Abstract
Background
Pulmonary arterial hypertension (PAH) is characterized, in part, by decreased endothelial nitric oxide (NO•) production and elevated levels of endothelin-1. Endothelin-1 is known to stimulate endothelial nitric oxide synthase (eNOS) via the endothelin-B receptor (ETB), suggesting that this signaling pathway is perturbed in PAH. Endothelin-1 also stimulates adrenal aldosterone synthesis; in systemic blood vessels, hyperaldosteronism induces vascular dysfunction by increasing endothelial reactive oxygen species (ROS) generation and decreasing NO• levels. We hypothesized that aldosterone modulates PAH by disrupting ETB-eNOS signaling through a mechanism involving increased pulmonary endothelial oxidant stress.
Methods and Results
In rats with PAH, elevated endothelin-1 levels were associated with elevated aldosterone levels in plasma and lung tissue and decreased lung NO• metabolites in the absence of left heart failure. In human pulmonary artery endothelial cells (HPAECs), endothelin-1 increased aldosterone levels via PGC-1α/steroidogenesis factor-1-dependent upregulation of aldosterone synthase. Aldosterone also increased ROS production, which oxidatively modified cysteinyl thiols in the eNOS-activating region of ETB to decrease endothelin-1-stimulated eNOS activity. Substitution of ETB-Cys405 with alanine improved ETB-dependent NO• synthesis under conditions of oxidant stress, confirming that Cys405 is a redox sensitive thiol that is necessary for ETB-eNOS signaling. In HPAECs, mineralocorticoid receptor antagonism with spironolactone decreased aldosterone-mediated ROS generation and restored ETB-dependent NO• production. Spironolactone or eplerenone prevented or reversed pulmonary vascular remodeling and improved cardiopulmonary hemodynamics in two animal models of PAH in vivo.
Conclusions
Our findings demonstrate that aldosterone modulates an ETB cysteinyl thiol redox switch to decrease pulmonary endothelium-derived NO• and promote PAH.
Keywords: endothelin, nitric oxide, pulmonary heart disease, aldosterone, redox biochemistry
Introduction
Pulmonary endothelial reactive oxygen species (ROS) have been implicated in the pathobiology of pulmonary arterial hypertension (PAH) and have been shown to disrupt nitric oxide (NO•)-dependent vasodilatory signaling pathways to promote pulmonary vasoconstriction, muscularization of pulmonary arterioles, and perivascular fibrosis.1,2 However, contemporary PAH pharmacotherapies that aim to restore pulmonary vascular NO• levels have waning long-term efficacy and do not maintain normal pulmonary vascular tone and pulmonary hemodynamics.3 This observation suggests that in PAH, perturbations to the redox milieu of pulmonary vascular tissue is sufficient to offset the vasodilatory effects of NO•, although the factor(s) that modulate this effect have not been fully elucidated.
Elevated levels of the mineralocorticoid hormone aldosterone are associated with a vasculopathy in systemic blood vessels that is characterized by mineralocorticoid receptor-dependent increases in endothelial ROS generation that decreases levels of bioavailable NO• resulting in vascular endothelial dysfunction, vascular fibrosis, and decreased vascular compliance.4 In patients with hyperaldosteronism and hypertension or congestive heart failure, mineralocorticoid receptor antagonism with spironolactone or eplerenone improves vascular reactivity and attenuates the adverse effects of aldosterone on blood vessel function and architecture.5 We hypothesized that hyperaldosteronism is present in PAH owing to increased circulating levels of endothelin-1 (ET-1), which is a potent stimulus of adrenal aldosterone synthesis,6 and/or overactivation of the renin-angiotensin-aldosterone axis. Together, these observations and the derivative hypothesis suggest the possibility that by increasing pulmonary endothelial ROS levels, hyperaldosteronism is an unrecognized contributor to the pathobiology of PAH.
The mechanism(s) by which ROS decreases pulmonary endothelial NO• levels in PAH is unresolved. In the systemic vasculature, ROS has been implicated in the oxidative modification of redox-sensitive cysteinyl thiols in regulatory proteins involved in NO•-dependent vasodilatory signaling to decrease NO• bioactivity.7 A key source of endogenous NO• generation in pulmonary endothelial cells is via endothelin type B receptor (ETB)-mediated activation of endothelial nitric oxide synthase (eNOS).8 ETB contains an intracellular cysteine-rich region near its carboxyterminal domain that includes Cys405, demonstrated previously to be a cysteinyl thiol that regulates ETB signal transduction.9 Taken together, we hypothesized that oxidative modification of ETB Cys405 by aldosterone-induced ROS serves as a redox switch that disables ETB-dependent synthesis of NO• to promote pulmonary vascular dysfunction and negative remodeling of pulmonary arterioles in PAH.
Methods
An expanded Methods section is located in the online supplement
Cell culture and treatments
Human pulmonary artery endothelial cells (HPAECs) (Lonza) (male donors) were grown to confluence using phenol-free EGM-2 medium supplemented with 5% fetal bovine serum at 37°C, 5% CO2. Cells were passaged twice-weekly using 0.5% trypsin/EDTA, and experiments were performed on cells from passages 4–10. Aldosterone (Steraloids) and ET-1 (1–100 nM) (Sigma-Aldrich) were dissolved in dimethylsufloxide (10 nmol/L) and deoxygenated water, respectively, which served as vehicle controls. Cells were treated with aldosterone (10−9–10−7 mol/L) for 24 h and in selected experiments co-incubated with the mineralocorticoid receptor inhibitor spironolactone (10 μM) (Sigma-Aldrich).
Western analysis to detect ETB disfulfide bond formation
Western analysis to detect ETB disulfide bond formation was performed as described previously.7 Briefly, protein extracts from cells were lysed in alkylating buffer containing 0.1 M Tris-HCL, pH 6.8, 1% SDS, 100 mM iodoacetamide, and 100 mM N-ethylmaleimide, and sonicated on ice for 5 min followed by a 30-min incubation at 25 °C. Alkylated proteins were then precipitated with acetone. Proteins were resuspended in 50 μl of 0.1 M Tris-HCl, pH 7.4, 1% SDS; and disulfides were reduced with 5mM tris(2-carboxyethyl)phosphine hydrochloride (TCEP). Following a 20-min incubation at 25 °C, TCEP was removed with a Micro Bio-Spin column 6 (Bio-Rad), and 1% SDS was added to the eluant. The cysteines previously participating in a disulfide bond, now reduced, were labeled with 1 mM polyethylene glycol-conjugated maleimide (molecular mass 10 kDa) (Fluka). After a 1-h incubation at 25 °C, proteins were precipitated with acetone, resuspended in 50 μl of non-reducing SDS electrophoresis buffer, and boiled for 10 min. Protein samples were then size-fractionated electrophoretically using SDS-PAGE, and transferred to a polyvinylidene fluoride membrane. The membrane was immunoblotted with an anti-ETB antibody to the region of ETB that contains Cys405 (amino acid sequence to which ETB antibody was raised: CLCCWCQSFEEKQSLEEKQSCLKFKANDHGYDNFRSSNKYSSS) (Santa Cruz Biotechnology). Bands were visualized using the ECL detection method.4
Animal model of PAH
Male Sprague-Dawley rats (age 12–14 weeks; Charles River Laboratories) were handled in accordance with US National Institutes of Health guidelines, and all procedures were approved by the local committee at Brigham and Women’s Hospital, Harvard Medical School. All surgeries were performed under ketamine/xylazine anesthesia. For the monocrotaline (MCT) model of PAH, rats were fed standard chow and treated with a 0.5 ml intraperitoneal injection of MCT (50 mg/ml) (Sigma-Aldrich) or 0.9% saline as control. Rats were randomized to spironolactone (25 mg/kg/d) (Henry Schein) or vehicle added to the drinking water. For the prevention study, treatment with spironolactone began immediately following administration of MCT and continued for 23–25 days until hemodynamic and tissue analyses were performed. For the reversal study, a second experiment was performed in which rats were randomized to spironolactone or vehicle that was initiated 14 days following the administration of MCT and continued until hemodynamic and tissue analyses were performed 10 days later.
For SU-5416/hypoxia-induced PAH, rats (~225 g) were administered a single subcutaneous injection of the vascular endothelial growth factor (VEGF)-2 inhibitor SU-5416 (20 mg/kg; Sigma) and exposed immediately to chronic hypoxia (barometric pressure, 410 mm Hg; inspired O2 tension 76 mm Hg) as described previously.10 Rats were randomized to either the selective mineralocorticoid receptor antagonist eplerenone (0.6 mg/ 1 gm standard chow; Test Diet Inc.) or standard chow as a control.11 Hemodynamic and tissue analyses were performed on all rats 21 days following exposure to chronic hypoxia.
Statistical analysis
Normality was tested using the Shapiro-Wilk test. When samples were normally distributed, results are expressed as mean ± SEM, and an unpaired t-test was used to compare two independent groups. Comparisons between multiple groups were made using a one-way analysis of variance (ANOVA) with post-hoc analysis performed using the protected Fisher LSD test. When data were not normally distributed, data are presented as median and range, and comparisons between two groups were made using the Mann-Whitney test. P <0.05 was considered significant.
Results
PAH is associated with increased plasma and lung tissue levels of ET-1 and aldosterone
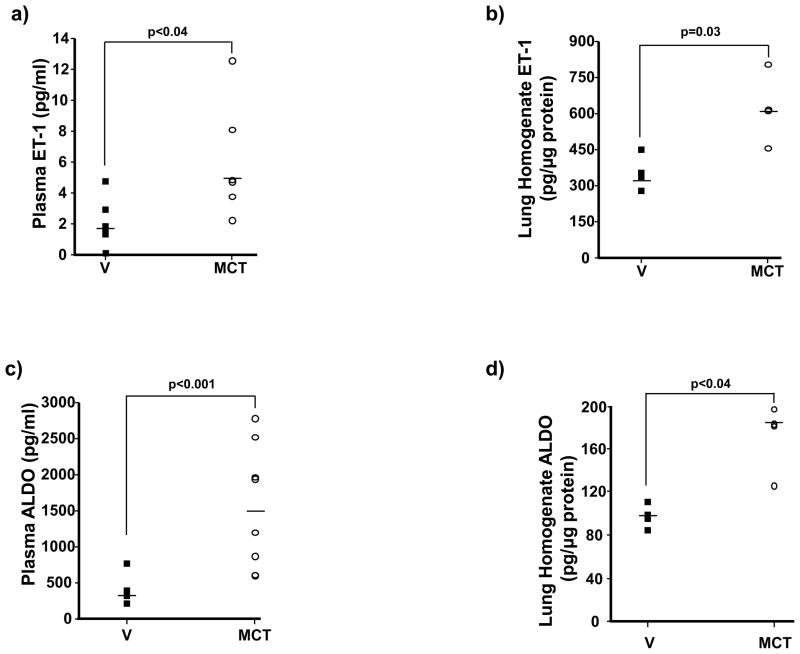
The Sprague Dawley rat monocrotaline (MCT) model of PAH was selected initially to test the hypothesis that hyperaldosteronism is present in PAH in vivo as MCT is believed to induce pulmonary hypertension through a mechanism that involves elevated levels of the aldosterone secretagogue ET-1.12 Transthoracic echocardiography demonstrated that compared to vehicle control (V)-treated rats, MCT decreased the pulmonary artery (PA) flow acceleration time (PAAT) (35.4 ± 2.6 vs. 14.1 ± 1.2 msec, p<0.005, n=6) and increased right ventricular (RV) free-wall thickness (0.58 ± 0.05 vs. 1.1 ± 0.05 mm, p<0.03, n=6). Right heart catheterization confirmed that MCT increased significantly pulmonary artery systolic pressure (PASP) (assumed to be equivalent to RV systolic pressure in the setting of a normal pulmonic valve) (28.3 ± 2.7 vs. 89.3 ± 5.3 mm Hg, p<0.01, n=6). In rats with PAH, there was a 274% increase in ET-1 levels in plasma (1.76 [non-detectable-4.82] vs. 4.83 [2.3–12.6] pg/ml, p<0.04, n=6) and a 183% increase in lung homogenates (335.5 [279.8–453.6] vs. 615.3 [458.4–806.5] pg/μg protein, p=0.03, n=4) (Figure 1a,b), which correlated with an increase in aldosterone levels of 442% (357.5 [223.0–784.0] vs. 1580.4 [611.4–2790.5] pg/ml, p<0.001, n=7–8 rats per condition) and 183% (100.0 [87.3–113.4] vs. 183.1 [126.1–197.4] pg/μg protein, p<0.04, n=4) in plasma and lung tissue, respectively (Figure 1c,d).
Figure 1.
Elevated levels of ET-1 are associated with hyperaldosteronism in PAH. (a,b) Levels of ET-1 (n=4–6) and (c,d) aldosterone (ALDO) (n=4–8) were measured in plasma and lung tissue homogenates of Sprague-Dawley rats 25 days following treatment with vehicle control (V) or monocrotaline (MCT) (50 mg/kg). Horizontal line represents the median for each condition.
The finding of increased aldosterone levels in lung tissue suggested that PAH may be associated with extraadrenal aldosterone synthesis. To determine if this occurred, we examined lungs for expression of the enzyme CYP11B2 (aldosterone synthase), which catalyzes the final and rate-limiting step in aldosterone steroidogenesis. Following saline perfusion of lungs prior to organ harvest, protein levels of CYP11B2 were increased significantly in lung tissue of rats with PAH compared to controls (483 ± 75 vs. 1319 ± 226 arb. units, p<0.03, n=4) (Supplemental Figure 1), indicating that it is plausible that elevated levels of aldosterone in lung tissue may also result from local synthesis of aldosterone in PAH.
Aldosterone increases pulmonary artery pressure and pulmonary vascular remodeling in PAH in vivo
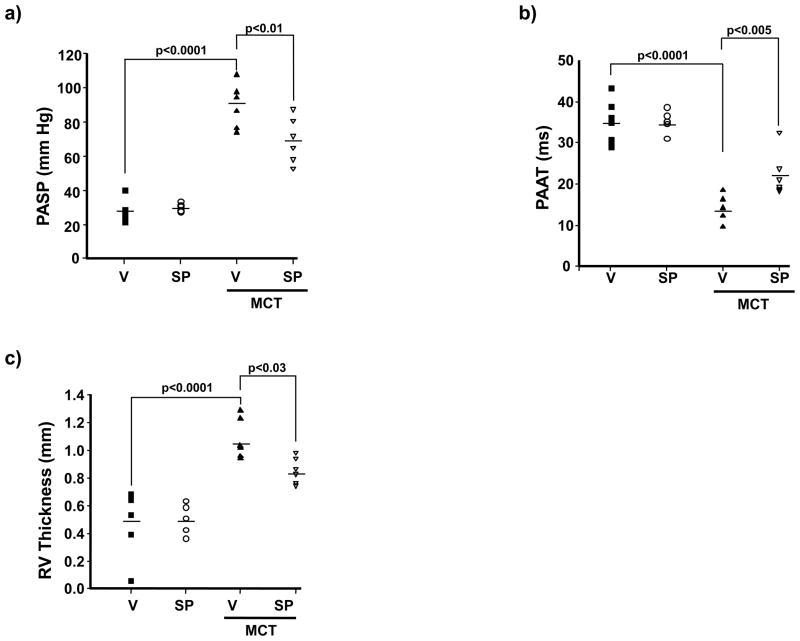
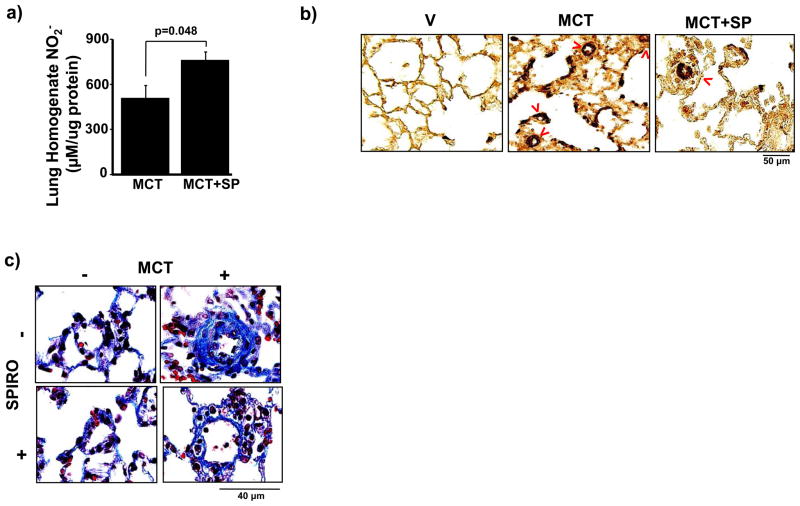
To determine if hyperaldosteronism contributes to increased pulmonary artery pressure in PAH in vivo and if mineralocorticoid receptor antagonism could prevent PAH, rats were treated with spironolactone (25 mg/kg/d) or V starting at the time of MCT injection. We observed that without significantly decreasing plasma ET-1 levels or influencing body weight, mean arterial pressure (MAP), or left ventricular end-diastolic pressure (LVEDP) (Supplemental Figure 2), spironolactone decreased PASP significantly in PAH (89.3 ± 5.2 vs. 69.5 ± 5.4 mm Hg, p<0.01, n=6) (Figure 2a), which was confirmed by an increase in PAAT (14.1 ± 1.2 vs. 22.3 ± 2.2 ms, p<0.005, n=6) (Figure 2b). Spironolactone also decreased RV free-wall thickness (1.07 ± 0.05 vs. 0.86 ± 0.03 mm, p<0.03, n=6) (Figure 2c) and RV weight (0.43 ± 0.07 vs. 0.35 ± 0.04 RV weight/LV septum weight, p=0.22, n=5) (Supplemental Figure 3). Notably, these findings were associated with increased levels of the stable NO• metabolite, nitrite (NO2−), in lung tissue specimens harvested from spironolactone-treated rats with PAH as compared to V-treated rats with PAH (759 ± 55 vs. 506 ± 86 μM/μg protein, p=0.048, n=4) indicating that spironolactone improved NO• bioavailability (Figure 3a).
Figure 2.
Aldosterone promotes PAH in vivo. Sprague-Dawley rats were treated with vehicle control (V) or monocrotaline (MCT) (50 mg/kg) and randomized immediately to V or spironolactone (SP) (25 mg/kg/d) for 25 days (n=6 rats per condition). The contribution of aldosterone to PAH was assessed by (a) right heart catheterization to measure pulmonary artery (assumed to be equivalent to right ventricular) systolic pressure (PASP); echocardiography to assess changes in (b) pulmonary artery acceleration time (PAAT); and, (c) right ventricular (RV) free-wall thickness. Horizontal line represents the mean for each condition.
Figure 3.
Spironolactone increases pulmonary vascular NO• levels and attenuates pulmonary vascular remodeling in PAH. (a) The effect of spironolactone (SP)(25 mg/kg/d) on pulmonary vascular NO• levels in PAH was assessed by measuring nitrite (NO2−) in lung tissue homogenates from Sprague-Dawley rats treated with vehicle control (V) or monocrotaline (MCT) (50 mg/kg) (n=4). (b) Tissue sections were stained with anti-smooth muscle cell α-actin antibody and the number of muscularized distal pulmonary arterioles (red arrows) was counted in 20 consecutive fields per section (100x magnification). Compared to V-treated rats with PAH, spironolactone decreased significantly the number of α-actin-stained muscularized distal pulmonary arterioles (76.0 [64–95] vs. 59.5 [59–61] muscularized pulmonary arterioles/20 high powered fields, p<0.005, n=5). (c) Gomori’s trichrome stain was performed on paraffin-embedded lung sections and perivascular collagen deposition in pulmonary arterioles measuring 20–50 μm located distal to terminal bronchioles (400x magnification) was measured. Compared to V-treated rats with PAH, spironolactone decreased perivascular collagen deposition by 77% (p<0.001, n=4–5 rats per condition). Representative photomicrographs are shown.
Spironolactone also prevented pathophenotypic changes to distal pulmonary arterioles [located distal to terminal bronchioles with diameters 20–50 μm13] as demonstrated by immunohistochemical staining for smooth muscle α-actin. Compared to V-treated rats with PAH, spironolactone decreased the number of α-actin-stained muscularized distal pulmonary arterioles (76.0 [64–95] vs. 59.5 [59–61] muscularized pulmonary arterioles/20 high powered fields, p<0.005, n=5) (Figure 3b), and increased significantly the cross-sectional luminal area of vessels (13.7 [12.7–16.1] vs. 36.8 [30.1–38.0] % cross sectional area, p<0.02, n=5). Furthermore, Gomori’s trichrome staining of rat lung sections revealed that, compared to V-treated rats with PAH, spironolactone decreased perivascular collagen deposition by 77% (p<0.001, n=4–5 rats per condition), similar to levels observed in rats without PAH (Figure 3c). Analysis using picrosirius red staining paralleled these findings, indicating that hyperaldosteronism contributed to perivascular collagen deposition (i.e., fibrillar collagen), which, in turn, is strongly associated with impaired vascular compliance in PAH (Supplemental Figure 4).1
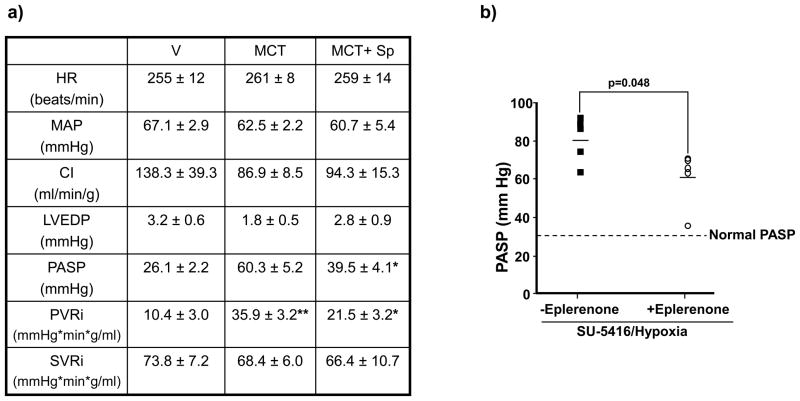
To determine if aldosterone antagonism reverses established PAH, a second study was performed in which V or spironolactone (25 mg/kg/d) was initiated 14 days following administration of MCT, a time point associated with histological evidence of MCT-induced inflammatory injury to distal pulmonary arterioles (Supplemental Figure 5). Compared to V-treated rats with PAH, spironolactone decreased levels of perivascular collagen by 71% (p=0.03, n=6), which was associated with a significant decrease in indexed pulmonary vascular resistance (PVRi) (35.9 ± 3.2 vs. 21.5 ± 3.2 mm Hg*min*g/ml, n=4, p<0.02) and PASP (60.3 ± 5.2 vs. 39.5 ± 4.1 mm Hg, n=6, p<0.005) without changes to heart rate, cardiac index (CI), LVEDP, MAP, or indexed systemic vascular resistance (SVRi) (Figure 4a).
Figure 4.
The effect of mineralocorticoid receptor antagonism on reversal or prevention of adverse cardiopulmonary hemodynamics in two models of experimental PAH. (a) In a reversal study, Sprague-Dawley rats were randomized to receive vehicle control (V) or spironolactone (SP) (25 mg/kg/d) 14 days following the administration of V or monocrotaline (MCT) (50 mg/kg), and cardiopulmonary hemodynamics were assessed by cardiac catheterization 10 days later. *p<0.02 vs. MCT, n=6 rats per condition; **p<0.04 vs. V, n=4 rats per condition. Data are presented as mean ± S.E. (b) In a prevention study, Sprague-Dawley rats were injected with SU-5416 and exposed to chronic hypoxia for 21 days. Immediately following exposure to hypoxia, rats were randomized to receive standard chow or eplerenone (0.6 gm/1 gm chow) until completion of the study. The effect of eplerenone on pulmonary artery systolic pressure (PASP) was assessed by cardiac catheterization (n=5 rats per condition). HR, heart rate; CI, cardiac index; LVEDP, left ventricular end-diastolic pressure; PVRi, pulmonary vascular resistance index; SVRi, systemic vascular resistance index.
Next, to confirm the role of aldosterone in a second animal model of PAH and to determine if there was a class effect for mineralocorticoid receptor antagonists, we studied the preventive effects of eplerenone on the development of abnormal cardiopulmonary hemodynamics in rats administered SU-5416 and exposed to chronic hypoxia for 21 days. Compared to normal rats, plasma aldosterone levels were increased by 397% in SU-5416/hypoxia-induced PAH (352.7 [223.0–557.2] vs. 1402.5 [542.3–2620.1], p<0.02, n=5). Eplerenone decreased perivascular collagen in SU-5416/hypoxia-induced PAH by 67% (n=5, p<0.02), which was associated with a decrease in PVRi (64.6 ± 21.4 vs. 43.9 ± 8.7 mm Hg*min*gm/ml, n=3–4 rats/condition, p=0.18) and PASP (80.5 ± 4.9 vs. 61.5 ± 6.5 mm Hg, p=0.048, n=5) (Figure 4b) without significantly influencing body weight, heart rate, MAP, CI, LVEDP, or SVRi. Collectively, our findings demonstrate that hyperaldosteronism modulates PAH and that a class effect exists among mineralocorticoid receptor antagonists for abrogating the adverse consequences of aldosterone on pulmonary vascular remodeling, PVRi, and PASP in two animal models of PAH in vivo.
ET-1 increases aldosterone levels in pulmonary artery endothelial cells
As ET-1 levels associated positively with lung CYP11B2 protein expression and aldosterone levels in MCT-induced PAH in vivo, we explored the possibility that ET-1 is an unrecognized stimulus of extraadrenal aldosterone synthesis in HPAECs in vitro. We first confirmed that compared to V-treated cells, ET-1 (1, 10, 100 nM) increased CYP11B2 protein expression levels (157.3 ± 27.5 vs. 180.4 ± 13.4 vs. 234.8 ± 4.3 % control, respectively, p<0.02, n=3) (Supplemental Figure 6a), which correlated with a concentration-dependent increase in aldosterone levels detected in the cell culture medium (241.1 ± 44.8 vs. 283.5 ± 94.7 vs. 396.0 ± 116.5 % control, respectively, p<0.04, n=4) (Supplemental Figure 6b). Consistent with prior reports in dispersed adrenal cortical cells,6,14 ET-1 increased aldosterone levels via activation of the ETB receptor in HPAECs (Supplemental Figure 7).
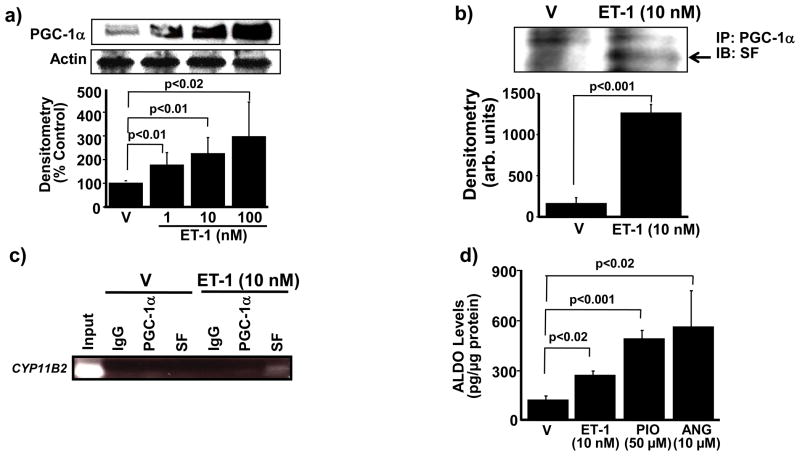
We next sought to determine the mechanism by which ET-1 increases aldosterone levels in HPAECs. In adrenal cortical Y-1 cells, the transcription factor PPAR-γ co-activator-1α (PGC-1α) interacts with the nuclear receptor protein steroidogenesis factor-1 (SF) to regulate CYP11B2 gene transcription and induce aldosterone synthesis.15 Therefore, to determine if ET-1 increased aldosterone synthase protein levels by this mechanism in HPAECs, we first explored the effect of ET-1 on PGC-1α and SF protein expression levels in these cells. Compared to V-treated cells, exposure to ET-1 (1, 10, 100 nM) for 24 h induced a concentration-dependent increase in PGC-1α protein expression levels (176. 5 ± 52.8 vs. 224.7 ± 68.1 vs. 296.7 ± 145.8 % control, respectively, p<0.02, n=3) (Figure 5a). ET-1 had no effect on SF protein levels; however, ET-1 did increase the association between PGC-1α and SF as demonstrated by co-immunoprecipitation (1260 ± 104 vs. 160 ± 71 arb. units, p<0.001, n=3) (Figure 5b).
Figure 5.
ET-1 stimulates PGC-1α-dependent association of SF with CYP11B2 to increase aldosterone levels. (a) The effect of ET-1 on PGC-1α expression was assessed by Western analysis (n=4). (b) Co-immunoprecipitation experiments demonstrated that incubation of HPAECs with ET-1 (10 nM) for 24 h induced the association of PGC-1α with steroidogenesis factor-1 (SF) (n=3). (c) Chromatin immunoprecipitation (n=3) of cell lysates using antibodies to PGC-1α, SF, and immunoglobulin-G (IgG) as a negative control was followed by PCR amplification of the proximal region of the CYP11B2 promoter region containing the gonadotrope-specific element. (d) The functional effect of PGC-1α stimulation on aldosterone production was assessed in cells treated with the selective PGC-1α agonist pioglitazone (50 μM) for 24 h (n=4), or with ET-1 (10 nM) or angiotensin II (ANG)(10 μM) for 24 h as positive controls. PGC-1α, PPAR-γ co-activator-1α; arb. units, arbitrary units; IP, immunoprecipitation, IB; immunoblot. Data are presented as mean ± S.E.M. Representative blots are shown.
We next performed a chromatin immunoprecipitation assay to assess the effect of ET-1 (10 nM) for 24 h on PGC-1α and/or SF association with the CYP11B2 promoter. PGC-1α alone did not bind to the CYP11B2 promoter in cells treated with either V or ET-1; however, compared to V, ET-1 induced a significant increase in SF binding to the CYP11B2 promoter (16.3 ± 9.8 vs. 61.6 ± 9.3 arb. units, p<0.03, n=3) (Figure 5c). Collectively, these data indicate that ET-1 stimulates PGC-1α binding with SF, which, in turn, promotes the association of SF to the promoter region of CYP11B2 to upregulate CYP11B2 protein expression levels. We confirmed that PGC-1α stimulation is linked functionally to aldosterone synthesis in cells treated with the selective PGC-1α agonist pioglitazone (50 μM) for 24 h, which, compared to V, increased aldosterone levels by 365% (p<0.001, n=3) (Figure 5d). Thus, ET-1 increases extraadrenal aldosterone synthesis in endothelial cells via upregulation of CYP11B2 in a PGC1-α/SF-dependent manner.
Aldosterone increases oxidant stress in HPAECs
Next, to determine if hyperaldosteronism in PAH could contribute to pulmonary vascular dysfunction akin to what we observed previously in the systemic vasculature,4,7 we investigated the effect of aldosterone on ROS levels in HPAECs. Cells were exposed to increasing concentrations of aldosterone (10−9, 10−8, 10−7 mol/L) for 12–36 h and H2O2 levels were measured by Amplex Red assay. Compared to V-treated cells, maximal H2O2 accumulation was observed in cells treated with aldosterone (10−7 mol/L) for 24 h (65.4 ± 1.6 vs. 100.6 ± 3.5 μM/mg protein, p<0.001, n=3); this effect was abrogated by 56% in aldosterone-treated cells coincubated with spironolactone (p<0.01, n=3), indicating that a majority of aldosterone-induced H2O2 formation was due to mineralocorticoid receptor activation (Supplemental Figure 8a). As no further H2O2 generation was observed in aldosterone-treated cells beyond 24 h, subsequent experiments were performed at this time point using (patho)physiologically relevant levels of aldosterone similar to those observed in MCT- or SU-5416/hypoxia-treated rats with PAH in vivo. Furthermore, the observed increase in ROS was due to aldosterone, and not ET-1, as ET-1 (10 nM) had no effect on H2O2 levels compared to V-treated cells (p=0.43, n=4).
NADPH oxidase type 4 (NOX4) is implicated as a key source of vascular ROS generation in pulmonary hypertension and human vascular endothelial cells exposed to pathophysiologic concentrations of aldosterone.16,17 The primary product of NOX4 activation is H2O2, and its formation is closely aligned to changes in NOX4 protein expression.17 Therefore, we examined the effect of aldosterone on NOX4 expression in HPAECs as a potential mechanism to explain the aldosterone-mediated increase in H2O2 formation. Compared to V-treated cells, aldosterone (10−9, 10−8, 10−7 mol/L) increased protein levels of NOX4 (134.6 ± 16.5 vs. 146.3 ± 12.4 ± vs. 157.0 ± 4.4 % control, respectively, p<0.02, n=3) and of p22phox (1009.4± 167.0 vs. 961 ± 226.2 vs. 829.5 ± 295.6 % control, respectively, p<0.01, n=3), a NOX4 subunit that is required for NOX4-mediated H2O2 formation, in a concentration-dependent manner (Supplemental Figure 8b,c).
Aldosterone decreases ETB-dependent activation of eNOS and NO• levels
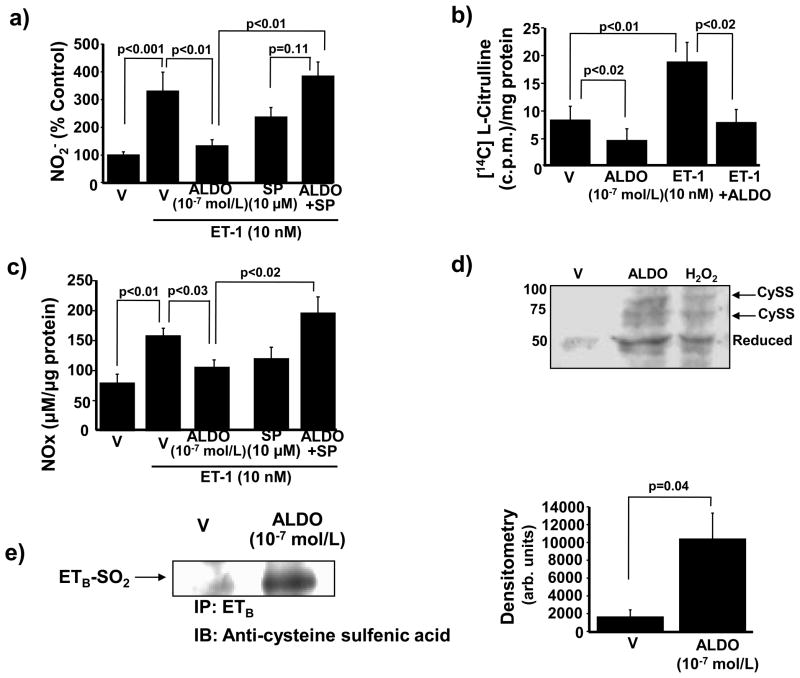
We next investigated the effect of ETB receptor activation by ET-1 (10 nM) on levels of the NO• metabolite nitrite (NO2−). Compared to V-treated cells, ET-1 increased NO2− generation with a maximum effect observed at 10 min (29.4 ± 3.6 vs. 139.4 ± 31.8 μM/μg protein, p<0.001, n=3). We then evaluated the effect of aldosterone on ETB-stimulated NO• levels. Without influencing protein expression of ETB, or inducing expression of ETA (which is not constitutively expressed in HPAECs)18 (Supplemental Figure 9), exposure to aldosterone (10−7 mol/L) for 24 h decreased ETB-mediated NO2− levels by 60.3% (p<0.01, n=3). Coincubation with spironolactone (10 μM) restored NO2− levels to those observed in cells stimulated with ET-1 in the absence of aldosterone (Figure 6a).
Figure 6.
Aldosterone decreases ETB-dependent synthesis of NO•. (a) HPAECs were exposed to vehicle (V) or aldosterone (ALDO) (10−7 mol/L) for 24 h in the presence or absence of spironolactone (SP) (10 μM) and NO2− formation was assessed. Prior to analysis, cells were exposed to ET-1 (10 nM) for 10 min to stimulate ETB signaling (n=4). (b) The effect of ALDO on ETB-dependent activation of eNOS was determined (n=4). c.p.m., counts per minute. (c) The effect of ALDO on ETB-dependent NO• generation was assessed by measuring total NO• metabolite levels (NOx: NO2− + NO3−) (n=3). (d) HPAECs were exposed to V, hydrogen peroxide (H2O2) (200 μM) for 20 min, or ALDO (10−7 mol/L) for 24 h to assess changes to the redox status and de novo disulfide bond formation by ETB cysteinyl thiols. For each disulfide formed, a 20-kDa shift in band location of the reduced ETB protein occurs on the Western blot using an antibody specific to the region of ETB containing Cys405 (n=4). Cyss, disulfide bond. A representative blot is shown. (e) The region of ETB containing Cys405 was immunoprecipitated from cells treated with V or ALDO (10−7 mol/L) for 24 h and immunoblotting was performed to detect differences in protein sulfenic acid levels (R-SOH) (n=3). IP, immunoprecipitation, IB; immunoblot. Data are presented as mean ± S.E.M. Representative blots are shown.
We and others have demonstrated previously that in the absence of oxidant stress, NO• metabolism to NO2− and nitrate (NO3−) occurs in a ratio that favors NO2− by approximately 2:1, but that this ratio shifts in favor of increased NO3− formation in the presence of superoxide anion (•O2−), owing to the interaction of NO2− with •O2− to generate peroxynitrate (O2NOO−)7 or via tautomerization of peroxynitrite (ONOO−) to NO3−.19 In HPAECs, ET-1 alone did not affect the NO2−/NO3− ratio significantly compared to V. In contrast, exposure to aldosterone decreased the NO2−/NO3− ratio by 62% in ET-1-stimulated cells, which was restored fully by coincubation of aldosterone with spironolactone (p<0.04, n=3) (Supplemental Figure 10a). This effect was likely mediated by increased ONOO− formation as aldosterone-treated HPAECs had increased levels of 3-nitrotyrosine, a marker of ONOO−, compared to cells stimulated with V or ET-1 alone (24.1 ± 3.3 vs. 31.2 ± 3.2 vs. 46.8 ± 6.6 arb. units, p<0.02, n=5) (Supplemental Figure 10b).
We also examined the effect of aldosterone on ET-1-stimulated eNOS activity. Without influencing eNOS protein levels, aldosterone decreased eNOS activity in ET-1 (10 nM) stimulated cells (18.5 ± 3.5 vs. 7.6 ± 2.4 [14C] L-citrulline c.p.m./mg protein, n=3, p<0.02) (Figure 6b), leading to a decrease in total NO• metabolite (NOx: NO2− + NO3−) formation (157.9 ± 12.7 vs. 103.4 ± 12.2 μM/μg protein, p<0.01, n=3). Coincubation with spironolactone increased NOx levels in aldosterone-treated cells stimulated with ET-1 by 87% (p<0.02, n=3) (Figure 6c). Taken together, these data demonstrate that aldosterone diminished levels of bioavailable NO• in ET-1-stimulated cells by decreasing ET-1-mediated eNOS activity to limit NO• generation, increasing ONOO− formation, and by oxidizing NO2− to NO3−. Aldosterone decreases ETB-dependent NO• levels by oxidative modification of Cys405.
Given that aldosterone decreased ET-1-stimulated eNOS activity and NO• generation, we postulated that aldosterone affected ETB receptor function. As aldosterone induced H2O2 formation and ETB contains functionally essential cysteinyl thiol residues in its eNOS-activating region, it is plausible that aldosterone may induce an oxidative post-translational modification of ETB that influences receptor function. To examine ETB for oxidation of cysteinyl thiols, protein extracts from HPAECs were treated with V, aldosterone (10−7 mol/L) for 24 h, or H2O2 (200 μmol/L) for 20 min, and free thiols were blocked with iodoacetamide and N-ethylmaleimide. Disulfides were reduced with TCEP hydrochloride, and previously oxidized (now reduced) cysteines were labeled with PEG-conjugated maleimide (molecular mass 10 kDa). In this way, each reduced disulfide bond yields a shift in the apparent molecular mass of the reduced protein by 20 kDa. Western analysis using an antibody specific to the region of ETB containing Cys405 revealed that only the reduced form of ETB was present (50 kDa) in V-treated cells; however, bands at 70 kDa and 90 kDa were evident in cells treated with H2O2 or aldosterone, indicating the de novo formation of one or two disulfide bonds under these conditions of increased oxidant stress (Figure 6d).
To support these findings, we determined if aldosterone modulates the formation of other higher oxidative intermediates of ETB Cys405. Cells were treated V or aldosterone (10−7 mol/L) for 24 h and the region of ETB containing Cys405 was immunoprecipitated using the specific ETB containing Cys405 antibody (Santa Cruz). Western analysis using an anti-sulfenic acid (R-SOH) antibody (derivatized with dimedone)20 (Millipore) revealed that compared to V-treated cells, aldosterone increased ETB-SOH protein expression levels by 639% (p=0.04, n=3)(Figure 6e).
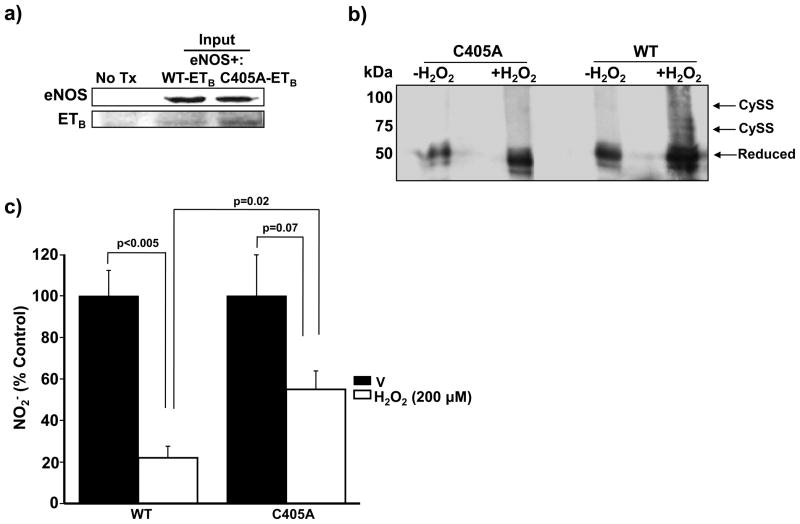
To confirm that oxidative modification of Cys405 has functional implications for ETB-dependent NO• generation, we transiently transfected COS-7 cells with human DNAs coding for wild type (WT)-eNOS and WT-ETB or a mutant ETB containing a substitution of cysteine with alanine, which is insensitive to oxidant stress, at position 405 (C405A-ETB). Expression of transiently transfected WT-eNOS and WT-ETB or C405A-ETB DNA was established by immunoblotting (Figure 7a). Additionally, immunoblotting of PEG-conjugated maleimide-labeled extracts confirmed that compared to WT-ETB, in which H2O2 (200 μmol/L for 20 min) induced the formation of one or two disulfide bonds, C405A-ETB was resistant to the formation of disulfide bonds (Figure 7b). Next, COS-7 cells expressing eNOS and WT-ETB or C405A-ETB were exposed to H2O2 (200 μmol/L) for 60 min and ETB-dependent NO• synthesis was assessed. This treatment time point was selected because activation of eNOS by H2O2 is time-dependent and attenuated fully within 60 min following exposure of eNOS to H2O2.21 After this time, the medium was replaced and cells were treated with ET-1 (10 nM) for 10 min to stimulate ETB signal transduction. Although exposure to H2O2 decreased ET-1-stimulated NO2− formation by 78.0% in WT-ETB transfected cells compared to V-treated cells (p<0.005, n=4), this effect was attenuated significantly in C405A-ETB-transfected cells in which H2O2 decreased nitrite levels by only 45.0% compared to V-treated cells (p=0.07, n=4) (Figure 7c). Taken together, these data confirm that Cys405 is a redox sensitive, functional cysteinyl thiol whose oxidation to sulfenic acid impairs ETB-dependent NO• generation.
Figure 7.
Oxidation of Cys405 impairs ETB-dependent NO• generation. (a) COS-7 cells were transiently transected with wild type (WT)-eNOS and WT-ETB or mutant ETB DNA containing a substitution of alanine for cysteine at position 405 (C405A-ETB) and protein expression was confirmed. No Tx, untransfected. (b) Disulfide bond formation was assessed by Western immunoblotting of PEG-conjugated maleimide-labeled cell extracts exposed to H2O2 (200 μmol/L for 20 min). Compared to WT-ETB-transfected cells, in which H2O2 (200 μmol/L for 20 min) induced the formation of 1 or 2 disulfide bonds, C405A-ETB was resistant to disulfide bond formation (n=4) Cyss, disulfide bond. (c) COS-7 cells expressing WT-eNOS and WT-ETB or C405A-ETB were exposed to vehicle (V) control or hydrogen peroxide (H2O2) (200 μmol/L) for 60 min. After that time, the cell culture medium was replaced and cells were treated with ET-1 (10 nM) for 10 min. and nitrite (NO2−) levels were measured (n=4). Data are presented as mean ± S.E.M. Representative blots are shown.
Discussion
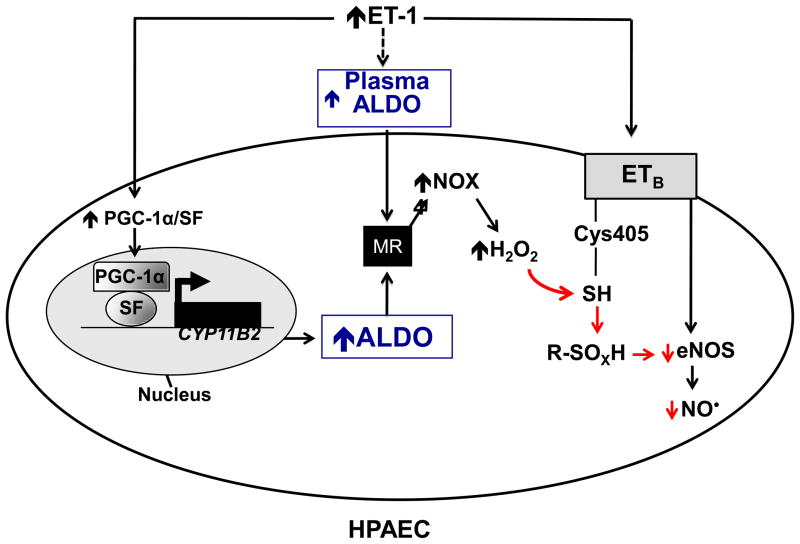
In this study, we found that elevated levels of ET-1 in PAH are associated with increased plasma and lung tissue levels of aldosterone, indicating that the pathophysiological effects attributed to ET-1 may, in part, occur as a result of systemic and local hyperaldosteronism. This conclusion was confirmed in vivo by demonstrating that the mineralocorticoid receptor antagonists spironolactone or eplerenone, given in the absence of ET-1 blockade, decreased PASP, RV hypertrophy, PVRi, and pulmonary vascular remodeling. These effects did not occur as a result of changes in left-sided hemodynamics or differences in plasma ET-1 levels as a result of mineralocorticoid receptor blockade. We demonstrated that ET-1 increases aldosterone levels through a mechanism that involves upregulation of CYP11B2, the rate-limiting enzyme in aldosterone synthesis, in a PGC-1α/SF-dependent manner. The functional consequences of elevated aldosterone levels include increased oxidant stress and decreased bioavailable NO•. Although diminished NO• levels resulted, in part, from its consumption by ROS as demonstrated by an increase in ONOO− formation, we also found a novel mechanism to explain the aldosterone-mediated decrease in ET-1-stimulated NO• formation: oxidation of cysteinyl thiols (Cys405) in the eNOS-activating region of the ETB receptor (to sulfenic acid and the disulfide form) (Figure 8). Thus, aldosterone contributes to high pulmonary vascular tone by oxidizing cysteinyl thiols in ETB, which, in turn, acts as a redox switch to impair ET-1-stimulated endothelial NO• generation.
Figure 8.
A proposed mechanism by which hyperaldosteronism decreases pulmonary endothelial eNOS activation and NO• generation in PAH. Hyperaldosteronism (ALDO) in pulmonary arterial hypertension (PAH) may occur via i) endothelin-1 (ET-1)-mediated activation of PPARγ coactivator-1α (PGC-1α)/steroidogenesis factor-1 (SF) to increase CYP11B2 (aldosterone synthase) gene transcription in HPAECs, and/or ii) upregulation of adrenal ALDO synthesis via ET-1 and/or overactivation of the renin-angiotensin pathway. Stimulation of the mineralocorticoid receptor (MR) in HPAECs by ALDO activates NADPH oxidase type 4 (NOX4) to increase levels of hydrogen peroxide (H2O2), which, in turn, oxidatively modifies redox sensitive, functional cysteinyl thiol(s) in the ETB receptor (Cys405) to impair ETB-dependent activation of eNOS and decrease synthesis of nitric oxide (NO•). eNOS, endothelial nitric oxide synthase; R-SOXH, higher oxidative intermediaries of cysteine.
Other studies have linked hyperaldosteronism to end-stage disease in idiopathic pulmonary hypertension.22 Although studies to date examining the role of mineralocorticoid receptor antagonism in PAH are limited to case reports,23 recently a clinical trial was announced to examine the hypothesis that secondary hyperaldosteronism modulates the adverse effects of PAH leading to RV failure. In this study, patients will be treated with spironolactone and the effect of mineralocorticoid receptor blockade on pulmonary hemodynamics and World Health Organization functional class will be examined.24 While this study focuses on the efficacy of aldosterone antagonism once PAH is established, our data suggest that aldosterone antagonism may also have benefit when started early in the disease course.
We implicate ET-1 as the stimulus for increased lung tissue and plasma aldosterone levels in PAH. Using the MCT rat model of PAH, we confirmed a 3-fold increase in plasma ET-1 levels, which supports prior studies that reported an increase in ET-1 levels and showed that ET-1 contributed to the pathogenesis of PAH.12 The levels of ET-1 that we observed were 1000-fold higher than that required to stimulate aldosterone secretion from adrenocortical cells in vitro.6 Furthermore, the levels of plasma ET-1 measured in this study, akin to those observed in patients with PAH,25 were sufficient to increase plasma aldosterone levels by 442%. These plasma aldosterone levels are similar to what have been observed in patients with left-sided congestive heart failure and secondary pulmonary hypertension.26,27 Moreover, our study likely underestimated the maximal level of hyperaldosteronism achieved in PAH as we measured plasma levels antecedent to advanced stage disease, which is associated with decreased cardiac output vis-à-vis cor pulmonale that results in a decline in PASP and compensatory (over)activation of the renin-angiotensin-aldosterone system.27
The mechanism by which ET-1 stimulates aldosterone secretion in HPAECs involved upregulation of the expression of CYP11B2, the rate-limiting enzyme in aldosterone biosynthesis. The concept of extraadrenal aldosterone synthesis by the vascular endothelium remains controversial. CYP11B2 expression in human pulmonary vascular endothelial and smooth muscle cells has been demonstrated and shown to be responsive to angiotensin II or potassium resulting in an increase in local aldosterone production.28 In contrast, other studies performed in HPAECs failed to show an effect of angiotensin II on CYP11B2 transcription or aldosterone production;29 however, these studies were performed on cells at passage 14 or older, which may adversely affect global vascular endothelial mRNA and protein expression levels. Moreover, this earlier study measured aldosterone production using an assay with a lower limit of detection reported to be 20 pg/ml. Our study utilized a more sensitive assay with a lower limit of detection of 7 pg/ml. Our observation that CYP11B2 expression was increased via upregulation of PGC-1α and its association with SF at the promoter region of the CYP11B2 gene confirms prior work in adrenal cortex-derived Y1 cells that demonstrated a similar mechanism of CYP11B2 upregulation.15 We were also able to provide additional evidence for this mechanism by PGC-1α agonism with the thiazolidinedione, pioglitazone. Notwithstanding this finding, the relationship between thioglitazone/PGC-1α and aldosterone remains unresolved. In one study performed in healthy volunteers, pioglitazone treatment for 6 weeks increased circulating aldosterone levels,30 whereas rosiglitazone has been linked to aldosterone-independent plasma volume expansion through inhibition of sodium transport in the renal collecting duct.31 More recently, studies performed in adrenocortical H295R cells demonstrated that pioglitazone suppressed CYP11B2 expression in angiotensin II-stimulated cells, and this effect was associated with a modest decrease in aldosterone secretion.32 Although we now report differing results, we believe they may be attributable to the cell types studied as well as the duration of exposure to pioglitazone and angiotensin II.
We and others have shown previously that the adverse effects of aldosterone on the systemic vasculature include increased oxidant stress and decreased bioavailable NO• that promotes endothelial dysfunction and impairs vascular reactivity.4, 7, 26, 33 Our finding of increased pulmonary endothelial oxidant stress is not surprising as others have reported an increase in reactive oxygen species production owing to increased NOX1 expression in the small muscularized arteries isolated from the MCT-rat model of PAH.34 Here, we focused selectively on oxidant stress in the endothelium and found an increase in expression of NOX4 and the NOX4 subunit p22phox, indicating that both NOX1 and NOX4 systems may be operative in PAH. Furthermore, our in vitro studies attribute this increase in NADPH oxidase activity to aldosterone and not to ET-1. Conversely, other studies have reported that ET-1 decreased H2O2 production in fetal pulmonary artery endothelial cells in an ETB-dependent manner;35 however, these studies were not performed in a timeframe that would afford upregulation of aldosterone synthesis by ET-1.
While there is a consensus opinion that PAH is associated with a decrease in eNOS activity and bioavailable NO•, several mechanisms have been demonstrated to explain this phenomenon. In the setting of increased oxidant stress, NO• reacts with superoxide to form ONOO−, which we observed in our study. Other mechanisms include uncoupling of eNOS to form superoxide in preference to NO•, upregulation of arginase II,36 oxidation of tetrahydrobiopterin,37 altered S-nitrosoglutathione reductase activity,38 and caveolin-1 deficiency.39
We now identify an additional mechanism to explain the decrease in eNOS activity and bioavailable NO•: dysfunctional ET-1/ETB-eNOS signaling in the setting of elevated aldosterone levels owing to oxidative posttranslational modification of redox-sensitive cysteinyl thiol(s) in the ETB receptor. Oxidation of cysteine residues to form higher oxidative intermediates of cysteine, including sulfenic acid and the disulfide form, is known to occur under conditions of oxidant stress and to regulate protein function.40 The ETB receptor is a 7 transmembrane domain G-coupled protein receptor with a carboxy-terminal cystoplasmic tail that contains 3 functional cysteine residues: Cys402, Cys403, and Cys405.9 Here, we report that these cysteines are oxidatively modified, which is associated with functional consequences for ETB-dependent eNOS activity. It is known that these cysteines are subject to posttranslational modification, such as palmitoylation, and site-directed mutagenesis has revealed that palmitoylation is required for coupling with Gi but not Gq subunits.41 In the current work, the relationship between ETB cytoplasmic tail cysteines, palmitoylation, and eNOS was not explored. Interestingly, an in vitro study using a synthetic peptide of ETB constructed to contain residues 390–409, and, therefore, including the 3 cytoplasmic tail cysteines, was shown to bind eNOS and inhibit its activity with an EC50 of 3 & 1.8 μM.42 While that study did not examine the cysteinyl residues directly for posttranslational modification(s), our observation that oxidative posttranslational modification of ETB Cys405 is associated with impaired ETB-dependent NO• generation suggests that this cysteine functions as a redox switch to modulate eNOS activity. In support of this concept is our observation that site-directed mutagenesis of Cys405 rendered ETB resistant to oxidant stress-induced sulfenic acid and disulfide formation, and, as a result, improved redox-sensitive signaling. It is, however, important to acknowledge that our methods did not utilize liquid chromatography-mass spectrometry, which is required to definitively characterize this effect. Moreover, our observation that site-directed mutagenesis of Cys405 alone restored ETB-dependent NO• generation incompletely in the presence of pathological concentrations of H2O2 suggests further that Cys402 and Cys403 may also be redox sensitive cysteinyl thiols involved in ETB-eNOS signal transduction. Our finding that cysteines in the ETB cytoplasmic tail are oxidatively modified is also supported by the observation that global sulfhydryl (-SH) levels are decreased in lung tissue isolated from rats with MCT-induced PAH compared to controls.43
Our results may account, in part, for limitations in the clinical efficacy of endothelin receptor antagonism for patients with PAH, viz., currently available ETreceptor antagonists are believed to improve pulmonary vascular tone primarily by attenuating ETA-mediated pulmonary vasoconstriction in pulmonary vascular smooth muscle cells, and, therefore, these drugs do not address the potential contribution of abnormal ETB signal transduction in HPAECs to pulmonary vascular dysfunction in PAH. Along these lines, our findings suggest that by preventing aldosterone-induced oxidation of ETB, mineralocorticoid receptor antagonism preserves normal ET-1-ETB vasodilatory signaling to maintain levels of NO• in HPAECs and attenuate pulmonary vascular remodeling in PAH in vivo (Figure 8). Importantly, however, the effect of spironolactone/eplerenone on the development of plexiform lesions was not specifically addressed in this study. Thus, the role of mineralocorticoid receptor antagonists in modulating cardiopulmonary hemodynamic improvements in forms of PAH that are characterized primarily by the plexiform arteriopathy remains unknown.
In summary, we identify aldosterone as an unrecognized biological intermediate that modulates the adverse vascular effects of ET-1 in PAH. We describe a novel mechanism by which to explain the defect in ET-1/ETB-eNOS signaling associated with PAH: oxidative posttranslational modification of the ETB receptor. Our observations demonstrate further that a class effect exists for mineralocorticoid receptor antagonists and that these agents ameliorate the PAH phenotype by improving pulmonary hemodynamics and (mal)adaptive pulmonary vascular remodeling. Collectively, these findings suggest that mineralocorticoid receptor antagonism in PAH may represent a novel pharmacotherapeutic strategy to improve pulmonary vascular dysfunction and its attendant sequelae in patients with PAH.
Supplementary Material
Clinical Summary.
Despite recent advances in diagnosis and treatment, pulmonary arterial hypertension (PAH) remains a devastating disease that is associated with a 10% mortality rate within the first year of diagnosis. Currently available pharmacotherapies based on known biological mediators of the disease are limited and in certain cases have waning long-term efficacy. In this study, we identify aldosterone as a novel contributor to the pathobiology of PAH and demonstrate that mineralocorticoid receptor antagonism is efficacious in the prevention and reversal of experimental PAH. We describe a novel mechanism for the increase in pulmonary aldosterone levels whereby elevated levels of endothelin-1, which have been observed in PAH, function as a potent stimulator of adrenal and extraadrenal aldosterone synthesis to modulate pulmonary vascular dysfunction. Our findings demonstrate that aldosterone-induced oxidant stress impairs endothelin-B (ETB) receptor signal transduction to diminish ETB-dependent nitric oxide synthesis in pulmonary artery endothelial cells in vitro and promote negative remodeling of pulmonary arterioles and pulmonary vascular dysfunction in two experimental rat models of PAH in vivo. Importantly, mineralocorticoid receptor antagonism with spironolactone or eplerenone prevented or reversed the adverse effects of hyperaldosteronism on pulmonary vascular remodeling and improved pulmonary vascular resistance, pulmonary artery pressure, and remodeling of the right ventricle. Moreover, our findings relating to the potential benefit of spironolactone or eplerenone in attenuating pulmonary vascular dysfunction in PAH may support future clinical trials and/or repurposing of mineralocorticoid receptor antagonists, which are already an accepted medical therapy in patients with certain cardiovascular diseases, to those patients afflicted with PAH and other pulmonary vascular diseases with similar pathobiology.
Acknowledgments
The authors thank Ms. Stephanie Tribuna for technical assistance with this manuscript.
Funding Sources: This work was supported in part by the US National Institutes of Health (grants HL105301 to J.A.L.; HL61795, HL48743, HL107192, HL070819, and HL108630 to J.L.; T32 HL007604 to B.A.M.) and the American Heart Association (11POST6720000 to B.A.M.).
Footnotes
Conflict of Interest Disclosures:
None.
References
- 1.Archer SL, Weir EK, Wilkins MR. Basic science of pulmonary arterial hypertension for clinicians: new concepts and experimental therapies. Circulation. 2010;121:2045–2066. doi: 10.1161/CIRCULATIONAHA.108.847707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Farber HW, Loscalzo J. Pulmonary arterial hypertension. N Engl J Med. 2004;351:1655–1665. doi: 10.1056/NEJMra035488. [DOI] [PubMed] [Google Scholar]
- 3.Michelakis ED. The role of the NO axis and its therapeutic implications in pulmonary arterial hypertension. Heart Fail Rev. 2003;8:5–21. doi: 10.1023/a:1022150819223. [DOI] [PubMed] [Google Scholar]
- 4.Leopold JA, Dam A, Maron BA, Scribner AW, Liao R, Handy DE, Stanton RC, Pitt B, Loscalzo J. Aldosterone impairs vascular reactivity by decreasing glucose-6-phosphate dehydrogenase activity. Nat Med. 2007;13:189–197. doi: 10.1038/nm1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Weber KT. Aldosterone in congestive heart failure. N Engl J Med. 2001;345:1689–1697. doi: 10.1056/NEJMra000050. [DOI] [PubMed] [Google Scholar]
- 6.Rossi GP, Albertin G, Neri G, Andreis PG, Hofmann S, Pessina AC, Nussdorfer GG. Endothelin-1 stimulates steroid secretion of human adrenocortical cells ex vivo via both ETA and ETB receptor subtypes. J Clin Endocrinol Metab. 1997;82:3445–3449. doi: 10.1210/jcem.82.10.4279. [DOI] [PubMed] [Google Scholar]
- 7.Maron BA, Zhang YY, Handy DE, Beuve A, Tang SS, Loscalzo J, Leopold JA. Aldosterone increases oxidant stress to impair guanylyl cyclase activity by cysteinyl thiol oxidation in vascular smooth muscle cells. J Biol Chem. 2009;284:7665–7672. doi: 10.1074/jbc.M809460200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hirata Y, Emori T, Eguchi S, Kanno K, Imai T, Ohta K, Marumo F. Endothelin receptor subtype B mediates synthesis of nitric oxide by cultured bovine endothelial cells. J Clin Invest. 1993;91:1367–1373. doi: 10.1172/JCI116338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Okamoto Y, Ninomiya H, Tanioka M, Sakamoto A, Miwa S, Masaki T. Palmitoylation of human endothelinB. Its critical role in G protein coupling and a differential requirement for the cytoplasmic tail by G protein subtypes. J Biol Chem. 1997;272:21589–21596. doi: 10.1074/jbc.272.34.21589. [DOI] [PubMed] [Google Scholar]
- 10.Oka M, Homma N, Taraseviciene-Stewart L, Morris KG, Kraskauskas D, Burns N, Voelkel NF, McMurty IF. Rho kinase-mediated vasoconstriction is important in severe occlusive pulmonary arterial hypertension in rats. Circ Res. 2007;100:923–929. doi: 10.1161/01.RES.0000261658.12024.18. [DOI] [PubMed] [Google Scholar]
- 11.Guo C, Ricchiuti V, Lian BQ, Yao TM, Coutinho P, Romero JL, Li J, Williams GH, Adler GK. Mineralocorticoid receptor blockade reverses obesity-related changes in expression of adiponectin, peroxisome proliferator-activated receptor-gamma, and proinflammatory adipokines. Circulation. 2008;117:2253–2261. doi: 10.1161/CIRCULATIONAHA.107.748640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang TT, Cui B, Dai DZ, Su W. CPU 86017, p-chlorobenzyltetrahydroberberine chloride, attenuates monocrotaline-induced pulmonary hypertension by suppressing endothelin pathway. Acta Pharmacol Sin. 2005;26:1309–1316. doi: 10.1111/j.1745-7254.2005.00214.x. [DOI] [PubMed] [Google Scholar]
- 13.Jones JE, Walker JL, Song Y, Weiss N, Cardoso WV, Tuder RM, Loscalzo J, Zhang YY. Effect of 5-lipoxygenase on the development of pulmonary hypertension in rats. Am J Physiol Heart Circ Physiol. 2004;286:H1775–1784. doi: 10.1152/ajpheart.00281.2003. [DOI] [PubMed] [Google Scholar]
- 14.Morishita R, Higaki J, Ogihara T. Endothelin stimulates aldosterone biosynthesis by dispersed rabbit adreno-capsular cells. Biochem Biophys Res Commun. 1989;160:628–632. doi: 10.1016/0006-291x(89)92479-0. [DOI] [PubMed] [Google Scholar]
- 15.Zhu L, Ke Y, Shao D, Cui Y, Qiao A, Liu X, Fang F, Chang Y. PPARgamma co-activator-1alpha co-activates steroidogenic factor 1 to stimulate the synthesis of luteinizing hormone and aldosterone. Biochem J. 2010;432:473–483. doi: 10.1042/BJ20100460. [DOI] [PubMed] [Google Scholar]
- 16.Hashikabe Y, Suzuki K, Jojima T, Uchida K, Hattori Y. Aldosterone impairs vascular endothelial cell function. J Cardiovasc Pharmacol. 2006;47:609–613. doi: 10.1097/01.fjc.0000211738.63207.c3. [DOI] [PubMed] [Google Scholar]
- 17.Touyz RM, Briones AM, Sedeek M, Burger D, Montezano AC. NOX isoforms and reactive oxygen species in vascular health. Mol Interv. 2011;11:27–35. doi: 10.1124/mi.11.1.5. [DOI] [PubMed] [Google Scholar]
- 18.Kedzierski RM, Yanagisawa M. Endothelin system: the double-edged sword in health and disease. Annu Rev Pharmacol Toxicol. 2001;41:851–876. doi: 10.1146/annurev.pharmtox.41.1.851. [DOI] [PubMed] [Google Scholar]
- 19.Gunaydin H, Houk KN. Mechanisms of peroxynitrite-mediated nitration of tyrosine. Chem Res Toxicol. 2009;22:894–898. doi: 10.1021/tx800463y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Maller C, Schröder E, Eaton P. Glyceraldehyde 3-phosphate dehydrogenase is unlikely to mediate hydrogen peroxide signaling: studies with a novel anti-dimedone sulfenic acid antibody. Antioxid Redox Signal. 2011;14:49–60. doi: 10.1089/ars.2010.3149. [DOI] [PubMed] [Google Scholar]
- 21.Hu Z, Chen J, Wei Q, Xia Y. Bidirectional actions of hydrogen peroxide on endothelial nitric-oxide synthase phosphorylation and function: co-commitment and interplay of Akt and AMPK. J Biol Chem. 2008;283:25256–25263. doi: 10.1074/jbc.M802455200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Martyniuk TV, Chazova IE, Masenko VP, Volkov VN, Belenkov Iu N. Activity of renin-angiotensin-aldosterone system (RAAS) and vasopressin level in patients with primary pulmonary hypertension. Ter Arkh. 1998;70:33–36. [PubMed] [Google Scholar]
- 23.Kokubu T, Kazatani Y, Hamada M, Matsuzaki K, Ito T, Nishimura K, Ochi T, Daimon F, Joh T. Is captopril effective in primary pulmonary hypertension? Circ J. 1982;46:1095–1097. doi: 10.1253/jcj.46.1095. [DOI] [PubMed] [Google Scholar]
- 24.Bansal S, Badesch D, Bull T, Schrier RW. Role of vasopressin and aldosterone in pulmonary arterial hypertension: A pilot study. Contemp Clin Trials. 2009;30:392–399. doi: 10.1016/j.cct.2009.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cacoub P, Dorent R, Nataf P, Carayon A. Endothelin-1 in pulmonary hypertension. N Engl J Med. 1993;329:1967–1968. doi: 10.1056/NEJM199312233292618. [DOI] [PubMed] [Google Scholar]
- 26.Rousseau MF, Gurne O, Duprez D, Van Mieghem W, Robert A, Ahn S, Galanti L, Ketelslegers JM. Beneficial neurohormonal profile of spironolactone in severe congestive heart failure: results from the RALES neurohormonal substudy. J Am Coll Cardiol. 2002;40:1596–1601. doi: 10.1016/s0735-1097(02)02382-3. [DOI] [PubMed] [Google Scholar]
- 27.Usui S, Yao A, Hatano M, Kohmoto O, Takahashi T, Nagai R, Kinugawa K. Upregulated neurohumoral factors are associated with left ventricular remodeling and poor prognosis in rats with monocrotaline-induced pulmonary arterial hypertension. Circ J. 2006;70:1208–1215. doi: 10.1253/circj.70.1208. [DOI] [PubMed] [Google Scholar]
- 28.Takeda Y, Miyamori I, Yoneda T, Hatakeyama H, Inaba S, Furukawa K, Mabuchi H, Takeda R. Regulation of aldosterone synthase in human vascular endothelial cells by angiotensin II and adrenocorticotropin. J Clin Endocrinol Metab. 1996;81:2797–2800. doi: 10.1210/jcem.81.8.8768832. [DOI] [PubMed] [Google Scholar]
- 29.Ahmad N, Romero DG, Gomez-Sanchez EP, Gomez-Sanchez CE. Do human vascular endothelial cells produce aldosterone? Endocrinology. 2004;145:3626–3629. doi: 10.1210/en.2004-0081. [DOI] [PubMed] [Google Scholar]
- 30.Zanchi A, Chiolero A, Maillard M, Nussberger J, Brunner HR, Burnier M. Effects of the peroxisomal proliferator-activated receptor-gamma agonist pioglitazone on renal and hormonal responses to salt in healthy men. J Clin Endocrinol Metab. 2004;89:1140–1145. doi: 10.1210/jc.2003-031526. [DOI] [PubMed] [Google Scholar]
- 31.Zhang H, Zhang A, Kohan DE, Nelson RD, Gonzalez FJ, Yang T. Collecting duct-specific deletion of peroxisome proliferator-activated receptor gamma blocks thiazolidinedione-induced fluid retention. Proc Natl Acad Sci U S A. 2005;102:9406–9411. doi: 10.1073/pnas.0501744102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Uruno A, Matsuda K, Noguchi N, Yoshikawa T, Kudo M, Satoh F, Rainey WE, Hui XG, Akahira J, Nakamura Y, Sasano H, Okamoto H, Ito S, Sugawara A. Peroxisome proliferator-activated receptor-{gamma} suppresses CYP11B2 expression and aldosterone production. J Mol Endocrinol. 2011;46:37–49. doi: 10.1677/JME-10-0088. [DOI] [PubMed] [Google Scholar]
- 33.Farquharson CA, Struthers AD. Aldosterone induces acute endothelial dysfunction in vivo in humans: evidence for an aldosterone-induced vasculopathy. Clin Sci (Lond) 2002;103:425–431. doi: 10.1042/cs1030425. [DOI] [PubMed] [Google Scholar]
- 34.Csiszar A, Labinskyy N, Olson S, Pinto JT, Gupte S, Wu JM, Hu F, Ballabh P, Podlutsky A, Losonczy G, de Cabo R, Mathew R, Wolin MS, Ungvari Z. Resveratrol prevents monocrotaline-induced pulmonary hypertension in rats. Hypertension. 2009;54:668–675. doi: 10.1161/HYPERTENSIONAHA.109.133397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wedgwood S, Black SM. Endothelin-1 decreases endothelial NOS expression and activity through ETA receptor-mediated generation of hydrogen peroxide. Am J Physiol Lung Cell Mol Physiol. 2005;288:L480–487. doi: 10.1152/ajplung.00283.2004. [DOI] [PubMed] [Google Scholar]
- 36.Watts JA, Marchick MR, Gellar MA, Kline JA. Up-regulation of arginase II contributes to pulmonary vascular endothelial cell dysfunction during experimental pulmonary embolism. Pulm Pharmacol Ther. 2011;24:407–413. doi: 10.1016/j.pupt.2011.01.009. [DOI] [PubMed] [Google Scholar]
- 37.Grobe AC, Wells SM, Benavidez E, Oishi P, Azakie A, Fineman JR, Black SM. Increased oxidative stress in lambs with increased pulmonary blood flow and pulmonary hypertension: role of NADPH oxidase and endothelial NO synthase. Am J Physiol Lung Cell Mol Physiol. 2006;290:L1069–1077. doi: 10.1152/ajplung.00408.2005. [DOI] [PubMed] [Google Scholar]
- 38.Brown-Steinke K, deRonde K, Yemen S, Palmer LA. Gender differences in S-nitrosoglutathione reductase activity in the lung. PLoS One. 2010;5:e14007. doi: 10.1371/journal.pone.0014007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhao YY, Zhao YD, Mirza MK, Huang JH, Potula HH, Vogel SM, Brovkovych V, Yuan JX, Wharton J, Malik AB. Persistent eNOS activation secondary to caveolin-1 deficiency induces pulmonary hypertension in mice and humans through PKG nitration. J Clin Invest. 2009;119:2009–2018. doi: 10.1172/JCI33338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wouters MA, Fan SW, Haworth NL. Disulfides as redox switches: from molecular mechanisms to functional significance. Antioxid Redox Signal. 2010;12:53–91. doi: 10.1089/ars.2009.2510. [DOI] [PubMed] [Google Scholar]
- 41.Elshourbagy NA, Adamou JE, Gagnon AW, Wu HL, Pullen M, Nambi P. Molecular characterization of a novel human endothelin receptor splice variant. J Biol Chem. 1996;271:25300–25307. doi: 10.1074/jbc.271.41.25300. [DOI] [PubMed] [Google Scholar]
- 42.Marrero MB, Venema VJ, Ju H, He H, Liang H, Caldwell RB, Venema RC. Endothelial nitric oxide synthase interactions with G-protein-coupled receptors. Biochem J. 1999;343:335–340. [PMC free article] [PubMed] [Google Scholar]
- 43.Mathew R, Yuan N, Rosenfeld L, Gewitz MH, Kumar A. Effects of monocrotaline on endothelial nitric oxide synthase expression and sulfhydryl levels in rat lungs. Heart Dis. 2002;4:152–158. doi: 10.1097/00132580-200205000-00004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.