Abstract
Monocytes/macrophages are innate immune cells that play a crucial role in the resolution of inflammation. In presence of Th2 cytokines interleukin-4 (IL-4) and interleukin-13 (IL-13), they display an anti-inflammatory profile and this activation pathway is known as alternative activation. In this study we compare and differentiate pathways mediated by IL-4 and IL-13 activation of human monocytes/macrophage. Here we report differential regulation of IL-4 and IL-13 signaling in monocytes/macrophages starting from IL-4/IL-13 cytokine receptors to Jak-Stat-mediated signaling pathways that ultimately control expression of several infl1ammatory genes. Our data demonstrate that while the receptor-associated tyrosine kinases Jak2 and Tyk2 are activated after the recruitment of IL-13 to its receptor (containing IL-4Rα and IL-13Rα1), IL-4 stimulates Jak1 activation. We further show that Jak2 is upstream of Stat3 activation and Tyk2 controls Stat1 and Stat6 activation in response to IL-13 stimulation. In contrast, Jak1 regulates Stat3 and Stat6 activation in IL-4-induced monocytes. Our results further reveal that while IL-13 utilizes both IL-4Rα-Jak2-Stat3 and IL-13Rα1-Tyk2-Stat1/Stat6 signaling pathways, IL-4 can only use the IL-4Rα-Jak1-Stat3/Stat6 cascade to regulate the expression of some critical inflammatory genes including 15-lipoxygenase (15-LO), monoamine oxidase A (MAO-A) and scavenger receptor CD36. Moreover, we demonstrate here that IL-13 and IL-4 can uniquely affect the expression of particular genes like dual specificity phosphatase 1 (DUSP1) and tissue inhibitor of metalloprotease-3 (TIMP3) and do so through different Jak kinaes. As evidence of differential regulation of gene function by IL-4 and IL-13, we further report that MAO-A-mediated reactive oxygen species (ROS) generation is influenced by different Jak kinases. Collectively, these results have major implications for understanding the mechanism and function of alternatively activated monocytes/macrophages by IL-4 and IL-13 and add novel insights into the pathogenesis and potential treatment of different inflammatory diseases.
Keywords: Monocytes, cytokines, Inflammation, Gene regulation, ROS, alternative activation
Introduction
IL-4 and IL-13 are Th2 cytokines in the immune system that exhibit a wide range of activities in regulating inflammatory responses and are thought to play significant roles during allergic reactions [1]. However, these cytokines primarily act as antiinflammatory molecules [2]. IL-13 shares many biological activities with IL-4. Both cytokines reduce production of IL-1, tumor necrosis factor-α (TNF-α), and other proinflammatory mediators, whereas they upregulate the expression of several markers like IL-1Rα, mannose receptor, Dectin-1, CD23 etc. by monocytes/macrophages [3]. Both IL-4 and IL-13 antagonize the actions of interferon-γ (IFN-γ) [3], significantly enhance the ability of activated human monocytes to oxidize LDL [4] and reduce inflammation in different animal models of arthritis [5–7]. In addition, IL-4 has been shown to downregulate the production of prostaglandin E2 by synovial macrophage-type cells through the inhibition of cyclooxygenase 2 [8], whereas IL-13 has been reported to induce tissue fibrosis by the stimulation and activation of TGFβ [9].
IL-4 and IL-13 are hallmark cytokines of Th2-associated diseases including asthma. IL-13 mediates central characteristics of allergic asthma including immunoglobulin E (IgE) synthesis, mucus hypersecretion, airway hyperresponsiveness (AHR), and fibrosis [10]. Both IL-4 and IL-13 signal through a complex receptor system including IL-4Rα chain. In human monocytes, IL-4 mediates its effect through the type I IL-4R, composed of the IL-4Rα and common γ-chain (IL-2Rγ) [11]. In contrary, IL-13 primarily exerts its IL-4Rα–dependent effect via type II IL-4R containing a different component IL-13Rα1[11]. Previously IL-13Rα1 was identified as a fundamental receptor mediating IL-13-and IL-4– induced AHR, mucus production, and fibrosis in response to the “classical” experimental asthma model [12]. Further studies have demonstrated a key role for IL-13Rα1 and the type II IL-4R in lung Th2 responses and asthma pathogenesis [12, 13]. Recent studies show the differential regulation of IL-13Rα1 in aeroallergen-induced lung responses [14]. Strategies targeting IL-13Rα1 for anti-asthma therapy are currently under way [15] and require further elucidation.
IL-4 and IL-13 have been classified as alternative macrophage (M2a-phenotype) activators [16–18]. Previous studies suggested that in human peripheral blood monocytes IL-4/13 significantly downregulate the expression of classical pro-inflammatory signal transducers, such as IL-1, IL-6, IL-8, IL-18, monocyte chemotactic protein-1 (MCP-1), TNFα [19]. Expression of enzymes involved in the biosynthesis of pro-inflammatory eicosanoids e.g. cyclooxygenase-2 (COX2), 5-lipoxygenase (5-LO) is also attenuated [19]. In contrast, expression of some gene products involved in inflammatory resolution (15-lipoxygenase (15-LO), Fibronectin (FN), Monoamine oxidase-A (MAO-A), coagulation factor XIII (FXIII), annexin 1, collagen 1α2, laminin α5, heme oxygenase-1, C–C motif chemokine 22 (CCL22), heat shock protein 8 (HSP8) etc.) were upregulated in monocytes upon exposure to IL-4/IL-13 [19]. Among the most strongly upregulated gene products in alternatively activated monocytes/macrophages with potential anti-inflammatory properties are 15-LO [19–21], MAO-A) [19, 22, 23], scavenger receptor CD36 [23–25], FN [19, 26] and FXIII [19].
Our recent studies also identify Hck as the essential Src kinase isoform which regulates the expression of a panel of gene including 15-LO, MAO-A and CD36 in alternatively activated monocytes/macrophages [23]. Moreover, our recent results present evidence that Stat transcription factors which regulate 15-LO expression are also involved in controlling both CD36 (Yakubenko, V. et al, 2012, manuscript submitted) and MAO-A (data presented in this manuscript) expression in IL-13-activated monocytes/macrophages.
In the present study we explore both IL-4 and IL-13 signaling in monocytes/macrophages starting from the level of the IL-4/IL-13 receptor to Jak-Stat-mediated signaling pathways and investigate the differential expression of several inflammatory genes mediated by these two cytokines. Our data demonstrate that while IL-13 utilizes both IL-4Rα-Jak2-Stat3 and IL-13Rα1-Tyk2-Stat1/Stat6 signaling cascades to regulate 15-LO, MAO-A and CD36 gene expression, IL-4 can only use the IL-4Rα-Jak1-Stat3/Stat6 axis to control the expression of these genes. Furthermore, we present evidence that IL-13 and IL-4 uniquely induces the gene expression of Dual specificity phosphatase 1 (DUSP1) and Tissue inhibitor of metalloprotease-3 (TIMP3) respectively. Our results further show that generation of MAO-A-mediated reactive oxygen species (ROS) in monocytes/macrophages is also regulated by different Jak kinases upon exposure to IL-4 and IL-13. These results add novel insights into the regulation of IL-4/IL-13-mediated asthma pathogenesis as well as in the control of inflammation and atherosclerosis.
Materials and Methods
Materials
Recombinant human IL-13 and IL-4 were purchased from Biosource International (Camarillo, CA). The rabbit reticulocyte 15-LO antibody, cross-reacting with human 15-LO, was raised in sheep and was obtained as a gift from Dr. Joseph Cornicelli (Molecular Imaging). Anti-phosphotyrosine-Stat (pY701-Stat1, pY705-Stat3 and pY641-Stat6) antibodies were purchased from Cell Signaling Technology (Beverly, MA). Stat6 antibody was purchased from BD Pharmingen (San Diego, CA). Anti-CD36 polyclonal antibody was from Cayman Chemical (Ann Arbor, MI). The other primary antibodies used in this study were: mouse anti-human p-Tyr (PY99), anti-Jak1, Jak2, Jak3, Tyk2, MAO-A and β-tubulin from Santa Cruz Biotechnology (Santa Cruz, CA). The ROS-sensitive fluorescent probe 6-carboxy-2′, 7′-dichlorodihydrofluorescein diacetate, diacetoxymethyl ester (H2DCFDA) was from Life Technologies (Carlsbad, CA). Amplex Red Monoamine Oxidase assay kit (Cat# A12214) was from Molecular Probes (Invitrogen, Eugene, OR). MAO-GLO™ assay kit (Cat# V1401) and MAO-A enzyme (Human recombinant enzyme expressed in Yeast, Cat# V1452) were purchased from Promega (Madison, WI). Tyramine and pharmacological inhibitors such as Pargyline (a pan-MAO inhibitor) and Moclobemide (specific inhibitor for MAO-A) were purchased from Sigma Chemical Co. (St. Louis, MO). The inhibitors were stored either at room temperature or at −20°Cas concentrated stock solutions (either in water or DMSO) according to manufacturer’s instructions.
Isolation of Human Monocytes
Human peripheral blood monocytes (PBM) were isolated either by separation of mononuclear cells followed by adherence to bovine calf serum (BCS)-coated flasks as described earlier [27] or by Ficoll-Hypaque sedimentation followed by countercurrent centrifugal elutriation [28, 29]. PBM purified by these two methods were identical in response to IL-13 and IL-4 and consistently >95% CD14+. These studies complied with all relevant federal guidelines and institutional policies regarding the use of human subjects.
Immunoprecipitation (IP) and Immunoblotting
PBM were routinely treated with IL-13 (2 nM) or IL-4 (670 pM) for various time intervals (These doses were chosen because they demonstrated comparable 15-LO induction in monocytes in numerous previous studies performed by our group). In some specific experiments, primary monocytes were stimulated with 2nM of both IL-13 and IL-4. and qualitatively similar results were observed to those reported here. Total and postnuclear extracts were prepared by previously published protocols [11, 30]. After determining the protein concentration using the Bio-Rad protein assay reagent (Hercules, CA), lysate proteins (50 μg/well) were resolved by 8% SDS-PAGE and subjected to immunoblotting as described previously [31]. 15-LO protein was detected on Western blots following a previously described protocol [27]. Immunoprecipitation experiments were performed according to our previously published method [32] using prewashed Protein A-Sepharose beads (Sigma, St. Louis, MO) at 4°C overnight. Immunoblots were stripped and reprobed to assess equal loading according to our previously published protocol [11].
RNA Extraction and Real-time RT-PCR
Monocytes (5×106 in 2 ml 10% BCS/DMEM) were plated in six-well culture plates, treated with antisense or decoy ODN and finally treated with IL-13 (2nM) or IL-4 (670pM) for 24 h. Total cellular RNA was extracted using the RNeasy mini kit from Qiagen (Valencia, CA) and real-time quantitative RT-PCR was performed according to established protocols [33]. The sequences of the primers used were: 15-LO forward 5′-GCTGGAAGGATCTAGATGACT-3′ and 15-LO reverse 5′-TGGCTACAGAGAATG ACGTTG-3′; CD36 forward 5′-CAGAGGCTGACAACTTCACAG-3′ and CD36 reverse 5′-AGGGTACGGAACCAAACTCAA-3′; MAO-A forward 5′-GCCAAGATTCAC TTCAGACCAGAG-3′ and MAO-A reverse 5′-TGCTCCTCACACCAGTTCTTCTC -3′; DUSP1 forward 5′-TTTGAGGGTCACTACCAG-3′ and DUSP1 reverse 5′-GAGATGATGCTTCGCC-3′ and TIMP3 forward 5′-CCAGGACGCCTTCTGCAAC-3′ and TIMP3 reverse 5′-CCTCCTTTACCAGCTTCTTCCC-3′. GAPDH was used as an internal control with the primer sequences of forward 5′-CACCAACTG CTTAGCACCCC-3′ and reverse 5′-TGGTCATGAGTCCTTCCACG-3′.
DNA-binding activity of Stat1 and Stat3
A TransAM™ Stat family kit (Active Motif) was used to evaluate the DNA-binding activity of Stat1 and Stat3 according to the instruction provided by the supplier. Briefly, nuclear extracts were incubated in a 96-well assay plate precoated with immobilized oligonucleotide containing the Stat consensus binding site (5′-TTCCCGGAA-3′). The active forms of Stat1 and Stat3 contained in nuclear extracts specifically bind the oligonucleotide. The wild-type (WT) consensus oligonucleotide was provided with the kit as a competitor for Stat binding in order to monitor the specificity of the assay by preventing Stat binding to the probe immobilized on the plate. Conversely, the mutated (MT) consensus oligonucleotide was provided as a control and acts as a non-competitor. The detection of activated Stats (Stat1 and Stat3) was carried out with specific Stat1 and Stat3 primary antibodies and an HRP-conjugated secondary antibody supplied in the kit. After incubation with the developing solution for the recommended time, the reaction was stopped and the colorimetric readout was taken at 450 nm with a reference wavelength of 655 nm using a Synergy™ HT Multi-Mode Microplate Reader from Biotek (Vermont, USA).
Treatment of Monocytes with Jak1, Jak2, Tyk2 and MAO-A antisense oligodeoxyribonucleotides (ODNs)
The antisense ODN sequences for human Jak1, Jak2 and Tyk2 were selected based on our previously published studies [27]. Control ODN for Jak2 and Tyk2 consisted of complementary sense ODN. The sense ODN sequence for Jak1 was predicted to possibly serve as an antisense for human γ adaptin mRNA. Hence we chose a scrambled ODN as a control for the Jak1 antisense. All ODNs were end-modified (phosphorothioated, three bases at the 5 and 3 ends) to limit DNA degradation and all were HPLC-purified before use (Invitrogen, Carlsbad, CA).
The sequences of the ODNs are as follows.
Jak2 antisense: 5′-TCTTAACTCTGTTCTCGTTC-3′.
Jak2 sense: 5′-GAACGAGAACAGAGTTAAGA-3′.
Tyk2 antisense: 5′-CCAACTTTATGTGCAATGTG-3′.
Tyk2 sense: 5′-CACATTGCACATAAAGTTGG-3′.
Jak1 antisense: 5′-GGTTGCATCTGGAATCTTT-3′.
Jak1 scrambled: 5′-TTGTGAACTGCCTGTGATT-3′.
MAO-A antisense: 5′-ATTTGTCAGCATGTTGAGCC-3′.
MAO-A sense: 5′-GGCTCAACATGCTGACAAAT-3′.
Primary human monocytes were transfected with Jak1, Jak2 and Tyk2 antisense ODNs along with the sense and scrambled ODN controls for 48 h with one re-feed at 24 h prior to the addition of IL-13 and IL-4. For MAO-A sense and antisense treatment, cells were pre-treated with MAO-A sense (S) or antisense (AS) ODN (10 μM) for 2 h prior to IL-13 or IL-4 addition and then re-fed with 10 μM ODNs at 24 h and incubated for another 24 h before harvesting the cells. In some experiments (to study Stat6 tyrosine phosphorylation), cells were transfected with Jak2 and Tyk2 antisense ODNs or their sense ODN controls (2 μM concentration) using Mirus TransIt-Oligo Transfection Reagent (Mirus Bio Corporation, Madison, WI) according to the manufacturer’s protocols and the incubation was continued for 48 h before IL-4 and IL-13 addition. For the transfection control, cells were incubated with the transfection reagent alone for 48 h. After the antisense treatments, monocytes were stimulated with IL-4 and IL-13 for another 15 min (to study the tyrosine phosphorylation of Stat1 and Stat3), 30 min (to check the tyrosine phosphorylation of Stat6) or 24h (for the induction of 15-LO).
Transfection of Stat1, Stat3 and Stat6 decoy and mismatched/scrambled ODNs into monocytes
The phosphorothioated ODNs used for Stat1, Stat3 and Stat6 decoys were purchased from Invitrogen (Carlsbad, CA). These decoys were used in previous studies and shown to provide specific inhibition of Stat1, Stat3 and Stat6 activities [33, 34]. The sequences for the decoys were 5′-ATATTCCTGTAAGTG-3′ and 3′-TATAAGGACATT CAC-5′ for Stat1; 5′-ATATTGGAGTAAGTG-3′ and 3′-TATAACCTCATTCAC-5′ for the mismatched Stat1 decoy; 5′-GATCCTTCTGGGAATTCCTAGATC-3′ and 3′-CTA GGAAGACCCTTAAGGATCTAG-5′ for the Stat3 decoy; 5′-GATCCTTCTGGGCCG TCCTAGATC-3′ and 3′-CTAGGAAGACCCGGCAGGATCTAG-5′ for the mismatched Stat3 decoy; 5′-GATCAAGACCTTTTCCCAAGAAATCTAT-3′ and 3′-CATGTTCTGGAAAAGGGTTC TTTAGATA-5′ for Stat6 decoy and 5′-CGAAAATTCGTTAAATCA CTAGCTTACC-3′ and 3′-GCTTTTAAGCAATTTAGTGATCGAATGG-5′ for the scrambled decoy ODN for Stat6. Stat1, Stat3 and Stat6 decoy, mismatched decoy and scrambled decoy ODN sequences were prepared by annealing the single-stranded phosphorothioate-modified ODNs as described previously [33, 34]. Human monocytes were then transfected with decoy ODNs using Superfect Transfection Reagent (Qiagen, Valencia, CA) according to the manufacturer’s instructions for 24 h. Monocytes were further incubated with IL-13 or IL-4 for another 24 h for 15-LO and MAO-A mRNA quantification or 48 h for 15-LO and MAO-A protein detection.
Native MAO-A activity assay
MAO-A activity was measured by a two-step bioluminescent assay method as described previously [35] using a MAO-GLO™ assay kit from Promega (Madison, WI). The amount of signal (light intensity) generated was detected using a microplate luminometer (Multilabel Counter Victor™, Perkin Elmer). Human recombinant MAO-A enzyme (Promega) was used as a positive control. The results are expressed as relative light units (RLU) after the background was subtracted.
Intracellular reactive oxygen species (ROS) determination
The generation of intracellular ROS was measured using the ROS-sensitive fluorescent probe H2DCFDA (5μM). After incubation with the probe for 30 min, cells were removed from the media, scraped off in PBS, washed and centrifuged. Cell pellet was resuspended in prewarmed PBS and the fluorescence was read (excitation. 495 nm; emission. 525 nm) as described previously [36, 37].
Amplex Red monoamine oxidase assay
This assay is based on the detection of H2O2 in an HRP-coupled reaction using Amplex Red as a sensitive and stable probe (for H2O2) and p-Tyramine as a substrate. The fluorometric assay was carried out following the manufacturer’s instructions. Each reaction contained 1mM p-Tyramine, 1U/ml HRP and 200 μM Amplex Red reagent. Reactions were incubated at room temperature for 60 min (protected from light) and fluorescence was measured with a fluorescence microplate reader using excitation. at 571 nm and emission at 585 nm.
Data analysis
The number of experiments analyzed is indicated in each figure. Band intensities were quantified by densitometric analyses using a laser densitometer (Microtek ScanMaker 8700, Cerritos, CA) and the NIH Image software program. Differences among experimental groups (for dose responses) were analyzed using one-way ANOVA. The significance of observations was calculated using unpaired Student’s t test analysis and p<0.05 was considered statistically significant.
Results
IL-4 and IL-13 signal through different receptor components and activate different Jak kinases in primary human monocytes
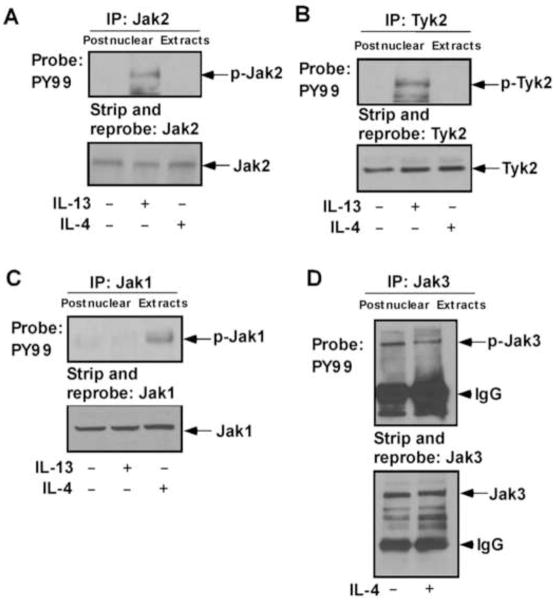
Previous results from our group and several others indicate that IL-4Rα is the common protein component for both IL-4 and IL-13 receptors [3, 11, 38] whereas IL-13Rα1 and IL-2Rγc are the other active constituents of IL-13 and IL-4 receptor signaling complexes, respectively [11, 39, 40]. Results from previous studies also indicate that while IL-4Rα is phosphorylated on tyrosine in response to both IL-4 and IL-13 [11, 38], IL-13Rα1 is phosphorylated only by IL-13 [11, 40] and IL-2Rγc phosphorylation is only induced by IL-4 [11]. Earlier we examined the association of several Jak kinases with the known receptor components, IL-4Rα, IL-13Rα1 and IL-2Rγc [11, 41, 42]. Here, we investigated whether these Jak kinases (Jak2, Tyk2, Jak1 and Jak3) are activated upon IL-13 and/or IL-4 stimulation. Results from our immunoprecipitation (IP) experiments with different Jak/Tyk antibodies followed by immunoblotting with antibody to phosphotyrosine, PY99 indicated that both Jak2 and Tyk2 are phosphorylated on tyrosine upon exposure to IL-13 but not by IL-4 in human blood monocytes (Fig. 1A and 1B). In contrast, Jak1 is phosphorylated on tyrosine by IL-4 but not by IL-13 (Fig. 1C). Our results further show that although Jak3 is known to associate with IL-2Rγc [41, 42], it is not activated (tyrosine phosphorylated) by IL-4 stimulation in monocytes (Fig. 1D).
Figure 1. IL-4 and IL-13 activates different Jak kinases in monocytes.
Human blood monocytes (10×106/group) were directly stimulated with IL-13 or IL-4 for 10 min or left untreated as indicated. Cells were lysed and the postnuclear extracts were immunoprecipitated (IP) with different Jak/Tyk antibodies as mentioned in panels A–D. The immune complexes were analyzed by immunoblotting with antibody to phosphotyrosine, PY99 and presented in the upper panels of A–D. The bottom panels represent stripping and reprobing the blot with the same individual antibody used for immunoprecipitation, to assess equal protein loading. Arrows indicate the positions of respective Jak kinases as mentioned, based on the migration of molecular weight markers that were run in adjacent lanes. The arrowhead marks the migration of the heavy chain of IgG. Data are from a representative experiment of three independent experiments that were performed.
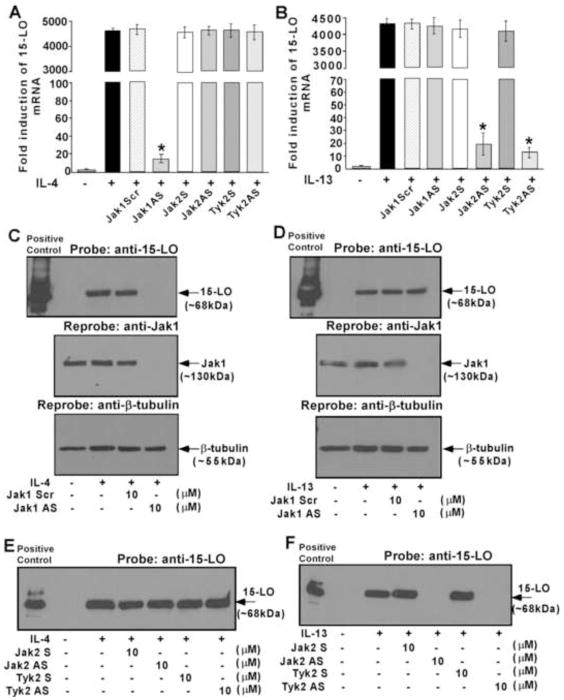
IL-4 induction of 15-LO expression requires Jak1 but not Jak2 and Tyk2
We next investigated whether Jak1 is critical in regulating IL-4-induced 15-LO mRNA expression in monocytes. To examine, that, we treated monocytes with the Jak1-specific antisense or control scrambled ODN along with our previously characterized antisense or sense ODN against Jak2 or Tyk2 kinases [27]. Results from our real-time quantitative PCR experiment indicated that the Jak1 antisense ODN significantly inhibited the IL-4-mediated induction of 15-LO mRNA expression (Fig. 2A) (*p<0.002), whereas the scrambled ODN control had no effect (Fig. 2A). The antisense ODN against either the Jak2 or Tyk2 kinases also caused no inhibition of IL-4-induced 15-LO mRNA expression (Fig. 2A).
Figure 2. Induction of 15-LO expression by IL-4 and IL-13 requires different Jak kinases in monocytes.
Monocytes (5×106/group) were pre-treated directly with antisense ODN to Jak1 or a scrambled ODN control (10 μM) (A, B, C, D) or Jak2 or Tyk2 antisense or sense ODNs (10 μM) (A, B, E, F) for 48 h, with one re-feed at 24 h, prior to the incubation with IL-4 (A, C, E) or IL-13 (B, D, F) for another 24h. In panels A–B, total cellular RNA extracts were prepared and subjected to real-time quantitative PCR analysis. After normalization with GAPDH amplification, the fold induction of 15-LO mRNA expression for different groups was plotted. Data are the means ± S.D. (n=3). Significant differences were determined by comparing the antisense (AS), scrambled (Scr) and sense (S) ODN treated groups to the IL-4 or IL-13-treated control (*p<0.002). In panels C–F, cells were lysed and 50μg of postnuclear extracts were separated by SDS-PAGE. The proteins were resolved, transferred to a PVDF membrane and immunoblotted with an antibody against 15-LO (upper panels of C and D, E, F). The same blots were then reprobed with Jak1 antibody to examine the effect of antisense ODN on Jak1 protein expression (middle panels of C and D). To confirm equal loading the blots were then stripped and reprobed with (β-tubulin antibody (lower panels of C and D). Arrows indicate the predicted migration of 15-LO, Jak1 and β-tubulin as determined by the migration of molecular weight markers in adjacent lanes. Recombinant 15-LO was used as a positive control. The data shown represent one of three separate experiments giving similar results.
Similar to IL-4-stimulated 15-LO mRNA expression, monocytes treated with antisense ODN to Jak1 substantially inhibited 15-LO protein expression whereas treatment with the control scrambled ODN caused no inhibition (Fig. 2C, upper panel). Antisense ODN inhibition of Jak1 protein expression was verified by reprobing the same blot with an antibody against Jak1 kinase (Fig. 2C, middle panel). As confirmation that Jak2 and Tyk2 are not phosphorylated by IL-4 and likely not required for 15-LO expression, we also examined whether inhibition of Jak2 or Tyk2 altered IL-4-stimulated expression of 15-LO. The antisense ODN against either the Jak2 or Tyk2 kinases showed no inhibition of IL-4-mediated induction of 15-LO protein (Fig. 2E).
Jak2 and Tyk2 but not Jak1 are involved in regulating the induction of 15-LO expression in response to IL-13
Jak1, in contrast to Jak2 and Tyk2, was not phosphorylated on tyrosine in response to IL-13 treatment and therefore would not be predicted to regulate IL-13-mediated signaling. We examined whether inhibition of Jak1 altered the expression of 15-LO after IL-13 stimulation. As expected, our results show that Jak1 antisense and scrambled ODNs caused no inhibition of IL-13-induced 15-LO mRNA expression (Fig. 2B). As a comparison, the substantial inhibition of Jak2 and Tyk2 antisense ODNs on 15-LO mRNA expression were also monitored in response to IL-13 stimulation (Fig. 2B).
We next examined the effect of Jak1 down-regulation on IL-13 induction of 15-LO protein expression. Our results indicated substantial inhibition of Jak1 expression in monocytes treated with antisense to Jak1 (Fig. 2D, middle panel), whereas IL-13-induced 15-LO protein expression was not inhibited when Jak1 expression was significantly reduced (Fig. 2D, upper panel). In contrast, IL-13 induction of 15-LO protein expression was markedly reduced either by Jak2 or Tyk2 antisense treatment whereas sense ODNs against Jak2 and Tyk2 (used as control) had no effect (Fig. 2F). These data clearly established the involvement of both Jak2 and Tyk2 but not Jak1 for IL-13-mediated 15-LO expression in monocytes.
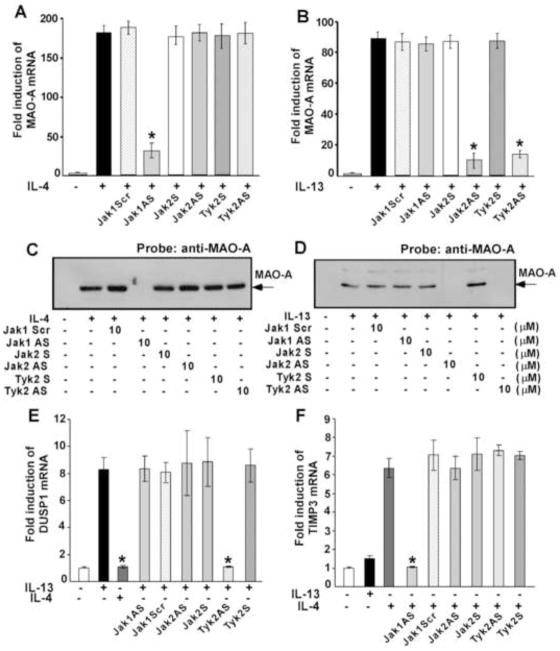
MAO-A and CD36 expression is regulated by different Jak kinases in alternatively activated human monocytes/macrophages by IL-4 and IL-13
As our previous studies demonstrated the induction of MAO-A and CD36 gene expression in alternatively activated monocytes/macrophages [23] and since CD36 gene expression is directly controlled by 15-LO expression/activity in IL-13-driven monocytes/macrophages (Yakubenko, V. et al, 2012, manuscript submitted), we further investigated the requirement of different Jak kinases in regulating MAO-A and CD36 expression in IL-4 and IL-13-activated human monocytes/macrophages. For these studies we treated monocytes with the same antisense ODNs described earlier. Our results demonstrate that Jak1 antisense ODN significantly inhibited the IL-4-mediated induction of MAO-A (Fig. 3A) (*p<0.002) and CD36 (Supplemental Fig. 1A) (*p<0.005) mRNA and protein (Fig. 3C and Supplemental Fig. 1B) expression, whereas the scrambled ODN control had no effect (Figs. 3A, C and Supplemental Figs. 1A, B). As a control we further showed that Jak2 and Tyk2 antisense ODN caused no inhibition of IL-4-induced expression of MAO-A and CD36 mRNA (Fig. 3A, Supplemental Fig. 1C and data not shown) or protein (Fig. 3C, Supplemental Fig. 1D and data not shown). These data thus confirm that similar to IL-4-stimulated 15-LO expression, Jak1 is the upstream regulator of both MAO-A and CD36 expression in IL-4-activated monocytes/macrophages.
Figure 3. IL-4 and IL-13-stimulated MAO-A expression as well as IL-13 and IL-4 specific induction of DUSP1 and TIMP3 is controlled by different Jak kinases in human monocytes/macrophages.
Monocytes (5×106/group) were pre-treated directly with antisense ODN to Jak1 or a scrambled ODN control (10 μM) (A–F) or Jak2 or Tyk2 antisense or sense ODNs (10 μM) (A–F) for 48 h, with one re-feed at 24 h, prior to the incubation with IL-4 (A, C, E, F) or IL-13 (B, D, E, F) for another 24h (A–D) or 48h (E, F). In panels A–B, total cellular RNA extracts were prepared and subjected to real-time quantitative PCR analysis. After normalization with GAPDH amplification, the fold induction of MAO-A mRNA expression for different groups was plotted. Data are the means ± S.D. (n=3). Significant differences were determined by comparing the antisense (AS), scrambled (Scr) and sense (S) ODN treated groups to the IL-4 or IL-13-treated control (*p<0.002) In panels C–D, cells were lysed and 50μg of postnuclear extracts were separated by SDS-PAGE and immunoblotted with an antibody against MAO-A. The data shown are representative of three separate experiments giving similar results.
For panels E and F, real-time PCR analyses was performed and fold induction of DUSP1 (E) and TIMP3 (F) mRNA expression for different groups was plotted after normalization with GAPDH. Data are the mean ± SD; (n=3). *p<0.003 compared to the IL-13 (E) or IL-4 (F)-treated controls.
The effect of Jak1, Tyk2 and Jak2-specific antisense and scrambled/sense ODNs were also monitored on both MAO-A and CD36 expression in response to IL-13 stimulation. Our results showed that both Jak2 and Tyk2 antisense ODNs significantly attenuated the expression of MAO-A (Fig. 3B) (*p<0.002) and CD36 (Supplemental Fig. 1E and data not shown) (*p<0.005) mRNA and protein (Fig. 3D and data not shown) expression after IL-13 activation, as compared to sense ODN controls (Figs. 3B, D, Supplemental Fig. 1E and data not shown). In contrast, Jak1 antisense ODN caused no inhibition of IL-13-activated MAO-A and CD36 mRNA (Fig. 3B, Supplemental Fig. 1E) and protein expression (Fig. 3D and data not shown). These results thus clearly demonstrate that both Jak2 and Tyk2 are required for the IL-13-mediated up regulation of MAO-A and CD36 gene expression in monocytes/macrophages. Differential regulation of MAO-A and CD36 gene expression in IL-4 and IL-13-activated monocytes/macrophages by distinct Jak kinases is entirely new and to our knowledge presented for the first time.
IL-13 and IL-4 unique signaling differentially regulates the induction of DUSP1 (dual specificity phosphatase 1) and TIMP3 (tissue inhibitor of metalloprotease-3) gene expression in alternatively activated human monocytes/macrophages
As IL-13 and IL-4 differ in their ability to activate different Jak kinases, we next explored whether IL-13 and IL-4 can uniquely induce a particular gene expression. Our results show that IL-13 can uniquely induce the expression of DUSP1 (Fig. 3E) whereas IL-4 specifically stimulates the expression of TIMP3 mRNA (Fig. 3F). Further results demonstrated that Tyk2 antisense ODN significantly down-regulated the expression of IL-13-induced DUSP1 mRNA (*p<0.003) as compared to the sense ODN control (Fig. 3E) whereas, Jak1 and Jak2 antisense ODNs had no effect on IL-13-activated DUSP1 mRNA (Fig. 3E). In contrast, IL-4-mediated induction of TIMP3 mRNA was substantially attenuated by Jak1 antisense ODN (*p<0.003) as compared to the scrambled ODN control (Fig. 3F) whereas, Jak2 and Tyk2 antisense ODNs had no effect on IL-4-stimulated TIMP3 mRNA (Fig. 3F). These novel data clearly demonstrate that IL-13 and IL-4 can uniquely affect the expression of a particular gene in alternatively activated monocytes/macrophages.
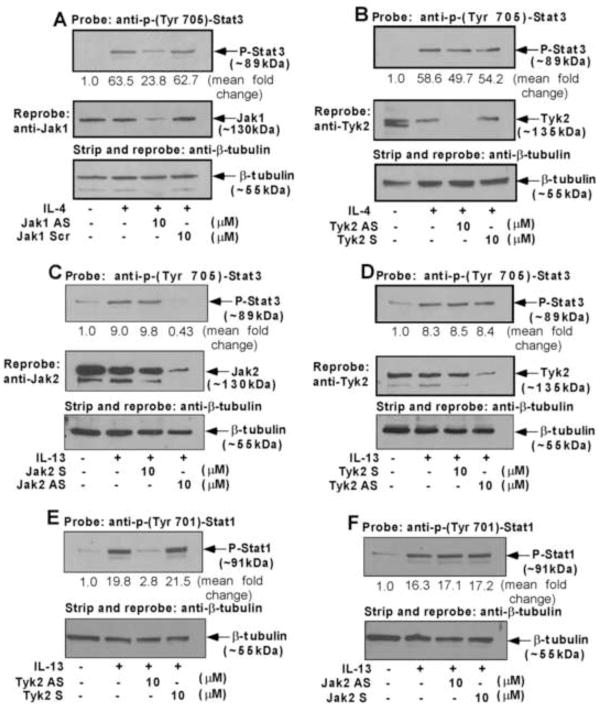
Stat3 activation by IL-4 requires Jak1 kinase in monocytes
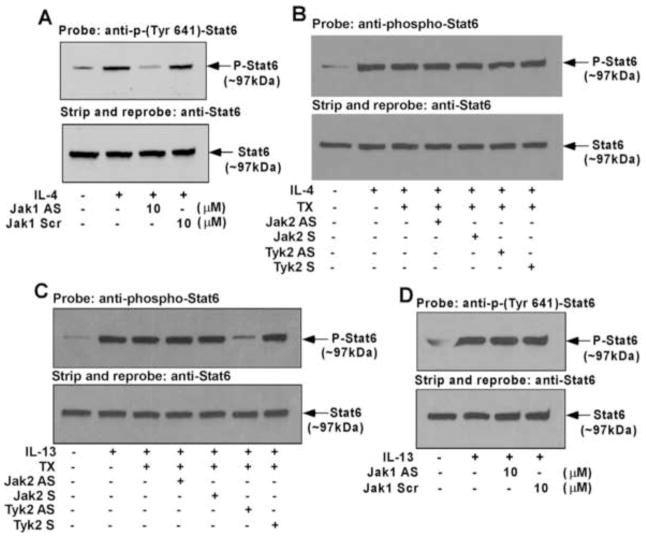
Previously we reported that both IL-4 and IL-13 stimulate the phosphorylation of Stats1, 3, and 5 in addition to Stat6. IL-4, in contrast to IL-13, induced very low levels of phosphorylation of Stats1 and 5 and a more robust phosphorylation of Stat3 and Stat6 [11]. Our results further suggested that Stat3 is one of the crucial regulators of 15-LO gene expression [33]. As we identified Jak1 as the Jak kinase that regulates IL-4-mediated signaling, we next investigated whether Jak1 is required for Stat3 activation. Our results indicate that the antisense ODN against Jak1 kinase inhibited IL-4-stimulated activation/phosphorylation of Stat3 on Tyr 705 residue whereas the scrambled ODN control had no effect (Fig. 4A, upper panel). Antisense ODN inhibition of Jak1 protein expression was also verified by reprobing the same blot with an antibody against Jak1 kinase (Fig. 4A, middle panel).
Figure 4. Jak2 and Tyk2 are the upstream regulators of Stat3 and Stat1 activation in IL-13-stimulated monocytes whereas Jak1 controls Stat3 activation in IL-4-treated monocytes.
Monocytes (5×106/group) were either pre-treated with antisense ODN to Jak1 or scrambled ODN control (A), Tyk2 antisense or sense ODNs (B, D, E) or with Jak2 antisense or sense ODNs (C, F) for 48 h, with one re-feed at 24 h, prior to the addition of IL-4 (A, B) and IL-13 (C–F) for 15 min. The cells were lysed and whole cell extracts were resolved by SDS-PAGE. Stat3 and Stat1 tyrosine phosphorylation was detected by immunoblotting using p-Tyr705 Stat3 (upper panels of A–D) and p-Tyr701 Stat1 (upper panels of E and F) antibodies. The same blots were then reprobed with Jak1 (middle panel of A), Tyk2 (middle panels of B and D) and Jak2 (middle panel of C) antibodies to examine the effect of antisense ODN on Jak1, Tyk2 and Jak2 protein expression levels. To assess equal loading the blots were then stripped and reprobed with (β-tubulin antibody (lower panels of A–F). The data shown are representative of three independent experiments giving similar results. Blots from three different experiments were quantified and normalized to the respective total protein loads (β-tubulin). Mean fold change data are provided for all panels after considering all untreated groups (no IL-4 and IL-13) as 1.0.
As a control experiment, we next examined whether blocking Tyk2 protein expression also inhibited the IL-4-induced activation of Stat3. Our results demonstrated that substantial inhibition of Tyk2 protein expression by Tyk2 antisense (Fig. 4B, middle panel) had no inhibitory effect on IL-4-induced tyrosine 705 phosphorylation of Stat3 (Fig. 4B, upper panel). These results thus confirm that Jak1 is the upstream regulator of Stat3 phosphorylation/activation whereas Tyk2 is not involved in Stat3 tyrosine phosphorylation/activation in alternatively activated monocytes/macrophages in response to IL-4 stimulation.
Jak2 is required for IL-13-mediated Stat3 activation
Since we demonstrated earlier that IL-13-induced Stat3 tyrosine 705 phosphorylation is critical for IL-13-stimulated 15-LO gene expression in human monocytes [32], we next investigated the requirement for Jak2 and Tyk2 kinases for the IL-13-induced activation/tyrosine phosphorylation of Stat3 in primary monocytes. Our results show that the antisense ODN against Jak2 kinase inhibited the IL-13-mediated tyrosine 705 phosphorylation of Stat3, whereas the sense ODN had no effect (Fig. 4C, upper panel). Antisense ODN inhibition of Jak2 protein expression was also verified by reprobing the same blot with an antibody against Jak2 kinase (Fig. 4C, middle panel). These data thus identify Jak2 as the upstream kinase of Stat3 tyrosine phosphorylation/activation in human monocytes in response to IL-13 stimulation. Although similar experiments with Tyk2 antisense ODN indicated substantial inhibition of Tyk2 protein expression in monocytes (Fig. 4D, middle panel), the reduced expression level of Tyk2 had no inhibitory effect on IL-13-induced tyrosine 705 phosphorylation of Stat3 (Fig. 4D, upper panel).
Tyk2 but not Jak2 is involved in regulating the IL-13-stimulated activation of Stat1
As we showed earlier that Stat1 is also a regulator of 15-LO gene expression in IL-13-treated monocytes [33], next we wanted to investigate the specific receptor-associated Jak kinase that is required for Stat1 activation in response to IL-13 stimulation. Our results, presented in Fig. 4E (upper panel), indicate that antisense ODN against Tyk2 almost completely blocked the IL-13-stimulated Stat1 phosphorylation at a specific tyrosine residue (Tyr701) which controls Stat1 activation. Tyk2 sense ODN did not inhibit Stat1 tyrosine-701 phosphorylation. By using the antisense ODN against Jak2, we further confirmed that Jak2 is not involved in IL-13-stimulated Stat1 phosphorylation/activation (Fig. 4F, upper panel). The specificity and effectiveness of these antisense ODNs has been tested previously by our lab and is also shown in Fig. 4C and 4D.
Jak1 and Tyk2 are required for Stat6 activation in monocytes stimulated with IL-4 and IL-13 respectively
Many studies provide evidence that Stat6 is a crucial player in the regulation of 15-LO gene expression [43–46]. We next explored whether Jak1 is upstream of IL-4-induced Stat6 activation (tyrosine phosphorylation of Tyr641 residue). Our results demonstrated substantial inhibition of Stat6 activation in monocytes treated with Jak1 antisense ODN, whereas treatment with the scrambled ODN control showed no inhibitory effect on Stat6 activation/tyrosine phosphorylation (Fig. 5A, upper panel). The bottom panel in Fig. 5A shows nearly equal Stat6 expression in all lanes. As a control, we also checked the effect of Jak2 and Tyk2 antisense ODN on IL-4-stimulated activation of Stat6. Our results confirm that antisense ODN against either the Jak2 or Tyk2 kinases do not inhibit IL-4-mediated activation of Stat6 (Fig. 5B). Our results, presented in Fig. 5C (upper panel), further reveal that antisense ODN against Tyk2 significantly blocked the IL-13-stimulated activation of Stat6 (phosphorylation at a specific tyrosine residue, Tyr 641) whereas Jak2 antisense, Jak2 sense and Tyk2 sense ODN did not inhibit Stat6 activation. Our results further indicate that the antisense ODN against Jak1 has no inhibitory effect on IL-13-stimulated Stat6 activation (Fig. 5D).
Figure 5. IL-4-stimulated Stat6 activation is mediated by Jak1 whereas Tyk2 is required for Stat6 activation in IL-13-treated monocytes.
Monocytes (5×106/group) were pre-treated with scrambled/sense control ODNs or antisense ODNs to Jak1 (A, D) or Jak2 or Tyk2 (B, C) according to protocols described under “Materials and Methods” prior to the addition of IL-4 (A, B) or IL-13 (C, D) for 30 min. The cells were lysed and immunoblotted with anti-phospho-Stat6 antibody (upper panels of A–D). The same blots were stripped and reprobed with an antibody recognizing total Stat6 to confirm equal loading (lower panels of A–D). Data in panels A–D are from a representative of three repeat experiments showing similar results.
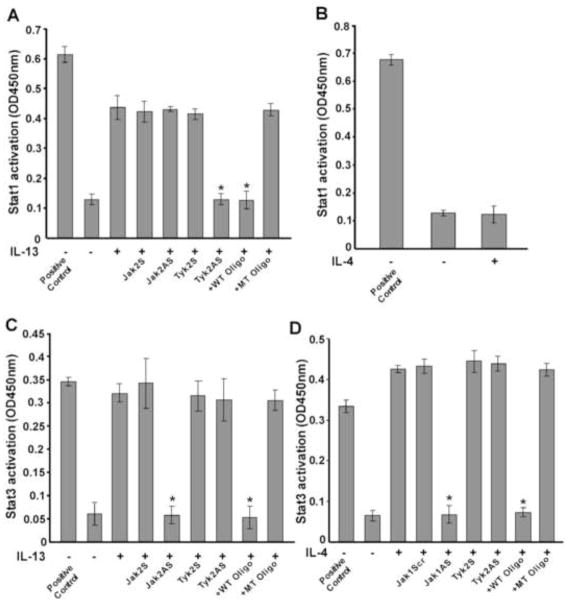
IL-13 induction of Stat1 DNA binding activity is regulated by Tyk2
Earlier we demonstrated that phosphorylation/activation of Stat1 by IL-13 is required for Stat1 DNA binding activity [11]. Since we showed that Tyk2 is required for Stat1 phosphorylation at a specific tyrosine (Tyr701, Fig. 4E), we investigated whether Tyk2 was required for IL-13-stimulated Stat1 DNA binding activity in primary human monocytes. Using an ELISA based TransAM™ method we demonstrated that IL-13 treatment enhanced Stat1 DNA binding activity in nuclear extracts of monocytes (Fig. 6A). Furthermore, pretreatment of monocytes with antisense ODN against Tyk2 significantly reduced the extent of Stat1 DNA binding activity whereas the sense ODN had no effect (Fig. 6A). By using Jak2 antisense ODN, we showed that Jak2 is not required for IL-13-induced Stat1 DNA binding activity (Fig. 6A). The wild-type (WT) consensus oligonucleotides also substantially reduced the DNA binding activity of Stat1. Conversely, the mutated (MT) consensus oligonucleotides showed negligible effects on Stat1 DNA binding activity (Fig. 6A). These results establish the involvement of Tyk2 in regulating Stat1 DNA binding in response to IL-13. We also verified the effect of IL-4 treatment on Stat1 DNA binding activity using the same ELISA based method. Our results revealed that IL-4 could not stimulate Stat1 DNA binding activity (Fig. 6B). This observation supports our previous result that IL-4 has a negligible effect on Stat1 phosphorylation/activation [11].
Figure 6. IL-13-induced Stat1 DNA binding activity requires Tyk2 whereas Jak1 and Jak2 are involved in regulating IL-4 and IL-13-induced Stat3 DNA binding activity in human monocytes.
Human monocytes (5×106/group) were either directly exposed to IL-13 (A, C) or IL-4 (B, D) for 1h or pretreated with control ODN or antisense ODN to Jak2 or Tyk2 (A, C) or Jak1 or Tyk2 (D) (10 μM) for 48 h, with one re-feed at 24 h followed by IL-13 or IL-4 stimulation for 1h. Nuclear extracts (5μg) were run in duplicate to perform an immunodetection of activated Stat1 (A, B) and Stat3 (C, D) using a TransAM™ Stat family kit. Nuclear extracts from IFNγ-stimulated COS-7 cells and IL-6-stimulated HepG2 cells were used as positive controls for activated Stat1 (A, B) and activated Stat3 (C, D) respectively. The wild-type (WT) and mutated (MT) consensus oligonucleotides were used to monitor the specificity of the assay. Values are mean ± SEM of three separate experiments giving similar results. Significant differences were determined by comparing each group to the IL-13 or IL-4-treated monocytes as the control (*p<0.004).
Jak2 regulates IL-13-stimulated Stat3 DNA binding activity whereas IL-4-induced Stat3 DNA binding activity is controlled by Jak1
Following the same ELISA based method we showed that IL-13 treatment facilitated DNA binding activity of Stat3 (Fig. 6C). Furthermore, pretreatment of monocytes with antisense ODN against Jak2 significantly attenuated the extent of DNA binding activity of Stat3 whereas the sense ODN control showed no inhibition (Fig. 6C). As a control we further showed that Tyk2 antisense ODN treatment did not affect Stat3 DNA binding activity in response to IL-13 stimulation (Fig. 6C). The wild-type (WT) oligonucleotides also significantly reduced the level of DNA binding activity of Stat3 (Fig. 6C). In contrast, the mutated (MT) oligonucleotides showed negligible effects on IL-13-induced DNA binding activity of Stat3 in primary monocytes (Fig. 6C). These results indicate the involvement of Jak2 in regulating Stat3 DNA binding activity in response to IL-13 stimulation.
Since we showed that Jak1 is required for IL-4-dependent Stat3 Tyr705 phosphorylation, we next examined whether Jak1 controls IL-4-stimulated Stat3 DNA binding activity. Our results demonstrated that IL-4 treatment enhanced Stat3 DNA binding activity in nuclear extracts of monocytes (Fig. 6D). Pretreatment of monocytes with antisense ODN against Jak1 markedly reduced the extent of IL-4-mediated Stat3 DNA binding activity whereas the scrambled ODN control or Tyk2 antisense ODN showed no inhibitory effect (Fig. 6D). These data support the concept that the IL-4-stimulated Stat3 DNA binding activity is dependent on Jak1.
Stat1 and Stat3 are both required for IL-13-induced expression of 15-LO and MAO-A whereas only Stat3 is involved in IL-4 induction of 15-LO and MAO-A
Previously we reported the contribution of both Stat1 and Stat3 in controlling IL-13-stimulated 15-LO expression in human monocytes by using transcription factor decoy technology [33]. To examine the involvement of Stat1 and Stat3 for other M2 gene expression in alternatively activated monocytes/macrophages by IL-13, we next checked the effect of both Stat1 and Stat3 decoy ODN treatment in regulating IL-13-induced MAO-A gene expression. Results from our Western blot analysis indicate that both Stat1 and Stat3 decoy ODNs significantly down-regulated the IL-13-mediated induction of MAO-A protein expression (*p<0.002) (Fig. 7A and 7B, upper panels), whereas transfection of cells with either Stat1 or Stat3 mismatched ODNs (used as controls) essentially had no effect on IL-13-stimulated MAO-A protein expression (Fig. 7A and 7B, upper panels). These results suggest that in addition to 15-LO, Stat1 and Stat3 transcription factors are also required for the expression of other alternative state (M2) genes like MAO-A in IL-13-stimulated monocytes/macrophages.
Figure 7. IL-13 stimulation requires both Stat1 and Stat3 whereas only Stat3 is required by IL-4 for the expression of 15-LO and MAO-A in alternatively activated monocytes.
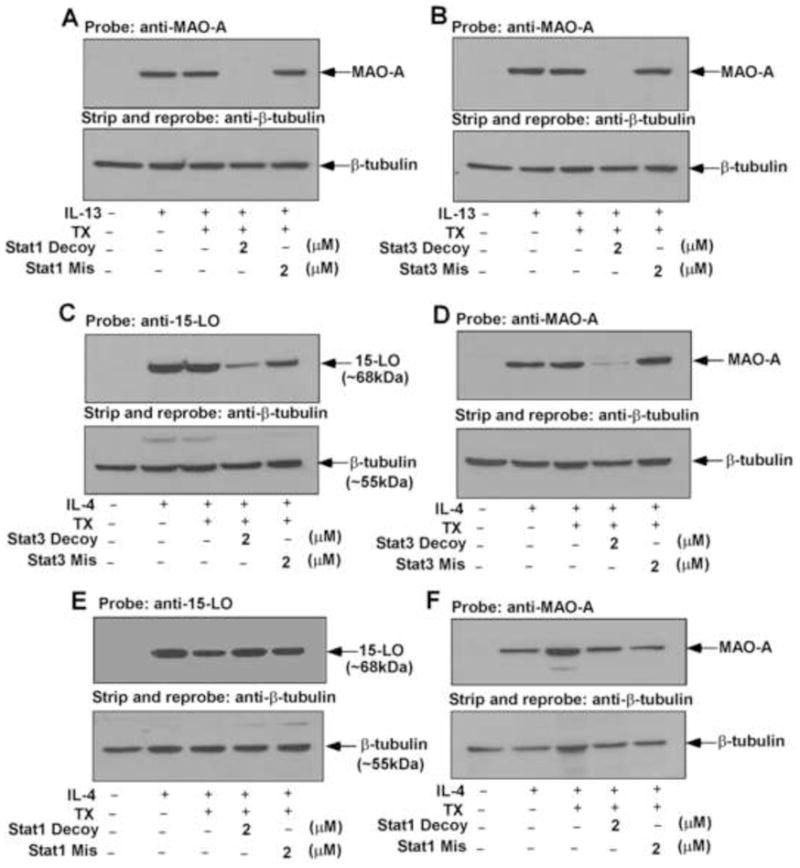
Monocytes (5×106/group) were transfected with or without decoy or mismatched decoy ODNs to Stat1 (A, E, F) or Stat3 (B, C, D) according to protocols described under “Materials and Methods” prior to the addition of IL-13 (A, B) or IL-4 (C–F) for 48 h. Monocyte lysates were resolved by SDS-PAGE and immunoblotted with MAO-A (upper panels of A, B, D, F) or 15-LO (upper panels of C and E) specific antibodies. The same blots were then stripped and reprobed with (β-tubulin antibody (lower panels of A–F) to assess equal loading. Data are from a representative of three repeat experiments showing similar results.
To investigate whether Stat1 and Stat3 also regulate IL-4-induced 15-LO and MAO-A gene expression, we used the same Stat1 and Stat3 decoy ODNs and their corresponding mismatched ODNs (used as a control) to transfect monocytes with them as described earlier [33]. Our results demonstrate that the Stat3 decoy ODN substantially inhibited the IL-4-mediated induction of both 15-LO (~80%) and MAO-A (~90%) protein expression (Fig. 7C and 7D, upper panels), whereas the Stat3 mismatched ODN control did not inhibit either (Fig. 7C or 7D, upper panels). In contrast, Stat1 decoy ODN transfection could not block either 15-LO or MAO-A protein expression in response to IL-4 stimulation (Fig. 7E and 7F, upper panels). These results indicate that Stat3 activation is required for the induction of both 15-LO and MAO-A expression whereas Stat1 is not required for 15-LO or MAO-A expression in alternatively activated monocytes/macrophages by IL-4.
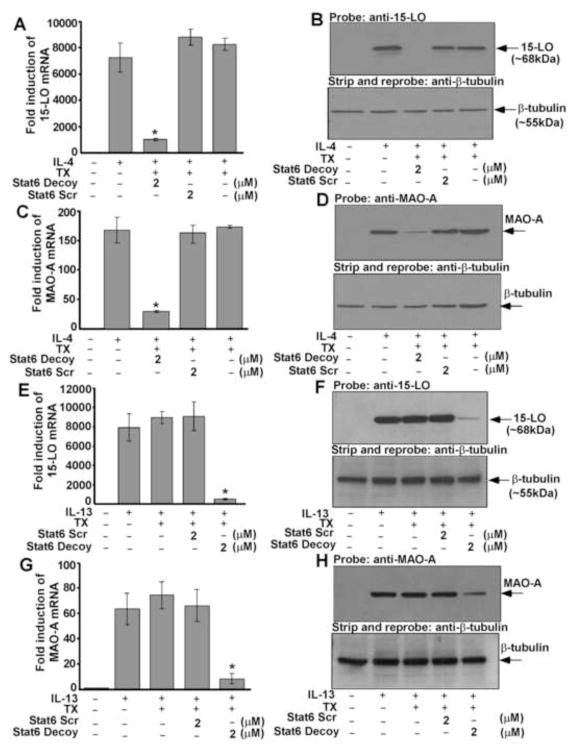
15-LO and MAO-A gene expression require Stat6 in alternatively activated monocytes/macrophages by IL-4 and IL-13
To directly assess the functional role of Stat6 in IL-4 and IL-13-induced 15-LO gene expression, we transfected monocytes with Stat6 decoy ODNs. Transfection of monocytes with Stat6 decoy markedly attenuated both IL-4 and IL-13-induced 15-LO mRNA expression as compared to IL-4 and IL-13-treated cells or transfection controls, respectively (Fig. 8A and 8E). Cells transfected with Stat6 scrambled decoy ODN showed no inhibition of IL-4 or IL-13-stimulated 15-LO mRNA induction (Fig. 8A and 8E). Transfection of Stat6 decoy ODN also inhibited both IL-4 as well as IL-13-induced 15-LO protein expression significantly as compared to the levels in untransfected monocytes (IL-4 and IL-13-treated controls) and transfection controls, respectively (p<0.001) (Fig. 8B and 8F, upper panels), whereas transfection of cells with Stat6 scrambled decoy ODN showed no inhibition. These results provide evidence that activation of Stat6 is required for 15-LO gene expression in alternatively activated monocytes by IL-4 and IL-13.
Figure 8. Stat6 regulates 15-LO and MAO-A gene expression in IL-4 and IL-13-activated monocytes.
Human blood monocytes (5×106/group) were transfected with or without decoy or scrambled ODN to Stat6 according to protocols described under “Materials and Methods” prior to the addition of IL-4 (A–D) or IL-13 (E–H) for 24 h. 15-LO (A, E) and MAO-A (C, G) mRNA expression was detected by real time quantitative PCR analysis. Data are the mean ± SD; (n=3). Significant differences were determined by comparing the decoy or scrambled decoy ODN (to Stat6) treated groups to the transfection control (IL-4 or IL-13-treated) (*p<0.006). In B, D, F and H, monocyte lysates were separated by SDS-PAGE and immunoblotted with 15-LO (upper panels of B and F) and MAO-A (upper panels of D and H) antibodies. The same blots were then stripped and reprobed with (β-tubulin antibody (lower panels of B, D, F and H) to assess equal loading. Data in panels A–H are from a representative of three independent experiments showing similar results.
We next investigated the involvement of Stat6 activation, in regulating both IL-4 and IL-13-induced MAO-A gene expression in monocytes/macrophages. Results from our real-time PCR experiment or Western blot analysis indicated that treatment with the Stat6 decoy significantly inhibited both the IL-4 and IL-13-mediated induction of MAO-A mRNA (Fig. 8C and 8G) (*p<0.006) and protein expression (Fig. 8D and 8H, upper panels) (p<0.002), whereas the Stat6 scrambled decoy ODN control had no effect on either IL-4 or IL-13-stimulated MAO-A mRNA (Fig. 8C and 8G) or protein expression (Fig. 8D and 8H, upper panels). These data thus suggest that in both IL-4 and IL-13-stimulated monocytes/macrophages, Stat6 activation is not restricted to the regulation of 15-LO pathway, but also required for the induction of other M2 genes like MAO-A.
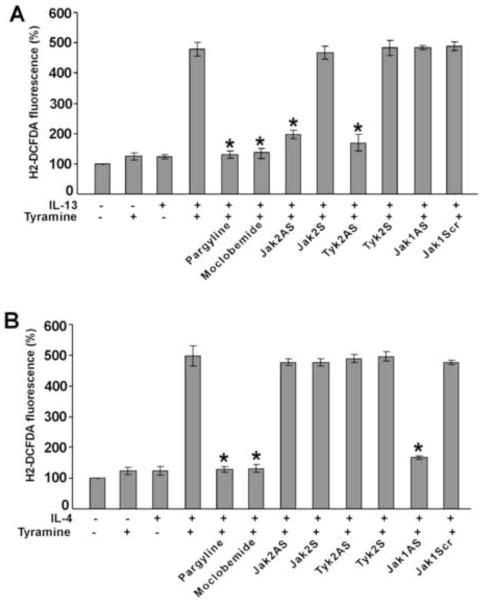
Tyramine-induced ROS generation requires Jak2 and Tyk2 in IL-13-activated monocytes but is controlled by only Jak1 in monocytes treated with IL-4
As MAO-A is induced by both IL-13 and IL-4 in alternatively activated monocytes/macrophages [19, 22, 23] and as previous studies report the generation of intracellular reactive oxygen species (ROS) during the oxidation of biogenic amines by MAO-A [37], we first investigated the requirement of MAO-A in tyramine-stimulated ROS generation in alternatively activated monocytes/macrophages by IL-13 and IL-4. Our data suggest that the MAO inhibitors Pargyline (a pan-MAO inhibitor) and Moclobemide (a specific inhibitor for MAO-A) strongly inhibited the tyramine induction of intracellular ROS in both IL-13 and IL-4-activated monocytes/macrophages (Fig. 9A and 9B). Furthermore, the involvement of different Jak kinases in regulating both IL-13 and IL-4-induced MAO-A-mediated ROS generation was also examined in monocytes/macrophages. Our results show that antisense ODN against both Jak2 and Tyk2 significantly blocked the tyramine-induced rise of intracellular ROS in IL-13-stimulated monocytes/macrophages (*p<0.004) (Fig. 9A) whereas Jak1 antisense ODN and control ODNs (sense ODN controls for Jak2 or Tyk2 and scrambled ODN control for Jak1) had no effect on ROS generation during tyramine oxidation of MAO-A in IL-13-activated monocytes/macrophages (Fig. 9A). In contrast, treatment of monocytes/macrophages by the same antisense ODNs show that tyramine-induced ROS generation in IL-4-activated monocytes/macrophages is controlled by Jak1 but not by Jak2 and Tyk2 (Fig. 9B). Altogether, these data strongly suggest that IL-13 and IL-4 use distinct Jak kinase-mediated signaling pathways to control the function of MAO-A in tyramine-induced ROS generation.
Figure 9. Tyramine-induced reactive oxygen species (ROS) generation is controlled by different Jak kinases in IL-13 versus IL-4 activated human monocytes/macrophages.
Monocytes (5×106/group) were pre-treated with control ODN or antisense ODNs to Jak1 or Jak2 or Tyk2 (A, B) according to protocols described under “Materials and Methods” or with the MAO inhibitors, pargyline (1 μM) (A, B) and Moclobemide (1 μM) (A, B) for 30 min prior to stimulation with IL-13 (2nM) (A) or IL-4 (2nM) (B) for 24h. Monocytes/macrophages were stimulated by tyramine (5 μM) for 30 min and then incubated with the fluorescent probe H2DCFDA (5 μM) for another 30 min before the fluorescence was measured (A, B). Data in panels A and B are expressed as percentage of unstimulated controls and represented as mean ± SD; (n=3, *p<0.004). Data are from a representative of three independent experiments.
MAO-A expression/activity controls tyramine-induced ROS generation in alternatively activated monocytes/macrophages
To examine the direct involvement of MAO-A in tyramine-induced ROS generation in IL-13 and IL-4-activated monocytes/macrophages, we treated monocytes with the MAO-A-specific antisense or control sense ODNs and then added IL-13 (Fig. 10A). Treatment with MAO-A-specific antisense ODN significantly inhibited the IL-13-mediated induction of MAO-A protein expression (Fig. 10A) and downregulated MAO-A enzyme activity (*p<0.002) in both IL-13 and IL-4-activated monocytes (Fig. 10B) whereas the sense ODN control was without any effect (Fig. 10B). Next we investigated the effect of MAO-A antisense ODN on tyramine-stimulated ROS generation in IL-13 and IL-4-activated monocytes. Our results demonstrate that MAO-A antisense ODN significantly blocked both IL-4 and IL-13-activated ROS generation in tyramine-stimulated monocytes (*p<0.004) whereas the MAO-A sense ODN control had no effect (Fig. 10C). We confirmed our data by introducing a second method. to measure MAO-A activity, the Amplex Red Monoamine Oxidase assay. Our results again show that MAO-A antisense ODN significantly down regulated both IL-4 and IL-13-activated H2O2 generation in p-Tyramine-stimulated monocytes/macrophages (*p<0.005) (Fig. 10D). Taken together these results show that ROS (H2O2) generation (using p-Tyramine as a substrate) due to MAO-A expression/activity is regulated by distinctly different Jak kinases in alternatively activated monocytes/macrophages by IL-4 and IL-13.
Figure 10. MAO-A regulates tyramine-induced ROS generation in IL-13 and IL-4-activated monocytes/macrophages.
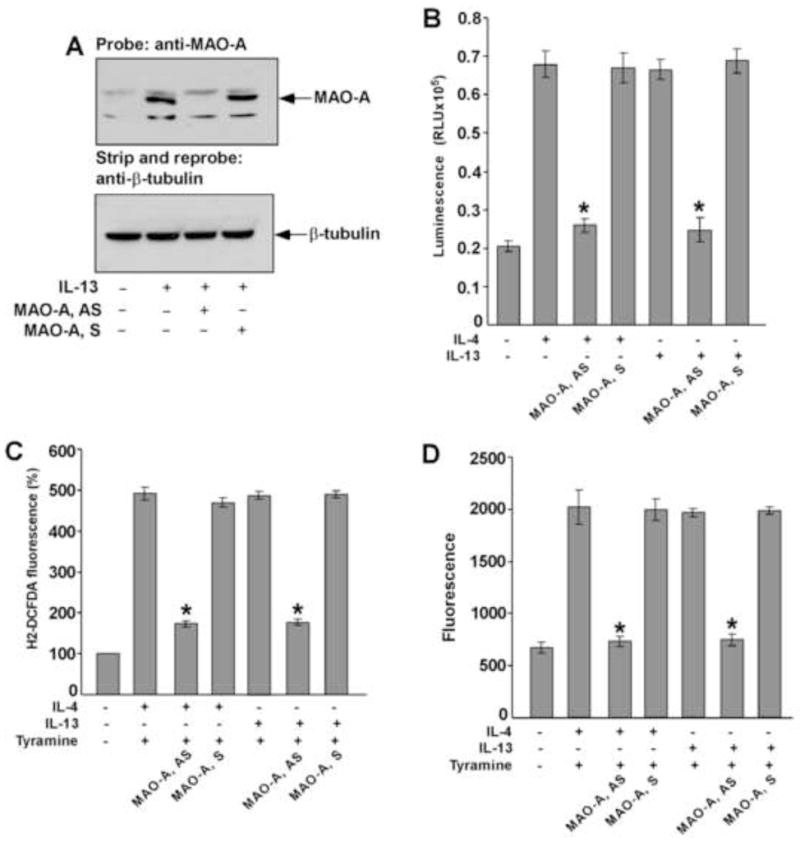
Monocytes (5×106/group) were pre-treated with MAO-A sense (S) or antisense (AS) ODN (A–D) according to protocols described under “Materials and Methods” and incubated with IL-13 (A–D) and IL-4 (B–D) (2nM) for 46h. In panel A, whole cell extracts (50 μg/lane) were resolved by SDS-PAGE. MAO-A protein expression was detected on Western blots with a MAO-A specific antibody (upper panel of A). The blot was stripped and reprobed with an antibody against (β-tubulin (lower panel of A) to assess equal loading. In panel B, native MAO-A enzyme activity was detected. In a total volume of 50 μl, 20 μl whole cell extract was incubated with substrate (final conc. 40 μM) in presence of MAO reaction buffer [100mM HEPES (pH 7.5); 5% glycerol]. After incubation at room temperature for 3h, 50μl luciferin detection reagent was added and the luminescent signal was measured after 20 min. Data represented as mean ± SD; (n=3, *p<0.002). In panel C, monocytes/macrophages were stimulated by tyramine (5 μM) for 30 min and then incubated with the fluorescent probe H2DCFDA (5 μM) for another 30 min before the fluorescence was measured. Data in panel C is expressed as percentage of unstimulated controls and represented as mean ± SD; (n=3, *p<0.004). In panel D, monocyte lysates (50μg/well) were incubated with Amplex Red reagent/HRP/p-Tyramine working solution for 60 min before the fluorescence was measured. Data represented as mean ± SD; (n=3, *p<0.005). Data in all panels are from a representative of three independent experiments.
Discussion
IL-4 and IL-13 have many similar modulating effects on immune responses and play important roles in the development of inflammatory diseases [47]; however, further studies demonstrate that IL-13 possesses several unique effector functions that differentiate it from IL-4 [10]. Recent studies reveal that IL-13 is a powerful regulator of tissue remodeling and fibrotic responses in vivo and in vitro [10]. IL-13 has also been implicated in the pathogenesis of asthma, parasitic infections and many other pathological conditions [38]. In an effort to determine whether the differential response is due to the differences in signaling pathways employed by IL-4 and IL-13, we investigate the direct effects of IL-4 and IL-13 on signal transduction in primary monocytes/macrophages by examining the activation of receptor-associated Jak kinases and Stat family members. Although our results show that these two cytokines activate different Jak kinases (Jak1 in the case of IL-4 stimulation and both Jak2 and Tyk2 in response to IL-13 stimulation), activation of Stat6 and Stat3 seems to be a common effect after IL-4 and IL-13 stimulation. In contrast, Stat1 is activated substantially by IL-13 and only to a negligible extent by IL-4. These results suggest that Stat1 activity is an integral part of IL-13 signaling and contributes in many situations where IL-13 appears to a play a more important role than IL-4.
IL-4, IL-13 and Stat6 are key components in the development of airway inflammation, mucus production and airway hyperresponsiveness(AHR) in asthma [48]. After binding of IL-4 and IL-13 to the receptor complex, signals are transduced via Jaks to activate Stat family members by phosphorylating the specific tyrosine residues [11, 49]. In both IL-4 and IL-13 signaling, phosphorylated Stat6 dimers translocated to the nucleus and activate the transcription of several genes. In experimental models, targeted gene disruption of Stat6 inhibited AHR, airway inflammation and fibrosis [50]. Several experimental approaches for the downregulation of Stat6 are under investigation, including small-molecule inhibitors, antisense therapy, siRNA and dominant-negative peptides. From our studies it is evident that in addition to the IL-4/IL-13/Stat6 pathway, IL-4/IL-13/Stat3 and IL-13/Stat1 pathways can also be targeted for the development of biological compounds that may provide a new therapeutic modality for patient populations with uncontrolled severe asthma and other chronic airway diseases.
Recent reports demonstrate that in addition to 15-LO and CD36 (both implicated in the pathogenesis of inflammation and atherosclerosis), expression of MAO-A is strongly upregulated in alternatively activated monocytes/macrophages [19, 22, 23, 51]. Although MAO-A has been implicated as a vital regulator of embryonic brain development [52] and its deficiency has been associated with abnormal behavior [53], the biological importance of IL-4/IL-13-stimulated upregulation of MAO-A for monocyte physiology remains to be investigated. Since the biogenic amine serotonin is a well-known inflammatory mediator and preferentially oxidized by MAO-A, removal of this pro-inflammatory mediator from the site of inflammation may contribute to switching monocytes from the pro- to antiinflammatory phenotype, which may contribute to resolution of inflammation.
Results from previously published reports and the data presented in this manuscript suggest that both 15-LO and MAO-A are induced in alternatively activated monocytes/macrophages [19, 22, 51]. Previous observations also indicated the co induction of MAO-A and 15-LO in a certain subpopulation of peripheral blood monocytes [22]. These findings suggest a common regulatory pathway for the expression of these two genes. In fact, our data in this manuscript indicate the involvement of the same Jak kinases in regulating MAO-A expression that are required for 15-LO gene expression in IL-4/IL-13-activated monocytes. Furthermore, the existence of Stat responsive elements in the promoter of 15-LO gene and their regulatory role in IL-4/IL-13-dependent upregulation of 15-LO expression (Yakubenko, V. et al, 2012, unpublished observations), [33, 44] led us to speculate that the promoter of MAO-A gene may also contain the cognate sequences for the members of Stat family. Although the in silico structural promoter analysis failed to detect any Stat-responsive sequences in the 5 -flanking region of MAO-A gene promoter [51], our results show that IL-4/IL-13-stimulated MAO-A gene expression in peripheral blood monocytes requires the involvement of Stat family members (Stat1, Stat3 and Stat6). The difference between these two observations can be explained by the fact that the Stat transcription factors may not be directly involved in regulating MAO-A gene expression. This hypothesis is supported by our recent unpublished observation that 15-LO antisense oligonucleotide transfection in primary human monocytes substantially inhibits IL-13-induced MAO-A expression (unpublished observations by Bhattacharjee, A. and Cathcart, M.K.), suggesting the direct involvement of 15-LO in controlling IL-13-activated MAO-A gene expression in monocytes.
In this study, we demonstrate for the first time that reactive oxygen species (ROS) are generated by MAO-A in IL-4 and IL-13-driven alternatively activated monocytes/macrophages. Inhibition of Tyramine-induced ROS production by both the MAO-A selective pharmacological inhibitors and MAO-A-specific antisense ODN strongly supports the role of MAO-A in this process. Moreover, we investigated the differential regulation of MAO-A-mediated ROS generation in response to IL-4 and IL-13 by demonstrating the involvement of cytokine-selective Jak kinases suggesting the functional relevance of different Jak kinases in ROS generation. A previous report examined the involvement of mitochondrial H2O2 in superoxide anion production and subsequent LDL oxidation via NADPH oxidase in human endothelial cells [54]. Furthermore, it was suggested that monocyte-derived superoxide anion production due to the activation of NADPH oxidase, is required for monocyte-mediated LDL oxidation and contributes to atherogenesis [55]. Hence the involvement of MAO-A-mediated intracellular ROS can not be excluded in the activation of monocyte NADPH oxidase and related superoxide anion production leading to atherogenesis but future experiments are needed to clarify these events.
In addition to neurodegenerative disorders, MAO-A has been shown to play a predominant role in myocardial injury [56] and also contributes to heart failure [57]. MAO-A can also cause cardiac cell apoptosis through ROS-dependent sphingosine kinase inhibition [58]. Moreover, MAO-A-mediated ROS generation has been shown to trigger mitogenic signaling in smooth muscle cells via MMP2/sphingolipid pathway which might contribute to vascular wall remodeling [37]. Hence inhibition of MAO-A is an important tool for the study of vascular pathologies and MAO-A-mediated signaling pathways may serve as future targets for therapeutic interventions in cardiovascular disorders.
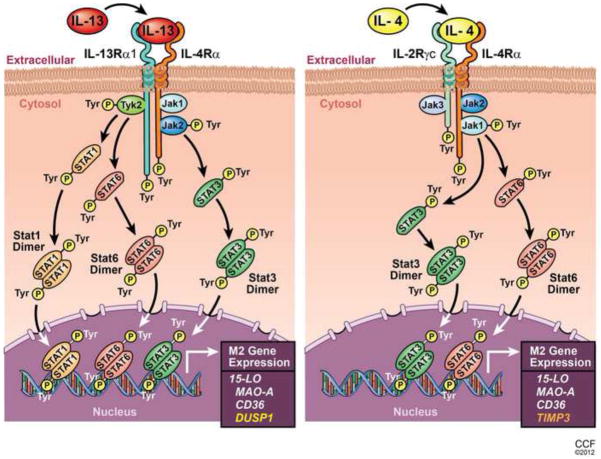
In summary, we report here the differential regulation of gene expression and function by IL-4 and IL-13-mediated signaling pathways in alternatively activated monocytes/macrophages. In Fig. 11, we present schematic models of both the IL-13 and IL-4 signaling pathways in monocytes/macrophages leading to the expression of genes of alternatively activated macrophages like 15-LO, CD-36 and MAO-A. The models are focused on differential Jak/Stat activation by these two related cytokines. Additionally we provide evidence that IL-13 and IL-4 uniquely affect the expression of particular genes like DUSP1 (induced only by IL-13 and by Tyk2-mediated pathways) and TIMP3 (induced only by IL-4 and by Jak1-mediated pathways). These results are novel in the context of differential gene regulation by IL-4 and IL-13 and add greater impact in this study.
Figure 11. Proposed model showing the differential regulation of gene expression in alternatively activated monocytes/macrophages by IL-13 and IL-4.
Schematic representations indicate that both IL-13 and IL-4 share and signal through a common receptor component IL-4Rα. For IL-13 the other receptor component is IL-13Rα1 whereas the IL-4 receptor contains IL-2Rγc. These receptor components are phosphorylated upon exposure of monocytes to IL-13 or IL-4. Different Jak kinases are associated with these receptor components and activated (tyrosine phosphorylated) in response to IL-13 or IL-4 stimulation as presented. Consequently Stat1 (for IL-13 only), Stat3 and Stat6 (for both IL-13 and IL-4) are activated, form dimmers and, translocate into the nucleus, bind DNA and facilitate the induction of 15-LO, MAO-A and CD36 gene expression. IL-13 and IL-4 differentially regulate the gene expression of DUSP1 and TIMP3 through distinct Jak/Stat pathways. Different color code for DUSP1 and TIMP3 is used to demonstrate the unique regulation of these genes by IL-13 and IL-4 respectively.
Our results indicate that IL-4 and IL-13 activate different Jak kinases which are upstream of several Stat transcription factors (Stats 1, 3 and 6 for IL-13 and Stats 3 and 6 for IL-4). We report here for the first time that Jak2 is upstream of Stat3 activation and Stat3 DNA binding activity while Tyk2 controls Stat1 and Stat6 activation and Stat1DNA binding activity in response to IL-13 stimulation. In contrast, Jak1 regulates Stat3 and Stat6 activation and Stat3 DNA binding activity in IL-4-induced monocytes. Finally, our observations that tyramine-induced ROS generation due to MAO-A expression/activity is regulated by distinctly different Jak kinases (Figs 9 and 10) add novel insights into the functional aspects of MAO-A in IL-13 and IL-4-activated monocytes/macrophages. These results thus increase our understanding of the unique signal transduction pathways utilized by IL-4 and IL-13 to affect gene expression, pathways previously presumed to be similar. Our findings have major implications for the management of allergy and inflammation.
Supplementary Material
Distinct Jak/Stat pathways regulate gene expression in alternatively activated monocytes
IL-13 and IL-4 uniquely affect expression of particular genes like DUSP1 and TIMP3
IL-4 and IL-13 activate different Jak kinases which are upstream of several Stats
MAO-A expression/activity is required for Tyramine-induced ROS generation
Different Jaks control MAO-A-mediated ROS in IL-4 and IL-13-activated monocytes
Acknowledgments
These studies were supported by Grant HL051068 and HL087018 from the National Institutes of Health to M.K.C and National Center for Research Resources, CTSA 1UL1RR024989. We thank GCRC/CTSA core at the Cleveland Clinic for providing elutriated monocytes. Authors are also thankful to David Schumick (Center for medical art and photography, Cleveland Clinic) for preparing the models.
Abbreviations used
- 15-LO
15-lipoxygenase
- ODN
oligodeoxyribonucleotide
- MAO-A
monoamine oxidase A
- ROS
reactive oxygen species
- Jak
janus kinase
- Stat
signal transducer and activator of transcription
Footnotes
The authors have no financial conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Iademarco MF, Barks JL, Dean DC. Regulation of vascular cell adhesion molecule-1 expression by IL-4 and TNF-alpha in cultured endothelial cells. J Clin Invest. 1995;95:264–271. doi: 10.1172/JCI117650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Miossec P, van den Berg W. Th1/Th2 cytokine balance in arthritis. Arthritis Rheum. 1997;40:2105–2115. doi: 10.1002/art.1780401203. [DOI] [PubMed] [Google Scholar]
- 3.Gordon S. Alternative activation of macrophages. Nat Rev Immunol. 2003;3:23–35. doi: 10.1038/nri978. [DOI] [PubMed] [Google Scholar]
- 4.Folcik VA, Aamir R, Cathcart MK. Cytokine modulation of LDL oxidation by activated human monocytes. Arterioscler Thromb Vasc Biol. 1997;17:1954–1961. doi: 10.1161/01.atv.17.10.1954. [DOI] [PubMed] [Google Scholar]
- 5.Allen JB, Wong HL, Costa GL, Bienkowski MJ, Wahl SM. Suppression of monocyte function and differential regulation of IL-1 and IL-1ra by IL-4 contribute to resolution of experimental arthritis. J Immunol. 1993;151:4344–4351. [PubMed] [Google Scholar]
- 6.Bessis N, Boissier MC, Ferrara P, Blankenstein T, Fradelizi D, Fournier C. Attenuation of collagen-induced arthritis in mice by treatment with vector cells engineered to secrete interleukin-13. Eur J Immunol. 1996;26:2399–2403. doi: 10.1002/eji.1830261020. [DOI] [PubMed] [Google Scholar]
- 7.Joosten LA, Lubberts E, Durez P, Helsen MM, Jacobs MJ, Goldman M, van den Berg WB. Role of interleukin-4 and interleukin-10 in murine collagen-induced arthritis. Protective effect of interleukin-4 and interleukin-10 treatment on cartilage destruction. Arthritis Rheum. 1997;40:249–260. doi: 10.1002/art.1780400209. [DOI] [PubMed] [Google Scholar]
- 8.Sugiyama E, Taki H, Kuroda A, Mino T, Yamashita N, Kobayashi M. Interleukin-4 inhibits prostaglandin E2 production by freshly prepared adherent rheumatoid synovial cells via inhibition of biosynthesis and gene expression of cyclo-oxygenase II but not of cyclo-oxygenase I. Ann Rheum Dis. 1996;55:375–382. doi: 10.1136/ard.55.6.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee CG, Homer RJ, Zhu Z, Lanone S, Wang X, Koteliansky V, Shipley JM, Gotwals P, Noble P, Chen Q, Senior RM, Elias JA. Interleukin-13 induces tissue fibrosis by selectively stimulating and activating transforming growth factor beta(1) J Exp Med. 2001;194:809–821. doi: 10.1084/jem.194.6.809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wynn TA. IL-13 effector functions. Annu Rev Immunol. 2003;21:425–456. doi: 10.1146/annurev.immunol.21.120601.141142. [DOI] [PubMed] [Google Scholar]
- 11.Roy B, Bhattacharjee A, Xu B, Ford D, Maizel AL, Cathcart MK. IL-13 signal transduction in human monocytes: phosphorylation of receptor components, association with Jaks, and phosphorylation/activation of Stats. J Leukoc Biol. 2002;72:580–589. [PubMed] [Google Scholar]
- 12.Munitz A, Brandt EB, Mingler M, Finkelman FD, Rothenberg ME. Distinct roles for IL-13 and IL-4 via IL-13 receptor alpha1 and the type II IL-4 receptor in asthma pathogenesis. Proc Natl Acad Sci U S A. 2008;105:7240–7245. doi: 10.1073/pnas.0802465105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ramalingam TR, Pesce JT, Sheikh F, Cheever AW, Mentink-Kane MM, Wilson MS, Stevens S, Valenzuela DM, Murphy AJ, Yancopoulos GD, Urban JF, Jr, Donnelly RP, Wynn TA. Unique functions of the type II interleukin 4 receptor identified in mice lacking the interleukin 13 receptor alpha1 chain. Nat Immunol. 2008;9:25–33. doi: 10.1038/ni1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rothenberg ME, Wen T, Shik D, Cole ET, Mingler MM, Munitz A. IL-13 receptor alpha1 differentially regulates aeroallergen-induced lung responses. J Immunol. 2011;187:4873–4880. doi: 10.4049/jimmunol.1004159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mitchell J, Dimov V, Townley RG. IL-13 and the IL-13 receptor as therapeutic targets for asthma and allergic disease. Curr Opin Investig Drugs. 2010;11:527–534. [PubMed] [Google Scholar]
- 16.Duffield JS. The inflammatory macrophage: a story of Jekyll and Hyde. Clin Sci (Lond) 2003;104:27–38. doi: 10.1042/. [DOI] [PubMed] [Google Scholar]
- 17.Goerdt S, Orfanos CE. Other functions, other genes: alternative activation of antigen-presenting cells. Immunity. 1999;10:137–142. doi: 10.1016/s1074-7613(00)80014-x. [DOI] [PubMed] [Google Scholar]
- 18.Maderna P, Godson C. Phagocytosis of apoptotic cells and the resolution of inflammation. Biochim Biophys Acta. 2003;1639:141–151. doi: 10.1016/j.bbadis.2003.09.004. [DOI] [PubMed] [Google Scholar]
- 19.Chaitidis P, O’Donnell V, Kuban RJ, Bermudez-Fajardo A, Ungethuem U, Kuhn H. Gene expression alterations of human peripheral blood monocytes induced by medium-term treatment with the TH2-cytokines interleukin-4 and -13. Cytokine. 2005;30:366–377. doi: 10.1016/j.cyto.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 20.Conrad DJ, Kuhn H, Mulkins M, Highland E, Sigal E. Specific inflammatory cytokines regulate the expression of human monocyte 15-lipoxygenase. Proc Natl Acad Sci U S A. 1992;89:217–221. doi: 10.1073/pnas.89.1.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nassar GM, Morrow JD, Roberts LJ, 2nd, Lakkis FG, Badr KF. Induction of 15-lipoxygenase by interleukin-13 in human blood monocytes. J Biol Chem. 1994;269:27631–27634. [PubMed] [Google Scholar]
- 22.Chaitidis P, Billett EE, O’Donnell VB, Fajardo AB, Fitzgerald J, Kuban RJ, Ungethuem U, Kuhn H. Th2 response of human peripheral monocytes involves isoform-specific induction of monoamine oxidase-A. J Immunol. 2004;173:4821–4827. doi: 10.4049/jimmunol.173.8.4821. [DOI] [PubMed] [Google Scholar]
- 23.Bhattacharjee A, Pal S, Feldman GM, Cathcart MK. Hck is a key regulator of gene expression in alternatively activated human monocytes. J Biol Chem. 2011;286:36709–36723. doi: 10.1074/jbc.M111.291492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yakubenko VP, Bhattacharjee A, Pluskota E, Cathcart MK. alphaMbeta Integrin activation prevents alternative activation of human and murine macrophages and impedes foam cell formation. Circ Res. 2011;108:544–554. doi: 10.1161/CIRCRESAHA.110.231803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Berry A, Balard P, Coste A, Olagnier D, Lagane C, Authier H, Benoit-Vical F, Lepert JC, Seguela JP, Magnaval JF, Chambon P, Metzger D, Desvergne B, Wahli W, Auwerx J, Pipy B. IL-13 induces expression of CD36 in human monocytes through PPARgamma activation. Eur J Immunol. 2007;37:1642–1652. doi: 10.1002/eji.200636625. [DOI] [PubMed] [Google Scholar]
- 26.Gratchev A, Guillot P, Hakiy N, Politz O, Orfanos CE, Schledzewski K, Goerdt S. Alternatively activated macrophages differentially express fibronectin and its splice variants and the extracellular matrix protein betaIG-H3. Scand J Immunol. 2001;53:386–392. doi: 10.1046/j.1365-3083.2001.00885.x. [DOI] [PubMed] [Google Scholar]
- 27.Roy B, Cathcart MK. Induction of 15-lipoxygenase expression by IL-13 requires tyrosine phosphorylation of Jak2 and Tyk2 in human monocytes. J Biol Chem. 1998;273:32023–32029. doi: 10.1074/jbc.273.48.32023. [DOI] [PubMed] [Google Scholar]
- 28.Wahl LM, Katona IM, Wilder RL, Winter CC, Haraoui B, Scher I, Wahl SM. Isolation of human mononuclear cell subsets by counterflow centrifugal elutriation (CCE). I. Characterization of B-lymphocyte-, T-lymphocyte-, and monocyte-enriched fractions by flow cytometric analysis. Cell Immunol. 1984;85:373–383. doi: 10.1016/0008-8749(84)90251-x. [DOI] [PubMed] [Google Scholar]
- 29.Wahl SM, Katona IM, Stadler BM, Wilder RL, Helsel WE, Wahl LM. Isolation of human mononuclear cell subsets by counterflow centrifugal elutriation (CCE). II. Functional properties of B-lymphocyte-, T- lymphocyte-, and monocyte-enriched fractions. Cell Immunol. 1984;85:384–395. doi: 10.1016/0008-8749(84)90252-1. [DOI] [PubMed] [Google Scholar]
- 30.Rosen RL, Winestock KD, Chen G, Liu X, Hennighausen L, Finbloom DS. Granulocyte-macrophage colony-stimulating factor preferentially activates the 94-kD STAT5A and an 80-kD STAT5A isoform in human peripheral blood monocytes. Blood. 1996;88:1206–1214. [PubMed] [Google Scholar]
- 31.Bhattacharjee A, Mulya A, Pal S, Roy B, Feldman GM, Cathcart MK. Monocyte 15-lipoxygenase gene expression requires ERK1/2 MAPK activity. J Immunol. 2010;185:5211–5224. doi: 10.4049/jimmunol.1000514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bhattacharjee A, Xu B, Frank DA, Feldman GM, Cathcart MK. Monocyte 15-lipoxygenase expression is regulated by a novel cytosolic signaling complex with protein kinase C delta and tyrosine-phosphorylated Stat3. J Immunol. 2006;177:3771–3781. doi: 10.4049/jimmunol.177.6.3771. [DOI] [PubMed] [Google Scholar]
- 33.Xu B, Bhattacharjee A, Roy B, Xu HM, Anthony D, Frank DA, Feldman GM, Cathcart MK. Interleukin-13 induction of 15-lipoxygenase gene expression requires p38 mitogen-activated protein kinase-mediated serine 727 phosphorylation of Stat1 and Stat3. Mol Cell Biol. 2003;23:3918–3928. doi: 10.1128/MCB.23.11.3918-3928.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang LH, Yang XY, Kirken RA, Resau JH, Farrar WL. Targeted disruption of stat6 DNA binding activity by an oligonucleotide decoy blocks IL-4-driven T(H)2 cell response. Blood. 2000;95:1249–1257. [PubMed] [Google Scholar]
- 35.Valley MP, Zhou W, Hawkins EM, Shultz J, Cali JJ, Worzella T, Bernad L, Good T, Good D, Riss TL, Klaubert DH, Wood KV. A bioluminescent assay for monoamine oxidase activity. Anal Biochem. 2006;359:238–246. doi: 10.1016/j.ab.2006.09.035. [DOI] [PubMed] [Google Scholar]
- 36.Robbesyn F, Garcia V, Auge N, Vieira O, Frisach MF, Salvayre R, Negre-Salvayre A. HDL counterbalance the proinflammatory effect of oxidized LDL by inhibiting intracellular reactive oxygen species rise, proteasome activation, and subsequent NF-kappaB activation in smooth muscle cells. Faseb J. 2003;17:743–745. doi: 10.1096/fj.02-0240fje. [DOI] [PubMed] [Google Scholar]
- 37.Coatrieux C, Sanson M, Negre-Salvayre A, Parini A, Hannun Y, Itohara S, Salvayre R, Auge N. MAO-A-induced mitogenic signaling is mediated by reactive oxygen species, MMP-2, and the sphingolipid pathway. Free Radic Biol Med. 2007;43:80–89. doi: 10.1016/j.freeradbiomed.2007.03.036. [DOI] [PubMed] [Google Scholar]
- 38.Gordon S, Martinez FO. Alternative activation of macrophages: mechanism and functions. Immunity. 32:593–604. doi: 10.1016/j.immuni.2010.05.007. [DOI] [PubMed] [Google Scholar]
- 39.Heller NM, Qi X, Junttila IS, Shirey KA, Vogel SN, Paul WE, Keegan AD. Type I IL-4Rs selectively activate IRS-2 to induce target gene expression in macrophages. Sci Signal. 2008;1:ra17. doi: 10.1126/scisignal.1164795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.LaPorte SL, Juo ZS, Vaclavikova J, Colf LA, Qi X, Heller NM, Keegan AD, Garcia KC. Molecular and structural basis of cytokine receptor pleiotropy in the interleukin-4/13 system. Cell. 2008;132:259–272. doi: 10.1016/j.cell.2007.12.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Miyazaki T, Kawahara A, Fujii H, Nakagawa Y, Minami Y, Liu ZJ, Oishi I, Silvennoinen O, Witthuhn BA, Ihle JN, et al. Functional activation of Jak1 and Jak3 by selective association with IL- 2 receptor subunits. Science. 1994;266:1045–1047. doi: 10.1126/science.7973659. [DOI] [PubMed] [Google Scholar]
- 42.Dawson CH, Brown BL, Dobson PR. A 70-kDa protein facilitates interleukin-4 signal transduction in the absence of the common gamma receptor chain. Biochem Biophys Res Commun. 1997;233:279–282. doi: 10.1006/bbrc.1997.6397. [DOI] [PubMed] [Google Scholar]
- 43.Conrad DJ, Lu M. Regulation of human 12/15-lipoxygenase by Stat6-dependent transcription. Am J Respir Cell Mol Biol. 2000;22:226–234. doi: 10.1165/ajrcmb.22.2.3786. [DOI] [PubMed] [Google Scholar]
- 44.Heydeck D, Thomas L, Schnurr K, Trebus F, Thierfelder WE, Ihle JN, Kuhn H. Interleukin-4 and -13 induce upregulation of the murine macrophage 12/15-lipoxygenase activity: evidence for the involvement of transcription factor STAT6. Blood. 1998;92:2503–2510. [PubMed] [Google Scholar]
- 45.Kuhn H, Heydeck D, Brinckman R, Trebus F. Regulation of cellular 15-lipoxygenase activity on pretranslational, translational, and posttranslational levels. Lipids. 1999;34:S273–279. doi: 10.1007/BF02562317. [DOI] [PubMed] [Google Scholar]
- 46.Shankaranarayanan P, Chaitidis P, Kuhn H, Nigam S. Acetylation by histone acetyltransferase CREB-binding protein/p300 of STAT6 is required for transcriptional activation of the 15-lipoxygenase- 1 gene. J Biol Chem. 2001;276:42753–42760. doi: 10.1074/jbc.M102626200. [DOI] [PubMed] [Google Scholar]
- 47.Brombacher F. The role of interleukin-13 in infectious diseases and allergy. Bioessays. 2000;22:646–656. doi: 10.1002/1521-1878(200007)22:7<646::AID-BIES7>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 48.Oh CK, Geba GP, Molfino N. Investigational therapeutics targeting the IL-4/IL-13/STAT-6 pathway for the treatment of asthma. Eur Respir Rev. 2010;19:46–54. doi: 10.1183/09059180.00007609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hebenstreit D, Wirnsberger G, Horejs-Hoeck J, Duschl A. Signaling mechanisms, interaction partners, and target genes of STAT6. Cytokine Growth Factor Rev. 2006;17:173–188. doi: 10.1016/j.cytogfr.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 50.Blease K, Schuh JM, Jakubzick C, Lukacs NW, Kunkel SL, Joshi BH, Puri RK, Kaplan MH, Hogaboam CM. Stat6-deficient mice develop airway hyperresponsiveness and peribronchial fibrosis during chronic fungal asthma. Am J Pathol. 2002;160:481–490. doi: 10.1016/S0002-9440(10)64867-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chaitidis P, Billett E, Kuban RJ, Ungethuem U, Kuhn H. Expression regulation of MAO isoforms in monocytic cells in response to Th2 cytokines. Med Sci Monit. 2005;11:BR259–265. [PubMed] [Google Scholar]
- 52.Wang CC, Borchert A, Ugun-Klusek A, Tang LY, Lui WT, Chu CY, Billett E, Kuhn H, Ufer C. Monoamine oxidase a expression is vital for embryonic brain development by modulating developmental apoptosis. J Biol Chem. 2011;286:28322–28330. doi: 10.1074/jbc.M111.241422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Brunner HG, Nelen M, Breakefield XO, Ropers HH, van Oost BA. Abnormal behavior associated with a point mutation in the structural gene for monoamine oxidase A. Science. 1993;262:578–580. doi: 10.1126/science.8211186. [DOI] [PubMed] [Google Scholar]
- 54.Mabile L, Meilhac O, Escargueil-Blanc I, Troly M, Pieraggi MT, Salvayre R, Negre-Salvayre A. Mitochondrial function is involved in LDL oxidation mediated by human cultured endothelial cells. Arterioscler Thromb Vasc Biol. 1997;17:1575–1582. doi: 10.1161/01.atv.17.8.1575. [DOI] [PubMed] [Google Scholar]
- 55.Cathcart MK. Regulation of superoxide anion production by NADPH oxidase in monocytes/macrophages: contributions to atherosclerosis. Arterioscler Thromb Vasc Biol. 2004;24:23–28. doi: 10.1161/01.ATV.0000097769.47306.12. [DOI] [PubMed] [Google Scholar]
- 56.Bianchi P, Kunduzova O, Masini E, Cambon C, Bani D, Raimondi L, Seguelas MH, Nistri S, Colucci W, Leducq N, Parini A. Oxidative stress by monoamine oxidase mediates receptor-independent cardiomyocyte apoptosis by serotonin and postischemic myocardial injury. Circulation. 2005;112:3297–3305. doi: 10.1161/CIRCULATIONAHA.104.528133. [DOI] [PubMed] [Google Scholar]
- 57.Kaludercic N, Carpi A, Menabo R, Di Lisa F, Paolocci N. Monoamine oxidases (MAO) in the pathogenesis of heart failure and ischemia/reperfusion injury. Biochim Biophys Acta. 2011;1813:1323–1332. doi: 10.1016/j.bbamcr.2010.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pchejetski D, Kunduzova O, Dayon A, Calise D, Seguelas MH, Leducq N, Seif I, Parini A, Cuvillier O. Oxidative stress-dependent sphingosine kinase-1 inhibition mediates monoamine oxidase A-associated cardiac cell apoptosis. Circ Res. 2007;100:41–49. doi: 10.1161/01.RES.0000253900.66640.34. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.