Abstract
Despite a century of debate over the existence of adult cortical neurogenesis, a consensus has not yet been reached. Here, we review evidence of the existence, origin, migration, and integration of neurons into the adult and neonatal cerebral cortex. We find that the lack of consensus likely stems from the low rate of postnatal cortical neurogenesis that has been observed, the fact that it may be limited to sub-types of interneurons, and variability in other conditions, both physiological and environmental. We emphasize that neurogenesis occurs in the neonatal cortex and neural stem cells exist into adulthood; perhaps these progenitors are dormant, but they may be reactivated, for example, with injury.
Keywords: neural stem cell, neurogenesis, hypoxia, Tuberous Sclerosis Complex, neural progenitor cell, subventricular zone
Introduction
The rapid and robust expansion of the cerebral cortex relative to other brain areas has been proposed as the crowning achievement of human evolution. Indeed, the cerebral cortex plays a critical role in all perceptions, thoughts, and behaviors that distinguish humans from other animals. Do these complex cognitive phenomena require a stable brain structure? Early experimental evidence suggested that cortical neurogenesis (Glossary) in primates occurred only during embryonic development1. These findings, coupled with the complexity of primate behavior, led to the development of a “central dogma”: that the postnatal (and thus, adult) cerebral cortex possesses a stable number of neurons, all generated prior to birth; and the lack of new neurons or other regenerative capacity was a trade-off for the complexity and diversity of cognitive functions performed by the cerebral cortex. However, a substantial number of recent studies have challenged these central concepts.
The central dogma was first called into serious question in 1999 by a study showing evidence for adult-born neurons in the neocortex of macaques2. This work was quickly followed by several other studies in different mammalian classes reporting conflicting data on adult neurogenesis in part due to the limitations of the labeling techniques. Work in adults has also called attention to the neonatal period, which was recently shown to display protracted neurogenesis to different cortical regions, including the prefrontal cortex (PFC) in infant humans3.
The occurrence of newborn neurons in the postnatal cortex implies the existence of neural progenitor cells (NPCs) or neural stem cells (NSCs). The brain contains two well-accepted postnatal NSC niches, the subventricular zone (SVZ) and hippocampal subgranular zone (SGZ), contributing to persistent neurogenesis in the olfactory bulb and hippocampus4. Additional niches close to the SVZ in the neonatal and adult brain have been reported, but the evidence of a neurogenic niche in the cortex is lacking. Nevertheless, several studies provided evidence of dormant bipotential progenitor cells in the cortical parenchyma. These different pools of progenitor cells are amenable to manipulated for generating neurons following brain repair.
Many studies have found postnatal neurogenesis in pathological conditions5 that arise from NPCs in the SVZ and/or the parenchyma. Two conditions, hypoxic insult and mTOR hyperactivity as observed in Tuberous Sclerosis Complex (TSC), will be discussed.
Neonatal and adult cortical neurogenesis has been the subject of several thorough reviews 1,4,6–8. Here, we focus mainly on recent work in adult and neonates related to neurogenesis and the sites of NPCs. In the interest of brevity, we have essentially limited our assessments to studies of neurogenesis in the neocortex and piriform cortex (Pir).
Evidence for and against adult-born cortical neurons
A widespread method to label dividing cells is the use of exogenous nucleotide analogs that are incorporated into DNA during its synthesis and thus cells in S-phase of the cell cycle. These nucleotide analogs include tritiated thymidine and the thymidine analog bromodeoxyuridine (BrdU)9. Use of BrdU, which can be visualized immunohistochemically, allows co-staining for neuronal markers. Advantages and limitations of the labeling methods are summarized in Table 1. We discuss studies reporting adult neurogenesis in the neocortex and Pir and several studies that did not detect it. The neocortex, which is the most evolutionary recent part of the cerebral cortex, is organized as six layers while the Pir is phylogenetically old and organized as three layers.
Table 1.
Advantages and disadvantages of the different approaches used to identify newborn neurons
| Technique | Specific approach | Advantages | Disadvantages |
|---|---|---|---|
| Anatomical | Cellular morphometry, Nissl and Golgi stainings, and electron microscopy |
|
|
| Cell Count |
|
|
|
| Birthdating |
|
|
|
| Tritiated Thymidine |
|
|
|
| Halogenated Thymidine Analogs (i.e. BrdU) |
|
|
|
| (14)C |
|
|
|
| Fluorescent Tracing |
|
|
|
| Lentiviral Adenoviral |
|
|
|
| Retroviral |
|
|
|
| Postnatal electroporation |
|
|
|
| Transgenic Mice |
|
|
The use of tritiated thymidine aided the identification of the two brain regions with the most prominent postnatal neurogenesis: the SVZ-olfactory bulb and the SGZ-hippocampal granule cell layer10,11. Tritiated thymidine also labeled a sparse population of proliferative cells in the neocortex of adult rats10,12 of presumed neuronal identity based on electron microscopy but at exceedingly low rates (0.011%)12.
More recently, supporting evidence for neocortical neurogenesis, albeit at an extremely low rate, has been published using BrdU labeling and co-immunostaining for the neuronal marker NeuN (Table 2 and Figure 1A)13–18. Though use of BrdU is widespread, the field lacks standard experimental paradigms and very few studies quantified the number of adult-born BrdU/NeuN+ cells13,17. Quantification revealed that the incidence of adult-born neurons was extremely low (between 0.0026% and 0.012% in Monkeys; 3 newborn neurons/mm3 in rats17). From these studies, two important characteristics of adult-born neurons emerged. First, their existence is likely transient13. Second, they are small, GABAergic interneurons, and not large, glutamatergic projection neurons17,19.
Table 2.
Reports of adult neurogenesis in the neocortex of mammalsa
| Species | Age with respect to BrdU labeling | BrdU labeling (and additional neuronal markers) | Additional details | Conclusions | Regions | Refs |
|---|---|---|---|---|---|---|
| Monkeys | ||||||
| Macaca fascicularis (male) | 5–6 years | BrdU/NeuN+ (at 9 wk: 12–36% of BrdU+ cells) |
|
Transient generation of small neurons | Frontal and temporal cortex | 13 |
| Macaca fascicularis (female) | 2–5 years | 26 days survival: 18 BrdU/NeuN+ cells in >500 cortical slices |
|
Very few. Presumably small interneurons | Principal sulcus and ventral part of area 14 | 63 |
| Macaca | 6–12 years | BrdU/TuJ1+ cells in a stream |
|
Very few NeuN/BrdU+ cells | Inferior temporal cortex and Pir | 14 |
| Squirrel monkeys | 3–6 years | 28 days survival NeuN in 27% of BrdU+ cells in the piriform cortex. | ||||
| Rabbits | ||||||
| New Zealand White rabbits | 4–6 month 1–2 year |
|
|
Very few BrdU/NeuN+ cells in the cortex. | 15,52 | |
| Rodents | ||||||
| Male golden hamster | 4–5 months | 7 weeks post- injection: BrdU/NeuN+ cells occasionally found in cortex |
|
|
Regions are unknown | 16 |
| Male Sprague- Dawley rats | 9–10 weeks |
|
|
|
Infralimbic, cingulate, somatosen sory and secondary motor V–VI cortex | 17 |
| Male rats | 12–13 weeks |
|
Frequency: 0.011% (1 in 10,000) 38 sections: 10 neurons |
|
|
12 |
| Mice (5HT3- EGFP transgenics) | 1–3 months | BrdU/NeuN+ cells | Small axonless GABAergic interneurons | Preferentially deep cortical layers, frontal cortex, AOC and orbital cortex | 86 | |
Abbreviations: AOC, anterior olfactory cortex; CB: calbindin; CR: calretinin
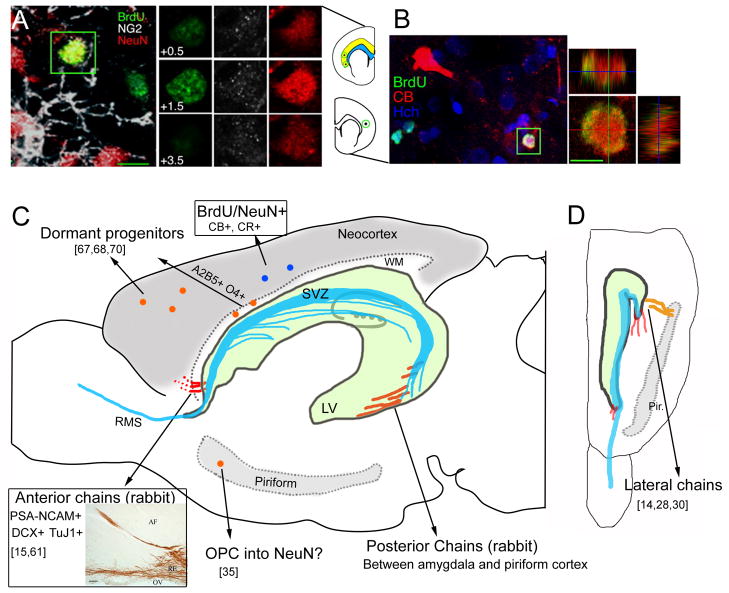
Figure 1. Sites of adult progenitor cells and adult born neuroblasts in cortical regions.
(A) A 4–5-wk-old cell in rat cortex labeled with BrdU and NeuN. Sample z-planes through the green boxed area are shown with color separation on the right, showing colocalization of BrdU and NeuN but not NG2 [17]. The location of the cell shown in A is circled in the diagram of a coronal section; the analyzed region of cortex is shown in yellow on the diagram, and the subcortical white matter, used as a boundary for the analysis, is shown in blue. (B) Image of an 11–12-wk-old BrdU/Calbindin+ CB) cell in a coronal section of rat brain. Higher magnification orthogonal views are shown on the right [17]. Hch: hoetsch (blue). (C and D) Diagram of a sagittal (C) and a horizontal (D) section of an adult mouse brain illustrating the main sites of NPCs. The neocortex and the piriform cortex are highlighted in grey. The blue line illustrates migratory chains of neuroblasts throughout the SVZ located along the lateral ventricle (green, LV). The LV is underrepresented as it folds until the level of the piriform cortex (Pir). The red line illustrates anterior and posterior chains of DCX and PSA-NCAM+ cells that have been observed in the rabbit. The anterior chains are immersed into the white matter (WM) of the anterior forcep of the WM. The inset depicts newborn cells labeled with PSA-NCAM in anterior chains [15]. Abbreviations: CB, calbindin; CR, calretinin; DCX, doublecortin; OPC, oligodendrocyte progenitor cells; RMS, rostral migratory stream. Adapted, with permission, from [17] (A and B), [15] (C, inset).
There are studies that did not find evidence of adult-born neurons in the neocortex20–24 (Table S1 in the Supplementary material online). One study, performed in humans, used a clever approach: the use of radioactive (14)C taken up during nuclear testing20. The biggest caveat to this study is the relatively low detection sensitivity of 1%, which is below the reported rate of adult cortical neurogenesis. They also calculated that they would miss neurons that survive for <4.2 months20 and may thus have missed short-lived adult-born neurons. Another study in mice analyzed only a few cells (50) and, thus, may have missed BrdU/NeuN+ cells22. A study in rats focused on pyramidal neurons elegantly showed that pyramidal neurons are not born during the postnatal period23, though not excluding the possibility that interneurons are born.
In addition to the neocortex, there are conflicting reports on neurogenesis in the adult Pir25. Such studies were motivated by the identification of layer II cells with a neuronal morphology expressing the immature neuronal markers, doublecortin (DCX) and the polysialylated form of the neural cell adhesion molecule PSA-NCAM26. However, labeled cells are now known to be post-mitotic and embryonic born26–28, suggesting that some of the early studies may have erroneously identified BrdU expression in PSA-NCAM and NeuN cells in adults15,29,30. Nevertheless, a stream of PSA-NCAM+ cells has been reported suggesting migration of immature neurons towards the cortex despite the lack of integration15,29–31. Genetic fate mapping studies using reporter mice [e.g. expressing yellow fluorescent protein (YFP) upon tamoxifen injection] expressed an inducible Cre (CreERT2) under a specific cell type promoter also reported the generation of YFP/NeuN+ neurons32–34 except in one study35. In this latter study, YFP was expressed in a few Pir neurons 1.5 days following tamoxifen injection suggesting that the promoter was abnormally turned on in neurons35. The most recent study clearly identified YFP/NeuN+ cells in the Pir, but these cells were not generated following proliferation (no staining for the BrdU analog EdU)36. This finding needs to be replicated in other lines of transgenic mice and perhaps using other approaches.
In conclusion, the majority of studies reported the presence of adult-born neurons, likely GABAergic interneurons, using BrdU and neuronal markers in the neocortex and neurons of unclear identity using BrdU and genetic mapping in the Pir. However, the rate was extremely low and the limitations of the labeling techniques have contributed to the controversy. Therefore, we discuss some of the technical parameters that may have affected the reported numbers.
Limitations of non-invasive birthdating techniques and immunostaining
Tritiated thymidine and BrdU likely underestimate the number of newborn neurons since they only label cells in S-phase. This may underlie false-negative reports. However, increasing dosage is not a solution because high doses of BrdU can lead to false-positive reports of neurogenesis37,38; BrdU can be taken up by neurons undergoing DNA repair or non-proliferative DNA synthesis after brain injury39 and BrdU labeling in NeuN+ cells has been found following transplantation of dead cells40. Thus, just because a cell is labeled with BrdU doesn’t necessarily mean that it is proliferative.
Any study using immunostaining has limitations. NeuN is thought to be a “pan-neuronal” marker, but it is not expressed in all neuronal populations41 and has been found in adult neocortical nestin-GFP/NG2+ cells42. The widely used marker of immature neurons DCX has been found in cells that differentiated into glial cells43 and in post-mitotic neurons thought to undergo structural plasticity26; similarly, some PDGFR+ and NG2+ oligodendroglial progenitor cells (OPCs) express DCX44–45–48; NG2 also labels pericytes on capillaries49 and neonatal NPCs48,50. Finally, PSA-NCAM is not a neuroblast marker, but is perhaps best described as a marker for migrating cells51. These examples emphasize the need for staining cells with multiple markers17. Identification of co-labeling by BrdU and NeuN is difficult. Satellite glia cells are tightly juxtaposed to neurons; thus demonstration of co-labeling requires optimizing the optical Z-section thickness, imaging entire cells for examining Z-projections; this was not routinely performed in earlier studies (e.g.2,14).
Several other factors could influence the number of adult-born neurons including gender, environment, health, stress levels, hormonal states (e.g. during pregnancy or mating seasons)16, as well as injury and disease. For example, one study in macaques was performed using individually housed animals exposed to a daily schedule of enrichment13. It is also intriguing that large variability in terms of animals exhibiting newborn neurons in the same study was reported15,52. This emphasizes the need for examining neurogenesis in different conditions and, in particular, in animals in a more “normal” environment than a cage such as adult feral rodents.
In conclusion, BrdU staining should be paired with staining for DNA repair markers and several neuronal markers to confirm that BrdU/NeuN+ cells were healthy, newborn neurons.
Sites of NSCs in the postnatal brain
In the early twentieth century, Allen identified mitotic cells along the lateral ventricle (LV) in adult rats53. Three decades later, the existence of NPCs in a germinal zone along the LV was proposed based on the observation of tumors near the ventricle54. Yet, it is only in the 1990’s that a direct proof of the existence of NSCs in the SVZ along the LV was provided for all adult mammalian species examined, including humans4,55–58. These NSCs result from the transformation of radial glia during the neonatal period and display astrocytic properties59,60. NSCs rarely divide, self-renew, and generate both neurons and glia in vivo. Evidence was also reported for the existence of radial glia-like NSCs in the hippocampal SGZ61,62. The SVZ and SGZ are the main postnatal neurogenic niches4. Although no neurogenic niche has been found in the neocortex, one has been reported in the white matter beneath the neocortex in rats63, named the temporal germinal layer (TGL). The TGL is close to the posterior part of the SVZ, but DiI injection into the LV suggested that the SVZ and TGL are disconnected. Despite co-staining of BrdU+ cells with the NPC transcription factor Pax6 or Olig264–66, and with DCX and NeuN, no BrdU/NeuN+ were found in the adjacent cortex; the majority of the newborn cells is thought to undergo apoptosis.
Several studies reported that NSCs of the SVZ contributed to anterior and posterior/temporal streams of migrating neuroblasts (Figure 1C and D). Streams containing BrdU/PSA-NCAM+ or β3 Tubulin+ (TuJ1) cells have been reported in the subcortical white matter of rabbits15,52. The anterior streams project beneath the frontal cortex and disintegrate upon entry into the cortex. Finding a few cortical BrdU/NeuN+ cells adjacent to the stream suggested that PSA-NCAM+ cells in the stream generated neurons. A temporal stream from the ventral SVZ along the LV inferior horn to the amygdala and Pir has also been reported in monkeys, rats, and bats using staining for immature neuronal markers, BrdU at different time points post-injection, or DiI labeling14,29,31. Despite the evidence of these streams, additional approaches (e.g. viral tracing) are required to show that BrdU/NeuN+ cells originated from SVZ cells.
Intriguingly, induction of the tyrosine kinase receptor ErbB2 in 6-week-old mature astrocytes using inducible transgenic mice led to re-expression of radial glial identity in NSCs of the dorsal SVZ but not in cortical astrocytes. This was accompanied with SVZ cell proliferation in vivo, and generation of GABAergic and glutamatergic neurons in vitro and NeuN+ cells in the cortex67. This study highlights a remarkable plasticity of NSCs in the SVZ that can be harvested upon genetic manipulation.
Despite the absence of a NSC niche in the cortex, the following studies suggest the existence of NPCs in the brain parenchyma. Indeed, NPCs were isolated from the adult human subcortical white matter by labeling dissociates with OPC markers (CNP2 or A2B5)68 (Figure 1C). Isolated cells formed neurospheres and generated functionally competent neurons and glia in vitro and after xenograft into fetal rat brain69. The same group reported the absence of cells with neurogenic potential from the temporal lobe of epileptic patients70. But in this study, culturing cells without bFGF may be an important difference because cells isolated from the adult rat neocortex generated neurons in vitro only after FGF exposure bFGF71. These studies suggest the existence of dormant NPCs in the white matter and neocortex that have the potential to generate neurons in vitro.
Based on these findings, the potential of OPCs to act as NPCs was investigated in vivo using genetic fate-mapping strategies in Rosa26R mice carrying CreERT2 under the Pdgfra or NG2 promoter34–36,72. YFP+ neurons were found in the anterior Pir34,36, but EdU expression in every OPC but not in YFP+ neurons suggested that these neurons were not generated from OPCs36. It remains to be examined whether an unidentified population of Pdgfra-expressing NPCs was labeled by YFP or whether YFP expression results from abnormal Cre expression. Of interest is the potential of reactive astrocytes to reacquire NSC features and generate neurons at least in vitro73. Indeed, using genetic fate mapping and cell type-specific viral targeting, a study showed that proliferating astrocytes (GFAP+) but not NG2 cells following a stab wound injury displayed multipotency and self-renewal in vitro although they generated only astrocytes in vivo73. Consistent with this finding, they subsequently showed that expression of neurogenic transcription factors in proliferative astrocytes in vitro led to neuron generation74–76. Collectively, these studies suggest that the adult cortex possesses anti-neurogenic properties preventing NPCs and proliferative astrocytes following insult from generating neurons.
The neonatal period displays protracted cortical neurogenesis and offers clues to NSC identity
The neonatal rat brain continues to grow by nearly 6 times in weight and in size from birth to adulthood77. An increase in the number of neocortical neurons has been reported in different animal species, including humans78–81. As a quick note, neonatal rodents (P7–P12) are the age-equivalents of in utero humans82. The increase in brain weight and size was hypothesized to be partly due to embryonically born neuroblasts remaining along the LV and then migrating through the cortical plate83. Although these descriptive studies rely on extrapolations of neuron numbers based on density and brain size, they highlight the possibility that some neurons are born during the neonatal period. Consistent with this idea, a recent finding is the identification of a stream of DCX+ cells in infants up to 8 month old3 that is reminiscent of that identified in adult rabbits15,52 (Figure 2A). The stream in humans emanates from cells migrating to the olfactory bulb and culminates within the ventromedial PFC. Whether neuroblasts detach from the stream, migrate into the cortex, and integrate into the circuitry are important questions to address in future studies.
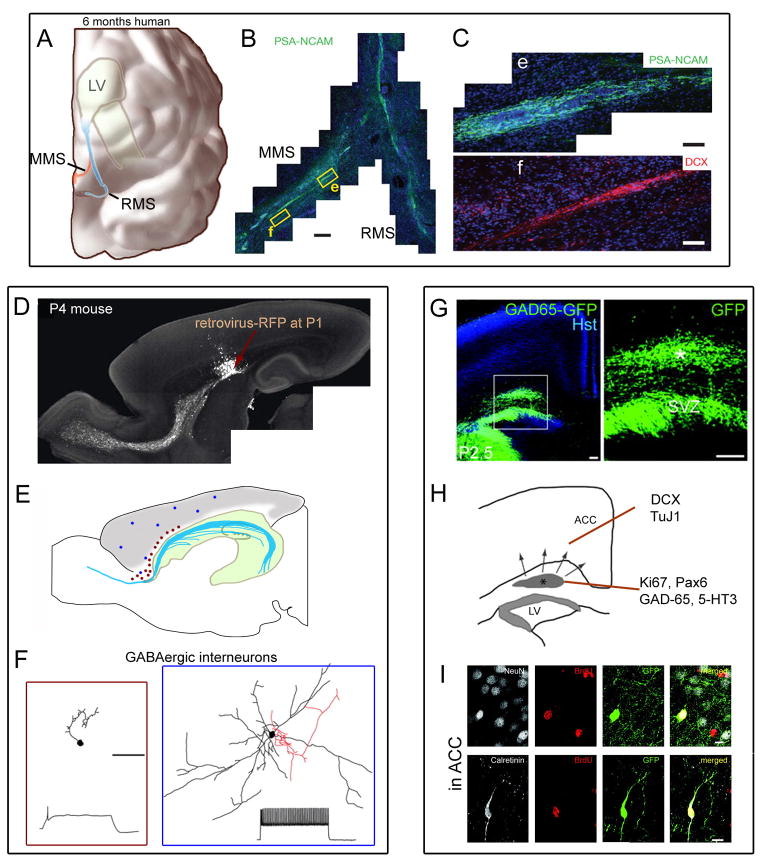
Figure 2. Major sites of neonatal neurogenesis in the cortex.
(A) Diagram of a hemisected immature (6 months old) human cortex containing the rostral migratory stream (RMS) and the medial migratory stream (MMS) originating from the SVZ along the lateral ventricle (LV) [3]. (B) Coronal reconstruction of a 6-month specimen showing PSA-NCAM+ cells in the MMS diverging from the RMS to reach the ventro-medial prefrontal cortex [3]. (C) Chains of PSA-NCAM+ cells (i) and DCX+ cells (ii) in the MMS [3]. The images correspond to the yellow boxes in (B). Scale bars: 150 μm (B) and 20 μm (C). (D–F) Generation of newborn neurons from the SVZ. (D) Red fluorescent protein (RFP)-labeled cells in a P4 mouse injected with a retrovirus encoding RFP at P1. The arrow points to the injection site [85]. (E) Location of newborn neurons (red and blue circles) in the cortex above the lateral ventricle (LV, green) and streams of migrating SVZ neuroblasts (blue). The red versus blue colors refer to two different categories of GABAergic newborn neurons (as described in F). (F) Newborn GABAergic neurons were divided in two categories, small axonless neurons (left, red square) and multipolar neurons (right, blue square) exhibiting different spiking patterns [85]. Scale bar 50 μm.(G–I) Generation of anterior cingulate neurons (ACC) in mice from a transient progenitor pool in the white matter above the SVZ. (G) Images at low (left) and higher (right) magnification showing at P2.5 a pool of GAD65-GFP+ cells in the white matter progenitor pool (white star) and dorsal SVZ [87]. Abbreviation: Hst, Hoechst. (H) Schematic coronal section depicting during the first postnatal week migration of GAD65-GFP+ cells from the white matter progenitor population (indicated with a star) toward cortical regions. A fraction of progenitor cells were Ki67+, Pax6+, and all were GAD-65 and 5-HT3+. Migrating cells were DCX and TuJ1+ [86]. Scale bars: 100 μm. (I) Confocal images of ACC layer VI at P10 showing GAD65-GFP+ interneurons labeled for NeuN or calretinin and BrdU. BrdU was injected postnatally at P0.5 (3× 20 mg/kg intraperitioneal) [86]. Scale bar: 10 μm. Adapted, with permission, from [3] (A–C), [85] (D, F) and [86] (G–I).
The following studies reported the presence of protracted neurogenesis during the neonatal period from NCSs in the SVZ, in the white matter, and the parenchyma (Table 3). Only one elegant study showed that neocortical pyramidal neurons are not generated from NSCs in the SVZ during neonatal life23. Although they identified BrdU/NeuN+ cells expressing GFP following intraventricular GFP retroviral labeling, they showed that GFP expression in pyramidal neurons resulted from fusion with infected microglia (Table 1). This study does not contradict those reporting neurogenesis of neocortical interneurons.
Table 3.
Reports of neonatal neurogenesis in the neocortex a
| Species | Age with respect to BrdU labeling | BrdU labeling (except human study) with additional neuronal labeling | Viral labeling or fate- mapping | Conclusions | Regions | Refs |
|---|---|---|---|---|---|---|
| Humans | ||||||
| Infants | < 8 months | DCX+ and PSA-NCAM+ cells | No | Existence of a stream of presumably migrating neuroblasts | Ventromedial prefrontal cortex | 3 |
| Mice | ||||||
| Wild-type | P10;P49 |
|
None |
|
Neocortex and cingulate cortex | 84,85 |
| 5HT3-EGFP transgenic | P1–4; P30 |
|
EGFP retrovirus injection in dorsal SVZ at P3-P4 |
|
Deep layers (VI) of mostly AON and orbital cortex In cortical layer II–VI |
18 86 |
| GAD65-GFP and 5HT3-GFP transgenics (and other lines) | Different neonatal ages |
|
No |
|
ACC | 87 |
| Plp-Cre x R 26R- YFP transgenics | P8 (tamoxifen); P60 |
|
Post-tamoxifen: P15; P60 |
|
Dorsal and ventral forebrain cortex | 44 |
Abbreviations: ACC: anterior cingulate cortex; AON: anterior olfactory nucleus; CR: calretinin; EGFP, enhanced green fluorescent protein; GAD65: glutamic acid decarboxylase 65; Plp, proteolipid protein.
Two recent studies reported the generation of neurons (~20,000) during the late neonatal period using BrdU in mice84,85. This is a relatively high number that was based on extrapolations. It was proposed that these newborn neurons originate from the SVZ based on the presence of DCX+ and Mash1+ cells84. It remains unclear whether these migrating neuroblasts contribute to the 20,000 neurons identified. Nevertheless, additional studies identified the neonatal SVZ as a source of cortical neurons. Mice expressing the 5HT3 receptor fused to GFP allowed the identification of GFP+ cells migrating to the cortex in neonates18. Retroviral labeling in or above the SVZ together with BrdU showed that a cohort of DCX+ cells migrates from the SVZ to frontal cortical structures and become GABAergic interneurons (Figure 2E–F). Considering the large difference between the number of migrating DCX+ cells and BrdU/NeuN+ neurons, it is possible that many of the newborn neurons have a transient life. Further investigation revealed two GABAergic neuronal populations and provided the first functional evidence that these neurons displayed action potentials and received synaptic inputs86. A small population of multipolar GABAergic neurons spread across all frontal cortical layers and corresponded to the tail end of embryonic neurogenesis. A second population of small axon-less GABAergic neurons integrated in the olfactory and orbital cortices with a peak generation around P1 in mice.
Another study recently reported the generation of interneurons during the neonatal period, but with a twist87. Using transgenic mice, they identified a pool of GABAergic and 5HT3+ precursors in the dorsal white matter (Figure 2G–I). These progenitors appear around embryonic day 19 and peak at P7. Some of these precursors expressed Pax6 and were proliferative. Importantly, they generated migrating DCX+ cells and GABAergic neurons in the anterior cingulate cortex. This finding questions whether some of the virally infected cells reported to generated GABAergic interneurons in the two previous studies were these transient white matter precursors.
One study explored the presence of neurogenesis from parenchymal OPCs. Using genetic fate mapping in Rosa26R-YFP mice carrying CreERT2 under the proteolipid promoter (in some OPCs and oligodendrocytes), they observed YFP+ cells expressing TuJ1, NeuN, and the vesicular glutamate transporter 1 identifying them as glutamatergic pyramidal neurons in the dorsal and ventral cortex, but at very low incidence44. It remains unknown whether these neurons would accumulate with time after tamoxifen, as one would predict if the permanently labeled progenitors persisted beyond the neonatal period.
Collectively, these studies identify three sources of newborn neurons in neonates: a transient pool of white matter progenitors, bipotential cortical progenitors, and NSCs in the SVZ. The function of these neonatally-born interneurons and their duration of life remain to be investigated.
Pathophysiological conditions that impact postnatal cortical neurogenesis
Our comparison of neonatal and adult neurogenesis has suggested that adult neurogenesis may be actively suppressed by strong anti-neurogenic molecules mediating a non-cell autonomous effect, in addition to a lack of supportive molecules. A pioneering study showed that cortical neurogenesis can be induced in adult mice by triggering cell death88. Here, we focus on two pathological conditions, hypoxia and TSC that are associated with increased and dysregulated neonatal neurogenesis, respectively. These studies emphasize that neonatal cortical neurogenesis can be manipulated and may impact neurological behavior.
Hypoxia
An estimated 1–8/1000 births are complicated by hypoxic ischemia, a condition in which oxygen levels drop below optimal levels. Approximately half of the neonates exposed to hypoxia have prolonged neurological delays. In perinatal mice, chronic hypoxia is associated with massive apoptosis of cortical neurons, decreased cortical volume, and, in some strains, neurological dysfunction84,89. In other strains, the decreased cell number and cortical volume recovered84,85. Part of this recovery seems to involve a doubling of newborn cortical neurons following hypoxia. This was assessed using stereology (see caveats in Table 1) and BrdU, which could incorporate into neurons during non-proliferative DNA synthesis following hypoxia. The source of these newborn BrdU+ neurons was originally speculated to be SVZ cells84, but new evidence using lineage analysis suggest that some are generated from neonatal parenchymal astrocytes85. This report is in agreement with a previous study showing that neonatal radial glia or immature parenchymal astrocytes retain neurogenic potential in vitro90. Collectively, these studies emphasize the presence of neonatal neurogenesis that is enhanced following a hypoxic insult and may contribute to neurological improvement.
Tuberous Sclerosis Complex
TSC is a monogenic, developmental disorder characterized by abnormal brain development associated with seizures, hydrocephalus, and severe neurological symptoms91–93. TSC is caused by mutations in TSC1 or TSC2 leading to hyperactivity of the mTOR pathway94,95. TSC patients display cortical malformations that are essentially generated during embryonic life and have been modeled in mice96,97. It has been reported that Tsc1 deletion in neonatal SVZ cells using inducible nestin-Cre mice generated SVZ nodules98,99. Intriguingly, ectopic Tsc1null neurons were also found in the cortex. Using targeted electroporation to delete Tsc1 selectively in NSCs of the SVZ (see caveats in Table 1)100, a study reported the presence of heterotopia along the migratory path to olfactory structures and ectopic cells in the nucleus accumbens and in the neocortex99. The identity and function of these Tsc1null cortical neurons remain to be examined. Collectively, these findings show that the production of newborn neurons from the neonatal SVZ can be enhanced or neurons can be re-routed to reach cortical structures upon mTOR activation. It remains to be examined whether such abnormal infiltration of neurons to forebrain structures contributes to network malfunction in TSC.
Concluding Remarks
In the adult cortex, one point of consensus is that the numbers of adult-born neurons are low and their lives are short. Thus, we need to search for them, like needles in a haystack, perhaps in many “haystacks” subject to different conditions. In addition, supplemental approaches need to be developed since BrdU may label neurons repairing their DNA; alternatively, markers of DNA repair need to be routinely performed. Due to their low number, it is difficult to conclude whether the identified BrdU/NeuN+ cells are the result of progenitor proliferation or neuronal repair. Another important finding is the presence of anterior and posterior streams of presumably migrating neuroblasts going towards the frontal cortex and Pir. Based on these studies, it seems that some neuroblasts are made and reach white matter, but die since the adult brain is not permissive for newborn neuron survival under normal conditions. These studies - as well as studies in injury and disease models - suggest that the adult brain may be actively suppressing neurogenesis. This suggests that these mechanisms can potentially be exploited to make new neurons for brain repair. Thus, the more critical question is whether one, or more, of the NPC populations would be amenable to manipulation and enhancement of their production of cells; or if, once we have more molecular details, de-differentiation of mature astrocytes into NPCs will be a more promising strategy (see Box 1 for additional questions).
Box 1. Outstanding questions.
Are BrdU/NeuN+ cortical neurons undergoing DNA repair? In other words, do the few adult-born neurons stain for markers of DNA repair?
What new approaches can be applied to study postnatal neurogenesis? For example, non-invasive in vivo imaging, in combination with transgenic mice in which specific neural populations are labeled, would be a valuable approach to take to track NSCs and newborn neurons longitudinally, for weeks or months.
Are neonate-born cortical neurons derived from diverse progenitor pools including those from the SVZ, the parenchyma, or white matter transient progenitors?
What is the identity and/or fate specification of SVZ stem cells, parenchymal stem cells and bipotential progenitors? Can the fate be altered by microenvironmental cues or it is predetermined?
What is (are) the source(s) of variability in the number of newborn neurons in the postnatal cortex?
What are the molecular pathways controlling neonatal neurogenesis from NSCs to neuronal integration? This is a critical question to be able to enhance or limit neuronal production when necessary.
What disorders/pathological states are associated with altered postnatal cortical neurogenesis?
What is the contribution of the newborn cortical neurons on network function in health and disease during postnatal life?
During the neonatal period, there is a clear generation of a subpopulation of GABAergic neurons in rodents. In this case, perhaps more controversial is the identity of the pool of progenitors that generate these GABAergic neurons. There is evidence for contributions from the SVZ, from transient white matter progenitors, and possibly from parenchymal progenitors. However, though it seems like it might be critical to generate the sheer numbers of cortical neurons, the function of neonatal neurogenesis isn’t much more defined than that of adult neurogenesis. Yet, two facts are undeniable: neural stem cells exist, perhaps they are latent or dormant, but they can be reactivated, for example, with injury. Identifying these cells and defining the molecules keeping them dormant is at the key of brain repair and tumor prevention.
Supplementary Material
Acknowledgments
The work was supported by grants from the Conneticut Stem Cell initiative, Department of Defense (Idea development award, W81XWH-10-1-0041) and the National Institutes of Health (R01 DC007681 to A.B. and NRSA 10668225 to D.M.F).
Glossary
- Bipotential/Neural progenitor cells
cells generated from NSCs that are bipotential (i.e. generate neurons and glia) and have self-renewal capacity but more limited than NSCs
- Neonatal electroporation
this technique allows cDNA to enter cells by applying voltage, which creates small pores in the cell membranes and pushes charged cDNA to enter cells. In neonatal mice, following pressure ejection of a cDNA plasmid into the LV, voltages are applied across the head of anesthetized pups allowing plasmid entry into NSCs lining the ventricle
- Neurogenesis
a process leading to the generation of neurons through several stages, rare NSC assymetric division and generation of bipotential progenitor cells; proliferation of progenitor cells leading to pool amplification and generation of neuroblasts, neuroblast proliferation, post-mitotic differentiation, migration, maturation, and synaptic integration
- NSCs
neural stem cells, which can generate both neurons and glia and self-renew indefinitely. In the adult, NSCs have a more restricted fate than embryonic NSCs and generate only certain types of neurons
- Transgenic floxed, inducible Cre (CreERT2), and Rosa26R reporter mice
floxed is used to describe the bordering of a DNA sequence between two LoxP sites and is abbreviated “flanked by LoxP.” Cre recombinase expression in cells expressing a floxed gene leads to gene sequence excision and deletion. In reporter Rosa26R mice, a Stop sequence is inserted in the Rosa26 locus between two LoxP sites preceding a reporter gene (e.g. YFP). Upon Cre expression, the Stop sequence is excised leading to the reporter expression. CreERT2 mice express a CreERT2 under a specific promoter that requires tamoxifen to be active. Upon tamoxifen injection, Cre is translated to the nucleus and excises any floxed gene in CreERT2 mice crossed with floxed mice. Using CreERT2-Rosa26R mice allows to label cells expressing a specific promoter driving CreERT2 and track their progeny
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Rakic P. Neurogenesis in adult primate neocortex: an evaluation of the evidence. Nat Rev Neurosci. 2002;3:65–71. doi: 10.1038/nrn700. [DOI] [PubMed] [Google Scholar]
- 2.Gould E, et al. Neurogenesis in the neocortex of adult primates. Science. 1999;286:548–552. doi: 10.1126/science.286.5439.548. [DOI] [PubMed] [Google Scholar]
- 3.Sanai N, et al. Corridors of migrating neurons in the human brain and their decline during infancy. Nature. 2011;478:382–386. doi: 10.1038/nature10487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bonfanti L, Peretto P. Adult neurogenesis in mammals--a theme with many variations. Eur J Neurosci. 2011;34:930–950. doi: 10.1111/j.1460-9568.2011.07832.x. [DOI] [PubMed] [Google Scholar]
- 5.Jin K. Adult Neurogenesis and Central Nervous System Diseases. Transworld Research Network; 2010. [Google Scholar]
- 6.Gould E. How widespread is adult neurogenesis in mammals? Nat Rev Neurosci. 2007;8:481–488. doi: 10.1038/nrn2147. [DOI] [PubMed] [Google Scholar]
- 7.Cameron HA, Dayer AG. New interneurons in the adult neocortex: small, sparse, but significant? Biol Psychiatry. 2008;63:650–655. doi: 10.1016/j.biopsych.2007.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mitchell BD, et al. Constitutive and induced neurogenesis in the adult mammalian brain: manipulation of endogenous precursors toward CNS repair. Dev Neurosci. 2004;26:101–117. doi: 10.1159/000082131. [DOI] [PubMed] [Google Scholar]
- 9.Miller MW, Nowakowski RS. Use of bromodeoxyuridine-immunohistochemistry to examine the proliferation, migration and time of origin of cells in the central nervous system. Brain Res. 1988;457:44–52. doi: 10.1016/0006-8993(88)90055-8. [DOI] [PubMed] [Google Scholar]
- 10.Altman J. Autoradiographic and histological studies of postnatal neurogenesis. IV Cell proliferation and migration in the anterior forebrain, with special reference to persisting neurogenesis in the olfactory bulb. J Comp Neurol. 1969;137:433–457. doi: 10.1002/cne.901370404. [DOI] [PubMed] [Google Scholar]
- 11.Kaplan MS, Hinds JW. Neurogenesis in the adult rat: electron microscopic analysis of light radioautographs. Science. 1977;197:1092–1094. doi: 10.1126/science.887941. [DOI] [PubMed] [Google Scholar]
- 12.Kaplan MS. Neurogenesis in the 3-month-old rat visual cortex. J Comp Neurol. 1981;195:323–338. doi: 10.1002/cne.901950211. [DOI] [PubMed] [Google Scholar]
- 13.Gould E, et al. Adult-generated hippocampal and neocortical neurons in macaques have a transient existence. Proc Natl Acad Sci U S A. 2001;98:10910–10917. doi: 10.1073/pnas.181354698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bernier PJ, et al. Newly generated neurons in the amygdala and adjoining cortex of adult primates. Proc Natl Acad Sci U S A. 2002;99:11464–11469. doi: 10.1073/pnas.172403999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Luzzati F, et al. Glia-independent chains of neuroblasts through the subcortical parenchyma of the adult rabbit brain. Proc Natl Acad Sci U S A. 2003;100:13036–13041. doi: 10.1073/pnas.1735482100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huang L, et al. Photoperiod regulates neuronal bromodeoxyuridine labeling in the brain of a seasonally breeding mammal. J Neurobiol. 1998;36:410–420. doi: 10.1002/(sici)1097-4695(19980905)36:3<410::aid-neu8>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 17.Dayer AG, et al. New GABAergic interneurons in the adult neocortex and striatum are generated from different precursors. J Cell Biol. 2005;168:415–427. doi: 10.1083/jcb.200407053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Inta D, et al. Neurogenesis and widespread forebrain migration of distinct GABAergic neurons from the postnatal subventricular zone. Proc Natl Acad Sci U S A. 2008;105:20994–20999. doi: 10.1073/pnas.0807059105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gabbott PL, Bacon SJ. Local circuit neurons in the medial prefrontal cortex (areas 24a, b, c, 25 and 32) in the monkey: I. Cell morphology and morphometrics. J Comp Neurol. 1996;364:567–608. doi: 10.1002/(SICI)1096-9861(19960122)364:4<567::AID-CNE1>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 20.Bhardwaj RD, et al. Neocortical neurogenesis in humans is restricted to development. Proc Natl Acad Sci U S A. 2006;103:12564–12568. doi: 10.1073/pnas.0605177103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kornack DR, Rakic P. Cell proliferation without neurogenesis in adult primate neocortex. Science. 2001;294:2127–2130. doi: 10.1126/science.1065467. [DOI] [PubMed] [Google Scholar]
- 22.Ehninger D, Kempermann G. Regional effects of wheel running and environmental enrichment on cell genesis and microglia proliferation in the adult murine neocortex. Cereb Cortex. 2003;13:845–851. doi: 10.1093/cercor/13.8.845. [DOI] [PubMed] [Google Scholar]
- 23.Ackman JB, et al. Fusion of microglia with pyramidal neurons after retroviral infection. J Neurosci. 2006;26:11413–11422. doi: 10.1523/JNEUROSCI.3340-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tamura Y, et al. Multi-directional differentiation of doublecortin- and NG2-immunopositive progenitor cells in the adult rat neocortex in vivo. Eur J Neurosci. 2007;25:3489–3498. doi: 10.1111/j.1460-9568.2007.05617.x. [DOI] [PubMed] [Google Scholar]
- 25.Arisi GM, et al. The role of olfactory stimulus in adult mammalian neurogenesis. Behav Brain Res. 2012;227:356–362. doi: 10.1016/j.bbr.2011.03.050. [DOI] [PubMed] [Google Scholar]
- 26.Bonfanti L, Nacher J. New scenarios for neuronal structural plasticity in non-neurogenic brain parenchyma: The case of cortical layer II immature neurons. Prog Neurobiol. 2012;98:1–15. doi: 10.1016/j.pneurobio.2012.05.002. [DOI] [PubMed] [Google Scholar]
- 27.Klempin F, et al. Properties of doublecortin-(DCX)-expressing cells in the piriform cortex compared to the neurogenic dentate gyrus of adult mice. PLoS ONE. 2011;6:e25760. doi: 10.1371/journal.pone.0025760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gomez-Climent MA, et al. A population of prenatally generated cells in the rat paleocortex maintains an immature neuronal phenotype into adulthood. Cereb Cortex. 2008;18:2229–2240. doi: 10.1093/cercor/bhm255. [DOI] [PubMed] [Google Scholar]
- 29.Shapiro LA, et al. Origin, migration and fate of newly generated neurons in the adult rodent piriform cortex. Brain Struct Funct. 2007;212:133–148. doi: 10.1007/s00429-007-0151-3. [DOI] [PubMed] [Google Scholar]
- 30.Pekcec A, et al. Neurogenesis in the adult rat piriform cortex. Neuroreport. 2006;17:571–574. doi: 10.1097/00001756-200604240-00003. [DOI] [PubMed] [Google Scholar]
- 31.Gatome CW, et al. Hippocampal neurogenesis and cortical cellular plasticity in Wahlberg’s epauletted fruit bat: a qualitative and quantitative study. Brain Behav Evol. 2010;76:116–127. doi: 10.1159/000320210. [DOI] [PubMed] [Google Scholar]
- 32.Chen J, et al. Inducible site-specific recombination in neural stem/progenitor cells. Genesis. 2009;47:122–131. doi: 10.1002/dvg.20465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Guo F, et al. Pyramidal neurons are generated from oligodendroglial progenitor cells in adult piriform cortex. J Neurosci. 2010;30:12036–12049. doi: 10.1523/JNEUROSCI.1360-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rivers LE, et al. PDGFRA/NG2 glia generate myelinating oligodendrocytes and piriform projection neurons in adult mice. Nat Neurosci. 2008;11:1392–1401. doi: 10.1038/nn.2220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kang SH, et al. NG2+ CNS glial progenitors remain committed to the oligodendrocyte lineage in postnatal life and following neurodegeneration. Neuron. 2010;68:668–681. doi: 10.1016/j.neuron.2010.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Clarke LE, et al. Properties and fate of oligodendrocyte progenitor cells in the corpus callosum, motor cortex, and piriform cortex of the mouse. J Neurosci. 2012;32:8173–8185. doi: 10.1523/JNEUROSCI.0928-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Breunig JJ, et al. Everything that glitters isn’t gold: a critical review of postnatal neural precursor analyses. Cell Stem Cell. 2007;1:612–627. doi: 10.1016/j.stem.2007.11.008. [DOI] [PubMed] [Google Scholar]
- 38.Kunz BA, Kohalmi SE. Modulation of mutagenesis by deoxyribonucleotide levels. Annu Rev Genet. 1991;25:339–359. doi: 10.1146/annurev.ge.25.120191.002011. [DOI] [PubMed] [Google Scholar]
- 39.Burns KA, et al. Nestin-CreER mice reveal DNA synthesis by nonapoptotic neurons following cerebral ischemia hypoxia. Cereb Cortex. 2007;17:2585–2592. doi: 10.1093/cercor/bhl164. [DOI] [PubMed] [Google Scholar]
- 40.Burns TC, et al. Thymidine analogs are transferred from prelabeled donor to host cells in the central nervous system after transplantation: a word of caution. Stem Cells. 2006;24:1121–1127. doi: 10.1634/stemcells.2005-0463. [DOI] [PubMed] [Google Scholar]
- 41.Mullen RJ, et al. NeuN, a neuronal specific nuclear protein in vertebrates. Development. 1992;116:201–211. doi: 10.1242/dev.116.1.201. [DOI] [PubMed] [Google Scholar]
- 42.Tanaka Y, et al. Excitatory GABAergic activation of cortical dividing glial cells. Cereb Cortex. 2009;19:2181–2195. doi: 10.1093/cercor/bhn238. [DOI] [PubMed] [Google Scholar]
- 43.Seidenfaden R, et al. Glial conversion of SVZ-derived committed neuronal precursors after ectopic grafting into the adult brain. Mol Cell Neurosci. 2006;32:187–198. doi: 10.1016/j.mcn.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 44.Guo F, et al. Early postnatal proteolipid promoter-expressing progenitors produce multilineage cells in vivo. J Neurosci. 2009;29:7256–7270. doi: 10.1523/JNEUROSCI.5653-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Trotter J, et al. NG2 cells: Properties, progeny and origin. Brain Res Rev. 2010;63:72–82. doi: 10.1016/j.brainresrev.2009.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ehninger D, et al. Enriched environment and physical activity reduce microglia and influence the fate of NG2 cells in the amygdala of adult mice. Cell Tissue Res. 2011;345:69–86. doi: 10.1007/s00441-011-1200-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Belachew S, et al. Postnatal NG2 proteoglycan-expressing progenitor cells are intrinsically multipotent and generate functional neurons. J Cell Biol. 2003;161:169–186. doi: 10.1083/jcb.200210110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Aguirre A, Gallo V. Postnatal neurogenesis and gliogenesis in the olfactory bulb from NG2-expressing progenitors of the subventricular zone. J Neurosci. 2004;24:10530–10541. doi: 10.1523/JNEUROSCI.3572-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Stallcup WB. The NG2 proteoglycan: past insights and future prospects. J Neurocytol. 2002;31:423–435. doi: 10.1023/a:1025731428581. [DOI] [PubMed] [Google Scholar]
- 50.Cesetti T, et al. Analysis of stem cell lineage progression in the neonatal subventricular zone identifies EGFR+/NG2- cells as transit-amplifying precursors. Stem Cells. 2009;27:1443–1454. doi: 10.1002/stem.74. [DOI] [PubMed] [Google Scholar]
- 51.Wang C, et al. Functional N-methyl-D-aspartate receptors in O-2A glial precursor cells: a critical role in regulating polysialic acid-neural cell adhesion molecule expression and cell migration. J Cell Biol. 1996;135:1565–1581. doi: 10.1083/jcb.135.6.1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ponti G, et al. Cellular composition and cytoarchitecture of the rabbit subventricular zone and its extensions in the forebrain. J Comp Neurol. 2006;498:491–507. doi: 10.1002/cne.21043. [DOI] [PubMed] [Google Scholar]
- 53.Allen E. Cessation of mitosis in central nervous system of the albino rat. J Comp Neurol. 1912;22:547–568. [Google Scholar]
- 54.Globus JH, Kuhlenbeck H. The subependymal plate (matrix) and its relationship to brain tumors of the ependymal type. J Neuropathol Exp Neurol. 1944;3:1–35. [Google Scholar]
- 55.Roy NS, et al. Promoter-targeted selection and isolation of neural progenitor cells from the adult human ventricular zone. J Neurosci Res. 2000;59:321–331. doi: 10.1002/(sici)1097-4547(20000201)59:3<321::aid-jnr5>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 56.Curtis MA, et al. Human neuroblasts migrate to the olfactory bulb via a lateral ventricular extension. Science. 2007;315:1243–1249. doi: 10.1126/science.1136281. [DOI] [PubMed] [Google Scholar]
- 57.Sanai N, et al. Unique astrocyte ribbon in adult human brain contains neural stem cells but lacks chain migration. Nature. 2004;427:740–744. doi: 10.1038/nature02301. [DOI] [PubMed] [Google Scholar]
- 58.van den Berge SA, et al. Longterm quiescent cells in the aged human subventricular neurogenic system specifically express GFAP-delta. Aging Cell. 2010;9:313–326. doi: 10.1111/j.1474-9726.2010.00556.x. [DOI] [PubMed] [Google Scholar]
- 59.Liu X, et al. GFAP-expressing cells in the postnatal subventricular zone display a unique glial phenotype intermediate between radial glia and astrocytes. Glia. 2006;54:394–410. doi: 10.1002/glia.20392. [DOI] [PubMed] [Google Scholar]
- 60.Merkle FT, et al. Radial glia give rise to adult neural stem cells in the subventricular zone. Proc Natl Acad Sci U S A. 2004;101:17528–17532. doi: 10.1073/pnas.0407893101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Eriksson PS, et al. Neurogenesis in the adult human hippocampus. Nat Med. 1998;4:1313–1317. doi: 10.1038/3305. [DOI] [PubMed] [Google Scholar]
- 62.Seri B, et al. Astrocytes give rise to new neurons in the adult mammalian hippocampus. J Neurosci. 2001;21:7153–7160. doi: 10.1523/JNEUROSCI.21-18-07153.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Takemura NU. Evidence for neurogenesis within the white matter beneath the temporal neocortex of the adult rat brain. Neuroscience. 2005;134:121–132. doi: 10.1016/j.neuroscience.2005.04.033. [DOI] [PubMed] [Google Scholar]
- 64.Ericson J, et al. Pax6 controls progenitor cell identity and neuronal fate in response to graded Shh signaling. Cell. 1997;90:169–180. doi: 10.1016/s0092-8674(00)80323-2. [DOI] [PubMed] [Google Scholar]
- 65.Lu QR, et al. Common developmental requirement for Olig function indicates a motor neuron/oligodendrocyte connection. Cell. 2002;109:75–86. doi: 10.1016/s0092-8674(02)00678-5. [DOI] [PubMed] [Google Scholar]
- 66.Zhou Q, Anderson DJ. The bHLH transcription factors OLIG2 and OLIG1 couple neuronal and glial subtype specification. Cell. 2002;109:61–73. doi: 10.1016/s0092-8674(02)00677-3. [DOI] [PubMed] [Google Scholar]
- 67.Ghashghaei HT, et al. Reinduction of ErbB2 in astrocytes promotes radial glial progenitor identity in adult cerebral cortex. Genes Dev. 2007;21:3258–3271. doi: 10.1101/gad.1580407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Nunes MC, et al. Identification and isolation of multipotential neural progenitor cells from the subcortical white matter of the adult human brain. Nat Med. 2003;9:439–447. doi: 10.1038/nm837. [DOI] [PubMed] [Google Scholar]
- 69.Roy NS, et al. Identification, isolation, and promoter-defined separation of mitotic oligodendrocyte progenitor cells from the adult human subcortical white matter. J Neurosci. 1999;19:9986–9995. doi: 10.1523/JNEUROSCI.19-22-09986.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kirschenbaum B, et al. In vitro neuronal production and differentiation by precursor cells derived from the adult human forebrain. Cereb Cortex. 1994;4:576–589. doi: 10.1093/cercor/4.6.576. [DOI] [PubMed] [Google Scholar]
- 71.Palmer TD, et al. Fibroblast growth factor-2 activates a latent neurogenic program in neural stem cells from diverse regions of the adult CNS. J Neurosci. 1999;19:8487–8497. doi: 10.1523/JNEUROSCI.19-19-08487.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zhu X, et al. Age-dependent fate and lineage restriction of single NG2 cells. Development. 2011;138:745–753. doi: 10.1242/dev.047951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Buffo A, et al. Origin and progeny of reactive gliosis: A source of multipotent cells in the injured brain. Proc Natl Acad Sci U S A. 2008;105:3581–3586. doi: 10.1073/pnas.0709002105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Berninger B, et al. Functional properties of neurons derived from in vitro reprogrammed postnatal astroglia. J Neurosci. 2007;27:8654–8664. doi: 10.1523/JNEUROSCI.1615-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Heinrich C, et al. Directing astroglia from the cerebral cortex into subtype specific functional neurons. PLoS Biol. 2010;8:e1000373. doi: 10.1371/journal.pbio.1000373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Blum R, et al. Neuronal network formation from reprogrammed early postnatal rat cortical glial cells. Cereb Cortex. 2011;21:413–424. doi: 10.1093/cercor/bhq107. [DOI] [PubMed] [Google Scholar]
- 77.A comparison of the norway rat with the albino rat in respect to body length, brain weight, spinal cord weight and the percentage of water in both the brain and the spinal cord. J Comp Neurol. 1911;21:417–458. [Google Scholar]
- 78.Sugita N. Comparative studies on the growth of the cerebral cortex. VI Part I On the increase in size and on the developmental changes of some nerve cells in the cerebral cortex of the albino rat during the growth of the brain Part II On the increase in size of some nerve cells in the cerebral cortex of the Norway rat (Mus norvegicus), compared with the corresponding changes in the albino rat. J Comp Neurol. 1918;29:119–162. [Google Scholar]
- 79.Bandeira F, et al. Changing numbers of neuronal and non-neuronal cells underlie postnatal brain growth in the rat. Proc Natl Acad Sci U S A. 2009;106:14108–14113. doi: 10.1073/pnas.0804650106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hou J, et al. The temporal pattern of postnatal neurogenesis found in the neocortex of the Gottingen minipig brain. Neuroscience. 2011;195:176–179. doi: 10.1016/j.neuroscience.2011.08.025. [DOI] [PubMed] [Google Scholar]
- 81.Shankle WR, et al. Approximate doubling of numbers of neurons in postnatal human cerebral cortex and in 35 specific cytoarchitectural areas from birth to 72 months. Pediatr Dev Pathol. 1999;2:244–259. doi: 10.1007/s100249900120. [DOI] [PubMed] [Google Scholar]
- 82.Quinn R. Comparing rat’s to human’s age: how old is my rat in people years? Nutrition. 2005;21:775–777. doi: 10.1016/j.nut.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 83.Zgraggen E, et al. Early postnatal migration and development of layer II pyramidal neurons in the rodent cingulate/retrosplenial cortex. Cereb Cortex. 2012;22:144–157. doi: 10.1093/cercor/bhr097. [DOI] [PubMed] [Google Scholar]
- 84.Fagel DM, et al. Cortical neurogenesis enhanced by chronic perinatal hypoxia. Exp Neurol. 2006;199:77–91. doi: 10.1016/j.expneurol.2005.04.006. [DOI] [PubMed] [Google Scholar]
- 85.Fagel DM, et al. Fgfr1 is required for cortical regeneration and repair after perinatal hypoxia. J Neurosci. 2009;29:1202–1211. doi: 10.1523/JNEUROSCI.4516-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Le MC, et al. “Small axonless neurons”: postnatally generated neocortical interneurons with delayed functional maturation. J Neurosci. 2011;31:16731–16747. doi: 10.1523/JNEUROSCI.4273-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Riccio O, et al. New pool of cortical interneuron precursors in the early postnatal dorsal white matter. Cereb Cortex. 2012;22:86–98. doi: 10.1093/cercor/bhr086. [DOI] [PubMed] [Google Scholar]
- 88.Magavi SS, et al. Induction of neurogenesis in the neocortex of adult mice. Nature. 2000;405:951–955. doi: 10.1038/35016083. [DOI] [PubMed] [Google Scholar]
- 89.Li Q, et al. Strain differences in behavioral and cellular responses to perinatal hypoxia and relationships to neural stem cell survival and self-renewal: Modeling the neurovascular niche. Am J Pathol. 2009;175:2133–2146. doi: 10.2353/ajpath.2009.090354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Laywell ED, et al. Identification of a multipotent astrocytic stem cell in the immature and adult mouse brain. Proc Natl Acad Sci U S A. 2000;97:13883–13888. doi: 10.1073/pnas.250471697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Curatolo P, et al. Infantile spasms in tuberous sclerosis complex. Brain Dev. 2001;23:502–507. doi: 10.1016/s0387-7604(01)00300-x. [DOI] [PubMed] [Google Scholar]
- 92.Holmes GL, Stafstrom CE. Tuberous sclerosis complex and epilepsy: recent developments and future challenges. Epilepsia. 2007;48:617–630. doi: 10.1111/j.1528-1167.2007.01035.x. [DOI] [PubMed] [Google Scholar]
- 93.Webb DW, et al. Morbidity associated with tuberous sclerosis: a population study. Dev Med Child Neurol. 1996;38:146–155. doi: 10.1111/j.1469-8749.1996.tb12086.x. [DOI] [PubMed] [Google Scholar]
- 94.Crino PB, et al. The tuberous sclerosis complex. N Engl J Med. 2006;355:1345–1356. doi: 10.1056/NEJMra055323. [DOI] [PubMed] [Google Scholar]
- 95.European Chromosome 16 Tuberous Sclerosis Consortium. Identification and characterization of the tuberous sclerosis gene on chromosome 16. Cell. 1993;75:1305–1315. doi: 10.1016/0092-8674(93)90618-z. [DOI] [PubMed] [Google Scholar]
- 96.Crino PB. Molecular pathogenesis of tuber formation in tuberous sclerosis complex. J Child Neurol. 2004;19:716–725. doi: 10.1177/08830738040190091301. [DOI] [PubMed] [Google Scholar]
- 97.Feliciano DM, et al. Single-cell Tsc1 knockout during corticogenesis generates tuber-like lesions and reduces seizure threshold in mice. J Clin Invest. 2011;121:1596–1607. doi: 10.1172/JCI44909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Zhou J, et al. Tsc1 mutant neural stem/progenitor cells exhibit migration deficits and give rise to subependymal lesions in the lateral ventricle. Genes Dev. 2011;25:1595–1600. doi: 10.1101/gad.16750211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Feliciano DM, et al. Postnatal neurogenesis generates heterotopias, olfactory micronodules and cortical infiltration following single-cell Tsc1 deletion. Hum Mol Genet. 2012;21:799–810. doi: 10.1093/hmg/ddr511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Lacar B, et al. Imaging and recording subventricular zone progenitor cells in live tissue of postnatal mice. Front Neurosci. 2010:4. doi: 10.3389/fnins.2010.00043. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.