Abstract
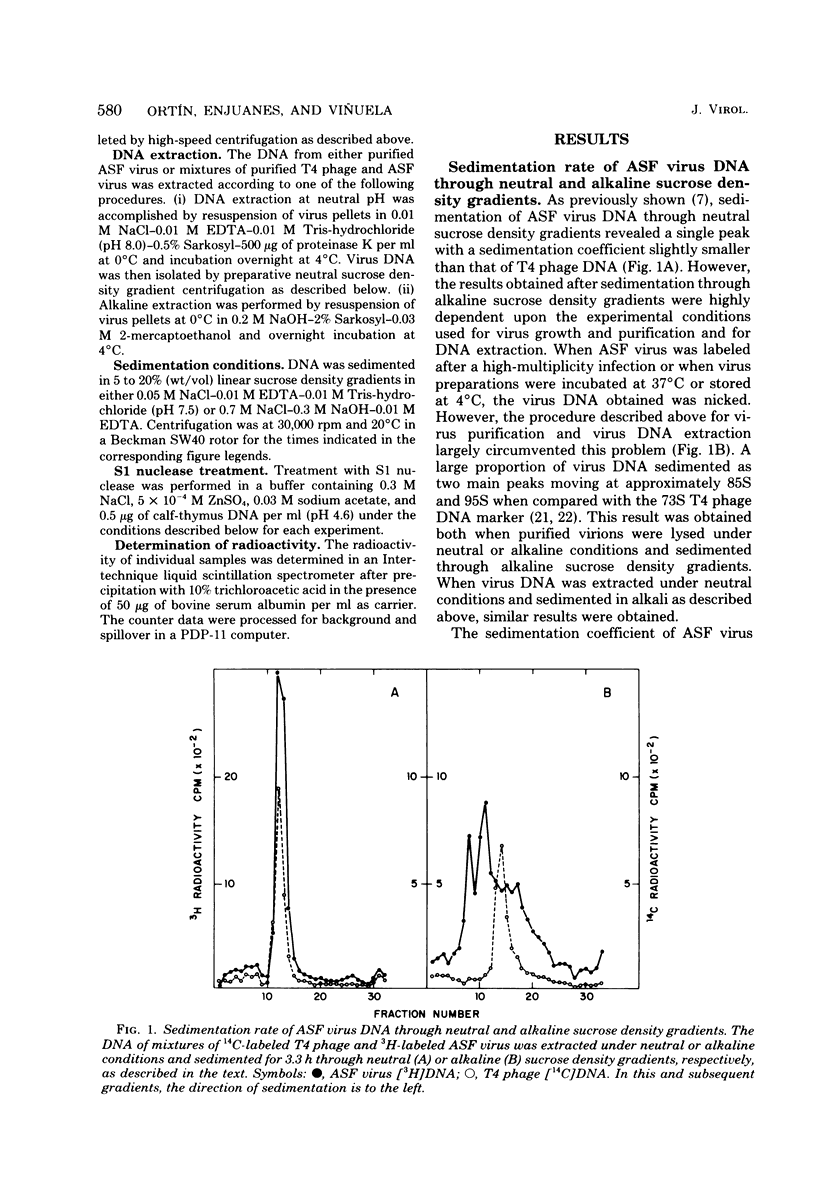
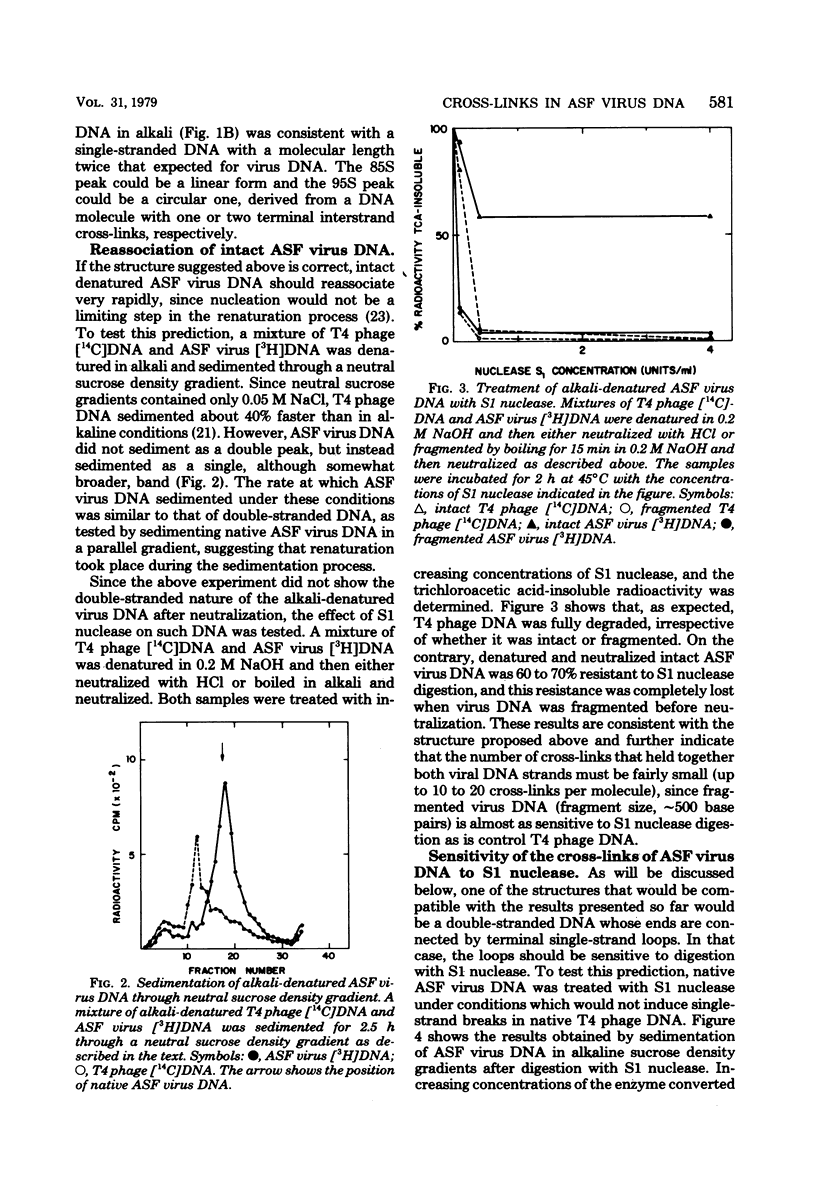
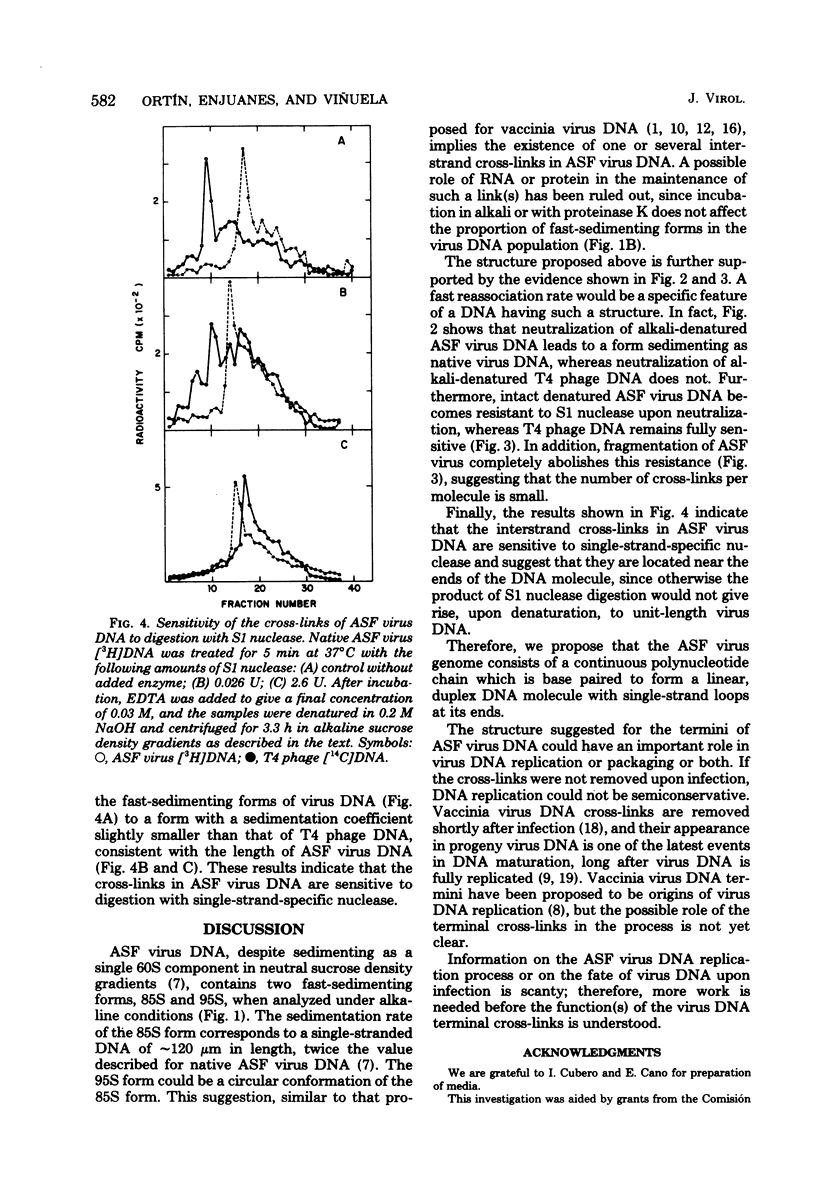
African swine fever virus DNA sediments in neutral sucrose density gradients as a single component with a sedimentation coefficient of 60S. In alkaline sucrose density gradients, this material shows two components with sedimentation coefficients of 85S and 95S, respectively. The sedimentation rate value of alkali-denatured virus DNA in neutral sucrose density gradients and the renaturation velocity of denatured DNA show that is reassociated much faster than expected from its genetic complexity. This behavior is compatible with the existence of interstrand cross-links in the molecule. We also present results which suggest that there are only a few such cross-links per molecule, that they are sensitive to S1 nuclease digestion, and that they are probably located next to the ends of the DNA.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berns K. I., Silverman C. Natural occurrence of cross-linked vaccinia virus deoxyribonucleic acid. J Virol. 1970 Mar;5(3):299–304. doi: 10.1128/jvi.5.3.299-304.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breese S. S., Jr, Pan I. C. Electron microscopic observation of African swine fever virus development in Vero cells. J Gen Virol. 1978 Aug;40(2):499–502. doi: 10.1099/0022-1317-40-2-499. [DOI] [PubMed] [Google Scholar]
- Coggins L. African swine fever virus. Pathogenesis. Prog Med Virol. 1974;18(0):48–63. [PubMed] [Google Scholar]
- DULBECCO R., FREEMAN G. Plaque production by the polyoma virus. Virology. 1959 Jul;8(3):396–397. doi: 10.1016/0042-6822(59)90043-1. [DOI] [PubMed] [Google Scholar]
- Enjuanes L., Carrascosa A. L., Moreno M. A., Viñuela E. Titration of African swine fever (ASF) virus. J Gen Virol. 1976 Sep;32(3):471–477. doi: 10.1099/0022-1317-32-3-471. [DOI] [PubMed] [Google Scholar]
- Enjuanes L., Carrascosa A. L., Viñuela E. Isolation and properties of the DNA of African swine fever (ASF) virus. J Gen Virol. 1976 Sep;32(3):479–492. doi: 10.1099/0022-1317-32-3-479. [DOI] [PubMed] [Google Scholar]
- Esteban M., Flores L., Holowczak J. A. Model for vaccinia virus DNA replication. Virology. 1977 Dec;83(2):467–473. doi: 10.1016/0042-6822(77)90197-0. [DOI] [PubMed] [Google Scholar]
- Esteban M., Holowczak J. A. Replication of vaccinia DNA in mouse L cells. I. In vivo DNA synthesis. Virology. 1977 May 1;78(1):57–75. doi: 10.1016/0042-6822(77)90078-2. [DOI] [PubMed] [Google Scholar]
- Geshelin P., Berns K. I. Characterization and localization of the naturally occurring cross-links in vaccinia virus DNA. J Mol Biol. 1974 Oct 5;88(4):785–796. doi: 10.1016/0022-2836(74)90399-4. [DOI] [PubMed] [Google Scholar]
- Hess W. R. African swine fever virus. Virol Monogr. 1971;9:1–33. doi: 10.1007/978-3-7091-3987-5_1. [DOI] [PubMed] [Google Scholar]
- Holowczak J. A. Poxvirus DNA. I. Studies on the structure of the vaccinia genome. Virology. 1976 Jul 1;72(1):121–133. doi: 10.1016/0042-6822(76)90317-2. [DOI] [PubMed] [Google Scholar]
- Moreno M. A., Carrascosa A. L., Ortín J., Viñuela E. Inhibition of African swine fever (ASF virus replication by phosphonoacetic acid. J Gen Virol. 1978 May;39(2):253–258. doi: 10.1099/0022-1317-39-2-253. [DOI] [PubMed] [Google Scholar]
- Nunes J. F., Vigário J. D., Terrinha A. M. Ultrastructural study of African swine fever virus replication in cultures of swine bone marrow cells. Arch Virol. 1975;49(1):59–66. doi: 10.1007/BF02175596. [DOI] [PubMed] [Google Scholar]
- Ortin J., Vińuela E. Requirement of cell nucleus for African swine fever virus replication in Vero cells. J Virol. 1977 Mar;21(3):902–905. doi: 10.1128/jvi.21.3.902-905.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkhurst J. R., Peterson A. R., Heidelberger C. Breakdown of HeLa cell DNA mediated by vaccinia virus. Proc Natl Acad Sci U S A. 1973 Nov;70(11):3200–3204. doi: 10.1073/pnas.70.11.3200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parr R. P., Burnett J. W., Garon C. F. Ultrastructural characterization of the Molluscum contagiosum virus genome. Virology. 1977 Sep;81(2):247–256. doi: 10.1016/0042-6822(77)90141-6. [DOI] [PubMed] [Google Scholar]
- Pogo B. G. Elimination of naturally occurring crosslinks in vaccinia virus DNA after viral penetration into cells. Proc Natl Acad Sci U S A. 1977 Apr;74(4):1739–1742. doi: 10.1073/pnas.74.4.1739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pogo B. G., O'Shea M. T. The mode of replication of vaccinia virus DNA. Virology. 1978 Jan;84(1):1–8. doi: 10.1016/0042-6822(78)90213-1. [DOI] [PubMed] [Google Scholar]
- Polatnick J., Hess W. R. Increased deoxyribonucleic acid polymerase activity in African swine fever virus-infected culture cells. Brief report. Arch Gesamte Virusforsch. 1972;38(4):383–385. doi: 10.1007/BF01262828. [DOI] [PubMed] [Google Scholar]
- STUDIER F. W. SEDIMENTATION STUDIES OF THE SIZE AND SHAPE OF DNA. J Mol Biol. 1965 Feb;11:373–390. doi: 10.1016/s0022-2836(65)80064-x. [DOI] [PubMed] [Google Scholar]
- THOMAS C. A., Jr, BERNS K. I. The physical characterization of DNA molecules released from T2 and T4 bacteriophage. J Mol Biol. 1961 Jun;3:277–288. doi: 10.1016/s0022-2836(61)80069-7. [DOI] [PubMed] [Google Scholar]
- Wetmur J. G., Davidson N. Kinetics of renaturation of DNA. J Mol Biol. 1968 Feb 14;31(3):349–370. doi: 10.1016/0022-2836(68)90414-2. [DOI] [PubMed] [Google Scholar]



