Abstract
Background and Objective
Cytolethal distending toxin (CDT) is a genotoxin produced by Aggregatibacter actinomycetemcomitans. In spite of its association with pathogenesis, little is known about the humoral immune response against the CDT. This study aimed to test whether subgingival colonization and humoral response to A. actinomycetemcomitans would lead to a response against CDT.
Material and Methods
Sera from periodontally healthy, localized and generalized aggressive periodontitis and chronic periodontitis subjects (n = 80) were assessed for immunoglobulin G titers to A. actinomycetemcomitans serotypes a/b/c and to each CDT subunit (CdtA, CdtB and CdtC) by ELISA. A. actinomycetemcomitans subgingival levels and neutralization of CDT activity were also analyzed.
Results
Sera from 75.0% localized and 81.8% generalized aggressive periodontitis patients reacted to A. actinomycetemcomitans. A response to serotype b was detected in localized (66.7%) and generalized aggressive periodontitis (54.5%). Reactivity to A. actinomycetemcomitans correlated with subgingival colonization (R = 0.75, p < 0.05). There was no correlation between A. actinomycetemcomitans colonization or response to serotypes and the immunoglobulin G response to CDT subunits. Titers of immunoglobulin G to CdtA and CdtB did not differ among groups; however, sera of all generalized aggressive periodontitis patients reacted to CdtC. Neutralization of CDT was not correlated with levels of antibodies to CDT subunits.
Conclusion
Response to CdtA and CdtB did not correlate with the periodontal status of the subject in the context of an A. actinomycetemcomitans infection. However, a response to CdtC was found in sera of generalized but not of localized aggressive periodontitis subjects. Differences in response to CdtC between generalized and localized aggressive periodontitis subjects indicate that CDT could be expressed differently by the infecting strains. Alternatively, the antibody response to CdtC could require the colonization of multiple sites.
Keywords: Aggregatibacter actinomycetemcomitans, cytolethal distending toxin, enzyme-linked immunosorbent assay (ELISA), immunoglobulin G, serotype
Aggregatibacter actinomycetemcomitans is a non-motile gram-negative facultative anaerobic coccobacillus associated with the etiology of aggressive periodontitis (1). The microorganism can also be detected in the oral cavity of chronic periodontitis patients and periodontally healthy subjects (2). A. actinomycetemcomitans is classified into six serotypes (a–f) based on surface O-polysaccharides, and serotype b is usually correlated with aggressive periodontitis (1), although other serotypes have been associated with diseased patients in certain populations (3,4).
Similar to other mucosa-associated gram-negative pathogens, A. actinomycetemcomitans produces a cytolethal distending toxin (CDT; 5,6). Cytolethal distending toxin is a secreted tripartite AB2 toxin [An AB2 toxin is a toxin that has an active toxic subunit (A) represented by CdtB, and a B subunit which binds to the target cell, and is formed in Cdt by the subunits CdtA and CdtC], in which CdtB is the active toxic subunit that exhibits both type I deoxyribonuclease-like and phosphatase activities while subunits CdtA and CdtC seem to bind to target cells and CdtC also aids the delivery of CdtB into cells (6). The CDT triggers a DNA-damage response resulting in cell cycle arrest in G2/M or G0/G1 phase in many epithelial and macrophage cell lines and in T lymphocytes. Interestingly, CDT was not able to affect human periodontal ligament cells, although other fibroblastic cell lines were susceptible (6,7).
Although the association of CDT with pathogenesis is not fully understood, this toxin may represent a bacterial adaptation which could affect the interaction between the bacterium and the host immune system in chronic diseases. Mice experimentally challenged with a CDT-deficient Helicobacter hepaticus mutant developed a significantly lower immunoglobulin (Ig)G2c response and failed to mount an IgG1 response (8). Cytolethal distending toxin can partly inhibit the production of proinflammatory cytokines by antigen-presenting cells, although A. actinomycetemcomitans CDT induced the production of interleukin (IL)-1β, IL-6, IL-8 and interferon-γ (IFN-γ) by human monocytes (9,10). In mice model, a Haemophilus ducreyi CDT-deficient mutant induced chancroid lesions similar to the wildtype strain (11). However, a Campylobacter jejuni CdtB-deficient mutant was less invasive in mouse tissues (12), leading to an attenuated inflammatory effect in the animal when compared with the wild-type (13). Furthermore, evidence suggested that the A. actinomycetemcomitans CDT inhibited nitric oxide (NO) production by murine macrophages (7).
Very little is known about the humoral immune response to CDT in subjects colonized by producing bacteria. Similar levels of IgG to H. ducreyi CDT complex were observed in the sera of chancroid patients and control subjects, as well as in the sera of periodontitis subjects and control subjects (14). Even though the association between A. actinomycetemcomitans and localized aggressive periodontitis is well established, sera of few localized aggressive periodontitis patients contained antibodies to the A. actinomycetemcomitans CDT (15).
We aimed to evaluate the association between the IgG response to A. actinomycetemcomitans serotypes and to CDT subunits in sera of subjects with different periodontal conditions. We also tested whether subgingival colonization by A. actinomycetemcomitans in generalized aggressive periodontitis subjects and induction of an antibody response against the organism would lead to an immune response to CDT subunits and to A. actinomycetemcomitans CDT neutralization by sera. The sera neutralization of localized aggressive periodontitis subjects against CDT activity was also tested.
Material and methods
Study population and samples
Fifty-two periodontitis subjects and 28 periodontally healthy subjects (total n = 80) were selected at the Federal University of São Paulo and Guarulhos University, Brazil. The subjects signal a written informed consent and the study was approved by the Ethical Committee of the Institute of Biomedical Sciences, University of São Paulo, Brazil. Twenty-four subjects were classified as having aggressive localized periodontitis (age range 13–28 years), 11 as generalized aggressive periodontitis (age range 18–30 years) and 17 as chronic periodontitis (age range 33– 57 years). Healthy subjects (age range 13–47 years) comprised a group with no evidence of loss of attachment and probing depth < 3 mm. The disease classification was based on clinical and radiographic measurements such as probing depth, bleeding on probing and alveolar bone loss (16,17). Exclusion criteria were as follows: previous periodontal therapy, pregnancy, nursing, and any systemic condition that could affect the progression of periodontal disease, such as diabetes, or that required antibiotic coverage for routine dental therapy, and antibiotic therapy in the previous 12 mo.
Peripheral blood samples were collected by venipuncture, and sera were stored at −20°C until analyzed. Subgingival plaque samples were collected from nine non-contiguous interproximal sites per subject in the generalized aggressive periodontitis group. The sites were selected according to probing depth category (three with probing depth ≤ 3 mm, three with probing depth between 4 and 6 mm and three with probing depth ≥ 7 mm) and randomized in different quadrants. The supragingival plaque was removed, and the subgingival samples were taken with individual sterile mini-Gracey curettes (11,12) and immediately placed in Tris EDTA buffer (10 mM Tris–HCl, 1 mM EDTA, pH 7.6) containing 0.5 M NaOH. The samples were dispersed using a vortex mixer and frozen at −20°C until use.
Recombinant CdtA and CdtB construction
Escherichia coli expressing CdtA and CdtB were constructed as follows. Oligonucleotide primer pairs, based on the CDT nucleotide sequence in A. actinomycetemcomitans FDCY4 (GenBank accession number AF00 6830) were used to amplify CdtA (CDTA1-SacI, 5′-GATGGAGCTCAGGAGAGGTACAATGA-3′; and CDTA2-XhoI, 5′-TCACTGCAGTCTCCTTAGCGATCATGA-3′) and CdtB (CDTB1-SacI, 5′-GCTAAGGAGCTCATATGCAATGGGTAA AGC-3′; and CDTB2-PstI, 5′-TCACTGCAGTCTCCTTAGCGATCATGA-3′) with SacI/XhoI and SacI/PstI restrictions sites, respectively. Plasmid pCDT1, which contains the CdtABC open reading frames of strain FDCY4 cloned in pBluescript II SK (+) (Stratagene, La Jollla, CA, USA), was used as DNA template (7). The amplification reactions contained 80 ng of DNA, 50 pM of each primer pair, 1.5 mM Mg2+, 10 mM dNTP and 2.5 U Taq DNA polymerase (Invitrogen, Carlsbad, CA, USA). The temperature profile included an initial step at 94°C for 1 min, followed by 30 cycles of 94°C for 1 min, 54°C for 1 min and 74°C for 45 s, and a final extension at 74°C for 5 min. The amplified products were digested and ligated into pRSETa (Invitrogen) for CdtA and pTHIOHISb (Invitrogen) for CdtB and transformed in E. coli BL21(DE) pLYS and E. coli TOP10, respectively. The transformants were selected on LB plates containing 50 μg/mL ampicillin and 35 μg/mL chloramphenicol (for pRSETacdtA), or 100 μg/mL ampicillin (for pTHIOHISbcdtB), and confirmed by restriction analysis and sequencing.
Cytolethal distending toxin subunit expression and purification
The subunit CdtC was obtained from a previously described recombinant strain of E. coli (18). Recombinant strains E. coli BL21(DE) pLYS (pRSETacdtA) and E. coli TOP10 (pTHIOHISbcdtB) were grown in LB medium with antibiotics (ampicillin and chloramphenicol or ampicillin) to an optical density (O.D.600 nm) of 0.5. Expression of CDT was induced with 1 mM isopropyl β-D-thiogalactoside (IPTG). Cells were resuspended in 5 mM imidazole buffer and then lysed by sonication (Ultrasonic Processor VCX 130PB; Sonics & Materials Inc., Newtown, CT, USA). Histidine-tagged proteins were examined on 10% SDS-PAGE gels (19), stained with the Invision Instain™ System (Invitrogen) and purified by passage through an affinity chromatography column (Ni-NDA resin; Invitrogen). The histidine tag was removed from rCdtB-His after enterokinase treatment (Invitrogen).
Cytolethal distending toxin toxic activity assay
The purified CDT fractions were incubated at different concentrations to reconstitute the holotoxin [rCdtA (15 μg), rCdtB (10 μg), rCdtC (15 μg); and rCdtA (30 μg), rCdtB (10 μg), rCdtC (15 μg)] for 1 h at 0°C in a gently rocking platform (18). Chinese hamster ovary (CHO) cells (240 cells/well) were plated in six-well plates in Dulbecco’s modified Eagle’s medium (DMEM, Cultilab, Campinas, SP, Brazil) containing penicillin (1664 U/mL), streptomycin (745 U/mL) and sodium bicarbonate (2.2 g/mL) supplemented with 10% fetal calf serum (FCS). The reconstituted CDT holotoxin was added to each well and kept in ice for 15 min. Cells were washed once, and 3 mL of culture medium were added, followed by incubation for 7 d at 37°C in an atmosphere of air containing 5% CO2. The colonies were fixed with 10% (v/v) formaldehyde for 1 min, stained with 10% (w/v) crystal violet and counted. The number of surviving cells, determined by colony-forming units (CFU), was calculated. All experiments were performed in triplicate. Controls consisted of addition of the nonreconstituted toxin, consisting of the three CDT subunits, without incubation and no addition of CDT.
Response levels of serum antibodies to A. actinomycetemcomitans antigens
An ELISA was used to detect A. actinomycetemcomitans serotypes a, b and c and A. actinomycetemcomitans CDT subunits (rCdtA; rCdtB and rCdtC)-specific serum IgG. Formalin-fixed whole bacterial cells of reference strains A. actinomycetemcomitans ATCC 29523, JP2 and SA1151, serotypes a, b and c, respectively, were used as antigens in separate assays as described previously (20). Ninety-six-well plates (Corning-Costar, Lowell, MA, USA) were coated with 200 μL per well of A. actinomycetemcomitans (O.D.580 nm = 0.3) in carbonate buffer (pH 9.6) and incubated overnight at 4°C. Plates were blocked with 1% bovine serum albumin in phosphate-buffered saline. Sera were diluted (1:10 000, 1:20 000 and 1:40 000), 100 μL added to each well and run in triplicate. Peroxidase-conjugated mouse anti-human IgG (Sigma-Aldrich, St Louis, MO, USA), diluted 1:5000, was used as the secondary antibody. Color development was achieved by the addition of o-phenylenediamine (Sigma-Aldrich). The end-point conversion of the enzyme substrate was measured at O.D.490 nm in a microplate reader (Bio-Rad, Hercules, CA, USA). A negative control (no serum sample) and five control sera samples were included in each plate.
The same protocol was used in an ELISA using rCdtA, rCdtB and rCdtC as antigens, except that the wells were covered with 100 μL of each purified CDT subunit (1 μg/mL) and tested sera were diluted to 1:50 and 1:150.
Optical density values were normalized among plates based on data obtained for controls. The average and standard deviation of O.D. values from triplicate wells of each serum dilution were determined, and the cut-off levels for reactivity to A. actinomycetemcomitans serotypes a, b, c (F_serotype) and to each CDT subunit (F_Cdt) were mean values for periodontally healthy control subjects + 2 SD (21).
Subgingival levels of A. actinomycetemcomitans
Subgingival colonization by Aggregatibacter actinomycetemcomitans was estimated in nine subgingival sites per subject from 11 generalized aggressive periodontitis subjects by the semiquantitative checkerboard DNA–DNA hybridization technique (22). The DNA from plaque samples was immobilized on positively charged nylon membranes and submitted to hybridization against A. actinomycetemcomitans total DNA probes obtained from strains A. actinomycetemcomitans serotype a (ATCC29523) and serotype b (ATCC43718). The probes were diluted to approximately 20 ng/mL in a 45% formamide hybridization solution, and hybridized for 16 h at 42°C. After stringent washings, hybrids were detected by exposing the membranes to antidigoxigenin antibody conjugated to alkaline phosphatase (dilution 1:50 000; Boehringer Mannheim, Indianapolis, IN, USA) for 30 min. Signals were detected by chemiluminescence and compared to a standard containing known amounts of bacterial DNA. Subgingival levels of A. actinomycetemcomitans were given by the mean number of bacteria in the nine samples per subject.
Neutralization of CDT activity by sera
The effect of sera on CDT activity was examined in a standard cell viability assay using CHO cells and sera from five generalized aggressive periodontitis subjects, five localized aggressive periodontitis subjects and one healthy control subject. Aliquots of the reconstituted CDT [20 μL containing rCdtA, rCdtB and rCdtC (75 μg/mL of each) in phosphate-buffered saline] were mixed with 5 μL of each serum and incubated for 20 min. The CHO cells were plated in 96-well plates (1 × 105 cells per well) in DMEM, and the toxin–serum mixtures were added to a concentration of 45 μg of reconstituted CDT per milliliter human serum, dilution 1:20. The amount of serum in each well was made up with FCS to achieve a 10% final concentration in a total volume of 100 μL. The negative controls consisted of cells with 10% FCS, and positive controls consisted of cells with 10% FCS and CDT. Controls using serum from each subject without the addition of CDT were considered as 100% cell viability. After incubation for 72 h in an atmosphere of air containing 5% CO2, at 37°C, the CDT activity was determined by assessing cell viability using MTT [3(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide; Sigma-Aldrich]. Briefly, 50 μL of MTT solution were added to each well and incubated for 3 h at 37°C. After addition of 100 μL of 10% sodium dodecyl sulfate (in 0.01 N HCl) and incubation for 20 h at 37°C, absorbance was measured at an O.D. of 540 nm. The viability of CDT-treated cells added to serum from each subject was calculated by comparing the absorbance values obtained in wells with the addition of serum from each subject and with no addition of CDT (100% of viability).
Cytolethal distending toxin activity was considered as the percentage of reduction in cell viability promoted by CDT added to serum from each subject compared with the reduction promoted by CDT alone (100%). The experiment was done in triplicate in two independent assays. The results are presented as percentages ± SD. Thus, the neutralization of CDT activity is inversely proportional to the CDT activity value.
Statistical analysis
The ELISA data were analyzed using a one-way analysis of variance with Tukey–Kramer multiple comparisons (ANOVA–Tukey). Spearman rank correlation was used to correlate IgG titers to A. actinomycetemcomitans serotypes, CDT subunits, periodontal condition, A. actinomycetemcomitans subgingival colonization, sera neutralization activity and sites presenting aggressive periodontitis. Fisher’s exact test was used to detect differences in distribution of sera reactive to each serotype and CDT subunit. p-values < 0.05 were considered significant.
Results
Subunits CdtA, CdtB and CdtC
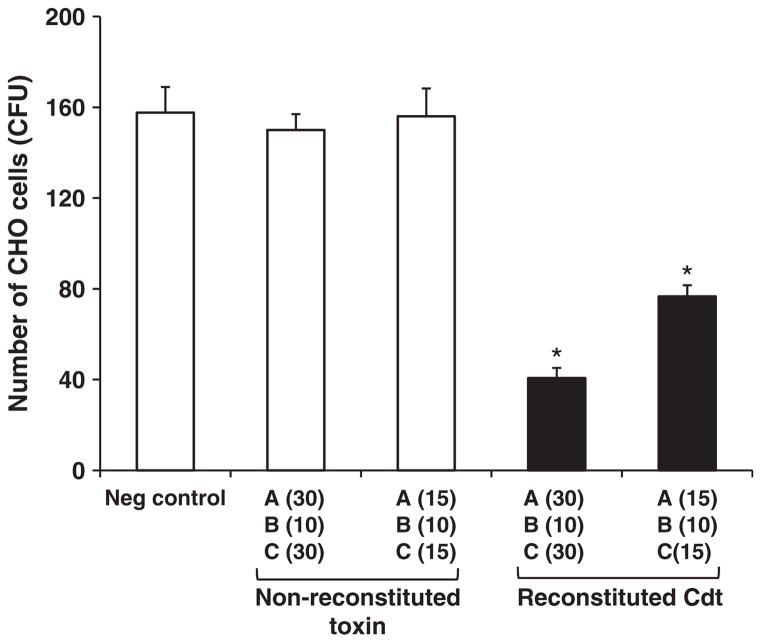
Recombinant CdtA, CdtB and CdtC were expressed in E. coli and purified by standard methods. The three proteins were used separately as antigens in ELISA, for analysis of the antibody response of sera from subjects with different periodontal conditions. The non-reconstituted and the reconstituted holotoxin were tested for growth inhibition in CHO cells and the results expressed as colony-forming units of surviving cells in relation to negative control cultures with no addition of CDT. The reconstituted but not the non-reconstituted toxin exhibited a strong cytotoxic effect, as shown in Fig. 1 (p < 0.001, ANOVA–Tukey).
Fig. 1.
Cytolethal distending toxin activity in CHO cells when reconstituted toxin was added to the cells. Reconstituted toxin formed by different amounts (in μg) of CDT subunits A, B and C was added to 240 cells, and the number of CFU was counted after 7 d of incubation. Filled bars represent cells inoculated with reconstituted CDT holotoxin. Open bars represent controls (Neg control, negative control without CDT addition, and non-reconstituted toxin). *p < 0.001 (ANOVA–Tukey) compared with negative control.
Levels of serum IgG antibodies to A. actinomycetemcomitans serotypes and CDT subunits
The sera from subjects with different periodontal conditions were tested for reactivity to several A. actinomycetemcomitans antigens. The cut-off point for reactivity to the different antigens was established as the mean O.D. values ± 2 SD obtained for healthy control subjects. All healthy subjects were considered as controls for the establishment of cut-off points for titers to A. actinomycetemcomitans serotypes. Since the IgG levels to CDT were more spread among healthy subjects, six healthy subjects were selected as controls because they exhibited the lowest response to all CDT subunits.
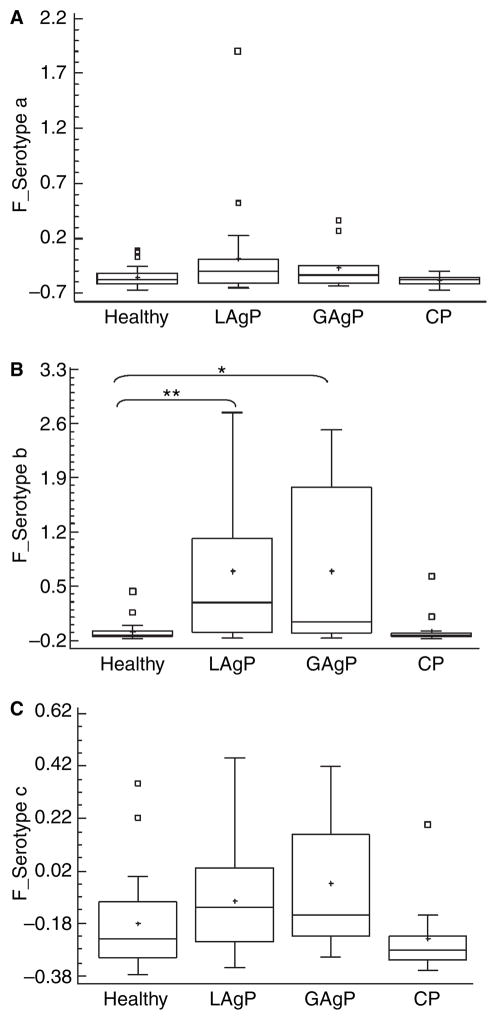
Thirty-eight (47.5%) of the 80 studied subjects had sera reactive to whole A. actinomycetemcomitans cells. Of these reactive subjects, the IgG response to serotype b was more prevalent (n = 25; 31.2%), followed by serotype c (n = 16; 20.0%) and serotype a (n = 12; 15.0%). Eleven subjects with aggressive periodontitis (n = 8 for localized aggressive periodontitis and n = 3 for generalized aggressive periodontitis) responded to two or more serotypes. Response to serotype a alone was found among healthy subjects but not in any diseased patient; in diseased patients, response to serotype a always presented together with response to serotype b and/or c. The data concerning reactivity to A. actinomycetemcomitans serotypes are shown in Table 1. Immunoglobulin G levels to A. actinomycetemcomitans serotype b were significantly higher in localized and generalized aggressive periodontitis subjects when compared with healthy subjects (ANOVA–Tukey, p < 0.001 and p < 0.01, respectively; Fig. 2).
Table 1.
Distribution of subjects classified as serum reactive to Aggregatibacter actinomycetemcomitans serotypes a, b and c among periodontally healthy, localized aggressive periodontitis (LAgP), generalized aggressive periodontitis (GAgP) and chronic periodontitis (CP) patients, based on cut-off levels for reactivity after O.D. corrected values (F_serotype) obtained by ELISA for total bacteria in sera dilution 1:20 000
| Periodontal diagnosis | Number of subjects (n) having sera reactive to A. actinomycetemcomitans (%) | Serotype a n (%) | Serotype b n (%) | Serotype c n (%) |
|---|---|---|---|---|
| Healthy n = 28 | 7 (25.0) | 3 (10.7) | 1 (3.6) | 3 (10.7) |
| LAgPa n = 24 | 18 (75.0) | 7 (29.2)* | 16 (66.7)* | 6 (25.0) |
| GAgPa n = 11 | 9 (81.8) | 2 (18.2) | 7 (63.6)* | 5 (45.4)* |
| CP n = 17 | 4 (23.5) | 0 (0.0) | 2 (11.8) | 2 (11.8) |
| Total n = 80 | 38 (47.5) | 12 (15.0) | 26 (32.5) | 16 (20.0) |
Certain subjects responded to two or more serotypes.
Statistically significant differences when compared with healthy subjects (p < 0.05, Fisher’s test).
Fig. 2.
Distribution of sera IgG levels from subjects with different periodontal conditions against A. actinomycetemcomitans serotypes a (A), b (B) and c (C) based on cut-off levels for reactivity after O.D. corrected values (F_serotype) obtained by ELISA. Non-reactive subjects: OD corrected values below 0. Sera dilution 1:20 000. Statistically significant differences between groups are indicated as *p < 0.01 and **p < 0.001 (ANOVA– Tukey). White boxes represent the outlier values (outside the cut-off, determined by the statistic program Statgraphics) and the crosses represent the means of the values.
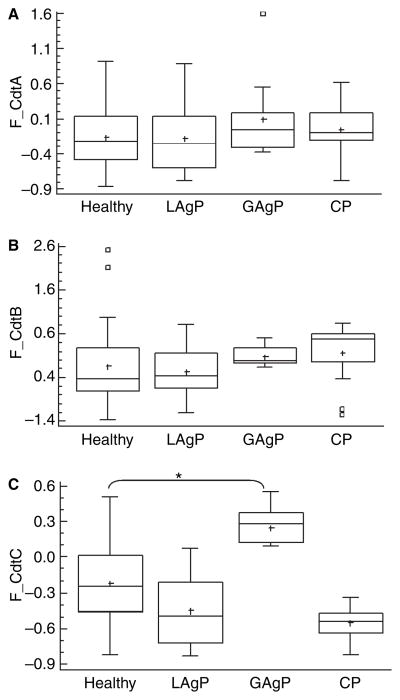
Data concerning the immune response to CDT subunits are shown in Table 2 and Fig. 3. When levels of antibodies were compared, serum IgG levels to CdtA and to CdtB did not differ according to the periodontal condition (ANOVA–Tukey, p > 0.05). However, IgG titers to CdtC (Fig. 3) were significantly higher in sera of generalized aggressive periodontitis subjects than in periodontally healthy subjects and in localized aggressive periodontitis subjects (ANOVA–Tukey p < 0.05).
Table 2.
Distribution of subjects classified as serum reactive to CdtA, CdtB and CdtC among periodontally healthy, localized aggressive periodontitis (LAgP), generalized aggressive periodontitis (GAgP) and chronic periodontitis (CP) subjects based on cut-off levels for reactivity after O.D. corrected values (F_Cdt) obtained by ELISA with sera dilution of 1:150
| Periodontal diagnosis | CdtA
|
CdtB
|
CdtC
|
|---|---|---|---|
| reactive n (%) | reative n (%) | reactive n (%) | |
| Healthy (n = 28) | 9 (32.1) | 8 (28.6) | 7 (25.0) |
| LAgP (n = 24) | 8 (33.3) | 9 (37.5) | 2 (8.3) |
| GAgP (n = 11) | 5 (45.4) | 5 (45.4) | 11 (100)* |
| CP (n = 17) | 7 (41.2) | 12 (70.6)* | 0 (0.0)* |
Statistically significant difference when compared with healthy subjects (p < 0.05, Fisher’s test).
Fig. 3.
Distribution of serum IgG levels from subjects with different periodontal conditions for CdtA (A), CdtB (B) and CdtC (C), based on cut-off levels for reactivity after O.D. corrected values (F_Cdt). Sera dilution 1:150. Statistically significant differences when compared with healthy subjects are indicated as *p < 0.05 (ANOVA–Tukey). White boxes represent the outlier values (outside the cut-off, determined by the statistic program Statgraphics) and the crosses represent the means of the values.
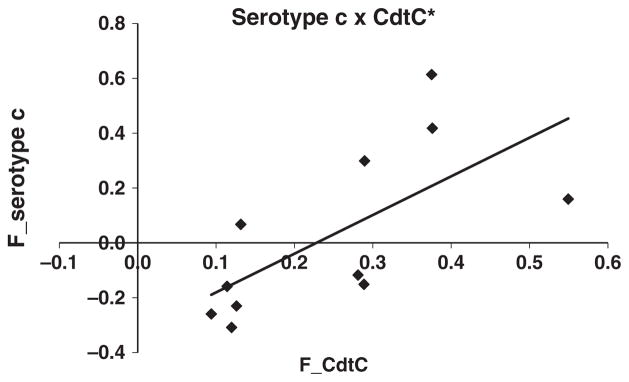
Despite the high prevalence of sera reactivity to A. actinomycetemcomitans serotype b in sera of aggressive periodontitis subjects, there were no correlations between the IgG response to serotype b and to any CDT subunit (CdtA, R = 0.30, p = 0.07; CdtB, R = 0.06, p = 0.07; and CdtC, R= −0.10, p = 0.53). In localized aggressive periodontitis subjects, there was no correlation between response to serotypes c and to CdtA (R = 0.16, p = 0.35), CdtB (R = 0.26, p = 0.11) and CdtC (R = 0.18, p = 0.29). However, a significant correlation between response to CdtC and response to A. actinomycetemcomitans serotype c (R = 0.85, p = 0.001) was shown in generalized aggressive periodontitis subjects (Fig. 4).
Fig. 4.
Sera IgG levels to A. actinomycetemcomitans serotype c (sera dilution 1:20 000) and to CdtC (sera dilution 1:150) in generalized aggressive periodontitis subjects based on cut-off levels for reactivity after O.D. corrected values (F_serotype c). *p < 0.001 (Spearman rank correlation).
Microbiological analysis was performed in 99 subgingival plaque samples obtained form 11 subjects with generalized aggressive periodontitis, since we did not have access to microbiological samples from the remaining subjects. Subgingival colonization by A. actinomycetemcomitans was demonstrated in 11 generalized aggressive periodontitis subjects studied in a semi-quantitative assay using serotype a and serotype b DNA probes (Table 3). The subgingival levels of A. actinomycetemcomitans correlated with response to A. actinomycetemcomitans serotype a (R = 0.75, p < 0.01) and serotype b (R = 0.70, p < 0.05), but not to serotype c, which was expected because this serotype was not used as a DNA probe. However, there was no correlation between bacterial colonization in subgingival sites and IgG response to any CDT subunit (CdtA, R = 0.34, p = 0.29; CdtB, R = 0.30, p = 0.35; and CdtC, R = 0.45, p = 0.16).
Table 3.
Analysis of number of A. actinomycetemcomitans in generalized aggressive periodontitis (GAgP) subjects obtained by DNA–DNA hybridization
| Number of A. actinomycetemcomitans cells | |
|---|---|
| GAgP1 | 0 |
| GAgP2 | 5714 |
| GAgP3 | 288 089 |
| GAgP4 | 8889 |
| GAgP5 | 1 402 222 |
| GAgP6 | 82 222 |
| GAgP7 | 36 667 |
| GAgP8 | 372 857 |
| GAgP9 | 0 |
| GAgP10 | 125 556 |
| GAgP11 | 74 444 |
The mean number of bacterial cells was determined in nine sites per subject. The DNA probe was from A. actinomycetemcomitans serotype a (ATCC29523) and serotype b (ATCC43718).
Sera neutralizing CDT activity
Owing to the small amount of serum obtained from each subject, only sera of five generalized aggressive periodontitis and five localized aggressive periodontitis subjects with different reactivity to CDT subunits and serum of one healthy subject were available for this assay. The addition of reconstituted CDT holotoxin induced a loss of cell viability of 60%, without the addition of human sera (negative control), and this value was considered as 100% CDT activity. As shown in Table 4, the addition of serum from the healthy subject to CDT resulted in 87.9 ± 3.0% of CDT activity (12.1 ± 3.0% of CDT activity inhibition). The CDT activity was only inhibited by the addition of serum from one localized aggressive periodontitis subject, whose serum was not reactive to A. actinomycetemcomitans serotypes or to CDT subunits.
Table 4.
Analysis of sera neutralizing activity in CHO cells against CDT holotoxin (% CDT activity ± SD) in five generalized aggressive periodontitis (GAgP), five localized aggressive periodontitis (LAgP) subjects and one healthy subject as a control
| % CDT activity ± SD | F_CdtA | F_CdtB | F_CdtC | F_serotype a | F_serotype b | F_serotype c | |
|---|---|---|---|---|---|---|---|
| Negative control | 0 | — | — | — | — | — | — |
| Healthy | 87.9 ± 3.0 | −0.267 | −0.347 | −0.612 | −0.327 | −0.230 | −0.149 |
| GAgP3 | 101.7 ± 8.9 | 0.185 | 0.064 | 0.281 | −0.163 | 1.701 | −0.117 |
| GAgP4 | 86.3 ± 18.4 | 0.557 | 0.397 | 0.290 | −0.129 | 0.008 | 0.299 |
| GAgP5 | 104.8 ± 12.2 | −0.053 | 0.300 | 0.376 | 0.361 | 2.518 | 0.418 |
| GAgP6 | 111.5 ± 6.2 | 1.594 | −0.037 | 0.289 | −0.086 | 0.085 | −0.151 |
| GAgP9 | 115.0 ± 2.7 | −0.369 | −0.119 | 0.120 | −0.222 | −0.166 | −0.308 |
| LAgP1 | −9.8 ± 6.9 | −0.361 | −0.546 | −0.666 | −0.217 | −0.175 | −0.068 |
| LAgP2 | 100.6 ± 3.1 | −0.240 | −0.262 | −0.198 | −0.234 | −0.026 | −0.368 |
| LAgP3 | 117.2 ± 0.9 | 0.820 | 0.480 | −0.074 | −0.172 | −0.168 | 0.167 |
| LAgP4 | 104.2 ± 2.8 | 0.168 | 0.123 | −0.735 | 1.904 | 0.153 | 0.452 |
| LAgP5 | 108.2 ± 3.1 | 0.367 | −0.599 | 0.071 | −0.097 | 1.980 | −0.110 |
Discussion
Sera from localized aggressive periodontitis subjects often contain markedly elevated IgG antibody titers against A. actinomycetemcomitans antigens, including repeats in toxin like leukotoxin, serospecific determinants in lipopolysaccharide (LPS) and outer membrane proteins (1).
Given the evidence that CDT may be involved in pathogenesis (6), we sought to define the antibody titers to this toxin in A. actinomycetemcomitans infected subjects. Previous infection by the bacterium was determined by evaluating IgG titers to LPS of the most prevalent serotypes in sera of all studied subjects. Reference strains were used as antigens for serotypes a, b and c, since A. actinomycetemcomitans reference strains are as efficient as the autologous strains to detection of IgG in sera by ELISA (23). As expected, sera IgG reactivity to A. actinomycetemcomitans serotypes was observed in sera from localized aggressive periodontitis subjects. Thus, the association of A. actinomycetemcomitans with localized aggressive periodontitis and its low prevalence in chronic periodontitis was confirmed, as shown by earlier studies (2). Most subjects with generalized aggressive periodontitis also exhibited sera IgG reactivity to A. actinomycetemcomitans LPS (Table 1 and Fig. 2), which is in accordance with finding that A. actinomycetemcomitans was highly prevalent in the subgingival dental plaque of these subjects (Table 3). A. actinomycetemcomitans was detected by DNA probes in most generalized aggressive periodontitis subjects, although the association between A. actinomycetemcomitans and generalized aggressive periodontitis is less clear than with the localized form of periodontitis (24). We have recently shown by 16S rRNA clonal analysis that the microbiota of these generalized aggressive periodontitis subjects included several yet to be cultivated bacteria and presented a high proportion of organisms belonging to the genus Selenomonas (17). The levels of A. actinomycetemcomitans were also significantly higher in the subgingival plaque of these generalized aggressive periodontitis subjects when compared with the periodontally healthy subjects, but the red complex species (Porphyromonas gingivalis, Tannerella forsythia and Treponema denticola) were the most numerous and prevalent periodontal pathogens in generalized aggressive periodontitis (22).
Most sera of localized aggressive periodontitis subjects reacted to A. actinomycetemcomitans serotype b, as indicated by other studies (25), whereas sera of generalized aggressive periodontitis subjects responded in a similar proportion to serotypes b and c (Table 1). The association of serotypes b and c with aggressive disease has been shown in Brazil (26). Differences in immunogenicity of distinct serotypes of A. actinomycetemcomitans and/or in their ability to reach high counts in the subgingival microenvironment and induce a robust humoral response may account for the different response to serotypes in localized and generalized aggressive periodontitis subjects.
Serum reactivity to A. actinomycetemcomitans serotypes correlated with subgingival colonization by this bacterium in the generalized aggressive periodontitis subjects, indicating that the immune response was not able to clear the organism. The protective role of antibodies in aggressive periodontitis is still controversial (27). Localized aggressive periodontitis sera contain IgG antibodies able to promote phagocytosis and killing of A. actinomycetemcomitans by human neutrophils (28). In addition, a concentration-dependent opsonic activity to A. actinomycetemcomitans in the presence of complement (29) and neutralization of leukotoxin activity (30) were shown in sera of localized aggressive periodontitis subjects. Moreover, the ability to invade epithelial cells (31) and to produce high levels of leukotoxin (2) may contribute to evasion of host surveillance by A. actinomycetemcomitans. Other mechanisms, such as the production of the CDT, could interfere with the generation of a protective immune response by the host. Animal studies revealed that CDT is involved in elevating a proinflammatory T helper 1-type response (12), and in experimental animal models a CDT-deficient strain was not able to mount a robust humoral response to bacterial antigens, in contrast to the wild-type, indicating a role for CDT in modulating the host response to the bacteria (6).
There were no differences in sera reactivity to CdtA or CdtB among the studied groups. Low but considered positive levels of IgG reactive to CdtB were more prevalent in chronic periodontitis subjects when compared with healthy control subjects. Since CdtB is the most conserved subunit among CDT-producing bacteria (5) and chronic periodontitis subjects were the oldest group among the studied subjects, the increased response to CdtB in this group could be the result of a prolonged exposure to other CDT-producing bacteria throughout life.
Despite the fact that most localized aggressive periodontitis subjects had sera reactive to A. actinomycetemcomitans, there was not a significant response to the CDT subunits, indicating that these proteins were not strong immunogens. In contrast, all subjects with generalized aggressive periodontitis presented positive sera antibody titers to CdtC, and there was correlation between sera reactivity to this subunit and response to A. actinomycetemcomitans serotype c (Fig. 4). In spite of differences in sera response to CdtC between localized and generalized aggressive periodontitis, there were no differences in IgG titers to A. actinomycetemcomitans serotypes b and c between these groups. These data are controversial, since sera reactivity was demonstrated in subjects with the more disseminated form of periodontitis (the generalized and not the localized form). In addition, no correlation was observed between the response to CdtC and to A. actinomycetemcomitans serotype b, the serotype most commonly associated with localized aggressive periodontitis, and with generalized aggressive periodontitis.
Cytolethal distending toxins are probably very poor immunogens, as shown in subjects with chancroid in the acute phase of the disease and in animal models (8). Repeated contact with bacteria or high doses of bacteria are required to obtain detectable antibody responses to cytotoxin in experimentally infected animals, and these result in serum neutralization of the toxic activity (32,33). Cytolethal distending toxin-neutralizing antibodies were detected in 66% of patients with chancroid, while only 4% of Swedish blood donors had detectable neutralizing antibodies (33). Other data had shown that 32% of chancroid patients had higher anti-Haemophilus ducreyi Cdt 2 antibodies, suggesting that chancroid may stimulate the production of antibodies against HdCDT in some cases (14). A study with localized aggressive periodontitis subjects revealed that antibodies to CDT were detected in only three of 23 subjects (12.5%; 15). Thus, our data are in agreement with others, indicating that infection by the producing bacteria often does not lead to an immune response to CDT.
The lack of an antibody response to CDT in some A. actinomycetemcomitans infected subjects may be due to differences in production of CDT by the infecting organism. A. actinomycetemcomitans positive to cdtABC genes were found in approximately one-third of aggressive periodontitis patients in a Chinese population (34). Other studies showed that most A. actinomycetemcomitans isolates present CDT activity in in vitro studies, although strain variations in CDT production and activity have been reported (35,36). Our previous data had shown that a higher cytotoxic effect is more often found among isolates of serotypes b and c than in serotype a, but differences in the cytotoxic effect among strains of the same serotype were shown (26). In addition, the ability of A. actinomycetemcomitans to express CDT in vivo has not been shown. However, our data indicate that serum antibodies to CDT are produced, at least in some subjects infected by A. actinomycetemcomitans.
The higher reactivity to CdtC in generalized than in localized aggressive periodontitis subjects could also suggest that production of antibodies to CdtC may require the colonization of A. actinomycetemcomitans in multiple sites for a prolonged time. This hypothesis is reinforced by comparing the number of periodontitis-affected teeth of generalized and localized aggressive periodontitis groups. Generalized aggressive periodontitis subjects exhibited at least 12 teeth affected by periodontitis, including six molars and/or incisors and six additional teeth, whereas in localized aggressive periodontitis subjects the disease was restricted to molars and/or incisors. In addition, members of the generalized aggressive periodontitis group were older than localized aggressive periodontitis subjects (mean age 19 years in localized aggressive periodontitis vs. 25 years in generalized aggressive periodontitis). The antibody response to CdtC in generalized aggressive periodontitis subjects may result in lower levels of subgingival colonization by A. actinomycetemcomitans than those achieved in localized aggressive periodontitis patients (22), suggesting that antibodies to CdtC might be beneficial for controlling A. actinomycetemcomitans overgrowth at individual sites. Whether this response would result in a lower disease progression after a period of aggressive disease must be elucidated.
Since CDT was associated with persistence of infection in animal models, and with increased expression of RANKL and consequently osteoclastogenesis, neutralizing antibodies against CDT would probably also affect the progression of periodontitis (37). The neutralization assay revealed that only the serum of a localized aggressive periodontitis subject was able to inhibit CDT activity. However, it should be noticed that IgG reactivity to neither CDT subunits nor A. actinomycetemcomitans serotypes was detected in this serum.
Patients with chancroid presented serum neutralizing antibodies to HdCDT in only 22% of cases, and this effect was not correlated with antibody titers to CDT subunits (14). Previous studies reported a low prevalence of serum neutralizing activity for CDT in chronic periodontitis patients (38), in periodontitis patients with no distinction of the form (aggressive or chronic; 14) and in healthy subjects (15). Antibodies to CDT were detected in three of 23 localized aggressive periodontitis subjects, and all these three subjects exhibited serum neutralizing activity (15). Thus, the present data, in accordance with others, suggested that CDT elicits a weak antibody production and that these antibodies do not seem to be protective. Sera of aggressive periodontitis subjects exhibit a high antibody response to outer membrane proteins, mainly OMP29 and OMP100 (39). It has been previously shown that IgG antibodies to A. actinomycetemcomitans in sera of localized aggressive periodontitis patients promoted phagocytosis and killing of A. actinomycetemcomitans (29). Thus, although sera of AgP subjects may not neutralize CDT activity, the high titers of IgG to A. actinomycetemcomitans whole cells would probably result in loss of bacterial cell viability.
Although the relationships between response to CDT and pathogenesis are still not conclusive, our data demonstrated that infection by A. actinomycetemcomitans in localized aggressive periodontitis subjects, determined by a strong serum IgG response toward LPS, does not result in a response to CDT. In contrast, infection by A. actinomycetemcomitans and subsequent serum IgG response to A. actinomycetemcomitans serotypes in generalized aggressive periodontitis subjects seemed to be associated with a response to CdtC. These data suggested that CDT may be expressed differently by strains of A. actinomycetemcomitans infecting generalized and localized aggressive periodontitis subjects. Alternatively, the antibody response to this antigen may require the colonization of multiple sites for a prolonged time, as seen in the generalized form of the disease.
Acknowledgments
This work was supported by FAPESP grants 03/03318-0 and 03/08598-0 and an USPHS grant, DE012593 (J.M.D.), from the National Institutes of Health. We thank Rosana Prisco for help with the statistical analysis.
References
- 1.Henderson B, Wilson M, Sharp L, Ward JM. Actinobacillus actinomycetemcomitans. J Med Microbiol. 2002;51:1013–1020. doi: 10.1099/0022-1317-51-12-1013. [DOI] [PubMed] [Google Scholar]
- 2.Fives-Taylor PM, Meyer DH, Mintz KP, Brissette C. Virulence factors of Actinobacillus actinomycetemcomitans. Periodontol 2000. 1999;20:136–167. doi: 10.1111/j.1600-0757.1999.tb00161.x. [DOI] [PubMed] [Google Scholar]
- 3.Wang D, Kawashima Y, Nagasawa T, et al. Elevated serum IgG titer and avidity to Actinobacillus actinomycetemcomitans serotype c in Japanese periodontitis patients. Oral Microbiol Immunol. 2005;20:172–179. doi: 10.1111/j.1399-302X.2005.00208.x. [DOI] [PubMed] [Google Scholar]
- 4.Rylev M, Kilian M. Prevalence and distribution of principal periodontal pathogens worldwide. J Clin Periodontol. 2008;8(Suppl):346–361. doi: 10.1111/j.1600-051X.2008.01280.x. [DOI] [PubMed] [Google Scholar]
- 5.Mayer MP, Bueno LC, Hansen EJ, Di-Rienzo JM. Identification of a cytolethal distending toxin gene locus and features of a virulence-associated region in Actinobacillus actinomycetemcomitans. Infect Immun. 1999;67:1227–1237. doi: 10.1128/iai.67.3.1227-1237.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Smith JL, Bayles DO. The contribution of cytolethal distending toxin to bacterial pathogenesis. Crit Rev Microbiol. 2006;32:227–248. doi: 10.1080/10408410601023557. [DOI] [PubMed] [Google Scholar]
- 7.Fernandes KP, Mayer MP, Ando ES, Ulbrich AG, Amarente-Mendes JG, Russo M. Inhibition of interferon-gammainduced nitric oxide production in endotoxin- activated macrophages by cytolethal distending toxin. Oral Microbiol Immunol. 2008;23:360–366. doi: 10.1111/j.1399-302X.2008.00434.x. [DOI] [PubMed] [Google Scholar]
- 8.Pratt JS, Sachen KL, Wood HD, Eaton KA, Young VB. Modulation of host immune responses by the cytolethal distending toxin of Helicobacter hepaticus. Infect Immun. 2006;74:4496–4504. doi: 10.1128/IAI.00503-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Akifusa S, Poole S, Lewthwaite J, Henderson B, Nair SP. Recombinant Actinobacillus actinomycetemcomitans cytolethal distending toxin proteins are required to interact to inhibit human cell cycle progression and to stimulate human leukocyte cytokine synthesis. Infect Immun. 2001;69:5925–5930. doi: 10.1128/IAI.69.9.5925-5930.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xu T, Lundqvist A, Ahmed HJ, Eriksson K, Yang Y, Lagergard T. Interactions of Haemophilus ducreyi and purified cytolethal distending toxin with human monocyte-derived dendritic cells, macrophages and CD4 + T cells. Microbes Infect. 2004;6:1171–1181. doi: 10.1016/j.micinf.2004.07.003. [DOI] [PubMed] [Google Scholar]
- 11.Lewis DA, Stevens MK, Latimer JL, et al. Characterization of Haemophilus ducreyi cdtA, cdtB, and cdtC mutants in in vitro and in vivo systems. Infect Immun. 2001;69:5626–5634. doi: 10.1128/IAI.69.9.5626-5634.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fox JG, Rogers AB, Whary MT, et al. Gastroenteritis in NF-kappaB-deficient mice is produced with wild-type Camplyobacter jejuni but not with C. jejuni lacking cytolethal distending toxin despite persistent colonization with both strains. Infect Immun. 2004;72:1116–1125. doi: 10.1128/IAI.72.2.1116-1125.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Purdy D, Buswell CM, Hodgson AE, McAlpine K, Henderson I, Leach SA. Characterization of cytolethal distending toxin (CDT) mutants of Campylobacter jejuni. J Med Microbiol. 2000;49:473–479. doi: 10.1099/0022-1317-49-5-473. [DOI] [PubMed] [Google Scholar]
- 14.Mbwana J, Ahmed HJ, Ahlman K, et al. Specificity of antibodies directed against the cytolethal distending toxin of Haemophilus ducreyi in patients with chancroid. Microb Pathog. 2003;35:133–137. doi: 10.1016/s0882-4010(03)00111-6. [DOI] [PubMed] [Google Scholar]
- 15.Xynogala I, Volgina A, DiRienzo JM, Korostoff J. Evaluation of the humoral immune response to the cytolethal distending toxin of Aggregatibacter actinomycetemcomitans Y4 in subjects with localized aggressive periodontitis. Oral Microbiol Immunol. 2009;24:116–123. doi: 10.1111/j.1399-302X.2008.00483.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Armitage GC. Development of a classification system for periodontal diseases and conditions. Ann Periodontol. 1999;4:1–6. doi: 10.1902/annals.1999.4.1.1. [DOI] [PubMed] [Google Scholar]
- 17.Faveri M, Mayer MP, Feres M, de Figueiredo LC, Dewhirst FE, Paster BJ. Microbiological diversity of generalized aggressive periodontitis by 16S rRNA clonal analysis. Oral Microbiol Immunol. 2008;23:112–118. doi: 10.1111/j.1399-302X.2007.00397.x. [DOI] [PubMed] [Google Scholar]
- 18.Mao X, DiRienzo JM. Functional studies of the recombinant subunits of a cytolethal distending holotoxin. Cell Microbiol. 2002;4:245–255. doi: 10.1046/j.1462-5822.2002.00186.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680– 685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 20.Ebersole JL, Frey DE, Taubman MA, Smith DJ. An ELISA for measuring serum antibodies to Actinobacillus actinomycetemcomitans. J Periodontal Res. 1980;15:621–632. doi: 10.1111/j.1600-0765.1980.tb00321.x. [DOI] [PubMed] [Google Scholar]
- 21.Deshpande SS. Assay Development, Evaluation, and Validation. In: Deshpande SS, editor. Enzyme Immunoassays from Concept to Product Development. New York: Chapman & Hall; 1996. pp. 343–349. [Google Scholar]
- 22.Faveri M, Figueiredo LC, Duarte PM, Mestnik MJ, Mayer MPA, Feres M. Microbiological profile of untreated subjects with localized aggressive periodontitis. J Clin Periodontol. 2009;36:739–749. doi: 10.1111/j.1600-051X.2009.01449.x. [DOI] [PubMed] [Google Scholar]
- 23.Vilkuna-Rautiainen T, Pussinen PJ, Mattila K, et al. Antigenically diverse reference strains and autologous strains of Actinobacillus actinomycetemcomitans are equally efficient antigens in enzyme-linked immunosorbent assay analysis. J Clin Microbiol. 2002;40:4640–4645. doi: 10.1128/JCM.40.12.4640-4645.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gmur R, Baehni PC. Serum immunoglobulin G responses to various Actinobacillus actinomycetemcomitans serotypes in a young ethnographically heterogeneous periodontitis patient group. Oral Microbiol Immunol. 1997;12:1–10. doi: 10.1111/j.1399-302x.1997.tb00360.x. [DOI] [PubMed] [Google Scholar]
- 25.Tinoco EM, Lyngstadaas SP, Preus HR, Gjermo P. Attachment loss and serum antibody levels against autologous and reference strains of Actinobacillus actinomycetemcomitans in untreated localized juvenile periodontitis patients. J Clin Periodontol. 1997;24:937–944. doi: 10.1111/j.1600-051x.1997.tb01215.x. [DOI] [PubMed] [Google Scholar]
- 26.Kawamoto D, Ando ES, Longo PL, Nunes ACR, Wikstrom M, Mayer MPA. Genetic diversity and toxic activity of Aggregatibacter actinomycetemcomitans isolates. Oral Microbiol Immunol. 2009;24:493–501. doi: 10.1111/j.1399-302X.2009.00547.x. [DOI] [PubMed] [Google Scholar]
- 27.Albandar JM, DeNardin AM, Adesanya MR, Diehl SR, Winn DM. Associations between serum antibody levels to periodontal pathogens and early-onset periodontitis. J Periodontol. 2001;72:1463–1469. doi: 10.1902/jop.2001.72.11.1463. [DOI] [PubMed] [Google Scholar]
- 28.Baker PJ, Wilson ME. Opsonic IgG antibody against Actinobacillus actinomycetemcomitans in localized juvenile periodontitis. Oral Microbiol Immunol. 1989;4:98–105. doi: 10.1111/j.1399-302x.1989.tb00106.x. [DOI] [PubMed] [Google Scholar]
- 29.Wilson ME, Hamilton RG. Immunoglobulin G subclass response of juvenile periodontitis subjects to principal outer membrane proteins of Actinobacillus actinomycetemcomitans. Infect Immun. 1995;63:1062–1069. doi: 10.1128/iai.63.3.1062-1069.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tsai CC, McArthur WP, Baehni PC, Evian C, Genco RJ, Taichman NS. Serum neutralizing activity against Actinobacillus actinomycetemcomitans leukotoxin in juvenile periodontitis. J Clin Periodontol. 1981;8:338–348. doi: 10.1111/j.1600-051x.1981.tb02043.x. [DOI] [PubMed] [Google Scholar]
- 31.Meyer DH, Mintz KP, Fives-Taylor PM. Models of invasion of enteric and periodontal pathogens into epithelial cells: a comparative analysis. Crit Rev Oral Biol Med. 1997;8:389–409. doi: 10.1177/10454411970080040301. [DOI] [PubMed] [Google Scholar]
- 32.Purven M, Frisk A, Lonnroth I, Lagergard T. Purification and identification of Haemophilus ducreyi cytotoxin by use of a neutralizing monoclonal antibody. Infect Immun. 1997;65:3496–3499. doi: 10.1128/iai.65.8.3496-3499.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lagergard T, Purven M. Neutralizing antibodies to Haemophilus ducreyi cytotoxin. Infect Immun. 1997;61:1589–1592. doi: 10.1128/iai.61.4.1589-1592.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tan KS, Song KP, Ong G. Cytolethal distending toxin of Actinobacillus actinomycetemcomitans Occurrence and association with periodontal disease. J Periodontal Res. 2002;37:268–272. doi: 10.1034/j.1600-0765.2002.01618.x. [DOI] [PubMed] [Google Scholar]
- 35.Fabris AS, DiRienzo JM, Wikstrom M, Mayer MP. Detection of cytolethal distending toxin activity and cdt genes in Actinobacillus actinomycetemcomitans isolates from geographically diverse populations. Oral Microbiol Immunol. 2002;17:231–238. doi: 10.1034/j.1399-302x.2002.170405.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yamano R, Ohara M, Nishikubo S, et al. Prevalence of cytolethal distending toxin production in periodontopathogenic bacteria. J Clin Microbiol. 2003;41:1391–1398. doi: 10.1128/JCM.41.4.1391-1398.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Belibasakis GN, Brage M, Lagergard T, Johansson A. Cytolethal distending toxin upregulates RANKL expression in Jurkat T-cells. APMIS. 2008;116:499–506. doi: 10.1111/j.1600-0463.2008.01017.x. [DOI] [PubMed] [Google Scholar]
- 38.Johansson A, Buhlin K, Koski R, Gustafsson A. The immunoreactivity of systemic antibodies to Actinobacillus actinomycetemcomitans and Porphyromonas gingivalis in adult periodontitis. Eur J Oral Sci. 2005;113:197–202. doi: 10.1111/j.1600-0722.2005.00218.x. [DOI] [PubMed] [Google Scholar]
- 39.Komatsuzawa H, Kawai T, Wilson ME, Taubman MA, Sugai M, Suginaka H. Cloning of the gene enconding the Actinobacillus actinomycetemcomitans serotype b OmpA-like outer membrane protein. Infect Immun. 1999;67:942–945. doi: 10.1128/iai.67.2.942-945.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]