Abstract
The semiallogenic fetus is tolerated by the maternal immune system through control of innate and adaptive immune responses. Trophoblast cells secrete nanometer scale membranous particles called exosomes, which have been implicated in modulation of the local and systemic maternal immune system. Here we investigate the possibility that exosomes secreted from the first trimester and term placenta carry HLA-G and B7 family immunomodulators. Confocal microscopy of placental sections revealed intracellular colocalization of B7-H1 with CD63, suggesting that B7-H1 associates with subcellular vesicles that give rise to exosomes. First trimester and term placental explants were then cultured for 24 hours. B7H-1 (CD274), B7-H3 (CD276) and HLA-G5 were abundant in pelleted supernatants of these cultures that contained microparticles and exosomes; the latter, however, was observed only in first trimester pellets and was nearly undetectable in term explant-derived pellets. Further purification of exosomes by sucrose density fractionation confirmed the association of these proteins specifically with exosomes. Finally, culture of purified trophoblast cells in the presence or absence of EGF suggested that despite the absence of HLA-G5 association with term explant-derived exosomes, it is present in exosomes secreted from mononuclear cytotrophoblast cells. Further, differentiation of cytotrophoblast cells reduced the presence of HLA-G5 in secreted exosomes. Together, the results suggest that the immunomodulatory proteins HLA-G5, B7-H1 and B7-H3, are secreted from early and term placenta, and have important implications in the mechanisms by which trophoblast immunomodulators modify the maternal immunological environment.
1. Introduction
In hemochorial placentation, trophoblast cells abut maternal immunocompetent tissues unimpeded by other barriers. Trophoblast populations contacting maternal tissues include extravillous trophoblast cells, which invade deeply into the decidualized endometrium and underlying myometrium, serving to anchor the placenta and extraplacental membranes to the uterine wall. Another subpopulation of extravillous trophoblast cells enters the uterine spiral arteries, eventually replacing the maternal endothelium. The syncytiotrophoblast of the villous component of the placenta, on the other hand, covers the chorionic villi that form the placental parenchyma, forming a vast interface between the fetus and the maternal blood. As the point of exchange of maternal nutrients and fetal waste, the syncytiotrophoblast is continually bathed in maternal blood through the latter two-thirds of pregnancy.
Although the intimacy with which these semiallogeneic tissues coexist permits an efficient system of placentation, it also likely permits maternal immunological recognition of the fetal alloantigens [1]. Indeed, it is clear that the maternal immune system responds both locally and systemically to the conceptus. The gravid uterus possesses an abundant and unique population of leukocytes, dominated by uterine natural killer cells, macrophages and in lower numbers, T cells; additionally, expanded populations of fetal antigen-specific T cells can be found in the blood of women during and after pregnancy [2–5]. Thus, multiple mechanisms must exist for maintaining these cells in a state that is not only tolerant to fetal antigens, but that is also beneficial to pregnancy. Importantly, the human trophoblast cells robustly express a number of immunomodulatory proteins, including members of the HLA-G and B7 families, that play an important role in modulating the functions of maternal leukocytes [6–8].
One mechanism of maternal immunomodulation that has recently received increased attention involves the release of shed material from the placenta [9–11]. Reminiscent of typical epithelial tissues, the syncytiotrophoblast undergoes a process of turnover in which cells and aggregates of aged nuclei are extruded, allowing for contribution of fresh nuclei and cytoplasm via fusion of underlying cytotrophoblast cells [12, 13]. In addition to these cellular structures, smaller micro- and nano-sized particles termed microvesicles and exosomes, respectively, are released directly into the maternal blood. While cells and syncytial knots lodge within the pulmonary capillary bed or are rapidly cleared from the maternal circulation [10, 14], the smaller material appears to circulate and therefore may have unrestricted access to the spleen and other lymphoid tissue [15]. Placental microparticles, also called syncytiotrophoblast membrane particles or STBM, have been defined as membrane-bound fragments of syncytiotrophoblast measuring between 300 nm and 1 μm [16], whereas exosomes originate from the endo-lysosomal pathway and measure 50–150 nm [17]. More precisely, exosomes are formed as a result of fusion of the late endosome/multivesicular body with the plasma membrane, resulting in the release of intralumenal vesicles into the extracellular space. Exosomes can arise from many different cell types, but their biological actions are not completely understood as they play complex and diverse roles in immunobiology. For example, exosomes secreted by dendritic cells can stimulate the immune system by participating in antigen presentation, while those secreted from tumor cells may either promote or inhibit tumor immunity, depending on the pathophysiological context [18–20]. It has recently become increasingly apparent that the placenta is a rich source of exosomes, and that placental exosomes may have a role in immunosuppression during pregnancy [21, 22].
Members of the B7 family, as well as HLA-G, are among the immunosuppressive proteins expressed by trophoblast cells. These proteins likely have a role in mediating local fetal trophoblast interactions with maternal immune cells including T cells, macrophages, and uterine CD56hiCD16− natural killer cells at the maternal-fetal interface [6, 23]. Additionally, HLA-G is produced in multiple isoforms, including soluble, secreted isoforms. Intriguingly, both HLA-G and B7-H1 have been suggested to exist in the maternal circulation in association with placenta-derived exosomes [22, 24], suggesting that they can also function systemically. The goal of the present study was to determine whether members of the B7 family of immunomodulators as well as HLA-G are associated with the secretory lysosomal pathway and secreted via exosomes.
2. Materials and Methods
2.1 Tissue collection
Normal term and first trimester placentas were collected from uncomplicated pregnancies following Cesarean section without labor or elective termination, respectively, in accordance with protocols approved by the Human Subjects Committee at the University of Kansas Medical Center and the University of Chicago. For isolation and culture of term trophoblast cells, 40–80 g villous tissue were dissected, rinsed and subjected to purification as previously described [25]. For explant culture, first trimester or term floating villi were dissected free of chorion and decidua into 30–50 mg pieces and washed in PBS. For immunofluorescence analysis, samples were fixed in 4% paraformaldehyde for 4 hours, 18% sucrose overnight, and embedded in Tissue-Tek O.C.T. freezing medium (Fisher) prior to cryosectioning.
2.2 Trophoblast and explant cultures and microparticle/exosome isolation
All trophoblast and explant cultures were performed at 37°C under 5% CO2. First trimester and term explants were cultured in 12-well plates on Netwell® (Corning) inserts with 74 μm pores. Explants were cultured for 24 hours in medium containing DMEM/F-12 medium containing 10% FBS, 5ng/ml EGF; 5 μg/ml insulin; 10 μg/ml transferrin; 20 nM sodium selenite; 400 U/l human chorionic gonadotropin; 100 U/ml penicillin; 100 μg/ml streptomycin and 0.25 μg/ml amphotericin B. For cytotrophoblast cell culture, 80×106 cells were cultured in 150 mm petri dishes in exosome-free medium, or medium containing 5ng/ml epidermal growth factor (EGF; Peprotech) for 24 hours or 3 days (see Figure 7A). To analyze extensively syncytialized cells, trophoblasts were cultured for 6 days in 5ng/ml EGF, the last 3 days of which occurred in exosome-free medium. FBS-containing medium was pre-cleared of exosomes prior to use in culture by centrifugation of 2x medium at 100,000xg for 15 hours at 4°C. The 2X medium was subsequently sterilized by filtration and diluted to 1X in DMEM/F-12.
Figure 7. EGF induces differential protein association with exosomes.

(A) Cytotrophoblast cells were cultured for 3 days in exosome-free medium (gray shading) in the absence or presence of EGF, or for 6 days in the presence of EGF. In 6 day cultures, EGF-supplemented medium containing standard FBS was replaced with EGF-suppliemented medium containing exosome-free FBS (gray shading). (B) Pelleted supernatants were subjected to Western blot analysis using antibodies specific for the proteins indicated. Asterisks on right side of figure indicate non-specific bands, most likely due to antibodies binding to residual serum albumin (~68 KDa).
Following culture, culture supernatants were collected for isolation of exosomes using previously described methods [26]. Supernatants were cleared by sequential centrifugation at 300xg, 2000xg, and 10,000xg for 10, 20 and 30 minutes, respectively. Following each centrifugation, pellets containing dead cells and debris were discarded. Supernatant was then ultracentrifuged at 100,000xg for 1.5 hours at 4°C to pellet small vesicles, including exosomes. The pellets were washed in a large volume of PBS to remove contaminating proteins and centrifuged at the same high speed for an additional 1.5 hours. The pellet was resuspended in PBS and the protein concentrations determined using the Detergent Compatible (DC) protein assay (Bio-Rad Laboratories, Hercules, CA) according to the manufacturer’s instructions.
For density gradient analysis of exosomes, pellets were dissolved in 100μl PBS, and loaded onto a continuous sucrose gradient (2.0-0.25M in 20 mM HEPES, pH 7.4) which was prepared using a Hoefer SG30 gradient maker (GE Healthcare, Piscataway, NJ). The gradients were ultracentrifuged at 210,000xg for 15 hours at 4°C using a SW41Ti swinging bucket rotor in an Optima-Max ultracentrifuge. Eleven 1 ml fractions were collected from the top of the gradients, and densities of each fraction were measured using a VeeGee 100 refractometer. Each fraction was washed with 20mM HEPES in PBS and ultracentrifuged at 110,000xg for 1.5 hours using a TLA-100.4 rotor. The pellets were resuspended in PBS and used for further analysis by electron microscopy and western blot.
2.3 Electron microscopy
Exosomes were fixed in 4% paraformaldehyde and adsorbed onto formvar-carbon coated grids. After washing in PBS they were again fixed in 1% glutaraldehyde and contrasted in uranyal oxalate and embedded and contrasted a second time in a mixture of methylcellulose and uranyl acetate on ice. Grids were observed using a JEOL 100CX II or a JEOL JEM-1400 transmission electron microscope.
2.4 Immunofluorescence and confocal microscopy
Paraformaldehyde/sucrose-fixed frozed placenta was cryosectioned at 10μm thickness and placed onto lysine-coated slides and blocked in 2% serum of the species in which the secondary antibody was generated and 50% Superblock (Pierce) in PBS. Slides were incubated overnight at 4°C or 1.5 hours at room tem perature with mouse anti-human B7-H1 (12 μg/ml) (Table 1). After several washes, Alexa Fluor (AF) 594-conjugated anti-mouse secondary antibody (1:400; Invitrogen) was added. For double labeling, this was followed by washing and a second primary antibody, either mouse anti-human CD63 (3 μg/ml) or mouse anti-human LAMP1 (1.5 μg/ml). AF 488-conjugated anti-mouse secondary antibody (1:2000; Invitrogen) was added. As controls, mouse IgG1 substituted for primary antibodies, and slides were counterstained with DAPI for nuclear localization. Because two mouse anti-human primary antibodies were used, additional steps were taken to ensure that their respective secondary antibodies could not bind the other. Anti-CD63 or anti-LAMP1 was omitted to ensure that added AF 488-conjugated secondary antibody did not bind anti-B7-H1, and anti-B7-H1 was omitted to ensure that no residual AF594 antibody could bind to anti-CD63/anti-LAMP1. Sections were viewed using a Zeiss Pascal confocal microscope, and 1-μm thick serial z-sections were captured over 8 μm.
Table 1.
Antibodies used.
| Antibody specificity | Host/clone | Company |
|---|---|---|
| CD63 | Mouse/MX-49.129.5 | Santa Cruz Biotechnology |
| LAMP1 | Mouse/H4A3 | Becton-Dickinson |
| Calnexin | Rabbit polyclonal | Santa Cruz Biotechnology |
| B7-H1 | Mouse/MIH1 | eBiosciences |
| B7-H3 | Rabbit polyclonal | R&D Systems |
| HLA-G | Mouse/MEM-G1 | Abcam |
| HLA-G | Mouse/16G1 | D. Geraghty; ref. 27 |
| MHC class I | Mouse/W632 | ATCC |
2.5 Peripheral blood mononuclear cell isolation
Peripheral venous blood samples (20 ml) was collected in heparinized tubes from a healthy donor and mixed with same volume of PBS. Diluted blood was gently layered onto the surface of Histopaque (Sigma-Aldrich, St. Louis, MO) and centrifuged at 400x g for 30 minutes at room temperature. The cells at the plasma/PBS:Histopaque interface were collected and washed twice with RPMI-1640 medium containing 10% FBS and antibiotics. Cells were counted using a hemacytometer and viability was determined using trypan blue dye. Cells were frozen in medium/10% DMSO until isolation of protein for Western blot analysis.
2.5 Protein isolation and western blot analysis
Protein from cultured cells was collected by lysis in RIPA buffer containing protease inhibitors (Aprotinin, 10 ug/ml; Leupeptin, 10 ug/ml; PMSF, 100 ug/ml). Protein concentrations were determined for each sample using the DC protein assay (Bio-Rad). Proteins from total cell lysate (10–20 μg) or microparticle/exosome preparations were electrophoresed under denaturing or non-denaturing conditions and transferred to a nitrocellulose membrane (Optitran BA-S 83, Whatman GmbH, Germany). The membranes were blocked in 5% nonfat milk in Tris-buffered saline containing 0.1% Tween-20 (TBS-T) for 2h at room temperature. The membranes were probed overnight at 4°C with gentle shaking with appropriate antibodies (Table 1): anti-CD63, anti-LAMP-1, anti-Calnexin, anti-MHC class I, anti-B7-H1, anti-B7-H3 and two different anti-human HLA-G antibodies (clone MEM-G/1, which recognizes all isoforms of HLA-G when denatured, and 16G1, which recognizes HLA-G5 through the uniquely retained intron 4-containing C-terminal peptide) [27, 28]. Membranes were then washed in TBS-T and incubated with species-specific horseradish peroxidase-labeled secondary antibody for 1 hour at room temperature. After washing with TBS-T, membranes were developed to detect bound antibodies using the ECL chemiluminescence detection system (Pierce).
3. Results
3.1 Co-localization of CD63 with B7-H1 in the placenta
Our previous studies identified B7-H1 as an immunomodulator that is highly expressed on populations of trophoblast in the human placenta, and we found that it is particularly prominent on the apical membrane of the syncytiotrophoblast [7, 29]. It has also been suggested that placenta-associated B7-H1 can be secreted via exosomes [24]. We reasoned that if B7-H1 is associated with exosomes, then it should co-localize intracellularly with markers of late endosomes, since exosomes arise from the internal vesicles of these organelles. Immunofluorescence staining combined with confocal microscopy revealed that in addition to its localization at the apical surface of the syncytium, B7-H1 protein can be detected intracellularly in both the first trimester and the term placenta (Figure 1). Intracellular staining was punctate in nature, suggesting association with intracellular vesicles (Figure 1D, 1J). Double labeling revealed that the B7-H1 and CD63, which was also often inside the cell and vesicular, exhibited both distinct and overlapping expression (Figure 1F, 1L). Similarly, a second marker of late endosomes, LAMP1, colocalized with B7-H1 in first trimester placenta (data not shown). Controls in which both AF488- and AF594-labeled anti-mouse secondary antibodies were included but CD63 (Figure 1M) or B7-H1 (Figure 1O) were omitted revealed exclusive binding of secondary antibody to the intended primary antibody. Additionally, substitution of primary antibodies with isotype controls yielded no immunospecific reactivity (Figure 1P).
Figure 1. B7-H1 colocalizes with CD63, a marker of late endosomes, in the placenta.
First trimester (A–F) and term (G–L) placentas were subjected to immunofluorescence staining followed by confocal microscopy using mouse anti-human B7-H1 (A, D, G, J, M) together with mouse anti-human-CD63 (B, E, H, K, O). Secondary anti-mouse antibodies conjugated with Alexafluor 594 (red) and Alexafluor 488 (green) were used sequentially following each primary antibody. DAPI-counterstained nuclei are shown in blue. Controls included omission of anti-CD63 (M) or anti-B7-H1 (O) primary antibodies together with inclusion of both fluorescently labeled secondary antibodies. Mouse IgG1 was used as a negative control (P). Arrowheads indicate double immunofluorescence (yellow) near the apical membrane of the syncytiotrophoblast; arrows indicate intracellular B7-H1 staining not associated with CD63.
3.2 Exosomes isolated from first trimester villous explant cultures
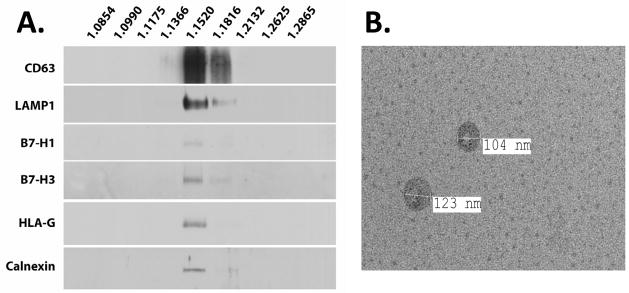
We next sought to determine whether first trimester explants were capable of secreting exosomes, and further, whether B7-H1 and other immunomodulatory proteins are present within exosomes secreted from cultured placental explants. First, we verified the presence of multivesicular bodies, wherein exosomes arise, at the apical surface of the syncytiotrophoblast by electron microscopy (Figure 2A). These results were very similar to those of Jones and Fox [30], who noted that these organelles predominantly localize at the apical surface of the syncytiotrophoblast. We next cultured first trimester explants (gestational ages 7–10 weeks) for 24 hours in medium pre-cleared of exosomes. Supernatant was collected and subjected to sequential centrifugation steps up to 100,000xg. Protein content of the final pellet was determined, and ranged from ~52–200 μg, depending on individual placentas. Electron microscopy revealed the presence of 50–150 nm-sized vesicles, which often displayed the cup-shaped morphology typical of exosomes [17, 31] (Figure 2B). Larger particles in the range of 300–500 nm, possibly representing microvesicles arising from the microvillous membrane, could also be observed in some preparations (Figure 2C) indicating that additional purification steps were warranted in order to definitively analyze exosomes. Nevertheless, pellets from all 6 first trimester explant cultures exhibited strong signals for the late endosome marker LAMP1, further confirming that pellets contained exosomes (Figure 2D). In addition, B7-H1 was nearly always associated with these vesicle preparations, as was a second B7 family member, B7-H3, and HLA-G. The molecular size of the latter was 37 KDa, which corresponds to the soluble form of the protein, HLA-G5. HLA-G5 and B7-H1 were observed in 5 of 6 pellets examined; one 7-week sample failed to yield a band corresponding to these proteins. B7-H3 was observed in all 6 preparations, although one 7-week sample exhibited only very faint reactivity; this was not the same preparation that lacked B7-H1 and HLA-G5.
Figure 2. Immunomodulatory proteins are associated with exosomes secreted from first trimester explant cultures.
(A) Transmission electron micrograph of apical surface of syncytiotrophoblast of a first trimester placenta. The left side of the image shows microvillus projections of the syncytiotrophoblast; asterisk, multivesicular body; arrows, intralumenal vesicles. (B – D) 24-hour first trimester explant supernatants were ultracentrifuged, and pellets subjected to electron microscopy (B, C) or Western blot analysis (D). Arrows in C demarcate exosomes; arrowheads show contaminating microvesicles. Numbers on left of (D) represent molecular weights (KDa).
Additional steps were taken to verify whether each of these proteins were specifically associated with exosomes. Initial pellets were subjected to ultracentrifugation over continuous 2.0–0.25M sucrose gradients, after which nine fractions were recovered. Refractive indices of each fraction were measured to determine sucrose density, and each fraction was washed and subjected to western blot. Signals for B7-H1, B7-H3, and HLA-G5 were restricted to densities corresponding to 1.15–1.18, consistent with the range of expected densities at which exosomes are known to float [32, 33] (Figure 3A). Furthermore, these immunomodulators co-localized with CD63 and LAMP1. Unexpectedly, calnexin also appeared within these fractions. Electron microscopy, however, confirmed the enrichment of exosomes within the 1.15–1.18 fractions, with exosomes ranging in size from ~100–200 nm in diameter (Figure 3B). No products appeared within fractions not associated with the density of exosomes.
Figure 3. Immunomodulators sediment to the density of exosomes.
Pellets derived from ultracentrifugation of supernatant from first trimester placental explants were layered over a sucrose gradient, and fractions were subjected to western blot analysis (A) and electron microscopy (B). (B) represents an image of exosomes collected from the 1.152 g/L fraction.
In addition to trophoblast, placental explants possess stromal support cells including Hofbauer cells, fibroblasts and endothelial cells. To determine whether trophoblast cells exclusively are responsible for exosome production in our culture system, we subjected exosome pellets to western blot using the conformation-dependent anti-HLA class I antibody, W6/32, which labels mesenchymal cells, but not trophoblast [34]. This antibody requires association of β2-microglobulin with the heavy chain of HLA; since villous cytotrophoblast and syncytiotrophoblast cells are devoid of β2-microglobulin [35], W6/32 should not recognize HLA-G5 secreted from these cells. Whereas W6/32 readily detected class I HLA from positive control peripheral blood mononuclear cells, this antibody did not detect any product from first-trimester explant-derived exosomes (Figure 4). Again, however, the anti-HLA-G-specific antibody MEM-G1, which recognizes denatured HLA-G1 and -G5 isoforms but is not dependent on β2-microglobulin [28], detected HLA-G5. CD63 and LAMP1 were also associated with the products secreted from these first trimester explants.
Figure 4. Exosomes lack β2-microglobulin-associated class I HLA.

Whole cell lysates of peripheral blood mononuclear cells (PBMC), first trimester explant-derived exosomes, and whole lysate of first trimester villous tissues were subjected to western blot using the conformation-dependent antibody, W6/32, the HLA-G-specific antibody, MEM-G/1, anti-CD63. Blots for W6/32 and CD63 were performed under non-denaturing electrophoresis conditions; blots for HLA-G and LAMP1 were performed under denaturing conditions.
3.3 Exosomes isolated from term villous explant cultures and cytotrophoblast cells
To determine whether term placenta could, like first trimester placenta, secrete B7-family members and HLA-G via exosomes, we cultured term placental explants identically to those derived from first trimester tissue. Pellets produced from supernatants subjected to sequential centrifugation contained readily detectable B7-H1 and B7-H3, but little or no HLA-G (Figure 5A). Electron microscopy confirmed the predominance of exosomes within the pellet, and sucrose gradient centrifugation followed by western blot analysis confirmed the association of B7-H1 and B7-H3, but only minimal HLA-G, with exosomes (Figure 5B, 5C).
Figure 5. Term placental explants secrete exosomes containing immunomodulators.
Term explants were cultured for 24 hours, and exosomes were pelleted and subjected to density gradient centrifugation, followed by analysis by western blot analysis. (A) Western blot analysis of pelleted supernatants; (B) electron microscopic image of pelleted material; (C) Western blot analysis of pelleted material following centrifugation over a sucrose gradient.
To begin to understand the regulation of secretion of exosome as well as their constituents, we purified term cytotrophoblast cells. These cells were maintained in culture for 3 days in exosome-free medium, and supernatants were subjected to sequential centrifugation. Although the protein yield from these cultures was too low in these preparations to be reliably quantified, we were routinely able to detect exosomes (Figure 6A) together with other larger particles, possibly microvesicles (not shown) within the pellets obtained from these cultures. Under basal culture conditions, B7-H1 and could consistently be detected in pellets of the culture supernatants by western blot analysis (Figure 6B). In addition, HLA-G5 was detected in the exosome/microparticle pellets from these cultures using two different monoclonal antibodies, MEM-G1 (not shown) and 16G1, despite this protein being low or undetectable in supernatant of term explant cultures (Figures 5 and 6B).
Figure 6. Cytotrophoblast cells secrete exosomes in culture.
Trophoblast cells were cultured for 3 days, at which time supernatants were subjected to sequential centrifugation. (A) Electron micrograph of pelleted vesicles; (B) Western blot of cell lysate (L1, 5 μg; L2, 10 μg) and pelleted vesicles/exosomes (Ex). Numbers on left of (B) represent molecular weights (KDa).
EGF promotes syncytialization of cytotrophoblast cells in vitro [36, 37], and we next asked whether syncytialization of trophoblast cells resulted in a difference in immunomodulatory protein content of exosome. Cells were cultured for 72 hours in medium alone, or medium supplemented with EGF (5ng/ml), or for 6 days in the presence of EGF (Figure 7A). This design was used to assess whether conditions that promote trophoblast differentation would alter the properties of exosomes/microparticles (least differentiated: untreated cells at 72 hours; most differentiated: EGF-treated cells at 6 days). Due to the requirement for high numbers of cells for these experiments, untreated cultures at 6 days were not included in the experimental design. At harvest, supernatants of each of these treatments were subjected to serial centrifugations to obtain a pellet. As expected, EGF-treated cells exhibited morphological characteristics of in vitro syncytialization: large multinucleate cells were increasingly observed in the presence of EGF, and further, with advancing duration of EGF treatment (data not shown). EGF treatment did not produce obvious changes in quantity of either B7-H1 or B7-H3 (Figure 7B). On the other hand, HLA-G5 was detected only in the control- and EGF-treated 72 hour cultures; no HLA-G was observed in the 6-day, EGF-treated trophoblast cultures. Similar results were observed using both antibodies MEMG1 and 16G1, and suggest that increased syncytialization of trophoblast cells diminishes their capability to secrete HLA-G5 within exosomes.
4. Discussion
In this study we have shown for the first time that the immunomodulators belonging to the B7 family, as well as the soluble HLA-G isoform, HLA-G5, are secreted from first trimester and term placentas, and that this secretion occurs via exosomes. Exosomes purified from first trimester and term placental explant cultures carry both B7-H1 and B7-H3; HLA-G5 was only found to be associated with exosomes produced by first trimester explants. Additionally, isolated cytotrophoblast cells and syncytiotrophoblast in culture were capable of secreting exosomes containing B7-H1 and -H3. Finally, cytotrophoblast cells, but not in vitro differentiated syncytiotrophoblast, also produced HLA-G5.
B7-H1 is constitutively transcribed and translated in the syncytiotrophoblast of the placenta [7, 38], a feature somewhat unique to trophoblast cells in that most other cell types do not translate the protein unless induced by proinflammatory stimuli. Similarly, B7-H3 is strongly expressed by the syncytiotrophoblast, particularly in the term placenta, but also to some extent in the first trimester placenta [8]. Because of their strong expression at the microvillous surface of the syncytiotrophoblast, it would not be surprising if these proteins are also associated with placental membrane microvesicles arising and/or shed syncytial ‘knots’ in addition to exosomes. Indeed, others have shown intriguing immunomodulatory qualities of cell surface-derived microvesicles, and that microvesicles are abundantly secreted from the placenta [39, 40] Here, we cannot rule out the possibility of association B7 family molecules with microvesicles, since these particles were found in the initial pellets from ultracentrifugation of culture supernatant, and further, since microvesicle size may overlap with those of exosomes [39]. To further assess whether these proteins were associated with exosomes, however, we further fractionated particles by centrifugation of these pellets over a continuous sucrose density gradient. The sedimentation of B7-H1, B7-H3, and HLA-G5 to the densities corresponding to that of exosomes, together with the apparent absence of contaminating material from these fractions as evaluated by electron microscopy, strongly suggests that these immunomodulators are indeed exosomal. Additional experiments to identify both distinct and overlapping functions of placental microvesicles and exosomes will be of primary interest.
Despite these results, we unexpectedly found the endoplasmic reticulum (ER)-membrane associated protein, calnexin, to be present in low levels in exosome preparations. Thus, despite predominance of exosome markers, we cannot at present rule out the possibility that some ER membrane particles represent a fraction of our exosome preparations. Alternatively, it is possible that calnexin is transiently associated with the trophoblast cell surface, as has been shown for other ER-associated proteins in trophoblast and other cells [41, 42]. Indeed, the ER contributes to membrane expansion during phagocytosis, which cytotrophoblast cells are well known to perform, and calnexin appears to play a critical role in this process [43–45]. Thus, there are two possible pathways by which calnexin could enter exosomes: from the cell surface via the endocytic pathway, or following generation of the phagosome. Indeed, the latter possibility is supported by the observation that after phagocytosis of Mycobacterium avium, pathogen-associated molecules traffic to the endocytic pathway and are subsequently secreted via exosomes [46]. Ongoing experiments in our lab are designed to resolve these possibilities.
HLA-G is generally considered to comprise a group of immunosuppressive molecules with an expression pattern is largely restricted to the placenta, and that influences both innate and adaptive immunity. The primary transcript of HLA-G is alternatively spliced and can give rise to 7 transcripts. Of these, four corresponding proteins have been identified, including the transmembrane proteins HLA-G1 and HLA-G2, and the soluble proteins HLA-G5 and HLA-G6, which correspond to truncated HLA-G1 and HLA-G2, respectively [6]. All four of these proteins are produced by extravillous trophoblast cells, and several studies suggest that although HLA-G1 and -G2 are not produced by villous cytotrophoblasts, the soluble isoforms are produced by these cells. HLA-G5 can be specifically identified in situ within cytotrophoblast cells, as well as within the supernatant of cultured cytotrophoblasts isolated from the term placenta [27, 47–49]. Our results strongly suggest that HLA-G5 is associated with placental exosomes derived from the placental villi. Using the well-characterized antibodies 16G1 and MEMG1, we detected an immunoreactive band that corresponds to the expected size of HLA-G5. Although 16G1 yielded several non-specific products by Western blot analysis, as has previously been shown for this antibody [50], it also revealed the specific immunoreactive band corresponding to 37 KDa HLA-G5. Additionally, the MEMG1 antibody yielded only a sole specific 37 KDa band. Furthermore, HLA-G5 and HLA-G6 can be detected in the serum of pregnant women throughout gestation [51, 52], and in one study, exosomes purified from the serum of pregnant women were reported to possess HLA-G [24], although which isoform of HLA-G in this finding was not indicated. Collectively, the results indicate that HLA-G5 may be secreted from the villous placenta either in free form or associated with exosomes.
The finding of HLA-G5 specifically in association with exosomes raises intriguing questions about the cellular path by which HLA-G5 exits the placenta. From our study, it is not clear whether HLA-G5 is associated with the interior or exterior of individual exosomes. As a secreted protein, the expectation would be that HLA-G5 associates with the exterior surface of the exosome, as the topology of this surface would be akin to the exterior plasma membrane of the cell and the limiting membrane of the multivesicular bodies. In this case, HLA-G5 would presumably require a binding protein to mediate its association with the exterior vesicle membrane. Alternatively, association with the interior of exosomes, which is consistent topologically with the cell cytoplasm, would necessitate transit of HLA-G5 into the cytoplasm after its biosynthesis. A second question is raised when considering how HLA-G-carrying exosomes access the maternal blood. Our data suggest that cytotrophoblast rather than syncytiotrophoblast-derived exosomes are a source of exosome-associated HLA-G5, which is consistent with the reported immunolocalization of the protein [27, 47]. Thus, HLA-G5 could be synthesized within the cytotrophoblast cells and transit through the syncytiotrophoblast en route to the maternal circulation. In addition, cytotrophoblast-derived exosomes with associated HLA-G5 could conceivably fuse with the overlying syncytiotrophoblast, effectively ‘donating’ the protein for future secretion. Altogether, future studies on the cell biology of HLA-G5 secretion will further our understanding of these intriguing questions.
B7-H1 and B7-H3 are both members of the B7 family and function to modulate effector functions of T lymphocytes. B7-H1, also called PD-L1, in particular has been implicated in maternal-fetal torlerance [29, 53, 54], and has been linked with exosomes secreted by tolerogenic dendritic cells [55]. B7-H3 can be associated with exosomes arising from prostate cancer and glioblastoma [56, 57]. However, the role of the latter in regulating the immune system is unclear, since both stimulatory and inhibitory properties of this protein are evident [58, 59]. In contrast to HLA-G5, the cellular mechanism by which B7-H1 and B7-H3 presumably associate with exosomes is more intuitive. As surface-associated transmembrane proteins, these proteins could be internalized from the cell surface and directed to the endosome-lysosome pathway, or alternatively, transported directly to this pathway following their biosynthesis. Regardless of which pathway(s) are followed, important future considerations will be the mechanisms by which all of these molecules are directed towards the exosome secretion pathway. Indeed, the content of exosomes secreted by mature dendritic cells differs from that of immature dendritic cells [60], suggesting that the signals inducing maturation trigger differential shuttling of proteins to this pathway.
The mechanisms by which placental exosomes and their associated macromolecules modulate the maternal immune system will require elucidation. Exosomes may exert their effects in multiple ways, including mediating receptor-ligand interactions, fusion with a target cell and donation of intravesicular and membrane-associated contents, and being engulfed by phagocytes. It is likely that all of these mechanisms are functional; indeed, receptor-ligand interactions seem to be at play in the effect of exosomes on NK and T cells, and others have shown that trophoblast-derived microvesicles and exosomes can be engulfed by phagocytes [21, 22, 61]. The latter raises the intriguing possibility that exosomes serve as vehicular shuttles for paternally-inherited placental antigens that are ultimately cross-presented to maternal T cells by antigen presenting cells. In conclusion, these results offer new insight into the potential for human placental exosomes to serve as important conveyors of the maternal immune response to the semiallogeneic fetus.
Acknowledgments
This work was supported by grants from the NIH (P01 HD049480 to J.S. Hunt and R01 HD045611 to M.G. Petroff). Further support and services were provided by the KUMC Center for Reproductive Sciences (NICHD), the Kansas IDeA Network of Biomedical Research Infrastructure (P20 RR016475), the KUMC Center of Biomedical Research Excellence (COBRE) program in Cell Development and Differentiation (P20 RR024214), and the Kansas State University COBRE program in Epithelial Function (P20 RR017686). The authors thank Barbara Fegley of the KUMC Electron Microscopy Research Laboratory for her assistance with electron microscopy studies, David Albertini and Darlene Limback for assistance with confocal microscopy, Lane Christiansen for critically reading the manuscript and Dan Geraghty for the gift of the 16G1 antibody.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Taglauer ES, Adams Waldorf KM, Petroff MG. The hidden maternal-fetal interface: events involving the lymphoid organs in maternal-fetal tolerance. Int J Dev Biol. 2010;54:421–30. doi: 10.1387/ijdb.082800et. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.James E, Chai JG, Dewchand H, Macchiarulo E, Dazzi F, Simpson E. Multiparity induces priming to male-specific minor histocompatibility antigen, HY, in mice and humans. Blood. 2003;102:388–93. doi: 10.1182/blood-2002-10-3170. [DOI] [PubMed] [Google Scholar]
- 3.Piper KP, McLarnon A, Arrazi J, Horlock C, Ainsworth J, Kilby MD, Martin WL, Moss PA. Functional HY-specific CD8+ T cells are found in a high proportion of women following pregnancy with a male fetus. Biol Reprod. 2007;76:96–101. doi: 10.1095/biolreprod.106.055426. [DOI] [PubMed] [Google Scholar]
- 4.Verdijk RM, Kloosterman A, Pool J, van de Keur M, Naipal AM, van Halteren AG, Brand A, Mutis T, Goulmy E. Pregnancy induces minor histocompatibility antigen-specific cytotoxic T cells: implications for stem cell transplantation and immunotherapy. Blood. 2004;103:1961–4. doi: 10.1182/blood-2003-05-1625. [DOI] [PubMed] [Google Scholar]
- 5.van Halteren AG, Jankowska-Gan E, Joosten A, Blokland E, Pool J, Brand A, Burlingham WJ, Goulmy E. Naturally acquired tolerance and sensitization to minor histocompatibility antigens in healthy family members. Blood. 2009;114:2263–72. doi: 10.1182/blood-2009-01-200410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hunt JS, Petroff MG, McIntire RH, Ober C. HLA-G and immune tolerance in pregnancy. FASEB J. 2005;19:681–93. doi: 10.1096/fj.04-2078rev. [DOI] [PubMed] [Google Scholar]
- 7.Petroff MG, Chen L, Phillips TA, Azzola D, Sedlmayr P, Hunt JS. B7 family molecules are favorably positioned at the human maternal-fetal interface. Biol Reprod. 2003;68:1496–504. doi: 10.1095/biolreprod.102.010058. [DOI] [PubMed] [Google Scholar]
- 8.Petroff MG, Kharatyan E, Torry DS, Holets L. The immunomodulatory proteins B7-DC, B7-H2, and B7-H3 are differentially expressed across gestation in the human placenta. Am J Pathol. 2005;167:465–73. doi: 10.1016/S0002-9440(10)62990-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Adams Waldorf KM, Yan Z, Stevens AM, Nelson JL. The changing maternal “self” hypothesis: a mechanism for maternal tolerance of the fetus. Placenta. 2007;28:378–82. doi: 10.1016/j.placenta.2006.07.003. [DOI] [PubMed] [Google Scholar]
- 10.Chamley LW, Chen Q, Ding J, Stone PR, Abumaree M. Trophoblast deportation: just a waste disposal system or antigen sharing? J Reprod Immunol. 2011;88:99–105. doi: 10.1016/j.jri.2011.01.002. [DOI] [PubMed] [Google Scholar]
- 11.Petroff MG. Fetal antigens - Identity, origins, and influences on the maternal immune system. Placenta. 2011;32:S176–81. doi: 10.1016/j.placenta.2010.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huppertz B, Kaufmann P, Kingdom J. Trophoblast turnover in health and disease. Fetal Maternal Med Rev. 2002;13:103–18. [Google Scholar]
- 13.Burton GJ, Jones CJP. Syncytial knots, sprouts, apoptosis, and trophoblast deportation from the human placenta. Taiwan J Obstet Gynecol. 2009;48:28–37. doi: 10.1016/S1028-4559(09)60032-2. [DOI] [PubMed] [Google Scholar]
- 14.Attwood HA, Park WW. Embolism to the lungs by trophoblast. J Obstet Gynaecol Br Comm. 1961;68:611–7. doi: 10.1111/j.1471-0528.1961.tb02778.x. [DOI] [PubMed] [Google Scholar]
- 15.Redman CWG, Sargent IL. Circulating microparticles in normal pregnancy and pre-eclampsia. Placenta. 2008;29(Supplement):73–7. doi: 10.1016/j.placenta.2007.11.016. [DOI] [PubMed] [Google Scholar]
- 16.Redman CW, Sargent IL. Microparticles and immunomodulation in pregnancy and pre-eclampsia. J Reprod Immunol. 2007;76:61–7. doi: 10.1016/j.jri.2007.03.008. [DOI] [PubMed] [Google Scholar]
- 17.Thery C, Zitvogel L, Amigorena S. Exosomes: composition, biogenesis and function. Nat Rev Immunol. 2002;2:569–79. doi: 10.1038/nri855. [DOI] [PubMed] [Google Scholar]
- 18.Zitvogel L, Regnault A, Lozier A, Wolfers J, Flament C, Tenza D, Ricciardi-Castagnoli P, Raposo G, Amigorena S. Eradication of established murine tumors using a novel cell-free vaccine: dendritic cell-derived exosomes. Nat Med. 1998;4:594–600. doi: 10.1038/nm0598-594. [DOI] [PubMed] [Google Scholar]
- 19.Wolfers J, Lozier A, Raposo G, Regnault A, Thery C, Masurier C, Flament C, Pouzieux S, Faure F, Tursz T, Angevin E, Amigorena S, Zitvogel L. Tumor-derived exosomes are a source of shared tumor rejection antigens for CTL cross-priming. Nat Med. 2001;7:297–303. doi: 10.1038/85438. [DOI] [PubMed] [Google Scholar]
- 20.Taylor DD, Gercel-Taylor C. Tumour-derived exosomes and their role in cancer-associated T-cell signalling defects. Br J Cancer. 2005;92:305–11. doi: 10.1038/sj.bjc.6602316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hedlund M, Stenqvist AC, Nagaeva O, Kjellberg L, Wulff M, Baranov V, Mincheva-Nilsson L. Human placenta expresses and secretes NKG2D ligands via exosomes that down-modulate the cognate receptor expression: evidence for immunosuppressive function. J Immunol. 2009;183:340–51. doi: 10.4049/jimmunol.0803477. [DOI] [PubMed] [Google Scholar]
- 22.Taylor DD, Akyol S, Gercel-Taylor C. Pregnancy-associated exosomes and their modulation of T cell signaling. J Immunol. 2006;176:1534–42. doi: 10.4049/jimmunol.176.3.1534. [DOI] [PubMed] [Google Scholar]
- 23.Petroff MG, Perchellet A. B7 family molecules as regulators of the maternal immune system in pregnancy. Am J Reprod Immunol. 2010;63:506–19. doi: 10.1111/j.1600-0897.2010.00841.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sabapatha A, Gercel-Taylor C, Taylor DD. Specific isolation of placenta-derived exosomes from the circulation of pregnant women and their immunoregulatory consequences. Am J Reprod Immunol. 2006;56:345–55. doi: 10.1111/j.1600-0897.2006.00435.x. [DOI] [PubMed] [Google Scholar]
- 25.Petroff MG, Phillips TA, Ka H, Pace JL, Hunt JS. Isolation and culture of term human trophoblast cells. Methods Mol Med. 2006;121:203–17. doi: 10.1385/1-59259-983-4:201. [DOI] [PubMed] [Google Scholar]
- 26.Thery C, Clayton A, Amigorena S, Raposo G. Isolation and characterization of exosomes from cell culture supernatants and biological fluids. Curr Prot Cell Biol. 2006;3:3.22.1–3..5. doi: 10.1002/0471143030.cb0322s30. [DOI] [PubMed] [Google Scholar]
- 27.Ishitani A, Sageshima N, Lee N, Dorofeeva N, Hatake K, Marquardt H, Geraghty DE. Protein expression and peptide binding suggest unique and interacting functional roles for HLA-E, F, and G in maternal-placental immune recognition. J Immunol. 2003;171:1376–84. doi: 10.4049/jimmunol.171.3.1376. [DOI] [PubMed] [Google Scholar]
- 28.Menier C, Saez B, Horejsi V, Martinozzi S, Krawice-Radanne I, Bruel S, Le Danff C, Reboul M, Hilgert I, Rabreau M, Larrad ML, Pla M, Carosella ED, Rouas-Freiss N. Characterization of monoclonal antibodies recognizing HLA-G or HLA-E: new tools to analyze the expression of nonclassical HLA class I molecules. Human Immunology. 2003;64:315–26. doi: 10.1016/s0198-8859(02)00821-2. [DOI] [PubMed] [Google Scholar]
- 29.Taglauer ES, Trikhacheva AS, Slusser JG, Petroff MG. Expression and function of PDCD1 at the human maternal-fetal interface. Biol Reprod. 2008;79:562–9. doi: 10.1095/biolreprod.107.066324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jones CJP, Fox H. Ultrastructure of the normal human placenta. Electron Microsc Rev. 1991;4:129–78. doi: 10.1016/0892-0354(91)90019-9. [DOI] [PubMed] [Google Scholar]
- 31.Ostrowski M, Carmo NB, Krumeich S, Fanget I, Raposo G, Savina A, Moita CF, Schauer K, Hume AN, Freitas RP, Goud B, Benaroch P, Hacohen N, Fukuda M, Desnos C, Seabra MC, Darchen F, Amigorena S, Moita LF, Thery C. Rab27a and Rab27b control different steps of the exosome secretion pathway. Nat Cell Biol. 2010;12:19–30. doi: 10.1038/ncb2000. [DOI] [PubMed] [Google Scholar]
- 32.Raposo G, Nijman HW, Stoorvogel W, Liejendekker R, Harding CV, Melief CJ, Geuze HJ. B lymphocytes secrete antigen-presenting vesicles. J Exp Med. 1996;183:1161–72. doi: 10.1084/jem.183.3.1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Thery C, Regnault A, Garin J, Wolfers J, Zitvogel L, Ricciardi-Castagnoli P, Raposo G, Amigorena S. Molecular characterization of dendritic cell-derived exosomes. Selective accumulation of the heat shock protein hsc73. J Cell Biol. 1999;147:599–610. doi: 10.1083/jcb.147.3.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Butterworth BH, Loke YW. Immunocytochemical identification of cytotrophoblast from other mononuclear cell populations isolated from first-trimester human chorionic villi. J Cell Sci. 1985;76:189–97. doi: 10.1242/jcs.76.1.189. [DOI] [PubMed] [Google Scholar]
- 35.Hunt JS, Pace JL, Morales PJ, Ober C. Immunogenicity of the soluble isoforms of HLA-G. Mol Hum Reprod. 2003;9:729–35. doi: 10.1093/molehr/gag087. [DOI] [PubMed] [Google Scholar]
- 36.Morrish DW, Bhardwaj D, Dabbagh LK, Marusyk H, Siy O. Epidermal growth factor induces differentiation and secretion of human chorionic gonadotropin and placental lactogen in normal human placenta. J Clin Endocrinol Metab. 1987;65:1282–90. doi: 10.1210/jcem-65-6-1282. [DOI] [PubMed] [Google Scholar]
- 37.Holets LM, Carletti MZ, Kshirsagar SK, Christenson LK, Petroff MG. Differentiation-induced post-transcriptional control of B7-H1 in human trophoblast cells. Placenta. 2009;30:48–55. doi: 10.1016/j.placenta.2008.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Holets LM, Hunt JS, Petroff MG. Trophoblast CD274 (B7-H1) is differentially expressed across gestation: influence of oxygen concentration. Biol Reprod. 2006;74:352–8. doi: 10.1095/biolreprod.105.046581. [DOI] [PubMed] [Google Scholar]
- 39.Redman CWG, Tannetta DS, Dragovic RA, Gardiner C, Southcombe JH, Collett GP, Sargent IL. Does size matter? Placental debris and the pathophysiology of preeclampsia. Placenta. 2012;33 (Suppl A):S48–S54. doi: 10.1016/j.placenta.2011.12.006. [DOI] [PubMed] [Google Scholar]
- 40.Southcombe J, Tannetta D, Redman C, Sargent I. The immunomodulatory role of syncytiotrophoblast microvesicles. PLoS One. 2011;6:e20245. doi: 10.1371/journal.pone.0020245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gonzalez-Gronow M, Selim MA, Papalas J, Pizzo SV. GRP78: a multifunctional receptor on the cell surface. Antioxid Redox Signal. 2009;11:2299–306. doi: 10.1089/ARS.2009.2568. [DOI] [PubMed] [Google Scholar]
- 42.Arnaudeau S, Arboit P, Bischof P, Shin-ya K, Tomida A, Tsuruo T, Irion O, Cohen M. Glucose-regulated protein 78: a new partner of p53 in trophoblast. Proteomics. 2009;9:5316–27. doi: 10.1002/pmic.200800865. [DOI] [PubMed] [Google Scholar]
- 43.Bevilacqua E, Hoshida MS, Amarante-Paffaro A, Albieri-Borges A, Zago Gomes S. Trophoblast phagocytic program: roles in different placental systems. Int J Dev Biol. 2010;54:495–505. doi: 10.1387/ijdb.082761eb. [DOI] [PubMed] [Google Scholar]
- 44.Gagnon E, Duclos S, Rondeau C, Chevet E, Cameron PH, Steele-Mortimer O, Paiement J, Bergeron JJ, Desjardins M. Endoplasmic reticulum-mediated phagocytosis is a mechanism of entry into macrophages. Cell. 2002;110:119–31. doi: 10.1016/s0092-8674(02)00797-3. [DOI] [PubMed] [Google Scholar]
- 45.Desjardins M. ER-mediated phagocytosis: a new membrane for new functions. Nat Rev Immunol. 2003;3:280–91. doi: 10.1038/nri1053. [DOI] [PubMed] [Google Scholar]
- 46.Bhatnagar S, Schorey JS. Exosomes released from infected macrophages contain Mycobacterium avium glycopeptidolipids and are proinflammatory. Cell Microbiol. 2007;8:25779–89. doi: 10.1074/jbc.M702277200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Morales PJ, Pace JL, Platt JS, Phillips TA, Morgan K, Fazleabas AT, Hunt JS. Placental cell expression of HLA-G2 isoforms is limited to the invasive trophoblast phenotype. J Immunol. 2003;171:6215–24. doi: 10.4049/jimmunol.171.11.6215. [DOI] [PubMed] [Google Scholar]
- 48.Solier C, Aguerre-Girr M, Lenfant F, Campan A, Berrebi A, Rebmann V, Grosse-Wilde H, Le Bouteiller P. Secretion of pro-apoptotic intron 4-retaining soluble HLA-G1 by human villous trophoblast. Eur J Immunol. 2002;32:3576–86. doi: 10.1002/1521-4141(200212)32:12<3576::AID-IMMU3576>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 49.Morales PJ, Pace JL, Platt JS, Langat DK, Hunt JS. Synthesis of b2-m-free, di-S linked HLA-G5 homodimers in human placental villous cytotrophoblast cells. Immunology. 2007;122:179–88. doi: 10.1111/j.1365-2567.2007.02623.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hunt JS, Geraghty DE. Soluble HLA-G isoforms: technical deficiencies lead to misinterpretations. Molecular Human Reproduction. 2005;11:715–7. doi: 10.1093/molehr/gah223. [DOI] [PubMed] [Google Scholar]
- 51.Hunt JS, Jadhav L, Chu W, Geraghty DE, Ober C. Soluble HLA-G circulates in maternal blood during pregnancy. American Journal of Obstetrics and Gynecology. 2000;183:682–8. doi: 10.1067/mob.2000.106762. [DOI] [PubMed] [Google Scholar]
- 52.Rebmann V, Pfeiffer K, Päßler M, Ferrone S, Maier S, Weiss E, Grosse-Wilde H. Detection of soluble HLA-G molecules in plasma and amniotic fluid. Tissue Antigens. 1999;53:14–22. doi: 10.1034/j.1399-0039.1999.530102.x. [DOI] [PubMed] [Google Scholar]
- 53.Taglauer ES, Yankee TM, Petroff MG. Maternal PD-1 regulates accumulation of fetal antigen-specific CD8+ T cells in pregnancy. J Reprod Immunol. 2009;80:12–21. doi: 10.1016/j.jri.2008.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Guleria I, Khosroshahi A, Ansari MJ, Habicht A, Azuma M, Yagita H, Noelle RJ, Coyle A, Mellor AL, Khoury SJ, Sayegh MH. A critical role for the programmed death ligand 1 in fetomaternal tolerance. J Exp Med. 2005;202:231–7. doi: 10.1084/jem.20050019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ruffner MA, Kim SH, Bianco NR, Francisco LM, Sharpe AH, Robbins PD. B7-1/2, but not PD-L1/2 molecules, are required on IL-10-treated tolerogenic DC and DC-derived exosomes for in vivo function. European Journal of Immunology. 2009;39:3084–90. doi: 10.1002/eji.200939407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lehmann BD, Paine MS, Brooks AM, McCubrey JA, Renegar RH, Wang R, Terrian DM. Senescence-associated exosome release from human prostate cancer cells. Cancer Research. 2008;68:7864–71. doi: 10.1158/0008-5472.CAN-07-6538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lemke D, Pfenning P-N, Sahm F, Klein A-C, Kempf T, Warnken U, Schnölzer M, Tudoran R, Weller M, Platten M, Wick W. Costimulatory protein 4IgB7H3 drives the malignant phenotype of glioblastoma by mediating immune escape and invasiveness. Clinical Cancer Research. 2012;18:105–17. doi: 10.1158/1078-0432.CCR-11-0880. [DOI] [PubMed] [Google Scholar]
- 58.Loos M, Hedderich D, Friess H, Kleeff J. B7-H3 and its role in antitumor immunity. Clin Dev Immunol. 2010;2010:1–7. doi: 10.1155/2010/683875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yi KH, Chen L. Fine tuning the immune response through B7-H3 and B7-H4. Immunol Rev. 2009;229:145–51. doi: 10.1111/j.1600-065X.2009.00768.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Segura E, Amigorena S, Théry C. Mature dendritic cells secrete exosomes with strong ability to induce antigen-specific effector immune responses. Blood Cells, Molecules, and Diseases. 2005;35:89–93. doi: 10.1016/j.bcmd.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 61.Atay S, Gercel-Taylor C, Suttles J, Mor G, Taylor DD. Trophoblast-derived exosomes mediate monocyte recruitment and differentiation. American Journal of Reproductive Immunology. 2011;65:65–77. doi: 10.1111/j.1600-0897.2010.00880.x. [DOI] [PubMed] [Google Scholar]