Abstract
Ca2+ has long been recognized as a conserved second messenger and principal mediator in plant immune and stress responses. How Ca2+ signals are sensed and relayed into diverse primary and global signaling events is still largely unknown. Comprehensive analyses of the plant-specific multigene family of Ca2+-dependent protein kinases (CDPKs) are unraveling the molecular, cellular and genetic mechanisms of Ca2+ signaling. CDPKs, which exhibit overlapping and distinct expression patterns, sub-cellular localizations, substrate specificities and Ca2+ sensitivities, play versatile roles in the activation and repression of enzymes, channels and transcription factors. Here, we review the recent advances on the multifaceted functions of CDPKs in the complex immune and stress signaling networks, including oxidative burst, stomatal movements, hormonal signaling and gene regulation.
Ca2+ sensor protein kinases in immune and stress signaling networks
Despite long-standing knowledge that Ca2+ mediates plant responses to a wide range of developmental and environmental stimuli through variations of its intracellular concentrations, the molecular, cellular and genetic links between Ca2+ signatures and the multiple downstream signaling events are largely obscure. Calcium signatures are defined by spatio-temporal features, including amplitude, frequency, duration and sub-cellular location, that are likely to contribute to Ca2+ signaling specificity along with the diverse proteins able to sense and decode these signals [1–9]. Plants possess three main families of calcium sensors: calmodulin (CaM), calcineurin B-like (CBL) and calcium-dependent protein kinases (CDPKs). Unlike CaM and CBL that must relay the Ca2+-induced conformational change to protein partners, CDPKs have the unique feature of both Ca2+ sensing and responding activities within a single protein to directly translate Ca2+ signals into phosphorylation events [2,6,10,11]. Recently, major progress has been made to uncover the central roles of CDPKs in triggering appropriate and diverse downstream responses in the plant immune and stress signaling networks. In this review, we examine the recent advances on the molecular activation mechanism of CDPKs, as well as their versatile roles in immune and stress signaling, such as the regulation of oxidative burst, cell death, stomatal movements, hormonal signaling and gene expression.
CDPK structure, regulation and Ca2+ signal relay
CDPK structure and regulation
CDPKs harbor an N-terminal variable domain, a Ser/Thr kinase domain, an auto-inhibitory junction region and a regulatory calmodulin-like domain (CaM-LD) [1,2,10,11]. The four EF-hand Ca2+-binding motifs of the CaM-LD are organized into two lobes that have distinct Ca2+ affinities resulting in different roles in CDPK regulation [12,13]. In the basal state, the intramolecular interaction between the junction region and the catalytic center maintains the kinase in an inactive state by a pseudosubstrate mechanism [1,2]. The C-terminal lobe of the CaM-LD exhibiting high Ca2+ affinity interacts with the auto-inhibitory region at low Ca2+ level to stabilize the structure [14]. Ca2+ binding to the low-affinity N-terminal lobe of the CaM-LD induces a conformational change that releases the auto-inhibition [1,2]. As a result, deleting both the autoinhibitory domain and the CaM-LD generates a constitutively active form that constitutes a powerful tool for studying CDPK functions in vivo [15–17].
The activation model, based on the in vitro characterization of recombinant proteins, is now supported by the in planta analysis of EF-hand-mutated variants of Arabidopsis (Arabidopsis thaliana) AtCPK21 [18]. Interestingly, the recent crystal structure of full-length apicomplexan (Toxoplasma gondii and Cryptosporidium parvum) CDPKs in both apo and Ca2+-bound forms confirms the essential role of the N-lobe of the CaM-LD in triggering CDPK activation [19]. Importantly, multiple CDPK isoforms from soybean (Glycine max) and Arabidopsis exhibit distinct Ca2+ sensitivities, which is consistent with their roles in decoding different Ca2+ signals [20,21]. However, several CDPKs, including Arabidopsis AtCPK13 and AtCPK23, were recently shown to be weakly or not sensitive to Ca2+ (Table 1) [21–23]. Despite alterations in some EF-hand motifs, the CDPKs with apparently lower Ca2+-sensitivity are able to bind Ca2+ in vitro [21], which triggers the same conformational change as in canonical CDPKs [24]. Moreover, most reported CDPK assays used generic but not specific and biologically relevant endogenous substrates to determine the Ca2+ sensitivity of CDPK activities, which may vary with different substrates [1,20,21]. Thus, it remains possible that all CDPKs exhibit Ca2+ activation in vivo with appropriate substrates. A high-throughput screen of synthetic peptides has identified many potential CDPK substrates for further characterization [25]. Nonetheless, Ca2+ also regulates other aspects of CDPKs, such as protein interactions [26,27] or sub-cellular localization [24]. Thus, CDPKs can sense various Ca2+ signals through the amplitude and sub-cellular location of Ca2+ rise, but how they may distinguish frequency and duration requires further investigation, such as elucidating the molecular mechanism of CDPK deactivation.
Table 1.
Functional characterization of Arabidopsis CPKs
| Protein (synonym) | Gene | Sub-group | Expression a | N-acylation predictionb | Localizationc | Ca2+ - depd | Functionc | Refs |
|---|---|---|---|---|---|---|---|---|
| CPK1 (AK1) | At5g04870 | I | R, S, GC | N-Myr | Peroxisomes, oil bodies | Yes | SA and defense, cold | [13,25,46,48,57,60, 79,87,90] |
| CPK2 | At3g10660 | I | R, P | N-Myr | ER and other membranes | Yes | ND | [21,40,42] |
| CPK3 (CDPK6) | At4g23650 | II | R, S, GC | N-Myr | Soluble, PM, tonoplast | Yes | Herbivore, salinity, stomata | [21,23,44,46,53,80, 88] |
| CPK4 | At4g09570 | I | R, S, P, GC | – | Cytosol, nucleus | Yes | MAMP, ABA, drought, salinity, stomata | [17,21,39,46,51,52, 88] |
| CPK5 | At4g35310 | I | R, S, GC | N-Myr | Membrane, cytosol, nucleus | Yes | MAMP | [17,21] |
| CPK6 (CDPK3) | At2g17290 | I | R, S, P, GC | N-Myr | Membrane, cytosol, nucleus | Yes | MAMP, ABA, drought, salinity, stomata | [17,42,74,80,81,83, 88] |
| CPK7 | At5g12480 | III | R, S, GC | N-Myr-Palm | PM | Unclear | Stomata | [21,46,88] |
| CPK8 (CDPK19) | At5g19450 | III | R, S, GC | N-Myr-Palm | PM | Unclear | Stomata | [21,46,88] |
| CPK9 | At3g20410 | II | R, S, GC | N-Myr-Palm | PM | Yes | ND | [21,42,46] |
| CPK10 (CDPK1) | At1g18890 | III | R, S, GC | N-Myr-Palm | PM | Unclear | ABA, drought, stomata | [15,21,25,27,88] |
| CPK11 (CDPK2) | At1g35670 | I | R, S, P, GC | – | Cytosol, nucleus | Yes | MAMP, ABA, drought, salinity, stomata | [17,21,39,51,52,88] |
| CPK12 (CDPK9) | At5g23580 | I | R, S | – | Cytosol, nucleus | Yes | ABA | [72] |
| CPK13 | At3g51850 | III | R, S, GC | N-Myr-Palm | Membrane, PM | Unclear | Herbivore | [21,23,42] |
| CPK14 | At2g41860 | III | P | N-Myr-Palm | ND | ND | ND | |
| CPK15 | At4g21940 | II | S | N-Myr-Palm | ND | ND | ND | |
| CPK16 | At2g17890 | IV | P | N-Myr-Palm | PM | ND | ND | [25,45,46] |
| CPK17 | At5g12180 | II | P | N-Myr-Palm | PM | ND | Pollen tube growth | [36] |
| CPK18 | At4g36070 | IV | P | N-Myr-Palm | ND | ND | ND | |
| CPK19 | At1g61950 | II | – | – | Membrane | Yes | ND | [21] |
| CPK20 | At2g38910 | I | P | N-Myr | ND | ND | ND | |
| CPK21 | At4g04720 | II | R, S, GC | N-Myr-Palm | PM | Yes | Osmotic stress, stomata | [18,22,46,82] |
| CPK22 | At4g04710 | II | R, S, GC | N-Myr-Palm | ND | ND | ND | |
| CPK23 | At4g04740 | II | – | N-Myr-Palm | PM | Unclear | Drought, salinity, stomata | [22,75,82] |
| CPK24 | At2g31500 | III | P | N-Myr-Palm | ND | ND | ND | |
| CPK25 | At2g35890 | I | P | N-Myr | Membrane | No | ND | [21] |
| CPK26 | At4g38230 | I | P | – | ND | ND | ND | |
| CPK27 | At4g04700 | II | R, S, GC | N-Myr-Palm | ND | ND | ND | |
| CPK28 | At5g66210 | IV | R, S, GC | N-Myr-Palm | PM | ND | ND | [46] |
| CPK29 | At1g76040 | II | R, S | – | ND | ND | ND | |
| CPK30 (CDPK1a) | At1g74740 | III | R, S, GC | N-Myr-Palm | Membrane | Unclear | ABA and abiotic stress | [15,21] |
| CPK31 | At4g04695 | II | – | N-Myr-Palm | ND | ND | ND | |
| CPK32 | At3g57530 | III | R, S, P, GC | N-Myr-Palm | Membrane, nucleus | Unclear | ABA, stomata | [21,47,88] |
| CPK33 | At1g50700 | II | R, S | N-Myr-Palm | ND | ND | ND | |
| CPK34 | At5g19360 | II | P | N-Myr-Palm | PM | Yes | Pollen tube growth | [25,36] |
The expression of CPKs in root (R), shoot (S), pollen (P) and guard cell (GC) was compiled from diverse microarray data (http://www.weigelworld.org/resources/microarray/AtGenExpress/). Several CPKs exhibit a low expression level in all organs (−).
The absence (−) or presence of acylation at the N-terminus, either a myristate (N-Myr) and/or a palmitate (Palm), was predicted by TermiNator program (http://www.isv.cnrs-gif.fr/terminator2/index.html).
Abbreviations: ER, endoplasmic reticulum; PM, plasma membrane; ND, not determined; SA, salicylic acid; MAMP, microbe-associated molecular pattern; ABA, abscisic acid.
Some CPKs are clearly Ca2+-dependent for their activity (Yes) whereas others exhibit no or low calcium stimulation on general and unspecific substrates despite effective calcium binding (Unclear). Only CPK25 lacking EF-hands is truly Ca2+-independent (No). ND, not determined.
Besides Ca2+, phosphorylation, lipids and interaction with 14-3-3 proteins have been reported to further modulate CDPKs in vitro [1,2,4,10,11]. Recent in vivo studies, including phosphoproteomic approaches [28,29], are starting to reveal the biological significance of these regulatory mechanisms in response to various signals. For instance, stress-dependent phosphorylations correlated with kinase activation have been reported for the tobacco (Nicotiana tabacum) NtCDPK2 and NtCDPK3 [30]. In maize (Zea mays), the lipid activation of ZmCPK11 by direct binding of phosphatidic acid probably occurs in response to wounding [31].
CDPK expression and localization
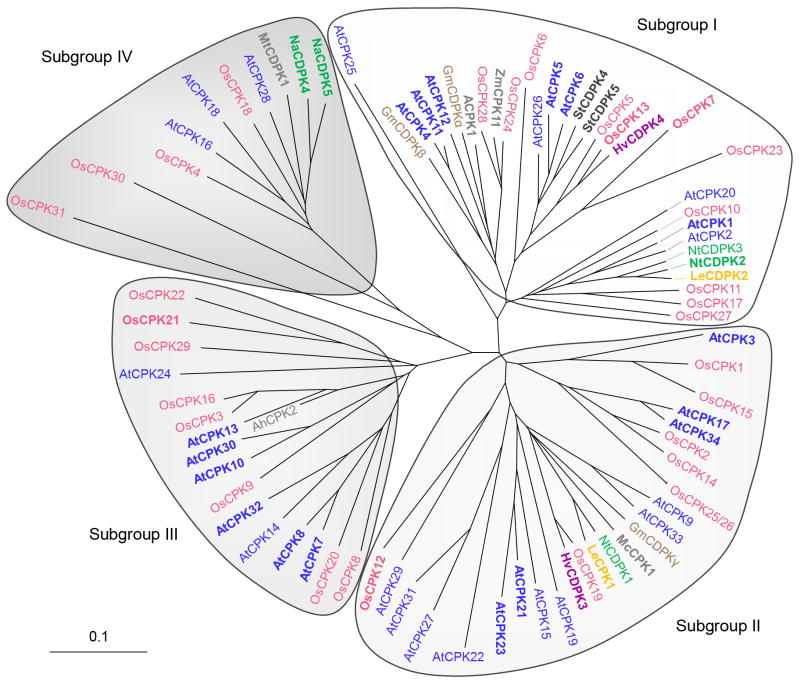
CDPKs are encoded by multigene families of 34 members in Arabidopsis [1,2,10], 31 in rice (Oryza sativa) [32,33] and at least 20 in wheat (Triticum aestivum) [34], and are divided into four subgroups (Figure 1, Table 1). Extensive transcriptomic analyses have revealed different expression patterns for each isoform, which contributes to the functional specificity of the CDPKs [1,33–35]. Some CDPKs are expressed in most organs whereas others are specific to some tissues. For example, AtCPK17 and AtCPK34 are preferentially expressed in mature pollen and regulate pollen tube growth [36]. Differential expression of CDPKs has been observed in response to diverse stimuli, including abscisic acid (ABA), cold, drought, salinity, heat, elicitors and pathogens [16,33–35], which correlates with the presence of stress-responsive cis-elements in rice CDPK gene promoters [35]. CDPK protein accumulation has also been reported after cold or ABA treatment, resulting in enhanced CDPK kinase activity [37–39].
Figure 1.
Relation tree of selected plant CDPKs. The full-length amino acid sequences of CDPKs (see supplementary material online) from Arabidopsis (At, blue), rice (Os, pink), soybean (Gm, brown), potato (St, black), barley (Hv, purple), tobacco (Nt, green), coyote tobacco (Na, green), tomato (Le, yellow) and grapevine (ACPK1, gray), maize (Zm, gray), alfalfa (Mt, gray), ice plant (Mc, gray) and peanut (Ah, gray) were aligned and analyzed with ClustalX and TreeView algorithms. The CDPK family is divided into four major subgroups (I–IV). The branched lengths are proportional to divergence and the scale of 0.1 represents 10% change. The CDPKs with known biological functions are highlighted in bold.
Most CDPKs have a predicted N-myristoylation site involved in membrane targeting (TermiNator, http://www.isv.cnrs-gif.fr/terminator2/index.html), which has been confirmed in vitro for some of them [12,40–45]. This irreversible co-translational acylation requires a second post-translational signal to maintain the membrane association, such as reversible palmitoylation [30,41], a polybasic domain that may be modified by phosphorylation [43] or protein interaction. As a result, most CDPKs are membrane anchored (Table 1) and the reversibility of the second signal may allow CDPKs to shuttle between membranes and the cytosol or nucleus. Diverse cellular localizations of CDPKs have been observed (Table 1), including the cytosol, nucleus, plasma membrane, endoplasmic reticulum (ER), tonoplast, mitochondria, chloroplasts, oil bodies and peroxisomes [17,27,34,36,38–40,42,44,46–48], indicating that CDPKs have access to a plethora of potential substrates throughout the cell. Moreover, stress-induced nuclear accumulation has been observed for the ice plant (Mesembryanthemum crystallinum) McCPK1 [43,49] and the groundnut (Arachis hypogaea) AhCPK2 [24].
Substrate specificity
Biochemical analyses based on known substrates have identified four distinct motifs that define CDPK target sites [2]. An extensive survey of 534 synthetic peptides tested for in vitro kinase assays with four recombinant Arabidopsis CDPKs (AtCPK1, AtCPK10, AtCPK16 and AtCPK34) revealed that CDPK specificity relies either on the substrate itself or on kinetic parameters for a common substrate [25]. This could result from different Ca2+ affinities that can affect substrate accessibility, as suggested by the crystal structure of apicomplexan CDPKs. Indeed, the N-lobe of the CaM-LD is located near the N-terminus of the kinase domain in resting conditions while the entire CaM-LD rotates around the kinase domain to release the substrate-binding site in the Ca2+-bound form [19]. Moreover, a domain-swap analysis between NtCDPK1 and AtCPK9 demonstrates the key role of the N-terminal variable domain in controlling substrate specificity [50].
Identifying in vivo substrates is an important challenge to unravel CDPK functions. To date, most substrates have been described using in vitro assays and are involved in diverse cellular processes, such as primary and secondary metabolism, stress responses, ion and water transport, transcription and signaling [2,4,10,25]. Interestingly, an analysis of 274 synthetic peptides harboring in vivo-mapped phosphorylation sites showed a 27% match with AtCPK substrates in an in vitro kinase reaction, suggesting potential biological relevance [25]. Yeast-two-hybrid screens with CDPK variants exhibiting altered kinase activity have facilitated the identification of putative substrates that require in vivo confirmation [49,51,52]. Several transcription factors, including ABF4 (ABA-responsive element-binding factor 4), RSG (repression of shoot growth) and HsfB2a (heat shock factor B2a), have been further characterized as in vivo CDPK substrates involved in ABA [47], gibberellin [26,50] and herbivore-induced signaling [23], respectively. Recently, an in planta random screen coupling bimolecular fluorescence complementation and flow cytometry has identified new AtCPK3 interactors that could not be revealed by yeast-two-hybrid assays, suggesting the potential to identify plant-specific protein interactions [53]. Future challenges include correlating the mutant phenotypes of particular CDPKs and their corresponding substrates to establish their biological significance.
In summary, the multigene family of CDPKs encodes key Ca2+ sensor protein kinases that differ by their expression pattern, sub-cellular localization, substrate specificities, Ca2+ sensitivities and regulation by lipids, phosphorylation and protein interactions. This huge diversity in molecular and biochemical properties of CDPKs is likely to provide functional specificity and redundancy in mediating plant Ca2+ signaling in immune and stress responses.
CDPKs in immune signaling
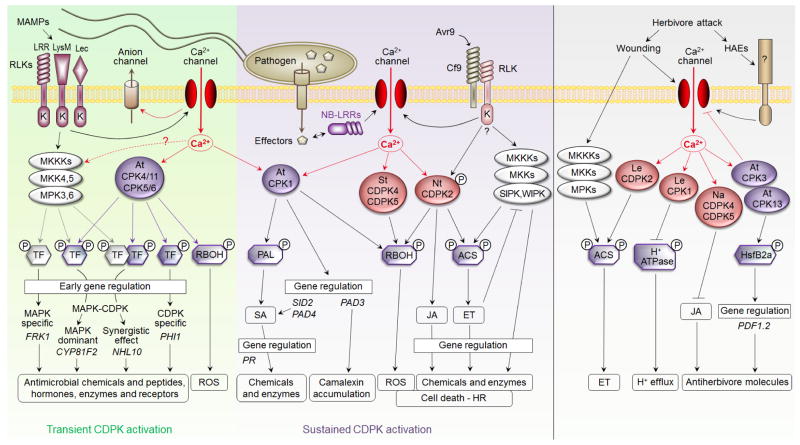
Plants sense potential pathogens through the recognition of microbe-associated molecular patterns (MAMPs) by cell-surface pattern-recognition receptors (PRRs) or effector proteins via intracellular nucleotide-binding leucine-rich repeat (NB-LRR) immune sensors to initiate overlapping and distinct signaling cascades, including protein kinase activation, Ca2+ influx, hormone biosynthesis, oxidative burst and transcriptional reprogramming. Recent studies have provided compelling evidence for the involvement of CDPKs in most of these signaling events (Figure 2).
Figure 2.
CDPK signaling network in immune responses. Microbe-associated molecular pattern (MAMP) perception by different cell-surface receptor kinases (RLKs) with distinct extracellular domains triggers transient CDPK activation to regulate transcription factors and early gene expression either independently or in coordination with MAPK cascades. Several CDPKs also activate NADPH oxidases (respiratory burst oxidase homologs, RBOHs) to induce early reactive oxygen species (ROS) production. By contrast, the sustained CDPK activation by extracellular (Avr9) or intracellular effector proteins leads to biosynthesis of salicylic acid (SA), jasmonic acid (JA) and ethylene (ET) through regulatory gene induction or enzyme activation such as phenylalanine ammonia-lyase (PAL) and ACC synthase (ACS). CDPKs also trigger a prolonged oxidative burst involved in cell death and hypersensitive response (HR). Constitutively active NtCDPK2 inhibits MAPK [salicylic acid-induced protein kinase (SIPK) and wound-induced protein kinase (WIPK)] activation by Avr9–Cf9 in an ET-dependent manner. Herbivores can be sensed through wounding or herbivore-associated elicitors (HAEs) by unknown receptors to activate MAPKs and Ca2+ influx. The co-regulation of ACS by MAPKs and CDPKs leads to ET production, whereas LeCPK1 inhibits the plasma membrane H+-ATPase to induce extracellular alkalinization. AtCPK3 and AtCPK13 mediate herbivore-induced gene expression by phosphorylating the transcription factor HsfB2a whereas only AtCPK3 negatively regulates Ca2+ channels. NaCDPK4 and NaCDPK5 negatively regulate defense against herbivores by inhibiting JA accumulation and subsequent production of defense metabolites. Abbreviations: MKKs, mitogen-activated protein kinase kinases; MKKKs, mitogen-activated protein kinase kinase kinases; MPKs, mitogen-activated protein kinases; TF, transcription factor.
Hormonal signaling and gene regulation in plant defense
The tobacco NtCDPK2 from subgroup I is the first CDPK identified for its role in race-specific plant defense. The extracellular Avr9 fungal effector strongly activates NtCDPK2 in tobacco leaves expressing the corresponding tomato (Lycopersicon esculentum) resistance gene Cf9 as a LRR receptor-like protein on the plasma membrane [16]. This activation requires both NtCDPK2 autophosphorylation and phosphorylation by an upstream kinase, revealing the complexity of fine-tuning defense signaling cascades [30]. Transient expression of constitutively active NtCDPK2 triggers jasmonic acid (JA) and ethylene (ET) accumulation, and subsequent induction of JA- and ET-regulated genes [54]. ET production could occur through stabilizing the rate-limiting enzyme ACC synthase (ACS) of ET biosynthesis by direct phosphorylation, as observed for the closest homolog LeCDPK2 in tomato [55] and purified maize CDPKs [56]. Interestingly, constitutively active NtCDPK2 blocks the Avr9–Cf9 activation of mitogen-activated protein kinases (MAPKs) in an ET-dependent manner, potentially serving as a negative feedback to reset the system after elicitation [54] (Figure 2). In Arabidopsis, overexpressing the closest homolog AtCPK1 confers broad-spectrum resistance to bacteria and fungi [48]. However, unlike NtCDPK2, which reduces salicylic acid (SA) levels and the expression of SA-regulated pathogenesis-related genes (PR1a and PR2a) [54], long-term AtCPK1 overexpression triggers SA accumulation through the induction of SA regulatory and biosynthesis genes, PAD4 (phytoalexin-deficient 4) and SID2/ICS1 (SA induction-deficient 2/isochorismate synthase 1), and consequently SA-regulated genes without affecting JA or ET [48]. Interestingly, AtCPK1 specifically phosphorylates phenylalanine ammonia-lyase (PAL) in vitro, which is a key enzyme in plant defense notably involved in an alternative pathway to produce SA [57]. Although the role of PAL phosphorylation in defense is not clear, this finding further supports the notion that AtCPK1 plays a crucial role in SA accumulation (Figure 2). Although we could expect that protein kinase orthologs play similar roles in various plant species, these results suggest that some closely related CDPK homologs might have evolved independently in different plant species or in different biological contexts. However, it is also crucial to distinguish transient and long-term CDPK overexpression and activation, which may lead to distinct direct and indirect physiological consequences.
In Arabidopsis leaf cells, the bacterial MAMP flg22 (a 22-amino acid peptide of flagellin) transiently activates multiple CDPK activities. Interestingly, a cell-based functional genomic screen with 25 constitutively active AtCPKs using a flg22-responsive reporter NHL10-LUC (NDR1/HIN1-like10-luciferase) identified four related CDPKs from subgroup I, AtCPK4, AtCPK5, AtCPK6 and AtCPK11, as early transcriptional regulators in MAMP signaling [17]. Unlike NtCDPK2, these four AtCPKs do not affect MAPK activation by flg22. Unexpectedly, CDPKs and MAPK cascades differentially regulate flg22-induced early genes in at least four regulatory programs, displaying CDPK-specific, MAPK-specific, CDPK/MAPK parallel or CDPK/MAPK synergistic regulation, which imply independent or co-regulation of common targeted transcription factors, transcription machinery and/or chromatin remodeling complexes [58,59] (Figure 2). Correlated with genome-wide analysis of redundant and specific target genes modulated by multiple MAMPs and by transiently expressed active AtCPK5 and AtCPK11, cpk5 cpk6 double mutant, cpk5 cpk6 cpk11 triple mutant and cpk4 cpk5 cpk6 cpk11 quadruple mutant exhibit gradually reduced flg22 responsiveness for gene expression and pathogen resistance, demonstrating the key positive roles of these CDPKs in convergent MAMP signaling [17].
In summary, various CDPKs from subgroup I mediate transient and sustained transcriptional reprogramming in plant innate immune responses, and play key roles in the regulation of SA, ET and JA hormonal signaling.
Oxidative burst and cell death
Transient expression of active NtCDPK2 also induces ROS (reactive oxygen species) production and HR (hypersensitive response)-like cell death upon exposure to a non-symptom-producing stress [54]. Consistently, silencing its orthologs in Nicotiana benthamiana reduces the HR elicited by the Avr4–Cf4 and Avr9–Cf9 interactions [16]. Interestingly, the closest homolog AtCPK1 also triggers ROS production by stimulating the NADPH oxidase activity in tomato protoplasts [60]. Active forms of potato (Solanum tuberosum) StCDPK4 and StCDPK5, close homologs of AtCPK5/AtCPK6, induce ROS production by directly phosphorylating the NADPH oxidase RBOHB (respiratory burst oxidase homolog B) in vivo at the site targeted by infection with Phytophthora infestans [61]. As a consequence, transgenic potato plants overexpressing the active variant of StCDPK5 display increased ROS production, HR-like cell death and resistance to the hemibiotrophic pathogen P. infestans, but higher susceptibility to the necrotrophic pathogen Alternaria solani [62]. Coherently, the Arabidopsis cpk5 cpk6 double mutant, cpk5 cpk6 cpk11 triple mutant and cpk4 cpk5 cpk6 cpk11 quadruple mutant show reduced early ROS production in response to flg22 [17]. These results suggest that multiple CDPKs from subgroup I play a key role in the defense-induced oxidative burst by activating NADPH oxidases through direct phosphorylation (Figure 2). Homologs of AtCPK5/AtCPK6 also mediate plant defense in monocots. The rice OsCPK13 induces cell death, accumulation of PR proteins and up-regulation of some defense genes when ectopically expressed in sorghum (Sorghum bicolor) [63], whereas the active form of barley (Hordeum vulgare) HvCDPK4 triggers cell death [64]. Thus, CDPKs display conserved defense functions among plant species in promoting cell death. However, genetic manipulation of CDPKs in crop protection may need specifically tailored strategies considering the site and duration of CDPK activation and the targeted pathogens.
By contrast, several CDPKs have been shown to play negative roles in plant defense. In barley, HvCDPK3 from subgroup II promotes host cell entry of the powdery mildew fungus during both compatible and incompatible interactions [64]. In rice, overexpressing OsCPK12 from subgroup II confers susceptibility to both virulent and avirulent blast fungus, potentially through ABA hypersensitivity and reduction in ROS production [65]. Interestingly, Ca2+- and CaM-regulated protein kinase (CCaMK) is a central signaling hub in root nodule and arbuscular mycorrhiza symbioses in plants [66]. However, plant defense must be shut down in the initial phase of the plant-microbe interaction, so that the symbiont is not recognized as a pathogen. In Medicago truncatula, MtCDPK1 from subgroup IV is likely to be involved in this process because the MtCDPK1-silenced plants are compromised in establishing symbiotic interactions and display enhanced ROS production induced by Nod factors and increased expression of cell wall biosynthesis and defense genes [67].
Responses to wounding and herbivore attacks
During insect attack, wounded tissues constitute entry sites for herbivory elicitors, making wounding perception a part of herbivore recognition. A recent screen of 19 cpk T-DNA insertion mutant lines identified two CDPKs, AtCPK3 and AtCPK13, as positive regulators of PDF1.2 induction by Spodoptera littoralis caterpillars [23]. This regulation probably occurs through phosphorylation-dependent activation of the transcription factor HsfB2a, without affecting the level of ET, JA or ABA. Moreover, AtCPK3, but not AtCPK13, also triggers a negative feedback on herbivore-induced Ca2+ signals, indicating that CDPKs can play redundant as well as specific functions in plant defense. Given that AtCPK3 can be activated by flg22 in protoplasts [44] and induce the flg22-responsive gene NHL10 [17], it may also be involved in MAMP signaling. By contrast, the tomato LeCDPK2 phosphorylates the ethylene biosynthesis enzyme LeACS2 at the site phosphorylated in vivo after wounding, suggesting that LeCDPK2 contributes to ethylene production in response to wounding [55]. Interestingly, MAPKs also phosphorylate LeACS2 at a different site, and both CDPK and MAPK phosphorylations are required simultaneously to stabilize LeACS2 in vivo [55]. Wounding also induces extracellular alkalinization through the inhibition of the plasma membrane H+-ATPase, which may be mediated in tomato by the membrane-anchored LeCPK1 [12] (Figure 2). In maize, ZmCPK11, which is closely related to AtCPK4/AtCPK11, is activated by wounding in a JA-dependent pathway; however, its precise biological function remains to be determined [68]. Recently, two redundant CDPKs from subgroup IV in coyote tobacco (Nicotiana attenuata), NaCDPK4 and NaCDPK5, were proposed to negatively regulate herbivore resistance by blocking JA and defense metabolite accumulation, without affecting the expression of JA biosynthesis enzymes [69].
Thus, various CDPKs from different subgroups mediate plant responses to wounding and herbivores through the regulation of hormone biosynthesis, such as ET and JA, and gene expression, either with a positive or a negative effect.
CDPKs in hormonal and abiotic stress signaling
Gene regulation in ABA, drought and salt stress signaling
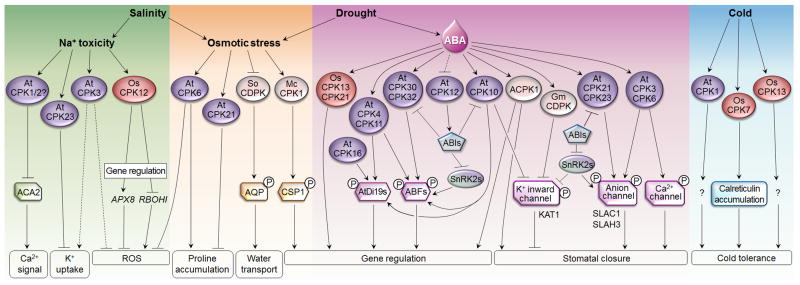
ABA is a key stress hormone that mediates plant responses to drought and salinity. AtCPK10 and AtCPK30 from subgroup III are the first CDPKs identified as positive regulators of the barley stress- and ABA-inducible HVA1 promoter in maize protoplasts [15]. Constitutively active AtCPK10 also induces endogenous stress- and ABA-inducible genes in Arabidopsis leaf cells (Y. Niu and J. Sheen, unpublished), suggesting a conservation of specific CDPK functions in dicots and monocots. This transcriptional induction is likely to occur through the ABA-responsive transcription factors, ABFs, which were identified as in vitro substrates for several CDPKs from subgroups I and III [39,47] (Figure 3). In particular, AtCPK32 activates ABF4 in vivo, resulting in the induction of ABF4 target genes [47]. AtCPK4 and AtCPK11 regulate both ABF1 and ABF4 and induce gene expression several hours after ABA elicitation, suggesting a role in long-term adaptation [39]. This modified gene expression correlates with ABA hypersensitivity and increased tolerance to drought and salt in plants overexpressing AtCPK4 or AtCPK11, whereas the cpk4 cpk11 double mutant exhibits the opposite phenotypes. Given that only selected ABA-responsive genes are partially affected in the cpk4 cpk11 double mutant, other regulators also contribute to ABA signaling [39]. Similar results were observed in rice transgenic plants overexpressing OsCPK21 [70] and OsCPK13 (OsCDPK7) [71], suggesting that ABA-induced transcriptional reprogramming via ABFs is likely to be a key feature of CDPK signaling in both monocots and dicots.
Figure 3.
CDPK signaling network in abiotic stress responses. Plants sense drought and salinity through Na+ toxicity, osmotic stress and ABA synthesis to activate CDPKs, which regulate K+ uptake, ROS production, accumulation of compatible osmolytes (proline), water transport (aquaporin, AQP) and gene expression. Redundant CDPKs modulate gene expression by activating the transcription factors ABFs and AtDi19s. AtCPK12 is a negative regulator that stimulates the protein phosphatase ABIs, inhibiting SnRK2- and CDPK-dependent transcriptional regulation. CDPKs also promote stomatal closure by inhibiting the K+ inward channel (KAT1), and activating slow-type anionic channels (SLAC1 and SLAH3). CDPKs and SnRK2s share common substrates (ABFs and channels) and common down-regulators (ABIs). Some CDPKs also inhibit permeable Ca2+ channels or Ca2+-pumps (ACA2) in a negative feedback loop. Several CDPKs have been shown to trigger cold tolerance; however, the molecular mechanism is not understood.
Although the role of CDPK phosphorylation has not been thoroughly investigated, other transcription factors involved in stress-induced gene regulation have also been identified as CDPK substrates in vitro. They include the Arabidopsis dehydration-inducible gene family AtDi19, which encodes nuclear zinc-finger proteins [25,51], and the ice plant pseudo-response regulator transcription factor CSP1 [49] (Figure 3). By contrast, AtCPK12, the closest homolog of AtCPK4/AtCPK11, inhibits ABA responses by stimulating the negative regulator ABI2 (ABA insensitive 2), although the role of ABI2 phosphorylation by AtCPK12 requires further studies [72].
Metabolic and transport regulation in drought and salt stress signaling
Drought and salinity trigger the production of ROS, which must be detoxified. Soybean GmCDPKα and GmCDPKγ have been shown to phosphorylate in vitro the serine acetyltranferase 2;1 (GmSerat2;1) involved in cysteine biosynthesis, at the site phosphorylated in vivo after oxidative stress [73]. Since the phosphorylation releases the feedback inhibition by cysteine, CDPKs may participate in anti-oxidant responses by providing cysteine for glutathione production. In rice, OsCPK12 regulates ROS homeostasis under salt stress conditions by inducing the expression of ROS scavenger genes OsAPX2/OsAPX8 and repressing the NADPH oxidase gene OsRBOHI, leading to increased salt tolerance [65]. Overexpressing AtCPK6 confers drought tolerance, correlated with enhanced gene expression and accumulation of the compatible osmolyte proline, but reduces lipid peroxidation, probably through decreased ROS production [74]. However, the cpk6 single mutant does not exhibit any stress phenotype, as observed in pathogen responses because of functional redundancy among CDPKs [17]. By contrast, AtCPK21 is a negative regulator of osmotic responses and inhibits proline accumulation [18]. The subtle enhanced drought tolerance in the cpk21 mutant is likely to result from the compensated overexpression of the closest homolog AtCPK23 [18], which also negatively regulates drought and salt resistance by inhibiting K+ uptake [75] (Figure 3). Interestingly, unlike in defense responses [17,23], AtCPK3 does not regulate gene expression in salt stress signaling but modulates the phosphoproteome independently of MAPK cascades [44]. In particular, two putative substrates, a glutathione-S-transferase and a subunit of a potassium channel, suggest a role of AtCPK3 in anti-oxidant responses and K+ uptake, respectively.
Limiting water loss is crucial during dehydration conditions and may occur through the down-regulation of aquaporin water channels, as observed in spinach (Spinacea oleracea) with the CDPK-stimulated aquaporin PM28A [76,77]. Regulating Ca2+ signals is also a key component of stress signaling to ensure that specific responses are induced by each stress. The Arabidopsis auto-inhibited calcium ATPase ACA2 has been shown to play a crucial role in generating the appropriate Ca2+ signal in yeast exposed to high salinity [78]. Interestingly, the active form of AtCPK1 inhibits the basal activity of ACA2 and blocks stimulation by CaM in yeast [79]. However, AtCPK1 is not localized in the ER, unlike its closest homolog AtCPK2 and ACA2, suggesting that AtCPK2 might regulate ACA2 in planta [40,48]. Thus, multiple CDPKs differentially modulate transcription factors, metabolic enzymes, ion and water transport to positively or negatively regulate drought and salt responses.
Regulation of stomatal movements
Ca2+ is an essential component of guard cell signaling, and regulates ion fluxes involved in stomatal movements, notably through CDPK-dependent phosphorylation of channels. The cpk3 cpk6 double mutant is impaired in ABA-activation of slow-type anionic channels and Ca2+ permeable channels, resulting in decreased ABA-induced stomatal closure [80]. Despite a weak in vivo interaction with the major guard cell Cl− channel SLAC1 [22], AtCPK6 but not AtCPK3 was recently shown to phosphorylate SLAC1 at S59 and to activate the channel in Xenopus (Xenopus laevis) oocytes [81]. Similarly, two close homologs from subgroup II, AtCPK21 and AtCPK23, also phosphorylate and activate SLAC1 and its related NO3− channel SLAH3 in response to ABA [22,82]. AtCPK23 preferentially regulates SLAC1 independently of Ca2+ whereas AtCPK21 mainly regulates SLAH3 in a Ca2+-dependent manner (Figure 3). Whether the three CDPKs target the same phosphosite remains to be determined to understand the biological relevance of such a redundancy. Surprisingly, cpk21 and cpk23 single mutants do not exhibit any stomata phenotype, unlike cpk3 and cpk6 [22,80,82]. It is thus possible that the stronger phenotypes observed in cpk3 and cpk6 result from the inhibition of Ca2+ currents that would impact global Ca2+-regulated responses rather than only inhibiting anionic channels. Interestingly, unlike other cpk mutants, cpk6 is also impaired in methyl jasmonate (MeJA)-induced S-type anionic current and stomatal closure [83]. Since MeJA and ABA differentially mediate pathogen and abiotic stress responses, distinct hormonal pathways may regulate stomatal closure upon biotic and abiotic stresses.
The cpk4 cpk11 double mutant is also partially compromised in ABA-induced stomatal closure with enhanced leaf water loss [39], whereas its closest homolog in grapevine (Vitis vinifera) ACPK1 confers the opposite phenotype when overexpressed in Arabidopsis [84]. The inhibition of K+ inward channels, such as KAT1, also contributes to stomatal closure. In the broad bean (Vicia faba), a CDPK phosphorylates KAT1 in vitro [85], which inhibits the channel activity [86]. Moreover, the ABA inhibition of K+ inward channels is abolished in the Arabidopsis cpk10 mutant, leading to reduced stomatal closure and drought hypersensitivity, suggesting that AtCPK10 may down-regulate KAT1 in vivo [27]. AtCPK1 stimulates a vacuolar Cl− channel, resulting in Cl− uptake into the vacuole and stomatal opening [87]. Despite some discrepancies in ABA-induced stomatal closure in some cpk mutants, the impaired Ca2+-induced stomatal closure in multiple cpk mutants from subgroups I, II and III, cpk4 cpk11 and cpk3 cpk6 double mutants, cpk10 single mutant and cpk7 cpk8 cpk32 triple mutant, demonstrates the crucial role of CDPKs in regulating stomatal movements, even though the molecular mechanism remains to be elucidated [88].
Cold tolerance
Ca2+-mediated early cold induction of the key transcriptional regulators, the CBFs (cold-responsive element-binding factors), is crucial for cold tolerance. Several CaM-interacting transcription activators (CAMTAs) have been shown to bind to the CBF2 promoter and the camta3 mutant is impaired in cold-induction of CBF1 and CBF2. The reduced freezing tolerance of the camta1 camta3 double mutant further supports the notion that CAMTAs play a role in cold-stimulated Ca2+ signaling [89]. However, the functions of CDPKs in cold stress signaling remain mostly elusive. Unlike in salt or drought signaling, OsCPK13 (OsCDPK7) confers cold resistance without affecting transcriptional regulation [71]. Similarly, AtCPK1 does not affect CBF induction but regulates the phosphoproteome after cold treatment [90]. A membrane-bound rice CDPK is activated by cold after 18–24h, suggesting a role in the adaptive process rather than early responses [91]. Likewise, overexpressing OsCPK7 (OsCDPK13), which is preferentially expressed in cold-tolerant rice varieties, triggers cold resistance and the accumulation of the cold-responsive chaperone calreticulin [37,92]. Thus, CDPKs are clearly involved in cold tolerance, but their molecular functions remain to be explored (Figure 3).
CDPKs at the crossroad of stress signaling networks
Recent advances have revealed CDPKs as central regulators of Ca2+-mediated immune and stress responses that are crucial for plant survival. Some key players, including AtCPK1, AtCPK3, AtCPK4, AtCPK6, AtCPK11, OsCPK12 and OsCPK13, represent crucial signaling nodes that mediate plant responses to both abiotic stress and pathogens (Table 1). Beside some specificity, several CDPKs also show functional redundancy that may provide robust plant responsiveness and adaptability to adverse environmental conditions. For instance, AtCPK4, AtCPK5, AtCPK6 and AtCPK11 co-regulate gene expression in early MAMP signaling [17], whereas AtCPK4, AtCPK11, AtCPK10, AtCPK30 and AtCPK32 all mediate ABA responses through ABFs [15,39,47]. There is also functional redundancy with other protein kinase families such as the SNF1-related kinases 2 (SnRK2s) [93,94] and MAPKs [17] (Figures 2 and 3). In particular, several effector proteins of guard cell signaling such as KAT1, SLAC1, RBOHs and ABFs are targeted by both CDPKs and SnRK2s, potentially at different sites, although the co-regulation has not been investigated [95]. Furthermore, analogous to the roles in antagonizing SnRK2s [95], the protein phosphatase 2C (PP2C) ABI1, ABI2 and/or related PP2Cs inhibit CDPKs in stress and ABA signaling, such as AtCPK10/AtCPK30-mediated gene regulation [15,96], or AtCPK21/AtCPK23 activation of anionic channels [22,82].
More complex interactions have been observed between CDPKs and MAPK cascades, from synergism [17,55] to independence [44] and antagonism [54]. The multiple cross-talks between CDPKs and SnRK2s or MAPKs provide additional layers of regulation to fine-tune plant immune and stress responses. Strikingly, some CDPKs may play opposite roles in different cell types. For instance, AtCPK21/AtCPK23 activate SLAC1/SLAH3 in guard cells to promote stomatal closure [22,82], whereas cpk21 and cpk23 mutants are drought tolerant at the whole plant level [18,75]. Distinct CDPK substrates are likely to be responsible for different physiological functions in diverse cellular contexts.
Concluding remarks and perspectives
Extensive research efforts with integrative approaches have provided conclusive evidence that CDPKs are versatile and evolutionarily conserved Ca2+-sensors/transducers that function in a diverse array of plant processes in response to environmental challenges. Combined with expression patterns, intracellular localizations/translocations and modulation by lipids, phosphorylation and interacting proteins, the broad ranges of Ca2+ sensitivity and substrate specificity of CDPKs dictate complex and sophisticated Ca2+ signaling networks via protein phosphorylation to coordinate the dynamic plant cellular processes. Future molecular, cellular, genetic, genomic and phosphoproteomic studies should lead to more precise understanding of specific and redundant roles of CDPKs in the immune and stress signaling networks in cooperation with other Ca2+ sensors and protein kinases, such as RLKs (receptor-like kinases), MAPKs, SnRK1s, SnRK2s and SnRK3s/CIPKs (CBL-interacting protein kinases) [5–7,9,17,25,88,93,94,97,98]. Considering that Ca2+ signals are restricted and localized inside the cells, establishing molecular, cellular and genetic links with specific Ca2+ channels, pumps and transporters [8] in a spatio-temporal analysis of CDPK activation and translocation is crucial to decipher Ca2+-mediated signaling networks in plants. Unlike the Ca2+-independent SnRK2s and MAPKs, analyzing the in vivo activation of CDPKs has been limited because of the difficulty in maintaining precise Ca2+ levels reflecting the physiological states in cell extracts. Developing specific anti-phosphopeptide antibodies raised against active CDPKs or their immediate substrates should facilitate the monitoring of CDPK activation in planta [17,25,30,99,100]. A complementary genetic approach by mutating the EF-hands should establish a functional link between CDPKs and Ca2+ signaling [18,36]. Finally, identifying in vivo substrates and the unique regulatory features of each isoform should provide new insights into CDPK signaling to understand their integrated roles in diverse biological responses, a prerequisite for genetic manipulation of agronomically valuable traits.
Supplementary Material
Acknowledgments
The projects on CDPK signaling have been supported by the National Science Foundation and the National Institutes of Health (J.S.). M.B. was supported by a Marie Curie International fellowship within the 6th European Community Framework Program.
Footnotes
Disclosure statement
The authors declare no conflict of interest.
Supplementary data associated with this article can be found at doi: XXXXXXX.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Harper JF, et al. Decoding Ca2+ signals through plant protein kinases. Annu Rev Plant Biol. 2004;55:263–288. doi: 10.1146/annurev.arplant.55.031903.141627. [DOI] [PubMed] [Google Scholar]
- 2.Harper JF, Harmon A. Plants, symbiosis and parasites: a calcium signalling connection. Nat Rev Mol Cell Biol. 2005;6:555–566. doi: 10.1038/nrm1679. [DOI] [PubMed] [Google Scholar]
- 3.Hepler PK. Calcium: a central regulator of plant growth and development. Plant Cell. 2005;17:2142–2155. doi: 10.1105/tpc.105.032508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Klimecka M, Muszynska G. Structure and functions of plant calcium-dependent protein kinases. Acta Biochim Pol. 2007;54:219–233. [PubMed] [Google Scholar]
- 5.Luan S. The CBL-CIPK network in plant calcium signaling. Trends Plant Sci. 2009;14:37–42. doi: 10.1016/j.tplants.2008.10.005. [DOI] [PubMed] [Google Scholar]
- 6.Boudsocq M, Sheen J. Stress signaling II: calcium sensing and signaling. In: Pareek A, et al., editors. Abiotic Stress Adaptation in Plants: Physiological, Molecular and Genomic Foundation. Springer; 2010. pp. 75–90. [Google Scholar]
- 7.Kudla J, et al. Calcium signals: the lead currency of plant information processing. Plant Cell. 2010;22:541–563. doi: 10.1105/tpc.109.072686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dodd AN, et al. The language of calcium signaling. Annu Rev Plant Biol. 2010;61:593–620. doi: 10.1146/annurev-arplant-070109-104628. [DOI] [PubMed] [Google Scholar]
- 9.Reddy AS, et al. Coping with stresses: roles of calcium- and calcium/calmodulin-regulated gene expression. Plant Cell. 2011;23:2010–2032. doi: 10.1105/tpc.111.084988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cheng SH, et al. Calcium signaling through protein kinases. The Arabidopsis calcium-dependent protein kinase gene family. Plant Physiol. 2002;129:469–485. doi: 10.1104/pp.005645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ludwig AA, et al. CDPK-mediated signalling pathways: specificity and cross-talk. J Exp Bot. 2004;55:181–188. doi: 10.1093/jxb/erh008. [DOI] [PubMed] [Google Scholar]
- 12.Rutschmann F, et al. LeCPK1, a calcium-dependent protein kinase from tomato. Plasma membrane targeting and biochemical characterization. Plant Physiol. 2002;129:156–168. doi: 10.1104/pp.000869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Christodoulou J, et al. Evidence for differing roles for each lobe of the calmodulin-like domain in a calcium-dependent protein kinase. J Biol Chem. 2004;279:29092–29100. doi: 10.1074/jbc.M401297200. [DOI] [PubMed] [Google Scholar]
- 14.Weljie AM, Vogel HJ. Unexpected structure of the Ca2+-regulatory region from soybean calcium-dependent protein kinase-alpha. J Biol Chem. 2004;279:35494–35502. doi: 10.1074/jbc.M311520200. [DOI] [PubMed] [Google Scholar]
- 15.Sheen J. Ca2+-dependent protein kinases and stress signal transduction in plants. Science. 1996;274:1900–1902. doi: 10.1126/science.274.5294.1900. [DOI] [PubMed] [Google Scholar]
- 16.Romeis T, et al. Calcium-dependent protein kinases play an essential role in a plant defence response. EMBO J. 2001;20:5556–5567. doi: 10.1093/emboj/20.20.5556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Boudsocq M, et al. Differential innate immune signalling via Ca2+ sensor protein kinases. Nature. 2010;464:418–422. doi: 10.1038/nature08794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Franz S, et al. Calcium-dependent protein kinase CPK21 functions in abiotic stress response in Arabidopsis thaliana. Mol Plant. 2011;4:83–96. doi: 10.1093/mp/ssq064. [DOI] [PubMed] [Google Scholar]
- 19.Wernimont AK, et al. Structures of apicomplexan calcium-dependent protein kinases reveal mechanism of activation by calcium. Nature Struct Mol Biol. 2010;17:596–601. doi: 10.1038/nsmb.1795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee JY, et al. Kinetic and calcium-binding properties of three calcium-dependent protein kinase isoenzymes from soybean. Biochemistry. 1998;37:6801–6809. doi: 10.1021/bi980062q. [DOI] [PubMed] [Google Scholar]
- 21.Boudsocq M, et al. Characterization of Arabidopsis calcium-dependent protein kinases: activated or not by calcium? Biochem J. 2012 doi: 10.1042/BJ20112072. [DOI] [PubMed] [Google Scholar]
- 22.Geiger D, et al. Guard cell anion channel SLAC1 is regulated by CDPK protein kinases with distinct Ca2+ affinities. Proc Natl Acad Sci U S A. 2010;107:8023–8028. doi: 10.1073/pnas.0912030107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kanchiswamy CN, et al. Regulation of Arabidopsis defense responses against Spodoptera littoralis by CPK-mediated calcium signaling. BMC Plant Biol. 2010;10:97. doi: 10.1186/1471-2229-10-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Raichaudhuri A, et al. Domain analysis of a groundnut calcium-dependent protein kinase: nuclear localization sequence in the junction domain is coupled with nonconsensus calcium binding domains. J Biol Chem. 2006;281:10399–10409. doi: 10.1074/jbc.M511001200. [DOI] [PubMed] [Google Scholar]
- 25.Curran A, et al. Calcium-dependent protein kinases from Arabidopsis show substrate specificity differences in an analysis of 103 substrates. Front Plant Sci. 2011;2:1–15. doi: 10.3389/fpls.2011.00036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ishida S, et al. A tobacco calcium-dependent protein kinase, CDPK1, regulates the transcription factor REPRESSION OF SHOOT GROWTH in response to gibberellins. Plant Cell. 2008;20:3273–3288. doi: 10.1105/tpc.107.057489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zou JJ, et al. Arabidopsis calcium-dependent protein kinase CPK10 functions in abscisic acid- and Ca2+-mediated stomatal regulation in response to drought stress. Plant Physiol. 2010;154:1232–1243. doi: 10.1104/pp.110.157545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Benschop JJ, et al. Quantitative phosphoproteomics of early elicitor signaling in Arabidopsis. Mol Cell Proteomics. 2007;6:1198–1214. doi: 10.1074/mcp.M600429-MCP200. [DOI] [PubMed] [Google Scholar]
- 29.Nakagami H, et al. Large-scale comparative phosphoproteomics identifies conserved phosphorylation sites in plants. Plant Physiol. 2010;153:1161–1174. doi: 10.1104/pp.110.157347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Witte CP, et al. Tobacco calcium-dependent protein kinases are differentially phosphorylated in vivo as part of a kinase cascade that regulates stress response. J Biol Chem. 2010;285:9740–9748. doi: 10.1074/jbc.M109.052126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Klimecka M, et al. Regulation of wound-responsive calcium-dependent protein kinase from maize (ZmCPK11) by phosphatidic acid. Acta Biochim Pol. 2011;58:589–595. [PubMed] [Google Scholar]
- 32.Asano T, et al. Genome-wide identification of the rice calcium-dependent protein kinase and its closely related kinase gene families: comprehensive analysis of the CDPKs gene family in rice. Plant Cell Physiol. 2005;46:356–366. doi: 10.1093/pcp/pci035. [DOI] [PubMed] [Google Scholar]
- 33.Ray S, et al. Expression analysis of calcium-dependent protein kinase gene family during reproductive development and abiotic stress conditions in rice (Oryza sativa L. ssp indica) Mol Genet Genomics. 2007;278:493–505. doi: 10.1007/s00438-007-0267-4. [DOI] [PubMed] [Google Scholar]
- 34.Li AL, et al. Evolutionary and functional study of the CDPK gene family in wheat (Triticum aestivum L.) Plant Mol Biol. 2008;66:429–443. doi: 10.1007/s11103-007-9281-5. [DOI] [PubMed] [Google Scholar]
- 35.Wan B, et al. Expression of rice Ca2+-dependent protein kinases (CDPKs) genes under different environmental stresses. FEBS Lett. 2007;581:1179–1189. doi: 10.1016/j.febslet.2007.02.030. [DOI] [PubMed] [Google Scholar]
- 36.Myers C, et al. Calcium-dependent protein kinases regulate polarized tip growth in pollen tubes. Plant J. 2009;59:528–539. doi: 10.1111/j.1365-313X.2009.03894.x. [DOI] [PubMed] [Google Scholar]
- 37.Abbasi F, et al. OsCDPK13, a calcium-dependent protein kinase gene from rice, is induced by cold and gibberellin in rice leaf sheath. Plant Mol Biol. 2004;55:541–552. doi: 10.1007/s11103-004-1178-y. [DOI] [PubMed] [Google Scholar]
- 38.Yu XC, et al. Abscisic acid stimulates a calcium-dependent protein kinase in grape berry. Plant Physiol. 2006;140:558–579. doi: 10.1104/pp.105.074971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhu SY, et al. Two calcium-dependent protein kinases, CPK4 and CPK11, regulate abscisic acid signal transduction in Arabidopsis. Plant Cell. 2007;19:3019–3036. doi: 10.1105/tpc.107.050666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lu SX, Hrabak EM. An Arabidopsis calcium-dependent protein kinase is associated with the endoplasmic reticulum. Plant Physiol. 2002;128:1008–1021. doi: 10.1104/pp.010770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Martin ML, Busconi L. Membrane localization of a rice calcium-dependent protein kinase (CDPK) is mediated by myristoylation and palmitoylation. Plant J. 2000;24:429–435. doi: 10.1046/j.1365-313x.2000.00889.x. [DOI] [PubMed] [Google Scholar]
- 42.Benetka W, et al. Experimental testing of predicted myristoylation targets involved in asymmetric cell division and calcium-dependent signalling. Cell Cycle. 2008;7:3709–3719. doi: 10.4161/cc.7.23.7176. [DOI] [PubMed] [Google Scholar]
- 43.Chehab EW, et al. Autophosphorylation and subcellular localization dynamics of a salt- and water deficit-induced calcium-dependent protein kinase from ice plant. Plant Physiol. 2004;135:1430–1446. doi: 10.1104/pp.103.035238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mehlmer N, et al. The Ca2+-dependent protein kinase CPK3 is required for MAPK-independent salt-stress acclimation in Arabidopsis. Plant J. 2010;63:484–498. doi: 10.1111/j.1365-313X.2010.04257.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stael S, et al. Protein N-acylation overrides differing targeting signals. FEBS Lett. 2011;585:517–522. doi: 10.1016/j.febslet.2011.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dammann C, et al. Subcellular targeting of nine calcium-dependent protein kinase isoforms from Arabidopsis. Plant Physiol. 2003;132:1840–1848. doi: 10.1104/pp.103.020008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Choi HI, et al. Arabidopsis calcium-dependent protein kinase AtCPK32 interacts with ABF4, a transcriptional regulator of abscisic acid-responsive gene expression, and modulates its activity. Plant Physiol. 2005;139:1750–1761. doi: 10.1104/pp.105.069757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Coca M, San Segundo B. AtCPK1 calcium-dependent protein kinase mediates pathogen resistance in Arabidopsis. Plant J. 2010;63:526–540. doi: 10.1111/j.1365-313X.2010.04255.x. [DOI] [PubMed] [Google Scholar]
- 49.Patharkar OR, Cushman JC. A stress-induced calcium-dependent protein kinase from Mesembryanthemum crystallinum phosphorylates a two-component pseudo-response regulator. Plant J. 2000;24:679–691. doi: 10.1046/j.1365-313x.2000.00912.x. [DOI] [PubMed] [Google Scholar]
- 50.Ito T, et al. Alteration of substrate specificity: the variable N-terminal domain of tobacco Ca(2+)-dependent protein kinase is important for substrate recognition. Plant Cell. 2010;22:1592–1604. doi: 10.1105/tpc.109.073577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rodriguez Milla MA, et al. A novel yeast two-hybrid approach to identify CDPK substrates: characterization of the interaction between AtCPK11 and AtDi19, a nuclear zinc finger protein. FEBS Lett. 2006;580:904–911. doi: 10.1016/j.febslet.2006.01.013. [DOI] [PubMed] [Google Scholar]
- 52.Uno Y, et al. Identification of proteins that interact with catalytically active calcium-dependent protein kinases from Arabidopsis. Mol Genet Genomics. 2009;281:375–390. doi: 10.1007/s00438-008-0419-1. [DOI] [PubMed] [Google Scholar]
- 53.Berendzen KW, et al. Screening for in planta protein-protein interactions combining bimolecular fluorescence complementation with flow cytometry. Plant Methods. 2012;8:25. doi: 10.1186/1746-4811-8-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ludwig AA, et al. Ethylene-mediated cross-talk between calcium-dependent protein kinase and MAPK signaling controls stress responses in plants. Proc Natl Acad Sci U S A. 2005;102:10736–10741. doi: 10.1073/pnas.0502954102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kamiyoshihara Y, et al. Turnover of LeACS2, a wound-inducible 1-aminocyclopropane-1-carboxylic acid synthase in tomato, is regulated by phosphorylation/dephosphorylation. Plant J. 2010;64:140–150. doi: 10.1111/j.1365-313X.2010.04316.x. [DOI] [PubMed] [Google Scholar]
- 56.Hernandez Sebastia C, et al. Identification of a new motif for CDPK phosphorylation in vitro that suggests ACC synthase may be a CDPK substrate. Arch Biochem Biophys. 2004;428:81–91. doi: 10.1016/j.abb.2004.04.025. [DOI] [PubMed] [Google Scholar]
- 57.Cheng SH, et al. Molecular identification of phenylalanine ammonia-lyase as a substrate of a specific constitutively active Arabidopsis CDPK expressed in maize protoplasts. FEBS Lett. 2001;503:185–188. doi: 10.1016/s0014-5793(01)02732-6. [DOI] [PubMed] [Google Scholar]
- 58.de Nadal E, Posas F. Multilayered control of gene expression by stress-activated protein kinases. EMBO J. 2010;29:4–13. doi: 10.1038/emboj.2009.346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Santos AP, et al. Transcription regulation of abiotic stress responses in rice: a combined action of transcription factors and epigenetic mechanisms. OMICS. 2011;15:839–857. doi: 10.1089/omi.2011.0095. [DOI] [PubMed] [Google Scholar]
- 60.Xing T, et al. Ectopic expression of an Arabidopsis calmodulin-like domain protein kinase-enhanced NADPH oxidase activity and oxidative burst in tomato protoplasts. Mol Plant Microbe Interact. 2001;14:1261–1264. doi: 10.1094/MPMI.2001.14.10.1261. [DOI] [PubMed] [Google Scholar]
- 61.Kobayashi M, et al. Calcium-dependent protein kinases regulate the production of reactive oxygen species by potato NADPH oxidase. Plant Cell. 2007;19:1065–1080. doi: 10.1105/tpc.106.048884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kobayashi M, et al. StCDPK5 confers resistance to late blight pathogen but increases susceptibility to early blight pathogen in potato via reactive oxygen species burst. New Phytol. 2012 doi: 10.1111/j.1469–8137.2012.04226.x. [DOI] [PubMed] [Google Scholar]
- 63.Mall TK, et al. Expression of the rice CDPK-7 in sorghum: molecular and phenotypic analyses. Plant Mol Biol. 2011;75:467–479. doi: 10.1007/s11103-011-9741-9. [DOI] [PubMed] [Google Scholar]
- 64.Freymark G, et al. Antagonistic control of powdery mildew host cell entry by barley calcium-dependent protein kinases (CDPKs) Mol Plant Microbe Interact. 2007;20:1213–1221. doi: 10.1094/MPMI-20-10-1213. [DOI] [PubMed] [Google Scholar]
- 65.Asano T, et al. A rice calcium-dependent protein kinase OsCPK12 oppositely modulates salt-stress tolerance and blast disease resistance. Plant J. 2012;69:26–36. doi: 10.1111/j.1365-313X.2011.04766.x. [DOI] [PubMed] [Google Scholar]
- 66.Singh S, Parniske M. Activation of calcium- and calmodulin-dependent protein kinase (CCaMK), the central regulator of plant root endosymbiosis. Curr Opin Plant Biol. 2012;15:444–453. doi: 10.1016/j.pbi.2012.04.002. [DOI] [PubMed] [Google Scholar]
- 67.Ivashuta S, et al. RNA interference identifies a calcium-dependent protein kinase involved in Medicago truncatula root development. Plant Cell. 2005;17:2911–2921. doi: 10.1105/tpc.105.035394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Szczegielniak J, et al. Maize calcium-dependent protein kinase (ZmCPK11): local and systemic response to wounding, regulation by touch and components of jasmonate signaling. Physiol Plant. 2012 doi: 10.1111/j.1399-3054.2012.01587.x. [DOI] [PubMed] [Google Scholar]
- 69.Yang DH, et al. Nicotiana attenuata Calcium-Dependent Protein Kinases, CDPK4 and CDPK5, Strongly Downregulate Wound- and Herbivory-Induced Jasmonic Acid Accumulations. Plant Physiol. 2012;159:1591–1607. doi: 10.1104/pp.112.199018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Asano T, et al. Functional characterisation of OsCPK21, a calcium-dependent protein kinase that confers salt tolerance in rice. Plant Mol Biol. 2011;75:179–191. doi: 10.1007/s11103-010-9717-1. [DOI] [PubMed] [Google Scholar]
- 71.Saijo Y, et al. Over-expression of a single Ca2+-dependent protein kinase confers both cold and salt/drought tolerance on rice plants. Plant J. 2000;23:319–327. doi: 10.1046/j.1365-313x.2000.00787.x. [DOI] [PubMed] [Google Scholar]
- 72.Zhao R, et al. The Arabidopsis Ca2+-dependent protein kinase CPK12 negatively regulates abscisic acid signaling in seed germination and post-germination growth. New Phytol. 2011;192:61–73. doi: 10.1111/j.1469-8137.2011.03793.x. [DOI] [PubMed] [Google Scholar]
- 73.Liu F, et al. Calcium-regulated phosphorylation of soybean serine acetyltransferase in response to oxidative stress. J Biol Chem. 2006;281:27405–27415. doi: 10.1074/jbc.M604548200. [DOI] [PubMed] [Google Scholar]
- 74.Xu J, et al. AtCPK6, a functionally redundant and positive regulator involved in salt/drought stress tolerance in Arabidopsis. Planta. 2010;231:1251–1260. doi: 10.1007/s00425-010-1122-0. [DOI] [PubMed] [Google Scholar]
- 75.Ma SY, Wu WH. AtCPK23 functions in Arabidopsis responses to drought and salt stresses. Plant Mol Biol. 2007;65:511–518. doi: 10.1007/s11103-007-9187-2. [DOI] [PubMed] [Google Scholar]
- 76.Johansson I, et al. The major integral proteins of spinach leaf plasma membranes are putative aquaporins and are phosphorylated in response to Ca2+ and apoplastic water potential. Plant Cell. 1996;8:1181–1191. doi: 10.1105/tpc.8.7.1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Johansson I, et al. Water transport activity of the plasma membrane aquaporin PM28A is regulated by phosphorylation. Plant Cell. 1998;10:451–459. doi: 10.1105/tpc.10.3.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Anil VS, et al. A plant Ca2+ pump, ACA2, relieves salt hypersensitivity in yeast. Modulation of cytosolic calcium signature and activation of adaptive Na+ homeostasis. J Biol Chem. 2008;283:3497–3506. doi: 10.1074/jbc.M700766200. [DOI] [PubMed] [Google Scholar]
- 79.Hwang I, et al. A calcium-dependent protein kinase can inhibit a calmodulin-stimulated Ca2+ pump (ACA2) located in the endoplasmic reticulum of Arabidopsis. Proc Natl Acad Sci U S A. 2000;97:6224–6229. doi: 10.1073/pnas.97.11.6224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Mori IC, et al. CDPKs CPK6 and CPK3 function in ABA regulation of guard cell S-type anion- and Ca2+-permeable channels and stomatal closure. PLoS Biol. 2006;4:e327. doi: 10.1371/journal.pbio.0040327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Brandt B, et al. Reconstitution of abscisic acid activation of SLAC1 anion channel by CPK6 and OST1 kinases and branched ABI1 PP2C phosphatase action. Proc Natl Acad Sci U S A. 2012;10(9):10593–10598. doi: 10.1073/pnas.1116590109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Geiger D, et al. Stomatal closure by fast abscisic acid signaling is mediated by the guard cell anion channel SLAH3 and the receptor RCAR1. Sci Signal. 2011;4:ra32. doi: 10.1126/scisignal.2001346. [DOI] [PubMed] [Google Scholar]
- 83.Munemasa S, et al. The Arabidopsis calcium-dependent protein kinase, CPK6, functions as a positive regulator of methyl jasmonate signaling in guard cells. Plant Physiol. 2011;155:553–561. doi: 10.1104/pp.110.162750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Yu XC, et al. Expression of a grape calcium-dependent protein kinase ACPK1 in Arabidopsis thaliana promotes plant growth and confers abscisic acid-hypersensitivity in germination, postgermination growth, and stomatal movement. Plant Mol Biol. 2007;64:531–538. doi: 10.1007/s11103-007-9172-9. [DOI] [PubMed] [Google Scholar]
- 85.Li J, et al. Guard cells possess a calcium-dependent protein kinase that phosphorylates the KAT1 potassium channel. Plant Physiol. 1998;116:785–795. doi: 10.1104/pp.116.2.785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Berkowitz G, et al. Co-expression of calcium-dependent protein kinase with the inward rectified guard cell K+ channel KAT1 alters current parameters in Xenopus laevis oocytes. Plant Cell Physiol. 2000;41:785–790. doi: 10.1093/pcp/41.6.785. [DOI] [PubMed] [Google Scholar]
- 87.Pei ZM, et al. A novel chloride channel in Vicia faba guard cell vacuoles activated by the serine/threonine kinase, CDPK. EMBO J. 1996;15:6564–6574. [PMC free article] [PubMed] [Google Scholar]
- 88.Hubbard KE, et al. Abscisic acid and CO2 signalling via calcium sensitivity priming in guard cells, new CDPK mutant phenotypes and a method for improved resolution of stomatal stimulus-response analyses. Ann Bot. 2012;109:5–17. doi: 10.1093/aob/mcr252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Doherty CJ, et al. Roles for Arabidopsis CAMTA transcription factors in cold-regulated gene expression and freezing tolerance. Plant Cell. 2009;21:972–984. doi: 10.1105/tpc.108.063958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Bohmer M, Romeis T. A chemical-genetic approach to elucidate protein kinase function in planta. Plant Mol Biol. 2007;65:817–827. doi: 10.1007/s11103-007-9245-9. [DOI] [PubMed] [Google Scholar]
- 91.Martin ML, Busconi L. A rice membrane-bound calcium-dependent protein kinase is activated in response to low temperature. Plant Physiol. 2001;125:1442–1449. doi: 10.1104/pp.125.3.1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Komatsu S, et al. Over-expression of calcium-dependent protein kinase 13 and calreticulin interacting protein 1 confers cold tolerance on rice plants. Mol Genet Genomics. 2007;277:713–723. doi: 10.1007/s00438-007-0220-6. [DOI] [PubMed] [Google Scholar]
- 93.Boudsocq M, et al. Identification of nine sucrose nonfermenting 1-related protein kinases 2 activated by hyperosmotic and saline stresses in Arabidopsis thaliana. J Biol Chem. 2004;279:41758–41766. doi: 10.1074/jbc.M405259200. [DOI] [PubMed] [Google Scholar]
- 94.Fujii H, et al. Arabidopsis decuple mutant reveals the importance of SnRK2 kinases in osmotic stress responses in vivo. Proc Natl Acad Sci U S A. 2011;108:1717–1722. doi: 10.1073/pnas.1018367108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Joshi-Saha A, et al. A brand new START: abscisic acid perception and transduction in the guard cell. Sci Signal. 2011;4:re4. doi: 10.1126/scisignal.2002164. [DOI] [PubMed] [Google Scholar]
- 96.Sheen J. Mutational analysis of protein phosphatase 2C involved in abscisic acid signal transduction in higher plants. Proc Natl Acad Sci U S A. 1998;95:975–980. doi: 10.1073/pnas.95.3.975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Baena-Gonzalez E, Sheen J. Convergent energy and stress signaling. Trends Plant Sci. 2008;13:474–482. doi: 10.1016/j.tplants.2008.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Tena G, et al. Protein kinase signaling networks in plant innate immunity. Curr Opin Plant Biol. 2011;14:519–529. doi: 10.1016/j.pbi.2011.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Romeis T, et al. Resistance gene-dependent activation of a calcium-dependent protein kinase in the plant defense response. Plant Cell. 2000;12:803–816. doi: 10.1105/tpc.12.5.803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Hegeman AD, et al. A phyloproteomic characterization of in vitro autophosphorylation in calcium-dependent protein kinases. Proteomics. 2006;6:3649–3664. doi: 10.1002/pmic.200500926. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.