Abstract
ApoA-I is the major protein of plasma high-density lipoproteins (HDL), macromolecular assemblies of proteins and lipids that remove cell cholesterol and protect against atherosclerosis. HDL heterogeneity, large size (7.7–12 nm) and ability to exchange proteins have prevented high-resolution structural analysis. Low-resolution studies showed that two apoA-I molecules form an antiparallel α-helical “double belt” around an HDL particle. Atomic-resolution structure of the C-terminal truncated lipid-free Δ(185–243)apoA-I, determined recently by Mei and Atkinson, provides unprecedented new insights into HDL structure-function. It allows us to propose a molecular mechanism for the adaptation of the full-length protein to increasing lipid load during cholesterol transport. ApoA-I conformations on the small, mid-size and large HDL are proposed based on the tandem α-helical repeats and the crystal structure of Δ(185–243)apoA-I, and are validated by comparison with extensive biophysical data reported by many groups. In our models, the central half of the double belt (“constant” segment 66–184) is structurally conserved while the N- and C-terminal half (“variable” segments 1–65 and 185–243) re-arranges upon HDL growth. This includes incremental unhinging of the N-terminal bundle around two flexible regions containing G39 and G65 to elongate the belt, along with concerted swing motion of the double belt around G65-P66 and G185–G186 hinges that are aligned on various-size particles, to confer 2D surface curvature to spherical HDL. The proposed conformational ensemble integrates and improves several existing HDL models. It helps provide a structural framework necessary to understand functional interactions with over 60 other HDL-associated proteins and, ultimately, improve cardioprotective function of HDL.
Keywords: Protein conformations on various HDL, amphipathic α-helical repeats, structural models, reverse cholesterol transport, cardiovascular disease
1. Introduction to HDL structure and function in reverse cholesterol transport
High-density lipoproteins (HDL, or Good Cholesterol) are non-covalent nanoassemblies of lipids and specific proteins, termed apolipoproteins (apos), that mediate lipid transport and metabolism in plasma and protect against atherosclerosis. Human plasma HDL form discrete subclasses differing in shape (nascent discoidal or mature spherical), size (7.7–12 nm), protein and lipid composition, and functions.1,2 Efforts to improve HDL functions are aimed at developing novel therapies for cardiovascular disease to complement statins and other lipid-lowering drugs.3–5 The cardioprotective action of HDL depends on their quality but not necessarily their quantity6 and is conferred through several mechanisms. HDL remove excess cholesterol from arterial macrophages and other peripheral cells to the liver for excretion in a complex pathway termed reverse cholesterol transport (RCT).1,7–9 Moreover, HDL protect against inflammation, oxidation and thrombosis, as well as transport proteins with other biological activities.1,9,10 Understanding structural basis underlying these diverse functions is essential for developing HDL-based therapies for cardiovascular disease.3–5
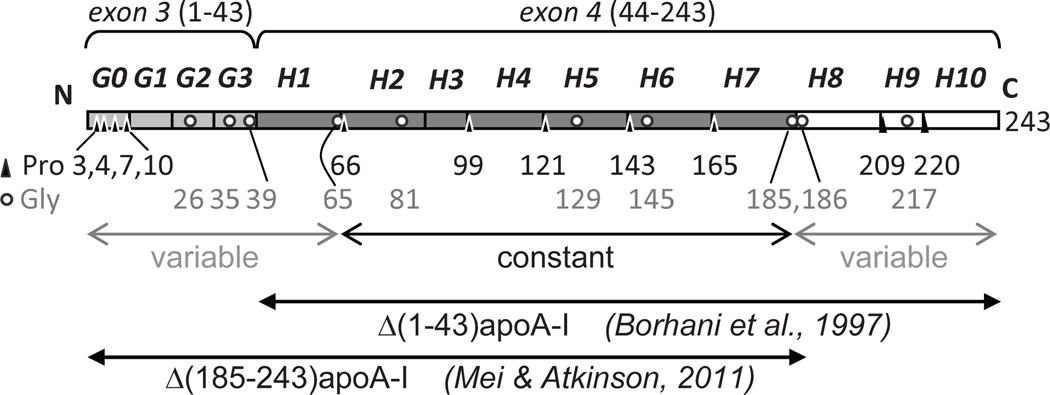
Apolipoprotein A–I (apoA-I) is a 243-residue single-chain polypeptide that comprises ~70% of the total HDL protein. ApoA-I stabilizes HDL assembly and directs its metabolism by interacting with lipophilic enzymes, lipid transport proteins, and HDL receptor.5–7 Structural plasticity of apoA-I enables it to exchange among various lipid-bound and lipid-poor or lipid-free states during RCT8 and is encoded in its amino acid sequence (Fig. 1). The sequence starts with exon 3-encoded residues 1–43, termed G-helix, subdivided into a Pro-rich G0 motif (residues 1–10) and three 10/11-mer motives G1–G3 (residues 11–43).11 Exon 4 encodes ten Pro-punctuated 11/22-mer tandem repeats, termed H1–H10 (residues 44–243), with high propensity to form class-A amphipathic α-helices that form the major lipid surface binding motif in apolipoproteins11, 12 (Fig. 1). During metabolism, apoA-I comes on and off the lipid surface and adapts its conformation to the increasing lipid load via a mechanism that is not clearly understood. Elucidation of this mechanism will help understand and, ultimately, improve the cardioprotective functions of HDL such as macrophage RCT.
Figure 1.
Sequence and structural repeats in apoA-I. Residues 1–43, encoded by exon 3, form four 10/11-mer repeats G0–G3 (light grey). Residues 44–243, encoded by exon 4, form ten 11/22-mer tandem repeats, H1–H10.41 H1–H7 are in dark grey and H8–H10 are in white. Positions of Pro (triangles) and Gly (circles) are shown by residue numbers. Segments that are proposed in the current work to have constant or variable structure in the double belts of different sizes are indicated. N- and C-terminally truncated protein fragments, Δ(1–43)apoA-I and Δ(185–243)apoA-I, whose x-ray crystal structures have been determined to 4 Å and 2.2 Å resolution, respectively,40,43 are indicated.
At the first step of RCT,1,8 apoA-I interacts with the plasma membrane and the lipid transporter ABCA14,13 to generate nascent “discoidal” HDL. These small particles are generally envisioned as a cholesterol-containing phospholipid bilayer surrounded by two copies of apoA-I (Figs 3C and 6A below), even though other models have also been proposed.* Following lecithin : cholesterol acyltransferase (LCAT) activation by apoA-I, nascent HDL acquire an apolar core of cholesterol esters and are thereby converted into mature “spheroid” particles. Continuous remodeling of these particles by LCAT and other plasma factors14 increases their lipid load and diameter, from small HDL (d~7.7 nm) containing two copies of apoA-I to large HDL (d~12 nm) containing four to five copies (Fig. 6 below). Finally, lipid-loaded HDL deliver their cargo via the preferential uptake of apolar lipids by the hepatic scavenger receptor, SR-BI ((15) and references therein). Structural details underlying functional reactions of HDL have been intensely investigated by using an array of biophysical techniques, including protein cross-linking and mass spectrometry (MS), protein labeling followed by electron paramagnetic resonance (EPR) or fluorescence resonance energy transfer (FRET), mutagenesis, antibody binding, electron microscopy, NMR, calorimetry, sequence alignment, and molecular dynamics, to name a few ((16–39) and references therein). Despite these efforts, the molecular details underlying many HDL functions remain unknown because of the paucity of the atomic-level structural information.
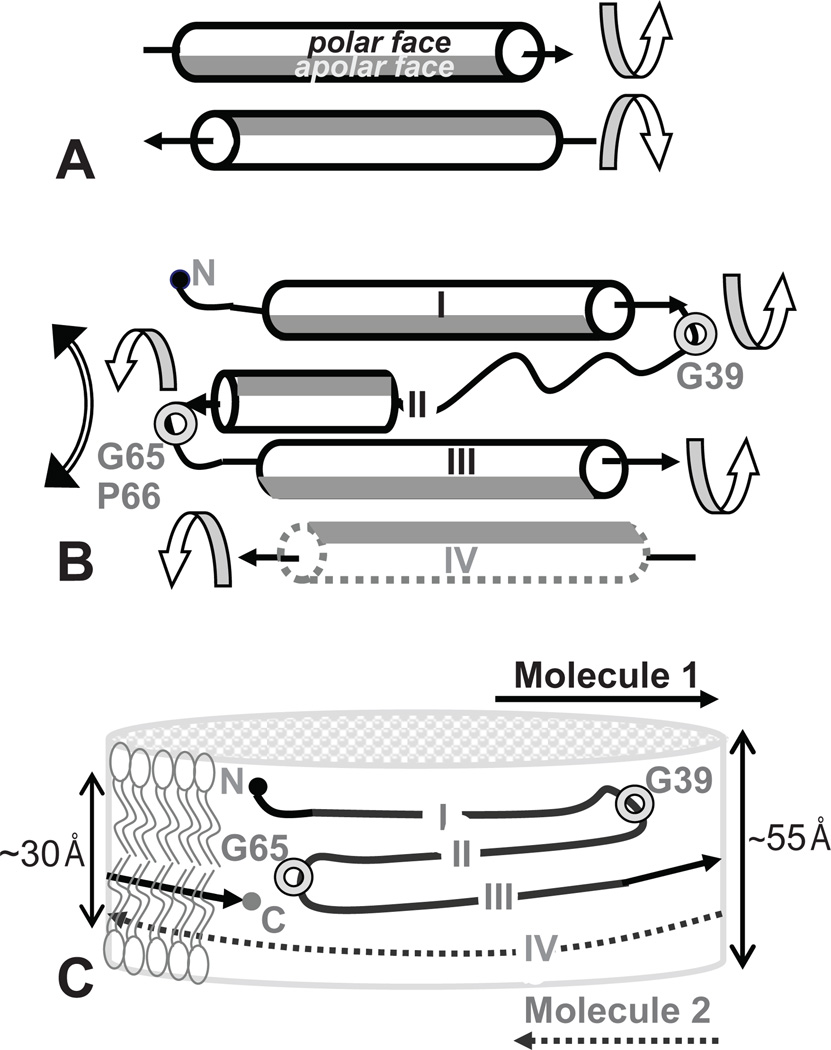
Figure 3.
Possible mechanism for the structural adaptation of apoA-I segments upon transfer from solution to the phospholipid surface at the edge of a nascent discoidal HDL. Arrows show helical orientation from the N- to the C-terminus. Apolar helical faces are in grey, polar faces are in white. The helices are viewed from the polar (aqueous) to the apolar (hydrocarbon) environment. (A) Paired amphipathic helices in the double-belt have been proposed to rotate around their axes (circular arrows) to expose their apolar faces to the lipid.45 (B) Segments I, II, III (from Molecule 1) and IV (from molecule II), which from a four-helix bundle in lipid-free C-terminal truncated apoA-I,43 rotate around two flexible linkers, one containing G39 and another G65, to separates segment pairs I–II from III–IV (circular double arrow) and expose their apolar faces to lipid. (C) Proposed arrangement of apoA-I with an N-terminal “belt buckle” around a nascent HDL. Total thickness and hydrophobic thickness (double arrows) of the idealized phospholipid bilayer are commensurate with the combined width of segments I–IV that form a four-segment “buckle” on the edge of a small disk.
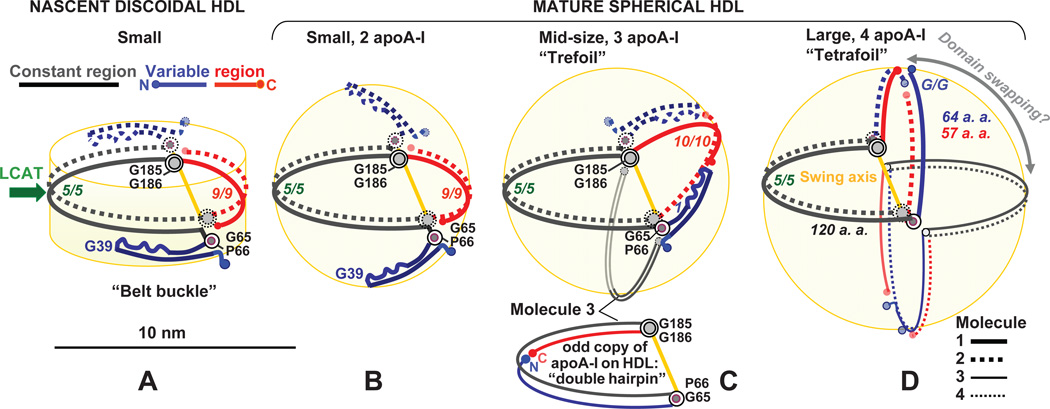
Figure 6.
Proposed conformational ensemble of apoA-I on HDL disks and spheres illustrates protein adaptation to the increasing lipid load during RCT. The two copies of apoA-I on nascent HDL form a double belt with a four-segment “buckle” (A). LCAT reaction (green arrow), which involves LL5/5 repeat pair forming the lipid presentation tunnel,48 converts nascent into small mature particles (B). HDL maturation is probably facilitated by an out-of-plane swing motion of apoA-I segment pair I–II (residues 1–65, in blue) around G65-P66-containing hinge, to confer 2D curvature to the surface of a small spherical HDL (B). Further remodeling by LCAT and other plasma factors converts small HDL into mid-size (C) and/or large spherical particles (D). We posit that on the mid-size spherical HDL (C), the “variable” region of the double belt rotates around G65-P66 and swings out of plane of the constant region around the axis connecting G65-P66 and G185–186 (yellow) to confer 2D surface curvature. The opposite hemisphere can accommodate the third apoA-I molecule, probably in a double-hairpin conformation (C, bottom). (D) Large HDL containing four-to-five copies of apoA-I can form upon remodeling and fusion of small and/or mid-size particles. Four apoA-I molecules are shown as two double belts bent around the swing axis connecting G65-P66 and G185–G186, with relative proximity of variable and constant (D) or constant-constant and variable-variable regions (not shown) from different dimers. Alternatively, these molecules can form one double belt and two double hairpins (not shown). Domain swapping among the variable and constant regions (circular double arrow) may be facilitated via the rotation around the swing axis (yellow).
High-resolution structural analysis of HDL has been precluded by particle heterogeneity and by their ability to exchange proteins and lipids.1,8 Until recently, structural flexibility of lipid-free apoA-I has hampered its atomic-resolution studies.** The low-resolution (4Å) crystal structure of the N-terminally truncated lipid-free protein, Δ(1–43)apoA-I,40 provided the first direct insight into the protein arrangement on HDL, prompting the development of the “double belt” model.40–42 In this model, amphipathic α-helices from two antiparallel copies of apoA-I wrap around the HDL perimeter to form a closed double belt. The belt curvature is imposed by the Pro-induced kinks that are in registry on the two juxtaposed molecules.40–42 The 2.2Å resolution X-ray crystal structure of the lipid-free Δ(185–243)apoA-I solved by Mei and Atkinson43 was a major breakthrough that not only substantiated the double-belt model but also allowed novel insights into the conformations of the lipid-free, lipid-poor and lipid-bound apoA-I, and in specific HDL functions such as LCAT activation.43–45 Here, we use the overall architecture of Δ(185–234)apoA-I as a starting model to propose a molecular mechanism for the protein adaptation to the increasing lipid load in HDL during RCT.
2. X-ray crystal structure of Δ(185–234)apoA-I as a starting model for HDL structures
The C-terminal truncated lipid-free apoA-I forms a crystallographic dimer stabilized by two four-segment bundles at its ends.43 Bundle segments I–III encompass G and H1–H4 from one copy of apoA-I, while segment IV encompasses H6–H7 from the second copy (Fig. 2B). Segment I (residues 7–34) is helical and is connected via a flexible G39-containing linker 37–41 to segment II (residues 44–64). Segment II contains an extended strand 44–55 followed by an α-helix 56–64 that is connected via a G65-P66-conatining linker to segment III (H2–H4). Segment III forms a long α-helix that is packed against another long α-helical segment IV. Thus, segments I, III and IV are helical, while segment II also contains an extended strand L44-S55 whose dihedral angles fall in the β-strand range.46 The latter is consistent with the sequence analysis12 and EPR assignments ((30) and references therein).
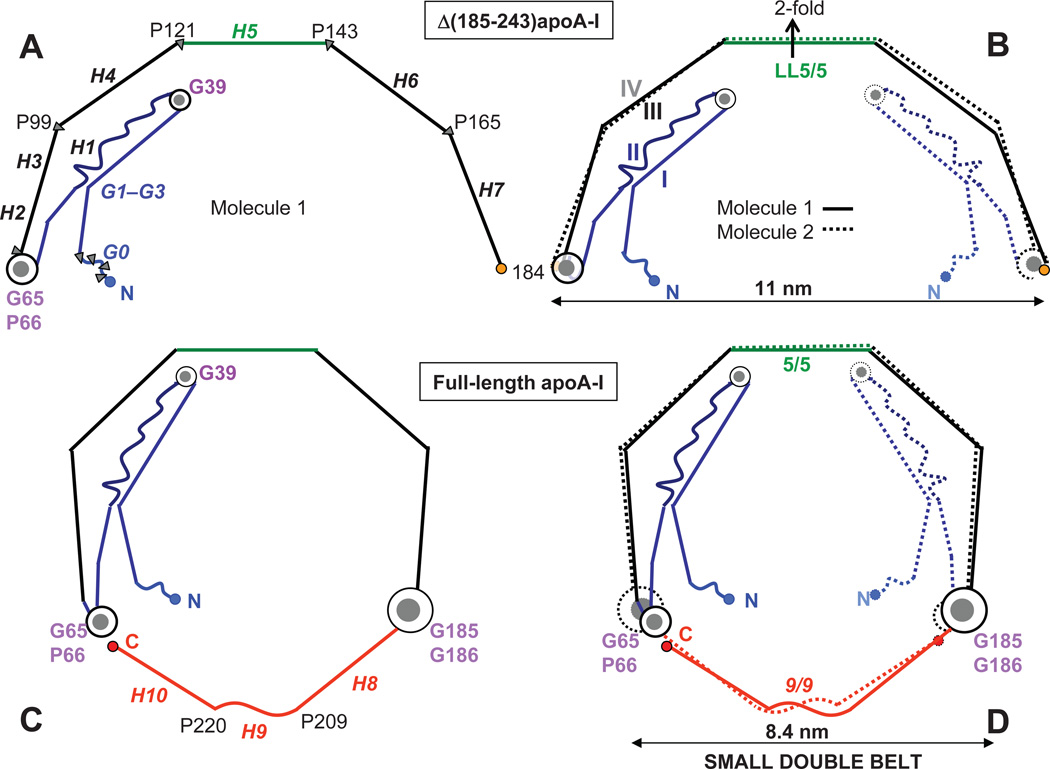
Figure 2.
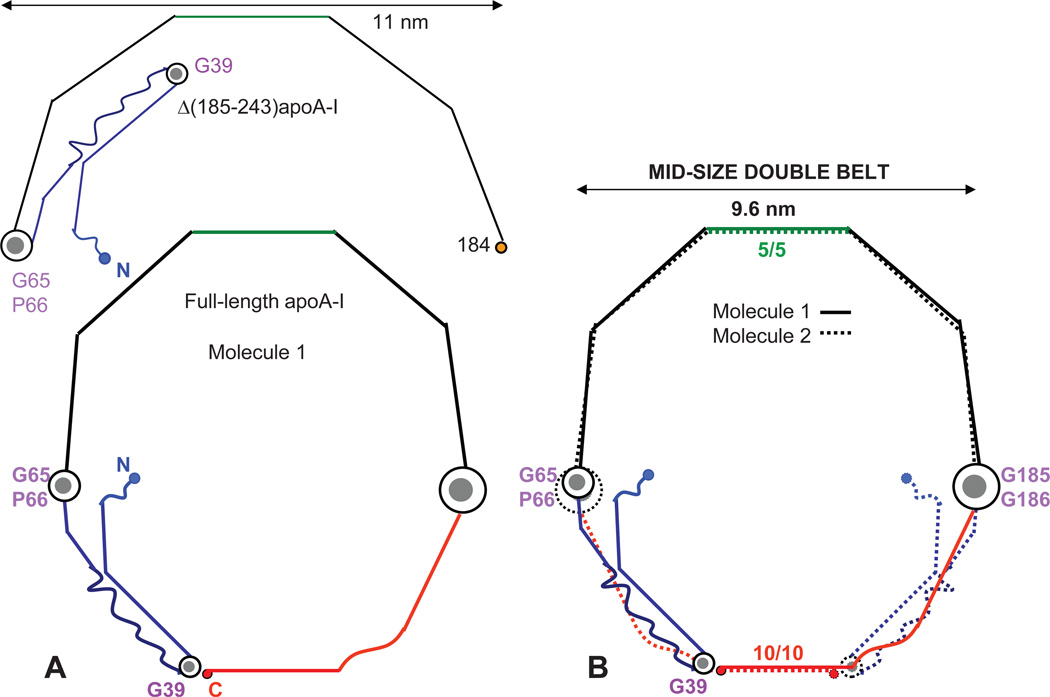
Proposed arrangement of full-length apoA-I in a small double belt (bottom) that was derived based on the crystal structure of Δ(185–243)apoA-I (top). Cartoon representation of the crystal structure of the C-terminally truncated apoA-I43 shows a monomer (A) and a crystallographic dimer (B) with 2-fold symmetry axis passing through the middle of H5 (indicated). Straight lines represent helical segments and waved lines the non-helical segments observed in the crystal structures of the N- and/or C-terminally truncated apoA-I. 40,43 In panel A, repeats G0–G3 (residues 1–43) and H1–H7 (residues 44–184) are in italics; Pro and key Gly locations are indicated. In panel B, segments I–IV that form the four-segment bundle in Δ(185– 243)apoA-I are indicated. The proposed overall arrangement of the full-length protein in a small belt shows one (C) or two copies of apoA-I (D) from the closed double-belt. Pro positions are shown. Repeat registry 5/5 and 9/9 in the small double belt is indicated (D). Color coding in this and other figures is as follows: red - C-terminal repeats H8–H10 that are absent from Δ(185–243)apoA-I; blue - N-terminal variable region (G and H1); black - constant region (H2–H7), except for the central repeats H5 that are in green.
The semi-circular shape of the Δ(185–234)apoA-I crystallographic dimer with diameter d=11 nm (Fig. 2B) suggests that it resembles key aspects of the full-length protein conformation on HDL.43 This resemblance is supported by the antiparallel orientation of the two copies of Δ(185–234)apoA-I in the dimer, with juxtaposed packing of the central helices H5 (residues 121–142) via their left (L) faces,43 termed LL5/5 registry.42 LL5/5 registry was first observed by Borhani, Brouillette and colleagues in the crystal structure of Δ(1–43)apoA-I40 and is now a well-established structural and functional motif in the full-length wild-type apoA-I on various HDL.37–42,47–52 Hence, our working hypothesis is that the structure of the lipid-free Δ(185–234)apoA-I reflects key aspects of the conformational ensemble of the full-length apoA-I on HDL.43 To assess what aspects of this ensemble are conserved and which ones are varied, we use basic structural principles to propose apoA-I models on various HDL, and validate these models by correlating them with the biophysical data reported by many groups.
3. Structural principles used to derive the apoA-I conformations on HDL
We take advantage of the modular protein structure (Fig. 1, Fig. 2A, B) and use the following constraints. First, we assume that the two copies of full-length apoA-I completely encircle HDL, leading to close proximity of the N- and C-termini, which was experimentally observed in various HDL.21,23,30,32,48,53 Second, to optimize helical packing in the double belt, the Pro-induced kinks maximize their registry in the juxtaposed molecules.30–43 Third, we posit that, to minimize free energy barriers between HDL subclasses54 and thereby facilitate their rapid remodeling during RCT,55 protein adaptation to the increasing lipid load in HDL is minimally disruptive. This is exemplified by gradual changes in the Pro-induced kink angles between the α-helices51,56 or by gradual rotation of the polypeptide chain around flexible hinges.25 Fourth, we hypothesize that formation of discrete HDL subclasses involves quantized structural changes in apoA-I requiring high mobility of its polypeptide chain. This probably involves three highly mobile regions43 which contain evolutionally conserved G39, G65 and G185–G186.33 Finally, we assume that protein-protein interactions are most extensive in lipid-free apoA-I and are progressively replaced with protein-lipid interactions upon increasing lipid load.25 Hence, the packing of the N-terminal bundle observed in the structure of lipid-free Δ(185–243)apoA-I (Fig. 2B) is expected to better represent small rather than large HDL, whereas the diameter of the crystallographic dimer, d=11 nm, resembles that of large HDL.
Here, we use these structural principles to propose molecular arrangements of the apoA-I dimer on discoidal particles of three distinct sizes, small (d~8 nm), medium (d~9.6 nm) and large (d~11.5 nm). Further, we adapt these arrangements to the surface of spherical HDL. Finally, we postulate a mechanism for sequential adaptation of the apoA-I conformation to HDL growth during RCT.
4. ApoA-I conformation in the small double belt
We use the structure of the Δ(185–243)apoA-I crystallographic dimer as a starting model to envision the arrangement of full-length apoA-I in the small double belt. The crystal structure shows H2–H7 forming a semi-circle with diameter d=11 nm, but lacking H8–H10 whose conformation on the lipid remains to be determined. These hydrophobic C-terminal repeats form the primary lipid binding site in apoA-I and are postulated to adopt a largely helical conformation on the lipid,57,58 with a possible exception of H9.40 Hence, our working hypothesis is that H8–H10 are predominantly helical in the full-length protein on HDL. To close the belt by combining the H2–H7 semi-circle with the H8–H10 helical extension, the average Pro-induced helical kink angle should be circa 135°, less than that observed in the structure of lipid-free Δ(185–243)apoA-I but well within the range reported for the Pro-induced helical kinks in other proteins.56
Fig. 2C shows the resulting apoA-I conformation in a small belt. For an idealized planar belt comprised of H2–H10, which contains ~175 amino acids in α-helical conformation, the perimeter is expected to be P~265 Å and the diameter d~84 Å. Since, in addition to the helical kinks, α-helices also “wobble” in and out of plane at Pro positions in globular proteins,56 in the two crystal structures of apoA-I40,43 and, probably, on HDL,51 the belt diameter is expected to be smaller than 84 Å. This arrangement, which we term “a small 8 nm belt”, may represent some of the smallest HDL with diameters circa 7.7 nm. Although alternative conformations that can reduce the belt size, such as the “looped belt” in the central repeats of apoA-I20,22 or folding back of both molecular termini23 have also been proposed in some, but not all studies,34 here we limit the discussion of the protein arrangement on the small HDL to that depicted in Figure 2D.***
Figure 2D shows the proposed arrangement of two antiparallel apoA-I molecules in a small double belt. Two copies of H2–H7 are in registry similar to that observed in the crystal structures of the N- or the C-terminally truncated apoA-I.40,43 Two antiparallel copies of H8–H10 are also packed in registry to close the double belt, resulting in an apparent 9/9 repeat overlap (Fig. 2D). Although the proposed H9-H9 proximity on small ~8 nm HDL is consistent with the previous cross-linking /MS studies,23 our model should be treated with caution until further experimental validation (work in progress).
5. Bundle opening upon apoA-I transfer from solution to the lipid surface
What conformational changes in the apoA-I double belt are involved in the protein transfer from solution to the lipid surface? Segrest proposed that paired antiparallel α-helices can rotate around their axes45 to orient the apolar faces towards the lipid (Fig. 3A). The helices can also slightly unwind, from the canonical 3.6 to 3.667 residues per turn, which straightens their apolar faces and optimizes them for lipid surface binding.41,45 In addition, helix bundle is expected to open on the lipid (Fig. 3B), as indicated by many spectroscopic studies ((28,37,38,54) and references therein). In the crystal, the antiparallel bundle segments I, II and III, IV are paired via extensive salt bridge and hydrophobic interactions that may be partially conserved on the lipid; in contrast, loose packing interactions between the pairs I–II and III–IV are expected to be disrupted.43 We posit that the latter can be accomplished by rotating segment pair I–II away from III–IV around the flexible hinge containing G65-P66 (Fig. 3B, double arrow). Further, we posit that rotation of segments I and II around their axes, facilitated by the flexible G39-containing linker, helps orient the apolar helical faces towards the lipid (Fig. 3B, circular arrows). The resulting structure can be envisioned as a stack of four antiparallel largely helical segments I–IV on the disk edge (Fig. 3C). The combined width of this stack is expected to be circa 45 Å (assuming α-helical diameter of about 12 Å and a somewhat smaller width of the extended segment L44-S55), which is between the total and the hydrophobic thickness of an idealized phospholipid bilayer (Fig. 3C). This comparison suggests that no more than four protein segments can be accommodated on the disk edge. Further, since segment pairs I–II and III–IV are expected to interact with the opposite monolayers (Fig. 3C), we speculate that pivoting of the I–II pair away from the II–IV pair around the G65-containing linker helps accommodate LCAT-generated cholesterol esters between the two monolayers and thereby augments HDL maturation.
In summary, we propose that the four-segment bundle partially opens on the edge of discoidal HDL, to separate the segment pair I–II from III–IV43 and orient the apolar helical faces towards the lipid (Fig. 3C). As a result, the aromatics residues in segments I–IV (which contain 12 out of 14 aromatic groups in apoA-I) are expected to re-pack on the lipid. Such aromatic re-packing upon apoA-I transfer from solution to the lipid surface has been well-documented by spectroscopy including near-UV circular dichroism, Trp fluorescence, and NMR ((28,59) and references therein). Studies using mutant and/or labeled apoA-I, calorimetry and molecular dynamics also indicate re-packing of the N-domain on the lipid.19,24,29–32,36 Taken together, these studies strongly support the opening of the four-segment bundle on the lipid, such as that depicted in Figure 3B, C.
6. ApoA-I conformation in the mid-size double belt
Mei and Atkinson proposed that unhinging of the I–II segment pair away from the III–IV pair by ~180° rotation around their flexible G65-containing linker helps elongate the double belt.43 Our model of the small double belt suggests that, even though such unhinging in one copy of apoA-I is sterically allowed, the unhinging in the two copies will lead to steric clashes among the six protein segments on the disk edge (two pairs of the bundle segments I–II and two C-terminal segments H8–H10, Fig. 2D). We hypothesize that, to alleviate these steric clashes, the unhinging of the segment pairs I–II must be accompanied by the overall expansion of the double belt. This can be accomplished by incremental sliding of the C-terminal helices H8–H10 to change their repeat registry in the double belt, and by a small <10° change in the average angle of the Pro-induced helical kinks to increase the belt perimeter from small to mid-size. Figure 4A shows the resulting conformation of one apoA-I molecule, and Figure 4B shows two such molecules in a mid-size double belt. This belt is approximately 9.6 nm in diameter; compared to the small 8 nm belt, this reflects a ~53 Å increase in the perimeter or ~35 residues in an α-helical conformation, which approximates the length of the segment pair I–II. In our model, the packing of H2–H7 is essentially conserved, which minimizes the structural perturbation upon increasing the belt size. In contrast, pairing of the juxtaposed H8–H10 repeats changes upon the belt expansion, from the apparent 9/9 repeat registry in the small (Figs. 2D) to 10/10 in the mid-size double belt (Fig. 4B). Further, our model suggests that the N-terminal stack gets thinner, from four segments on the small to three segments on the mid-size particle. Thus, the conversion from the small to the mid-size double belt leaves the packing of the “constant” half of the molecule (H2–H7, residues 66–185) essentially intact but causes major re-arrangements in the “variable” N- and C-terminal half (G-H1 and H8–H10, residues 1–65 and 186–243).
Figure 4.
Proposed arrangement of full-length apoA-I on HDL in the mid-size double belt. The conformation of Δ(185–243)apoA-I43 is shown for comparison (top). (A) Molecule I forms a closed belt via the unhinging of the segment pair I–II (G and H1, blue) around a linker containing G65-P66. (B) Two molecules form a closed double belt, with 5/5 and 10/10 repeat registry.
The 9.6 nm discoidal particles containing two copies of apoA-I are the best-studied reconstituted HDL (rHDL), and these studies strongly support our model (Fig. 4B). First, Tian and Jonas reported that A232C substitution produces an intermolecular disulfide link that locks the particle in the 9.6 nm diameter and prevents its re-arrangement to larger or smaller sizes.17 Notably, A232C is located in the middle of H10 in the “left” helical face,42 which facilitates S-S link formation between the two copies of the juxtaposed H10 while maintaining Pro kink registry in the double belt. Therefore, the results of Tian and Jonas indicate that LL10/10 repeat registry is uniquely suited for the 9.6 nm rHDL, in strong support of our model. Second, 10/10 helix registry was reported in the low-resolution structure of Δ(1–43)apoA-I40 whose belt dimensions are comparable to our mid-size belt (see part 10). Third, Lys cross-linking studies of the 9.6 nm discoidal rHDL identified several relatively short intermolecular distances, including K118–K140 (near H4–H5 junction), K12–K182 (between the ends of segments I and IV), and K40–K239 (belt-closing contact between the N- and C-terminal parts).21,23 In addition, FRET studies showed that the distance between the residues 40 and 240 on the 9.6 nm rHDL is ~30 Å.53 Our mid-size double belt readily accommodates these distances (supplemental Fig. S1).
On the basis of their cross-linking studies, Bhat and colleagues proposed the “belt-buckle” model for the 9.6 nm particle.21 This model is very similar to ours (Fig. 4B), with two small differences. In Bhat’s model, i) the “buckle” is longer by about 2 helical turns, and ii) the C-terminal region folds back around the N-terminus of the second apoA-I molecule to accommodate the intermolecular cross-link K40–K239. As a result, in Bhat’s model the helices are bent at L44 (start of H1) and S231 (middle of H10). Such bending appears energetically unfavorable since these positions contain no helix-breaking or bending motives such as Gly, Pro or discontinuous apolar helical faces; moreover, no helical bending at L44 or S231 was observed in the two available x-ray crystal structures.39,43 In contrast, in the crystal structure of Δ(185–243)apoA-I and in our model, the antiparallel segments I and II are bent at the G39-containing linker (residues 37–41). Another argument in favor of our model follows from the spin-labeling / EPR study of the first 98 residues in apoA-I on rHDL disks reporting that residues 34–41 (tip of the “buckle” in our model) are particularly sensitive to changes in the particle size and have increased molecular accessibility on larger disks.30 This result is in excellent agreement with our model (supplemental Fig. S2) but not with Bhat’s model, in which the tip of the “buckle” is centered at L44. Apart from these small differences, our model of the mid-size double belt is not only in excellent agreement with the belt-buckle model of the 9.6 nm rHDL,21 but is also strongly supported by the results of the S-S linking, EPR and other studies.17,30,39
7. ApoA-I double belt on the large particles
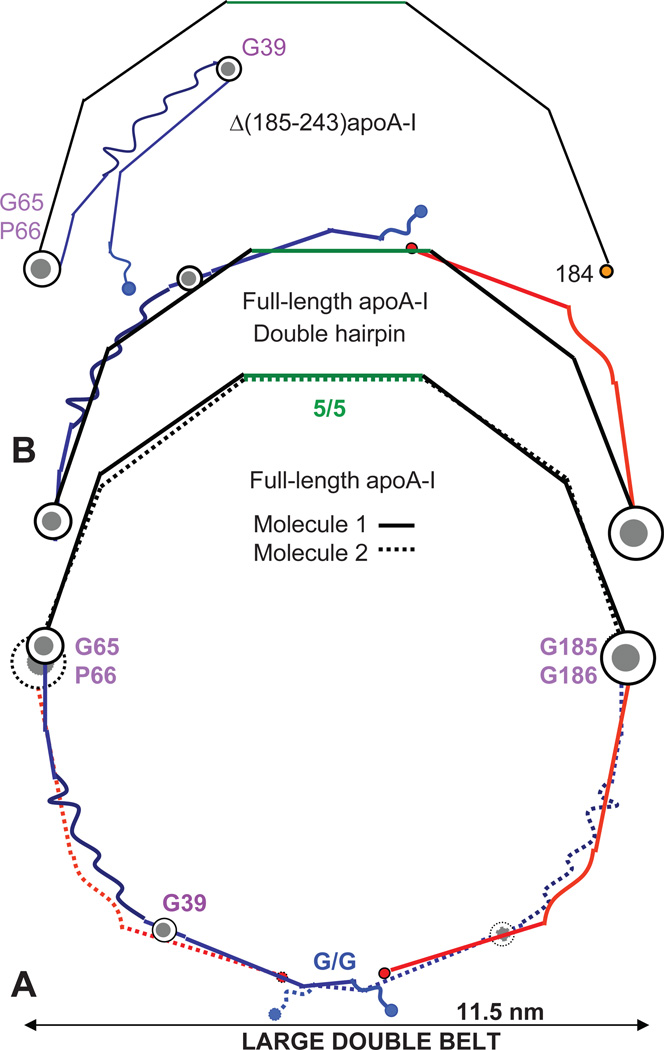
Mei and Atkinson proposed that the double belt can fully expand upon unhinging of segment I around the G39-containing linker.43 We hypothesize that, on large HDL, this unhinging must be accompanied by change in registry of the C-terminal H8–H10 repeats and by the belt expansion via changes in the Pro-induced kinks, from an average angle of ~140° on mid-size to ~146° on large HDL. The resulting belt diameter is about 11.5 nm (Fig. 5A), in excellent agreement with that observed in the largest HDL (11.5–12 nm).
Figure 5.
Proposed arrangement of full-length apoA-I in fully expanded conformations: large double belt (dimer) and double-hairpin (monomer). The conformation of Δ(185–243)apoA-I is shown for comparison (top). (A) Two copies of apoA-I form a double belt. The belt-closing contacts involve N-terminal G-repeats, such as G1/G1 that appear in registry. (B) ApoA-I monomer on HDL in a fully expanded double-hairpin conformation formed from the large fully expanded belt by a ~180° rotation of the polypeptide chain around two flexible hinges containing G65-P66 and G185–G186. We posit that this double-hairpin conformation helps accommodate additional copies of apoA-I on the mid-size and large HDL.
In our double-belt models of various sizes, the repeat registry in the constant region and in the G65-P66 and G185–G186 hinges is conserved (Figs. 2D, 4B, 5A). In contrast, the variable N- and C-terminal regions re-arrange upon conversion of the mid-size to the large fully-expanded double belt, resulting in a diminished overlap between the two molecules at the belt-closing contacts. In the large double belt these overlapping regions probably involve N-terminal repeats; their exact locations are difficult to pinpoint because of the irregular structure in G repeat.43 Figure 5A illustrates a possible arrangement in the large double belt; the belt-closing contacts involve overlapping G1/G1 pair flanked by the non-helical G0 repeats that were proposed to form “molecular Velcro” sealing the belt.60 Regardless of the exact repeat overlap, it is clear that the possible G/G repeat registry in the large double belt differs from the 10/10 registry observed in the mid-size double belt or from the putative 9/9 registry in the small double belt (Figs. 5A, 4B, and 2D).
8. Conformation of the variable N- and C-terminal regions modulates HDL stability and function
Comparison of the double belts in Figures 2D, 4B and 5A suggests that the incremental increase in particle size leads to a progressive decrease in the belt-closing contacts formed by the overlapping C-and/or N-terminal segments from the two copies of apoA-I. This is consistent with the cross-linking / MS studies by the team of Sorci-Thomas and Thomas, suggesting that the double-belt interactions involving the N- and C-termini of apoA-I incrementally decrease as the belt diameter increases.23 Our models suggest that the overlapping belt-closing segments encompass H8–H10 (the total of 55 residues) in a small double belt, H10 (22 residues) in a mid-size double belt, or G1 (11 residues) in a large double belt (Figs. 2D, 4B, 5A). We expect that the less extensive the belt-closing contacts are, the less stable the particle is. Hence, in our models larger double belts have fewer stabilizing interactions involving their variable N- and C-terminal regions, which helps explain the reduction in stability upon increasing particle size observed in human plasma HDL.61
The belt-closing contacts are expected to form the weakest link in the double belt. Their disruption is expected to open access to the core of HDL and facilitate its reactions with the metabolic partners that traffic apolar lipids, such as cholesterol ester transfer protein or SR-BI receptor. Productive interaction of HDL with SR-BI is postulated to depend upon formation of a receptor-apolipoprotein complex with specific requirements for conformation and/or dynamics of apoA-I.15 We speculate that the size-specific conformation of the variable N- and C-terminal regions together with the reduced stability in large HDL61 modulate HDL-receptor interactions.
9. Evidence for the re-arrangement of the variable region on HDL of various sizes
Figures 2–5 show that the key to the conformational adaptation of apoA-I to particles of various sizes is incremental unhinging of the N-terminal bundle segments I and II (residues 1–65). Multiple lines of evidence support this idea. First, mutagenesis studies by Phillips and colleagues suggest the importance of both the hydrophobic C-terminal domain and the stability of the N-terminal helix bundle for the apoA-I – lipid interactions.31,32 Second, the cross-linking / MS studies by the team of Thomas and Sorci-Thomas conclude that “the incremental changes in the interaction between the N- and C-terminal ends of apoA-I… allow it to unfold and sequester discrete amounts of phospholipids”.23, 39 Third, EPR studies by Oda’s group report the key role of the N-terminal part in the adaptation of apoA-I to various-size particles ((30) and references therein). Additional support comes from the earlier immunochemical studies16 as well as the electron microscopic studies reporting that apoA-I adopts a relatively compact globular conformation on small HDL but expands on larger particles.28 The NMR analysis of the latter work shows that such expansion involves tertiary but not secondary structural changes.28 Thus, multiple lines of evidence support the idea that the double belt adapts to increasing particle size via the incremental unhinging of the N-terminal bundle. The structure of Δ(185–243)apoA-I suggests strongly that the critical hinge regions in this adaptation include G39 and G65.43 Consistent with this idea, G39 and G65 are highly conserved in vertebrates, indicating the importance of these locations for molecular flexibility.33
10. ApoA-I dimer forms a bent double belt on spherical HDL
A striking feature emerging from the comparison of the small, mid-size and large double belts is the conserved alignment of the two flexible hinges, G65-P66 and G185–G186, from the two antiparallel apoA-I molecules (Figs. 2D, 4B, 5A, supplemental Fig. S2). Such alignment facilitates concerted swing motion of the two molecules around these hinges while preserving molecular packing in the double belt (Fig. 6). The hinges demarcate two halves of the apoA-I molecule, each containing about 120 amino acids: the structurally conserved constant region (H2–H7, residues 66–185) and the variable region (G-H1 and H8–H10, residues 1–65 and 186–243). This idea is supported by the sequence alignment showing that the most evolutionally conserved region in apoA-I encompasses H2–H7 and the Gly-containing flexible hinges demarcating these helices.33 We posit that this sequence conservation reflects the importance of the structural conservation of the constant region and the key flexible hinges during protein adaptation to increasing lipid load.
We hypothesize that the key hinge regions containing G65-P66 and G185–G186 are critical in several steps of the apoA-I adaptation to the changing shape and size of HDL. First, we propose that particle maturation and growth involve out-of-plane swing motion of the variable region around the axis connecting G65-P66 and G185–G186 (Fig. 6C, D). The resulting bent double-belt geometry helps confer positive curvature to the phospholipid surface in two dimensions, which can augment HDL maturation from disk to sphere. In fact, phospholipid monolayer has near-zero spontaneous curvature and must be bent by the protein in 2D to encapsulate apolar lipids.62 Second, the two halves of the apoA-I molecule can swing around the hinges at G65-P66 and G185–G186 to fold back upon themselves in a double-hairpin conformation (Fig. 5B). We posit that this monomer conformation provides a means to accommodate the third copy of apoA-I on the mid-size HDL (Fig. 6C). Furthermore, on large HDL that contain four to five copies of apoA-I per particle, additional protein copies can also be accommodated in a double-hairpin conformation. Alternatively, four copies of apoA-I can form two bent double belts in the opposite quadrants on the sphere (Fig. 6D). The presence of two distinct conformations of apoA-I, such as the double-hairpin monomer and the double-belt dimer, helps explain the presence of two distinct populations of apoA-I on human HDL, one more labile (double hairpin) and the other more tightly bound to the lipid (double belt), which was inferred from the spectroscopic studies.63,64
Our proposed models of HDL disks and spheres are supported by the results of the cross-linking / MS studies by Silva and colleagues suggesting that the overall conformation of apoA-I on HDL is similar regardless of the particle shape and size.25 In fact, our models show that the packing in the constant half of the double belt is conserved on HDL of various shapes and sizes. Moreover, the protein arrangement depicted in Figure 6C, D is reminiscent of the “trefoil / tetrafoil” models of spherical HDL proposed by Silva et al., with three important differences. First, in Silva’s model, the closed double belt encompasses residues 44–243 (H1–H10) in a fully expanded highly α-helical conformation with 10/10 repeat registry, whereas in our model 10/10 registry is limited to the mid-size “belt-buckle”. Since Silva’s model lacks residues 1–43 while our model includes full-length protein, the perimeter of the fully expanded belt in Silva’s model is comparable to our mid-size belt, which explains similar 10/10 repeat registry. Second, the hinge axis in Silva’s model passes through the centers of H5 and H10 near residues 133 and 233;25,51 this is nearly orthogonal to the hinge axis connecting G65-P66 and G185–G186 in our model. We propose that the hinge axis connecting H5 and H10 is potentially suited only for the mid-size particles, since only the mid-size double belt shows both 5/5 and 10/10 repeat registry (Fig. 6). In contrast, the hinge axis connecting G65-P66 and G185–G186 is compatible with the double belts of any size and hence, it is uniquely suited for the protein adaptation to the dynamic changes in HDL geometry (Fig. 6). Third, Silva’s model postulates domain swapping between the parts of adjacent apoA-I molecules on the spherical HDL. This idea is consistent with our models, with several caveats.
First, domain swapping (circular arrow in Fig. 6D) should change the pairing of the two halves of apoA-I, from constant/constant and variable/variable in the double belt to variable/constant, as in the double-hairpin conformation (Fig. 6C, bottom). Domain swapping is also expected to change the packing of the juxtaposed helices, from left (LL) to right (RR) faces. Further, since only protein domains with similar structures can be swapped for each other, we posit that the swapping between the variable and constant regions in apoA-I is possible only if these regions adopt similar fully expanded semi-circular conformations, which is characteristic of large spherical HDL (Fig. 6D). If such domain swapping between the two halves of the double belt is experimentally confirmed, it may have interesting functional implications that are beyond the scope of the current work.
These details notwithstanding, our models of spherical HDL not only support the “trefoil / tetrafoil” concept originally proposed for the N-terminally truncated apoA-I on the mid-size HDL,25 but also help apply this concept to full-length apoA-I on HDL of various sizes.
11. ApoA-I adaptation to changing lipid load in HDL during cholesterol transport
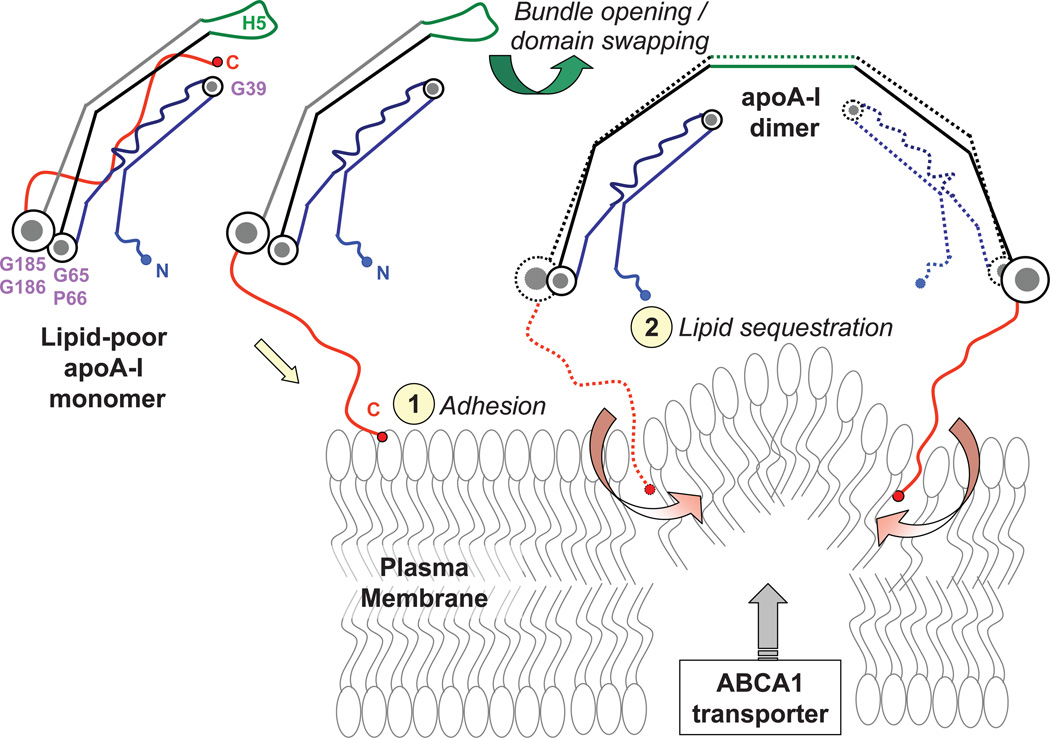
A hypothetical sequence of molecular events driving HDL remodeling in RCT is depicted in Figures 6 and 7. First, globular lipid-poor/free apoA-I monomer,18 which is generated either de-novo or upon HDL remodeling by plasma factors,14 readily adsorbs to the plasma membrane and is recruited for HDL biogenesis18 (Fig. 7). ApoA-I dimerization via domain swapping around H553 and insertion of the hydrophobic C-terminal repeats H8–H10 into the lipid, aided by ABCA1 transporter,4,13 facilitates lipid sequestration (Fig. 7). This results is formation of small nascent HDL that, we posit, contain two copies of apoA-I in a compact four-segment “belt-buckle” conformation (Fig. 6A). These nascent HDL form preferred substrates for LCAT. Productive interaction of HDL with LCAT involves the pair of antiparallel H5 repeats.43 We speculate that location of these flexible repeats in the middle of the constant region, H2–H7, helps lock 5/5 registry to optimize HDL structure for the LCAT reaction. Second, cholesterol esters produced by LCAT move between the phospholipid monolayers to form the HDL core.50 This is accompanied by separation of the two monolayers, which is augmented by the separation of the I–II and III–IV segment pairs via the rotation around the G65-conatining linker. We posit that, as the apolar lipid core grows through the action of LCAT and other plasma factors, progressive pivoting moves the segment pair I–II out of the plane of the double belt. This helps confer 2D curvature to the surface of a small nascent HDL particle during its maturation (Fig. 6B). We speculate that the curved shape of the I–II segment pair observed in Δ(185–243)apoA-I (Fig. 2A, B), which results from the helical kink at V21 and the non-helical secondary structure in L44-S55,43 helps impose 2D surface curvature to small spherical HDL (Fig. 6B) that contain two copies of apoA-I as their major if not sole apolipoprotein.65
Figure 7.
Proposed structural changes in the protein and lipid during HDL biogenesis. Lipid-poor / free apoA-I monomer, whose compact conformation has been described previously,43,44 is recruited to the plasma membrane via the hydrophobic C-terminal H8–H10 repeats (red) that form the primary lipid binding site.57,58 Protein adhesion to the lipid surface is followed by dimerization via domain swapping around H5 (green) and insertion of the C-terminal repeats into the membrane. The insertion is facilitated by ABCA1 transporter that perturbs lipid packing in the bilayer. This results is lipid sequestration by apoA-I dimer and formation of small nascent HDL containing two copies of apoA-I. This mechanism is reminiscent of the previously proposed opening of the four-helix bundle upon lipid binding by apoA-I.36
Further, our models suggest that pivoting of the segment pair I–II around G65 and away from the III–IV pair perturbs the ring-closing contacts formed by G65-P66 and the N- and C-termini of apoA-I on the small particles. In one hypothetical scenario, gradual changes in these contacts upon increasing lipid load culminate in the unhinging of the I–II segment pair around the G65-containing linker, which is accompanied by an overall expansion of the double belt. Such unhinging is expected to elongate the double belt by nearly 35 residues to form a three-segment N-terminal “buckle” on the mid-size HDL. Further increase in the lipid load can be accommodated via the second unhinging whereby segment I separates from segment II by rotating around the G39-containing linker, which converts the mid-size into a large fully expanded double belt. In this process, small HDL can potentially be converted first to mid-size and then to large HDL. Alternatively, small HDL may be converted directly into large HDL, consistent with our electron microscopic studies of HDL fusion.54 Such a direct conversion is expected to involve polypeptide chain opening around G65-P66 and G39 to increase the belt perimeter by up to 64 a. a. while maintaining close proximity of the N- and C-termini.
A important new feature of our models is that unhinging of the N-terminal bundle upon HDL growth is accompanied by change in H8–H10 registry, leading to changes the belt-closing contacts. In our models (Figs. 2, 4, 5, 6), these contacts always involve the C-terminus. In addition, they involve the N-terminus and/or the G65-containing hinge on small particles, G39-containing hinge on the mid-size particles, or the N-terminus on the large particle (Figs. 2D, 4B, 5A). Thus, probing the proximity of the molecular termini and the key Gly-containing hinge regions in apoA-I can be used as a future test for the proposed models.
Another important new feature of our models is that, to confer 2D surface curvature to the mid-size or large HDL, the variable region of apoA-I moves out of plane of the constant region by rotating around the swing axis between G65-P66 and G185–G186 (Fig. 6C). As the variable region of the double belt swings to one side of the sphere, the opposite vacant side accommodates additional protein (Fig. 6C). We speculate that this protein is either an apoA-I monomer in a semi-circular double-hairpin conformation (Figs. 5B, 6C bottom) or a dimer in a bent double-belt conformation. Together, these multiple copies of apoA-I probably form a “trefoil / tetrafoil / pentafoil” arrangement on the mid-size and large HDL (Fig. 6C, D). Alternatively, in addition to the apoA-I double belt, the mid-size HDL can also contain one or more copies of apoA-II. ApoA-II is the second-major HDL protein that forms disulfide-linked dimers in humans and circulates almost exclusively on the mid-size spherical HDL.65 We postulate that endogenous human apoA-II can selectively stabilize apoA-I in the mid-size belt-buckle conformation, and thereby modulate HDL interactions with its metabolic partners in RCT.62 Thus, our apoA-I models of can help better understand functions of other HDL-associated proteins.
12. Summary and future studies
In the proposed structural ensemble, the apoA-I double belt undergoes both continuous and quantized conformational changes enabling it to adapt to the increasing lipid load during RCT, from small nascent “discoid” to small and, eventually, large “spheroid” HDL. We propose that during this process, the constant region of the apoA-I double belt undergoes minimal structural re-arragnements, such as a gradual change in the interhelical angles at Pro postitions. In contrast, the variable region undergoes large quantized re-arrangements during HDL maturation and growth, from a four-segment stack on the small nascent particles to a fully expanded double belt on large mature HDL (Figs. 2–5). We posit that these quantized structural changes help explain the presence of distinct subclasses in plasma HDL1,8 and contribute to the free energy barriers separating these subclasses.54,55
The proposed models encompass a subset of apoA-I conformations that are critically hinged upon molecular flexibility at G39, G65-P66, and G185–G186. We speculate that other conformations of apoA-I on HDL, such as the putative “hinge domain”20 or the “looped belt” in the central repeats,22 may be facilitated by molecular flexibility in other Gly-containing protein segments, e.g. G129 and G145 in the central repeats (Fig. 1). Also, “twisting” of the double belt, which was proposed to facilitate its adaptation to spherical HDL of various sizes,51 may occur via the gradual changes in the Pro-induced angles between the helices combined with the flexibility of the Gly-containing hinge regions. Thus, the conformational ensemble reported here is consistent with the “twisted belt” model.
Although the proposed conformational ensemble of apoA-I on HDL is probably incomplete, it has predictive power and helps postulate new testable hypotheses. For example, sequential unhinging of the N-terminal bundle around G39- and G65-containing hinges can be probed by Gly substitutions and/or by engineering the structure-based disulfides in a approach similar to that used by Weisgraber’s team to test sequential unhinging of the four-helix bundle in the N-domain of apoE.66 Close proximity of the N- and C-termini on the small and large but not the mid-size particles can be probed in the cross-linking / MS or protein labeling / EPR or FRET studies. Other tests, such as engineered disulfides in H9, H10 and G1, can be used to probe for the proposed 9/9, 10/10 or G1/G1 repeat registry on the small, mid-size and large HDL, respectively.
In summary, the proposed conformations of apoA-I on HDL of various shapes and sizes help refine the “belt-buckle” and the “trefoil / tetrafoil” models and integrate them in a coherent ensemble. In this ensemble, the N-terminal “belt-buckle” conformations are postulated for the small and mid-size HDL (Fig. 6A–C); the “trefoil / tetrafoil” arrangements are proposed for the mid-size and large spherical HDL (Fig. 6C, D), but not for the small particles (Fig. 6A, B). We posit that domain swapping25 is potentially possible only if the variable and constant regions adopt similar fully expanded semi-circular conformations (Figs. 5B), which is limited to larger particles (Fig. 6C, D). Notably, our models help reconcile the studies reporting similar apoA-I conformations on HDL of various shapes and sizes25 with the evidence for substantial N-terminal re-packing upon changes in the particle size ((23,30,32) and references therein). We propose that the former concept applies to the constant and the latter to the variable half of the apoA-I double belt.
The proposed models of apoA-I have already allowed new insights into the structural and functional role of the second-major HDL protein, human apoA-II.42 We expect that, if confirmed, our models will provide a structural framework necessary for understanding the interactions of HDL with their metabolic partners in RCT and with a wide range of other HDL-associated proteins, including those involved in immune response, anti-oxidant and anti-thrombolytic action.10,68,69 Ultimately, this should help design rational approaches for improving cardioprotective functions of HDL.
Supplementary Material
Highlights.
Plasma HDL are heterogeneous nanoparticles that remove cell cholesterol
Crystal structure of the truncated HDL protein, apoA-I, is used as a starting model
Conformations of full-length apoA-I on various HDL are proposed
This conformational ensemble is supported by the existing biophysical data
It provides structural basis for understanding cardioprotective function of HDL
ACKNOWLEDGEMENTS
The author is grateful to her colleagues in the Department of Physiology and Biophysics at Boston University School of Medicine for many stimulating discussions, particularly to Drs. Xiaohu Mei, Shobini Jayaraman, and David Atkinson. This work was supported by the National Institutes of Health grants HL026355 and GM067260.
Glossary
- apo
apolipoprotein
- HDL
high-density lipoprotein
- rHDL
reconstituted HDL
- RCT
reverse cholesterol transport
- LCAT
lecithin cholesterol acyltransferase
- MS
mass spectrometry
- EPR
electron paramagnetic resonance
- NMR
nuclear magnetic resonance
- FRET
fluorescence resonance energy transfer
- ABCA1
ATP-binding cassette transporter A1
- SR-BI
scavenger receptor class B type 1
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Sorci-Thomas and colleagues used negative stain electron microscopy to conclude that nascent HDL have predominantly spheroid rather than discoid shape (Sorci-Thomas et al. (2012). J. Lipid Res. 53(9), 1890–1909). In our opinion, this is difficult to justify solely on the basis of the negative stain technique. Further, micelle-like lipid packing in spheroid HDL appears inconsistent with differential scanning calorimetry data of nascent HDL clearly showing acyl chain melting transition characteristic of lipid bilayers. Other HDL models were proposed by Hazen and colleagues, including “solar flare” in which large portions of the protein protrude from HDL perimeter (Wu et al. (2007). Nat. Struct. Mol. Biol. 14, 861–868) and “double super helix” in which two antiparallel copies of apoA-I wrap in an open twisted shape around an elongated lipid micelle rather than a bilayer disk (Wu et al. (2009). J. Biol. Chem. 284, 36605–36619). These models have been reported to collapse in molecular dynamic simulations (Shih, Sligar, & Schulten (2008). Biophys. J. 94(12), L87–L99; Jones et al. (2010). J. Biol. Chem. 285(52), 41161–41171; Gogonea et al. (2010). Biochemistry 49(34), 7323–7343). These models also appear inconsistent with each other and with the results of FRET, electron microscopic and other studies,53 including structural studies by H/D exchange 26,34 and the crystal structure of the C-terminal truncated apoA-I.43
The previously reported high-resolution x-ray crystal structure of apoA-I (Ajees et al. (2006). Proc. Natl. Acad. Sci. USA 103(7):2126–2131, PDB access code 2A01), together with 11 other PDB entries submitted by HM Murphy, have been discredited and have been or will be withdrawn from the journals and from the Protein Data Bank. For details see: http://main.uab.edu/Sites/reporter/articles/71570/ http://classic.the-scientist.com/blog/display/56226/
Notably, most inter- or intramolecular distances identified by Lys cross-linking MS/MS in 8 nm rHDL23 are consistent with our model as shown (Fig. 2) or upon slight modifications involving rotations around key flexible hinges. For example, rotation of the N-terminal segment pair I–II around G65 hinge, or of the C-terminal H8–H10 repeats around G185–G186 hinge, facilitates intermolecular cross-link 40–239;23 such C-terminal rotation also facilitates 94–239 cross-link.23 Further, 118-118 and 118–140 cross-links23 are consistent with H5/H5 registry on these (Fig. 2) and other HDL.
REFERENCES
- 1.Rothblat GH, Phillips MC. High-density lipoprotein heterogeneity and function in reverse cholesterol transport. Curr. Opin. Lipidol. 2010;21:229–238. doi: 10.1097/mol.0b013e328338472d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Asztalos BF, Tani M, Schaefer EJ. Metabolic and functional relevance of HDL subspecies. Curr. Opin. Lipidol. 2011;22(3):176–185. doi: 10.1097/MOL.0b013e3283468061. [DOI] [PubMed] [Google Scholar]
- 3.Duffy D, Rader DJ. Update on strategies to increase HDL quantity and function. Nat. Rev. Cardiol. 2009;6:455–463. doi: 10.1038/nrcardio.2009.94. [DOI] [PubMed] [Google Scholar]
- 4.Yvan-Charvet L, Wang N, Tall AR. Role of HDL, ABCA1, and ABCG1 transporters in cholesterol efflux and immune responses. Arterioscler. Thromb. Vasc. Biol. 2010;30:139–143. doi: 10.1161/ATVBAHA.108.179283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gao X, Yuan S. High density lipoproteins-based therapies for cardiovascular disease. J. Cardiovasc. Dis. Res. 2010;1(3):99–103. doi: 10.4103/0975-3583.70898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Voight BF, et al. Plasma HDL cholesterol and risk of myocardial infarction: a Mendelian randomisation study. Lancet. 2012;380(9841):572–580. doi: 10.1016/S0140-6736(12)60312-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fielding CJ, Fielding PE. Molecular physiology of reverse cholesterol transport. J. Lipid Res. 1995;36:211–228. [PubMed] [Google Scholar]
- 8.Lund-Katz S, Phillips MC. High density lipoprotein structure-function and role in reverse cholesterol transport. Subcell. Biochem. 2010;51:183–227. doi: 10.1007/978-90-481-8622-8_7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rosenson RS, Brewer HB, Jr, Davidson WS, Fayad ZA, Fuster V, Goldstein J, Hellerstein M, Jiang XC, Phillips MC, Rader DJ, Remaley AT, Rothblat GH, Tall AR, Yvan-Charvet L. Cholesterol efflux and atheroprotection: advancing the concept of reverse cholesterol transport. Circulation. 2012;125(15):1905–1919. doi: 10.1161/CIRCULATIONAHA.111.066589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gordon SM, Hofmann S, Askew DS, Davidson WS. High density lipoprotein: it's not just about lipid transport anymore. Trends Endocrinol. Metab. 2010;22(1):9–15. doi: 10.1016/j.tem.2010.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Segrest JP, Garber DW, Brouillette CG, Harvey SC, Anantharamaiah GM. The amphipathic alpha helix: a multifunctional structural motif in plasma apolipoproteins. Adv. Protein Chem. 1994;45:303–369. doi: 10.1016/s0065-3233(08)60643-9. [DOI] [PubMed] [Google Scholar]
- 12.Nolte RT, Atkinson D. Conformational analysis of apolipoprotein A–I and E-3 based on primary sequence and circular dichroism. Biophys. J. 1992;63:1221–1239. doi: 10.1016/S0006-3495(92)81698-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mulya A, Lee JY, Gebre AK, Boudyguina EY, Chung SK, Smith TL, Colvin PL, Jiang XC, Parks JS. Initial interaction of apoA-I with ABCA1 impacts in vivo metabolic fate of nascent HDL. J. Lipid Res. 2008;49:2390–23401. doi: 10.1194/jlr.M800241-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barter PJ. Hugh Sinclair lecture: The regulation and remodelling of HDL by plasma factors. Atheroscler. Suppl. 2002;3(4):39–47. doi: 10.1016/s1567-5688(02)00041-7. [DOI] [PubMed] [Google Scholar]
- 15.Liu T, Krieger M, Kan HY, Zannis VI. The effects of mutations in helices 4 and 6 of ApoA-I on scavenger receptor class B type I (SR-BI)-mediated cholesterol efflux suggest that formation of a productive complex between reconstituted high density lipoprotein and SR-BI is required for efficient lipid transport. J. Biol. Chem. 2002;277:21576–21584. doi: 10.1074/jbc.M112103200. [DOI] [PubMed] [Google Scholar]
- 16.Calabresi L, Meng QH, Castro GR, Marcel YL. Apolipoprotein A–I conformation in discoidal particles: evidence for alternate structures. Biochemistry. 1993;32:6477–6484. doi: 10.1021/bi00076a023. [DOI] [PubMed] [Google Scholar]
- 17.Tian S, Jonas A. Structural and functional properties of apolipoprotein A–I mutants containing disulfide-linked cysteines at positions 124 or 232. Biochim. Biophys. Acta. 2002;1599:56–64. doi: 10.1016/s1570-9639(02)00377-1. [DOI] [PubMed] [Google Scholar]
- 18.Li L, Chen J, Mishra VK, Kurtz JA, Cao D, Klon AE, Harvey SC, Anantharamaiah GM, Segrest JP. Double belt structure of discoidal high density lipoproteins: molecular basis for size heterogeneity. J. Mol. Biol. 2004;343:1293–1311. doi: 10.1016/j.jmb.2004.09.017. [DOI] [PubMed] [Google Scholar]
- 19.Saito H, Lund-Katz S, Phillips MC. Contributions of domain structure and lipid interaction to the functionality of exchangeable human apolipoproteins. Prog. Lipid Res. 2004;43:350–380. doi: 10.1016/j.plipres.2004.05.002. [DOI] [PubMed] [Google Scholar]
- 20.Maiorano JN, Jandacek RJ, Horace EM, Davidson WS. Identification and structural ramifications of a hinge domain in apolipoprotein A–I discoidal high-density lipoproteins of different size. Biochemistry. 2004;43:11717–11726. doi: 10.1021/bi0496642. [DOI] [PubMed] [Google Scholar]
- 21.Bhat S, Sorci-Thomas MG, Alexander ET, Samuel MP, Thomas MJ. Intermolecular contact between globular N-terminal fold and C-terminal domain of ApoA-I stabilizes its lipid-bound conformation: studies employing chemical cross-linking and mass spectrometry. J. Biol. Chem. 2005;280:33015–33025. doi: 10.1074/jbc.M505081200. [DOI] [PubMed] [Google Scholar]
- 22.Martin DD, Budamagunta MS, Ryan RO, Voss JC, Oda MN. Apolipoprotein A–I assumes a "looped belt" conformation on reconstituted high density lipoprotein. J. Biol. Chem. 2006;281:20418–20426. doi: 10.1074/jbc.M602077200. [DOI] [PubMed] [Google Scholar]
- 23.Bhat S, Sorci-Thomas MG, Tuladhar R, Samuel MP, Thomas MJ. Conformational adaptation of apolipoprotein A–I to discretely sized phospholipid complexes. Biochemistry. 2007;46:7811–7821. doi: 10.1021/bi700384t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kono M, Okumura Y, Tanaka M, Nguyen D, Dhanasekaran P, Lund-Katz S, Phillips MC, Saito H. Conformational flexibility of the N-terminal domain of apolipoprotein A–I bound to spherical lipid particles. Biochemistry. 2008;47:11340–11347. doi: 10.1021/bi801503r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Silva RA, Huang R, Morris J, Fang J, Gracheva EO, Ren G, Kontush A, Jerome WG, Rye KA, Davidson WS. Structure of apolipoprotein A–I in spherical high density lipoproteins of different sizes. Proc. Natl. Acad. Sci. USA. 2008;105:12176–12181. doi: 10.1073/pnas.0803626105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chetty PS, Mayne L, Lund-Katz S, Stranz D, Englander SW, Phillips MC. Helical structure and stability in human apolipoprotein A–I by hydrogen exchange and mass spectrometry. Proc. Natl. Acad. Sci. USA. 2009;106:19005–19010. doi: 10.1073/pnas.0909708106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Koyama M, Tanaka M, Dhanasekaran P, Lund-Katz S, Phillips MC, Saito H. Interaction between the N- and C-terminal domains modulates the stability and lipid binding of apolipoprotein A–I. Biochemistry. 2009;48:2529–2537. doi: 10.1021/bi802317v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen B, Ren X, Neville T, Jerome WG, Hoyt DW, Sparks D, Ren G, Wang J. Apolipoprotein AI tertiary structures determine stability and phospholipid-binding activity of discoidal high-density lipoprotein particles of different sizes. Protein Sci. 2009;18:921–935. doi: 10.1002/pro.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gu F, Jones MK, Chen J, Patterson JC, Catte A, Jerome WG, Li L, Segrest JP. Structures of discoidal high density lipoproteins: a combined computational-experimental approach. J. Biol. Chem. 2010;285(7):4652–4665. doi: 10.1074/jbc.M109.069914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lagerstedt JO, Cavigiolio G, Budamagunta MS, Pagani I, Voss JC, Oda MN. Structure of apolipoprotein A–I's N-terminus on nascent high density lipoprotein. J. Biol. Chem. 2011;286(4):2966–2975. doi: 10.1074/jbc.M110.163097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vedhachalam C, Chetty PS, Nickel M, Dhanasekaran P, Lund-Katz S, Rothblat GH, Phillips MC. Influence of apolipoprotein (Apo) A-I structure on nascent high density lipoprotein (HDL) particle size distribution. J. Biol. Chem. 2010;285(42):31965–31973. doi: 10.1074/jbc.M110.126292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tanaka M, Dhanasekaran P, Nguyen D, Nickel M, Takechi Y, Lund-Katz S, Phillips MC, Saito H. Influence of N-terminal helix bundle stability on the lipid-binding properties of human apolipoprotein A–I. Biochim. Biophys. Acta. 2011;1811:25–30. doi: 10.1016/j.bbalip.2010.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bashtovyy D, Jones MK, Anantharamaiah GM, Segrest JP. Analyses of sequence conservation of apolipoprotein A–I afford novel insights into HDL structure-function. J. Lipid Res. 2011;52(3):435–450. doi: 10.1194/jlr.R012658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sevugan Chetty P, Mayne L, Kan ZY, Lund-Katz S, Englander SW, Phillips MC. Apolipoprotein A–I helical structure and stability in discoidal high-density lipoprotein (HDL) particles by hydrogen exchange and mass spectrometry. Proc. Natl. Acad. Sci. USA. 2012;109(29):11687–11192. doi: 10.1073/pnas.1209305109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Roberts LM, Ray MJ, Shih TW, Hayden E, Reader MM, Brouillette CG. Structural analysis of apolipoprotein A–I: limited proteolysis of methionine-reduced and oxidized lipid-free and lipid-bound human apo A-I. Biochemistry. 1997;36(24):7615–7624. doi: 10.1021/bi962952g. [DOI] [PubMed] [Google Scholar]
- 36.Rogers DP, Roberts LM, Lebowitz J, Datta G, Anantharamaiah GM, Engler JA, Brouillette CG. The lipid-free structure of apolipoprotein A–I: effects of amino-terminal deletions. Biochemistry. 1998;37(34):11714–11725. doi: 10.1021/bi973112k. [DOI] [PubMed] [Google Scholar]
- 37.Li H, Lyles DS, Thomas MJ, Pan W, Sorci-Thomas MG. Structural determination of lipid-bound ApoA-I using fluorescence resonance energy transfer. J. Biol. Chem. 2000;275(47):37048–37054. doi: 10.1074/jbc.M005336200. [DOI] [PubMed] [Google Scholar]
- 38.Li HH, Lyles DS, Pan W, Alexander E, Thomas MJ, Sorci-Thomas MG. ApoA-I structure on discs and spheres. Variable helix registry and conformational states. J. Biol. Chem. 2002;277(42):39093–39101. doi: 10.1074/jbc.M206770200. [DOI] [PubMed] [Google Scholar]
- 39.Sorci-Thomas MG, Owen JS, Fulp B, Bhat S, Zhu X, Parks JS, Shah D, Jerome WG, Gerelus M, Zabalawi M, Thomas MJ. Nascent high density lipoproteins formed by ABCA1 resemble lipid rafts and are structurally organized by three apoA-I monomers. J. Lipid Res. 2012;53(9):1890–1909. doi: 10.1194/jlr.M026674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Borhani DW, Rogers DP, Engler JA, Brouillette CG. Crystal structure of truncated human apolipoprotein A–I suggests a lipid-bound conformation. Proc. Natl. Acad. Sci. USA. 1997;94:12291–12296. doi: 10.1073/pnas.94.23.12291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Segrest JP, Jones MK, Klon AE, Sheldahl CJ, Hellinger M, De Loof H, Harvey SC. A detailed molecular belt model for apolipoprotein A–I in discoidal high density lipoprotein. J. Biol. Chem. 1999;274:31755–31758. doi: 10.1074/jbc.274.45.31755. [DOI] [PubMed] [Google Scholar]
- 42.Brouillette CG, Anantharamaiah GM, Engler JA, Borhani DW. Structural models of human apolipoprotein A–I: a critical analysis and review. Biochim. Biophys. Acta. 2001;1531:4–46. doi: 10.1016/s1388-1981(01)00081-6. [DOI] [PubMed] [Google Scholar]
- 43.Mei X, Atkinson D. Crystal structure of C-terminal truncated apolipoprotein A–I reveals the assembly of HDL by dimerization. J. Biol. Chem. 2011;286(44):38570–38582. doi: 10.1074/jbc.M111.260422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jayaraman S, Cavigiolio G, Gursky O. Folded functional lipid-poor apolipoprotein A–I obtained by heating of high-density lipoproteins: Relevance to HDL biogenesis. Biochem. J. 2012;442(3):703–712. doi: 10.1042/BJ20111831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Segrest JP, Jones MK, Catte A, Thirumuruganandham SP. Validation of previous computer models and MD simulations of discoidal HDL by a recent crystal structure of apoA-I. J. Lipid Res. 2012 doi: 10.1194/jlr.M026229. (In press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gursky O, Mei X, Atkinson D. The crystal structure of the C-terminal truncated apolipoprotein A–I sheds new light on the amyloid formation by the N-terminal segment. Biochemistry. 2012;51(1):10–18. doi: 10.1021/bi2017014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Davidson WS, Thompson TB. The structure of apolipoprotein A–I in high density lipoproteins. J. Biol. Chem. 2007;282:22249–22253. doi: 10.1074/jbc.R700014200. [DOI] [PubMed] [Google Scholar]
- 48.Thomas MJ, Bhat S, Sorci-Thomas MG. Three-dimensional models of HDL apoA-I: implications for its assembly and function. J. Lipid Res. 2008;49:1875–1883. doi: 10.1194/jlr.R800010-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sorci-Thomas MG, Bhat S, Thomas MJ. Activation of lecithin:cholesterol acyltransferase by HDL apoA-I central helices. Clin. Lipidol. 2009;4:113–124. doi: 10.2217/17584299.4.1.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jones MK, Catte A, Li L, Segrest JP. Dynamics of activation of lecithin:cholesterol acyltransferase by apolipoprotein A–I. Biochemistry. 2009;48:11196–11210. doi: 10.1021/bi901242k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Huang R, Silva RAGD, Jerome WG, Kontush A, Chapman MJ, Curtiss LK, Hodges TJ, Davidson WS. Apolipoprotein A–I structural organization in high-density lipoproteins isolated from human plasma. Nat. Struct. Mol. Biol. 2011;18:416–422. doi: 10.1038/nsmb.2028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Catte A, Patterson JC, Bashtovyy D, Jones MK, Gu F, Li L, Rampioni A, Sengupta D, Vuorela T, Niemela P, Karttunen M, Marrink SJ, Vattulainen I, Segrest JP. Structure of spheroidal HDL particles revealed by combined atomistic and coarse-grained simulations. Biophys J. 2008;94:2306–2319. doi: 10.1529/biophysj.107.115857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jones MK, Zhang L, Catte A, Li L, Oda MN, Ren G, Segrest JP. Assessment of the validity of the double superhelix model for reconstituted high density lipoproteins: a combined computational-experimental approach. J. Biol. Chem. 2010;285(52):41161–41171. doi: 10.1074/jbc.M110.187799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mehta R, Gantz DL, Gursky O. Human plasma high-density lipoproteins are stabilized by kinetic factors. J. Mol. Biol. 2003;328:183–192. doi: 10.1016/s0022-2836(03)00155-4. [DOI] [PubMed] [Google Scholar]
- 55.Guha M, Gao X, Jayaraman S, Gursky O. Correlation of structural stability with functional remodeling of high-density lipoproteins: The importance of being disordered. Biochemistry. 2008;47(44):11393–11397. doi: 10.1021/bi8014746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Visiers I, Braunheim BB, Weinstein H. Prokink: a protocol for numerical evaluation of helix distortions by proline. Protein Eng. 2000;13(9):603–606. [Google Scholar]
- 57.Tanaka M, Dhanasekaran P, Nguyen D, Ohta S, Lund-Katz S, Phillips MC, Saito H. Contributions of the N- and C-terminal helical segments to the lipid-free structure and lipid interaction of apolipoprotein A–I. Biochemistry. 2006;45:10351–10358. doi: 10.1021/bi060726t. [DOI] [PubMed] [Google Scholar]
- 58.Zhu HL, Atkinson D. Conformation and lipid binding of a C-terminal (198–243) peptide of human apolipoprotein A–I. Biochemistry. 2007;46:1624–1634. doi: 10.1021/bi061721z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gursky O, Atkinson D. Thermal unfolding of human high-density apolipoprotein A-1: Implications for a lipid-free molten globular state. Proc. Natl. Acad. Sci. USA. 1996;93:2991–2995. doi: 10.1073/pnas.93.7.2991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Jones MK, Gu F, Catte A, Li L, Segrest JP. "Sticky" and "promiscuous", the yin and yang of apolipoprotein A–I termini in discoidal high-density lipoproteins: a combined computational-experimental approach. Biochemistry. 2011;50(12):2249–2263. doi: 10.1021/bi101301g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gao X, Yuan S, Jayaraman S, Gursky O. Differential stability of high-density lipoprotein subclasses: effects of particle size and protein composition. J. Mol. Biol. 2009;387:628–638. doi: 10.1016/j.jmb.2009.02.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gao X, Yuan S, Jayaraman S, Gursky O. Effect of apolipoprotein A–II on the structure and stability of human high-density lipoprotein: Implications for the role of apoA-II in HDL metabolism. Biochemistry. 2012;51(23):4633–4641. doi: 10.1021/bi300555d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Reijngoud DJ, Phillips MC. Mechanism of dissociation of human apolipoprotein A–I from complexes with dimyristoylphosphatidylcholine as studied by guanidine hydrochloride denaturation. Biochemistry. 1982;21:2969–2976. doi: 10.1021/bi00541a026. [DOI] [PubMed] [Google Scholar]
- 64.Jayaraman S, Gantz DL, Gursky O. Effects of salt on the thermal stability of human plasma high-density lipoprotein. Biochemistry. 2006;45:4620–4628. doi: 10.1021/bi0524565. [DOI] [PubMed] [Google Scholar]
- 65.Gauthamadasa K, Rosales C, Pownall HJ, Macha S, Jerome WG, Huang R, Silva RA. Speciated human high-density lipoprotein protein proximity profiles. Biochemistry. 2010;49(50):10656–10665. doi: 10.1021/bi1015452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lu B, Morrow JA, Weisgraber KH. Conformational reorganization of the four-helix bundle of human apolipoprotein E in binding to phospholipid. J. Biol. Chem. 2000;275:20775–20781. doi: 10.1074/jbc.M003508200. [DOI] [PubMed] [Google Scholar]
- 67.Cavigiolio G, Shao B, Geier EG, Ren G, Heinecke JW, Oda MN. The interplay between size, morphology, stability, and functionality of high-density lipoprotein subclasses. Biochemistry. 2008;47:4770–4779. doi: 10.1021/bi7023354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hoofnagle AN, Heinecke JW. Lipoproteomics: using mass spectrometry-based proteomics to explore the assembly, structure, and function of lipoproteins. J. Lipid Res. 2009;50:1967–1975. doi: 10.1194/jlr.R900015-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Davidson WS, Silva RA, Chantepie S, Lagor WR, Chapman MJ, Kontush A. Proteomic analysis of defined HDL subpopulations reveals particle-specific protein clusters: Relevance to antioxidative function. Arterioscler. Thromb. Vasc. Biol. 2009;29:870–876. doi: 10.1161/ATVBAHA.109.186031. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.