Abstract
Objectives
Identifying common clinical and environmental factors that influence newborn metabolic biomarkers will improve the utilization of metabolite panels for clinical diagnostic medicine.
Design and Methods
Environmental effects including gender, season of birth, gestational age, birth weight, feeding method and age at time of collection were evaluated for over 50 metabolites collected by the Iowa Neonatal Metabolic Screening Program on 221,788 newborns over a six year period.
Results
We replicated well known observations that low birth weight and preterm infants have higher essential amino acids and lower medium and long chain acylcarnitine levels than their term counterparts. Smaller, but still significant, differences were observed for gender and timing of sample collection, specifically the season in which the infant was born. Most intriguing were our findings of higher thyroid stimulating hormone in the winter months (P<1×10−40) which correlated with an increased false positive rate of congenital hypothyroidism in the winter (0.9%) compared to summer (0.6%). Previous studies, conducted globally, have identified an increased prevalence of suspected and confirmed cases of congenital hypothyroidism in the winter months. We found that the percentage of unresolved suspected cases were slightly higher in the winter (0.3% vs 0.2%).
Conclusions
We identified differences in metabolites by gestational age, birth weight, gender and season. Some are widely reported such as gestational age and birth weight, while others such as the effect of seasonality are not as well studied.
Keywords: newborn screening, 17-hydroxyprogesterone, amino acids
Introduction
Newborn screening (NBS) is an extremely successful public health program for the detection of numerous, often rare, disorders at birth. A dried blood spot card (DBS) is used to screen for over 40 disorders by quantitatively measuring amino acids, acylcarnitines and various hormones. Approximately four million newborns are screened each year in the United States and as many as 3,000 are identified with a severe medical condition [1]. These neonates benefit from early detection and treatment of disorders that otherwise would have caused severe morbidities or death. Although the sensitivity of the tests used in NBS is high, many of the metabolites and enzymes have a broad distribution of concentrations among newborns, particularly those born sick or preterm that impacts false positive rates and potentially false negative rates [2-6]. For example a study of the national records for phenylketonuria (PKU), galactosemia (GAL), biotinidase deficiency, congenital hypothyroidism (CH) and congenital adrenal hyperplasia (CAH) found that for every reported true-positive there were approximately fifty reported false positives [7]. The comprehensive panel of metabolites obtained from the DBS may also be essential in the neonatal intensive care unit for monitoring, diagnosing and treating common complex conditions as they develop [8-10]. There are several considerations for the use of dried blood spots for monitoring more subtle changes in newborn metabolic biomarkers including the strong effect of blood volume and hematocrit on the precision and accuracy of measurements [11]. However, the benefits including the small amount of biological material needed, which is particularly important in small and sick infants makes the use of DBS for diagnostic and clinical monitoring, in addition to NBS, appealing.
In addition to blood volume and hematocrit, other factors such as gestational age, birth weight and gender are observed to influence NBS analytes such as thyroid stimulating hormone (TSH) the biomarker for CH, 17-hydroxyprogesterone (17-OHP) the biomarker for CAH and acylcarnitines that serve as biomarkers for rare fatty acid oxidation disorders and organic acidemias [12-16]. However, there has not been a systematic examination of the effect of routine screening procedures and demographic factors on metabolomic-scale analyte concentrations obtained through newborn screening. We analyzed common demographic and environmental factors that potentially influence newborn metabolic biomarkers at time of routine newborn screening. Identifying factors that influence newborn metabolites may be important not only for improving newborn screening programs but also for applying metabolic profiling for monitoring or diagnosing common complex conditions as they develop in the NICU [11].
Materials and Methods
Study Population
We present data from the Iowa Neonatal Metabolic Screening Program (INMSP) on 2 enzymes (immunoreactive trypsinogen – IRT, galactose-1-phosphate uridyl transferase – GALT), 2 hormones (thyroid stimulating hormone – TSH, 17-hydroxyprogesterone – 17-OHP), 14 amino acids, 36 acylcarnitines and 23 ratios (Supplemental Table 1). Dried blood spot specimens were collected, dried and handled according to the Clinical Laboratory Standards Institute (CLSI) guideline [17]. Quantification of 17-OHP, TSH and IRT were determined by solid phase, time-resolved fluoroimmunoassay from dried newborn blood spots using PerkinElmer’s AutoDELFIA® platform (Waltham, MA, USA). Galactose-1-phosphate uridyl transferase (GALT) was determined by a semi-quantitative enzymatic assay by PerkinElmer (Waltham, MA, USA) based on the Beutler method. Tandem mass spectrometry is performed with Waters Quattro Micro triple quadrupole tandem mass spectrometers, equipped with an electrospray ionization source operated in the positive ion mode Screening procedures in Iowa are based on previously established methodology [18,19]. Briefly, a derivatization method is used in which butyl esters of acylcarnitines and amino acids are prepared from the extracts. Multiple reaction monitoring (MRM) mode is used to scan for specific mass ion intensities. Concentrations are obtained from the ratio of ion intensity at the mass that represents a specific analyte compared to its isotopically labeled internal standard and correcting for blood volume in a 1/8 inch DBS punch. Both internal and external spiked control specimens, a normal control specimen, and a blank are analyzed with each batch of specimens. The external spiked control specimens are obtained from the Newborn Screening Quality Assurance Program at the Centers for Disease Control.
The intra-assay imprecision, based on manufacturer’s guidelines, for TSH and 17-OHP is ~6% (PerkinElmer), for IRT is ~9% (PerkinElmer) and, in general, is 20% for the metabolites measured by tandem mass spectrometry [11]. Infant demographics and clinical data were obtained from the NBS requisition form and included gestational age, weight at time of sample collection (which is a close approximation of birth weight), gender, timing of sample collection including age at time of sample collection and month in which the sample was collected and feeding method. Multiple gestation births were identified by the State Hygienic Laboratory (SHL) based on infants having the same birth date, gestational age, mother’s first name and facility identification number. Approval for use of the de-identified data was obtained from the Iowa Department of Public Health. The data were de-identified by the SHL and provided for this study after a waiver of consent was obtained from the Institutional Review Board at the University of Iowa (IRB#200908793). Data for this study include measurements and demographics on 238,809 infants that received their initial NBS between 2004 and 2009. Exclusion criteria included infants that received a transfusion prior to collection, those with a poor quality specimen and specimens not collected between 24 and 72 hours after birth. Additionally, only infants with complete gestational age and birth weight information that were born between 24 and 42 weeks gestation and were between 500 and 6,000 grams were considered for analysis. A total of 15,621 subjects (6.5%) did not meet inclusion criteria. Extreme discrepancies between gestational age and birth weight were considered as potential database entry errors. Therefore, an additional 1,400 infants (0.63%) were excluded because their birth weight was greater or less than three standard deviations from the mean for each gestational age week. A total of 221,788 infants were included in the analysis.
Statistical Analysis
Differences in relative analyte and ratio concentrations were individually evaluated by gestational age, birth weight, gender, age at time of sample collection, season, twin status, feeding method and total parenteral nutrition (TPN) status. Analyte measurements were transformed using the Box Cox transformation [20], briefly, this algorithm uses maximum-likelihood estimates to transform the original data to improve correlations for variables that are not normally distributed. Several of the analytes were not screened past 2005 (GLY, 5-OXOPRO, ORN, C8-DC, C10-DC, C10:2). SUAC screening did not begin until 2009, screening for C4-OH began midway through 2005 and IRT screening began in 2006. The remaining analytes were screened for all 6 years of the study period (2004-2009). Several analytes including, ASA, C14:2, C14-OH, C16:1-OH, C16-OH, C18:1-OH, C18:2-OH, C18-OH, had low variability (standard deviation≤ 0.01umol/L) and are excluded from the tables, results and discussion. Ratios C4/C0, C5/C2, C5-DC/C16 and C16-OH/C16 also had low variability. These results were excluded as they likely arose due to the large sample size that increases the power to detect extremely small effect size differences. As there is little to no variability for these analytes the results are difficult to interpret biologically and clinically. Results for the analyte ratios are presented only in the supplemental material as these were secondary markers for this study. Multiple statistical modeling methods were performed and few differences between these methods were observed (Supplemental Table 2). A single model including the following categorical covariates was evaluated with analysis of variance (ANOVA): gestational age (37-42 weeks; 32-<37 weeks; 24-<32 weeks), birth weight (>=2,250 g; 1,750-<2,250 g; 1,250-<1,750 g; <1,250 g), gender, age at time of sample collection (24-36 hours; >36-48 hours; >48-60 hours; >60-72 hours), season (spring: March-May; summer: June-August; fall: September-November; winter: December-February), multiple gestations (singleton; multiple), feeding method (breast; bottle; breast and bottle; nothing by mouth: NPO), total parenteral nutrition (TPN) status, year of sample collection and specific assay lot. The birth weight categories were chosen based on the four weight classes used in Iowa to adjust 17-OHP measurements when reporting positive screens for CAH. Each ANOVA model was examined for outliers using standardized residuals and measurements that were < −3.5 or > 3.5 were removed. A Bonferroni threshold of P < 8×10−5 was used to correct for 616 tests (77 analytes × 8 primary covariates). While we did not have access to follow-up testing in the genetics clinics for presumptive positive findings, we did have additional repeat NBS screens, if taken. From this we were able to identify initial positive results that were subsequently resolved with re-sampling of the infant at a later time point and a repeat of the screening test; however since not all repeat screens were resolved and we have no information on additional follow-up testing, we were not able to identify true cases of a given disease. All analyses were performed in STATA version 12.0 (College Station, Texas).
Results
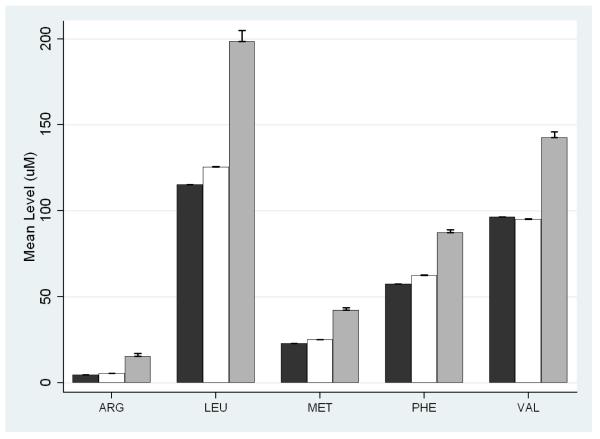
Study demographics are presented in Table 1. As expected the majority of infants (91.1%) were born term (>37 weeks) with a birth weight ≥2,250 grams. Most samples were obtained between 24 and 48 hours after birth. The majority of analytes significantly differed by gestational age and birth weight (Supplemental Table 3). The most significant difference (P<1×10−40) was higher 17-OHP in very preterm (24-32 weeks) neonates compared to term (>37 weeks) (Table 2). This corresponded to a false positive rate of 4.1% in infants born 32-36 weeks and 8.5% in infants born <32 weeks compared to 0.1% in infants born at term (Table 2). Similar trends were observed for birth weight, which is of note, as the State of Iowa laboratory has set distinct thresholds for 17-OHP as a function of birth weight in order to reduce the false positive rate (Table 2). Other notable differences included amino acids, arginine (ARG), leucine (LEU), methionine (MET), phenylalanine (PHE) and valine (VAL), that were significantly (P<1×10−40) higher in neonates born preterm (Figure 1 and Supplemental Table 4). In infants not on TPN at time of screening, gestational age contributed significantly to the false positive rate of 9.9% and 0.9% in very preterm (24-32 weeks) and preterm (32-36 weeks) infants, respectively, compared to 0.2% in term infants (Table 2).
Table 1.
Demographic Characteristics of the Study Cohort.
| Variable | No. Obs. | % |
|---|---|---|
| Birth Weight | ||
| >=2250 | 213,888 | 96.44 |
| 1750-<2250 | 5,199 | 2.34 |
| 1250-<1750 | 1,692 | 0.76 |
| <1250 | 1,009 | 0.45 |
| Gestational Age | ||
| 37-42 | 203,171 | 91.61 |
| 32-<37 | 16,756 | 7.55 |
| 22-<32 | 1,861 | 0.84 |
| Gender | ||
| Female | 108,030 | 48.71 |
| Male | 112,829 | 50.87 |
| Unknown | 929 | 0.42 |
| Twin Status | ||
| singleton | 217,118 | 98.16 |
| twin | 3,909 | 1.76 |
| triplet | 157 | 0.07 |
| quadruple | 4 | <0.01 |
| Feeding Method | ||
| Bottle | 60,482 | 27.27 |
| Breastfeed | 128,180 | 57.79 |
| Bottle and Breast | 23,653 | 10.66 |
| NPO | 4,520 | 2.04 |
| Unknown | 4,953 | 2.23 |
| TPN | ||
| No | 219,287 | 98.87 |
| Yes | 2,501 | 1.13 |
| Time of Collection | ||
| 24-36 | 136,405 | 61.50 |
| >36-48 | 60,901 | 27.46 |
| >48-60 | 15,399 | 6.94 |
| >60-72 | 9,083 | 4.10 |
| Season of Sample Collection | ||
| winter | 52,858 | 23.83 |
| spring | 55,844 | 25.18 |
| summer | 57,738 | 26.03 |
| fall | 55,348 | 24.96 |
Table 2.
Analysis of False Positive Rate and Demographic Covariates
| Normal | Resolved – False Positive | # Unresolved |
||||
|---|---|---|---|---|---|---|
| Analyte | Variable | Mean (1-99%tile) | # (%) | Mean (1-99%tile) | # (%) | |
| 17-OHP1 | Gestational Age | |||||
| 24-32 weeks | 68.7 (11-182.5) | 1,695 (91.1.8%) | 188.4 (54.5-230) | 158 (8.5%) | 8 (0.4%) | |
| 32-36 weeks | 28.1 (6-105) | 16,037 (95.7%) | 102.5 (46.5-207) | 680 (4.1%) | 38 (0.2%) | |
| 37-42 weeks | 14.7 (5-43) | 202,850 (99.8%) | 89.4 (39-185) | 277 (0.1%) | 38 (0.02%) | |
| Birth Weight | ||||||
| <=1,250 grams | 74.6 (12.5-187) | 900 (89.2%) | 202.7 (137.5-230) | 103 (10.2%) | 6 (0.6%) | |
| 1,250g-1749.9 | 51.4 (7-146.5) | 1,633 (96.5%) | 167.7 (90.5-230) | 57 (3.4%) | 2 (0.1%) | |
| 1,750g-2,249.9 | 32.7 (6-124) | 5,116 (98.4%) | 132.5 (59.5-230) | 81 (1.6%) | 2 (0.04%) | |
| >=2,250 grams | 15.2 (5-48.5) | 212,933 (99.6%) | 95.0 (44-195.5) | 874 (0.4%) | 74 (0.03%) | |
| Gender | ||||||
| Male | 17.2 (5-65.5) | 112,062 (99.3%) | 110.2 (50-230) | 711 (0.63%) | 54 (0.05%) | |
| Female | 14.9 (5-61.5) | 107,596 (99.6%) | 113.6 (44-230) | 402 (0.37%) | 27 (0.02%) | |
| MS/MS2 | Gestational Age | |||||
| PHE | 24-32 weeks | 73.2 (38.7-125.7) | 1,139 (89.8%) | 137.4 (57.3-415.2) | 126 (9.9%) | 4 (0.3%) |
| 32-36 weeks | 60.2 (37.1-100.6) | 15,302 (98.9%) | 115.3 (43.4-428.8) | 144 (0.9%) | 33 (0.2%) | |
| 37-42 weeks | 57.3 (37.7-88.9) | 202,015 (99.8%) | 84.1 (41.5-423.1) | 337 (0.2%) | 171 (0.1%) | |
| C0 | Gender | |||||
| Male | 20.2 (8.7-43.1) | 110,934 (99.6%) | 28.1 (9.7-78.7) | 343 (0.31%) | 122 (0.11%) | |
| Female | 18.4 (8.1-38.9) | 106,599 (99.7%) | 24.4 (4.5-83.4) | 264 (0.25%) | 86 (0.08%) | |
| C4 | Gender | |||||
| Male | 0.25 (0.09-0.71) | 110,934 (99.6%) | 0.42 (0.12-1.6) | 343 (0.31%) | 122 (0.11%) | |
| Female | 0.26 (0.09-0.71) | 106,599 (99.7%) | 0.46 (0.10-1.9) | 264 (0.25%) | 86 (0.08%) | |
| C16 | Gender | |||||
| Male | 3.1 (1.3-5.9) | 110,934 (99.6%) | 3.3 (0.63-9.1) | 343 (0.31%) | 122 (0.11%) | |
| Female | 2.9 (1.2-5.5) | 106,599 (99.7%) | 2.8 (0.64-7.3) | 264 (0.25%) | 86 (0.08%) | |
| C12 | Gender | |||||
| Male | 0.27 (0.08-0.77) | 110,934 (99.6%) | 0.31 (0.05-1.4) | 343 (0.31%) | 122 (0.11%) | |
| Female | 0.25 (0.08-0.70) | 106,599 (99.7%) | 0.27 (0.05-0.98) | 264 (0.25%) | 86 (0.08%) | |
| TSH | Season | |||||
| Winter (Dec-Feb) | 8.3 (2.2-22.1) | 52,215 (98.8%) | 28.4 (25-53.3) | 464 (0.9%) | 175 (0.3%) | |
| Spring (Mar-May) | 8.0 (2.2-21.5) | 55,389 (99.2%) | 28.1 (25-52.1) | 326 (0.6%) | 128 (0.2%) | |
| Summer (Jun-Aug) | 7.9 (2.2-21.2) | 57,310 (99.3%) | 27.9 (25-46.4) | 329 (0.6%) | 94 (0.2%) | |
| Fall (Sep-Nov) | 8.1 (2.2-21.8) | 54,830 (99.1%) | 27.9 (25-52.6) | 368 (0.7%) | 145 (0.3%) | |
threshold values for an abnormal test result for 17-OHP are based on birth weight thresholds and changed in 2007.
False positive results are grouped for all disorders tested on MS/MS including fatty acid, amino acid and organic acid disorders.
Figure 1.
Mean concentrations of the essential amino acids, arginine (ARG), leucine (LEU), methionine (MET), phenylalanine (PHE) and valine (VAL) by gestational age. Black bars represent the mean concentration for gestational age ≥ 37 weeks, white bars represent gestational ages 33-36 and gray bars represent gestational ages 24-32 weeks. Standard errors of the mean are given above each bar.
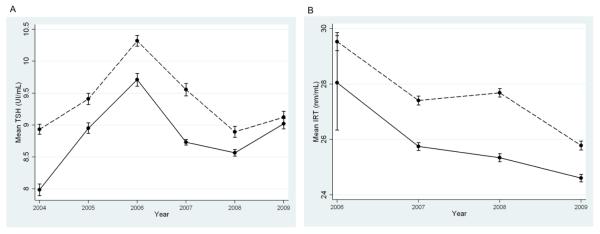
There were many significant differences for gender, age at time of sample collection, feeding method, total parenteral nutrition, multiple gestations and season of birth; however, most of these while significant after correction for multiple testing, were of extremely small effect (Supplemental Table 3). For example, free carnitine (C0) and long chain acylcarnitines (C12, C12:1, C14, C14:1, C16, C16:1, C18, C18:1, C18:2) were lower in females compared to males (Supplemental Table 4). Most had very small differences between genders (i.e. C12) while others had larger effects (i.e. C0, C16) (Table 2). This is in contrast to short and medium chain acylcarnitines biomarkers (C4, C5, C5-DC, C6, C8, C10, C10:1) where some analytes were higher in females compared to males (i.e. C4) and all were of extremely small effect (Table 2 and Supplemental Table 4). There were also several notable differences including higher levels of 17-OHP in males compared to females (17.2 vs 14.9 ng/mL, respectively, P<1×10−40). This correlated with more false positive results in males (0.63% compared to 0.37% in females; Table 2). There were significant differences in TSH and IRT by season of birth, both of which were higher in the winter months (Figure 2). Mean concentrations of TSH and IRT fluctuated each year; however, the difference in the mean values between the summer and winter seasons remained relatively constant. There was an increased false positive rate in the winter (0.9%) compared to the summer (0.6%).
Figure 2.
Mean concentrations of thyroid stimulating hormone (TSH) (A) and immunoreactive trypsinogen (IRT) (B) by year. Dotted lines represent the means for winter months (Dec-Feb) and solid lines represent summer months (Jun-Aug). Standard errors are given for each mean.
Discussion
It is well known that term and preterm infants have different metabolic profiles [21]; this has largely been attributed to fetal stress, sickness or immature kidney, liver and adrenal function. However, several studies have suggested this difference is mostly a result of intravenous nutrition [22,23]. We accounted for total parenteral nutrition (TPN), as it is recorded on the newborn screening card, and still found significant differences in the concentration and false positive rate for most of the amino acid metabolites. Unrecorded TPN may nonetheless have remained as a strong driving force in the large differences observed for the very preterm (<32 weeks) and very low birth weight infants. However, there was still a significant difference in the concentration and false positive rate for late preterm infants, where TPN is less common. This indicates there are still underlying metabolic differences even in the late preterm infants compared to their term counterparts that are unrelated to TPN.
In addition to the differences in amino acid concentrations, 17-hydroxyprogesterone (17-OHP) was also extremely elevated in preterm and very preterm infants. 17-OHP is the biomarker for congenital adrenal hyperplasia (CAH) a rare (1:10-18,000 infants) disorder of the adrenal gland. CAH can cause virilization and in extreme cases a salt wasting crisis can occur which if left untreated can be fatal. In Iowa there are four different thresholds based on birth weight ranges (<1,250 g, 1,250-<1,750 g, 1,750-<2,250 g and >=2,250 g) for identifying abnormal CAH results. We observed that even with the adjusted thresholds 17-OHP was higher in very preterm and preterm infants and this resulted in higher false positive rates. Several studies have suggested that thresholds based on either gestational age or algorithms that account for both gestational age and birth weight would result in a more accurate screening measurement in preterm infants [24,25]. Our study supports that conclusion as even with the current thresholds adjusted for birth weight, gestational age is still contributing significantly to the false positive rate of the CAH test.
In addition to gestational age and birth weight we also observed higher 17-OHP in males compared to females which replicates previous findings [15]. This contributes to the slightly higher false positive rate in males (0.63%) compared to females (0.37%) for the CAH test. There are several reasons for this observation. Most likely the 2 ng/mL lower average 17-OHP level observed in females for our study and others [15] prevents females from reaching the presumptive positive threshold. Another, albeit rare explanation, is that virilized female infants may be initially misidentified as males. While the difference in 17-OHP level between males and females is small it does indicate that careful consideration may need to be taken in relation to potential false negative results in females.
We also observed that free carnitine (C0) and long chain acylcarnitines (e.g. C14, C16, C18) were lower in females compared to males. This is in contrast to short-chain acylcarnitines biomarkers (e.g. C4, C5, C6, C8) where the difference was either in the opposite direction or very small. This is consistent with several studies that have described lower total carnitine in adult females compared to males [26-28]. A possible explanation is estradiol levels, which have been shown to have an inverse relationship with plasma carnitine concentrations in rats [29].
It has been previously been demonstrated that timing of sample collection can influence analyte results for NBS, to our knowledge few studies have extensively examined the relationship between seasonality and newborn metabolism. The relative concentration changes for this effect are extremely small and likely will not aid in the adjustment of analyte ranges to improve newborn screening tests. However, this finding is of interest as it indicates that either an environmental or biological mechanism is responsible for the variation in metabolite measurements related to temperature. One likely explanation for these observations is that ambient temperature is affecting the properties of specific analytes. This has been previously reported for immunoreactive trypsinogen (IRT) the biomarker for cystic fibrosis (CF) [30]. We observed the same trend as described in Wisconsin, a state that is similar in climate to Iowa, where concentrations of IRT are lower in the summer months. In Iowa temperatures range from average highs of 86°F (30°C) in July to 28°F (−2°C) in January and samples are transported by road to one central analysis site that may result in prolonged stays in unregulated temperature environments. IRT is stable stored at temperature ranges of −20°C to 4°C [31] which explains why IRT values would be lower in the summer months where IRT destabilizes due to higher ambient temperatures. In our study this did not impact the presumptive positive cystic fibrosis cases, nor did the IRT thresholds for a positive test result change during our study period, indicating that no adjustments to the NBS thresholds are needed in regards to season. Individual states may vary in this effect based on temperature and other transport characteristics if these differences are due to weather. Additionally, genetic testing of the CFTR gene is performed on infants with high IRT values and on any clinically suspected cases. We did not observe any changes in positive CFTR results by season.
Another explanation for seasonal associations with analyte concentrations include biological influences directed by environmental stimulates such as temperature which has been documented for CH [32-35]. CH is found in approximately 1 out of 4,000 newborns and results in thyroid hormone deficiency at birth. Interestingly, we observed similar trends to studies demonstrating that the incidence of suspected and confirmed cases of congenital hypothyroidism is increased in the winter. Studies that have demonstrated an increase in CH in the winter months have been conducted in Japan, Britain, Finland and Iran, indicating this is an effect that is observed globally in geographic areas with varying climates [32-36]. While permanent CH is due to genetic inheritance that is not affected by temperature, it is possible that intrauterine viral infection, seasonal influences on food products and influence of temperature, acting through exposure to seasonally-affected environmental factors, on gene expression patterns or human leukocyte antigen backgrounds contribute to this finding [32,33].
While we were able to examine several important demographic and environmental variables that may affect the metabolite patterns of newborns we were limited in relation to other relevant covariates. IIn particular, ethnic and racial information is not captured by the Iowa newborn screening program. This covariate may be important for defining analyte specific thresholds that differ due to ancestrally different genetic backgrounds. We had no information on infection or health status present at birth. It would have been informative to compare infants on TPN to those of matched illness severity to determine whether TPN is merely a marker of illness. Additionally, studies with access of true positive results is needed to determine the utility of incorporating information on gender, seasonality and gestational age into the thresholds used for newborn screening. Unfortunately, we did not have access to follow-up data as our subjects were de-identified and we were not able to link specific patient information to databases of congenital and inherited metabolic diseases. Therefore, while we are not able to assess the impact of these variables on current screening thresholds we believe that these data are still beneficial for screening programs to better inform which factors should be examined when evaluating and readjusting thresholds for newborn screening.
Supplementary Material
Acknowledgements
We would like to express our thanks to the Congenital and Inherited Disorders Advisory Committee, particularly Kim Piper for her enthusiastic support and management. We thank Sara Copeland at the Health Resources Services Administration for her guidance and support on this project. We would also like to express our gratitude to the State University Hygienic Laboratory staff including a special thanks to Dari Shirazi and Frank Delin. We would also like to thank Susie McConnell, Nancy Davin and Erin Brothers-Smith for administrative support. This work was supported by the March of Dimes (1-FY05-126 and 6-FY08-260) and the National Institute of Health (R01 HD-52953, R01 HD-57192). Dr. Ryckman’s postdoctoral fellowship and research was supported in part by a NIH/NRSA T-32 training grant (5T32 HL 007638-24) and the Eunice Kennedy Shriver National Institute of Child Health & Human Development (K99 HD-065786). The content is solely the responsibility of the authors and does not necessarily represent the official views of the Eunice Kennedy Shriver National Institute of Child Health & Human Development or the National Institutes of Health.
Funding Sources: This work was supported by the March of Dimes (1-FY05-126 and 6-FY08-260), National Institute of Health (R01 HD-52953, R01 HD-57192) and the Children’s Miracle Network through the University of Iowa (Grant #2224). Dr. Ryckman’s postdoctoral fellowship and research was supported in part by a NIH/NRSA T-32 training grant (5T32 HL 007638-24) and the Eunice Kennedy Shriver National Institute of Child Health & Human Development (K99 HD-065786). The content is solely the responsibility of the authors and does not necessarily represent the official views of the Eunice Kennedy Shriver National Institute of Child Health & Human Development or the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- [1].CDC Using Tandem Mass Spectrometry for Metabolic Disease Screening Among Newborns. MMWR. 2001;50:1–22. [PubMed] [Google Scholar]
- [2].Laroche D, Travert G. Abnormal frequency of delta F508 mutation in neonatal transitory hypertrypsinaemia. Lancet. 1991;337:55. doi: 10.1016/0140-6736(91)93377-l. [DOI] [PubMed] [Google Scholar]
- [3].Schreiner F, Brack C, Salzgeber K, Vorhoff W, Woelfle J, Gohlke B. False negative 17-hydroxyprogesterone screening in children with classical congenital adrenal hyperplasia. Eur. J. Pediatr. 2008;167:479–481. doi: 10.1007/s00431-007-0505-0. [DOI] [PubMed] [Google Scholar]
- [4].Schreiner F, Tozakidou M, Maslak R, Holtkamp U, Peter M, Gohlke B, Woelfle J. Functional glucocorticoid receptor gene variants do not underlie the high variability of 17-hydroxyprogesterone screening values in healthy newborns. Eur. J. Endocrinol. 2009;160:667–673. doi: 10.1530/EJE-08-0639. [DOI] [PubMed] [Google Scholar]
- [5].Therrell BL, Jr., Berenbaum SA, Manter-Kapanke V, Simmank J, Korman K, Prentice L, Gonzalez J, Gunn S. Results of screening 1.9 million Texas newborns for 21-hydroxylase-deficient congenital adrenal hyperplasia. Pediatrics. 1998;101:583–590. doi: 10.1542/peds.101.4.583. [DOI] [PubMed] [Google Scholar]
- [6].Votava F, Torok D, Kovacs J, Moslinger D, Baumgartner-Parzer SM, Solyom J, Pribilincova Z, Battelino T, Lebl J, Frisch H, Waldhauser F. Estimation of the false-negative rate in newborn screening for congenital adrenal hyperplasia. Eur. J. Endocrinol. 2005;152:869–874. doi: 10.1530/eje.1.01929. [DOI] [PubMed] [Google Scholar]
- [7].Kwon C, Farrell PM. The magnitude and challenge of false-positive newborn screening test results. Arch. Pediatr. Adolesc. Med. 2000;154:714–718. doi: 10.1001/archpedi.154.7.714. [DOI] [PubMed] [Google Scholar]
- [8].Atzori L, Antonucci R, Barberini L, Griffin JL, Fanos V. Metabolomics: a new tool for the neonatologist. J. Matern. Fetal. Neonatal Med. 2009;22(Suppl 3):50–53. doi: 10.1080/14767050903181500. [DOI] [PubMed] [Google Scholar]
- [9].Antonucci R, Atzori L, Barberini L, Fanos V. Metabolomics: the “new clinical chemistry” for personalized neonatal medicine. Minerva Pediatr. 2010;62:145–148. [PubMed] [Google Scholar]
- [10].Fanos V, Barberini L, Antonucci R, Atzori L. Metabolomics in neonatology and pediatrics. Clin. Biochem. 2011;44:452–454. doi: 10.1016/j.clinbiochem.2011.03.006. [DOI] [PubMed] [Google Scholar]
- [11].Chace DH, Spitzer AR. Altered metabolism and newborn screening using tandem mass spectrometry: lessons learned from the bench to bedside. Curr. Pharm. Biotechnol. 2011;12:965–975. doi: 10.2174/138920111795909104. [DOI] [PubMed] [Google Scholar]
- [12].Allen DB, Hoffman GL, Fitzpatrick P, Laessig R, Maby S, Slyper A. Improved precision of newborn screening for congenital adrenal hyperplasia using weight-adjusted criteria for 17-hydroxyprogesterone levels. J. Pediatr. 1997;130:128–133. doi: 10.1016/s0022-3476(97)70321-4. [DOI] [PubMed] [Google Scholar]
- [13].Pang S. Newborn screening for congenital adrenal hyperplasia. Pediatr. Ann. 2003;32:516–523. doi: 10.3928/0090-4481-20030801-09. [DOI] [PubMed] [Google Scholar]
- [14].Pang S, Shook MK. Current status of neonatal screening for CAH. Curr. Opin. Pediatr. 1997;9:419–423. doi: 10.1097/00008480-199708000-00018. [DOI] [PubMed] [Google Scholar]
- [15].Varness TS, Allen DB, Hoffman GL. Newborn screening for congenital adrenal hyperplasia has reduced sensitivity in girls. J. Pediatr. 2005;147:493–498. doi: 10.1016/j.jpeds.2005.04.035. [DOI] [PubMed] [Google Scholar]
- [16].Kaye CI. Committee on Genetics, Newborn Screening Fact Sheets. Pediatrics. 2006;118:e934–63. doi: 10.1542/peds.2006-1783. [DOI] [PubMed] [Google Scholar]
- [17].Hannon WH, Whitley RJ, Davin B, Fernhoff P, Halonen T, Lavochkin M, Miller J, Ojodu J, Therrell BL., Jr. Blood Collection on Filter Paper for Newborn Screening Programs; Approved Standard-Fifth Edition. Clinical and Laboratory Standards Institute document LA04-A5. 2007;27:1–13. [Google Scholar]
- [18].Chace DH, DiPerna JC, Mitchell BL, Sgroi B, Hofman LF, Naylor EW. Electrospray tandem mass spectrometry for analysis of acylcarnitines in dried postmortem blood specimens collected at autopsy from infants with unexplained cause of death. Clin. Chem. 2001;47:1166–1182. [PubMed] [Google Scholar]
- [19].Turgeon C, Magera MJ, Allard P, Tortorelli S, Gavrilov D, Oglesbee D, Raymond K, Rinaldo P, Matern D. Combined newborn screening for succinylacetone, amino acids, and acylcarnitines in dried blood spots. Clin. Chem. 2008;54:657–664. doi: 10.1373/clinchem.2007.101949. [DOI] [PubMed] [Google Scholar]
- [20].Lo K, Gottardo R. Flexible mixture modeling via the multivariate t distribution with the Box-Cox transformation: an alternative to the skew-t distribution. Stat. Comput. 2012;22:33–52. doi: 10.1007/s11222-010-9204-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Atzori L, Antonucci R, Barberini L, Locci E, Marincola FC, Scano P, Cortesi P, Agostiniani R, Defraia R, Weljie A, Gazzolo D, Lai A, Fanos V. 1H NMR-based metabolomic analysis of urine from preterm and term neonates. Front. Biosci. (Elite Ed) 2011;3:1005–1012. doi: 10.2741/e306. [DOI] [PubMed] [Google Scholar]
- [22].Clark RH, Chace DH, Spitzer AR. Pediatrix Amino Acid Study Group, Effects of two different doses of amino acid supplementation on growth and blood amino acid levels in premature neonates admitted to the neonatal intensive care unit: a randomized, controlled trial. Pediatrics. 2007;120:1286–1296. doi: 10.1542/peds.2007-0545. [DOI] [PubMed] [Google Scholar]
- [23].Kelleher AS, Clark RH, Steinbach M, Chace DH, Spitzer AR. Pediatrix Amino-Acid Study Group, The influence of amino-acid supplementation, gestational age and time on thyroxine levels in premature neonates. J. Perinatol. 2008;28:270–274. doi: 10.1038/jp.2008.5. [DOI] [PubMed] [Google Scholar]
- [24].Lee JE, Moon Y, Lee MH, Jun YH, Oh KI, Choi JW. Corrected 17-alpha-hydroxyprogesterone values adjusted by a scoring system for screening congenital adrenal hyperplasia in premature infants. Ann. Clin. Lab. Sci. 2008;38:235–240. [PubMed] [Google Scholar]
- [25].van der Kamp HJ, Oudshoorn CG, Elvers BH, van Baarle M, Otten BJ, Wit JM, Verkerk PH. Cutoff levels of 17-alpha-hydroxyprogesterone in neonatal screening for congenital adrenal hyperplasia should be based on gestational age rather than on birth weight. J. Clin. Endocrinol. Metab. 2005;90:3904–3907. doi: 10.1210/jc.2004-2136. [DOI] [PubMed] [Google Scholar]
- [26].Borum PR. Plasma carnitine compartment and red blood cell carnitine compartment of healthy adults. Am. J. Clin. Nutr. 1987;46:437–441. doi: 10.1093/ajcn/46.3.437. [DOI] [PubMed] [Google Scholar]
- [27].Harper P, Wadstrom C, Cederblad G. Carnitine measurements in liver, muscle tissue, and blood in normal subjects. Clin. Chem. 1993;39:592–599. [PubMed] [Google Scholar]
- [28].Reuter SE, Evans AM, Chace DH, Fornasini G. Determination of the reference range of endogenous plasma carnitines in healthy adults. Ann. Clin. Biochem. 2008;45:585–592. doi: 10.1258/acb.2008.008045. [DOI] [PubMed] [Google Scholar]
- [29].Borum PR. Regulation of the carnitine deficiency syndromes. In: Frenkel RA, McGarry JD, editors. Carnitine Biosynthesis, Metabolism and Functions. Academic Press; New York: 1980. pp. 115–126. [Google Scholar]
- [30].Kloosterboer M, Hoffman G, Rock M, Gershan W, Laxova A, Li Z, Farrell PM. Clarification of laboratory and clinical variables that influence cystic fibrosis newborn screening with initial analysis of immunoreactive trypsinogen. Pediatrics. 2009;123:e338–46. doi: 10.1542/peds.2008-1681. [DOI] [PubMed] [Google Scholar]
- [31].Li L, Zhou Y, Bell CJ, Earley MC, Hannon WH, Mei JV. Development and characterization of dried blood spot materials for the measurement of immunoreactive trypsinogen. J. Med. Screen. 2006;13:79–84. doi: 10.1258/096914106777589623. [DOI] [PubMed] [Google Scholar]
- [32].Aminzadeh M, Chomeili B, Riahi K, Dehdashtian M, Cheraghian B, Valavi E. Effect of temperature changes on the occurrence of congenital hypothyroidism. J. Med. Screen. 2010;17:121–124. doi: 10.1258/jms.2010.010026. [DOI] [PubMed] [Google Scholar]
- [33].Miyai K, Inaoka K, Miyagi T, Committee for Newborn and Infant Screening in Osaka (CONISO) Further studies on episodic occurrence of congenital dysgenetic hypothyroidism in Osaka, Japan. Endocr. J. 2005;52:599–603. doi: 10.1507/endocrj.52.599. [DOI] [PubMed] [Google Scholar]
- [34].Nakamizo M, Toyabe S, Asami T, Akazawa K. Seasonality in the incidence of congenital hypothyroidism in Japan. J. Paediatr. Child Health. 2005;41:390–391. doi: 10.1111/j.1440-1754.2005.00644_1.x. [DOI] [PubMed] [Google Scholar]
- [35].Virtanen M, Maenpaa J, Pikkarainen J, Pitkanen L, Perheentupa J. Aetiology of congenital hypothyroidism in Finland. Acta Paediatr. Scand. 1989;78:67–73. doi: 10.1111/j.1651-2227.1989.tb10889.x. [DOI] [PubMed] [Google Scholar]
- [36].Gu YH, Kato T, Harada S, Inomata H, Saito T, Aoki K. Seasonality in the incidence of congenital hypothyroidism in Japan: gender-specific patterns and correlation with temperature. Thyroid. 2007;17:869–874. doi: 10.1089/thy.2006.0317. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.