Abstract
BACKGROUND
Carvedilol and its analogues suppress delayed afterdepolarizations (DADs) and catecholaminergic polymorphic ventricular tachycardias by direct action on the cardiac ryanodine receptor (RyR2).
OBJECTIVE
We tested a hypothesis that carvedilol analogue may also prevent triggered activities (TAs) through the suppression of early afterdepolarizations (EADs).
METHODS
Intracellular Ca2+ and membrane voltage were simultaneously recorded using optical mapping technique in Langendorff-perfused mouse and rabbit hearts to study the effect of carvedilol analogue, VK-II-36 that does not have significant beta-blocking effects.
RESULTS
Spontaneous intracellular Ca2+ elevations (SCaEs) during diastole was induced by rapid ventricular pacing and isoproterenol infusion in intact rabbit ventricles. Systolic and diastolic SCaEs were simultaneously noted in Langendorff-perfused RyR2 R4496+/− mouse hearts after creating atrioventricular block. VK-II-36 effectively suppressed SCaEs and eliminated TAs observed in both mouse and rabbit ventricles. We tested the effect of VK-II-36 on EADs using a rabbit model of acquired long QT syndrome in which phase-2 and phase-3 EADs were observed in association with systolic SCaEs. VK-II-36 abolished the systolic SCaEs and phase-2 EADs, and greatly decreased the dispersion of repolarization and the amplitude of phase-3 EADs. VK-II-36 completely prevented EAD-mediated TAs in all ventricles studied.
CONCLUSION
A carvedilol analogue, VK-II-36 inhibits ventricular tachyarrhythmias in intact mouse and rabbit ventricles by suppression of SCaEs, independent of beta-blocking activity. The RyR2 may be a potential target for treating focal ventricular arrhythmias triggered by either EADs or DADs.
Keywords: afterdepolarization, intracellular calcium, long-QT syndrome
Introduction
Afterdepolarization is defined by depolarizing changes in membrane potential following the upstroke of a preceding action potential (AP). Afterdepolarizations can occur during the repolarization phase of the AP (early afterdepolarization, EAD) or during diastole after completion of repolarization (delayed afterdepolarization, DAD). Both EADs and DADs can induce triggered activity (TA), a major mechanism of arrhythmogenesis in mammalian hearts1-3 including failing human hearts.4 It is known that beta-blockers reduce mortality in patients with heart failure,5, 6 but their effectiveness varies.7, 8 Carvedilol, a third-generation beta-blocker with well-proven clinical benefits in heart failure patients,8, 9 seems to have more antiarrhythmic potentials compared with other beta-blockers.10 Various pleiotropic actions may account for the superior benefit of carvedilol.11 Recently, Zhou et al12 demonstrated a unique effect of carvedilol on spontaneous Ca2+ release during sarcoplasmic reticulum (SR) Ca2+ overload, also known as store overload-induced Ca2+ release (SOICR). It is well known that SOICR can activate the inward Na+-Ca2+ exchange current (INCX), causing DADs.3 Carvedilol suppressed SOICR independently of its beta-blocking effect and prevented catecholaminergic polymorphic ventricular tachycardia (CPVT) in mice heterozygous for the R4496C mutation (R4496+/−) in the cardiac ryanodine receptor type 2 (RyR2) gene that likely gives rise to DAD-mediated tachyarrhythmias.12 In addition to causing DADs, spontaneous SR Ca2+ release or SOICR might also play a key role in EADs occurring at the plateau phase of the AP (i.e. phase-2 EAD) in long QT syndrome.13, 14 The phase-2 EADs can induce phase-3 EADs through electrotonic interactions in drug-induced long QT conditions.15 VK-II-36 is a carvedilol analogue that does not have significant beta-blocking effects. We studied VK-II-36 using Langendorff-perfused mouse and rabbit hearts. The purpose of these studies was to test the hypotheses that VK-II-36 can suppress not only DADs, but also EADs.
Methods
All animal studies were approved by the Animal Care Committee of the University of Calgary or the Institutional Animal Care and Use Committee of Indiana University School of Medicine, and complied with the Guide for the Care and Use of Laboratory Animals. Detailed experimental methods and the synthesis of carvedilol analogue, VK-II-36, are described in the online Data Supplement.
Optical mapping of Langendorff-perfused rabbit and mouse ventricles
These studies were done at Indiana University. The excised heart from New Zealand White female adult rabbits (n = 18) or from R4496+/−knock-in (n = 11) and wild-type littermate (n = 8) mice were perfused with oxygenated normal Tyrode’s solution using a Langendorff-perfusion system. Intracellular Ca2+ (Cai) and membrane voltage (Vm) optical signals with ECG were simultaneously recorded, as described previously.16 Bipolar electrodes were used for ventricular pacing. We performed endocardial cryoablation for a rabbit model of acquired long QT syndrome to create a quasi 2-dimensional model of rabbit ventricles.15 Atrioventricular block was created in a rabbit model with DAD-related arrhythmias16 and R4496C+/− mouse hearts.
Data Analysis
The detail of the methods for optical data processing were reported elsewhere.16 We defined the amplitude of baseline Vm and Cai transient as 1 arbitrary unit (AU). Action potential duration (APD) was measured at 70% repolarization (APD70) and APD70 dispersion was defined as a time difference between the maximal and minimal APD70 in the mapped area. Spontaneous Cai elevation (SCaE) was defined as an increase in Cai not preceded by an upstroke of the AP (i.e. not electrically-driven). Systolic and diastolic SCaEs were defined as SCaEs that occurred during the phase before and after completion of repolarization of the AP, respectively. The former was designated as Cai reelevation in our previous study.15 We defined phase-2 EAD as depolarizing afterpotentials occurring at the plateau phase of the AP. Phase-3 EAD was defined as afterpotentials that deviated the expected course of phase-3 repolarization of the AP in a depolarizing direction. The amplitude of phase-3 EAD was measured as the difference between the resting Vm and the first deviation from smooth contour during phase-3 repolarization.15
Continuous variables were expressed as mean ± SEM. Statistical analysis for continuous variables was performed by paired Student’s t-test, or by one-way repeated measure ANOVA for multiple comparisons. Fisher’s exact test was used to analyze categorical data. P ≤ 0.05 was considered statistically significant.
Results
VK-II-36 suppresses DAD-mediated arrhythmias
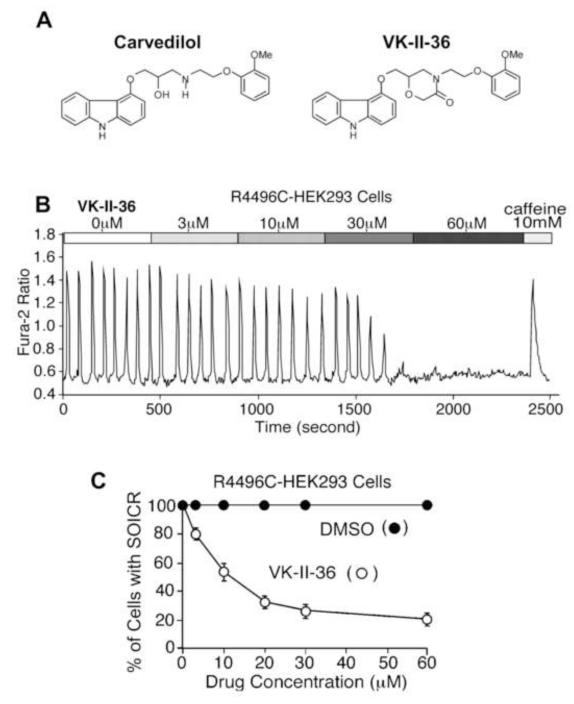
We have recently shown that several carvedilol analogues inhibit SOICR and prevent CPVT in RyR2 R4496C+/− knock-in mice.12 Here, we tested another carvedilol analogue, VK-II-36 (Figure 1A). Like the other carvedilol analogues, VK-II-36 had little beta-blocking effect. VK-II-36 had no effect on heart rate in anesthetized mice, isoproterenol-enhanced Ca2+ transient in isolated mouse ventricular cardiomyocytes, or isoproterenol-induced phosphorylation of RyR2 (Supplementary Figure panels A to C). Figure 1B shows an example of SOICR induced at an extracellular Ca2+ (1 mM) in a HEK293 cell stably expressing RyR2 with the R4496C mutation. VK-II-36 reduced the fraction of HEK293 cells that display SOICR in a dose-dependent manner (n = 403, P < 0.01). Also, VK-II-36 inhibited Ca2+ waves in ventricular cardiomyocytes isolated from R4496C+/− heterozygous mutant mice (Supplementary Figure panel D). VK-II-36 dose-dependently reduced the fraction of myocytes that display Ca2+ waves (n = 287, P < 0.01). Spontaneous diastolic Ca2+ waves are considered arrhythmogenic since resulting increase in Cai can activate Cai-sensitive transient inward currents such as INCX which in turn induce DADs and TAs.3 We have previously shown that diastolic SCaEs can be induced by a prolonged rapid ventricular pacing (cycle length 200 ms for 200 beats) under isoproterenol infusion (0.01 to 0.3 μM) in the rabbit ventricle.16 Since diastolic SCaEs represent the sum of the Ca2+ waves at the tissue level, this rabbit model was used to determine whether VK-II-36 prevents DAD-mediated ventricular arrhythmias (n = 12). Optical mapping of Cai revealed that diastolic SCaEs were observed following prolonged rapid ventricular pacing under isoproterenol infusion in all 12 hearts studied (arrowheads, Figure 2A). VK-II-36 (30 μM) suppressed the diastolic SCaE (0.139 ± 0.017 AU to 0.007 ± 0.004 AU, P < 0.001), and DAD (0.035 ± 0.011 AU to 0.00 ± 0.00 AU, P = 0.008). Ventricular arrhythmias were reproducibly induced in 7 of 12 hearts (single TAs in 2 hearts, VTs in 5 hearts). VK-II-36 (30 μM) abolished all episodes of the ventricular arrhythmias (P = 0.005, Figure 2B). Thus, VK-II-36 effectively prevented DAD-mediated ventricular arrhythmias in intact rabbit hearts.
Figure 1.
Carvedilol analogue, VK-II-36. A, Chemical structures of carvedilol and VK-II-36. B, Fura-2 ratios of a representative RyR2-R4496C expressing HEK293 cell perfused with KRH buffer containing 1 mM extracellular Ca2+ and increasing dose of VK-II-36 followed by the addition of 10 mM caffeine. Note that VK-II-36 dose-dependently inhibited spontaneous Cai oscillations (i.e. SOICR) in HEK293 cells. C, The fraction of HEK293 cells that display spontaneous Cai oscillations in the presence of various concentration of VK-II-36 (white circles, n = 403) or DMSO (black circles, n=10). P < 0.01.
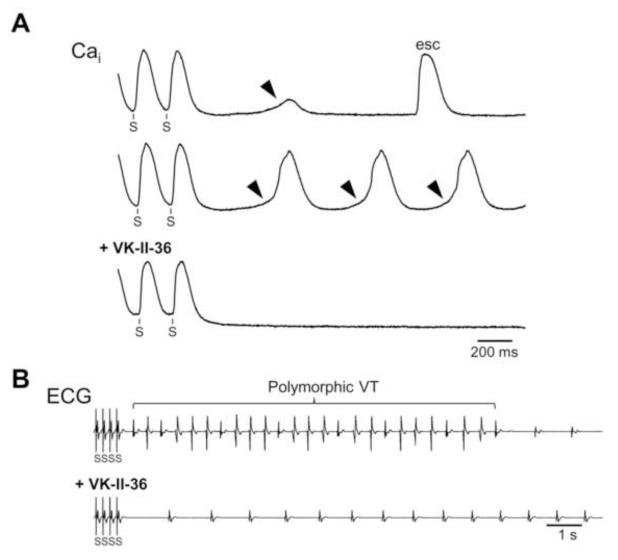
Figure 2.
Effect of carvedilol analogue on diastolic SCaEs in perfused rabbit ventricles. A, Cai optical recordings during the period following the cessation of rapid ventricular pacing (S) under isoproterenol infusion (0.1 μM). (Top) Note the transient increase in Cai level (diastolic SCaE, arrowhead) after pacing. esc = escape beat. (Middle) Cai recording during VT observed following rapid pacing. This tracing was recorded at the site of the VT origin. Note that the diastolic SCaE preceded each VT beat. (Bottom) VK-II-36 (30 μM) abolished the diastolic SCaEs and VT. B, ECGs show that VK-II-36 suppressed polymorphic VT induced after the cessation of rapid ventricular pacing under isoproterenol infusion.
VK-II-36 suppresses EAD-mediated arrhythmias and reduces dispersion of repolarization
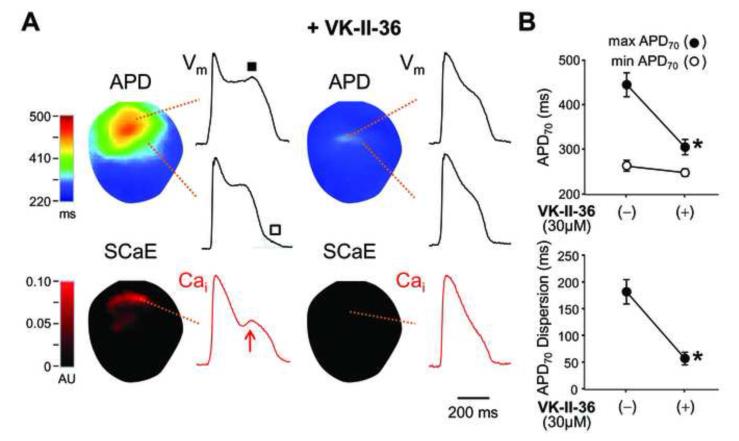
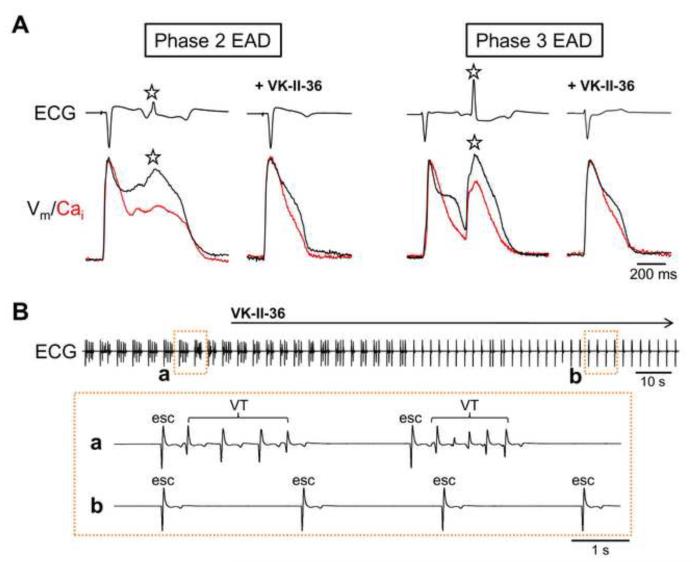
Recent evidence that phase-2 EADs and DADs share the same mechanism (i.e. inward INCX activation secondary to spontaneous SR Ca2+ release via RyR2)13, 14 prompted us to study the effect of VK-II-36 on EADs. We induced EADs by a selective IKr blocker E-4031 (0.5 μM) and 50% reduction in extracellular K+ and Mg2+ in Langendorff-perfused rabbit ventricles with endocardial cryoablation (n = 6), simulating acquired long QT syndrome.14, 15 Figure 3 shows a typical example of simultaneous optical recordings of Vm and Cai, APD map, and systolic SCaE map. The APD map shows that APDs were heterogeneously prolonged during IKr blockade with reduced extracellular K+ and Mg2+. This greatly enhanced spatial dispersion of repolarization, which is known to provide a substrate necessary for the perpetuation of lethal arrhythmias.17 As reported previously,15 phase-2 EADs (filled square) were observed only in sites with a long APD, while large phase-3 EADs (unfilled square) were noted in the boundary zone between sites with long and short APDs. Systolic SCaEs (red arrow) were also noted in the long APD sites, and phase-2 EADs were invariably accompanied by systolic SCaEs. VK-II-36 (30 μM) consistently eliminated the systolic SCaEs (0.08 ± 0.03 AU to 0.00 ± 0.00 AU, P = 0.04) and phase-2 EADs (0.03 ± 0.01 AU to 0.00 ± 0.00 AU, P = 0.067). Also, VK-II-36 decreased phase-3 EADs (0.23 ± 0.02 AU to 0.05 ± 0.02 AU, P = 0.003). Since phase-2 EADs developed only at sites with a long APD, the elimination of phase-2 EADs shortened the maximal APD70 (458 ± 37 ms to 310 ± 19 AU, P = 0.002), but did not significantly change the minimal APD70 (263 ± 12 ms to 244 ± 10 ms, P = 0.10). As a result, VK-II-36 dramatically reduced the spatial dispersion of repolarization (APD70 dispersion: 195 ± 33 ms to 66 ± 14 ms, P = 0.002; standard deviation of APD70: 48 ± 10 ms to 15 ± 4 ms, P = 0.005). The decrease in the spatial dispersion of repolarization was accompanied by a decrease in Vm gradient during repolarization (0.33 ± 0.02 AU/mm to 0.18 ± 0.03 AU/mm, P = 0.003), which accounts for the suppression of phase-3 EADs since electrotonic depolarization across a high Vm gradient underlies the mechanism of phase-3 EAD.15 With Ikr blockade and 50% reduction in extracellular K+ and Mg2+, both phase-2 and phase-3 EAD developed TAs leading to polymorphic VT (Figure 4A). VK-II-36 (30 μM) abolished the TAs and VTs induced by EADs in all hearts studied (Figure 4B). Therefore, VK-II-36 directly abolished phase-2 EADs with indirect suppression of phase-3 EADs, which eliminated the EAD-mediated ventricular arrhythmias in a rabbit model of acquired long QT syndrome.
Figure 3.
Effect of carvedilol analogue on EADs and dispersion of repolarization in a rabbit model of acquired long QT syndrome. A, APD map and systolic SCaE map are shown with a local Vm and Caioptical signals obtained at sites as indicated. Phase-2 EAD (filled square) is noted at the long APD site, while phase-3 EAD (unfilled square) was detected at the boundary zone between the long and short APD sites. The sites where systolic SCaEs (red arrow) are observed correspond to the long APD sites. Note that VK-II-36 (30 μM) abolished the systolic SCaE and phase-2 EAD, resulting in a marked reduction of repolarization heterogeneity. B, Mean ± SEM values of maximal APD70, minimal APD70, and APD70 dispersion before and after the addition of VK-II-36 (n = 6). *P < 0.01.
Figure 4.
Carvedilol analogue suppresses EAD-mediated ventricular arrhythmias. A, ECG and simultaneous Vm and Cai optical recordings in the acquired long QT syndrome model. TA (asterisk) was triggered by phase-2 EAD (left panel) or phase-3 EAD (right panel). VK-II-36 (30 μM) suppressed the TAs mediated by both phase-2 and phase-3 EADs. B, ECG during VK-II-36 administration in this model. Expanded ECGs are also shown. Polymorphic VTs disappeared immediately after initiation of VK-II-36 infusion. esc = escape beat.
VK-II-36 suppresses both systolic and diastolic SCaEs
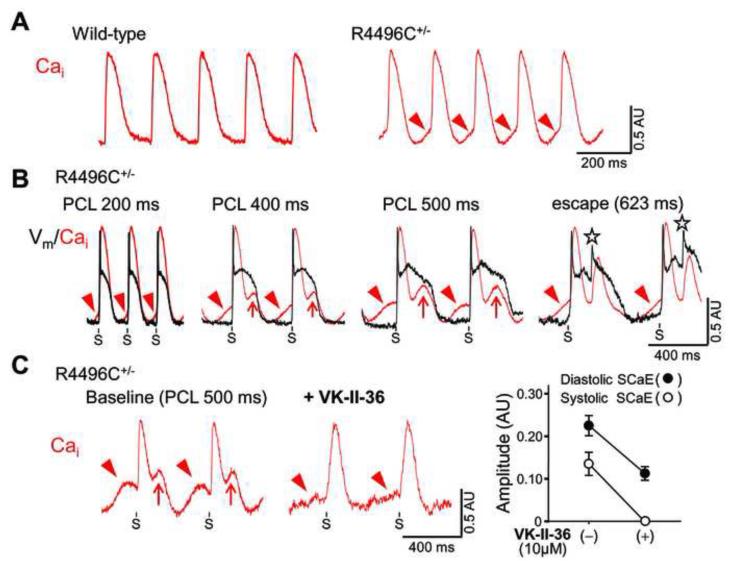
Finally, we optically mapped Cai and Vm in the epicardial surface of Langendorff-perfused ventricles isolated from RyR2 R4496C+/− mutant mice (n = 11) and wild-type littermates (n = 8). We found diastolic SCaEs during sinus rhythm at baseline in 7 of 11 (64%) RyR2 R4496C+/− mutant hearts, but not in wild-type hearts (P = 0.013, Figure 5A). Since spontaneous Ca2+ release from RyR2 more frequently occurs in ventricular myocytes with RyR2 R4496C+/− mutation,12, 18 we hypothesize that RyR2 R4496C+/− mutant ventricles may predispose to systolic SCaEs and EADs as well as diastolic SCaEs and DADs. To test this hypothesis, we created AV block in RyR2 R4996C+/− mutant hearts which showed diastolic SCaEs at baseline (n = 4) to facilitate bradycardia-dependent APD prolongation that is necessary for induction of systolic SCaEs in the rabbit model of long QT syndrome. Interestingly, bradycardia alone induced systolic SCaEs and TAs in all 4 hearts even in the absence of any drugs reducing repolarization reserve (Figure 5B). VK-II-36 (10 μM) suppressed both systolic and diastolic SCaEs (0.14 ± 0.03 AU to 0.00 ± 0.00 AU, and 0.23 ± 0.02 AU to 0.11 ± 0.01 AU, respectively, both P < 0.05, Figure 5C), indicating that carvedilol analogue has the potential for the treatment of cardiac arrhythmias associated with TA induced by either EADs or DADs.
Figure 5.
Systolic and diastolic SCaEs in perfused R4496C+/− RyR2 mouse ventricles. A, Cai optical tracings at baseline (sinus rhythm) in wild-type littermate control and R4496C+/− knock-in mice. Note diastolic SCaEs (red arrowheads) only in the R4496C+/− mouse ventricle. B, Simultaneous Cai and Vm tracings during ventricular pacing at various pacing cycle lengths (PCL) after creating AV block in the R4496C+/− mouse ventricle with diastolic SCaEs at baseline. As the cycle length was prolonged, systolic SCaEs (red arrows) became larger and TAs (asterisks) developed during escape rhythm at a cycle length of 623 ms. C, Effect of VK-II-36 (10 μM) on systolic and diastolic SCaEs. (Left panels) Representative Cai tracings before and after VK-II-36 in the R4496C+/− mouse ventricle. (Right panel) Mean ± SEM values of the amplitudes of diastolic and systolic SCaEs at a PCL of 500 ms before and after the addition of VK-II-36 (n = 4, both P < 0.05).
Discussion
Major findings of the present study include the following: 1) A carvedilol analogue with little beta-blocking effect, VK-II-36 inhibited SOICR in HEK293 cells expressing the RyR2 R4496C mutation and Ca2+ waves in isolated ventricular cardiomyocytes with the RyR2 R4496C+/− mutation. 2) VK-II-36 inhibited diastolic SCaEs and prevented DAD-mediated ventricular arrhythmias in intact rabbit hearts. 3) VK-II-36 eliminated systolic SCaEs and suppressed EAD-mediated ventricular arrhythmias in intact rabbit hearts. 4) Prolonged cycle length induced systolic and diastolic SCaEs in R4496C+/− mouse hearts. 5) VK-II-36 suppressed both systolic and diastolic SCaEs in R4496C+/− mouse hearts.
Effect of Carvedilol Analogue on DAD
Spontaneous Ca2+ waves resulting from diastolic Ca2+ release from the SR have been considered as the arrhythmogenic mechanism in some pathological conditions such as digitalis toxicity, CPVT, and heart failure, which activates the inward INCX causing DADs and TAs.3 We recently showed that carvedilol or its analogues suppressed SOICR and effectively prevented Ca2+ waves at the cellular level and CPVTs at the animal level.12 In this study, we experimentally confirmed that a carvedilol analogue suppressed DAD-mediated arrhythmias at the tissue-organ level. Diastolic SCaEs likely represent averaged signals of Ca2+ waves.16 In line with this finding, diastolic SCaEs were observed at baseline only in the R4496C+/− mutant mice (Figure 5A). VK-II-36 consistently eliminated isoproterenol-induced DAD and ventricular arrhythmias in intact rabbit hearts by the suppression of the diastolic SCaEs. Direct inhibition of Ca2+ waves with VK-II-36 is the likely mechanism of its antiarrhythmic effect, since VK-II-36 has little beta-blocking activity. How does VK-II-36 suppress arrhythmogenic Ca2+ waves? Our previous study showed that carvedilol analogues shortened the duration but increases the frequency of RyR2 channel opening.12 This type of action (i.e. open state block) diminishes Ca2+ spark mass without significant changes in SR Ca2+ leak or SR Ca2+ content. It prevents Ca2+ waves by reducing the probability of salutatory Ca2+ wave propagation between adjacent Ca2+ release units.19 Some class I antiarrhythmic drugs have been reported to also block RyR2 in an open state.20 We recently demonstrated that a class I antiarrhythmic drug, (R)-propafenone greatly inhibited diastolic SCaE and DAD in intact rabbit hearts.21
Effect of Carvedilol Analogue on EAD
In congenital or acquired long QT syndrome, life-threatening ventricular arrhythmias can develop and result in sudden cardiac death. It is thought that TAs induced by phase-2 or phase-3 EAD underlie the mechanism of ventricular arrhythmias in the long QT syndrome. Phase-2 EAD is believed to result from reactivation of L-type Ca2+ current,22 but recent experimental data showed that spontaneous SR Ca2+ release during the plateau phase of the AP (i.e. systolic SCaE) plays a critical role in the genesis of phase-2 EAD.13, 14 In addition, several observations indicate that the dispersion of repolarization, rather than absolute QT interval prolongation, is essential for the ventricular arrhythmogenesis.23, 24 The increased dispersion of repolarization not only creates a functional substrate for reentry, but also sets the stage for phase-3 EAD that is caused by electrotonic reexcitation occurring at the sites with a steep Vm gradient.15In this study, carvedilol analogue, VK-II-36 abolished systolic SCaEs and phase-2 EADs, which affirms the importance of the SR in the genesis of phase-2 EAD. The abolition of phase-2 EADs shortened APDs only in the long APD region but not in the short APD region, indicating that APD shortening was secondary to the effect of VK-II-36 on systolic SCaEs that occur only in the long APD region, but not due to non-specific effects of VK-II-36 on ionic currents responsible for repolarization. Notably, this site-selective APD-shortening effect of VK-II-36 dramatically decreased the dispersion of repolarization and Vm gradient during repolarization, thereby indirectly attenuating phase-3 EADs despite its Cai-independence.15 As a consequence, VK-II-36 completely suppressed ventricular arrhythmias in this rabbit model of acquired long QT syndrome. Thus, the systolic SCaE represents a therapeutic target for cardiac arrhythmias in long QT conditions. The inhibition of both types of EAD seems to account for the potent antiarrhythmic effects of VK-II-36.
SCaE as a Therapeutic Target for Ventricular Arrhythmias
It has been reported that EADs and DADs can occur simultaneously in the same cell or tissue under some particular conditions.25, 26 This is consistent with the notion that spontaneous SR Ca2+ release is responsible for both EADs and DADs.13, 14, 16 Furthermore, spontaneous SR Ca2+ release during late diastole can promote EADs indirectly by affecting Cai-sensitive ionic currents during the subsequent AP (i.e. DADs beget EADs).27 Hence, an upstream approach to these afterdepolarizations is theoretically preferable, especially in certain cardiac diseases such as heart failure.1-3 Induction of systolic SCaEs in the R4496C+/− mice with diastolic SCaEs confirms that both types of SCaEs are mechanistically linked and they can be the common potential therapeutic target. Consistent with this hypothesis, several recent studies documented that RyR2 inhibition may play an important role of antiarrhythmic action of multiple antiarrhythmic agents, including flecainide,28 carvedilol,12 (R)-propafenone21 and ranolazine.29
Clinical implications
Our results obtained from mouse and rabbit models suggest that inhibition of SOICR may be a promising strategy for the treatment of triggered arrhythmias. Because a relatively high dose of carvedilol is necessary to elicit the inhibitory effect on SOICR,12 the potent beta-blocking effect of carvedilol may limit its clinical use due to negative inotropic, chronotropic, and dromotropic actions when it is administered to patients who have severe cardiac dysfunction, sinus node dysfunction, or conduction disturbances. VK-II-36, or other carvedilol analogues with little beta-blocking effects12 may provide new promising antiarrhythmic agents without affecting cardiac performance and heart rate.
Conclusion
In summary, our results demonstrate that carvedilol analogue with little beta-blocking effect suppresses ventricular arrhythmias with focal mechanisms due to TAs mediated not only by DADs, but also EADs. A limitation of this study is that the carvedilol analogue may have other actions on electrical excitability in addition to the direct effect on RyR2. However, our results suggest that suppression of spontaneous SR Ca2+ release is the likely mechanism underlying their antiarrhythmic effects. Our previous study12 showed that high doses of carvedilol inhibit SOICR, but such high doses of beta-blocker could produce excessive bradycardia that may exacerbate EAD-mediated arrhythmias. Carvedilol analogues can be promising agents for treating cardiac arrhythmias of either EAD or DAD etiology which are observed in patients with heart diseases such as CPVT, long QT syndromes, and heart failure.
Supplementary Material
Acknowledgments
Sources of financial support This study was supported in part by National Institutes of Health grants P01 HL78931, R01 HL78932, 71140 (Dr. P.-S. Chen), R01 HL075210 (Dr. SRW Chen); a Nihon Kohden/St Jude Medical electrophysiology fellowship (Dr Maruyama); an American Heart Association Established Investigator Award (Dr Lin), a Medtronic-Zipes endowment (Dr. P.-S. Chen) and the Indiana University Health-Indiana University School of Medicine Strategic Research Initiative. The authors would also like to thank the generous donations from the King family and the Libin Cardiovascular Institute of Alberta, Canada.
ABBREVIATIONS
- AP
action potential
- APD
Action potential duration
- AU
arbitrary unit
- CPVT
catecholaminergic polymorphic ventricular tachycardia
- DAD
delayed afterdepolarization
- EAD
early afterdepolarization
- HEK
human embryonic kidney
- INCX
Na+-Ca2+ exchange current
- RyR2
cardiac ryanodine receptor
- SCaE
Spontaneous Cai elevation
- SOICR
store overload-induced Ca2+ release
- SR
sarcoplasmic reticulum
- TA
triggered activity
Footnotes
Conflicts of interest: none
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Fozzard HA. Afterdepolarizations and triggered activity. Basic Res Cardiol. 1992;87(Suppl 2):105–113. doi: 10.1007/978-3-642-72477-0_10. [DOI] [PubMed] [Google Scholar]
- 2.Janse MJ. Electrophysiological changes in heart failure and their relationship to arrhythmogenesis. Cardiovasc Res. 2004;61:208–217. doi: 10.1016/j.cardiores.2003.11.018. [DOI] [PubMed] [Google Scholar]
- 3.Pogwizd SM, Bers DM. Cellular basis of triggered arrhythmias in heart failure. Trends Cardiovasc Med. 2004;14:61–66. doi: 10.1016/j.tcm.2003.12.002. [DOI] [PubMed] [Google Scholar]
- 4.Pogwizd SM, McKenzie JP, Cain ME. Mechanisms underlying spontaneous and induced ventricular arrhythmias in patients with idiopathic dilated cardiomyopathy. Circulation. 1998;98:2404–2414. doi: 10.1161/01.cir.98.22.2404. [DOI] [PubMed] [Google Scholar]
- 5.Effect of metoprolol CR/XL in chronic heart failure: Metoprolol CR/XL randomised intervention trial in congestive heart failure (MERIT-HF) Lancet. 1999;353:2001–2007. [PubMed] [Google Scholar]
- 6.The cardiac insufficiency bisoprolol study II (CIBIS-II) A randomised trial. Lancet. 1999;353:9–13. [PubMed] [Google Scholar]
- 7.A trial of the beta-blocker bucindolol in patients with advanced chronic heart failure. N Engl J Med. 2001;344:1659–1667. doi: 10.1056/NEJM200105313442202. [DOI] [PubMed] [Google Scholar]
- 8.Poole-Wilson PA, Swedberg K, Cleland JG, et al. Comparison of carvedilol and metoprolol on clinical outcomes in patients with chronic heart failure in the carvedilol or metoprolol European trial (COMET): Randomised controlled trial. Lancet. 2003;362:7–13. doi: 10.1016/S0140-6736(03)13800-7. [DOI] [PubMed] [Google Scholar]
- 9.Packer M, Bristow MR, Cohn JN, et al. The effect of carvedilol on morbidity and mortality in patients with chronic heart failure. U.S. Carvedilol heart failure study group. N.Engl.J.Med. 1996;334:1349–1355. doi: 10.1056/NEJM199605233342101. [DOI] [PubMed] [Google Scholar]
- 10.Kowey PR. A review of carvedilol arrhythmia data in clinical trials. J Cardiovasc Pharmacol Ther. 2005;10(Suppl 1):S59–68. doi: 10.1177/10742484050100i407. [DOI] [PubMed] [Google Scholar]
- 11.El-Sherif N, Turitto G. Electrophysiologic effects of carvedilol: Is carvedilol an antiarrhythmic agent? Pacing Clin Electrophysiol. 2005;28:985–990. doi: 10.1111/j.1540-8159.2005.00200.x. [DOI] [PubMed] [Google Scholar]
- 12.Zhou Q, Xiao J, Jiang D, et al. Carvedilol and its new analogs suppress arrhythmogenic store overload-induced Ca(2+) release. Nat Med. 2011;17:1003–1009. doi: 10.1038/nm.2406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Volders PG, Vos MA, Szabo B, et al. Progress in the understanding of cardiac early afterdepolarizations and torsades de pointes: Time to revise current concepts. Cardiovasc Res. 2000;46:376–392. doi: 10.1016/s0008-6363(00)00022-5. [DOI] [PubMed] [Google Scholar]
- 14.Choi BR, Burton F, Salama G. Cytosolic Ca2+ triggers early afterdepolarizations and torsade de pointes in rabbit hearts with type 2 long QT syndrome. J Physiol. 2002;543:615–631. doi: 10.1113/jphysiol.2002.024570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maruyama M, Lin SF, Xie Y, et al. Genesis of phase 3 early afterdepolarizations and triggered activity in acquired long-QT syndrome. Circ Arrhythm Electrophysiol. 2011;4:103–111. doi: 10.1161/CIRCEP.110.959064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maruyama M, Joung B, Tang L, et al. Diastolic intracellular calcium-membrane voltage coupling gain and postshock arrhythmias: Role of Purkinje fibers and triggered activity. Circ Res. 2010;106:399–408. doi: 10.1161/CIRCRESAHA.109.211292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kuo CS, Munakata K, Reddy CP, Surawicz B. Characteristics and possible mechanism of ventricular arrhythmia dependent on the dispersion of action potential durations. Circulation. 1983;67:1356–1367. doi: 10.1161/01.cir.67.6.1356. [DOI] [PubMed] [Google Scholar]
- 18.Fernandez-Velasco M, Rueda A, Rizzi N, et al. Increased Ca2+ sensitivity of the ryanodine receptor mutant RYR2 R4496C underlies catecholaminergic polymorphic ventricular tachycardia. Circ Res. 2009;104:201–209. doi: 10.1161/CIRCRESAHA.108.177493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hilliard FA, Steele DS, Laver D, et al. Flecainide inhibits arrhythmogenic Ca2+ waves by open state block of ryanodine receptor Ca2+ release channels and reduction of Ca2+ spark mass. J Mol Cell Cardiol. 2010;48:293–301. doi: 10.1016/j.yjmcc.2009.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hwang HS, Hasdemir C, Laver D, et al. Inhibition of cardiac Ca2+ release channels (RYR2) determines efficacy of class I antiarrhythmic drugs in catecholaminergic polymorphic ventricular tachycardia. Circ Arrhythm Electrophysiol. 2011;4:128–135. doi: 10.1161/CIRCEP.110.959916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee YS, Maruyama M, Chang PC, et al. Ryanodine receptor inhibition potentiates the activity of na channel blockers against spontaneous calcium elevations and delayed afterdepolarizations in langendorff-perfused rabbit ventricles. Heart Rhythm. 2012;9:1125–1132. doi: 10.1016/j.hrthm.2012.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Clancy CE, Rudy Y. Linking a genetic defect to its cellular phenotype in a cardiac arrhythmia. Nature. 1999;400:566–569. doi: 10.1038/23034. [DOI] [PubMed] [Google Scholar]
- 23.Antzelevitch C. Arrhythmogenic mechanisms of QT prolonging drugs: Is QT prolongation really the problem? J Electrocardiol. 2004;(37 Suppl):15–24. doi: 10.1016/j.jelectrocard.2004.08.004. [DOI] [PubMed] [Google Scholar]
- 24.Vos MA, van Opstal JM, Leunissen JD, Verduyn SC. Electrophysiologic parameters and predisposing factors in the generation of drug-induced torsade de pointes arrhythmias. Pharmacol Ther. 2001;92:109–122. doi: 10.1016/s0163-7258(01)00162-0. [DOI] [PubMed] [Google Scholar]
- 25.Patterson E, Szabo B, Scherlag BJ, Lazzara R. Early and delayed afterdepolarizations associated with cesium chloride-induced arrhythmias in the dog. J Cardiovasc Pharmacol. 1990;15:323–331. doi: 10.1097/00005344-199002000-00021. [DOI] [PubMed] [Google Scholar]
- 26.Priori SG, Corr PB. Mechanisms underlying early and delayed afterdepolarizations induced by catecholamines. Am J Physiol. 1990;258:H1796–H1805. doi: 10.1152/ajpheart.1990.258.6.H1796. [DOI] [PubMed] [Google Scholar]
- 27.Weiss JN, Garfinkel A, Karagueuzian HS, Chen PS, Qu Z. Early afterdepolarizations and cardiac arrhythmias. Heart Rhythm. 2010;7:1891–1899. doi: 10.1016/j.hrthm.2010.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Watanabe H, Chopra N, Laver D, et al. Flecainide prevents catecholaminergic polymorphic ventricular tachycardia in mice and humans. Nat Med. 2009;15:380–383. doi: 10.1038/nm.1942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Parikh A, Mantravadi R, Kozhevnikov D, et al. Ranolazine stabilizes cardiac ryanodine receptors: A novel mechanism for the suppression of early afterdepolarization and torsades de pointes in long QT type 2. Heart Rhythm. 2012;9:953–960. doi: 10.1016/j.hrthm.2012.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.