Abstract
Some tissues from metal-on-metal (MoM) hip arthroplasty revisions have shown evidence of adaptive-immune reactivity (i.e., excessive peri-implant lymphocyte infiltration/activation). We hypothesized that, prior to symptoms, some people with MoM hip arthroplasty will develop quantifiable metal-induced lymphocyte reactivity responses related to peripheral metal ion levels. We tested 3 cohorts (Group-1: n=21 prospective longitudinal MoM hip arthroplasty; Group-2: n=17 retrospective MoM hip arthroplasty; and Group-3: n=20 controls without implants). We compared implant position, metal-ion release, and immuno-reactivity. MoM cohorts had elevated (p<0.01) amounts of serum Co and Cr compared to controls as early as 3 mos post-op (Group-1:1.2ppb-Co, 1.5ppb-Cr; Group-2: 3.4ppb-Co,, 5.4ppb-Cr; Group-3: 0.01ppb-Co, 0.1ppb-Cr). However, only after 1 to 4 yrs post-op did 56% of Group-1 develop metal-reactivity (vs. 5%pre-op, metal-LTT, SI>2), compared with 76% of Group-2 and 15% of Group-3 controls (patch testing was a poor diagnostic indicator with only 1/21 Group-1 positive). Higher cup-abduction angles (50° vs. 40°) in Group-1 were associated with higher serum Cr (p<0.07). However, sub-optimal cup-anteversion angles (9° vs. 20°) had higher serum Co (p<0.08). Serum Cr and Co were significantly elevated in reactive vs. non-reactive Group-1 participants (p<0.04). CD4+CD69+ T-helper lymphocytes (but not CD8+) and IL-1β, IL-12 and IL-6 cytokines were all significantly elevated in metal-reactive vs. non-reactive Group-1 participants. Our results showed that lymphocyte reactivity to metals can develop within the first 1 to 4 years after MoM arthroplasty in asymptomatic patients and lags increases in metal ion levels. This increased metal reactivity was more prevalent in those individuals with extreme cup angles and higher amounts of circulating metal.
INTRODUCTION
Adaptive immune responses have been implicated in the failure of metal-on-metal (MoM) hip arthroplasties as evidenced histologically by elevated peri-implant lymphocyte infiltrations/accumulations.1–4 Factors independent of implant debris-induced responses can contribute to the pathogenesis of adverse soft tissue reactions or osteolysis, such as surgical technique, patient activity levels, and implant design. Nonetheless, variability in innate and adaptive immune responses to metal debris was reported as a key contributor to poor implant performance in specific individuals.4,5
Metal-implant related adaptive immune reactivity (metal-hypersensitivity) is well documented in case and group studies; however, overall it remains incompletely understood in orthopedics.1–3,6,7 Traditionally, the terms “metal sensitivity”, “metal allergy”, and “metal hypersensitivity” are used interchangeably to refer to adaptive immune responses to metal antigens by lymphocytes. These responses are typically characterized as a Type IV delayed type hypersensitivity (DTH) response mediated by T-cells.8 And this characterization is independent of orthopedic clinical relevance. The “hyper” in hypersensitivity is misleading; it does not mean “excessive” adaptive immune response, but simply identifies the antigen specific reactivity of an adaptive immune response. While metal hypersensitivity reactions can manifest as skin hives, eczema, redness, and itching8, for MoM arthroplasties, symptoms such as aseptic pain and swelling are more typical.1,9,10 Metal induced sensitivity responses can be sources of potent cytokines (e.g., TNF, IFN-γ, IL-2, and IL-17), and evidence is mounting that activated T-cells can affect bone remodeling and cause osteolysis.11,12
Past investigations of metal hypersensitivity in metal-on-polymer (MoP) implants generally indicate a higher incidence in symptomatic (45%) vs. asymptomatic patients (25%).1,10,13,14 In contrast, only a 10 to 15% incidence of metal-sensitivity exists in the general population.8 Of concern is the metal hypersensitivity-like reactivity in symptomatic patients with failing MoM implants, reported to be as high as 76 to 100%.10 This incidence of metal reactivity suggests a mechanistic link between metal exposure and metal adaptive immune responses.2,3,10 However, cause and effect have not been proven, and it remains unknown if and when metal-specific lymphocyte responses develop in MoM patients. Does metal reactivity accompany increases in serum metal? To answer this question, we initially hypothesized that prior to symptoms of poor implant performance (e.g., pain, effusions and adverse tissue reactions) people with MoM hip arthroplasty will develop metal-reactivity that accompanies elevated metal ion levels. We tested this hypothesis in two groups (a prospective longitudinal cohort and a retrospective cohort of patients with MoM hip arthroplasties) by comparing implant performance/position and metal ion release with measures of immuno-reactivity to metals, including in vivo patch testing, in vitro metal-lymphocyte transformation testing [metal-LTT], cytokine analysis, and flow cytometry analysis.
MATERIALS AND METHODS
Study Groups
38 participants with MoM implants and 20 no-implant controls were enrolled into 3 investigational groups, after obtaining informed consent and Institutional Review Board approval (Table 1). In Group 1, 21 participants were enrolled prospectively prior to receiving a MoM resurfacing implant (Conserve® Plus, Wright Medical Tech., Arlington, TN) and were followed pre-operatively and post-operatively at 3 mos, and 1 to 4 yrs (average follow-up was 2.26 yrs). All 21 (100%) patients were diagnosed with osteoarthritis. The average femoral head size was 50mm (range: 46 to 56mm), and the average acetabular shell size was 56mm (range 52 to 62mm). Five Group 1 participants had bilateral surgeries. Pre- and post-operative range of motion and SF-12 functional measurements are shown in Table 2. All surgeries were single-center, single-surgeon. In Group 2, 17 participants were enrolled retrospectively (Conserve® Plus). Five Group 2 participants had bilateral surgeries (separate analysis provided). In Group 3, 20 participants were used as healthy controls without any implant (average age 63.5 range: 44 to 74 yrs) with 10 males/10 females, with no history of metal sensitivity. Similar, but smaller groups n=7 to 12 were used in previous studies.15 None of the subjects in Groups 1 and 2 had known histories of metal allergy.
Table 1.
Summary of Groups 1–3 demographics.
| # Participants (n) | Male | Female | Age (range) (yrs) | Implant Age (yrs) | Actetab. Cup Abduction° | Actetab. Cup Anteversion° | |
|---|---|---|---|---|---|---|---|
| Group 1 | 21 | 19 | 2 | 49 (23–63) | 2.3 (1–4) | 44 (31–58) | 14 (3–24) |
| Group 2 | 17 | 11 | 6 | 43 (23–83) | 5.4 (2–11) | 47 (37–61) | 16 (2–40) |
| Group 3 | 20 | 10 | 10 | none | none | none | none |
Table 2.
Group 1 Pre vs Post operative range of motion measurements demonstrate significantly increased ranges of motion following hip arthroplasty.
| Group 1 (n=21) | Pre-Op | Post-Op* | P value** |
|---|---|---|---|
| Flexion | 104 (70–140) | 128 (110–145) | p<0.0001 |
| Flexion contracture | 19 (0–65) | 1 (0–5) | p<0.0001 |
| Abd (in extension) | 27 (−20–60) | 48 (30–60) | p<0.0001 |
| Add (in extension) | 20 (5–45) | 32 (10–45) | p<0.0001 |
| Internal Rotation | 3 (−10–30) | 48 (15–60) | p<0.01 |
| External Rotation | 11 (−10–35) | 47 (30–90) | p<0.01 |
| Rotation Arc | 23 (0–75) | 93 (65–145) | p<0.0001 |
| SF-12 | 36.7 (25.0–56.2) | 53.7 (29.6–66.7) | P<0.0008 |
| SF-12 (Group 2) | 29.6 (17.0–41.4) | 49.5 (28.7–59.4) | p<0.0001 |
At last follow-up average 2.26 (1–4) years post operative
Wilcoxon (one-tailed, matched pair) statistical comparison.
Implant Position
All post-op radiographs were analyzed as previously described where cup abduction angle and anteversion angle were calculated with use of Einzel-Bild-Roentgen-Analysis cup software (EBRA-CUP Digital; University of Innsbruck, Innsbruck, Austria).16,17 Contact patch to rim (CPR) distance was calculated using the mean position of the articular contact patch to the cup rim with the patient in standing weight-bearing position.18 EBRA is not well established for measuring version angles for large diameter MoM hips.
Cell Preparation
Human primary peripheral blood mononuclear cells (PBMCs) were isolated from 30 mLs of blood (15–30 × 106 cells per subject) and incubated with DMEM and 10% autologous serum with either no metal (plain media) as a negative control or 0.01 mg/ml phytohemagglutinin (PHA) as a positive control, and Cobalt (Co+2), Chromium (Cr+3), and Nickel (Ni+2) chloride solutions at final concentrations of 0.01mM and 0.1mM were used as metal challenge agents in all immuno-assays. These high concentrations (equivalent to 500 ppb and 5,000 ppb Cr and 580ppb and 5,800ppb Co) only exist in peri-implant fluids/tissues19 and were previously established as baseline concentrations needed to provoke cellular immune responses.20,21 We previously reported that the viability of lymphocytes challenged with Co, Cr, and Ni at 0.1mM was >75% and for 0.01mM viability was >80% at 0.01mM using propidium iodide (PI) based viability assays (flow cytometry analysis of cell membrane integrity).20,21
Patch Testing
16 Group 1 participants were recruited for patch testing post-op, at an average follow-up of ~4 yrs. Patch testing was conducted using the True Test® (GlaxoSmithKline) for Co, Cr, and Ni and applied to the midline of the back at the time of clinical follow-up and examined 48 hrs later for signs of skin reaction such as redness or blistering (scored on a 0, +1, +2, or +3 severity scale).
Metal Ion Analysis
Metal ion content was measured using inductively coupled plasma-mass spectrometry (ICP-MS) analysis of serum using methods previously detailed.22 All vessels and utensils used for specimen collection were verified to be free of metal contamination. Methods to reduce contamination were used. The serum was separated within 4 hrs of collection and frozen at −80°C until analysis. Samples were tested using detection limits for Cr of 0.03 ng/mL or ppb and for Co of 0.3 ng/mL or ppb. All preparation was conducted in a class II biological safety cabinet.
Proliferation assays
All cell-based testing was performed within 24 hrs of blood draw. The amount of lymphocyte reactivity to each metal was measured using proliferation assays (also known as Lymphocyte Transformation Testing, LTT) conducted using [3H]-thymidine (Amersham International, Arlington Heights, IL) that incorporates into new DNA upon cell division. [3H]-Thymidine-based LTT assays are the only proliferation detection assays that are highly accurate (linear) over several orders of magnitude (0.1–10,000x increases for a lymphocyte population) and were performed using PBMCs cultured in 96-well cell-culture plates (Sigma) with metal treatments, at a density of ~0.2×106 cells/well with [3H]-Thymidine (1 μCi [3H]-thymidine/well) for 6 days in 150μL of DMEM/well at 37°C and 0.5% CO2 with metal challenge agents. Co+2, Cr+3, and Ni+2 chloride solutions were used at 0.01mM and 0.1mM (4 wells/metal). [3H]-Thymidine (0.5uCi/well) was added during the last 12 hrs of a 6-day incubation and was measured using liquid scintillation Beta plate analysis (Wollac, Gatesburg, MD). The stimulation index was calculated using measured radiation counts/min (cpm) as follows: Stimulation Index = (mean cpm with treatment)/(mean cpm without treatment). SIs were averaged for group comparisons.
The term “reactivity” was used to refer to these specific metal-induced T-cell/lymphocyte reactions; it does not indicate/imply excessive peri-implant hypersensitivity, EPH. The reactivity index (SI) only measures proliferating cells at day 5. A sampling size of >150,000 cells/96-well is required. Other techniques that measure total numbers of cells without discriminating proliferating from non-proliferating cells are not accurate enough to readily discriminate proliferating subsets (e.g., colorimetric live/dead cell kits).
Flow Cytometry
Activation of CD4 and CD8 (T-helper and cytotoxic cells) by CD25 (T-cell activation) and CD69 (early T-cell activation) was measured using PBMCs cultured in 48-well plates at a density of 1×106 cells/well and 1 metal challenge agent/well after 48 hrs. Cells collected from each of the 6 wells/subject were stained with CD markers to determine the ratio of activated T-helper cells (CD4/CD25 and CD4/CD69), and the ratio of activated T-cytolitic/cytotoxic-cells (CD8/CD25 and CD8/CD69). 10,000 cells were counted per each CD marker pair FACScan flow cytometer (BD Biosciences, San Diego, CA) (Supplementary Fig. S-1).
Measurement of cytokines in culture media
Cytokine concentrations in supernatants of PBMC cultures (>1×106 PBMCs/well per metal in 48-well plates) were obtained after 48 hrs of incubation with metal challenge agents and were measured by sandwich enzyme-linked immunosorbent assays (ELISA) in 96-well microtitration plates following the manufacturer’s instructions (R&D Systems, Minneapolis, MN) for IL-1β, IL-2, IL-4, IL-6, IFN-gamma, IL-12p70, IL-17, IL-1ra, and TNF-α (assay range from 0.5 to 2000 pg/ml). All cytokine measurements were conducted in triplicate for each concentration of each metal treatment.
Statistical analysis was determined using Mann-Whitney testing (two-tailed for metal-LTT reactivity and one-tailed for metal ion measurements22) or Wilcoxon (one-tailed, matched pairs) used after Kolmogorov-Smirnov (with Dallal-Wilkinson-Lillie for p values) normality testing. By convention, below detection limit ion values were assigned one half the detection limits to facilitate data presentation (Graphpad Prism 5).
RESULTS
Implant Function
Group 1 participants had improved range of motion and SF-12 measures (Table 2).
Metal Ion Levels: Prospective MoM levels 1–4 years
The pre-op ion levels for Group 1 participants were <1ppb (ng/mL) for Co and Cr. Post-op levels were increased significantly (p<0.001)at all intervals from 3 mos to 4yrs to ~1.2 ppb Co and 1.5 ppb Cr (ng/mL); no difference existed in ion levels among post-op time intervals. Group 1 and 2 participants demonstrated a linear relationship between Cr and Co (Cr=1.4*Co, r=0.85).
Group 2 had an average serum metal level of 3.4 ppb Co and 5.4 ppb Cr, a 3-fold increase over the levels at last follow-up in Group 1; however, this increase was not significant (p>0.2). Serum metal levels of Group 3 controls were indistinguishable from pre-op Group 1 levels.
Metal-Induced Lymphocyte Proliferation (Stimulation Index)
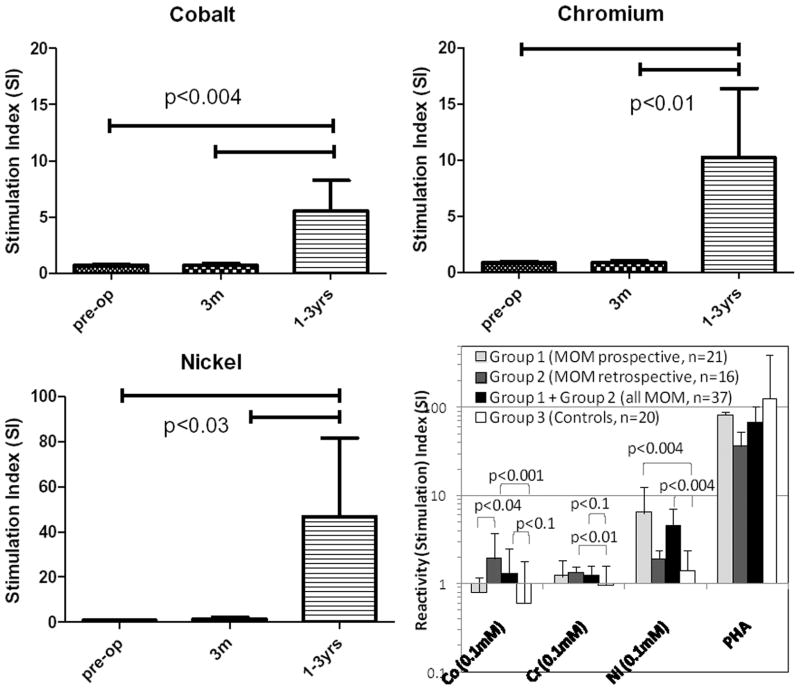
There were significant increases in lymphocyte reactivity between pre-op and post-op time points in prospective Group 1 MoM participants. These averaged reactivity levels to Co, Cr, and Ni increased significantly post-op (p<0.03, Figs 1a–c). Reactivities to Co, Cr, and Ni were also significantly elevated in MoM Groups 1 and 2 over that of Group 3 controls (p<0.04, Fig. 1d). Group 2 retrospective-MoM had the highest reactivity levels to Co and Cr, while Group 1 prospective-MoM had the greatest reactivity to Ni. Group 3 controls did not demonstrate significant elevated reactivity (SI>2) to Co, Cr, or Ni.
Figure 1.
Significant acquired increases occurred in the average lymphocyte proliferations (SI) over time for all prospective Group 1 MoM participants (n=21) when challenged with (a) Co, (b) Cr, and (c) Ni at 1 to 4 yrs compared to 3 mos and pre-op, p<0.03. (d) Comparison of combined groups 1, 2, 1+2, and 3 metal reactivity: generally, Co, Cr, and Ni were significantly elevated in Groups 1 and 2 over Group 3 (p<0.04).
Incidence of Metal Sensitivity
The percentage of people within the groups that could be classified as “metal reactive” are based on well established threshold levels (i.e. stimulation index, SI>2).4,23–25 This threshold has not been established as clinically meaningful for predicting MoM pathogenesis, so we calculated the incidences of metal reactivity at a range of thresholds. Of Group 1 participants there was a 56% incidence at SI>2, which increased over time from 5% pre-op to 19% at 3mos and 56% at 1 to 4 yrs to any metal (Table 3). 13/17 (76%) Group 2 participants were metal reactive. The incidence of sensitivity to Co and Cr was significantly greater than controls and that of Group 1 was significantly less than that of Group 2 for Cr but significantly greater for Ni (p<0.1). There was a significant 40% increase from 19% pre-op to 56% at 1 at 4years post-op. However, the incidence in reactivity to Co and Cr increased from 0% to ~33% and 25% at 3mos and 1 to 4 yrs, respectively.
Table 3.
Incidence of metal reactivity (SI>2) for Group 1 (prospective metal-on-metal.hip arthroplasty) measured over time and incidence of reactive of Group 1 compared to Group 2 and 3.
| Incidence (% metal reactive) of Reactivity in Group 1 (n=21) | |||||
|---|---|---|---|---|---|
| Cobalt | Chromium | Nickel | Any | ||
| SI>2 | Pre-op | 0 | 0 | 5 | 5 |
| 3 months | 5 | 10 | 19 | 19 | |
| 1–4 years | 38* | 13* | 31* | 56* | |
|
| |||||
| SI>3 | Pre-op | 0 | 0 | 5 | 5 |
| 3 months | 0 | 0 | 10 | 10 | |
| 1–4 years | 19 | 13 | 25 | 31 | |
|
| |||||
| SI>4 | Pre-op | 0 | 0 | 5 | 5 |
| 3 months | 0 | 0 | 5 | 5 | |
| 1–4 years | 19 | 13 | 19 | 25 | |
|
| |||||
| SI>5 | Pre-op | 0 | 0 | 0 | 0 |
| 3 months | 0 | 0 | 10 | 10 | |
| 1–4 years | 6 | 6 | 13 | 13 | |
| Incidence (% metal reactive) of Reactivity (using SI>2)
| ||||
|---|---|---|---|---|
| Cobalt | Chromium | Nickel | Any | |
| Group 1 (n=21) | 38* | 13 | 31* | 56* |
| Group 2 (n=17) | 35* | 33*,** | 29 | 76* |
| Group 3 (n=20) | 10 | 15 | 15 | 35 |
Note:
p<0.05, z-test compared to pre-op values
Note:
p<0.05, z-test compared to Group 3,
=p<0.05 for Group 1 vs Group 2
Level of Metal Reactivity vs. Metal Ion Levels
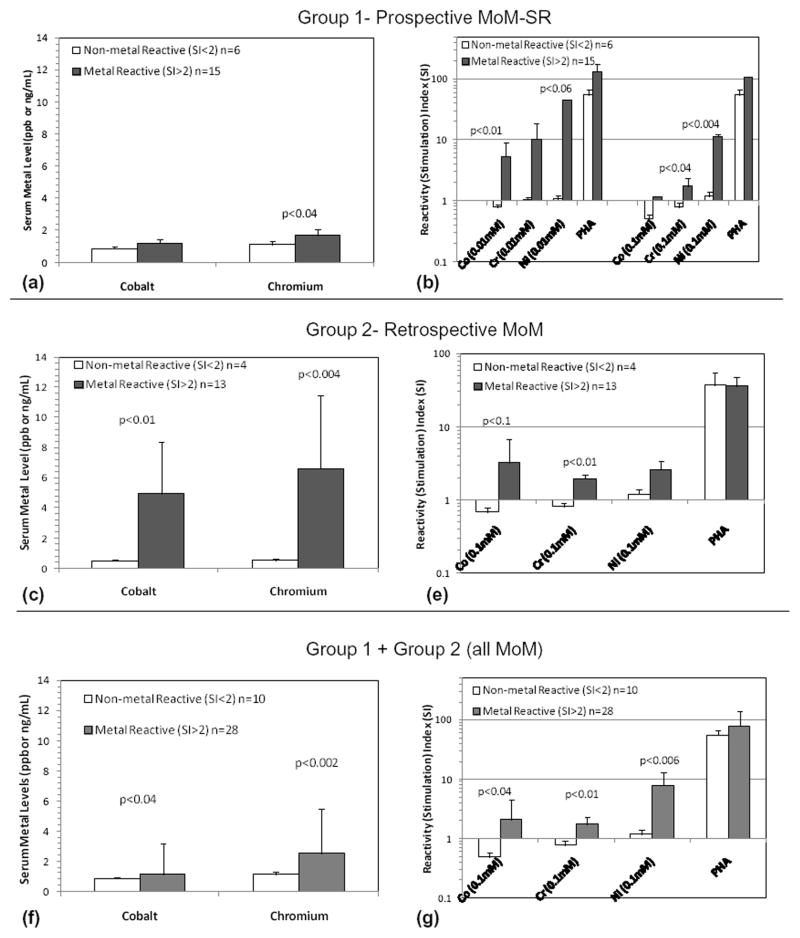
Group 1 reactive vs non-reactive subjects had similar SF-12 scores (p>0.1). The absolute difference between the SI of these subjects and non-reactive subGroups 1a and 1b (reactive vs non-reactive, prospective MoM) for Co, Cr, and Ni) is shown in Figure 2. Not all individuals with high metal levels demonstrated high metal LTT responses (and vice versa); thus implications of these data are limited to comparison of group data for high and low reactivity levels. While acquired metal reactivity is generally related to increased metal ion levels, this was not true for every individual.
Figure 2.
Metal ion levels in reactive vs. non-reactive participants in (a) Group 1, (c) Group 2, and (f) Group 1+2 demonstrated that serum metal levels were significantly elevated in metal-reactive individuals. Average SI’s of reactive vs. non-reactive individuals in (b) Group 1 (at 2 concentrations of metal challenge), (e) Group 2, and (g) Group 1+2 showed metal-reactivity differences were greatest for Ni (at 0.01mM) in Group 1 participants.
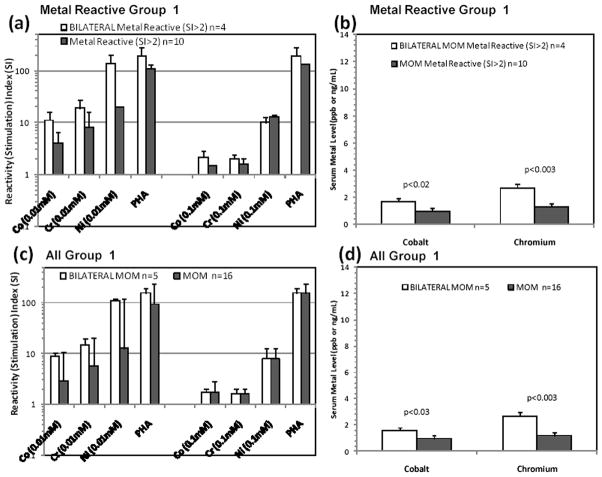
Cr serum metal contents were significantly elevated in the Group 1 reactive subgroup (Fig. 2a, p<0.04). Reactivity increased significantly from 5% pre-op to 56% at 1 to 4 yrs (Fig. 2d, p<0.1 Co and p<0.01 Cr). Co and Cr serum ion levels were elevated in metal-reactive Group 2 participants (Fig. 2c). Combined Groups 1 and 2 (all MoM) were similarly analyzed on a reactive vs non-reactive basis; Co and Cr serum metal levels were significantly greater (p<0.04) in metal-reactive individuals with MoM implants. 4 of 5 Group 1 participants with bilateral MoM were metal reactive. When these 5 participants with bilateral MoM were compared to unilateral MoM participants, non-significant 10 fold elevations were found in the Ni reactivity (Fig. 3). Similarly, no significant increases in the 4 reactive bilateral MoM individuals existed over that of the reactive Group 1 subjects with one MoM hip. Serum ion levels were significantly higher for Group 1 subjects (p<0.03) with bilateral MoM compared to people with one MoM hip arthroplasty (Fig. 3).
Figure 3.
Bilateral MoM vs. unilateral MoM arthroplasty: Lymphocyte reactivity and metal ion levels of Group 1 participants with bilateral MoM vs. unilateral MOM hip arthroplasty were compared based on (a–b) all Group 1 participants and (c–d) metal reactive Group 1 participants. SI>2 was used to indicate reactive individuals. Despite ~10 fold elevations in Ni reactivity associated with participants with bilateral MoM (a) and (c), the increase was not significant due to the small number of bilateral MoM subjects (n=5) in Group 1. Serum Co and Cr ion levels were significantly higher for Group 1 subjects with bilateral MoM compared to people with one MoM hip arthroplasty.
Patch Test
Only 1 of 16 individuals tested positive for mild reactivity to Ni (+1 response).
Flow Cytometry
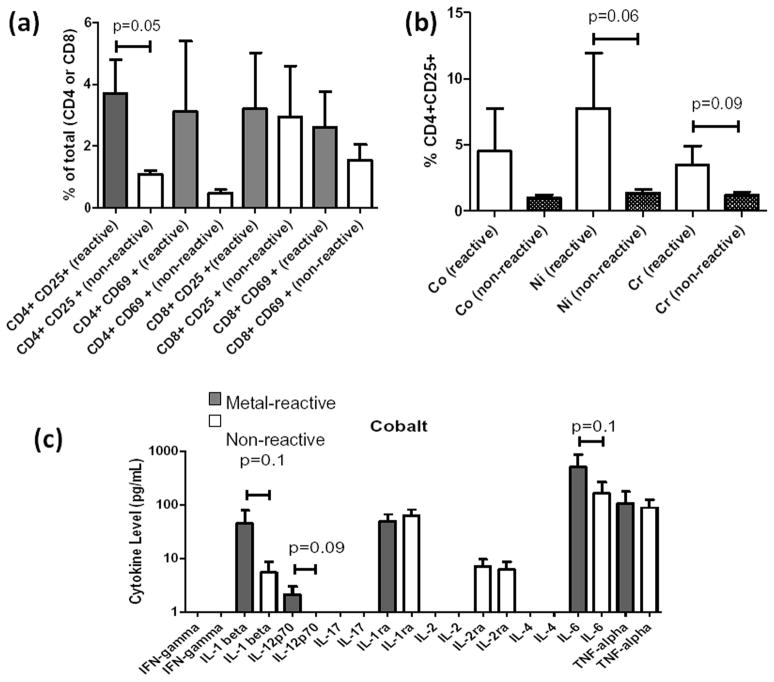
17 of 21 Group 1 participants samples contained enough cells for flow analysis, where 8 were metal-reactive and 9 were not. The metal-reactive participants had significantly increased CD4+CD25+ activation over that of non-metal-reactive participants (p<0.05, Fig. 4). CD8+ T-cells did not demonstrate differential activation (Fig. 4a). CD4+CD25+ activation was only detected for Cr and Ni (p<0.09, Fig. 4b). These data indicate that MoM lymphocyte reactivity is more likely to result from CD4 T-helper cell activation than CD8+ T-cytotoxic cell activation.
Figure 4.
Flow cytometry of Group 1 individuals (at 3.75 yrs post-op, last follow-up) PBMC’s after 48 hrs of challenge with Co, Cr, and Ni (0.1mM) at 1×106 cells per 96-well conducted in triplicate for each individual and analyzed for CD4+ and CD8+ T-cell activation using CD25+ and CD69+ activation markers. (a) 8 of 17 metal-reactive Group 1 participants determined by LTT (SI>2) demonstrated significantly increased CD4+CD25+ over the average of 9 of 17 non-reactive individuals (p=0.05). (b) On an individual metal basis, Group 1 metal-reactive individuals were more CD25+ compared to non-reactive subjects for Cr and Ni. (c) Supernatants of these challenged PBMCs were analyzed for 10 cytokines; only IL-1b, IL-12 and IL-6 were significantly elevated in LTT reactive vs. non-reactive individuals (p<0.1).
Cytokine Analysis
Cytokine analysis was performed at the last follow-up date on 17 of 21 Group 1 MoM participants; 8 of 17 were metal-reactive (SI>2, metal-LTT). 6 of 9 cytokines (IL-1β, IL-12, IL-1ra, IL-2ra, IL-6 and TNF-α) were detectably elevated in the supernatants of metal-challenged PBMCs in vitro. When Group 1 PBMCs were challenged with Co, significant increases occurred in IL-1β, IL-12 and IL-6 (p<0.1) of metal-reactive Group 1 vs non-metal-reactive Group 1 participants (Fig. 4c). IL-1ra was elevated in non-reactive Group 1 participants (p<0.01). When cytokine Co, Cr, and Ni responses were pooled, only IL-1β was significantly increased in metal-reactive individuals.
Acetabular Cup Angle and Metal Ion Levels
A positive correlation was found between abduction angle (AAb) and Co and Cr ion levels, and a negative correlation between acetabular cup anteversion angle (AAA) and ion levels. Midrange values for AA and AAA were used for segregating low vs high angle groups. Cr was significantly higher (p<0.07) in Group 1 participants with higher AAs (avg. of 50 vs. 40°). Group 1 participants with lower AAAs (avg. 9°) had significantly elevated Co (p<0.08) when compared to higher AAAs (avg. 20°).
Level of Metal reactivity vs. Acetabular Cup Angles
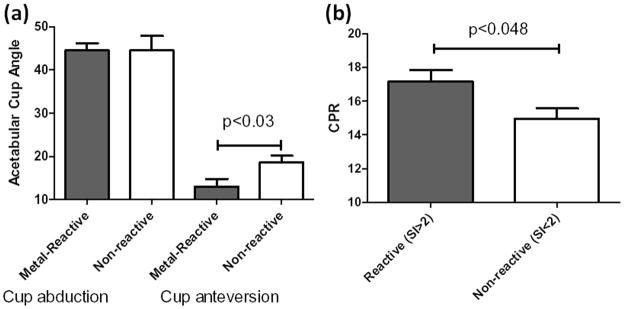
Metal-reactive Group 1participants demonstrated significantly less (p<0.03) cup AAA (13.1° vs 18.7°) compared to Group 1 non-reactive individuals (Fig. 5a); however, no difference in the AA was found for Group 1 metal-reactive vs. non-reactive participants. This was surprising given the correlation between AA and metal ion level was higher than AAA (Fig. 5). A significant difference in CPR distance was found for reactive vs non-reactive Group 1 participants (Fig. 5b), in agreement with the cup angle vs. reactivity data.
Figure 5.
(a) Acetabular Cup Angle (AA, as determined by EBRA) was determined for reactive vs. non-reactive Group 1 participants (as determined by LTT SI>2). Anteversion angles (AAA) (but not AAs) were significantly less for reactive individuals in Group 1 (p<0.03, average AAA of 13.1° vs. 18.7°), indicative of sub-optimal AAAs. (b) The contact patch to acetabular cup rim (CPR) distance was significantly greater in reactive vs. non-reactive participants in Group 1 (measured at last follow-up, average 2.3 yrs).
DISCUSSION
Our results indicated a significant increase in metal reactivity with time in an asymptomatic MoM hip arthroplasty cohort. Moreover, a retrospective asymptomatic MoM group had an even higher average incidence of metal reactivity at 76%, presumably because the implants were in situ longer (2 to 11 yrs). While these levels seem high, they are less than previously reported in symptomatic MoM patient.2 This pattern of increasing metal reactivity with time in situ strongly supports a causal or contributing relationship between local adaptive immune responses and the pathogenesis of MoM failure. Our results generally support our hypothesis that metal reactivity develops prior to poor performance in concert with metal ion levels. However, we did not expect to find a time lag or sensitization phase of >3 mos, where ion levels were elevated, but there was little to no increase in metal reactivity. A similar relationship between elevated ions, acquired adaptive immune responses, and more specific MoM pathologies was reported by Kwon et al, where MoM patients with pseudotumors had a nearly 2-fold increase (80% vs 45%) in incidence of metal reactivity to Ni (LTT, SI>2) while at the same time showed >5 fold increases in Co and Cr serum ion levels when compared to non-pseudotumor MoM patients.4 Other investigations of aseptically failed MoM hip joints found histologic lymphocyte accumulations associated with implant failure.9,10,26–28 For example, Milosev et al29 found that 76% of retrieved MoM THAs at an average of 7 yrs post op “demonstrated hypersensitivity-like reaction(s) with aseptic inflammatory changes accompanied by moderate to extensive diffuse and perivascular infiltration of lymphocytes”.
Our results demonstrated that elevated lymphocyte reactivity to Co and Cr was not apparent at 3 mos, but developed after 1 to 4 yrs (Fig. 1). This “slow” increase is in stark contrast to fast elevations in Co and Cr levels at 3 mos, suggesting the presence of a lag/sensitization phase of >3mos to a year after the start of elevated metal exposure. Patch testing did not correlate with ion levels or measures of hypersensitivity (metal-LTT, flow cytometry, or cytokine analysis), and thus may not accurately reflect adaptive immune responses in the deep-tissue peri-implant environment.
CD4+ T-helper lymphocytes were significantly more activated by metal challenge agents when compared to CD8+ T-cytotoxic cells. Flow cytometric analysis of PBMC activation to metals was highly variable and was not consistent among participants due to single time-point sampling.
The complex interplay between monocytes and lymphocytes in PBMCs when challenged with Co, Cr, or Ni in vitro resulted in increased cytokine expression in some metal-reactive individuals with MoM hip arthroplasty. It is not surprising that elevated in vitro metal-reactivity was reflected by increased IL-1β, IL-12 and IL-6 (Fig. 5). Elevated IL-1β and TNF-α expression was reported in the peri-implant tissues of revised MoM implants by others.30 We did not find evidence of generally increased IL-2 and IL-17 in response to metals in Group 1 participants.
There are important limitations to our study. Metal chlorides in serum as challenge agents and metal-LTT are well established13,31 However, the degree to which metal ion-serum solutions mimic the antigenicity of soluble metal in vivo is incompletely understood. Generally, metal-LTT was a far more sensitive measure of immune reactivity than flow cytometry, cytokine analysis, or patch testing. Other practical limitations, such as a maximum numbers of collected PBMCs, precluded testing of both dose and timing. Group 1 and 2 participants were accepted on an all-comer basis; group demographics resulted in only 10 to 35% women in groups 1 and 2 (limited number of women precluded co-variate analysis). However, because the rates of metal-reactivity are generally higher in women,32,33 and females have double the risk of failure of MoM resurfacing as men34(National Joint Registry, 5thAnn. Report, www.njrcentre.org.uk), future studies of symptomatic/failing MoM implants should include enough women to demonstrate if a connection exists between this increased sensitivity and increased failure rates. Different individuals clearly have different levels of metal reactivity, and this reactivity varies with time and metal exposure. Generally all MoM revision patients show evidence of peri-implant lymphocyte infiltration, consistent with our results2. However, at what point these lymphocyte accumulations begin to significantly contribute to poor implant outcome remains unknown. Despite our in vitro evidence of acquired metal reactivity, the prospective MoM cohort continues to be asymptomatic, warranting continued follow-up studies on these and other MoM groups to help determine if and when clinically relevant thresholds are reached for LTT and metal ions in asymptomatic MoM patients.
Our investigation indicated a high percentage of individuals developed lymphocyte metal-reactivity responses from 5% preoperative to 56% post-operative at 2.3yrs as measured by LTT. The degree to which these responses play into long term implant failure has yet to be determined. The incidence levels in the control group were identical to previous large-scale studies for each metal, but had an overall incidence of 35% to any metal, which was significantly below Groups 1 and 2), but higher than the 15% incidence levels to any one of the metal challenge agents. All the MoM arthroplasties in Groups 1 and 2 were asymptomatic at 2.3 and 5.4 years, respectively. While our findings indicate the propensity of MoM hip arthroplasty to activate a metal-induced adaptive immune response, the degree to which these lymphocyte responses contribute to implant pathogenesis is unknown. Just as innate immune responses to polymeric debris slowly progresses over time into granuloma-mediated implant failure in some people, so too is it likely that these observed acquired adaptive reactions to implant metals may similarly lead to clinical pathology via a different path. Further investigation is needed to determine whether the participants whom we identified as metal-reactive will be more likely than other group members to progress to failure with clinically relevant levels of hypersensitivity (excessive peri-implant lymphocyte infiltration). Thus follow-up of these patients continues.
Supplementary Material
Acknowledgments
Support was provided by NIH/NIAMS AR039310-18S1 and the Crown Family Chair of Orthopedics. We thank and acknowledge Michelle LeDuff and Dr. Patricia Campbell for their time and effort in helping conduct this research.
References
- 1.Aroukatos P, Repanti M, Repantis T, et al. Immunologic adverse reaction associated with low-carbide metal-on-metal bearings in total hip arthroplasty. Clin Orthop Relat Res. 2010;468:2135–42. doi: 10.1007/s11999-009-1187-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Thomas P, Braathen LR, Dorig M, et al. Increased metal allergy in patients with failed metal-on-metal hip arthroplasty and peri-implant T-lymphocytic inflammation. Allergy. 2009;64:1157–65. doi: 10.1111/j.1398-9995.2009.01966.x. [DOI] [PubMed] [Google Scholar]
- 3.Willert HG, Buchhorn GH, Fayyazi A, et al. Metal-on-metal bearings and hypersensitivity in patients with artificial hip joints. A clinical and histomorphological study. J Bone Joint Surg Am. 2005;87:28–36. doi: 10.2106/JBJS.A.02039pp. [DOI] [PubMed] [Google Scholar]
- 4.Kwon YM, Thomas P, Summer B, et al. Lymphocyte proliferation responses in patients with pseudotumors following metal-on-metal hip resurfacing arthroplasty. J Orthop Res. 2010;28:444–50. doi: 10.1002/jor.21015. [DOI] [PubMed] [Google Scholar]
- 5.Gordon A, Greenfield EM, Eastell R, et al. Individual susceptibility to periprosthetic osteolysis is associated with altered patterns of innate immune gene expression in response to pro-inflammatory stimuli. J Orthop Res. 2010;28:1127–35. doi: 10.1002/jor.21135. [DOI] [PubMed] [Google Scholar]
- 6.Davies AP, Willert HG, Campbell PA, et al. An unusual lymphocytic perivascular infiltration in tissues around contemporary metal-on-metal joint replacements. J Bone Joint Surg Am. 2005;87:18–27. doi: 10.2106/JBJS.C.00949. [DOI] [PubMed] [Google Scholar]
- 7.Kwon YM, Ostlere S, Thomas P. Lymphocyte Proliferation Responses in Patients With Pseudotumours Following Metal-on-Metal Hip Resurfacings. Trans 55th Annual Meeting of the Orthopaedic Research Society Poster#441; 2009. [DOI] [PubMed] [Google Scholar]
- 8.Hallab N, Merritt K, Jacobs JJ. Metal sensitivity in patients with orthopaedic implants. J Bone Joint Surg Am. 2001;83-A:428–36. doi: 10.2106/00004623-200103000-00017. [DOI] [PubMed] [Google Scholar]
- 9.Campbell P, Ebramzadeh E, Nelson S, et al. Histological features of pseudotumor-like tissues from metal-on-metal hips. Clin Orthop Relat Res. 2010;468:2321–7. doi: 10.1007/s11999-010-1372-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Korovessis P, Petsinis G, Repanti M, Repantis T. Metallosis after contemporary metal-on-metal total hip arthroplasty. Five to nine-year follow-up. J Bone Joint Surg Am. 2006;88:1183–91. doi: 10.2106/JBJS.D.02916. [DOI] [PubMed] [Google Scholar]
- 11.Roato I, Caldo D, D’Amico L, et al. Osteoclastogenesis in peripheral blood mononuclear cell cultures of periprosthetic osteolysis patients and the phenotype of T cells localized in periprosthetic tissues. Biomater. 2010;31:7519–25. doi: 10.1016/j.biomaterials.2010.06.027. [DOI] [PubMed] [Google Scholar]
- 12.Arora A, Song Y, Chun L, et al. The role of the TH1 and TH2 immune responses in loosening and osteolysis of cemented total hip replacements. J Biomed Mater Res A. 2003;64:693–7. doi: 10.1002/jbm.a.10200. [DOI] [PubMed] [Google Scholar]
- 13.Kwon YM, Thomas P, Summer B, et al. Lymphocyte proliferation responses in patients with pseudotumors following metal-on-metal hip resurfacing arthroplasty. J Orthop Res. 2009 doi: 10.1002/jor.21015. [DOI] [PubMed] [Google Scholar]
- 14.Hallab NJ. A review of the biologic effects of spine implant debris: Fact from Fiction. SAS Journal. 2009;3:143–60. doi: 10.1016/j.esas.2009.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hallab NJ, Anderson S, Stafford T, et al. Lymphocyte responses in patients with total hip arthroplasty. J Orthop Res. 2005;23:384–91. doi: 10.1016/j.orthres.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 16.Le Duff MJ, Wang CT, Wisk LE, et al. Benefits of thin-shelled acetabular components for metal-on-metal hip resurfacing arthroplasty. J Orthop Res. 2010;28:1665–70. doi: 10.1002/jor.21176. [DOI] [PubMed] [Google Scholar]
- 17.Biedermann R, Tonin A, Krismer M, et al. Reducing the risk of dislocation after total hip arthroplasty: the effect of orientation of the acetabular component. J Bone Joint Surg Br. 2005;87:762–9. doi: 10.1302/0301-620X.87B6.14745. [DOI] [PubMed] [Google Scholar]
- 18.Langton DJ, Jameson SS, Joyce TJ, et al. Early failure of metal-on-metal bearings in hip resurfacing and large-diameter total hip replacement: A consequence of excess wear. J Bone Joint Surg Br. 2010;92:38–46. doi: 10.1302/0301-620X.92B1.22770. [DOI] [PubMed] [Google Scholar]
- 19.Davda K, Lali FV, Sampson B, et al. An analysis of metal ion levels in the joint fluid of symptomatic patients with metal-on-metal hip replacements. J Bone Joint Surg Br. 2011;93:738–45. doi: 10.1302/0301-620X.93B6.25804. [DOI] [PubMed] [Google Scholar]
- 20.Caicedo M, Jacobs JJ, Reddy A, Hallab NJ. Analysis of metal ion-induced DNA damage, apoptosis, and necrosis in human (Jurkat) T-cells demonstrates Ni(2+) and V(3+) are more toxic than other metals: Al(3+), Be(2+), Co(2+), Cr(3+), Cu(2+), Fe(3+), Mo(5+), Nb(5+), Zr(2+) J Biomed Mater Res A. 2007;86:905–13. doi: 10.1002/jbm.a.31789. [DOI] [PubMed] [Google Scholar]
- 21.Hallab NJ, Anderson S, Caicedo M, et al. Effects of soluble metals on human peri-implant cells. J Biomed Mater Res A. 2005;74:124–40. doi: 10.1002/jbm.a.30345. [DOI] [PubMed] [Google Scholar]
- 22.Jacobs JJ, Skipor AK, Patterson LM, et al. Metal release in patients who have had a primary total hip arthroplasty. A prospective, controlled, longitudinal study. J Bone Joint Surg [Am] 1998;80:1447–58. doi: 10.2106/00004623-199810000-00006. [DOI] [PubMed] [Google Scholar]
- 23.Carando S, Cannas M, Rossi P, Portigliatti-Barbos M. The lymphocytic transformation test (L.T.T.) in the evaluation of intolerance in prosthetic implants. Ital J Orthop Traumatol. 1985;11:475–81. [PubMed] [Google Scholar]
- 24.Everness KM, Gawkrodger DJ, Botham PA, Hunter JA. The discrimination between nickel-sensitive and non-nickel-sensitive subjects by an in vitro lymphocyte transformation test. Br J Dermatol. 1990;122:293–8. doi: 10.1111/j.1365-2133.1990.tb08276.x. [DOI] [PubMed] [Google Scholar]
- 25.Pichler WJ, Tilch J. The lymphocyte transformation test in the diagnosis of drug hypersensitivity. Allergy. 2004;59:809–20. doi: 10.1111/j.1398-9995.2004.00547.x. [DOI] [PubMed] [Google Scholar]
- 26.Guyer RD, Shellock J, MacLennan B, et al. Early failure of metal-on-metal artificial disc prostheses associated with lymphocytic reaction: diagnosis and treatment experience in four cases. Spine (Phila Pa 1976 ) 2011;36:E492–E497. doi: 10.1097/BRS.0b013e31820ea9a2. [DOI] [PubMed] [Google Scholar]
- 27.Hart AJ, Skinner JA, Winship P, et al. Circulating levels of cobalt and chromium from metal-on-metal hip replacement are associated with CD8+ T-cell lymphopenia. J Bone Joint Surg Br. 2009;91:835–42. doi: 10.1302/0301-620X.91B6.21844. [DOI] [PubMed] [Google Scholar]
- 28.Fujishiro T, Moojen DJ, Kobayashi N, et al. Perivascular and diffuse lymphocytic inflammation are not specific for failed metal-on-metal hip implants. Clin Orthop Relat Res. 2011;469:1127–33. doi: 10.1007/s11999-010-1649-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Milosev I, Trebse R, Kovac S, et al. Survivorship and retrieval analysis of Sikomet metal-on-metal total hip replacements at a mean of seven years. J Bone Joint Surg Am. 2006;88:1173–82. doi: 10.2106/JBJS.E.00604. [DOI] [PubMed] [Google Scholar]
- 30.Park YS, Moon YW, Lim SJ, et al. Early osteolysis following second-generation metal-on-metal hip replacement. J Bone Joint Surg Am. 2005;87:1515–21. doi: 10.2106/JBJS.D.02641. [DOI] [PubMed] [Google Scholar]
- 31.Thyssen JP, Menne T. Metal allergy--a review on exposures, penetration, genetics, prevalence, and clinical implications. Chem Res Toxicol. 2010;23:309–18. doi: 10.1021/tx9002726. [DOI] [PubMed] [Google Scholar]
- 32.Hinsch A, Vettorazzi E, Morlock MM, et al. Sex differences in the morphological failure patterns following hip resurfacing arthroplasty. BMC Med. 2011;9:113. doi: 10.1186/1741-7015-9-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Thyssen JP, Jakobsen SS, Engkilde K, et al. The association between metal allergy, total hip arthroplasty, and revision. Acta Orthop. 2009;80:646–52. doi: 10.3109/17453670903487008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hart AJ, Skinner JA, Henckel J, et al. Insufficient acetabular version increases blood metal ion levels after metal-on-metal hip resurfacing. Clin Orthop Relat Res. 2011;469:2590–7. doi: 10.1007/s11999-011-1930-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.