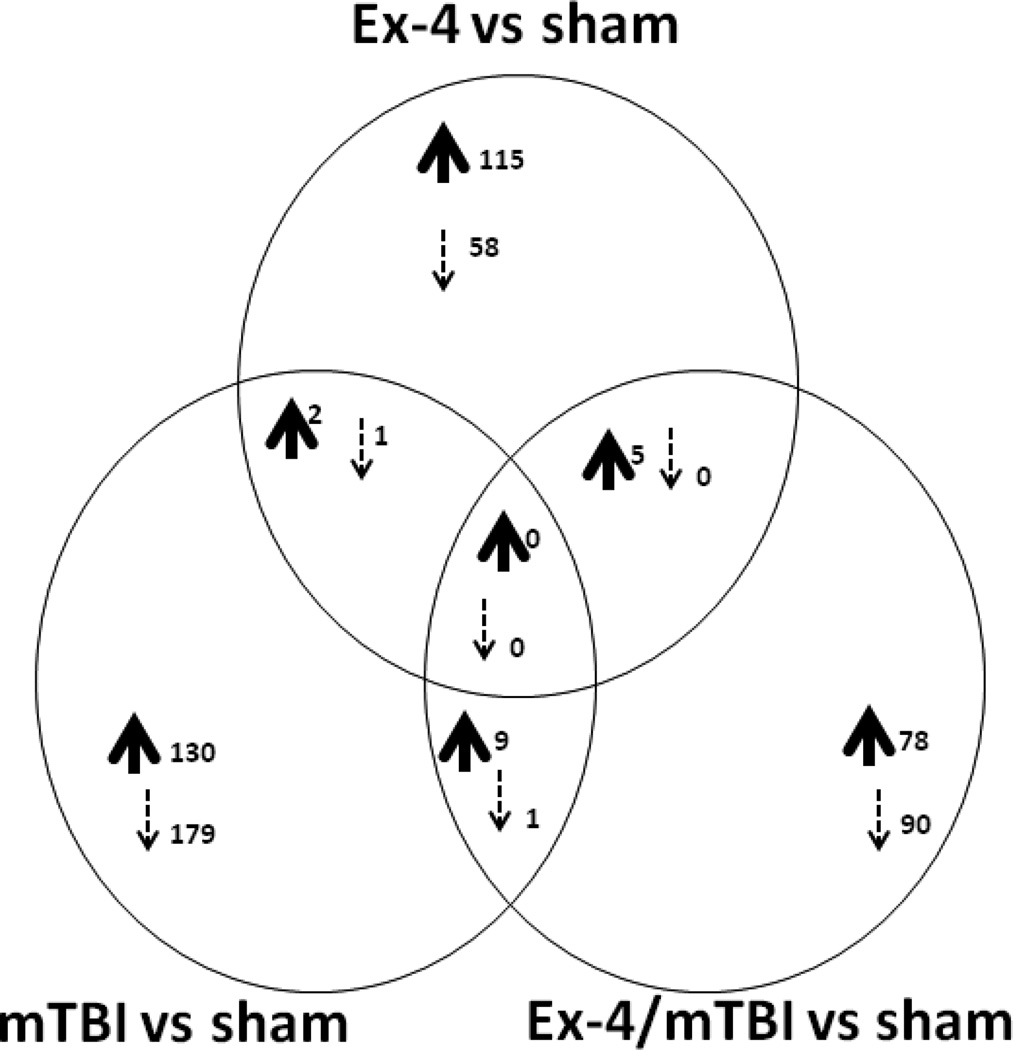
All tissues were obtained from the same side of the brain that received the trauma in the mTBI and the Ex-4/mTBI animals. We used analytical tools that allowed us to identify changes in genes altered in Ex-4; mTBI and Ex-4/mTBI mice compared to sham animals. The numbers of genes exclusively regulated and co-regulated by each treatment are illustrated by a Venn diagram in Fig. 2. There were no genes co-regulated in all three comparison groups; however, there were small subsets of genes co-regulated in two data set comparisons (i.e. Ex-4 vs. sham compared with mTBI vs. sham: 2 genes were up regulated and 1 gene was down regulated in both data sets). The identities of the co-regulated genes are indicated in Fig. 2. When comparing all 3 datasets many genes presented a more complex form of regulation (i.e. some genes were up regulated in two datasets, yet the same genes were down regulated in the third dataset); the numbers and gene identities for some of the genes are shown in Table 2. The specific details of the large numbers of genes regulated by Ex-4, mTBI, and Ex-4/mTBI all compared to sham animals are presented in supplemental Tables 1, 2, and 3: accession number; gene symbol; fold change and Z-ratios are shown.
Fig. 2.
Venn diagram illustration of the number of up and down co-regulated genes observed in hippocampus tissue from Ex-4 vs. sham mice, mTBI vs. sham mice, and Ex-4/mTBI vs. sham mice. Common co-regulated genes between Ex-4 vs. sham and mTBI vs. Sham include Atp13a1 and Dctn4 (up, n=2), and BC048546 (down, n=1). Common co-regulated genes between mTBI vs. Sham and Ex-4/mTBI vs. sham include Actb; Ap1s1; Apoe; Arl6ip4; D11Bwg0517e, Ergic3; Fkbp1s; Med25; Mlf2 (up, n=9), and Shprh (down, n=1). Common co-regulated genes between Ex-4 vs. Sham and Ex-4/mTBI vs. sham include Edrnrb; Gpm6a; Hspd1; Rnf11; Zfp238 (up, n=5).
Table 2.
Numbers of genes exhibiting non uniform gene expression changes comparing Ex-4 vs. sham mice, mTBI vs. sham mice, and Ex-4/mTBI vs. sham mice
| Group | Ex4 vs. sham | mTBI vs. sham | sham vs. Ex- 4/mTBI |
total number of genes |
|---|---|---|---|---|
| 1 | Up | Up | Down | 8 |
| 2 | Up | Down | no change | 2 |
| 3 | Up | Down | Down | 2 |
| 4 | Up | no change | Down | 217 |
| 5 | no change | Up | Down | 4 |
| 6 | Down | Up | no change | 2 |
| 7 | Down | no change | Up | 43 |
| 8 | Down | Down | Up | 3 |
| 9 | no change | Down | Up | 28 |
For all: Up refers to up regulated expression; Down refers to down regulated expression; no change refers to no change in expression between specific groups (i.e. Ex-4 vs. sham group 5). Gene identities have been provided for regulation groups where the numbers of genes were less than 10. Gene identities for group 1: BC030476; Eif3k; Mrps10; Ndufb9; Prf1; Ptprs; Wnk2; Zxda (n=8). Gene identities for group 2: Armcx1; Fgf1 (n=2). Gene identities for group 3: Rcan2; Sumo1 (n=2). Gene identities for group 5: Bai2; Ifngr2; Rpl24; Rpl29 (n=4). Gene identities for group 6: Mrpl53; Rprml (n=2). Gene identities for group 8: Pde1a; Scrg1; Tbc1d20 (n=3).
Treatment with Ex-4 induced an increased expression in hippocampus gene transcripts for over 300 genes (Fig. 2, supplemental Table 1); the largest detected fold change in gene expression was +2.60 for the gene Stmn1 (stathmin 1). Ex-4 treatment reduced the expression of approximately 100 genes (Fig. 2, supplemental Table 1), the largest detected fold change was −2.03 for the gene Kras (v-Ki-ras2 Kirsten rat sarcoma viral oncogene homolog). In mTBI tissues, more than 120 genes were up regulated compared to sham animals (Fig. 2, supplemental Table 2); the largest detected fold change was +1.59 for the gene Hist1h2bm (histone cluster 1, H2bm). More than 170 genes were down regulated (Fig. 2, supplemental Table 2); the largest detected fold change was −2.42 for the gene Ccdc53 (coiled-coil domain containing 53). Comparing sham with Ex-4/mTBI gene expression indicated that there was a difference of approximately 150 and 320 genes up and down regulated, respectively. The accompanying fold changes were +2.72 (Mtap1b; microtubule-associated protein 1B) and −2.49 (Stmn1 (stathmin 1), respectively. In addition we have compared Ex-4/mTBI mice to mTBI animals (supplemental Table 4). The largest fold change in gene expression observed was +3.10 for the gene Ccng2 (Cyclin G2); the largest down regulation was −2.70 for the gene Mtap1b (microtubule-associated protein 1B). In all over 490 genes were up regulated and 280 genes were down regulated comparing the two groups.
Gene ontology analysis
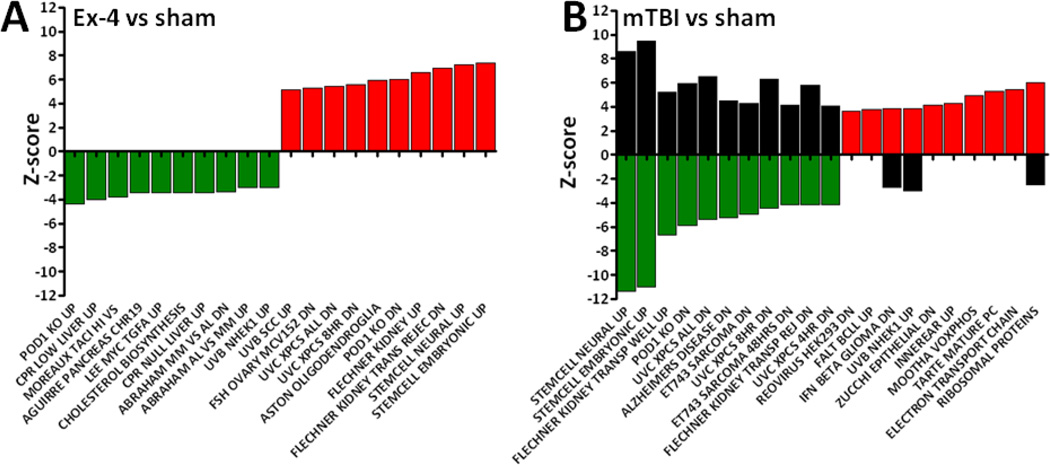
Due to the large numbers of genes regulated by the treatments we chose to illustrate data for the 10 most up and down regulated groupings for Gene Ontology; Molecular Function; Cellular Component and Biological Process for the Ex-4, mTBI groups relative to sham animals. The lists are provided in a graphical form in supplemental Figs. 1 and 2, the figures display the Z-score, y-abscissa, and the GO term description on the x-abscissa; for these and all subsequent graphs green bars indicate down regulated gene sets and red bars indicate up regulated gene sets. Supplemental Fig. 2 displays the effects of mTBI on gene sets and additionally the changes in gene sets observed in Ex-4/mTBI animals compared to mTBI animals, (black bars). In this and all subsequent graphs, black bars indicate Ex-4 effects on mTBI. Interestingly, where Ex-4 treatment altered gene sets compared to mTBI, the drug did not potentiate any mTBI induced changes; typically Ex-4 prevented the mTBI induced changes in gene set Z-scores. However, the absence of a black bar indicates a lack of effect of Ex-4 treatment on mTBI-induced gene sets. For all figures CNS and neuronal related data sets are indicated with an asterisk *.
CNS gene ontology terms regulated by Ex-4 vs. sham
Compared to sham animals, Ex-4 treatment caused reductions in expression of gene sets associated with the following nervous tissue related GO terms: olfactory receptor activity (GO0004984) and sensory perception of smell (GO0007608); neuropeptide Y receptor activity (GO0004983); and synaptic vesicle membrane (GO0030672). In contrast Ex-4 treatment increased the expression of gene sets related to myelination (GO0042552) and behavior (GO0007610); and dopamine D1 receptor (GO0001590) and response to amphetamine (GO0001975).
CNS gene ontology terms regulated by mTBI vs. sham
Compared to sham animals, mTBI reduced the expression of nervous tissue related gene sets associated with: GABAA receptor activity (GO0004890); gamma aminobutyric acid signaling pathway (GO0007214); extracellular ligand gated ion channel activity (GO0005230); neurotransmitter receptor activity (GO0030594); nicotinic acetylcholine gated receptor channels (GO0005892); aspects of the autonomic nervous system development (GO0048483), and neurogenesis (GO0022008) were down regulated. In contrast, mTBI induced an up regulation in gene sets for: forebrain development (GO0030900); axongenesis (GO0007409); negative regulation of neurogenesis (GO0050768); regulation of circadian sleep or wake cycle (GO0045187); and olfactory receptor activity (GO0004984).
Pathway analysis
The biological relevance of the altered gene sets associated with behavioral deficits and drug treatment benefits on behavior may be better understood when considered in light of changes seen in pathway mapping. Pathway mapping is a tool that helps to group genes into functionally related categories. Pathway mapping data are shown in Fig. 3, the magnitude of the change in a pathway gene set, the Z-score, is shown on the y-abscissa, and the pathway name is shown on the x-abscissa. Ex-4 induced changes in pathway mapping for the 10 most altered pathways are summarized in Fig. 3A. Pathway gene sets for mTBI tissues are shown in Fig. 3B; gene changes for Ex-4/mTBI treatment on pathways are shown as black bars, where appropriate. Treatment with Ex-4 caused a marked up regulation of genes associated with pathways related to ‘stemcell neuronal up’, this effect is opposite to what was seen in the mTBI animals. Interestingly, the increase in expression of the ‘stemcell neuronal up’ pathway induced by Ex-4 was apparent even after the induction of mTBI, (Fig. 3B, black bars). Pretreatment with Ex-4 prior to the induction of trauma largely prevented the down regulation of pathway gene sets regulated by mTBI (green), as indicated by a shift in gene expression Z-score in the opposite direction to that obtained for the mTBI vs. sham group, (Fig. 3B, black bars).
Fig. 3.
Effects of treatment with Ex-4 vs. sham and mTBI vs. sham on pathway Z-scores are presented. A: The effects of Ex-4 treatment vs. sham and B mTBI vs. sham on the 10 most down regulated (green) and up regulated (red) pathway gene sets in mouse hippocampus are illustrated. In panel B, the presence of a black bar indicates where Ex-4 treatment prior to mTBI induced a change in gene sets relative to the mTBI group. Where no black bar is present, Ex-4 had no effect on gene sets relative to mTBI. Treatment with Ex-4 prevented the down regulation of the 10 most affected pathways associated with mTBI, while Ex-4 treatment had beneficial effects upon three of 10 up regulated pathways.
Effects of mTBI and Ex-4 treatment prior to trauma upon the ‘stemcell neural up’ pathway
Fig 4 illustrates a gene expression heat map of significant changes in the gene expression Z-ratio of genes altered in the ‘stemcell neural up’ pathway, that are common to both mTBI vs. sham mice and Ex-4/mTBI vs. mTBI mice. Genes in this pathway were mostly down regulated by mTBI compared to sham animals to give a Z-score of −11.36. However, Ex-4/mTBI animals displayed an alteration in gene expression that gave a Z-score of +8.60, compared to mTBI (Fig. 3B and Fig. 4). The majority of genes altered by mTBI displayed a reduced level of expression of variable magnitude; the ratios were towards the green end of the scale. However, a subset of genes manifested an elevation in expression compared to sham tissues as shown in Fig. 4; the ratios were towards the red end of the scale. The changes in gene expression observed in Ex-4/mTBI mice are almost the reverse of those observed in mTBI mice (Fig. 4). Where genes were down regulated by mTBI, the effects of Ex-4 largely prevented or reversed the mTBI-induced shift in the Z-ratios. Examples of such EX-4/mTBI-induced shifts include Sdcbp (syndecan binding protein (syntenin): −1.33 to +0.56 Z-ratio); Akap7 (A kinase (PRKA) anchor protein 7: −2.87 to +0.93 Z-ratio); Ide (insulin-degrading enzyme: −1.60 to +0.47 Z-ratio); Vbp1 (von Hippel-Lindau binding protein 1: −2.04 to +4.61 Z-ratio). Likewise in the subset of genes up regulated by mTBI, Ex-4 treatment prevented or reversed those effects: Mrps10 (mitochondrial ribosomal protein S10: +2.20 to +0.12 Z-ratio); Pgls (6-phosphogluconolactonase: +3.18 to −1.38 Z-ratio); Polr2f (polymerase (RNA) II (DNA directed) polypeptide F: +2.51 to −2.57 Z-ratio) and Dbi (diazepam binding inhibitor): +0.65 to −0.79 Z-ratio).
Fig. 4.
Ex-4 treatment largely reversed any changes in gene expression induced by mTBI. Common significant gene expression changes in ‘stemcell neuronal up’ pathway observed in mTBI and Ex-4/mTBI animals are shown. A heat map illustrating an up regulation of genes is shown as red and a down regulation of genes as green. Gene identities are shown as gene symbol, the scale for expression level is shown on the upper right; the group comparisons are shown at the bottom of each section on the heat map.
mTBI and Ex-4 pretreatment effect upon ‘Alzheimer’s disease dn’ pathway
Additional pathways of significant interest are related to AD. TBI has been associated with the onset of AD and our data support this link as we, herein, identified mTBI induced changes in genes linked with several AD pathways. Importantly we determined that pretreatment with Ex-4 demonstrated a robust opposite effect on many genes regulated by mTBI (Fig. 5), additionally Ex-4 was able to restore multiple gene expression levels to that observed in sham animals (Fig. 6).
Fig. 5.
Ex-4 treatment largely blocked any changes in gene expression induced by mTBI. Common significant gene expression changes in ‘alzheimers disease dn’ pathway observed in mTBI vs. sham and Ex-4/mTBI vs. mTBI animals are shown. A heat map illustrating an up regulation of genes is shown as red and down regulation of genes as green. Gene identities are shown as gene symbol, the scale for expression level is shown on the upper right; the group comparisons are shown at the bottom of each section on the heat map.
Fig. 6.
Ex-4 treatment largely restored gene expression levels induced by mTBI close to those of sham animals. Common significant gene expression changes in ‘alzheimers disease dn’ pathway observed in mTBI vs. sham, Ex-4/mTBI vs. mTBI and Ex-4/mTBI vs. sham animals are shown. A heat map illustrating up and down regulated genes is shown as red and green, respectively. Where gene expression levels were found to be not different between Ex-4/mTBI and sham comparisons (Z-ratios close to zero) they appear dark brown/green in the heat map. Gene identities are shown as gene symbol, the scale for expression level is shown on the upper right; the group comparisons are shown at the bottom of each section on the heat map.
Fig. 5 illustrates, in the form of a gene expression Z-ratio heat map, a list of genes regulated in both mTBI and Ex-4/mTBI animals, where in the Ex-4/mTBI mice a strong opposite effect in gene expression vs. mTBI was found. The effects of mTBI vs. sham and Ex-4/mTBI vs. mTBI are presented. In this pathway mTBI altered gene expressions to generate a Z-score of −5.25; while pretreatment with Ex-4 largely induced an opposite effect to that observed in the mTBI group; gene changes in Ex-4/mTBI animals generated a Z-score of +4.52 (Fig. 3B). mTBI induced increases or decreases in gene expression were by and large reversed by treatment with Ex-4 (Fig. 5). A hallmark protein associated with AD is the product of the gene APP. This gene codes for amyloid-β precursor protein (APP); mTBI up regulated the expression of this gene while Ex-4 treatment prevented the mTBI-induced increased expression (APP +1.40 to −1.76 Z-ratio, Fig. 5). The most striking change in Z-ratio was observed for a gene whose protein product is a member of a class of proteins classically believed to be involved in cell cycle processes; Cyclin G2, (Ccng2 −6.46 in mTBI to +5.91 for Ex-4/mTBI Z-ratio, Fig. 5).
Treatment with Ex-4 prior to injury restored the levels of gene expression for multiple genes associated with the ‘Alzheimer’s disease dn’ pathway, to close to sham levels. An additional data set of gene expressions common to all three comparison groups is represented by Z-ratio heat map (Fig. 6). A third comparison between Ex-4/mTBI vs. sham animals shows that the gene expression of multiple AD related genes from Ex-4/mTBI mice is more akin to the expression profile of sham animals (represented by the Ex-4/mTBI vs. sham data column (Fig. 6)). Many genes show marked changes by mTBI (i.e. mTBI vs. sham) and Ex-4/mTBI (i.e. Ex-4/mTBI vs. mTBI). However in the Ex-4/mTBI vs. sham group no overt changes in gene expression were detected (as indicated by Z-ratios close to zero). This restorative effect of Ex-4 treatment was clearly observed for the following genes: Cck (cholecystokinin) where mTBI induced an up regulation of expression compared to sham, the Z-ratio was +2.68; Ex-4/mTBI induced a down regulation in expression compared to mTBI animals, the Z-ratio was −1.87); a comparison of Ex-4/mTBI to sham animals showed no marked changes in levels of expression where the Z-ratio was −0.37. Additional genes that strongly presented this form of regulation are: Armcx1 (armadillo repeat containing, X-linked 1; (Z-ratios are: −2.33 (mTBI v.s. sham), +2.15 (Ex-4/mTBI v.s mTBI), +0.94 (Ex-4/mTBI v.s. sham)); Ube1c (ubiquitin-like modifier activating enzyme 3; (Z-ratios are: −3.53 (mTBI v.s. sham), +1.89 (Ex-4/mTBI v.s mTBI), −0.16 (Ex-4/mTBI v.s. sham)); Orc5l (origin recognition complex, subunit 5; (Z-ratios are: −1.72 (mTBI v.s. sham), +0.95 (Ex-4/mTBI v.s mTBI), −0.05 (Ex-4/mTBI v.s. sham)); Ttc1 (tetratricopeptide repeat domain 1; (Z-ratios are: −2.11 (mTBI v.s. sham), +1.67 (Ex-4/mTBI v.s mTBI), +0.53 (Ex-4/mTBI v.s. sham)); Camk2a (calcium/calmodulin-dependent protein kinase II alpha; (Z-ratios are: +2.34 (mTBI v.s. sham), −1.03 (Ex-4/mTBI v.s mTBI), +0.37 (Ex-4/mTBI v.s. sham)); Atp6v1e1 (ATPase, H+ transporting, lysosomal 31kDa, V1 subunit E1; (Z-ratios are: +1.77 (mTBI v.s. sham), −1.04 Ex-4/mTBI v.s mTBI), −0.02 (Ex-4/mTBI v.s. sham)) and Nrn1 (neuritin 1; (Z-ratios are: +1.52 (mTBI v.s. sham), −1.44 Ex-4/mTBI v.s mTBI), −0.66 (Ex-4/mTBI v.s. sham)), for a more complete list see Fig. 6.
Other pathways related to AD were identified; however, they did not fall into the category of 10 most up or down regulated by mTBI. They include ‘Alzheimer’s disease up’ and ‘Alzheimer’s disease incipient dn’ and the ‘p35 Alzheimer’s pathway’ genes. For the ‘Alzheimer’s disease up’ pathway gene expression was altered by mTBI providing a Z-score of +2.89, while the pretreatment with Ex-4 generated a Z-score of −2.37. The ‘Alzheimer’s disease Incipient dn’ pathway demonstrated mTBI–induced changes in expression to yield a Z-score of −2.10, and pretreatment with Ex-4 reversed the gene expression to a Z-score of +2.86. The ‘p35 Alzheimer’s pathway’ was not regulated by mTBI, yet it was altered by Ex-4/mTBI giving a Z-score of −2.82. It is of importance to note that many of the AD related genes displaying regulation by mTBI, were all largely reversed, or restored to near sham levels by treatment with Ex-4.
Q-RT-PCR validation of array data
Fig. 7 illustrates Q-RT-PCR gene expression data for four genes investigated to validate our cDNA array gene expression data. Shown are the changes in expression levels of the appropriate genes of interest for the two relevant treatment groups; specifically, Ex-4 vs. sham and mTBI vs. sham. Additionally the fold change in gene expression obtained by both the Q-RT-PCR and the array methods are presented for comparison. All genes examined presented gene expression changes between sham and the appropriate treatment groups with nearly identical fold changes comparing the array and Q-RT-PCR methodology, those genes were: Mal (T-cell differentiation protein or myelin and lymphocyte protein); Hexb (hexosaminidase B (beta polypeptide)) Apoe (apolipoprotein E) and Junb (jun B proto-oncogene).
Discussion
Behavioral data described in this report is similar to prior published findings where a primary mTBI event induces secondary deficits in one of several indices of rodent cognition observed in our weight drop model (Pan et al. 2003, Zohar et al. 2003, Milman et al. 2005; Baratz et al. 2011). We also identified many genes that were found to be both exclusively regulated and co-regulated in our treatment groups two weeks after the primary trauma event; additionally there were many genes that displayed a more complex form of treatment-dependent expression profile (Fig. 2, Table 2). Potential caveats to this study are (1): the choice to use of this form of the closed head weight drop mTBI model regarding aspects of injury reproducibility. Our model differs with other commonly used head injury models, such as the fluid percussion injury (FPI) and the controlled cortical impact injury (CCI) models of head trauma in several aspects. Both the FPI and CCI models of head trauma rely upon the use of stereotaxic placement of devices to apply an injury via a craniectomy, directly to the exposed dura of the brain (Dixon et al. 1987; 1991; Lighthall, 1988; Lighthall et al. 1990; Sullivan et al. 1976; Saunders et al. 1979). The injuries are generated by a fluid compression of the brain or a probe penetration of the brain dura for the FPI or CCI models, respectively. The use of these models thereby allow for the generation of highly reproducible brain injuries of a moderate to severe form. Interestingly, weight drop models of head trauma can be either closed or open head in nature, similar to the FPI and CCI models with an exposed dura (Dial et al. 1981; Feeney et al. 1981). In open head injury models a craniectomy is performed with a stereotaxic frame which allows for a highly reproducible form of injury. With this injury and CCI, a small necrotic cavity forms at the injury site, which over time increases in size generating a somewhat more severe form of TBI. However, with the closed head weight drop model, as utilized in this study, there is no stereotaxic head restraint used to guide the weight to the site of impact. A possible disadvantage of this system is that there may be a higher degree of variability as to the point of impact of the weight on the mouse head. However, importantly for our study, due to the lack of overt damage to the mouse skin, skull and underlying brain tissues, this model can be considered as a mild TBI. Additionally, our model may more closely represent the clinical nature of the majority of human TBIs, as most civilian clinical incidents are considered mild in nature (De Kruijck et al. 2001), and are generated by diverse physical traumas and varying degrees of force occurring at different head sites. Similar to human mTBI, a diffuse neuronal damage occurs in our model, rather than a focal lesion. Hence, although no single animal model of mTBI is fully representative of the diversity in man (Marklund and Hillered 2011), our non invasive mTBI mouse model, like that used in the rat (Marmarou et al., 1994), has numerous features relevant to traffic accidents, sports and battle injuries and falls.
We chose to focus upon the 10 most up and down regulated gene ontologies and pathways regulated by the treatments. We could have missed more subtle, yet biologically important changes in gene regulations; however, we believe that we were able to identify highly relevant ontology and pathway data related to both mTBI and pretreatment of mTBI animals with Ex-4. The relatively small fold changes in gene expression observed in our array study made array validation more difficult; nonetheless, our Q-RT-PCR data illustrates that there was strong agreement between the two methods even with low fold changes in gene expression.
The question arises about whether the changes in genes observed in mTBI animals are linked to the observed secondary cognitive changes observed in these animals. Importantly, treatment of animals with Ex-4, a GLP-1 receptor agonist, prior to the induction of trauma abolished the memory deficit and mostly prevented the mTBI-induced changes in gene pathways, and to a lesser extent, gene ontology classifications. This implies that the cellular and molecular signaling events initiated by Ex-4 are both relevant to, and beneficial in, the setting of rodent mild head trauma, and that Ex-4 treatment may possess favorable effects in managing secondary events observed in human head trauma. Of note, our Ex-4 dose (3.5 pM/kg/min equivalent to 21 µg/kg/day) has not only proven efficacious in both AD and amyotrophic lateral sclerosis preclinical mouse models (Li et al. 2010, 2012), but also compares favorably with Ex-4 dosing in human type 2 diabetes mellitus studies (once weekly exenatide LAR: 2 mg/week administration delivers 5.7 µg/kg/day for a 50 kg person; Drucker et al., 2008). In addition, Ex-4 treatment was initiated 48 hours prior to the mTBI event to allow the development of steady-state plasma and brain levels of compound prior to mTBI and, to allay the known acute transient actions of GLP-1 receptor agonists on body temperature (O’Shea et al., 1996; Griffioen et al. 2011), since hypothermia has been shown to reduce mortality and improve outcome in human TBI (Sadaka and Veremakis 2012). In light of reports that systemic (intraperitoneal) administration of Ex-4 (3.0 µg/kg (Hayes et al. 2008)) and GLP-1 (300 µg/250 g (O’Shea et al. 1996)) have been described to transitorily induce hypothermia (approximately 1°C) in rats that is fully reversed within 4 hours, core body temperature was evaluated in our studies prior to and following Ex-4 and sham treatments (Table 1). No significant changes in core body temperature were evident, thereby ruling out the potential for drug-induced hypothermia to account for mTBI amelioration. In a similar manner, cessation of systemic Ex-4 administration in our study occurred 48 hours prior to behavioral studies to provide a sufficient washout period to ensure that potential direct actions of Ex-4 on cognition could not confound the neuroprotective/regenerative actions of the agent associated with mTBI. Likewise, gene array analyses were undertaken days following the behavioral studies to mitigate potentially confounding effects of any transient changes in immediate early genes, such as Arc (activity-regulated cytoskeleton-related protein (Guzowski et al. 1999)), induced by the behavioral paradigm.
The GLP-1 receptor is expressed in several regions of rodent and human brain, validating the utility of CNS mediated GLP-1 receptor agonist actions as a viable mode of treatment in clinical neurological disorders (Göke et al. 1995a, 1995b; Perry and Greig 2003; Baggio and Drucker 2007). The efficacy of GLP-1 receptor signaling observed here is in line with literature describing GLP-1 and Ex-4 brain entry (Kastin and Akerstrom, 2003), and GLP-1 receptor-mediated actions in various CNS neurological conditions (for review see, Holsher 2010; Holst et al. 2011; Bak et al. 2011; Salcedo et al. 2012). The utility of GLP-1 receptor stimulation in diverse disease states such as AD, PD, HD, ALS, stroke and peripheral neuropathy signifies commonalities in what are likely to be the latter stages of neuronal cell death observed in these conditions (Li et al. 2009, 2010, 2012; Martin et al. 2009; Perry et al. 2007). The GLP-1 peptide and Ex-4 both act through a class B family of 7-transmembrane-spanning, heterotrimeric G-protein-coupled receptors. Receptor activation stimulates adenylyl cyclase via the G-protein Gα; this in turn causes an increase in cAMP levels that regulates many diverse cellular responses including the activity of pro-survival factors (Li et al. 2010b;Salcedo et al. 2012). A primary biological consequence of GLP-1 receptor signaling in the peripheral system is to regulate glucose metabolism by facilitating the insulin dependent transfer of glucose into cells. The novel object recognition assessment has been shown to be sensitive to glucose (Messier 1997). Perhaps a part of the improvement seen in the novel object recognition assessment here is mediated by GLP-1 receptor mediated actions on brain glucose metabolism. However, considering the large number of genes regulated in the Ex-4 and Ex-4/mTBI animals, it is unlikely that the observed benefits are solely due to improved brain glucose metabolism.
In a prior study utilizing the same mTBI model that focused upon gene expression changes, it was reported that 3 days post trauma in neocortex, gene sets connected with stress responses, inflammation, immunity and defense responses were all up regulated compared to sham animals (Israelsson et al. 2009). Supporting this, we recently demonstrated a role of the potent pro-inflammatory cytokine TNF-α in mediating mTBI-induced behavioral deficits that were reversed by administration of a small molecule TNF-α synthesis inhibitor (Baratz et al. 2011). Interestingly, in the Baratz study mouse behavioral deficits were minimal at 3 days post trauma, yet became significant by 7 days post trauma with a 50 g weight drop. This signifies the importance of the time interval between the primary trauma and the onset of secondary behavioral disturbances that are associated with mTBI. There are a few contrasts between our study and the Israelsson et al. (2009) study, related to the numbers of genes regulated by mTBI and the fold change in gene expression. Israelsson et al. (2009) presented data on 37 genes that were all up regulated with fold changes in gene expression ranging from +3.0 up to +20.9 fold. In contrast, we have found more than 200 genes to be regulated by mTBI, but with smaller fold changes. The largest up and down regulation determined in our study was +1.59 and −2.42 fold, respectively. The discrepancy in the numbers of genes and the degree of expression is likely attributed to the longer time interval between the primary and secondary events utilized in this study. However, an important similarity between the two studies relates to the up regulation of a set of genes associated with the ‘acute phase response’ ontology classification. The ‘acute phase response’ is defined as an acute inflammatory response that involves non-antibody proteins whose concentrations in the plasma increase in response to infection or injury in homeothermic animals. In our study this ontology grouping was up regulated in the mTBI tissues compared to sham even at 14 days post injury (supplemental Fig. 2 D, Biological Process). Importantly, pretreatment with Ex-4 prior to induction of trauma prevented the associated up regulation of this immunological ontology, indicating that Ex-4 treatment possesses anti-inflammatory actions, as has been suggested by others (Darsalia et al. 2012; Kim-Chung et al. 2009).
Additionally, this study highlights a marked down regulation in GABA signaling induced by mTBI; again these findings are in accord with prior studies (Bonislawski et al. 2007; Gibson et al. 2010). It is thought that a lack of GABA-ergic inhibitory action in the dentate gyrus region of the brain makes the hippocampus more prone to aberrant excitability and possibly seizures. In a more severe model of TBI, involving fluid percussion in mouse, an impairment of GABAA-ergic signaling was found to be due to a reduced function of the cell membrane chloride channel protein, (KCC2, K-Cl co-transporter-2). Gibson and co-workers (2010) demonstrated time-dependent changes. There were also both increases and reductions in the protein components of GABAA receptors in a rat fluid percussion model of TBI, where receptor protein components were evaluated over a 3 hour to 7 day time period after the injury. In our study it is interesting to note that while Ex-4 caused a marked improvement in the novel object recognition memory assessment, there were no marked changes in GABA signaling gene sets. This suggests that, at least at the 14 day time interval, Ex-4 treatment had no direct action on events responsible for the alteration in GABA-ergic transmission. The role of GABA-ergic transmission in brain is complex; impairments in GABA signaling have been linked to learning and memory deficits in mice as was seen here (Andrews-Zwilling et al. 2010), and somewhat counter-intuitively it is also known that GABA signaling is responsible for anti-proliferative effects in rodent brain in vivo, thus negatively impacting neurogenesis (Liu et al. 2005; Fernando et al. 2011). A better understanding of the precise role of impaired GABA signaling in this particular model of mTBI will require additional experiments.
Herein, pathways associated with neural stem cells were markedly down regulated by mTBI; the ensuing inflammatory response triggered by mTBI as described by Israelsson and co-workers (2009) may be responsible for this effect, as inflammation is believed to impair neurogenesis (Monje et al. 2003; Russo et al. 2011a, 2011b). Inflammation associated with early events in this model may alter the responses of neuronal precursor cells to endogenous stimulation via the activation of cell surface receptors. Under normal physiological conditions activation of GLP-1 receptors in vivo has been reported to induce cell proliferation, specifically neural cell proliferation in brain tissues, which has been observed in association with an enhanced memory function in rodents (Belsham et al. 2009; Bertilsson et al. 2008; Hamilton et al. 2011; Isacson et al. 2011; Li et al. 2010; McClean et al. 2011). However, given the time for a new neuron to differentiate from a precursor cell into a fully functional neuron (van Praag et al. 2002), it is unlikely that the behavioral benefit observed here was specifically due to the functional integration of new neurons induced by treatment with Ex-4. We have no data to illustrate what, if any, changes in adult hippocampal neural precursor cell differentiation took place in these animals; therefore we cannot be definitive regarding a possible involvement of immature neurons generated by Ex-4 treatment in this model. However, as clear benefits in behavior and changes in gene sets involved in neural stem cells were observed, it is feasible that GLP-1 receptor stimulation triggered CNS tissues to generate neurotrophic factors responsible for the activation of neural stem cell gene transcripts. Alternatively, or in addition to direct effects of Ex-4 on neural stem cell pathways, the suppression of mTBI-induced inflammation may ameliorate any anti-neurogenic effects of inflammation, thereby facilitating neurogenesis in these mice.
Lastly, there is an established link between head trauma and the subsequent development of neurodegenerative diseases exemplified by AD (Costanza et al. 2011; Johnson et al. 2010; Kiraly and Kiraly 2007; Uryu et al. 2003). Our data support this linkage as in this study mTBI animals displayed significant changes in gene sets related to AD. The most marked treatment-induced change in gene expression was for the gene Ccng2, which codes for the protein Cyclin G2. Classically, Cyclin proteins have been shown to have a major role in the regulation of signaling events associated with cell division in cells actively undergoing mitosis. While mature neuronal tissue no longer undergoes mitosis the inappropriate reactivation of cell cycle processes in adult neurons has been proposed as a possible mechanism of cell death in neurodegenerative diseases exemplified by AD (Currais et al. 2009; Sultana and Butterfield 2007). The level of a closely related Cyclin protein family member, Cyclin G1 has been demonstrated to be elevated in human, non-pathological AD brain tissue and in regenerating hypoglossal nerve tissue after experimental transection (Morita et al. 1996; Jordan-Sciutto et al. 1999). However, the Cyclin G2 protein has been linked with suppression of the progression of the cell cycle (Bennin et al. 2002; Martínez-Gac et al. 2004); thus low protein levels in neurons may allow the inappropriate reactivation of the cell cycle and ultimately cell death, while elevated levels would be predicted to block this phenomenon. Our data illustrate an mTBI-induced loss of Cyclin G2 expression and importantly an Ex-induced recovery of expression that may play a role in blocking cell cycle reactivation induced-cell death. Currently, little is known about the role of Cyclin G2 in CNS neurodegeneration and further studies are required to elucidate any potential role in neuronal cell death. Interestingly, marked changes in glial cell activation are considered a hallmark feature of TBI (Saijo and Glass, 2011). Accordingly, due to the high brain content of glial cells (Azevedo et al., 2009) the observed alterations in cell cycle genes may in part reflect alterations in glial cell activation. Additional studies would be required to define the respective identities of glial over neuronal, cell cycle markers.
In our study we observed an up regulation of the gene that codes for APP, which can be proteolytically cleaved to form the pro-aggregatory neurotoxic Aβ peptide, a hallmark protein involved in the pathology of AD (Fig. 5). Importantly, while mTBI up regulated APP expression, treatment with Ex-4 prevented the mTBI-induced increase (APP +1.40 vs −1.76 Z-ratio). This is in agreement with our previous finding utilizing Ex-4 treatment in a mouse model of AD and in vitro cell culture studies (Li et al. 2010a). Additionally, a gene thought to be involved in AD is the insulin degrading enzyme (Ide), a protease that can target and degrade the neurotoxic Aβ peptide. mTBI induced a down regulation of the expression of this gene whereas Ex-4 treatment prevented this shift in expression (Ide −1.60 vs +0.47 Z-ratio). A potential biological benefit of these Ex-4 mediated effects on APP and Ide may translate to a reduction in altered Aβ peptide levels in brain, as has been reported in AD animal models (Perry et al. 2003; Li et al. 2010b; Bomfim et al. 2012) as well as in TBI (Uryu et al. 2002; Goldstein et al. 2012). Another important gene involved in AD is apolipoprotein (ApoE), more specifically the presence of the ApoE4 allele. The presence of the ApoE4 allele is associated with an enhanced risk for the development of AD; the presence of two ApoE4 alleles both increases the risk and lowers the age of onset of AD (Lahiri et al. 2004; Farrer et al. 2007; Zhong and Weisgraber 2009). In our study the gene expression of ApoE was found to be up regulated by mTBI in both the array and Q-RT-PCR validation experiments (Fig. 6), while Ex-4 treatment prevented this trauma induced change (ApoE +2.90 vs −3.20 Z-ratio). While it is important to realize that the tissues utilized in this study were from mouse, the implications for human mTBI may be important as Ex-4 treatment prevented the enhanced expression of the ApoE gene.
Conclusion
In summary, treatment of mice with Ex-4 induces positive effects on gene expression for several well defined proteins involved in Alzheimer’s disease. We have shown that treatment of mice with exendin-4 prior to an mTBI event prevented the manifestation of a trauma associated behavioral deficit and largely reversed, or restored to sham levels, TBI-induced changes in expression of many hippocampal genes. Based on these data one could predict that the utility of such treatments, mediated through the activation of GLP-1 receptors, may have substantial benefits in preventing TBI associated secondary illness in man. It is also reasonable to hypothesize a favorable outcome regarding the development of dementia-related diseases at later stages in life even decades after an initiating primary brain insult. As an aside, perhaps neurological data obtained from diabetic patients who are currently prescribed Ex-4 (known as Byetta®), or other GLP-1 peptide mimetics for type II diabetes, will generate valuable data relating to any possible changes in the incidence of neurodegenerative disease in later stages of life. Only through further research, using longer time frames involving TBI events in animal models susceptible to AD and other dementias, will we be able to test this hypothesis.
Supplementary Material
The effects of Ex-4 treatment vs. sham on the 10 most down regulated (green) and up regulated (red) gene sets in mouse hippocampus are illustrated. * - indicates a CNS or neuronal related gene set. Treatment effects on Z-score data for Gene Ontology (A); Molecular Function (B); Cellular Component (C) and Biological Process (D) are shown.
The effects of mTBI vs. sham on the 10 most down regulated (green) and up regulated (red) gene sets in mouse hippocampus are illustrated. The presence of a black bar indicates where Ex-4 treatment prior to mTBI induced an expression change in gene sets relative to the mTBI group. Where no black bar is present, Ex-4 had no effect on gene sets relative to mTBI. Treatment with Ex-4 prevented some but not all changes in gene sets altered by mTBI. * - indicates a CNS or neuronal related gene set. Treatment effects on Z-score data for Gene Ontology (A); Molecular Function (B); Cellular Component (C) and Biological Process (D) are shown.
Highlights.
Mild traumatic brain injury is associated with cognitive defects in visual memory in mice.
Mild traumatic brain injury induces marked changes in the regulation of hippocampus genes.
Treatment with exendin-4 prevents injury related changes in mouse behavior and gene regulation.
Changes in Alzheimer’s disease genes were prevented by treatment with exendin-4.
Acknowledgments
Acknowledgments and role of the funding source
This research was supported in part by the Sackler School of Medicine, Tel-Aviv University, and the Intramural Research Programs of both the National Institute on Aging and National Institute on Drug Abuse, National Institutes of Health.
Abbreviations
- TBI
traumatic brain injury
- mTBI
mild traumatic brain injury
- AD
Alzheimer’s disease
- CNS
central nervous system
- Ex-4
exendin-4
- GLP-1
glucagon-like peptide-1
- PD
Parkinson’s disease
- ALS
amyotrophic lateral sclerosis
- HD
Huntington’s disease
- DMSO
dimethyl sulfoxide
- cDNA
complementary DNA
- cRNA
complementary RNA
- PAGE
parametric analysis of gene set enrichment
- Q-RT-PCR
quantitative reverse transcriptase PCR
- GO term
gene ontology term
- APP
amyloid-beta precursor protein
- GOI
gene of interest
- TNF-alpha
tumor necrosis factor alpha
- GABA
gamma-aminobutyric acid
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author Disclosure Statement
No actual or potential conflicts of interest are present for any of the authors and work presented in this report.
Contributor Information
D. Tweedie, Email: tweedieda@grc.nia.nih.gov.
L. Rachmany, Email: litalrac@post.tau.ac.il.
V. Rubovitch, Email: rubovitc@post.tau.ac.il.
E. Lehrmann, Email: elehrman@mail.nih.gov.
Y. Zhang, Email: zhangyon@grc.nia.nih.gov.
K.G. Becker, Email: BeckerK@grc.nia.nih.gov.
E. Perez, Email: perezev@grc.nia.nih.gov.
J. Miller, Email: Jonathan.Miller@UHhospitals.org.
B.J. Hoffer, Email: bjh82@case.edu.
N.H. Greig, Email: greign@grc.nia.nih.gov.
C.G. Pick, Email: pickc@post.tau.ac.il.
References
- Andrews-Zwilling Y, Bien-Ly N, Xu Q, Li G, Bernardo A, Yeon Yoon S, Zwilling D, Xue Yan T, Chen L, Huang Y. Apolipoprotein E4 Causes Age- and Tau-Dependent Impairment of GABAergic Interneurons, Leading to Learning and Memory Deficits in Mice. J. Neuroscience. 2010;30:13707–13717. doi: 10.1523/JNEUROSCI.4040-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azevedo FA, Carvalho LR, Grinberg LT, Farfel JM, Ferretti RE, Leite RE, Jacob Filho W, Lent R, Herculano-Houzel S. Equal numbers of neuronal and nonneuronal cells make the human brain an isometrically scaled-up primate brain. J. Comp. Neurology. 2009;513:532–541. doi: 10.1002/cne.21974. [DOI] [PubMed] [Google Scholar]
- Baggio LL, Drucker DJ. Biology of incretins: GLP-1 and GIP. Gastroenterology. 2007;132:2131–2157. doi: 10.1053/j.gastro.2007.03.054. [DOI] [PubMed] [Google Scholar]
- Bak AM, Egefjord L, Gejl M, Steffensen C, Stecher CW, Smidt K, Brock B, Rungby J. Targeting amyloid-beta by glucagon-like peptide -1 (GLP-1) in Alzheimer's disease and diabetes. Expert. Opin. Ther. Targets. 2011;10:1153–1162. doi: 10.1517/14728222.2011.600691. [DOI] [PubMed] [Google Scholar]
- Baratz R, Tweedie D, Rubovitch V, Luo W, Yoon JS, Hoffer BJ, Greig NH, Pick CG. Tumor necrosis factor-α synthesis inhibitor, 3,6'-dithiothalidomide, reverses behavioral impairments induced by minimal traumatic brain injury in mice. J. Neurochem. 2011;118:1032–1042. doi: 10.1111/j.1471-4159.2011.07377.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belsham DD, Fick LJ, Dalvi PS, Centeno ML, Chalmers JA, Lee PK, Wang Y, Drucker DJ, Koletar MM. Ciliary neurotrophic factor recruitment of glucagon-like peptide-1 mediates neurogenesis, allowing immortalization of adult murine hypothalamic neurons. FASEB J. 2009;23:4256–4265. doi: 10.1096/fj.09-133454. [DOI] [PubMed] [Google Scholar]
- Bennin DA, Don AS, Brake T, McKenzie JL, Rosenbaum H, Ortiz L, DePaoli-Roach AA, Horne MC. Cyclin G2 associates with protein phosphatase 2A catalytic and regulatory B' subunits in active complexes and induces nuclear aberrations and a G1/S phase cell cycle arrest. J. Biol. Chem. 2002;277:27449–27467. doi: 10.1074/jbc.M111693200. [DOI] [PubMed] [Google Scholar]
- Bertilsson G, Patrone C, Zachrisson O, Andersson A, Dannaeus K, Heidrich J, Kortesmaa J, Mercer A, Nielsen E, Rönnholm H, Wikström L. Peptide hormone exendin-4 stimulates subventricular zone neurogenesis in the adult rodent brain and induces recovery in an animal model of Parkinson’s disease. J. Neurosci. Res. 2008;86:326–338. doi: 10.1002/jnr.21483. [DOI] [PubMed] [Google Scholar]
- Bomfim TR, Forny-Germano L, Sathler LB, Brito-Moreira J, Houzel JC, Decker H, Silverman MA, Kazi H, Melo HM, McClean PL, Holscher C, Arnold SE, Talbot K, Klein WL, Munoz DP, Ferreira ST, De Felice FG. An anti-diabetes agent protects the mouse brain from defective insulin signaling caused by Alzheimer's disease-associated Aβ oligomers. J Clin Invest. 2012 doi: 10.1172/JCI57256. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonislawski DP, Schwarzbach EP, Cohen AS. Brain injury impairs dentate gyrus inhibitory efficacy. Neurobiol. Dis. 2007;25:163–169. doi: 10.1016/j.nbd.2006.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clifton GL, Miller ER, Choi SC, Levin HS, McCauley S, Smith KR, Jr, Muizelaar JP, Wagner FC, Jr, Marion DW, Luerssen TG, Chesnut RM, Schwartz M. Lack of effect of induction of hypothermia after acute brain injury. N. Engl. J. Med. 2001;344:556–563. doi: 10.1056/NEJM200102223440803. [DOI] [PubMed] [Google Scholar]
- Clifton GL, Coffey CS, Fourwinds S, Zygun D, Valadka A, Smith KR, Jr, Frisby ML, Bucholz RD, Wilde EA, Levin HS, Okonkwo DO. Early induction of hypothermia for evacuated intracranial hematomas: a post hoc analysis of two clinical trials. J. Neurosurg. 2012 doi: 10.3171/2012.6.JNS111690. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Clifton GL, Valadka A, Zygun D, Coffey CS, Drever P, Fourwinds S, Janis LS, Wilde E, Taylor P, Harshman K, Conley A, Puccio A, Levin HS, McCauley SR, Bucholz RD, Smith KR, Schmidt JH, Scott JN, Yonas H, Okonkwo DO. Very early hypothermia induction in patients with severe brain injury (the National Acute Brain Injury Study: Hypothermia II): a randomised trial. Lancet Neurol. 2011;10:131–139. doi: 10.1016/S1474-4422(10)70300-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costanza A, Weber K, Gandy S, Bouras C, Hof PR, Giannakopoulos P, Canuto A. Contact sport-related chronic traumatic encephalopathy in the elderly: clinical expression and structural substrates. Neuropathol. Appl. Neurobiol. 2011;37:570–584. doi: 10.1111/j.1365-2990.2011.01186.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Currais A, Hortobágyi T, Soriano S. The neuronal cell cycle as a mechanism of pathogenesis in Alzheimer's disease. Aging (Albany NY) 2009;1:363–371. doi: 10.18632/aging.100045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dail WG, Feeney DM, Murray HM, Linn RT, Boyeson MG. Responses to cortical injury: II. Widespread depression of the activity of an enzyme in cortex remote from a focal injury. Brain Res. 1981;211:79–89. doi: 10.1016/0006-8993(81)90068-8. [DOI] [PubMed] [Google Scholar]
- Darsalia V, Mansouri S, Ortsäter H, Olverling A, Nozadze N, Kappe C, Iverfeldt K, Tracy LM, Grankvist N, Sjöholm Å, Patrone C. Glucagon-like peptide-1 receptor activation reduces ischaemic brain damage following stroke in Type 2 diabetic rats. Clin. Sci. 2012;122:473–483. doi: 10.1042/CS20110374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeKosky ST, Ikonomovic MD, Gandy S. Traumatic brain injury-football, warfare, and long-term effects. N. Engl. J. Med. 2010;363:1293–1296. doi: 10.1056/NEJMp1007051. [DOI] [PubMed] [Google Scholar]
- De Kruijk JR, Twijnstra A, Meerhoff S, Leffers P. Management of mild traumatic brain injury: lack of consensus in Europe. Brain Inj. 2001;15:117–123. doi: 10.1080/026990501458353. [DOI] [PubMed] [Google Scholar]
- Dietrich WD, Bramlett HM. The evidence for hypothermia as a neuroprotectant in traumatic brain injury. Neurotherapeutics. 2010;7:43–50. doi: 10.1016/j.nurt.2009.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon CE, Lyeth BG, Povlishock JT, Findling RL, Hamm RJ, Marmarou A, Young HF, Hayes RL. A fluid percussion model of experimental brain injury in the rat. J. Neurosurg. 1987;67:110–119. doi: 10.3171/jns.1987.67.1.0110. [DOI] [PubMed] [Google Scholar]
- Dixon CE, Clifton GL, Lighthall JW, Yaghmai AA, Hayes RL. A controlled cortical impact model of traumatic brain injury in the rat. J Neurosci. Methods. 1991;39:253–262. doi: 10.1016/0165-0270(91)90104-8. [DOI] [PubMed] [Google Scholar]
- Drucker DJ, Buse JB, Taylor K, Kendall DM, Trautmann M, Zhuang D, Porter L for the DURATION-1 Study Group. Exenatide once weekly versus twice daily for the treatment of type 2 diabetes: a randomised, open-label, non-inferiority study. Lancet. 2008;372:1240–1250. doi: 10.1016/S0140-6736(08)61206-4. [DOI] [PubMed] [Google Scholar]
- Edut S, Rubovitch V, Schreiber S, Pick CG. The intriguing effects of ecstasy (MDMA) on cognitive function in mice subjected to a minimal traumatic brain injury (mTBI) Psychopharmacology (Berl) 2010;214:877–889. doi: 10.1007/s00213-010-2098-y. [DOI] [PubMed] [Google Scholar]
- Faul M, Xu L, Wald MM, Coronado VG. Traumatic Brain Injury in the United States: Emergency Department Visits, Hospitalizations and Deaths 2002–2006. Atlanta (GA): Centers for Disease Control and Prevention, National Center for Injury Prevention and Control; 2010. [Google Scholar]
- Farrer LA, Cupples LA, Haines JL, Hyman B, Kukull WA, Mayeux R, Myers RH, Pericak-Vance MA, Risch N, van Duijn CM. Effects of age, sex, and ethnicity on the association between apolipoprotein E genotype and Alzheimer disease. A meta-analysis. APOE and Alzheimer Disease Meta Analysis Consortium. JAMA. 1997;278:1349–1356. [PubMed] [Google Scholar]
- Feeney DM, Boyeson MG, Linn RT, Murray HM, Dail WG. Responses to cortical injury: I. Methodology and local effects of contusions in the rat. Brain Res. 1981;211:67–77. doi: 10.1016/0006-8993(81)90067-6. [DOI] [PubMed] [Google Scholar]
- Fernandoa RN, Eleuteria B, Abdelhadya S, Nussenzweigb A, Andänga M, Ernforsa P. Cell cycle restriction by histone H2AX limits proliferation of adult neural stem cells. Proc. Natl. Acad. Sci. USA. 2011;108:5837–5842. doi: 10.1073/pnas.1014993108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson CJ, Meyer RC, Hamm RJ. Traumatic brain injury and the effects of diazepam, diltiazem, and MK-801 on GABA-A receptor subunit expression in rat hippocampus. J. Biomed. Sci. 2010;17:38. doi: 10.1186/1423-0127-17-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Göke R, Larsen PJ, Mikkelsen JD, Sheikh SP. Identification of specific binding sites for glucagon-like peptide-1 on the posterior lobe of the rat pituitary. Neuroendocrinology. 1995a;62:130–134. doi: 10.1159/000126997. [DOI] [PubMed] [Google Scholar]
- Göke R, Larsen PJ, Mikkelsen JD, Sheikh SP. Distribution of GLP-1 binding sites in the rat brain: Evidence that exendin-4 is a ligand of brain GLP-1 binding sites. Eur. J. Neurosci. 1995b;7:2294–2300. doi: 10.1111/j.1460-9568.1995.tb00650.x. [DOI] [PubMed] [Google Scholar]
- Goldstein LE, Fisher AM, Tagge CA, Zhang XL, Velisek L, Sullivan JA, Upreti C, Kracht JM, Ericsson M, Wojnarowicz MW, Goletiani CJ, Maglakelidze GM, Casey N, Moncaster JA, Minaeva O, Moir RD, Nowinski CJ, Stern RA, Cantu RC, Geiling J, Blusztajn JK, Wolozin BL, Ikezu T, Stein TD, Budson AE, Kowall NW, Chargin D, Sharon A, Saman S, Hall GF, Moss WC, Cleveland RO, Tanzi RE, Stanton PK, McKee AC. Chronic traumatic encephalopathy in blast-exposed military veterans and a blast neurotrauma mouse model. Sci. Transl. Med. 2012;4 doi: 10.1126/scitranslmed.3003716. 134ra60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffioen KJ, Wan R, Okun E, Wang X, Lovett-Barr MR, Li Y, Mughal MR, Mendelowitz D, Mattson MP. GLP-1 receptor stimulation depresses heart rate variability and inhibits neurotransmission to cardiac vagal neurons. Cardiovasc. Res. 2011;89:72–78. doi: 10.1093/cvr/cvq271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzowski JF, McNaughton BL, Barnes CA, Worley PF. Environment-specific expression of the immediate-early gene Arc in hippocampal neuronal ensembles. Nat. Neurosci. 1999;2:1120–1124. doi: 10.1038/16046. [DOI] [PubMed] [Google Scholar]
- Hamilton A, Patterson S, Porter D, Gault VA, Holscher C. Novel GLP-1 mimetics developed to treat type 2 diabetes promote progenitor cell proliferation in the brain. J. Neurosci. Res. 2011;89:481–489. doi: 10.1002/jnr.22565. [DOI] [PubMed] [Google Scholar]
- Hayes MR, Skibicka KP, Grill HJ. Caudal brainstem processing is sufficient for behavioral, sympathetic, and parasympathetic responses driven by peripheral and hindbrain glucagon-like-peptide-1 receptor stimulation. Endocrinology. 2008;149:4059–4068. doi: 10.1210/en.2007-1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoge CW, McGurk D, Thomas JL, Cox AL, Engel CC, Castro CA. Mild traumatic brain injury in U.S. Soldiers returning from Iraq. N. Engl. J. Med. 2008;358:453–463. doi: 10.1056/NEJMoa072972. [DOI] [PubMed] [Google Scholar]
- Holst JJ, Burcelin R, Nathanson E. Neuroprotective properties of GLP-1: theoretical and practical applications. Curr. Med. Res. Opin. 2011;27:547–558. doi: 10.1185/03007995.2010.549466. [DOI] [PubMed] [Google Scholar]
- Hölscher C. The role of GLP-1 in neuronal activity and neurodegeneration. Vitam. Horm. 2010;84:331–354. doi: 10.1016/B978-0-12-381517-0.00013-8. [DOI] [PubMed] [Google Scholar]
- Honeybul S. An update on the management of traumatic brain injury. J. Neurosurg. Sci. 2011;55:343–355. [PubMed] [Google Scholar]
- Isacson R, Nielsen E, Dannaeus K, Bertilsson G, Patrone C, Zachrisson O, Wikström L. The glucagon-like peptide 1 receptor agonist exendin-4 improves reference memory performance and decreases immobility in the forced swim test. Eur. J. Pharmacol. 2011;650:249–255. doi: 10.1016/j.ejphar.2010.10.008. [DOI] [PubMed] [Google Scholar]
- Israelsson C, Wang Y, Kylberg A, Pick CG, Hoffer BJ, Ebendal T. Closed head injury in a mouse model results in molecular changes indicating inflammatory responses. J. Neurotrauma. 2009;26:1307–1314. doi: 10.1089/neu.2008.0676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson VE, Stewart W, Smith DH. Widespread Tau and Amyloid-Beta Pathology Many Years After a Single Traumatic Brain Injury in Humans. Brain Pathol. 2012;22:142–149. doi: 10.1111/j.1750-3639.2011.00513.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan-Sciutto KL, Morgan K, Bowser R. Increased Cyclin G1 Immunoreactivity During Alzheimer's Disease. J. Alzheimers Dis. 1999;1:409–417. doi: 10.3233/jad-1999-1605. [DOI] [PubMed] [Google Scholar]
- Kastin AJ, Akerstrom V. Entry of exendin-4 into brain is rapid but may be limited at high doses. Int. J. Obes. Relat. Metab. Disord. 2003;27:313–318. doi: 10.1038/sj.ijo.0802206. [DOI] [PubMed] [Google Scholar]
- Kim-Chung LT, Hosaka T, Yoshida M, Harada N, Sakaue H, Sakai T, Nakaya Y. Exendin-4, a GLP-1 receptor agonist, directly induces adiponectin expression through protein kinase A pathway and prevents inflammatory adipokine expression. Biochem. Biophys. Res. Commun. 2009;390:613–618. doi: 10.1016/j.bbrc.2009.10.015. [DOI] [PubMed] [Google Scholar]
- Kiraly M, Kiraly SJ. Traumatic brain injury and delayed sequelae: a review--traumatic brain injury and mild traumatic brain injury (concussion) are precursors to later-onset brain disorders, including early-onset dementia. Scientific World Journal. 2007;7:1768–1776. doi: 10.1100/tsw.2007.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahiri DK, Sambamurti K, Bennett DA. Apolipoprotein gene and its interaction with the environmentally driven risk factors: molecular, genetic and epidemiological studies of Alzheimer's disease. Neurobiol Aging. 2004;25:651–660. doi: 10.1016/j.neurobiolaging.2003.12.024. [DOI] [PubMed] [Google Scholar]
- Li H, Lee CH, Yoo KY, Choi JH, Park OK, Yan BC, Byun K, Lee B, Hwang IK, Won MH. Chronic treatment of exendin-4 affects cell proliferation and neuroblast differentiation in the adult mouse hippocampal dentate gyrus. Neurosci. Lett. 2010;486:38–42. doi: 10.1016/j.neulet.2010.09.040. [DOI] [PubMed] [Google Scholar]
- Li Y, Perry T, Kindy MS, Harvey BK, Tweedie D, Holloway HW, Powers K, Shen H, Egan JM, Sambamurti K, Brossi A, Lahiri DK, Mattson MP, Hoffer BJ, Wang Y, Greig NH. GLP-1 receptor stimulation preserves primary cortical and dopaminergic neurons in cellular and rodent models of stroke and Parkinsonism. Proc. Natl. Acad. Sci. USA. 2009;106:1285–1290. doi: 10.1073/pnas.0806720106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Duffy KB, Ottinger MA, Ray B, Bailey JA, Holloway HW, Tweedie D, Perry T, Mattson MP, Kapogiannis D, Sambamurti K, Lahiri DK, Greig NH. GLP-1 receptor stimulation reduces amyloid-beta peptide accumulation and cytotoxicity in cellular and animal models of Alzheimer's disease. J. Alzheimers Dis. 2010;19:1205–1219. doi: 10.3233/JAD-2010-1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Tweedie D, Mattson MP, Holloway HW, Greig NH. Enhancing the GLP-1 receptor signaling pathway leads to proliferation and neuroprotection in human neuroblastoma cells. J. Neurochem. 2010b;113:1621–1631. doi: 10.1111/j.1471-4159.2010.06731.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Chigurupati S, Holloway HW, Mughal M, Tweedie D, Bruestle DA, Mattson MP, Wang Y, Harvey BK, Ray B, Lahiri DK, Greig NH. Exendin-4 ameliorates motor neuron degeneration in cellular and animal models of amyotrophic lateral sclerosis. PLoS ONE. 2012;7(2):e32008. doi: 10.1371/journal.pone.0032008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lighthall JW. Controlled cortical impact: a new experimental brain injury model. J. Neurotrauma. 1988;5:1–15. doi: 10.1089/neu.1988.5.1. [DOI] [PubMed] [Google Scholar]
- Lighthall JW, Goshgarian HG, Pinderski CR. Characterization of axonal injury produced by controlled cortical impact. J. Neurotrauma. 1990;7:65–76. doi: 10.1089/neu.1990.7.65. [DOI] [PubMed] [Google Scholar]
- Liu X, Wang Q, Haydar TF, Bordey A. Nonsynaptic GABA signaling in postnatal subventricular zone controls proliferation of GFAP-expressing progenitors. Nature Neuroscience. 2005;8:1179–1187. doi: 10.1038/nn1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marklund N, Hillered L. Animal modeling of traumatic brain injury in preclinical drug development: where do we go from here? Br. J. Pharmacol. 2011;164:1207–1229. doi: 10.1111/j.1476-5381.2010.01163.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marmarou A, Foda MA, van den Brink W, Campbell J, Kita H, Demetriadou K. A new model of diffuse brain injury in rats. Part I: pathophysiology and biomechanics. J. Neurosurg. 1994;80:291–300. doi: 10.3171/jns.1994.80.2.0291. [DOI] [PubMed] [Google Scholar]
- Martin B, Golden E, Carlson OD, Pistell P, Zhou J, Kim W, Frank BP, Thomas S, Chadwick WA, Greig NH, Bates GP, Sathasivam K, Bernier M, Maudsley S, Mattson MP, Egan JM. Exendin-4 improves glycemic control, ameliorates brain and pancreatic pathologies, and extends survival in a mouse model of Huntington's disease. Diabetes. 2009;58:318–328. doi: 10.2337/db08-0799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martínez-Gac L, Marqués M, García Z, Campanero MR, Carrera AC. Control of cyclin G2 mRNA expression by forkhead transcription factors: novel mechanism for cell cycle control by phosphoinositide 3-kinase and forkhead. Mol. Cell. Biol. 2004;24:2181–2189. doi: 10.1128/MCB.24.5.2181-2189.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClean PL, Parthsarathy V, Faivre E, Hölscher C. The diabetes drug liraglutide prevents degenerative processes in a mouse model of Alzheimer's disease. J. Neurosci. 2011;31:6587–6594. doi: 10.1523/JNEUROSCI.0529-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendez CV, Hurley RA, Lassonde M, Zhang L, Taber KH. Mild traumatic brain injury: neuroimaging of sports-related concussion. J. Neuropsychiatry Clin. Neurosci. 2005;17:297–303. doi: 10.1176/jnp.17.3.297. [DOI] [PubMed] [Google Scholar]
- Messier C. Object recognition in mice: improvement of memory by glucose. Neurobiol. Learn. Mem. 1997;67:172–175. doi: 10.1006/nlme.1996.3755. [DOI] [PubMed] [Google Scholar]
- Milman A, Rosenberg A, Weizman R, Pick CG. Mild traumatic brain injury induces persistent cognitive deficits and behavioral disturbances in mice. J. Neurotrauma. 2005;22:1003–1010. doi: 10.1089/neu.2005.22.1003. [DOI] [PubMed] [Google Scholar]
- Monje ML, Toda H, Palmer TD. Inflammatory blockade restores adult hippocampal neurogenesis. Science. 2003;302:1760–1765. doi: 10.1126/science.1088417. [DOI] [PubMed] [Google Scholar]
- Moore EM, Nichol AD, Bernard SA, Bellomo R. Therapeutic hypothermia: benefits, mechanisms and potential clinical applications in neurological, cardiac and kidney injury. Injury. 2011;42:843–854. doi: 10.1016/j.injury.2011.03.027. [DOI] [PubMed] [Google Scholar]
- Morita N, Kiryu S, Kiyama H. p53-independent cyclin G expression in a group of mature neurons and its enhanced expression during nerve regeneration. J. Neurosci. 1996;16:5961–5966. doi: 10.1523/JNEUROSCI.16-19-05961.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Shea D, Gunn I, Chen X, Bloom S, Herbert J. A role for central glucagon-like peptide-1 in temperature regulation. Neuroreport. 1996;7:830–832. doi: 10.1097/00001756-199602290-00035. [DOI] [PubMed] [Google Scholar]
- Pan W, Kastin AJ, Rigai T, McLay R, Pick CG. Increased hippocampal uptake of tumor necrosis factor alpha and behavioral changes in mice. Exp. Brain. Res. 2003;149:195–199. doi: 10.1007/s00221-002-1355-7. [DOI] [PubMed] [Google Scholar]
- Perry T, Greig NH. The glucagon-like peptides: A double-edged therapeutic sword? Trends Pharmacol. Sci. 2003;24:377–383. doi: 10.1016/S0165-6147(03)00160-3. [DOI] [PubMed] [Google Scholar]
- Perry T, Lahiri DK, Sambamurti K, Chen D, Mattson MP, Egan JM, Greig NH. Glucagon-like peptide-1 decreases endogenous amyloid-beta peptide (Abeta) levels and protects hippocampal neurons from death induced by Abeta and iron. J Neurosci. Res. 2003;72:603–612. doi: 10.1002/jnr.10611. [DOI] [PubMed] [Google Scholar]
- Perry T, Holloway HW, Weerasuriya A, Mouton PR, Duffy K, Mattison JA, Greig NH. Evidence of GLP-1-mediated neuroprotection in an animal model of pyridoxine-induced peripheral sensory neuropathy. Exp. Neurol. 2007;203:293–301. doi: 10.1016/j.expneurol.2006.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo I, Amornphimoltham P, Weigert R, Barlati S, Bosetti F. Cyclooxygenase-1 is involved in the inhibition of hippocampal neurogenesis after lipopolysaccharide-induced neuroinflammation. Cell Cycle. 2011a;10:2568–2573. doi: 10.4161/cc.10.15.15946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo I, Barlati S, Bosetti F. Effects of neuroinflammation on the regenerative capacity of brain stem cells. J. Neurochem. 2011b;116:947–956. doi: 10.1111/j.1471-4159.2010.07168.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadaka F, Veremakis C. Therapeutic hypothermia for the management of intracranial hypertension in severe traumatic brain injury: a systematic review. Brain Inj. 2012;26:899–908. doi: 10.3109/02699052.2012.661120. [DOI] [PubMed] [Google Scholar]
- Saijo K, Glass CK. Microglial cell origin and phenotypes in health and disease. Nat. Rev. Immunol. 2011;11:775–787. doi: 10.1038/nri3086. [DOI] [PubMed] [Google Scholar]
- Salcedo I, Tweedie D, Li Y, Greig NH. Neuroprotective and neurotrophic actions of glucagon-like peptide-1: an emerging opportunity to treat neurodegenerative and cerebrovascular disorders. Br. J. Pharmacol. 2012 doi: 10.1111/j.1476-5381.2012.01971.x. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders ML, Miller JD, Stablein D, Allen G. The effects of graded experimental trauma on cerebral blood flow and responsiveness to CO2. J. Neurosurg. 1979;51:18–26. doi: 10.3171/jns.1979.51.1.0018. [DOI] [PubMed] [Google Scholar]
- Sullivan HG, Martinez J, Becker DP, Miller JD, Griffith R, Wist AO. Fluid-percussion model of mechanical brain injury in the cat. J. Neurosurg. 1976;45:521–534. [PubMed] [Google Scholar]
- Sultana R, Butterfield DA. Regional expression of key cell cycle proteins in brain from subjects with amnestic mild cognitive impairment. Neurochem. Res. 2007;32:655–662. doi: 10.1007/s11064-006-9123-x. [DOI] [PubMed] [Google Scholar]
- Tashlykov V, Katz Y, Gazit V, Zohar O, Schreiber S, Pick CG. Apoptotic changes in the cortex and hippocampus following minimal brain trauma in mice. Brain Res. 2007;1130:197–205. doi: 10.1016/j.brainres.2006.10.032. [DOI] [PubMed] [Google Scholar]
- Tashlykov V, Katz Y, Volkov A, Gazit V, Schreiber S, Zohar O, Pick CG. Minimal traumatic brain injury induce apoptotic cell death in mice. J. Mol. Neurosci. 2009;37:16–24. doi: 10.1007/s12031-008-9094-2. [DOI] [PubMed] [Google Scholar]
- Tweedie D, Milman A, Holloway HW, Li Y, Harvey BK, Shen H, Pistell PJ, Lahiri DK, Hoffer BJ, Wang Y, Pick CG, Greig NH. Apoptotic and behavioral sequelae of mild brain trauma in mice. J. Neurosci. Res. 2007;85:805–815. doi: 10.1002/jnr.21160. [DOI] [PubMed] [Google Scholar]
- Uryu K, Giasson BI, Longhi L, Martinez D, Murray I, Conte V, Nakamura M, Saatman K, Talbot K, Horiguchi T, McIntosh T, Lee VM, Trojanowski JQ. Age-dependent synuclein pathology following traumatic brain injury in mice. Exp. Neurol. 2003;184:214–224. doi: 10.1016/s0014-4886(03)00245-0. [DOI] [PubMed] [Google Scholar]
- Uryu K, Laurer H, McIntosh T, Praticò D, Martinez D, Leight S, Lee VM, Trojanowski JQ. Repetitive mild brain trauma accelerates Abeta deposition, lipid peroxidation, and cognitive impairment in a transgenic mouse model of Alzheimer amyloidosis. J. Neurosci. 2002;22:446–454. doi: 10.1523/JNEUROSCI.22-02-00446.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Praag H, Schinder AF, Christie BR, Toni N, Palmer TD, Gage FH. Functional neurogenesis in the adult hippocampus. Nature. 2002;415:1030–1034. doi: 10.1038/4151030a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong N, Weisgraber KH. Understanding the basis for the association of apoE4 with Alzheimer's disease: opening the door for therapeutic approaches. Curr Alzheimer Res. 2009;6:415–418. doi: 10.2174/156720509789207921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zohar O, Rubovitch V, Milman A, Schreiber S, Pick CG. Behavioral consequences of minimal traumatic brain injury in mice. Acta Neurobiol Exp (Wars) 2011;71(1):36–45. doi: 10.55782/ane-2011-1821. [DOI] [PubMed] [Google Scholar]
- Zohar O, Schreiber S, Getslev V, Schwartz JP, Mullins PG, Pick CG. Closed-head minimal traumatic brain injury produces long-term cognitive deficits in mice. Neuroscience. 2003;118:949–955. doi: 10.1016/s0306-4522(03)00048-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The effects of Ex-4 treatment vs. sham on the 10 most down regulated (green) and up regulated (red) gene sets in mouse hippocampus are illustrated. * - indicates a CNS or neuronal related gene set. Treatment effects on Z-score data for Gene Ontology (A); Molecular Function (B); Cellular Component (C) and Biological Process (D) are shown.
The effects of mTBI vs. sham on the 10 most down regulated (green) and up regulated (red) gene sets in mouse hippocampus are illustrated. The presence of a black bar indicates where Ex-4 treatment prior to mTBI induced an expression change in gene sets relative to the mTBI group. Where no black bar is present, Ex-4 had no effect on gene sets relative to mTBI. Treatment with Ex-4 prevented some but not all changes in gene sets altered by mTBI. * - indicates a CNS or neuronal related gene set. Treatment effects on Z-score data for Gene Ontology (A); Molecular Function (B); Cellular Component (C) and Biological Process (D) are shown.