Introduction
Asthma is at a historically high prevalence among children in the U.S., with significant implications for health and public health systems. Pervasive disparities in the prevalence, severity and morbidity of this disease have been identified among minority populations and research suggests that multiple interrelated factors contribute to these health disparities [1-4]. Among these factors, evidence is sufficient to infer a causal relationship between parental smoking and ever having asthma among children of school age and is suggestive of a causal relationship between second hand smoke (SHS) exposure from parental smoking and the onset of childhood asthma [5]. Evidence also indicates that SHS exposure reduction is associated with fewer episodes of poor asthma control, respiratory related emergency room visits, and hospitalizations[6]. An estimated 11% of children under six years of age are reported to be regularly exposed to household SHS; exposure is significantly higher in households at and below the poverty level[7]; and many children with asthma continue to experience household exposure to SHS[5, 8].
Recognizing that reducing this exposure can confer substantial health benefits has led to the design and testing of interventions to reduce household SHS. Some studies utilizing intensive strategies (e.g., multiple clinic or home counseling sessions, follow-up phone calls, culturally tailored educational materials) have been found to reduce smoking in the home and children’s exposure, including among racially and ethnically diverse low-income households with healthy children[5, 9-11] and children with asthma,[12-14] and to reduce asthma health-care utilization[15]. Other studies testing multiple sessions led by trained research personnel[16] or bilingual/bicultural lay personnel[17], or where programs targeted a range of asthma triggers[18], have found no impact. Where successful, these approaches are encouraging, although it is unclear that they can be disseminated and maintained, particularly in care and educational settings serving low-income and minority children.
Brief educational interventions, including in clinical settings, have achieved short-term willingness to take protective steps in the homes of sick children[19], but, for the most part, have not resulted in reduced SHS exposure[20, 21] including among parents of children with asthma[22]. Strategies are needed that are effective and likely to be successfully implemented in diverse settings for this at-risk population. We report on a randomized controlled trial conducted between 1996 and 2001 to evaluate a minimal contact behavioral counseling intervention to decrease household SHS exposure among children with asthma from low-income, predominantly ethnic minority families. All study procedures were approved by the UCLA Office for the Protection of Human Subjects.
Methods
Participants
Study participants were children with asthma and a parent or guardian from each child’s household. A convenience sample was recruited of 242 children 2-14 years of age with asthma who, when a parent/guardian was screened, were identified as living in households with at least one current smoker and where smoking had occurred at home, either indoors or outdoors, at least once in the previous month. Affirmative responses to any of the following items (modified from Severson, et al.[23]) resulted in inclusion in the pool of eligible participants: (1) Do you smoke? (2) Does anyone else who lives in your household smoke? (3) Has anyone actually smoked at your home in the previous month? Recruitment occurred at 15 outpatient clinics, mobile asthma vans, schools, community agencies and hospital emergency rooms throughout Los Angeles serving predominantly low-income, medically underserved populations. Potential participants were screened for eligibility by a bi-lingual (Spanish/English) study coordinator either in-person at the recruitment site or by telephone. Eligible subjects were advised of the study’s objectives, procedures, risks and benefits; offered a potential total honorarium of up to $135 for participation; and if they chose to participate, guided through the UCLA Institutional Review Board’s informed consent procedure. Study participants received partial payment at time of completion of each study component. All field procedures were conducted by trained bicultural/bilingual (Spanish/English) staff members.
Intervention
This is a multi-dimensional conceptual/theoretical framework that synthesizes major theoretical formulations, taking socio-cultural, environmental, and individual factors into account. The Framework has been utilized in community-based research among diverse populations, reported in the research literature, and guided our preliminary work in the study on which we currently are reporting [24-26]. This preliminary work involved two 2-hour focus groups conducted among 8-10 children with asthma and their parents who were not included in the main study. These participants were recruited through a Los Angeles area school district serving a low-income predominantly minority (Latino, African American) population. In the focus groups, the children and parents separately completed draft baseline surveys, and parents participated in an audio-taped group discussion of potential intervention themes and strategies.
The intervention included a tailored Spanish/English video that addressed implications of SHS exposure for children with asthma; possible efficacy of household SHS exposure reductions on the child’s health and frequency of asthma attacks; and strategies to reduce household SHS exposure. A companion Spanish/English workbook was provided to reinforce the messages in the DVD and encourage discussion among household members, including among family members not participating in the study, regarding implementation of household change. Brief counseling consisted of asking participants to use the DVD and workbook, Field staff was specifically advised that no other information should be provided, and therefore no script was used. Parents were also instructed regarding data collection, placement of monitors, and collection of urine samples. Booster elements, successful in asthma self-management interventions, [13, 27] were provided (refrigerator magnet, mug, “no smoking” signs to place in rooms designated as “off limits” to smoking and in the family car) as reminders. Control group participants received standard brochures describing the importance of SHS exposure as an asthma trigger.
Data Collection and Measures
Sealed envelopes holding randomized group assignments were opened by the field coordinator following baseline data collection.
Baseline data were collected in-person at the time of recruitment or at a later scheduled appointment at the recruitment site according to participant preference. At the baseline visit, parents completed a survey administered by a field coordinator in the language (Spanish/English) preferred by the participant, with the coordinator reading each question to the participant and recording the participant’s response. As part of the survey, each parental respondent was asked to create a household roster that included all people residing in the home, including family, friends, relatives, boarders, and any visitors who “spend time in your home and smoke on a regular basis, such as relatives, in-laws, other children, friends, babysitters or others, at least 4 days a week or more.” The smoking status of each person on the roster was determined. For each smoker, the parent was asked the following questions: (1) On the days that (you smoke/PERSON smokes), about how many cigarettes (do you/does PERSON) usually smoke at home per day? (2) When (you are/PERSON is) at home, where (do you/does PERSON) usually smoke? Would you say (you smoke/PERSON smokes) outdoors only, indoors only, both indoors and outdoors, or (do you/does PERSON) not smoke at home? (3) In a typical week, what is your best estimate about the number of hours (you smoke/PERSON smokes) (inside your home/outside your home/in the car) in a week. Remember to think about both weekends and weekdays, evenings, and daytime. (4) Thinking about the number of hours (you smoke/ PERSON smokes) (inside your home/outside your home/in the car) per week, about how many of these hours do you think your child was (in the same room/in the same place/in the car) when (you were/PERSON was) smoking?
We used the parental survey information to calculate summary measures of household smoking, including total number of smokers in the home; paternal and maternal smoking status; total number of cigarettes smoked per day in the home, summed across all household smokers; total number of hours smoked per week (summed across all household smokers, at home indoors, at home outdoors, and in the car); and total number of hours the child with asthma was thought to be exposed to SHS in the home (indoors at home, outdoors at home, and in the car).
Each parent was asked to read a series of statements and mark those that described how they handled smoking inside their homes: “People are allowed to smoke anywhere and anytime inside my home”; “People are allowed to smoke only in certain areas or rooms inside my home”; “People are allowed to smoke only at certain times inside my home”; “Smoking inside my home is allowed only for special guests or on special occasions”; and/or “No one is allowed to smoke at all inside my home.” The responses were used to categorize households dichotomously as having either (1) total household smoking restrictions (“full smoking ban”) or (2) permitting smoking in the home, either at some places or times (e.g., when the child is not present) or with no restriction whatsoever (“partial/no smoking ban”) [28-30].
Other survey items included household demographics, child’s asthma severity, asthma-related quality of life, and attitudes towards smoking. For Spanish-speaking Latino participants, a five-item language usage scale was administered to assess acculturation [31]. A separate survey administered to children 7 years or older assessed the child’s perception of quality of life relating to asthma and of household smoking behavior (estimates of time household members smoked in the home weekly, number of hours of SHS exposure weekly, inside/outside the home and in the car, attitudes towards this exposure). For these children, urine samples were collected for analysis of urine cotinine, a biomarker for SHS exposure. The urine samples were collected in sterile specimen cups at time of survey, transported in coolers for immediate freeze-up, and stored frozen (-20 C) at the UCLA study office. The samples were then batch mailed to the Centers for Disease Control and Prevention, Division of Environmental Health Laboratory Sciences, for analyses using isotope dilution high performance liquid chromatography/atmospheric pressure chemical ionization tandem mass spectrometry (ID HPLC-APCI MS/MS) [32].
Air nicotine monitor readings have been found to correspond closely with self-reported measures of household smoking exposure [33-35]. Therefore at the time of the in-person data collection, parents/guardians were provided with two passive air nicotine monitors, each consisting of a sodium bisulfate-treated filter held in a 37 mm polystyrene cassette with a windscreen and relying on passive diffusion of nicotine to the filter [36] and asked to place the filters in a “major activity room” in their home (e.g., where family watch television) for two consecutive seven-day periods, with each filter used for a one week period. Each monitor was provided in a cup with a label that included the participant’s study ID number and the specific dates on which the air monitors were to be placed in the home and mailed back to UCLA in a provided mailer envelope. A phone call was made to remind participants to return the first week filter. When this filter was received at UCLA, a call was made to acknowledge receipt and remind participants to place and then return the second week monitor. All monitors were returned to UCLA and batch mailed to the University of California, Berkeley, School of Public Health where filter contents were desorbed in water and analyzed using gas chromatography. Cotinine and air monitor analyses were blind to condition assignment and questionnaire results.
Follow-up data were collected in-person at six months at the clinic, school or home according to participant preference, and by telephone at 12 months following baseline. At both assessments, in addition to the baseline survey domains, parents/guardians were asked about changes in household smoking practices and SHS exposure. Urine cotinine analysis and air nicotine monitoring were repeated at the 6-month follow-up.
Analysis
Intervention/control group differences in child and parent/guardian demographic characteristics were evaluated using t and chi-square tests. SHS measures included child’s urine cotinine levels (ng/mL); household average nicotine level (g/m3) over the two-week monitoring period; total number of hours of smoking weekly in the household; total number of hours of child’s exposure to second-hand smoke weekly; total number of cigarettes smoked in the household daily; and whether or not a full household smoking ban was in place. Differences in the distributions of these measures between the intervention and control groups at baseline and between subjects retained and not retained at follow-up were assessed using chi-square tests (full household smoking ban) or Wilcoxon rank sum tests (all other measures). Changes in SHS measures between baseline and follow-up within groups were assessed using McNemar’s test (full household smoking bans) or one-sample t-tests on change variables (follow-up minus baseline; all other measures). For SHS measures other than full household smoking bans, differences in change from baseline to follow-up between groups were assessed using two-sample t-tests on change variables. Differences between groups in the proportions of respondents with full household smoking bans at each follow-up assessment were compared using chi-square tests.
To assess the validity of self-report of household smoking bans, air nicotine levels between households reporting full versus partial/no smoking bans were compared using Wilcoxon signed-rank tests. To assess child SHS exposure outside the household, we examined the urine cotinine levels of children in households with zero air nicotine levels.
A two-sided significance level of 0.05 was used for all tests. Analyses were performed using Stata Version 11.
Results
Table 1 provides demographic characteristics of participants. The children were on average approximately nine years old (range = 2-14) and most were male. No children who smoked were identified. Most parent respondents were mothers of the child participant, Latino, married/cohabiting, had less than high school education, and had annual income less than $10,000. There were no significant differences in demographic characteristics between the intervention and control groups (all p-values >.05).
Table 1.
Characteristics of child and parent participants (N=242)
| Intervention | Control | Total | |
|---|---|---|---|
| Child demographics | N=118 n (%) |
N=124 n (%) |
N=242 n (%) |
| Age of child (years): Mean (SD) | 8.9 (3.0) | 8.5 (3.0) | 8.7 (3.0) |
| Child’s gender | |||
| Male (%) | 75 (64) | 74 (60) | 149 (62) |
| Female (%) | 43 (36) | 50 (40) | 93 (38) |
| Parent demographics | |||
| Relationship to child | |||
| Father | 5 (4) | 9 (7) | 14 (6) |
| Mother | 110 (93) | 107 (86) | 217 (90) |
| Other1 | 3 (3) | 8 (6) | 11 (4) |
| Ethnicity | |||
| Latino (%) | 73 (62) | 68 (56) | 141 (60) |
| Black (%) | 27 (23) | 32 (26) | 59 (25) |
| White (%) | 16 (14) | 20 (17) | 36 (15) |
| Other (%) | 2 (2) | 1 (1) | 3 (1) |
| Marital status | |||
| Married/cohabitation (%) | 73 (62) | 74 (60) | 147 (61) |
| No partner in home (%) | 45 (38) | 50 (40) | 95 (39) |
| Education | |||
| Less than high school (%) | 53 (45) | 58 (47) | 111 (46) |
| Completed high school (%) | 34 (29) | 33 (27) | 67 (28) |
| More than high school (%) | 31 (26) | 33 (27) | 64 (26) |
| Income | |||
| < $10,000 (%) | 54 (48) | 47 (40) | 101 (44) |
| $10-20,000 (%) | 36 (32) | 34 (29) | 70 (30) |
| > $20,000 (%) | 22 (20) | 38 (32) | 60 (26) |
Some numbers do not sum to the total due to missing data. Comparisons between the intervention and control groups were conducted using t- tests (age) and chi-square tests (all other variables). All p-values were >0.05.
Other parent includes grandmother, aunt, foster mother, legal guardian, and stepfather.
Table 2 reports baseline SHS measures, overall and by group. Child’s urine cotinine and household nicotine level were collected for only 60% and 67% of respondents, respectively. No significant intervention and control group differences were found for any baseline SHS measure (p-value >.05, Wilcoxon rank sum test). Respondents reported a median of 7 hours of household smoking per week and 0.5 hours per week of child exposure to SHS. Forty-eight percent of respondents reported full household smoking bans.
Table 2.
Baseline secondhand smoke measures
| Intervention participants | Control participants | All participants | |||||
|---|---|---|---|---|---|---|---|
| Measure | N=118 | N=124 | N=242 | ||||
| Response N |
Median (Range) | Response N |
Median (Range) | P-value* | Response N |
Median (Range) | |
| Child’s urine cotinine (ng/mL) |
72 | 0.80 (0.07-27.3) | 74 | 0.65(0.1-33.2) | 0.96 | 146 | 0.76 (0.07-33.2) |
| Air nicotine concentration (g/m3) |
83 | 0.04 (0-9.6) | 79 | 0.05 (0-12.7) | 0.49 | 162 | 0.05 (0-12.7) |
| Hours of weekly household smoking |
111 | 8.0 (0.25-132) | 116 | 6.0 (0-128) | 0.37 | 227 | 7 (0-132) |
| Hours of child’s weekly exposure to SHS |
117 | 0.75 (0-83) | 124 | 0.5 (0-100) | 0.81 | 241 | 0.5 (0-100) |
| Number of household cigarettes smoked daily |
114 | 6.8 (1-120) | 117 | 10 (0-120) | 0.25 | 231 | 8 (0-120) |
| N (%) | N (%) | N (%) | |||||
| Household smoking ban | 116 | 53 (46%) | 124 | 61 (49%) | 0.30 | 240 | 114 (48%) |
Differences between groups were assessed using the Wilcoxon rank sum test.
Differences from 0.5 were tested using two-sided t-test for household smoking bans in each intervention/control condition.
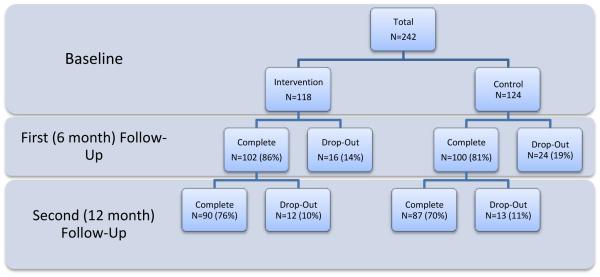
Figure 1 shows participant flow. Due to attrition, the sample was reduced to 202 households at first follow-up and 177 at second follow-up. The proportions of dropouts in each condition were similar (p-values >.05 by chi-square test). Child participants who dropped out had higher baseline cotinine (n = 27, median = 2.13 ng/mL, range = 0.13-14.7 ng/mL) than those retained at first follow-up (n = 119, median=0.675 ng/mL, range = .067-33.2 ng/mL; p= .024 by Wilcoxon rank sum test); no differences were found in demographic or baseline parental smoking characteristics or other baseline SHS measures between these groups.
Figure 1.
Flow chart of study retention (N=242).
Changes in SHS measures assessed at first and second follow-up time points are reported in Table 3. No intervention/control group differences were found in change between baseline and either first or second follow-up for any measure. Significant changes within both groups were found for number of smoking hours and number of hours of child’s exposure to SHS.
Table 3.
Changes in secondhand smoke measures at follow-up assessments
| Intervention participants | Control participants | All participants | |||||
|---|---|---|---|---|---|---|---|
| Response N |
Mean change from baseline (SD) or percentage point change |
Response N |
Mean change from baseline (SD) or percentage point change |
P-value, difference in change over time between groups |
Response N |
Mean change (SD) or N (%) |
|
| 6-month follow-up | |||||||
| Child’s urine cotinine (ng/mL) |
39 | −0.13 (2.4) | 33 | +0.10 (3.0) | 0.72 | 72 | −0.02 (2.7) |
| Air nicotine concentration (g/m3) |
83 | +0.14 (2.6) | 79 | −0.23 (1.7) | 0.29 | 162 | −0.04 (2.2) |
| Hours of weekly household smoking |
95 | −7.4 (27.0)* | 88 | −5.5 (22.3)* | 0.61 | 183 | −6.5 (24.8) |
| Hours of child’s weekly exposure to SHS |
102 | −2.5 (8.2)* | 100 | −4.0 (15.6)* | 0.38 | 202 | −3.2 (12.4) |
| Household smoking ban | 100 | +17%* | 99 | +16%* | 0.54 | 199 | +16% |
| 12-month follow-up | |||||||
| Hours of weekly household smoking |
85 | −11.1 (26.6)* | 80 | −6.6 (19.9)* | 0.25 | 165 | −9.0 (23.6) |
| Hours of child’s weekly exposure to SHS |
90 | −4.2 (10.9)* | 87 | −5.5 (16.8)* | 0.53 | 177 | −4.8 (14.0) |
| Number of household cigarettes smoked daily |
87 | −10.3 (14.0)* | 78 | −10.8 (20.5)* | 0.85 | 165 | −10.5 (17.3) |
| Household smoking ban | 88 | +25%* | 87 | +30%* | 0.59 | 175 | +28% |
Intervention/control group differences were assessed using chi-square tests (household smoking ban) or two-sample t-tests on change variables (all other variables). Change over time within group was assessed using McNemar’s test (household smoking ban) or one-sample t-tests on change variables (all other variables).
Indicates change over time within group (intervention/control) is different from zero at p<0.05.
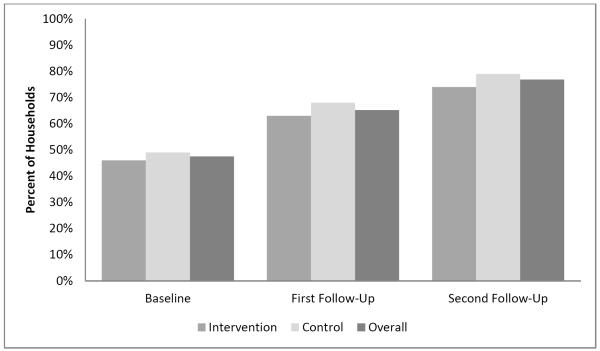
Figure 2 shows the proportion of households reporting full household smoking restrictions by intervention and control group and overall, at baseline and first and second follow-ups. The overall proportion reporting full smoking bans increased from 48% (114/240) at baseline to 65% (130/199) at first follow-up, and 76% (133/174) at second follow-up. No significant intervention/control group differences were found in the proportion of households reporting full smoking bans at baseline, first, or second follow-up (all p-values >.05 by chi-square tests). There were also no significant differences between groups in percentage of households reporting having changed restrictions, either at first or second follow-up (all p-values >.05 by chi-square tests).
Figure 2.
Rates of full household smoking ban at baseline, first, and second follow-up.
Air nicotine levels were significantly lower for households reporting full smoking bans as compared to partial/no bans at baseline (full bans: n=82, median = 0.0275, range = 0 -0.775; partial bans: n=78, median = 0.115, range = 0-12.685; Wilcoxon signed rank p-value <0.0001) and at 6-month follow-up (full bans: n=108, median = 0.04, range = 0-2.635; partial bans: n=53, median = 0.16, range = 0.01 – 16.69, Wilcoxon signed rank p-value < 0.0001).
Of the 102 children at baseline with both urine cotinine and air nicotine data, 26% (27/102) have mean air nicotine levels of zero. These 27 children had a median urine cotinine of 0.255 ng/mL and a range of 0.07 to 2.2 ng/mL, suggesting some source of SHS exposure outside the area where the air monitors were placed.
Discussion
Our goal was to develop and test a minimal intervention aimed at reducing household SHS exposure among low-income, race/ethnic minority children with asthma that could easily be incorporated and maintained in usual care and education at modest cost. Results of the randomized trial were disappointing. The intervention was unsuccessful with respect to reduction in household smoking and household SHS exposure of these children. In addition, while most households that remained in the study reported full household smoking bans by the end of the study, we found no difference in control/intervention households citing a ban on smoking.
A number of factors may have contributed to these results. Levels of initial exposure were already low, leaving limited opportunity for further reduction. Reflecting California’s tobacco control efforts [37], for many years the state has had the highest rate of household smoking bans in the nation [38]. Therefore, the number of households with high levels of smoking and a child with asthma may have been limited, and adults from high exposure households may have been unwilling to take part. This may particularly have been the case for the Latino families in our study, approximately 60% of participants. While there is considerable tobacco use among men in this population, tobacco use among Latinas is low, there is a cultural value of familismo, emphasis on family, including emphasis on children’s health [17, 39], and children’s household SHS exposure is lower among Latino families than for other race/ethnic groups[40]. Further, as in other unsuccessful trials [17, 21, 22], we likely lacked statistical power to detect intervention/group differences. Recruitment was difficult and attrition rates were high, possibly related to the extensive data collection protocol. Simply taking part in the study and completing the challenging data collection process may have overwhelmed the intervention’s impact, alerting all participants to the need for household change. This effect may have been increased by provision of some information to control group families [14, 20, 21]. Finally, to enhance the study’s cultural competence [17, 41], data collection was conducted by community members, lay Latinas who were extremely simpatico and supportive of study participants. An unanticipated consequence of this sympathetic tone may have been to overwhelm the minimal intervention’s impact and encourage control group participants to also reduce household SHS exposure, thus biasing results. Likewise, this sympathetic setting may have resulted in inaccurate reports of household change, a concern given continued evidence of household smoking.
We recognize the importance of children’s SHS exposure in places other than the household setting (e.g., friends’ homes, day care settings, outdoor venues) [42]. We considered asking participants for information regarding this exposure but decided against this out of concern for respondent burden, already considerable in the data collection process identifying exposure indoors and outdoors in the home setting and in the car. Moreover, major importance has been placed on household SHS exposure in the literature.
Conclusions
Despite these results, our study provided several important insights. The “null” results of our trial suggest that a minimal intervention with limited contact may not suffice to achieve a reduction in household tobacco exposure among low income minority families that have children with asthma. However, although no intervention/control group differences were found, our study suggests that it is possible to implement household restrictions that were not previously in place, including among low-income and minority families, since most households reported implementing full smoking bans by the end of the study (136 of the original 242 at baseline, 56%). This is significant in light of the implications of such restrictions for reduced exposure [43] and the association of bans with smoking cessation and decreased cigarette consumption. Our research also points to the need for further study of the process by which change occurs, i.e., what changes families believe they are making and actually achieve.
Support for smoke free homes is increasing, especially when children are present, [5, 44] and smoke free homes have increased in the past decade nationally and in every state [38]. Nevertheless, considerable household SHS exposure still occurs, including among children with asthma [5, 8], and reports of household bans or the extent of exposure are not always consistent [29, 45]. Although protecting the health of householders and particularly children, including those with asthma and other diseases, is a consideration as families decide to adopt household restrictions, [43, 46] a diagnosis of asthma in a child does not necessarily alter parental smoking behavior [47]. There continues to be a need for effective culturally and linguistically appropriate strategies that support reduction of household SHS exposure, including among children with asthma. Efficacy trials are needed to determine whether intensive programs can be successfully disseminated and maintained, particularly in settings serving low-income and minority youth [48]. Likewise, more research is needed to identify less intensive, minimal contact strategies that might increase the likelihood of uptake and maintenance in health care and other settings.
Acknowledgements
Support for this work was provided by National Institutes of Health grants HL53957 from the National Heart, Lung and Blood Institute, Division of Lung Diseases, and CA16042 from the National Cancer Institute. Approval was obtained from the UCLA Office for Protection of Research Subjects, UCLA IRB #G94-09-1000-34. We wish to thank the adults and children who participated in the research, and the administrative and clinical staffs at our recruitment sites. We also express appreciation to Ms. Connie Sosnoff, Mr. Alberto Febo, Charles Perrino, Tuyen Hoang, Ph.D., Darlene Goldstein, Ph.D., Ms. Guadelupe Escobar, Ms. Claudia Huezo, Ms. Yanscy Flores, Ms. Melissa Aguayo, and Ms. Laura Hoyos for assistance in data collection and analysis; K. Michael Cummings, Ph.D., Mary Ann Lewis, R.N., Dr.P.H., F.A.A.N., Maria Hayes-Bautista, R.N., M.P.H., Lesa Walden, M.D. (VitalSigns, Inc. Santa Monica, CA), and Antronette K.Yancey, M.D., M.P.H. for assistance in developing the intervention materials and survey instruments.
References
- 1.Akinbami LJ, Moorman JE, Garbe PL, Sondik EJ. Status of childhood asthma in the United States, 1980-2007. Pediatrics. 2009;123(Suppl 3):S131–S145. doi: 10.1542/peds.2008-2233C. [DOI] [PubMed] [Google Scholar]
- 2.Canino G, McQuaid EL, Rand CS. Addressing asthma health disparities: A multilevel challenge. J Allergy Clin Immunol. 2009;123(6):1209–1217. doi: 10.1016/j.jaci.2009.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bryant-Stephens T. Asthma disparities in urban environments. J Allergy Clin Immunol. 2009;123(6):1199–1206. doi: 10.1016/j.jaci.2009.04.030. [DOI] [PubMed] [Google Scholar]
- 4.Koinis-Mitchell D, McQuaid EL, Kopel SJ, Esteban CA, Ortega AN, Seifer R, et al. Cultural-related, contextual, and asthma-specific risks associated with asthma morbidity in urban children. J Clin Psychol Med Settings. 2010;17(1):38–48. doi: 10.1007/s10880-009-9178-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.U.S. Department of Health and Human Services . The Health Consequences of Involuntary Exposure to Tobacco Smoke: A Report of the Surgeon General. U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, Coordinating Center for Health Promotion, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; Atlanta, GA: 2006. [Google Scholar]
- 6.Gerald LB, Gerald JK, Gibson L, Patel K, Zhang S, McClure LA. Changes in environmental tobacco smoke exposure and asthma morbidity among urban school children. Chest. 2009;135(4):911–916. doi: 10.1378/chest.08-1869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.U.S. Environmental Protection Agency [Retrieved December 10, 2011];Fact sheet: National survey on environmental management of asthma and children’s exposure to ETS. from, http://www.epa.gov/smokefree/pdfs/survey_fact_sheet.pdf.
- 8.Halterman JS, Fagnano M, Conn KM, Szilagyi PG. Do parents of urban children with persistent asthma ban smoking in their homes and cars? Ambul Pediatr. 2006;6(2):115–9. doi: 10.1016/j.ambp.2005.10.004. [DOI] [PubMed] [Google Scholar]
- 9.Hovell MF, Zakarian JM, Matt GE, Hofstetter CR, Bernert JT, Pirkle J. Effect of counselling mothers on their children’s exposure to environmental tobacco smoke: randomised controlled trial. BMJ. 2000;321(7257):337–342. doi: 10.1136/bmj.321.7257.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Greenberg RA, Strecher VJ, Bauman KE, et al. Evaluation of a home-based intervention program to reduce infant passive smoking and lower respiratory illness. J Behav Med. 1994;17(3):273–290. doi: 10.1007/BF01857953. [DOI] [PubMed] [Google Scholar]
- 11.Emmons KM, Hammond SK, Fava JL, Velicer WF, Evans JL, Monroe AD. A randomized trial to reduce passive smoke exposure in low-income households with young children. Pediatrics. 2001;108(1):18–24. doi: 10.1542/peds.108.1.18. [DOI] [PubMed] [Google Scholar]
- 12.Hovell MF, Meltzer SB, Zakarian JM, et al. Reduction of environmental tobacco smoke exposure among asthmatic children: a controlled trial. Chest. 1994;106(2):440–446. doi: 10.1378/chest.106.2.440. [DOI] [PubMed] [Google Scholar]
- 13.Hovell MF, Meltzer SB, Wahlgren DR, et al. Asthma management and environmental tobacco smoke exposure reduction in Latino children: A controlled trial. Pediatrics. 2002;110(5):946–956. doi: 10.1542/peds.110.5.946. [DOI] [PubMed] [Google Scholar]
- 14.Wahlgren DR, Hovell MF, Meltzer SB, Hofstetter CR, Zakarian JM. Reduction of environmental tobacco smoke exposure in asthmatic children. A 2-year follow-up. Chest. 1997;111(1):81–88. doi: 10.1378/chest.111.1.81. [DOI] [PubMed] [Google Scholar]
- 15.Wilson SR, Yamada EG, Sudhakar R, et al. A controlled trial of an environmental tobacco smoke reduction intervention in low-income children with asthma. Chest. 2001;120(5):1709–1722. doi: 10.1378/chest.120.5.1709. [DOI] [PubMed] [Google Scholar]
- 16.Hughes DM, McLeod M, Garner B, Goldbloom RB. Controlled trial of a home and ambulatory program for asthmatic children. Pediatrics. 1991;87(1):54–61. [PubMed] [Google Scholar]
- 17.Conway TL, Woodruff SI, Edwards CC, Hovell MF, Klein J. Intervention to reduce environmental tobacco smoke exposure in Latino children: null effects on hair biomarkers and parent reports. Tob Control. 2004;13(1):90–92. doi: 10.1136/tc.2003.004440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Krieger JW, Takaro TK, Song L, Weaver M. The Seattle-King County Healthy Homes Project: a randomized, controlled trial of a community health worker intervention to decrease exposure to indoor asthma triggers. Am J Public Health. 2005;95(4):652–659. doi: 10.2105/AJPH.2004.042994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chan S, Lam TH. Protecting sick children from exposure to passive smoking through mothers’ actions: a randomized controlled trial of a nursing intervention. J Adv Nurs. 2006;54(4):440–449. doi: 10.1111/j.1365-2648.2006.03842.x. [DOI] [PubMed] [Google Scholar]
- 20.Irvine L, Crombie IK, Clark RA, et al. Advising parents of asthmatic children on passive smoking: randomised controlled trial. BMJ. 1999;318(7196):1456–1459. doi: 10.1136/bmj.318.7196.1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Priest N, Roseby R, Waters E, et al. Family and carer smoking control programmes for reducing children’s exposure to environmental tobacco smoke. Cochrane Database Syst Rev. 2008;(4):CD001746. doi: 10.1002/14651858.CD001746.pub2. [DOI] [PubMed] [Google Scholar]
- 22.Wakefield M, Banham D, McCaul K, et al. Effect of feedback regarding urinary cotinine and brief tailored advice on home smoking restrictions among low-income parents of children with asthma: a controlled trial. Prev Med. 2002;34:58–65. doi: 10.1006/pmed.2001.0953. [DOI] [PubMed] [Google Scholar]
- 23.Curry SJ, Emmons KM. Theoretical models for predicting and improving compliance with breast cancer screening. Ann Behav Med. 1994;16(4):302–316. [Google Scholar]
- 24.Bastani R, Glenn BA, Taylor VM, et al. Integrating theory into community interventions to reduce liver cancer disparities. The Health Behavior Framework. Prev Med. 2010;501(1-2):63–67. doi: 10.1016/j.ypmed.2009.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bastani R, Glenn BA, Tsui J, et al. Understanding suboptimal human papillomavirus vaccine uptake among ethnic minority girls. Cancer Epidemiol Biomarkers Prev. 2011;20:1463–1472. doi: 10.1158/1055-9965.EPI-11-0267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lozano P, Finkelstein JA, Carey VJ, et al. A multisite randomized trial of the effects of physician education and organizational change in chronic-asthma care - Health outcomes of the Pediatric Asthma Care Patient Outcomes Research Team II Study. Arch Pediatr Adolesc Med. 2004;158(9):875–883. doi: 10.1001/archpedi.158.9.875. [DOI] [PubMed] [Google Scholar]
- 27.Severson HH, Zoref L, Andrews J, Lichtenstein E, Wall M. Reducing environmental tobacco smoke (ETS) exposure for infants: a cessation intervention for mothers of newborns. Am J Health Promot. 1994;8(4):252–253. doi: 10.4278/0890-1171-8.4.252. [DOI] [PubMed] [Google Scholar]
- 28.Berman BA, Wong GC, Bastani R, et al. Household smoking behavior and ETS exposure among children with asthma in low-income, minority households. Addict Behav. 2003;28(1):111–128. doi: 10.1016/s0306-4603(01)00221-0. [DOI] [PubMed] [Google Scholar]
- 29.Wong GC, Berman BA, Hoang T, Bernaards C, Jones C, Bernert JT. Children’s exposure to environmental tobacco smoke in the home: comparison of urine cotinine and parental reports. Arch Environ Health. 2002;57(6):584–590. doi: 10.1080/00039890209602092. [DOI] [PubMed] [Google Scholar]
- 30.Wong GC, Bernaards CA, Berman BA, Jones C, Bernert JT. Do children with asthma and their parents agree on household ETS exposure? Implications for asthma management. Patient Educ Couns. 2004;53(1):19–25. doi: 10.1016/S0738-3991(03)00123-X. [DOI] [PubMed] [Google Scholar]
- 31.Cuellar I, Harris LC, Jasso R. An acculturation scale for Mexican American normal and clinical populations. Hisp J Behav Sci. 1980;2(3):199–217. [Google Scholar]
- 32.Bernert JT, Jr, Turner WE, Pirkle JL, et al. Development and validation of sensitive method for determination of serum cotinine in smokers and nonsmokers by liquid chromatography/atmospheric pressure ionization tandem mass spectrometry. Clin Chem. 1997;43(12):2281–2291. [PubMed] [Google Scholar]
- 33.Emerson JA, Hovell MF, Meltzer SB, et al. The accuracy of environmental tobacco smoke exposure measures among asthmatic children. J Clin Epidemiol. 1995;48(10):1251–1259. doi: 10.1016/0895-4356(95)00021-u. [DOI] [PubMed] [Google Scholar]
- 34.Gehring U, Leaderer BP, Heinrich J, et al. Comparison of parental reports of smoking and residential air nicotine concentrations in children. Occup Environ Med. 2006;63(1):766–772. doi: 10.1136/oem.2006.027151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Glasgow RE, Foster LS, Lee ME, Hammond SK, Lichtenstein E, Andrews JA. Developing a brief measure of smoking in the home: description and preliminary evaluation. Addict Behav. 1998;23(4):567–571. doi: 10.1016/s0306-4603(98)00008-2. [DOI] [PubMed] [Google Scholar]
- 36.Hammond SK, Leaderer BP. A diffusion monitor to measure exposure to passive smoking. Environ Sci Technol. 1987;21:494–497. doi: 10.1021/es00159a012. [DOI] [PubMed] [Google Scholar]
- 37.Martinez-Donate AP, Johnson-Kozlow M, Hovell MF, Perez GJ Gonzalez. Home smoking bans and SHSs exposure in Mexico and the US. Prev Med. 2009;48(3):207–212. doi: 10.1016/j.ypmed.2008.12.011. [DOI] [PubMed] [Google Scholar]
- 38.Centers for Disease Control and Prevention (CDC) State-specific prevalence of smoke-free home rules --- United States, 1992-2003. MMWR Morb Mortal Wkly Rep. 2007;56(20):501–504. [PubMed] [Google Scholar]
- 39.Marin B, Marin G. Research with Hispanic Populations. Sage Publications; Thousand Oaks, California: 1991. [Google Scholar]
- 40.Gergen PJ, Fowler JA, Maurer KR, Davis WW, Overpeck MD. [Retrieved November 13, 2011];The burden of environmental tobacco smoke exposure on the respiratory health of children 2 months through 5 years of age in the United States: Third National Health and Nutrition Examination Survey, 1988-1994. Pediatrics. 1998 101(2):E8. doi: 10.1542/peds.101.2.e8. from, http://www.pediatrics.org/cgi/content/full/101/2/e8. [DOI] [PubMed] [Google Scholar]
- 41.Flores G, Fuentes-Afflick E, Barbot O, et al. The health of Latino children: urgent priorities, unanswered questions, and a research agenda. JAMA. 2002;288:82–90. doi: 10.1001/jama.288.1.82. [DOI] [PubMed] [Google Scholar]
- 42.Chen X, Stanton B, Hopper J, Khankari N. Sources, locations, and predictors of environmental tobacco smoke exposure among young children from inner-city families. J Pediatr Health Care. 2011;25:365–372. doi: 10.1016/j.pedhc.2010.04.014. [DOI] [PubMed] [Google Scholar]
- 43.Wamboldt FS, Balkissoon RC, Rankin AE, et al. Correlates of household smoking bans in low-income families of children with and without asthma. Fam Process. 2008;47(1):81–94. doi: 10.1111/j.1545-5300.2008.00240.x. [DOI] [PubMed] [Google Scholar]
- 44.Ashley MJ, Cohen J, Ferrence R, et al. Smoking in the home: changing attitudes and current practices. Am J Public Health. 1998;88(5):797–800. doi: 10.2105/ajph.88.5.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mumford EA, Levy DT, Romano EO. Home smoking restrictions. Problems in Classification. Am J Prev Med. 2004;27(2):126–131. doi: 10.1016/j.amepre.2004.04.001. [DOI] [PubMed] [Google Scholar]
- 46.Kegler MC, Escoffery C, Groff A, Butler S, Foreman A. A qualitative study of how families decide to adopt household smoking restrictions. Fam Community Health. 2007;30(4):328–341. doi: 10.1097/01.FCH.0000290545.56199.c9. [DOI] [PubMed] [Google Scholar]
- 47.Liem JJ, Kozyrskyj AL, Benoit CM, Becker AB. Asthma is not enough: Continuation of smoking among parents with an asthmatic child. Can Respir J. 2007;14(6):349–353. doi: 10.1155/2007/178789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zakarian JM, Hovell MF, Sandweiss RD, et al. Behavioral counseling for reducing children’s ETS exposure: implementation in community clinics. Nicotine Tob Res. 2004;6(6):1061–74. doi: 10.1080/1462220412331324820. [DOI] [PubMed] [Google Scholar]