Abstract
Disruption of cholesterol homeostasis in the central nervous system (CNS) has been associated with neurological, neurodegenerative, and neurodevelopmental disorders. The CNS is a closed system with regard to cholesterol homeostasis, as cholesterol-delivering lipoproteins from the periphery cannot pass the blood-brain-barrier and enter the brain. Different cell types in the brain have different functions in the regulation of cholesterol homeostasis, with astrocytes producing and releasing apolipoprotein E and lipoproteins, and neurons metabolizing cholesterol to 24(S)-hydroxycholesterol. We present evidence that astrocytes and neurons adopt different mechanisms also in regulating cholesterol efflux. We found that in astrocytes cholesterol efflux is induced by both lipid-free apolipoproteins and lipoproteins, while cholesterol removal from neurons is triggered only by lipoproteins. The main pathway by which apolipoproteins induce cholesterol efflux is through ABCA1. By upregulating ABCA1 levels and by inhibiting its activity and silencing its expression, we show that ABCA1 is involved in cholesterol efflux from astrocytes but not from neurons. Furthermore, our results suggest that ABCG1 is involved in cholesterol efflux to apolipoproteins and lipoproteins from astrocytes but not from neurons, while ABCG4, whose expression is much higher in neurons than astrocytes, is involved in cholesterol efflux from neurons but not astrocytes. These results indicate that different mechanisms regulate cholesterol efflux from neurons and astrocytes, reflecting the different roles that these cell types play in brain cholesterol homeostasis. These results are important in understanding cellular targets of therapeutic drugs under development for the treatments of conditions associated with altered cholesterol homeostasis in the CNS.
Keywords: Neurons, Astrocytes, ABCA1, ABCG1, ABCG4, cholesterol efflux
1. Introduction
The regulation of cholesterol homeostasis in the brain has lately become an area of intense investigation, as cholesterol and regulators of cholesterol homeostasis have been implicated in the pathogenesis of genetic and neurodegenerative diseases such as Niemann-Pick Type C and Alzheimer’s diseases [1]. Furthermore, we recently proposed that developmental neurotoxicants, such as alcohol and retinoic acid, may exert some of their deleterious effects in the brain by affecting cholesterol homeostasis [2].
Cholesterol levels and trafficking in the brain are regulated by endogenous mechanisms, as the blood-brain-barrier is impermeable to lipoproteins, preventing blood cholesterol from reaching the brain parenchyma. While both astrocytes and neurons synthesize cholesterol de novo (though in different amounts), they greatly differ in the mechanisms they adopt to control intracellular cholesterol levels and cholesterol trafficking within the brain [3]. As in the periphery, cholesterol is circulated in the brain associated with lipoproteins, which are produced by astrocytes, but not neurons; astrocytes and microglia, but not neurons, also express apolipoprotein E (apo E). Lipoproteins produced and released by astrocytes are discoidal in shape and contain apo E, phospholipids, and cholesterol, but lack in the core lipids (cholesterol esters or triglycerides). In contrast, lipoproteins found in the cerebrospinal fluid are round, contain a cholesterol ester core, are similar to plasma high density lipoproteins (HDL) [4], and are derived from the remodeling of astrocyte-secreted lipoproteins after they extract cholesterol from other cell types [5]. Lipoproteins can exit the brain through the cerebrospinal fluid, which thus represents an important route of brain cholesterol elimination [6]. Astrocyte-released lipoproteins can extract cholesterol from neurons (Fig. 1)[7], but can also interact with lipoprotein receptors on the neuronal membrane and trigger neuritogenesis and synaptogenesis; whether this effect is due to the activation of signaling pathways activated by the binding of lipoproteins to lipoprotein receptors or to the uptake, by neurons, of cholesterol and other lipids from lipoproteins remains controversial [1, 8, 9]. Excess intracellular cholesterol in neurons activates the enzyme cholesterol 24-hydroxylase (a cytochrome P450, CYP46A1), which is expressed in neurons, but not in astrocytes [10, 11], thereby metabolizing cholesterol to 24(S)-hydroxycholesterol (24(S)-HC) which freely exits neurons and the brain. Hydroxysterols, such as 24(S)-HC, through the activation of Liver X Receptors (LXR), inhibit cholesterol synthesis in many cell types, and upregulate the levels of the cholesterol transporters ATP binding cassette A1 (ABCA1) and ABCG1 and cholesterol efflux [12, 13]. Interestingly, it has been reported that neuronal 24(S)-HC increases the levels of apo E, ABCA1 and ABCG1, and cholesterol efflux in astrocytes [14, 15].
Fig.1.
Cholesterol acceptor-mediated cholesterol efflux from neurons and astrocytes. Primary rat cortical neurons and astrocytes were labeled with 1 µCi/ml [3H]cholesterol for 24 h followed by a 6 h incubation with or without apo A-I (10 µg/ml), HDL (50 µg/ml), apo E3 (15 µg/ml) or ACM. [3H]Cholesterol was quantified in the medium and in the cellular lipids. In the absence of acceptors, 1.36% +/−0.137 and 1.403% +/−0.102 of total [3H]cholesterol was found in the medium of astrocytes and neurons respectively. Data, expressed as percent of control, represent the mean (± SE) of 6–9 independent determinations; *, p < 0.05; **, p < 0.01; ***, p < 0.001 compared to control by the Dunnett’s post hoc test.
From this brief overview it is apparent that several functions regulating cholesterol levels in the brain are compartmentalized in either neurons or astrocytes, that the behavior of one cell type affects the response of the other, and that both cell types together contribute to the regulation of cholesterol levels in the brain. In the present study we investigated whether cholesterol transporter-mediated cholesterol efflux is also differentially regulated in astrocytes and neurons.
The ATP binding cassette (ABC) transporter proteins have been described as key players in regulating cholesterol efflux in the periphery. ABCA1 facilitates cholesterol efflux to lipid-free apo A-I and apo E and is involved in the biogenesis of lipoproteins, while the half-transporters ABCG1 and ABCG4 are involved in the further lipidation of nascent lipoproteins [16]. Various studies have shown that ABCA1, ABCG1 and ABCG4 are expressed in both astrocytes and neurons [17, 18].
The regulation of cholesterol efflux in the brain has been extrapolated from the model of reversed cholesterol transport characterized in the cardiovascular system; however, several aspects of cholesterol efflux specific to the brain are not fully elucidated. Of particular relevance is the fact that no detailed studies have been carried out on the role of cholesterol transporters in mediating cholesterol efflux in primary neurons in cultures. It has been generally assumed that the presence of ABCA1 and ABCG1 cholesterol transporters in cells indicate their involvement in cholesterol efflux; we show here that, while this is indeed the case in astrocytes, neurons represent an exception to this rule, and that the upregulation of ABCA1 and ABCG1 transporters in neurons does not result in upregulation of cholesterol efflux.
In a previous study, we found that cholesterol efflux in cortical astrocytes is regulated by ABCA1 and ABCG1 and that ethanol and the LXR plus Retinoid X Receptors (RXR) agonists up-regulate ABCA1 and ABCG1, and increase cholesterol efflux, leading to cellular cholesterol depletion [14, 15, 19]. In the present study we compared ABCA1- and ABCG1-mediated cholesterol efflux in astrocytes and neurons by upregulating the levels of these two cholesterol transporters with LXR/RXR agonists and with ethanol, by inhibiting ABCA1 activity and by silencing ABCA1 and ABCG1 expression. Interestingly, we found that in cortical neurons, in contrast to what observed in astrocytes, ABCA1 and ABCG1 are not involved in acceptor-mediated cholesterol efflux. In addition, we found that while the expression of ABCA1 and ABCG1 was comparable in neurons and astrocytes, the expression of another cholesterol transporter, ABCG4, is much higher in neurons than in astrocytes. Silencing ABCG4 did not affect cholesterol efflux in astrocytes but decreased lipoprotein-mediated cholesterol efflux in neurons. Cholesterol efflux thus appears to be differentially regulated in astrocytes and neurons.
2. Material and Methods
2.1. Materials
ABCA1 and ABCG1 antibodies were from Novus Biologicals (Littleton, CO). Glial fibrillary acidic protein (GFAP), neuronal specific enolase (NSE) antibodies, Alexa fluor-488 and Alexa fluor-555 secondary antibodies, TRIzol reagent, Amplex red cholesterol assay kit, Stealth RNAi ™ siRNAs selectively targeting rat ABCG1 and ABCG4, tissue culture medium, fetal bovine serum (FBS), B27 supplements, lipofectamine RNAiMAX Transfection Reagent, and Opti-MEM I were from Invitrogen (Carlsbad, CA). βII-tubulin antibody was from Chemicon International (Tamecula, CA).Lipoprotein-deficient serum (LPDS) was from Biomedical Technologies, Inc. (Stoughton, MA). Sulfo-N-hydroxysuccinimide-biotin, non-targeting and ABCA1 specific small interfering RNA (siRNA) were from Thermo Fisher Scientific (Pittsburgh, PA). Protein agarose A immunoprecipitation kit was from Roche Applied Science (Indianapolis, IN). GeneAmp® RNA PCR kit and rat ABCA1, ABCG1 and ABCG4 primers were from Applied Biosystems (Carlsbad, CA). [3H]Cholesterol and [3H]acetate were from Perkin Elmer (Covina, CA). Apo A-I, HDL and low density lipoprotein (LDL) were from EMD Biosciences (La Jolla, CA). Recombinant apo E3 was from Leinco Technologies, Inc. (St. Louis, Missouri). TLC plates were from Macherey-Nagel (Bethlehem, PA). Rat astrocyte nucleofector kit and rat neuron nucleofector kit were from Lonza (Walkersville, MD). i-Fect™ siRNA Transfection Reagent was from Neuromics (Edina, MN). All other chemicals were from Sigma Chemical Co. (St. Louis, MO). Time-pregnant Sprague-Dawley rats were purchased from Charles River (Wilmington, MA).
2.2. Cell culture
Primary cortical astrocytes were prepared from E21 Sprague-Dawley fetuses, as previously described [20]. Astrocytes were grown in Dulbecco’s Modified Eagle Medium (DMEM) containing 10% FBS, 100 units/ml penicillin, and 100 µg/ml streptomycin (FBS/DMEM medium). The treatments were carried out in serum-free DMEM supplemented with 0.1% Bovine Serum Albumin (BSA) and antibiotics.
Primary cortical neurons were prepared from E21 Sprague-Dawley fetuses following a method optimized in our laboratory by modifying a previously described method [21]. Briefly, cortices were dissected and incubated in 2 mg/ml papain with 80 µg/ml DNAase and 5 mM MgCl2 for 30 min at 37 °C. Cells were then centrifuged at 150×g, the medium containing papain was removed, and cells were resuspended in Neurobasal medium supplemented with 2% B27, 2 mM glutamax, 10 mM glucose, 0.1 mg/ml gentamicin and 1.25 µg/ml fungizone (B27/Neurobasal medium). Cells were further dissociated by pipetting, and the cell suspension was filtered through a 40 µm sterile cell strainer. Neurons were then pre-plated in 20 µg/ml poly-D-lysine (PDL)-coated flasks for 30 min. The fast-attaching cells, representing mostly glial cells, were discarded and the medium containing neurons in suspension was collected, counted, and plated on 100 µg/ml PDL-coated 60 mm2 dishes or 24-well plates at 0.4 million cells/cm2 in B27/Neurobasal medium. Unless otherwise described, cells were cultured for 6 days before treatments. The treatments were carried out in Neurobasal medium supplemented with 1% LPDS and glutamax, glucose and antibiotics at the concentration described above (LPDS/Neurobasal medium). Cortical neuronal cultures have less than 5% astrocytes, as determined by immunolabeling neuronal cultures with the astrocyte marker Glial Fibrillary Acidic Protein (GFAP) antibody and staining the nuclei with Hoechst 33258.
2.3. Western Blot Analysis
After treatment, cells were lysed in cell lysis buffer (20 mM Tris, pH 7.5, 150 mM NaCl, 1.0 mM EDTA, 1.0 mM EGTA, 1.0% Triton X-100, 50 mM sodium fluoride, 10 mM sodium pyrophosphate, 10 mM sodium β-glycerophosphate, 1.5 mM sodium orthovanadate for astrocytes or the same lysis buffer supplemented with 0.5 % sodium dodecyl sulfate for neurons) supplemented with a protease inhibitor cocktail as previously described [19]. Total protein content was quantified and equal amounts of proteins were subjected to electrophoresis, transferred to polyvinylidene difluoride membranes, and labeled with polyclonal antibodies against ABCA1 and ABCG1, followed by the appropriate horseradish peroxidase-conjugated IgG. Membranes were then re-probed for β-actin for normalization of the results. After detection, films were scanned and bands were quantified by densitometry.
2.4. Measurement of Surface ABCA1
Surface ABCA1 was measured as previously described [19]. Briefly, surface proteins of cells were labeled with sulfo-N-hydroxysuccinimide-biotin and then solubilized in lysis buffer in the presence of protease inhibitors. Total ABCA1 was then immunoprecipitated followed by Western blot analysis. The biotinylated ABCA1 was detected using a streptavidin-horseradish peroxidase conjugate. After detection, films were scanned and bands were quantified by densitometry. The sulfo-N-hydroxysuccinimide-biotin purchased from Thermo Fisher Scientific for these studies was characterized as cell membrane-impermeable, accordingly to the company specifications; we verified that this chemical is indeed cell membrane-impermeable by carrying out the following experiment: astrocytes were plated, labeled with sulfo-N-hydroxysuccinimide- biotin and solubilized in lysis buffer in the presence of protease inhibitors as described above; cell lysates were incubated with 200 µl streptavidin beads and mixed by inversion at 4 °C for 1 h; in this way, we precipitated all the biotinylated proteins. In order to verify whether intracellular proteins were found among the biotinylated proteins, we separated proteins precipitated with streptavidin beads by SDS-PAGE, transferred to PVDF membranes and then labeled for the intracellular β-actin followed by an HRP-conjugated secondary antibody and detected by chemiluminescence; the same membrane was then stripped and re-probed with streptavidin-horseradish peroxidase conjugate. While the labeling of the membranes with β-actin antibody did not show any band, the streptavidin-HRP labeling resulted in several bands, corresponding to biotinylated proteins (not shown), indicating that indeed the sulfo-N-hydroxysuccinimide-biotin is cell membrane-impermeable and bind only to cell-surface proteins.
2.5. RNA Extraction and Real Time PCR Analysis
RNA was extracted using the TRIzol reagent as per the manufacturer's optimized protocol. Total RNA concentration, purity and integrity were determined as previously described [19]. Relative mRNA expressions were determined by RT real-time PCR using ABCA1, ABCG1, and ABCG4 TaqMan® reverse transcription kits and an ABI 7900 HS sequence detection (University of Washington; UW) or a Stratagene MX 3000p system (University of Illinois at Chicago; UIC). β-Actin was used as reference gene except in the experiments in which cholesterol transporter levels were compared in astrocytes and neurons, when hypoxanthine phosphoribosyltransferase 1 (HPRT1) was used as its levels in neurons and astrocytes were comparable.
2.6. Measurement of Exogenous Cholesterol Efflux
Cholesterol efflux was measured as previously described [19]. Briefly, cells labeled with 1 µCi/ml [3H]cholesterol in the presence of 50 µg/ml LDL were treated with 75 mM ethanol, or 1 µM 22(R)-HC plus 9-cisRA for 24 h followed by a 6 h chase in fresh medium supplemented with cholesterol acceptors, apo A-I (10 µg/ml), HDL (50 µg/ml), apo E3 (15 µg/ml) or astrocyte conditioned medium (ACM). The medium was collected and centrifuged to remove detached cells, and [3H]cholesterol content in the medium and in the cellular lipids, extracted in hexane/isopropyl alcohol (3:2, v/v), was measured using a scintillation counter (Beckman LS 6000SC, UW; or Packard 1600TR, UIC). Cholesterol efflux was calculated by dividing medium [3H]cholesterol from total (medium+cell) [3H]cholesterol and is expressed as a percentage of the control.
2.7. Cholesterol Synthesis and Endogenous Cholesterol Efflux
Cholesterol synthesis and endogenous cholesterol efflux were measured as previously described [15]. Briefly, cells labeled with 25 µCi/ml [3H]acetate were treated with 75 mM ethanol, or 1 µM 22(R)-HC plus 9-cisRA in the presence or absence of HDL (50 µg/ml). Lipids were extracted from the medium and from the cell monolayer and were then separated by TLC. [3H]Cholesterol was identified by co-migration with reference standards and was quantified by a scintillation counter. An aliquot of the lipid extract from the cell monolayer was counted to assess total [3H]lipids. The relative rate of cholesterol synthesis was expressed as the sum of the [3H]radioactivity associated with intracellular and medium cholesterol divided by the [3H]radioactivity associated with the total lipid mixture. The relative efflux of endogenously synthesized cholesterol was expressed as a percentage of total cholesterol by dividing medium [3H]cholesterol from total (medium+cell) [3H]cholesterol.
2.8. Cholesterol mass
Cholesterol mass was measured by an enzymatic method using the cholesterol kit Amplex Red Cholesterol Assay according to the direction of the manufacturer, and read with a fluorescence microplate reader (563 nm absorbance and 587 nm emission) as previously described [15]. Cholesterol content was normalized to protein content and expressed as µg cholesterol/mg protein measured by the BCA protein assay.
2.9. siRNA Transfection
ABCA1 SiRNA transfections: Neurons and astrocytes were transfected using the Nucleofector™ technology (Lonza/Amaxa; Walkersville, MD) as per the manufacturer's optimized protocol. In brief, primary neurons immediately after isolation, or astrocytes harvested after 7–10 days in vitro (DIV), were resuspended in Nucleofector solution. Aliquots of neurons or astrocytes were mixed with 200 pmol ABCA1 siRNA or non-targeting siRNA and were transfected using the Nucleofector programs O-007 and T-20 respectively. Exogenous cholesterol efflux was measured 96 h post transfection. ABCA1 down-regulation in ABCA1 siRNA-transfected cells was verified by Western blot.
ABCG1 and ABCG4 Stealth RNAi™ siRNA transfections: on the day of transfection primary astrocytes were switched to a medium (DMEM with 10% FBS) without antibiotics and supplemented with 50 nM ABCG1 or ABCG4 siRNA, lipofectamine RNAiMAX Transfection Reagent, and Opti-MEM I according to the manufacturer’s instruction for 24 h followed by the removal of the medium containing transfection reagents. Six days after preparation, primary cortical neurons were shifted to a medium (Neurobasal/B27) without antibiotic; transfection was carried out by adding to the cultures a solution containing 12nM ABCG1 or ABCG4 SiRNA, i-Fect™ siRNA Transfection Reagent, and Opti-MEM I for 24 h. Exogenous cholesterol efflux was measured 48 h after the removal of the transfection reagents. The specific silencing of ABCG1 in astrocytes and neurons was confirmed by Western blot and by qRT-PCR; silencing of ABCG4 was confirmed only by qRT-PCR because no specific antibody to ABCG4 is available.
2.10. Exposure of Cells to Ethanol
To reduce ethanol evaporation, ethanol incubations were carried out in sealed chambers, which prevent alcohol evaporation, under an atmosphere of 5% CO2 and 95% air as previously described [19].
2.11. Statistical Analysis
Student’s t-test or ANOVA followed by Dunnett’s test were used to determine significant differences from controls.
3. Results
The main objective of this study was to compare mechanisms of cholesterol efflux in primary astrocytes and neurons with particular emphasis on the role of ABC cholesterol transporters.
As the purity of neuronal cultures was critical for the purpose of this study, we took extra care to verify the number of astrocytes present in neuronal cultures by assessing GFAP (astrocytes), NSE (neurons), and Hoechst (nuclei) in neurons prepared using different purification approaches. After 9 days in culture (corresponding to the maximal length of culture in the present experiments) neuronal cultures prepared with an additional step called pre-plating (in which the suspension of mixed cortex cells was pre-plated for 30 min in flasks coated with 20 µg/ml poly-D-lysine, a concentration too low to allow adequate neuronal attachment, leading to the selective attachment of astrocytes and therefore their removal from the neuronal suspension), consistently contained less than 5% astrocytes (data not shown).
3.1. Cholesterol acceptors-mediated cholesterol efflux from astrocytes and neurons
To directly compare cholesterol efflux in neurons and astrocytes, after labeling cells with [3H]cholesterol, the following cholesterol acceptors were added to astrocyte and neuronal cultures for 6 h: HDL, apo A-I, apo E3 or astrocyte-conditioned medium (ACM). Lipid-free apolipoproteins induce cholesterol efflux through their interaction with ABCA1, leading to the formation of lipoproteins [22, 23], though mechanisms of cholesterol efflux independent from ABCA1 have also been described for apo E [24]. Lipoproteins extract cholesterol by ABCA1, ABCG1, and ABCG4, but also by mechanisms independent from ABC cholesterol transporters [25]. Astrocytes release nascent discoidal lipoproteins containing apo E [4]; therefore, the use of ACM prepared from cultures of astrocytes never exposed to any of the treatments is intended to assess the effect of brain endogenous lipoproteins on cholesterol efflux in astrocytes and neurons. We found that HDL and ACM increased cholesterol efflux from both cell types (Fig. 1); in contrast, the response of astrocytes and neurons to lipid-poor apolipoproteins was very different. Indeed, apo A-I (10 µg/ml) did not increase cholesterol efflux from neurons, while it induced a 2.15-fold increase in cholesterol efflux from astrocytes. Similarly, apo E3 (15 µg/ml) increased cholesterol efflux from neurons by only 40%, compared to 260% from astrocytes (Fig. 1). These surprising results suggest that ABCA1 may be involved in cholesterol efflux from astrocytes but not from neurons. Further experiments were then aimed at investigating the role of ABC cholesterol transporters in cholesterol efflux from neurons and astrocytes.
3.2. Relative expression of ABCA1, ABCG1, and ABCG4 cholesterol transporters in astrocytes and neurons
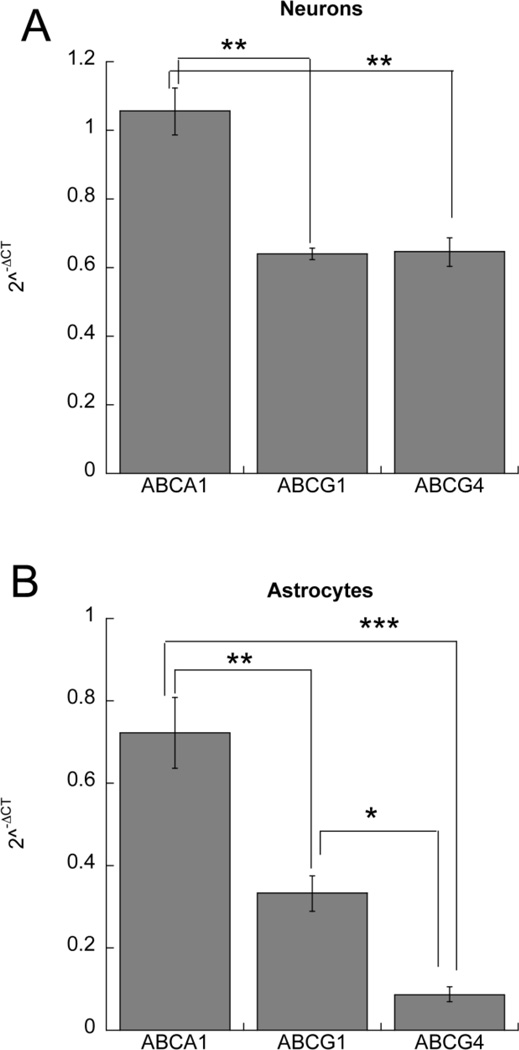
Three cholesterol transporters implicated in cholesterol efflux and lipoprotein remodeling have been described in the brain, namely ABCA1, ABCG1, and ABCG4. We compared the relative mRNA expression of these transporters in neurons and astrocytes. We first identified a reference gene that is similarly expressed in neurons and astrocytes. A reference gene commonly used in expression experiments, β-actin, was expressed at much higher levels in astrocytes than in neurons; indeed β-actin expression in neurons was 0.475 ± 0.023 fold its expression in astrocytes (p<0.001; n=4). Another reference gene, HPRT1, had similar levels of expression in these two cell types (the expression of HPRT1 in neurons was 0.92 ± 0.06 fold its expression in astrocytes; p=0.2921; n=4). For this reason, HPRT1 was used to normalize cholesterol transporter levels when comparing their expression within the same cell type and across astrocytes and neurons. In neurons, ABCA1 was the highest expressed transporter, while ABCG1 and ABCG4 expression was 60% and 63% of ABCA1 respectively (Fig. 2 A). In astrocytes, ABCA1 was also the highest expressed transporter with ABCG1 and ABCG4 at 45 % and 12% of ABCA1 respectively (Fig. 2 B). All transporters were expressed to a higher level in neurons than in astrocytes; however, the differential expression was modest for ABCA1 (ratio neurons/astrocytes = 1.46 ± 0.09-fold; p = 0.0682; n=3) and for ABCG1 (1.92 ± 0.05-fold; p = 0.0025; n=3), while expression of ABCG4 was much higher in neurons (7.39 ± 0.47-fold higher; p < 0.001; n=3). The efficiencies of the ABCA1, ABCG1, and ABCG4 primers were very high and very similar to each other (96.3%, 100.3%, and 97.3% respectively), therefore allowing for an accurate determination of the relative levels of expression of these genes in astrocytes and neurons.
Fig.2.
Relative expression of ABCA1, ABCG1 and ABCG4 in astrocytes and neurons. A: Relative mRNA expression of ABCA1, ABCG1 and ABCG4 in neurons. B: Relative mRNA expression of ABCA1, ABCG1 and ABCG4 in astrocytes. Data are expressed as 2−ΔCT (ΔCT=target gene CT - HPRT1 CT) and represent the mean (± SE) from 3 independent experiments; *, p < 0.05; **, p<0.01; ***, p<0.001 by Bonferroni’s post hoc test.
3.3. Effect of ABCA1 and ABCG1 upregulation on cholesterol efflux in astrocytes and neurons
The role of ABCA1 and ABCG1 cholesterol transporters in cholesterol efflux was evaluated in astrocytes and neurons by assessing the effect of their upregulation by ethanol (Figs. 3 and 4) or the LXR agonist 22(R)-HC in combination with the RXR agonist 9-cisRA (Figs. 5 and 6). We had previously shown that in astrocytes ethanol increases the levels of ABCA1 and ABCG1, as well as cholesterol efflux mediated by apolipoproteins and lipoproteins; the effect of ethanol was due to an increase of ABCA1 transcription and a decrease of its degradation [19]. Activation of LXR/RXR has been shown to greatly induce the transcription of ABCA1 and ABCG1 in several cell types [26, 27], including neurons and astrocytes [15, 28].
Fig.3.
Effect of ethanol on ABCA1 and ABCG1 mRNA and protein levels in neurons and astrocytes. Primary rat cortical neurons were incubated for 24 h in the presence or absence of 75 mM ethanol. Representative immunoblots from total cellular (A) and membrane-bound (B) ABCA1 and total cellular ABCG1 (C) are shown. D: Average optical density of ABCA1, ABCG1 (normalized to β-actin) and membrane-bound ABCA1 expressed as percent of control (n=4). E: Primary rat cortical neurons were incubated for 4 h, 8 h, 18 h, 24 h and 42 h in the presence or absence of 75 mM ethanol. Total RNA was extracted and ABCA1, ABCG1, and ABCG4 mRNA levels were quantified by RT real-time PCR. The results were normalized to β-actin mRNA and expressed as fold change relatively to time-matched controls (n=3). Inset: Representative immunoblots of the effect of 75 mM ethanol on ABCA1 and ABCG1 protein levels in primary cortical astrocytes and average optical density of ABCA1 and ABCG1 protein levels normalized to β-actin and expressed as % of control (n=3–5). *, p < 0.05; **, p < 0.01; ***, p<0.001 by Student’s t test.
Fig.4.
Effect of ethanol on cholesterol efflux from neurons and astrocytes. Primary rat cortical neurons (A) and astrocytes (B) labeled with 1 µCi/ml [3H]cholesterol for 24 h were incubated for an additional 24 h in the presence or absence of 75 mM ethanol followed by incubation with apo A-I (10 µg/ml), HDL (50 µg/ml), apo E3 (15 µg/ml) and ACM for 6 h. [3H]Cholesterol was quantified in the medium and in the cellular lipids. Data, expressed as percent of control, represent the mean (± SE) of 6–9 independent determinations; #, p < 0.05; ##, p < 0.01 compared to acceptor-matched controls. C: Endogenously synthesized cholesterol efflux. Primary rat cortical neurons and astrocytes pre-labeled with 25 µCi/ml [3H]acetate were incubated with ethanol (75 mM) in the presence or absence of HDL (50 µg/ml). Endogenous cholesterol efflux was calculated after lipid extraction from the medium and the cell monolayer and separation by TLC and was expressed as percent of total [3H]cholesterol. Data represent the mean ± (SE) of 3 independent determinations; **, p < 0.01; ***, p < 0.001 compared to control without acceptors; ##, p < 0.01 compared to acceptor-matched controls by Student’s t test.
Fig.5.
Effect of LXR and RXR agonists on ABCA1 and ABCG1 mRNA expression and protein levels in neurons and astrocytes. Primary fetal cortical neurons were incubated for 24 h in the presence or absence of 1µM 22(R)-HC and 1 µM 9-cisRA. Representative immunoblots from cellular (A) and membrane-bound (B) ABCA1 protein levels and ABCG1 protein levels (C) are shown. D: Average optical density of ABCA1, ABCG1 (normalized to β-actin) and membrane-bound ABCA1 expressed as percent of control (n=3). E: Primary rat cortical neurons were incubated for 4 h, 8 h, 18 h, 24 h and 42 h in the presence or absence of 1 µM 22(R)-HC plus 1 µM 9-cisRA. Total RNA was extracted and ABCA1, ABCG1, and ABCG4 mRNA levels were quantified by RT real-time PCR. The results were normalized to β-actin mRNA and expressed as fold changes relatively to time-matched controls (n=3). Inset: Representative immunoblots of the effect of 22(R)-HC plus 9-cisRA on ABCA1 cellular and membrane protein levels and ABCG1 protein levels in primary cortical astrocytes and average optical density of ABCA1 and ABCG1 protein levels normalized to β-actin and expressed as % of control (n=3–5). *, p < 0.05; **, p < 0.01; ***, p < 0.001 compared to control by Student’s t test.
Fig.6.
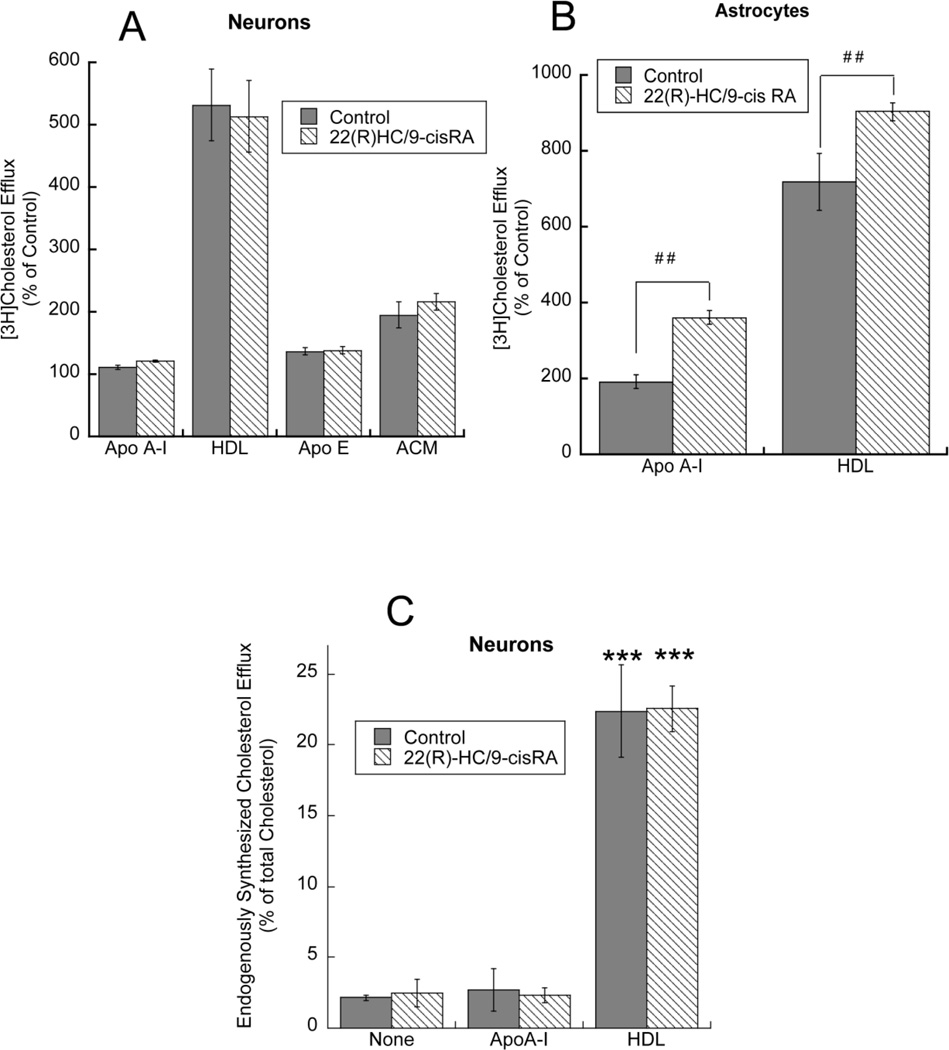
Effect of LXR and RXR agonists on cholesterol efflux from neurons and astrocytes. Primary rat neurons (A) and astrocytes (B) labeled with 1 µCi/ml [3H]cholesterol for 24 h were incubated for an additional 24 h in the absence or presence of 1 µM 22(R)-HC plus 1 µM 9-cisRA followed by incubation with apo A-I (10 µg/ml), HDL (50 µg/ml), apo E3 (15 µg/ml) or ACM for 6 h. [3H]Cholesterol was quantified in the medium and in the cellular lipids. Data are expressed as percent of control (n=6–9); ##, p < 0.01 compared to acceptor-matched controls. C: Endogenously synthesized cholesterol efflux. Primary rat cortical neurons, pre-labeled with 25 µCi/ml [3H]acetate, were incubated with 1 µM 22(R)-HC plus 1 µM 9-cisRA in the presence or absence of apo A-I (10 µg/ml) or HDL (50 µg/ml). Endogenous cholesterol efflux was calculated after lipid extraction from the medium and the cell monolayer and separation by TLC and was expressed as percent of total [3H]cholesterol. Data represent the mean ± (SE) of 3 independent determinations; ***, p < 0.001 compared to control without acceptors by Student’s t test;
Confirming our previous results, we found that ethanol (75 mM) upregulates expression of ABCA1 and ABCG1 in astrocytes (Fig. 3, inset). Similarly, in cortical neurons, ethanol upregulates total and membrane-bound (biotinylated) levels of ABCA1 and total levels of ABCG1, as well as ABCA1 and ABCG1 (but not ABCG4) mRNA levels (Fig. 3 A–E). Interestingly, the effect of ethanol on the membrane-bound levels of ABCA1 was greater than its effect on total ABCA1 levels; this finding is consistent with our previous observations in astrocytes, indicating that ethanol increases ABCA1 transcription and inhibits ABCA1 degradation at the plasma membrane [19, 29].
Ethanol has been previously shown to increase cholesterol efflux (measured after incubating [3H]cholesterol-labeled cells for 24 h with 75 mM ethanol followed by ethanol removal and incubation of cells in the presence of cholesterol acceptors apo A-I, HDL, apo E3, and ACM) in astrocytes [19], and this was confirmed in the present study (Fig. 4 B). In contrast, ethanol did not increase cholesterol efflux from cortical neurons (Fig. 4 A). Since the efflux of cholesterol derived from intracellular pools may be differentially regulated than the efflux from the plasma membrane cholesterol [30, 31], we also measured the effect of ethanol on the efflux of cholesterol endogenously synthesized from [3H]acetate. As for exogenously added cholesterol, endogenously synthesized cholesterol efflux was significantly induced by HDL in both neurons and astrocytes (Fig. 4C); however, while ethanol treatments increased the efflux of cholesterol to HDL in astrocytes, this was not the case in neurons (Fig. 4 C). The small increase in cholesterol efflux observed in astrocytes (but not neurons) after ethanol alone (Fig. 4 C) in the absence of acceptors, is likely due to the fact that ethanol stimulates the release of lipoproteins from astrocytes (Chen et al., unpublished), which act as “endogenous” cholesterol acceptors [19].
Experiments with LXR/RXR agonists provided similar results. In both cell types, LXR/RXR agonists increased the protein levels of ABCA1 and ABCG1, as well as the membrane-bound ABCA1, and the levels of ABCA1 and ABCG1 (but not ABCG4) mRNA (Fig. 5 A–E; inset); [19]. At difference with ethanol, LXR/RXR agonists greatly increased total levels of ABCA1, while membrane-bound ABCA1 was increased to a lower extent, suggesting that different mechanisms may be involved in the effects of these two treatments on ABCA1.
As found with ethanol, LXR/RXR agonists did not affect the efflux of exogenous cholesterol in neurons (Fig. 6 A), while they did so in astrocytes (Fig. 6 B), confirming our previous observations [19]. In neurons, efflux of endogenously synthesized cholesterol was stimulated by HDL but not by apo A-I and the incubation with 22(R)-HC in combination with 9-cisRA did not enhance acceptor-induced efflux (Fig. 6C).
Cholesterol efflux measured by quantifying the distribution of [3H]cholesterol in the medium and in the cells after chasing cells with cholesterol acceptors assumes that, before the addition of acceptors, cholesterol mass is the same in the tested cells. Twenty-four hour treatments with ethanol or 22(R)-HC in combination with 9-cisRA may affect cholesterol synthesis and/or cholesterol mass in neurons and/or astrocytes therefore resulting in different amounts of cholesterol present in cultures exposed to different treatments before cholesterol efflux determinations. To test whether this was the case, cholesterol mass (normalized to protein content) and cholesterol synthesis were quantified after 24 h incubations with ethanol or 22(R)-HC/9-cisRA. None of these treatments affected these parameters. Indeed, astrocyte cholesterol mass was 23.54±0.342, 24.31±0.673, and 25.11±0.667 µg cholesterol/mg proteins in control, 22(R)-HC/9-cisRA-, and ethanol-treated astrocytes respectively [p=0.755 control vs. 22(R)-HC/9-cisRA and p=0.1145 control vs. ethanol, by Dunnett’s post hoc test]. Neuronal cholesterol mass was 25.05±1.708, 27.47±0.578, and 23.94±1.075 µg cholesterol/mg in control, 22(R)-HC/9-cisRA-, and ethanol-treated neurons [p=0.3311 control vs. 22(R)-HC/9-cisRA and p=0.7384 control vs. ethanol, by Dunnett’s post hoc test]. Astrocyte cholesterol synthesis was 13.79±1.962%, 12.89±1.653%, and 12.55±3.419% [3H]cholesterol/[3H]total lipids in control, 22(R)-HC/9-cisRA-, and ethanol-treated astrocytes respectively [p=0.9488 control vs. 22(R)-HC/9-cisRA and p=0.9054 control vs. ethanol, by Dunnett’s post hoc test]. Neuronal cholesterol synthesis was 19.13±1.737%, 19.015±3.045%, and 18.54±1.133% [3H]cholesterol/[3H]total lipids in control, 22(R)-HC/9-cisRA-, and ethanol-treated neurons respectively [p=0.9988 control vs. 22(R)-HC/9-cisRA and p=0.9758 control vs. ethanol, by Dunnett’s post hoc test].
Taken together, these data establish that cholesterol content was not modified by 24 h ethanol or 22(R)-HC/9-cisRA treatments preceding the quantification of cholesterol efflux; therefore, the reported changes in cholesterol efflux are not due to preexisting differences in cholesterol content but to actual differences in the rate by which cholesterol is effluxed from cells.
Altogether these results suggest that the upregulation of ABCA1 and ABCG1 by ethanol and by LXR/RXR agonists increases cholesterol efflux from astrocytes but not from neurons; this supports the hypothesis that different mechanisms may regulate cholesterol efflux from these two cell types.
3.4. Effect of ABCA1 silencing and inhibition on acceptor-mediated cholesterol efflux from astrocytes and neurons
To further elucidate the role of ABCA1 in cholesterol efflux from astrocytes and neurons, we investigated the effects of ABCA1 inhibition on acceptor-mediated cholesterol efflux in these cell types. First, we down-regulated ABCA1 protein expression using a specific ABCA1 siRNA transfected into astrocytes and neurons (Fig. 7). Silencing ABCA1 expression in neurons did not affect cholesterol efflux to HDL and apo E in neurons (Fig. 7 A). In contrast, ABCA1 down-regulation by siRNA in astrocytes significantly decreased cholesterol efflux to several acceptors (apo A-I, apo E3, and HDL) (Fig. 7 B). The transfection of neurons and astrocytes with a non-targeting siRNA did not affect cholesterol efflux (Fig. 7 A, B). The down-regulation of ABCA1 levels in ABCA1 siRNA-transfected neurons and astrocytes was verified by Western blot analysis (Fig. 7 C, D).
Fig.7.
Effect of ABCA1 silencing and inhibition on cholesterol efflux from neurons and astrocytes. Primary rat cortical neurons (A) and astrocytes (B) were transfected with a non-target (NT siRNA) or an ABCA1 specific siRNA (ABCA1 siRNA) using the Amaxa Nucleofactor system. Seventy-two hours after transfection, cells were labeled with 1 µCi/ml [3H]cholesterol for 24 h followed by a 6 h incubation with cholesterol acceptors. [3H]Cholesterol was quantified in the medium and in the cellular lipids (n=3). #, p < 0.05; ##, p < 0.01 compared to acceptor-matched controls by Student’s t test. Representative immunoblots of ABCA1 levels (upper blots) and β-actin levels (lower blots) in neurons (C) and astrocytes (D) transfected with NT siRNA and ABCA1 siRNA. E, F: Effect of the ABCA1 inhibitor probucol on cholesterol efflux from neurons and astrocytes. Neurons (E) and astrocytes (F) labeled with 1 µCi/ml [3H]cholesterol were treated with 10 µM probucol for 4 h and with cholesterol acceptors apo A-I (10 µg/ml), HDL (50 µg/ml), apo E3 (15 µg/ml) or ACM in the presence of probucol for an additional 6 h. Cholesterol efflux was measured at the end of incubation and expressed as percent of control (n=6–9). ##, p < 0.01 compared to acceptor-matched controls by Student’s t test.
Additional experiments were carried out using probucol, an inhibitor of ABCA1 activity [32, 33]. Probucol did not affect cholesterol efflux to HDL, apo E, and ACM in neurons (Fig. 7 E), while cholesterol efflux was significantly inhibited in astrocytes (Fig. 7 F). These results confirm those obtained with siRNA and suggest that ABCA1 is involved in cholesterol efflux from astrocytes but not from neurons.
While ABCA1 siRNA transfection dramatically decreased ABCA1 levels in astrocytes (Fig. 7 D), its effect on cholesterol efflux was not as pronounced. This fact can be explained by two reasons: first, siRNA prevents the biosynthesis of new ABCA1; it is possible that the remaining ABCA1 is for the most part the membrane-bound, active ABCA1, which may be temporally the last ABCA1 pool to be affected by siRNA inhibition of biosynthesis. Second, other mechanisms, besides ABCA1, may play a role in cholesterol efflux, in astrocytes as in neurons. Indeed, ABCA1-independent mechanisms of apo A-I- and apo E-induced cholesterol efflux have been reported in other cell types. Data presented in Fig. 7 E, F support these hypotheses; indeed, when ABCA1 activity is inhibited by probucol, we observe a decrease in cholesterol efflux from astrocytes greater than the one observed after siRNA transfection; on the other hand, even probucol fails to completely inhibit cholesterol efflux from apo A-I, apo E, HDL, and ACM, confirming that other mechanisms are also involved.
3.5. Effect of ABCG1 and ABCG4 silencing on acceptor-mediated cholesterol efflux from astrocytes and neurons
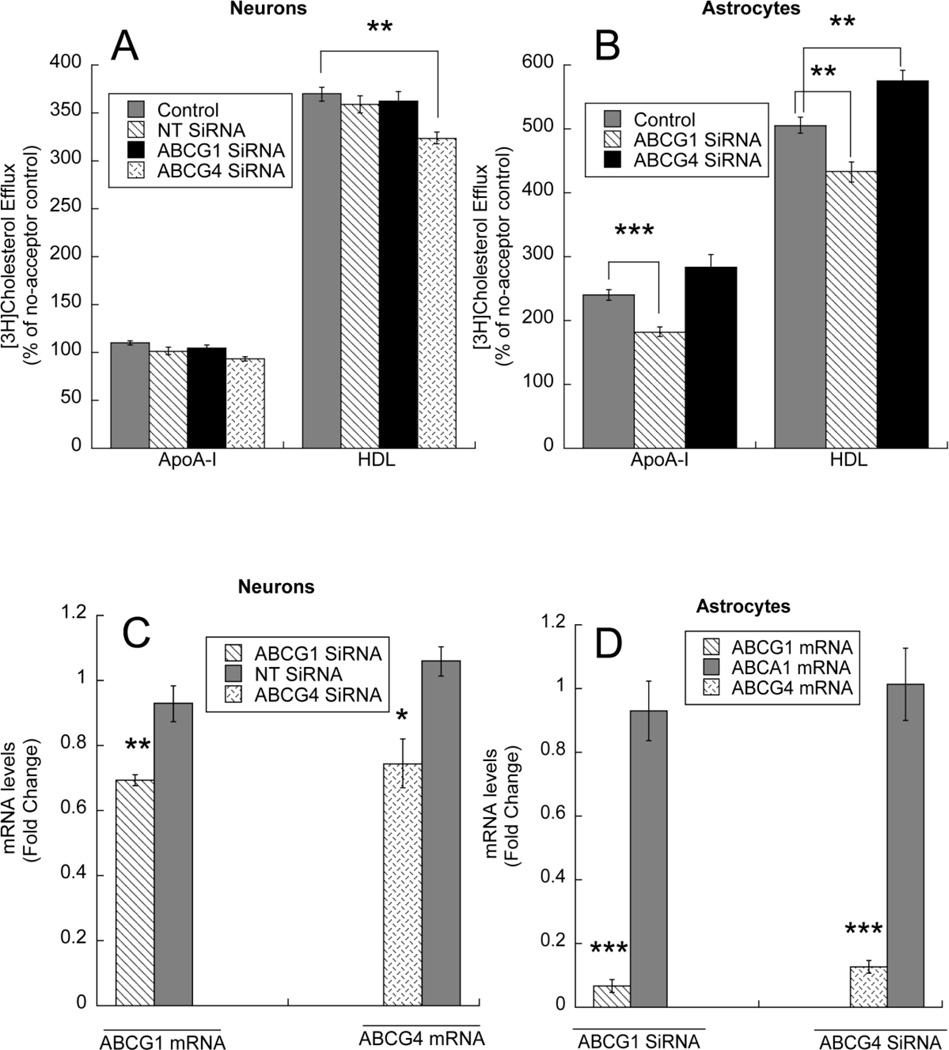
We also investigated the role of the two additional cholesterol transporters expressed in the brain, ABCG1 and ABCG4. Transfection with ABCG1 siRNA and NT siRNA did not affect cholesterol efflux from neurons, while transfection with ABCG4 siRNA significantly inhibited cholesterol efflux stimulated by HDL; as indicated earlier, apo A-I did not induce cholesterol efflux in neurons (Fig. 8 A). In contrast, transfection with ABCG1 siRNA significantly decreased cholesterol efflux induced by apo A-I and HDL in astrocytes; transfection with ABCG4 siRNA did not affect cholesterol efflux to apo A-I and increased cholesterol efflux to HDL (Fig. 8 B). The efficiency of transfection was verified by measuring ABCG1 and ABCG4 mRNA levels in cells transfected with ABCG1 siRNA and ABCG4 siRNA, respectively (Fig. 8 C, D); the specificity of siRNAs was assessed in neurons by transfecting NT siRNA and in astrocytes by testing the effect of ABCG1 siRNA and ABCG4 siRNA on ABCA1 levels. ABCG1 down-regulation was also verified by Western blot (Fig. 8 E, F, G), while ABCG4 protein levels could not be determined as no specific antibodies are available.
Fig.8.
Effect of ABCG1 and ABCG4 silencing on cholesterol efflux from neurons and astrocytes. A: Primary rat cortical neurons were transfected with a non-target (NT siRNA), an ABCG1 specific siRNA (ABCG1 siRNA) or an ABCG4 siRNA using the i-Fect™ siRNA transfection reagent. B: Primary rat cortical astrocytes were transfected with an ABCG1 siRNA or an ABCG4 siRNA using lipofectamine RNAiMAX. Twenty-four hours after transfection, cells were labeled with 1 µCi/ml [3H]cholesterol for 24 h followed by a 6 h incubation with cholesterol acceptors. [3H]Cholesterol was quantified in the medium and in the cellular lipids (n=11–12). **, p < 0.01; ***, p < 0.001 compared to acceptor-matched controls by Student’s t test. C: ABCG1 (left) and ABCG4 (right) mRNA levels were quantified by qPCR in neurons transfected with NT siRNA, ABCG1 siRNA or ABCG4 siRNA (n=4). D: the levels of ABCG1, ABCG4 and ABCA1 mRNA were determined by qPCR in ABCG1 siRNA-transfected (left) and ABCG4 siRNA transfected astrocytes (n=4). Representative immunoblots of ABCG1 levels (upper blots) and β-actin levels (lower blots) in neurons transfected with NT siRNA and ABCG1 siRNA (E) and astrocytes transfected with ABCG1 siRNA (F) and the densitometric quantification of ABCG1 levels normalized to β-actin from 4 independent determination (G) are shown.
4. Discussion
Understanding how cholesterol homeostasis is maintained and regulated in the CNS is important, as dysregulation of brain cholesterol homeostasis has been associated with neurological, neurodegenerative, and neurodevelopmental effects [34–36]. Cholesterol homeostasis in the brain is independent from the circulation as the blood-brain-barrier prevents the passage of blood lipoproteins into the brain parenchyma [37, 38].
Functions regulating the homeostasis of the metabolically active pool of cholesterol in the brain are highly segregated, with some functions present exclusively in glial cells (i.e. production and release of lipoproteins), and others exclusively in neurons (i.e. cholesterol metabolism by CYP46A1). Further differences between neurons and astrocytes were identified with regard to cholesterol synthesis; indeed neurons differ from glial cells in the levels and profiles of cholesterol biosynthetic enzymes, precursors, and metabolites, and this causes them to produce cholesterol less efficiently [11].
The present study identifies cholesterol efflux as another mechanism of brain cholesterol homeostasis that is differentially regulated in neurons and astrocytes. Indeed, we confirmed that ABCA1 is involved in cholesterol efflux from rat cortical astrocytes, while demonstrating that this transporter does not play a role in facilitating cholesterol efflux from rat cortical neurons. Our results further indicate that also ABCG1 plays a role in cholesterol efflux from astrocytes but not from neurons. A number of findings support these conclusions: 1) apo A-I did not induce cholesterol efflux under any of the test condition, while apo E slightly increased cholesterol efflux from neurons which was not affected by ABCA1 and ABCG1 upregulation, suggesting the involvement of mechanisms independent from these transporters (Figs. 1, 4, 5); in contrast, both acceptors induced cholesterol efflux from astrocytes; 2) ethanol, which significantly increased both ABCA1 and ABCG1 levels in neurons and astrocytes (Fig. 3), increased acceptor-mediated cholesterol efflux in astrocytes but not in neurons (Fig. 4); 3) the LXR/RXR agonists 22(R)-HC and 9-cisRA, which greatly increased both ABCA1 and ABCG1 levels in both neurons and astrocytes (Fig. 5), increased cholesterol efflux in astrocytes but not in neurons (Fig. 6); 4) ABCA1 and ABCG1 silencing did not affect cholesterol efflux from neurons while it decreased cholesterol efflux from astrocytes (Figs. 7,8); 5) pharmacological inhibition of ABCA1 activity did not affect cholesterol efflux in neurons while it inhibited cholesterol efflux from astrocytes (Fig. 7 E,F).
Cholesterol efflux to lipid-free apolipoproteins, namely apo A-I and apo E, is mediated by ABCA1 in many cell types including astrocytes [24, 39–41], although ABCA1-independent cholesterol efflux to both apo A-I and apo E has been reported [24, 39]. Several groups, including ours, have shown the involvement of ABCA1 in cholesterol efflux from cortical [19, 24, 42], but not cerebellar [43] astrocytes. The role of ABCA1 in cholesterol efflux from astrocytes is not surprising, as astrocytes also produce and release apo E-containing lipoproteins [4]. ABCA1 mediates the transfer of cholesterol and phospholipids from cells to lipid-poor apolipoproteins leading to the formation of nascent lipoproteins [40].
The finding that ethanol and LXR/RXR ligands upregulated total and membrane-bound ABCA1 levels without inducing cholesterol efflux in neurons was surprising. Indeed, increased levels of membrane-bound ABCA1 are associated with increased efflux of cholesterol in many cell types [12, 19, 40]. Our results suggest, instead, that the expression of ABCA1 alone is not sufficient to promote cholesterol efflux in neurons, but that additional factors are also needed. In support to this hypothesis it has been shown that ABCA1 activity is regulated by a number of kinases (such as protein kinase A, protein kinase CK2, Janus kinase 2, and protein kinase C), by partner proteins (such as the GTPase CDC42), and by factors controlling the intracellular trafficking of lipids (such as ARF-like proteins) [44]. Furthermore, increased ABCA1 expression alone may not be sufficient to increase ABCA1-mediated cholesterol efflux in adipocytes [45].
Neurons, but not astrocytes, express the cholesterol-metabolizing enzyme CYP46A1, which generates the blood-brain-barrier-permeable 24(S)-HC which can exits the brain. This represents an important pathway for the removal of excess of cholesterol from neurons and the brain [46]. In addition, 24(S)-HC released by neurons activates LXR receptors in astrocytes and induces ABCA1, ABCG1 and apo E-mediated cholesterol efflux from these cells [14]. Why ABCA1 and ABCG1 are expressed in neurons and are modulated by LXR and RXR agonists although, under physiological conditions, they do not appear to be active in mediating cholesterol efflux, remains elusive. This “insensitivity” to LXR/RXR stimulation may play a role in the overall maintenance of cholesterol homeostasis in neurons and in the whole brain, though it cannot be excluded that neurons express excess of ABCA1 and ABCG1 transporters, and therefore, further upregulation does not affect cholesterol efflux.
Two additional studies have shown that RXR and/or LXR stimulation does not increase cholesterol efflux from neurons [42] or neuroblastoma cells [14], but significantly increases cholesterol efflux from astrocytes or astrocytoma cells. On the other hand, two other studies reported an involvement of ABCA1 in facilitating cholesterol efflux from both neurons and astrocytes [28, 47]. One methodological factor that may explain these differences consists in culturing conditions; for instance, we took great care in implementing neuronal cultures with minimal astrocyte contamination (see Methods and Results). Additional differences in culturing conditions, such as the age of the cultures at the time of testing, may also be responsible for some of the discrepancies.
In contrast to apolipoproteins, we found that lipoproteins (HDL and ACM) increased cholesterol efflux with a similar potency in neurons and astrocytes (Fig. 1). In the cardiovascular system, ABCA1 is mostly involved in the initial lipidation of lipid-poor apolipoproteins, while the half-transporters ABCG1 and ABCG4 are mostly involved in transferring lipids from cells to lipoproteins [16]. According to this model, the use of different acceptors emphasizes the preferential (although not exclusive) contribution of different cholesterol transporters to cholesterol efflux. Similarly to what reported by others [17], we found that ABCG1 transcription is also upregulated by ethanol and by LXR/RXR ligands both in neurons and in astrocytes (Figs. 3 and 5). Since these treatments increased cholesterol efflux to acceptors from astrocytes but not from neurons (Figs. 4 and 6), and since ABCG1 siRNA inhibits cholesterol efflux from astrocytes but not from neurons (Fig. 8), we conclude that, similarly to ABCA1, ABCG1 is involved in cholesterol efflux from astrocytes but not from neurons.
Cholesterol efflux from peripheral tissues to lipoproteins or apolipoproteins is mediated by several mechanisms: by passive, bidirectional aqueous diffusion driven by cholesterol concentration gradient [48]; by the scavenger receptor class B type I (SR-BI) [49]; and by three ABC transporters, ABCA1 [50], ABCG1, and ABCG4 [51]. In the brain, SR-BI has been reported to be expressed in glial cells but not in neurons [52], and our present findings suggest that ABCA1 and ABCG1 are not involved in cholesterol efflux from neurons. While it is likely that a portion of lipoprotein-induced cholesterol efflux both from neurons and astrocytes may be mediated by passive diffusion, the robust cholesterol efflux induced by lipoproteins in neurons (similar to what was observed in astrocytes; Fig. 1), suggests the involvement of an active mechanism of cholesterol transport. We found that ABCG4, at difference with ABCA1 and ABCG1, is expressed at much higher levels in neurons than astrocytes (Fig. 2); furthermore, cholesterol efflux to HDL is inhibited in neurons transfected with ABCG4 siRNA but not in astrocytes (Fig. 8), indicating that this transporter, whose expression is not affected by LXR/RXR ligands and by ethanol (Fig. 3 and 5), is involved in lipoprotein-mediated cholesterol efflux from neurons, as also hypothesized by others [17].
In conclusion, the present study demonstrates that cholesterol efflux is differentially regulated in neurons and astrocytes. While ABCA1 and, likely, ABCG1 play an important role in cholesterol efflux from glial cells, the mechanism by which lipoproteins induce cholesterol efflux from neurons is not fully elucidated, though ABCG4 may play a role. These findings need to be kept in consideration in the development of therapeutic drugs for the treatments of diseases associated with disturbances of cholesterol homeostasis.
While in the case of ABCA1, siRNA findings were substantiated by experiments using the pharmacological inhibitor probucol, specific pharmacological inhibitors for ABCG1 and ABCG4 are not currently available. Because the efficiency of transfection in our experimental conditions is higher in astrocytes than in neurons, it is possible that the lack of an effect of ABCG1 siRNA on cholesterol efflux from neurons may be due to the fact that ABCG1 is not sufficiently down-regulated in these cells. However, the upregulation of ABCA1 and ABCG1 transporters by LXR/RXR agonists and ethanol does not increase cholesterol efflux from neurons, while doing so in astrocytes, supporting the proposed hypothesis that ABCG1 may not play a role in cholesterol efflux from neurons. Finally, ABCG4 siRNA reduced cholesterol efflux in neurons but not in astrocytes; this effect was significant in spite of the fact that, also in this case, the efficiency of transfection was lower in neurons than in astrocytes, suggesting that the role of ABCG4 on cholesterol efflux from neurons may be underestimated under our experimental conditions.
This study was conducted on primary cortical cultures of astrocytes and neurons and all the conclusions are based on results obtained in these in vitro systems. It is possible that, in vivo, neurons and astrocytes display a different behavior in regulating cholesterol homeostasis. Therefore, our results should be confirmed by studies carried out in more complex systems.
Highlights.
ABC cholesterol transporters are differentially expressed in neurons and astroglia.
ABCA1/ABCG1 induction increases cholesterol efflux from glia but not from neurons.
ABCA1 silencing and inhibition reduce cholesterol efflux from glia but not neurons.
ABCG1 silencing inhibits cholesterol efflux from astrocytes but not from neurons.
ABCG4 silencing reduces cholesterol efflux from neurons but not from astrocytes.
Acknowledgements
This work was supported by grant AA017180 from the National Institute of Alcoholism and Alcohol Abuse. We thank Dr. Gennaro Giordano for his help in optimizing cortical neuronal cultures, Ms. Khoi Dao for her assistance in some experiments, and Ms. Chunyan Zhou for assistance in some Western blot experiments. We remember Dr. John F. Oram (deceased) who initiated us to the investigation of cholesterol transporters.
Abbreviation used
- ABC
ATP-binding cassette
- ACM
astrocyte conditioned medium
- Apo
apolipoprotein
- DIV
day in culture
- HC
hydroxycholesterol
- LXR
liver X receptor
- PDL
poly-D-lysine
- RA
retinoic acid
- RXR
retinoid X receptor
- siRNA
small interfering RNA
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Vance JE, Karten B, Hayashi H. Lipid dynamics in neurons. Biochem Soc Trans. 2006;34:399–403. doi: 10.1042/BST0340399. [DOI] [PubMed] [Google Scholar]
- 2.Guizzetti M, Chen J, Costa LG. Disruption of cholesterol homeostasis in developmental neurotoxicity. In: Gupta RC, editor. Reproductive and developmental toxicology. New York: Elsevier; 2011. pp. 855–862. [Google Scholar]
- 3.Bjorkhem I, Leoni V, Meaney S. Genetic connections between neurological disorders and cholesterol metabolism. J Lipid Res. 2010;51:2489–2503. doi: 10.1194/jlr.R006338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.LaDu MJ, Gilligan SM, Lukens JR, Cabana VG, Reardon CA, Van Eldik LJ, Holtzman DM. Nascent astrocyte particles differ from lipoproteins in CSF. J Neurochem. 1998;70:2070–2081. doi: 10.1046/j.1471-4159.1998.70052070.x. [DOI] [PubMed] [Google Scholar]
- 5.Yu C, Youmans KL, LaDu MJ. Proposed mechanism for lipoprotein remodelling in the brain. Biochim Biophys Acta. 2010;1801:819–823. doi: 10.1016/j.bbalip.2010.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pitas RE, Boyles JK, Lee SH, Hui D, Weisgraber KH. Lipoproteins and their receptors in the central nervous system. Characterization of the lipoproteins in cerebrospinal fluid and identification of apolipoprotein B,E(LDL) receptors in the brain. J Biol Chem. 1987;262:14352–14360. [PubMed] [Google Scholar]
- 7.Kim WS, Rahmanto AS, Kamili A, Rye KA, Guillemin GJ, Gelissen IC, Jessup W, Hill AF, Garner B. Role of ABCG1 and ABCA1 in regulation of neuronal cholesterol efflux to apolipoprotein E discs and suppression of amyloid-beta peptide generation. J Biol Chem. 2007;282:2851–2861. doi: 10.1074/jbc.M607831200. [DOI] [PubMed] [Google Scholar]
- 8.Mauch DH, Nagler K, Schumacher S, Goritz C, Muller EC, Otto A, Pfrieger FW. CNS synaptogenesis promoted by glia-derived cholesterol. Science. 2001;294:1354–1357. doi: 10.1126/science.294.5545.1354. [DOI] [PubMed] [Google Scholar]
- 9.Matsuo M, Campenot RB, Vance DE, Ueda K, Vance JE. Involvement of low-density lipoprotein receptor-related protein and ABCG1 in stimulation of axonal extension by apoE-containing lipoproteins. Biochim Biophys Acta. 2011;1811:31–38. doi: 10.1016/j.bbalip.2010.10.004. [DOI] [PubMed] [Google Scholar]
- 10.Ramirez DM, Andersson S, Russell DW. Neuronal expression and subcellular localization of cholesterol 24-hydroxylase in the mouse brain. J Comp Neurol. 2008;507:1676–1693. doi: 10.1002/cne.21605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nieweg K, Schaller H, Pfrieger FW. Marked differences in cholesterol synthesis between neurons and glial cells from postnatal rats. J Neurochem. 2009;109:125–134. doi: 10.1111/j.1471-4159.2009.05917.x. [DOI] [PubMed] [Google Scholar]
- 12.Chawla A, Boisvert WA, Lee CH, Laffitte BA, Barak Y, Joseph SB, Liao D, Nagy L, Edwards PA, Curtiss LK, Evans RM, Tontonoz P. A PPAR gamma-LXR-ABCA1 pathway in macrophages is involved in cholesterol efflux and atherogenesis. Mol Cell. 2001;7:161–171. doi: 10.1016/s1097-2765(01)00164-2. [DOI] [PubMed] [Google Scholar]
- 13.Bates SR, Tao JQ, Yu KJ, Borok Z, Crandall ED, Collins HL, Rothblat GH. Expression and biological activity of ABCA1 in alveolar epithelial cells. Am J Respir Cell Mol Biol. 2008;38:283–292. doi: 10.1165/rcmb.2007-0020OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Abildayeva K, Jansen PJ, Hirsch-Reinshagen V, Bloks VW, Bakker AH, Ramaekers FC, de Vente J, Groen AK, Wellington CL, Kuipers F, Mulder M. 24(S)-hydroxycholesterol participates in a liver X receptor-controlled pathway in astrocytes that regulates apolipoprotein E-mediated cholesterol efflux. J Biol Chem. 2006;281:12799–12808. doi: 10.1074/jbc.M601019200. [DOI] [PubMed] [Google Scholar]
- 15.Chen J, Costa LG, Guizzetti M. Retinoic acid isomers up-regulate ATP binding cassette A1 and G1 and cholesterol efflux in rat astrocytes: implications for their therapeutic and teratogenic effects. J Pharmacol Exp Ther. 2011;338:870–878. doi: 10.1124/jpet.111.182196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vaughan AM, Oram JF. ABCA1 and ABCG1 or ABCG4 act sequentially to remove cellular cholesterol and generate cholesterol-rich HDL. J Lipid Res. 2006;47:2433–2443. doi: 10.1194/jlr.M600218-JLR200. [DOI] [PubMed] [Google Scholar]
- 17.Tarr PT, Edwards PA. ABCG1 and ABCG4 are coexpressed in neurons and astrocytes of the CNS and regulate cholesterol homeostasis through SREBP-2. J Lipid Res. 2008;49:169–182. doi: 10.1194/jlr.M700364-JLR200. [DOI] [PubMed] [Google Scholar]
- 18.Kim WS, Guillemin GJ, Glaros EN, Lim CK, Garner B. Quantitation of ATP-binding cassette subfamily-A transporter gene expression in primary human brain cells. Neuroreport. 2006;17:891–896. doi: 10.1097/01.wnr.0000221833.41340.cd. [DOI] [PubMed] [Google Scholar]
- 19.Guizzetti M, Chen J, Oram JF, Tsuji R, Dao K, Moller T, Costa LG. Ethanol induces cholesterol efflux and up-regulates ATP-binding cassette cholesterol transporters in fetal astrocytes. J Biol Chem. 2007;282:18740–18749. doi: 10.1074/jbc.M702398200. [DOI] [PubMed] [Google Scholar]
- 20.Guizzetti M, Costa LG. Inhibition of muscarinic receptor-stimulated glial cell proliferation by ethanol. J Neurochem. 1996;67:2236–2245. doi: 10.1046/j.1471-4159.1996.67062236.x. [DOI] [PubMed] [Google Scholar]
- 21.Brewer GJ. Serum-free B27/neurobasal medium supports differentiated growth of neurons from the striatum, substantia nigra, septum, cerebral cortex, cerebellum, and dentate gyrus. J Neurosci Res. 1995;42:674–683. doi: 10.1002/jnr.490420510. [DOI] [PubMed] [Google Scholar]
- 22.Gelissen IC, Harris M, Rye KA, Quinn C, Brown AJ, Kockx M, Cartland S, Packianathan M, Kritharides L, Jessup W. ABCA1 and ABCG1 synergize to mediate cholesterol export to apoA-I. Arterioscler Thromb Vasc Biol. 2006;26:534–540. doi: 10.1161/01.ATV.0000200082.58536.e1. [DOI] [PubMed] [Google Scholar]
- 23.Oram JF, Lawn RM. ABCA1. The gatekeeper for eliminating excess tissue cholesterol. J Lipid Res. 2001;42:1173–1179. [PubMed] [Google Scholar]
- 24.Hirsch-Reinshagen V, Zhou S, Burgess BL, Bernier L, McIsaac SA, Chan JY, Tansley GH, Cohn JS, Hayden MR, Wellington CL. Deficiency of ABCA1 impairs apolipoprotein E metabolism in brain. J Biol Chem. 2004;279:41197–41207. doi: 10.1074/jbc.M407962200. [DOI] [PubMed] [Google Scholar]
- 25.Yancey PG, Bortnick AE, Kellner-Weibel G, de la Llera-Moya M, Phillips MC, Rothblat GH. Importance of different pathways of cellular cholesterol efflux. Arterioscler Thromb Vasc Biol. 2003;23:712–719. doi: 10.1161/01.ATV.0000057572.97137.DD. [DOI] [PubMed] [Google Scholar]
- 26.Costet P, Luo Y, Wang N, Tall AR. Sterol-dependent transactivation of the ABC1 promoter by the liver X receptor/retinoid X receptor. J Biol Chem. 2000;275:28240–28245. doi: 10.1074/jbc.M003337200. [DOI] [PubMed] [Google Scholar]
- 27.Murthy S, Born E, Mathur SN, Field FJ. LXR/RXR activation enhances basolateral efflux of cholesterol in CaCo-2 cells. J Lipid Res. 2002;43:1054–1064. doi: 10.1194/jlr.m100358-jlr200. [DOI] [PubMed] [Google Scholar]
- 28.Koldamova RP, Lefterov IM, Ikonomovic MD, Skoko J, Lefterov PI, Isanski BA, DeKosky ST, Lazo JS. 22R-hydroxycholesterol and 9-cis-retinoic acid induce ATP-binding cassette transporter A1 expression and cholesterol efflux in brain cells and decrease amyloid beta secretion. J Biol Chem. 2003;278:13244–13256. doi: 10.1074/jbc.M300044200. [DOI] [PubMed] [Google Scholar]
- 29.Wang Y, Oram JF. Unsaturated fatty acids phosphorylate and destabilize ABCA1 through a phospholipase D2 pathway. J Biol Chem. 2005;280:35896–35903. doi: 10.1074/jbc.M506210200. [DOI] [PubMed] [Google Scholar]
- 30.Slotte JP, Oram JF, Bierman EL. Binding of high density lipoproteins to cell receptors promotes translocation of cholesterol from intracellular membranes to the cell surface. J Biol Chem. 1987;262:12904–12907. [PubMed] [Google Scholar]
- 31.Oram JF, Mendez AJ, Slotte JP, Johnson TF. High density lipoprotein apolipoproteins mediate removal of sterol from intracellular pools but not from plasma membranes of cholesterol-loaded fibroblasts. Arterioscler Thromb. 1991;11:403–414. doi: 10.1161/01.atv.11.2.403. [DOI] [PubMed] [Google Scholar]
- 32.Favari E, Zanotti I, Zimetti F, Ronda N, Bernini F, Rothblat GH. Probucol inhibits ABCA1-mediated cellular lipid efflux. Arterioscler Thromb Vasc Biol. 2004;24:2345–2350. doi: 10.1161/01.ATV.0000148706.15947.8a. [DOI] [PubMed] [Google Scholar]
- 33.Wu CA, Tsujita M, Hayashi M, Yokoyama S. Probucol inactivates ABCA1 in the plasma membrane with respect to its mediation of apolipoprotein binding and high density lipoprotein assembly and to its proteolytic degradation. J Biol Chem. 2004;279:30168–30174. doi: 10.1074/jbc.M403765200. [DOI] [PubMed] [Google Scholar]
- 34.Treiber-Held S, Distl R, Meske V, Albert F, Ohm TG. Spatial and temporal distribution of intracellular free cholesterol in brains of a Niemann-Pick type C mouse model showing hyperphosphorylated tau protein. Implications for Alzheimer's disease. J Pathol. 2003;200:95–103. doi: 10.1002/path.1345. [DOI] [PubMed] [Google Scholar]
- 35.Guizzetti M, Costa LG. Cholesterol homeostasis in the developing brain: a possible new target for ethanol. Hum Exp Toxicol. 2007;26:355–360. doi: 10.1177/0960327107078412. [DOI] [PubMed] [Google Scholar]
- 36.Blain JF, Poirier J. Cholesterol homeostasis and the pathophysiology of Alzheimer's disease. Expert Rev Neurother. 2004;4:823–829. doi: 10.1586/14737175.4.5.823. [DOI] [PubMed] [Google Scholar]
- 37.Bjorkhem I, Meaney S. Brain cholesterol: long secret life behind a barrier. Arterioscler Thromb Vasc Biol. 2004;24:806–815. doi: 10.1161/01.ATV.0000120374.59826.1b. [DOI] [PubMed] [Google Scholar]
- 38.Dietschy JM, Turley SD. Thematic review series: brain Lipids. Cholesterol metabolism in the central nervous system during early development and in the mature animal. J Lipid Res. 2004;45:1375–1397. doi: 10.1194/jlr.R400004-JLR200. [DOI] [PubMed] [Google Scholar]
- 39.Walter M, Forsyth NR, Wright WE, Shay JW, Roth MG. The establishment of telomerase-immortalized Tangier disease cell lines indicates the existence of an apolipoprotein A-I-inducible but ABCA1-independent cholesterol efflux pathway. J Biol Chem. 2004;279:20866–20873. doi: 10.1074/jbc.M401714200. [DOI] [PubMed] [Google Scholar]
- 40.Oram JF, Vaughan AM. ABCA1-mediated transport of cellular cholesterol and phospholipids to HDL apolipoproteins. Curr Opin Lipidol. 2000;11:253–260. doi: 10.1097/00041433-200006000-00005. [DOI] [PubMed] [Google Scholar]
- 41.Yu B, Wu J, Zhang XY, Chen LH, Yang GR, Wei MF, Xing CY, Guan YF. Liver X receptors mediate cholesterol efflux in mouse glomerular mesangial cells. Beijing Da Xue Xue Bao. 2006;38:244–248. [PubMed] [Google Scholar]
- 42.Whitney KD, Watson MA, Collins JL, Benson WG, Stone TM, Numerick MJ, Tippin TK, Wilson JG, Winegar DA, Kliewer SA. Regulation of cholesterol homeostasis by the liver X receptors in the central nervous system. Mol Endocrinol. 2002;16:1378–1385. doi: 10.1210/mend.16.6.0835. [DOI] [PubMed] [Google Scholar]
- 43.Karten B, Campenot RB, Vance DE, Vance JE. Expression of ABCG1, but not ABCA1, correlates with cholesterol release by cerebellar astroglia. J Biol Chem. 2006;281:4049–4057. doi: 10.1074/jbc.M508915200. [DOI] [PubMed] [Google Scholar]
- 44.Chait A, Han CY, Oram JF, Heinecke JW. Thematic review series: The immune system and atherogenesis. Lipoprotein-associated inflammatory proteins: markers or mediators of cardiovascular disease? J Lipid Res. 2005;46:389–403. doi: 10.1194/jlr.R400017-JLR200. [DOI] [PubMed] [Google Scholar]
- 45.Howard AD, Verghese PB, Arrese EL, Soulages JL. Characterization of apoA-I-dependent lipid efflux from adipocytes and role of ABCA1. Mol Cell Biochem. 2010;343:115–124. doi: 10.1007/s11010-010-0505-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lund EG, Xie C, Kotti T, Turley SD, Dietschy JM, Russell DW. Knockout of the cholesterol 24-hydroxylase gene in mice reveals a brain-specific mechanism of cholesterol turnover. J Biol Chem. 2003;278:22980–22988. doi: 10.1074/jbc.M303415200. [DOI] [PubMed] [Google Scholar]
- 47.Minagawa H, Gong JS, Jung CG, Watanabe A, Lund-Katz S, Phillips MC, Saito H, Michikawa M. Mechanism underlying apolipoprotein E (ApoE) isoform-dependent lipid efflux from neural cells in culture. J Neurosci Res. 2009;87:2498–2508. doi: 10.1002/jnr.22073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Johnson WJ, Mahlberg FH, Rothblat GH, Phillips MC. Cholesterol transport between cells and high-density lipoproteins. Biochim Biophys Acta. 1991;1085:273–298. doi: 10.1016/0005-2760(91)90132-2. [DOI] [PubMed] [Google Scholar]
- 49.Williams DL, Connelly MA, Temel RE, Swarnakar S, Phillips MC, de la Llera-Moya M, Rothblat GH. Scavenger receptor BI and cholesterol trafficking. Curr Opin Lipidol. 1999;10:329–339. doi: 10.1097/00041433-199908000-00007. [DOI] [PubMed] [Google Scholar]
- 50.Oram JF. ATP-binding cassette transporter A1 and cholesterol trafficking. Curr Opin Lipidol. 2002;13:373–381. doi: 10.1097/00041433-200208000-00004. [DOI] [PubMed] [Google Scholar]
- 51.Wang N, Lan D, Chen W, Matsuura F, Tall AR. ATP-binding cassette transporters G1 and G4 mediate cellular cholesterol efflux to high-density lipoproteins. Proc Natl Acad Sci U S A. 2004;101:9774–9779. doi: 10.1073/pnas.0403506101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rigotti A, Miettinen HE, Krieger M. The role of the high-density lipoprotein receptor SR-BI in the lipid metabolism of endocrine and other tissues. Endocr Rev. 2003;24:357–387. doi: 10.1210/er.2001-0037. [DOI] [PubMed] [Google Scholar]