Abstract
HIV-infected women with excessive alcohol consumption are at risk for adverse health outcomes, but little is known about their long-term drinking trajectories. This analysis included longitudinal data, obtained from 1996–2006, from 2791 women with HIV from the Women’s Interagency HIV Study. Among these women, the proportion in each of five distinct drinking trajectories was: continued heavy drinking (3%), reduction from heavy to non-heavy drinking (4%), increase from non-heavy to heavy drinking (8%), continued non-heavy drinking (36%), and continued non-drinking (49%). Depressive symptoms, other substance use (crack/cocaine, marijuana, and tobacco), co-infection with HCV, and heavy drinking prior to enrollment were associated with trajectories involving future heavy drinking. In conclusion, many women with HIV change their drinking patterns over time. Clinicians and those providing alcohol-related interventions might target those with depression, current use of tobacco or illicit drugs, HCV infection, or a previous history of drinking problems.
Keywords: Alcohol consumption, women, HIV-infection, trajectories
BACKGROUND
Approximately half of US women with HIV infection report any alcohol consumption in the past month, and at specific time points, approximately 12% – 22% exceed recommended limits as established by the National Institute of Alcohol Abuse and Alcoholism (1–4). Exceeding recommended consumption amounts, defined for women as drinking >7 drinks per week or >3 drinks per occasion, is associated with poorer medication adherence, increased risky sexual behavior, increased HIV viral load, and more rapid disease progression (5–11). Most previous assessments of alcohol consumption and related outcomes in HIV-infected women have focused on assessments at single time points. However, because individuals often change their drinking patterns over time, assessments at single time points likely do not accurately reflect their long-term drinking exposure, or their risk of drinking in the future. Alcohol trajectory analysis can identify long-term drinking patterns that may be associated with greater long-term consequences. Identification of factors that are associated with the most severe long-term trajectories might identify current drinkers at highest risk for continued long-term drinking, or those who are current non-drinkers (or low-level drinkers) who are at greatest risk for increasing their drinking over time, and therefore might be the target of concentrated preventive interventions.
Because alcohol consumption can have such significant health impacts in persons with HIV infection, it is important to determine their drinking patterns over time and to infer whether there are any differences in long-term drinking trajectories that are unique to women with HIV. However, few if any assessments of long-term alcohol drinking trajectories have been reported in HIV-infected women. Here, our objectives were to identify long-term drinking trajectories during longitudinal follow-up in women with HIV infection, to identify baseline characteristics of these women associated with the most serious long-term drinking trajectories, and to compare long-term drinking trajectories in HIV-positive and HIV-negative women.
METHODS
Data were obtained from 3766 women participating in the Women’s Interagency HIV Study (WIHS), a prospective observational cohort study of HIV-positive and HIV-negative women in the United States. The WIHS enrolled participants in 1994–1995 (n=2623) and again in 2000–2001 (N=1143). Participating sites are based in the Bronx and Brooklyn, New York City; Washington DC, Los Angeles, the San Francisco Bay area, and Chicago. Institutional Review Boards at each of the participating centers approved the WIHS study protocols, and informed written consent was obtained from all participants. The study design has been described previously (12, 13) and study information is available at https://statepiaps.jhsph.edu/wihs/.
Data Collection
The baseline questionnaire, completed at study enrollment, included items to assess age, race, educational attainment, marital status, and employment status. On the baseline questionnaire, participants also indicated the quantity and frequency of use of the following substances in the previous six months: tobacco, marijuana, cocaine, crack or freebase cocaine, heroin, and methadone. Current drug use was defined as taking the drug at least once a month. Participants also reported whether they had ever injected any drugs and whether they were currently injecting drugs. HIV serostatus was determined by ELISA antibody testing and an approved confirmatory test and CD4 cell count was categorized as <=200/mm3, 201–500/mm3, and >500/mm3. Information on the time since seroconversion was not available. Depression was assessed using the 20-item CES-D scale; women were classified as having clinically significant symptoms of depression if they had a CES-D score of >=16 (14). Baseline blood specimens for the 1994/1995 recruits were tested in 2000 for hepatitis C virus (HCV) infection, and the 2001/2002 recruits were tested in real time. Thus, all women in active follow-up eventually learned their HCV status, although women enrolled in 1994–95 were not given their HCV test results until later.
Alcohol assessment
WIHS has assessed alcohol quantity and frequency at enrollment and at each successive 6-month visit. WIHS uses several strategies to maximize the validity of the alcohol assessment information, including showing pictures of various drink types to participants and creating a non-judgmental atmosphere. At the enrollment and each six-month follow-up visit, WIHS participants were asked about the average number of days per week that they had a drink of alcohol, defined as “one can, bottle or glass of beer, a glass of wine, a shot of liquor, a mixed drink with that amount of liquor, or any other kind of alcoholic beverage.” Response options were every day, 5–6 days a week, 3–4 days a week, 1–2 days a week, less than once a week, and none. Next, they were asked about the usual number of drinks they consumed per day, with open-ended responses elicited during 1996 – 2004 and categorized choices for 2005 onward (0, 1–2, 3–4, 5–6, or 7 or more drinks per day). Open-ended responses such as “a pint of vodka” were converted to numbers of standard drinks. The number of drinks per week was determined by multiplying the reported quantity by the reported frequency per week. For these analyses, “heavy” drinking refers to consumption of >7 drinks per week, an amount defined as exceeding recommended limits by the National Institute on Alcohol Abuse and Alcoholism (4).
Past drinking behavior and treatment for drinking was assessed at study enrollment. Specifically, women provided information about the highest amounts of alcohol they ever regularly consumed in the past (categorized as past heavy, moderate, or non-drinking based on National Institute of Alcohol Abuse and Alcoholism criteria), and on whether they ever had received any type of treatment for alcohol problems (such as Alcoholics Anonymous, inpatient or outpatient detoxification, medication prescriptions).
Statistical analyses
The WIHS data from 23 semiannual study visits (1996 – 2006) were included in these analyses. The primary analyses included the women with HIV infection at enrollment, although we also conducted a separate analysis among women without HIV and among the entire sample combined (HIV-positive and HIV-negative). A semi-parametric group-based logistic model was used to identify groups of homogenous drinking trajectories. Specifically, the SAS macro PROC TRAJ was used to fit a linear mixture model that quantified alcohol intake patterns over study follow up and classified them into groups of homogenous drinking trajectories (15). This procedure sorts individual drinking curves into clusters of similar shape by taking each person’s response of number of drinks/week over all 23 visits and estimates a single model. Models with four to nine categories were considered and we chose the five-category model because it had the best fit to the data by the Baysean Information Criterion (BIC). The results for these trajectory analyses were similar when we excluded participants who had fewer than 3 follow-up visits, and thus we included data from all enrolled women. We also ran PROC TRAJ separately in women with and without HIV infection. The general trajectory patterns were similar.
Multivariable logistic regression models were used to predict specific long-term drinking trajectories using risk factors assessed at baseline. Two separate analyses were run, based on women’s drinking status at study enrollment. First, among women with heavy drinking at baseline, we compared those who continued to drink at heavy levels over time, to those who decreased their drinking (or stopped completely). Second, among women with lower levels of drinking (or no drinking) at baseline, we compared women who increased to heavy drinking over time to those who remained non-drinkers or low-level drinkers. Stepwise modeling was used to identify factors most strongly associated with specific drinking trajectories. Potential variables considered included the enrollment values for participant age, race, marital status, education, employment, study recruitment site, period of enrollment, depressive symptoms, HCV status, other illicit and licit substance use, past drinking amount, and past alcohol treatment. We examined the potential impact of HIV serostatus and HIV disease stage on long-term drinking trajectories using data from the entire sample (HIV-positive and HIV-negative). In this analysis, HIV disease stage at study enrollment was included in the multivariable model as a 4- category variable (HIV-negative, HIV+/CD4 <200 cells/mm3, HIV+/CD4 200–500 cells/mm3, and HIV+/CD4 >500 cells/ mm3), with other variables found to be significant in the HIV-stratified analysis also included in the multivariable model.
RESULTS
Of the 3766 study participants, most (74%) were HIV-positive. Among the 2791 HIV-positive women, mean age at enrollment was 35 years; 58% were African American, 24% were Hispanic, and the remainder were primarily white (Table 1). Fewer than half were married (37%), employed at enrollment (24%), or had greater than a high school education (32%). About 1 in 5 (21%) had HCV antibodies. Other substance use at enrollment was relatively common, and included tobacco (51%), marijuana (21%), crack/freebase (16%), cocaine (12%), and heroin (10%). About a third of the women (35%) had a previous history of regular heavy drinking (>7 drinks per week); and 20% reported a history of participation in an alcohol treatment program. The HIV-negative women in WIHS had fairly similar characteristics, although the HIV-negative women were more likely to be employed, to be enrolled in the 2001/2002 cohort (vs. the 1994/1995 cohort), to have HCV antibodies, and to use marijuana (see Table 1).
Table 1.
Baseline characteristics of 3766 women included in alcohol trajectories analysis, overall and according to HIV serostatus.
| Socio-Demographic Factors | All (N=3766) N (%) |
HIV+ (N=2791) N (%) |
HIV− (N=975) N (%) |
p-value* |
|---|---|---|---|---|
| Age Group (years) | ||||
| <30 | 1039 (28) | 652 (23) | 387 (40) | <.0001 |
| 30–40 | 1720 (46) | 1343 (48) | 377 (39) | |
| >40 | 1007 (27) | 796 (28) | 211 (22) | |
| Race | ||||
| White | 566 (15) | 426 (15) | 140 (14) | 0.72 |
| African American | 2174 (58) | 1617 (58) | 557 (57) | |
| Hispanic | 901 24) | 658 (24) | 243 (25) | |
| Other | 125 (3.3) | 90 (3.2) | 35 (3.6) | |
| Marital Status | ||||
| Single | 1315 (36) | 915 (33) | 400 (42) | <.0001 |
| Married | 1381 (37) | 1020 (37) | 361 (38) | |
| Sep/Div/Widowed | 998 (27) | 810 (30) | 188 (20) | |
| Employed | 999 (27) | 677 (24) | 322 (33) | <.0001 |
| Education > High School | 1204 (32) | 887 (32) | 317 (33) | 0.60 |
| Site | ||||
| Bronx | 772 (20 ) | 547 (20) | 225 (23) | 0.06 |
| Brooklyn | 611 (16) | 455 (16) | 156 (16) | |
| Washington DC | 568 (15) | 417 (15) | 151 (16) | |
| Los Angeles | 762 (20) | 572 (20) | 190 (20) | |
| San Francisco | 580 (15) | 427 (15) | 153 (16) | |
| Chicago | 473 (12) | 373 (13) | 100 (10) | |
| Year of Enrollment | ||||
| 1994/1995 | 2623 (70) | 2054 (74) | 569 (58) | <.0001 |
| 2001/2002 | 1143 (30) | 737 (26) | 406 (42) | |
| Clinical Characteristics | ||||
| CD4 Group | ||||
| <200/mm3 | 665 (25) | 665 (25) | --- | --- |
| 200–500/mm3 | 1171 (43) | 1171 (43) | --- | |
| >500/mm3 | 868 (32) | 868 (32) | --- | |
| HCV Antibody Positive | 1152 (31) | 197(21) | 955 (35) | <.0001 |
| Depressive Symptoms (CESD>=16) | 1953 (53) | 1476 (54) | 477 (50) | 0.02 |
| Drug Use at Baseline (at least monthly) | ||||
| cocaine | 460 (12) | 324 (12) | 136 (14) | 0.06 |
| heroin | 395 (10) | 284 (10) | 111 (11) | 0.29 |
| crack/freebase cocaine | 626 (17) | 442 (16) | 184 (19) | 0.03 |
| (illicit) methadone | 66 (1.8) | 38 (1.4) | 28 (2.9) | 0.002 |
| marijuana/hash | 912 (24) | 594 (21) | 318 (33) | <.0001 |
| injected drug | 307 (8) | 225 (8.1) | 82 (8.4) | 0.74 |
| tobacco | 1986 (53) | 1424 (51) | 562 (58) | 0.0004 |
| Peak Drinking History (prior to baseline) | ||||
| Non drinker | 1985 (54) | 1499 (55) | 486 (51) | 0.07 |
| Moderate drinker | 377 (10) | 274 (10) | 103 (11) | |
| Heavy drinker | 1329 (36) | 957 (35) | 372 (39) | |
| Ever Had Alcohol Treatment (prior to baseline) | 782 (21) | 554 (20) | 228 (24) | 0.02 |
P-values for comparisons between HIV + and HIV − women.
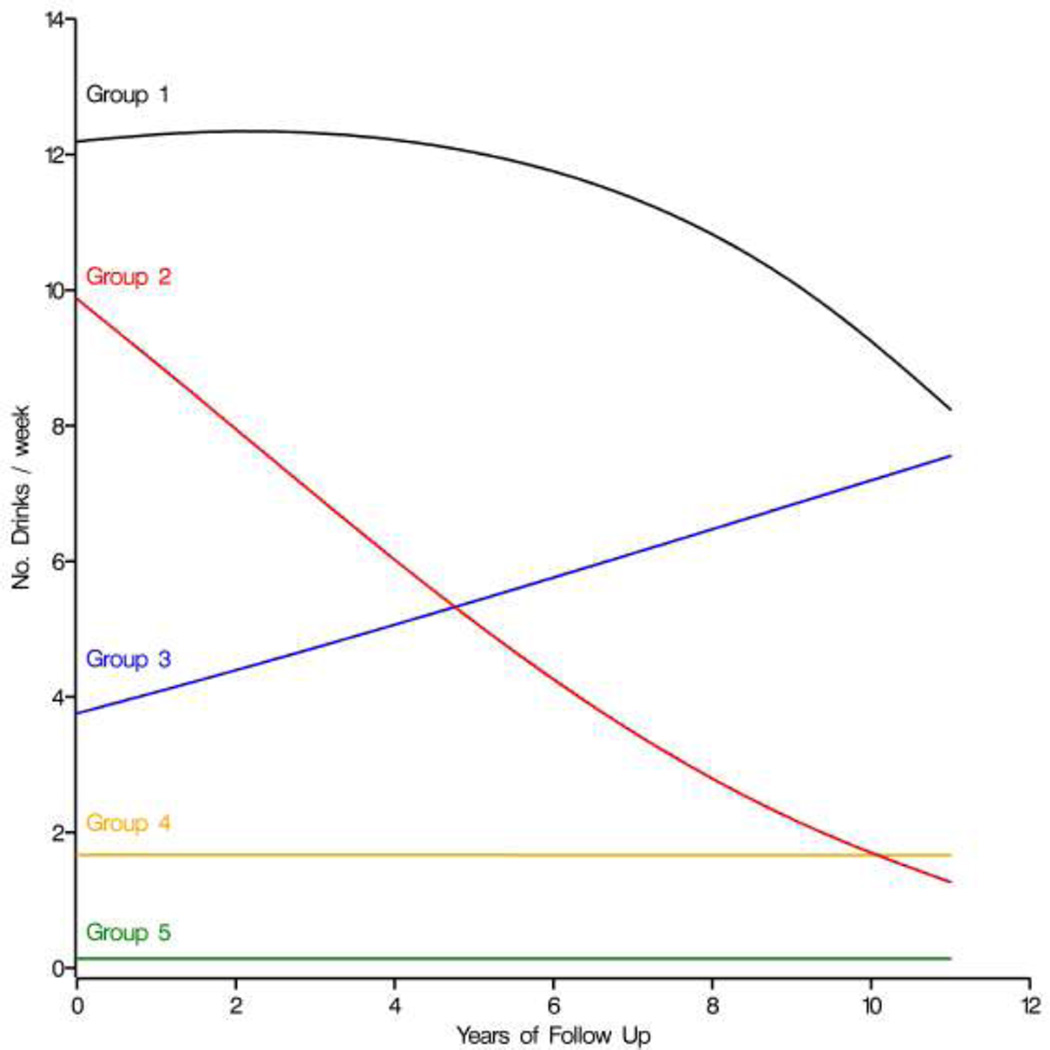
The analyses revealed five distinct long-term drinking trajectories (Figure 1). The proportion of HIV-positive women in each group was: Group 1) continued heavy drinking (3%); Group 2) reduction from heavy to non-heavy drinking (4%); Group 3) increase from non-heavy to heavy drinking (8%); Group 4) continued non-heavy drinking (36%), and Group 5) continued non-drinking (49%). Thus, while many women changed their drinking patterns over time, the majority of women with HIV infection either continued to drink but kept their alcohol consumption below heavy levels (Group 4) or remained non-drinkers throughout follow-up (Group 5).
Figure 1.
Drinking trajectory patterns for 3768 women: Women’s Interagency HIV Study, 1994 – 2006. The five groups include (1) women who were persistent heavy drinkers (3%); (2) women who cut back from heavy drinking to non-heavy drinking (4%); (3) women who increased to heavy drinking over time (8%); (4) women who remained non-heavy drinkers during the entire follow-up (36%); and (5) women who were non-drinkers (49%).
Factors associate with distinct drinking trajectories in HIV-positive women
Among HIV-positive women with heavier drinking at baseline (Groups 1 and 2), those with continued heavy drinking over time (Group 1) were significantly more likely to report depressive symptoms (OR 1.6, 95% CI 1.0 – 2.8), crack/cocaine use at baseline (OR 3.6, 95% CI 2.1 – 6.0), or positive HCV antibody status (OR 1.8, 95% CI 1.1 – 3.0), compared to women who reduced their heavy drinking over time (Group 2) (Table 2). The findings were similar in HIV-uninfected women, other than depressive symptoms (Table 2).
Table 2.
In women with heavy drinking at baseline, factors associated with a persistent heavy drinking trajectory, compared to women who reduced drinking over time: multivariable analysis.
| HIV-positive | HIV-uninfected | |||||||
|---|---|---|---|---|---|---|---|---|
| Baseline Variable |
(n = 329) | (n = 148) | ||||||
| Odds Ratio |
95% CI |
Wald χ2 |
P | Odds Ratio |
95% CI |
Wald χ2 |
p | |
| Depression | ||||||||
| Yes (vs. no) | 1.6 | 1.0–2.8 | 3.5 | 0.06 | ||||
| Crack/Cocaine Use | ||||||||
| Yes (vs. no) | 3.6 | 2.1–6.0 | 23.7 | <.001 | 5.8 | 2.6–12.9 | 18.3 | <.001 |
| HCV Seropositive | ||||||||
| Yes (vs. no) | 1.8 | 1. 1–3.0 | 4.7 | 0.03 | 2.4 | 1.0–5.8 | 4.8 | 0.03 |
Among HIV-positive women with non-heavy or no drinking at baseline (Groups 3, 4 and 5), women were significantly more likely to increase to heavy drinking (Group 3) if they engaged in other illicit or licit substance use at baseline (cocaine, marijuana or tobacco), had a history of heavy drinking prior to enrollment, or had ever participated in alcohol treatment prior to enrollment (Table 3). Hepatitis C antibody status was not associated with drinking trajectories in these women without heavy drinking at baseline. The results were similar in women who were HIV-uninfected (Table 3).
Table 3.
Among women without heavy drinking at baseline, factors associated with a trajectory increasing to heavy drinking over time (compared to trajectories that remained non-heavy drinking or non-drinking): multivariable analysis.
| Baseline Variable |
HIV-positive (n = 2303) | HIV-uninfected (n = 779) | ||||||
|---|---|---|---|---|---|---|---|---|
| Odds Ratio |
95% CI |
Wald χ2 |
p | Odds Ratio |
95% CI | Wald χ2 |
p | |
| Education | ||||||||
| > High School (vs. <= high school) | 0.65 | 0.41–1.04 | 3.3 | 0.07 | - | - | ||
| Crack/Cocaine Use | ||||||||
| Yes (vs. no) | 3.48 | 2.33 –5.19 | 37.3 | <.001 | 3.17 | 1.88–5.37 | 21.0 | <.001 |
| Marijuana Use | ||||||||
| Yes (vs. no) | 2.79 | 1.89–4.10 | 27.0 | <.001 | 3.06 | 1.86–5.05 | 24.8 | <.001 |
| Tobacco Use | ||||||||
| Yes (vs. no) | 2.91 | 1.74–4.87 | 16.5 | <.001 | 2.27 | 1.25–4.12 | 6.6 | 0.01 |
| Peak Drinking History (prior to baseline) | ||||||||
| Non-drinker (reference) | 1 | 1 | ||||||
| Moderate drinker | 0.36 | 0.13–1.03 | 5.4 | 0.02 | 0.44 | 0.15–1.33 | 5.4 | 0.02 |
| Heavy drinker | 1.51 | 1.00–2.28 | 22.0 | <.001 | 2.40 | 1.45–3.97 | 14.4 | <.001 |
| Ever Had Alcohol Treatment (prior to baseline) | ||||||||
| Yes (vs. no) | 2.66 | 1.77–3.99 | 22.0 | <.001 | - | - | ||
| HCV Seropositive | ||||||||
| Yes (vs. no) | 0.97 | 0.65–1.43 | 0.0 | 0.86 | 0.97 | 0.56–1.65 | 0.0 | 0.90 |
In multivariable analyses that included the entire sample (HIV-positive and HIV-uninfected), HIV infection status was not itself significantly associated with any of the distinct trajectory patterns (data not shown).
DISCUSSION
Examination of long-term drinking trajectories in women with HIV infection is important, as heavy alcohol consumption is associated with a wide range of behavioral and biologic health outcomes including more rapid disease progression (5–11). Our investigation identified five distinct long-term drinking trajectories in U.S. women with HIV infection. About a fifth of these women had substantial changes in their drinking behavior over time, whereas the remaining 80% continued either to not drink or to drink moderately. Drinking trajectory patterns were similar among HIV-positive and HIV-uninfected women. Previous research, including some from the same cohort,(3) has demonstrated that 12– 22% of HIV-infected women meet criteria for heavy drinking at single time points (1–3, 7). However, many women change their drinking behavior over time, and this longitudinal analysis suggest that fewer than 5% will continue to drink at heavy levels over a longer period of time, whereas another 10% will initiate (or re-initiate) drinking at a heavy level. Thus, clinicians caring for adult women with HIV infection need to include repeated assessments of alcohol consumption over time.
Most previous studies that examined drinking trajectories have presented results for trends among adolescents as they emerge into adulthood (16–21), or reported studies that combined men and women (22, 23), although some newer reports describe drinking trajectories in adult U.S. women over age 50 (24, 25). It is difficult to compare trajectory patterns across studies because the age groups, gender, or the number of trajectory categories were different. However, the results from our study, showing changing drinking patterns over time, are generally consistent with the findings from other cohorts of U.S. women.
In our study, women with depressive symptoms, who used other drugs such as crack/cocaine, marijuana, and tobacco, or who had a past history of heavy drinking, were most likely to be on trajectories associated with long-term heavy drinking. The few other studies that examined specific risk factors associated with long-term trajectories also found that previous drinking history, tobacco and drug use, and depression are associated with heavy drinking over time (22, 23, 25). The finding that women who used tobacco and other drugs were the most resistant to long-term moderation in drinking, suggests that intervention strategies that address multiple substances concurrently may be needed (rather than targeting single behaviors). The overlapping of several types of substance use mirror what we previously found in different analyses of this cohort, in which both chronic problem drinking and chronic crack use were associated with high viral load among women with HIV infection (26), and polysubstance use was associated with decreased success in smoking cessation in HIV-infected women (27).
This study has several strengths, including the repeated alcohol measures over time, use of strategies to improve accuracy of alcohol consumption reporting, and an HIV-negative control group. However, several study limitations should be noted. Although the WIHS study used several strategies to enhance self-reporting of alcohol, alcohol consumption is subject to measurement error and most likely is under-reported. Thus, the actual proportion of women with heavy drinking is likely to be somewhat higher. Some women were missing data at some time points or may have dropped out of the cohort. We found similar results in trajectories after excluding subjects with fewer than 3 outcome assessments, which suggests that the data are robust to these issues.
We focused our analysis on whether baseline characteristics are associated with long-term trajectories, although we recognize that several of the measures we assessed can change over time and might influence future alcohol trajectory patterns. A focus on baseline characteristics is most consistent with information a clinician might have when considering the likelihood of future drinking trajectories (initial risk). Thus, the findings have relevance for clinical screening and intervention programs. However, because many variables do change over time, future analyses of this topic might consider an analytic approach that incorporates behavior change over time in order to better understand the possible causal associations between these factors and changes in drinking.
Health care providers caring for women with HIV infection should assess for current alcohol consumption as well as identify women at increased risk for long-term drinking problems. The findings from this study may help clinicians and public health providers to identify women in need of more aggressive monitoring and possible intervention, regardless of their current drinking behavior. For example, women with a previous history of heavy drinking are at greater risk for long-term heavy drinking, even if they are not drinking heavily at the current visit. At the same time, health care providers can provide positive reinforcement towards the great majority of women who are not drinking at harmful levels, with more emphasis on those factors associated with resiliency.
In summary, these findings suggest that over time, the majority of HIV-infected and at-risk women will have long-term trajectories involving lower levels of drinking (or non-drinking). However, approximately 1 in 10 adult women will either continue to drink at heavy levels or increase their drinking to levels that exceed recommended guidelines. Past alcohol consumption behavior, current use of tobacco and/or other drugs, and depressive symptoms were the characteristics most strongly associated with long-term heavy drinking trajectories in this analysis.
Acknowledgments
Data in this manuscript were collected by the Women's Interagency HIV Study (WIHS) Collaborative Study Group with centers (Principal Investigators) at New York City/Bronx Consortium (Kathryn Anastos); Brooklyn, NY (Howard Minkoff); Washington, DC, Metropolitan Consortium (Mary Young); The Connie Wofsy Study Consortium of Northern California (Ruth Greenblatt); Los Angeles County/Southern California Consortium (Alexandra Levine); Chicago Consortium (Mardge Cohen); Data Coordinating Center (Stephen Gange). The WIHS is funded by the National Institute of Allergy and Infectious Diseases (UO1-AI-35004, UO1-AI-31834, UO1-AI-34994, UO1-AI-34989, UO1-AI-34993, and UO1-AI-42590) and by the Eunice Kennedy Shriver National Institute of Child Health and Human Development (UO1-HD-32632). The study is co-funded by the National Cancer Institute, the National Institute on Drug Abuse, and the National Institute on Deafness and Other Communication Disorders. Funding is also provided by the National Center for Research Resources (UCSF-CTSI Grant Number UL1 RR024131). The contents of this publication are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health. R Cook was supported in part by NIH grants R01-AA018934 and U01 –AA-020797; S Cole was supported in part through NIH grant R01-AA-01759; and K Weber was supported in part by NIH grant P30 AI082151.
REFERENCES
- 1.Galvan FH, Bing EG, Fleishman JA, London AS, Caetano R, Burnam MA, et al. The prevalence of alcohol consumption and heavy drinking among people with HIV in the United States: results from the HIV Cost and Services Utilization Study. J Stud Alcohol. 2002;63:179–186. doi: 10.15288/jsa.2002.63.179. [DOI] [PubMed] [Google Scholar]
- 2.Theall KP, Clark RA, Powell A, Smith H, Kissinger P. Alcohol consumption, ART usage and high-risk sex among women infected with HIV. AIDS Behav. 2007;11:205–215. doi: 10.1007/s10461-006-9159-6. [DOI] [PubMed] [Google Scholar]
- 3.Cook RL, Zhu F, Belnap BH, Weber K, Cook JA, Vlahov D, et al. Longitudinal trends in hazardous alcohol consumption among women with human immunodeficiency virus infection, 1995–2006. Am J Epidemiol. 2009;169:1025–1032. doi: 10.1093/aje/kwp004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.NIAAA: National Institute of Alcohol Abuse and Alcoholism. Helping patients who drink too much: a clinician's guide. Rockville, MD: NIAAA Publications Distribution Center; 2005. [accessed July 7, 2012]. Available from: http://pubs.niaaa.nih.gov/publications/practitioner/cliniciansguide2005/guide.pdf. [Google Scholar]
- 5.Braithwaite RS, McGinnis KA, Conigliaro J, Maisto SA, Crystal S, Day N, et al. A temporal and dose-response association between alcohol consumption and medication adherence among veterans in care. Alcohol Clin Exp Res. 2005;29:1190–1197. doi: 10.1097/01.alc.0000171937.87731.28. [DOI] [PubMed] [Google Scholar]
- 6.Chander G, Lau B, Moore RD. Hazardous alcohol use: a risk factor for non-adherence and lack of suppression in HIV infection. J Acquir Immune Defic Syndr. 2006;43:411–417. doi: 10.1097/01.qai.0000243121.44659.a4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cook RL, Sereika SM, Hunt SC, Woodward WC, Erlen JA, Conigliaro J. Problem drinking and medication adherence among persons with HIV infection. J Gen Intern Med. 2001;16:83–88. doi: 10.1111/j.1525-1497.2001.00122.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Samet JH, Horton NJ, Traphagen ET, Lyon SM, Freedberg KA. Alcohol consumption and HIV disease progression: are they related? Alcohol Clin Exp Res. 2003;27:862–867. doi: 10.1097/01.ALC.0000065438.80967.56. [DOI] [PubMed] [Google Scholar]
- 9.Wilson TE, Massad LS, Riester KA, Barkan S, Richardson J, Young M, et al. Sexual contraceptive, and drug use behaviors of women with HIV and those at high risk for infection: results from the Women’s Interagency HIV Study. AIDS. 1999;13:591–598. doi: 10.1097/00002030-199904010-00008. [DOI] [PubMed] [Google Scholar]
- 10.Neblett RC, Hutton HE, Lau B, McCaul ME, Moore RD, Chander G. Alcohol consumption among HIV-infected women: impact on time to antiretroviral therapy and survival. J Women’s Health. 2011;20:279–286. doi: 10.1089/jwh.2010.2043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Marianna K, Baum C, Lai S, Sales S, Page JB, Campa A. Alcohol use accelerates HIV disease progression. AIDS Res Hum Retroviruses. 2010;26:511–518. doi: 10.1089/aid.2009.0211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bacon MC, von Wyl V, Alden C, Sharp G, Robison E, Hessol N, et al. The Women's Interagency HIV Study: an observational cohort brings clinical sciences to the bench. Clin Diagn Lab Immunol. 2005;12:1013–1019. doi: 10.1128/CDLI.12.9.1013-1019.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Barkan SE, Melnick SL, Preston-Martin S, Weber K, Kalish LA, Miotti P, et al. The Women's Interagency HIV Study. Epidemiology. 1998;9:117–124. [PubMed] [Google Scholar]
- 14.Radloff LS. The CES-D Scale: A self-report depression scale for research in the general population. Applied Psychological Measurement. 1977;1:385–401. [Google Scholar]
- 15.Jones B, Nagin D, Roeder K. A SAS procedure based on mixture models for estimating developmental trajectories. Social Method Res. 2001;29:374–393. [Google Scholar]
- 16.Li F, Duncan T, Hops H. Examining developmental trajectories in adolescent alcohol use using piecewise growth mixture modeling analysis. J Stud Alcohol. 2001;62:199–210. doi: 10.15288/jsa.2001.62.199. [DOI] [PubMed] [Google Scholar]
- 17.Chung T, Maisto SA, Cornelius JR, Martin CS, Jackson KM. Joint trajectory analysis of treated adolescents' alcohol use and symptoms over 1 year. Addict Behav. 2005;30:1690–1701. doi: 10.1016/j.addbeh.2005.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jacob T, Bucholz K, Sartor C, Howell DN, Wood PK. Drinking trajectories from adolescence to the mid-forties among alcohol dependent males. J Stud Alcohol. 2005;66:745–755. doi: 10.15288/jsa.2005.66.745. [DOI] [PubMed] [Google Scholar]
- 19.Wiesner M, Weichold K, Silbereisen R. Trajectories of alcohol use among adolescent boys and girls: Identification, validation, and sociodemographic characteristics. Psychol Addict Behav. 2007;21(1):62–75. doi: 10.1037/0893-164X.21.1.62. [DOI] [PubMed] [Google Scholar]
- 20.Warner LA, White HR, Johnson V. Alcohol initiation experiences and family history of alcoholism as predictors of problem-drinking trajectories. J Stud Alcohol Drugs. 2007;68:56–65. doi: 10.15288/jsad.2007.68.56. [DOI] [PubMed] [Google Scholar]
- 21.Van Der Vorst H, Vermulst AA, Meeus WH, Dekovic M, Engels RC. Identification and prediction of drinking trajectories in early and mid-adolescence. J Clin Child Adolesc Psychol. 2009;38(3):329–341. doi: 10.1080/15374410902851648. [DOI] [PubMed] [Google Scholar]
- 22.Karlamangla A, Zhou K, Reuben D, Greendale G, Moore A. Longitudinal trajectories of heavy drinking in adults in the United States of America. Addiction. 2006;101:91–99. doi: 10.1111/j.1360-0443.2005.01299.x. [DOI] [PubMed] [Google Scholar]
- 23.Platt A, Sloan F, Costanzo P. Alcohol-consumption trajectories and associated characteristics among adults older than age 50. J Stud Alcohol Drugs. 2010;71:169–179. doi: 10.15288/jsad.2010.71.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bobo JK, Greek AK, Klepinger DH, Herting JR. Alcohol use trajectories in two cohorts of U.S women ages 50 to 65 at baseline. J am Geriatr Soc. 2010;58:2375–2380. doi: 10.1111/j.1532-5415.2010.03180.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bobo JK, Greek AK. Increasing and decreasing alcohol use trajectories among older women in the U.S. across a 10-year interval. Int J Enrivon Res Public Health. 2011;8:3263–3276. doi: 10.3390/ijerph8083263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cook JA, Burke-Miller JK, Cohen MH, Cook RL, Vlahov D, Wilson TE, et al. Crack cocaine, disease progression, and mortality in a multicenter cohort of HIV-1 positive women. AIDS. 2008;22:1355–1363. doi: 10.1097/QAD.0b013e32830507f2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Goldberg D, Weber K, Orsi J, Hessol NA, D’souza G, Watts DM, et al. Smoking cessation among women with and at risk for HIV: are they quitting? J Gen Intern Med. 2010;25:39–44. doi: 10.1007/s11606-009-1150-2. [DOI] [PMC free article] [PubMed] [Google Scholar]