Abstract
The study aim was to describe the temporal course of cognitive decline in Alzheimer‘s disease (AD). We selected 226 persons from 2 longitudinal clinical-pathological studies who were cognitively healthy at baseline, followed at least 4 years (mean = 10.2, SD = 3.5), and clinically diagnosed with AD at some point during follow-up. Each evaluation included a battery of 17 cognitive tests from which a previously established composite measure of global cognition was derived. In those who died, a uniform neuropathologic examination established the pathological diagnoses of Alzheimer's disease and other common conditions that impair cognition. Mixed-effects models with 2 change points were used to assess trajectories of cognitive decline. In the main analysis, there was no change in cognitive function until a mean of 7.5 years before dementia was diagnosed (95% confidence interval [CI]: -8.3, -6.7). The global cognitive measure declined a mean of 0.087-unit per year (95% CI: -0.099, -0.073) until a mean of 2.0 years before the diagnosis (95% CI: -2.2, -1.7) when it increased more than fourfold to a mean loss of 0.370-unit per year (95% CI: -0.417, -0.334). Of 126 individuals who died and underwent autopsy, 101 (80%) met pathologic criteria for AD of whom 67 had at least one other pathologic condition. Pathologic measures of AD and cerebral infarction were not strongly related to cognitive trajectories. The results indicate that cognitive decline in AD begins many years before dementia is diagnosed and accelerates during the course of the disease.
Keywords: longitudinal study, cognitive decline, Alzheimer's disease, mild cognitive impairment, neuropathologic examination
Progressive decline in cognitive function is the primary clinical manifestation of Alzheimer's disease (AD). Because cognitive decline in the disease begins several years before individuals develop dementia (Hall, Lipton, Sliwinski, & Steward, 2000; Amieva et al., 2008; Johnson, Storandt, Morris, & Galvin, 2009; Small & Bäckman, 2007; Grober et al., 2008) and continues to progress thereafter (Wilson, Leurgans, Boyle, & Bennett, 2011; Wilson, Beckett, Bennett, Albert, & Evans, 1999; Wilson, Aggarwal, et al., 2010), the transition from cognitive health through dementia can take a decade or more. The lengthy duration of cognitive symptoms in the disease makes it difficult to track individuals across the full spectrum from cognitive health through dementia, limiting knowledge about the temporal course of cognitive decline in AD.
The aim of the current study was to describe the temporal course of cognitive decline in AD. We used data from two longitudinal cohort studies that began in the 1990s and include annual administration of a battery of 18 cognitive performance tests. We selected a subgroup of 226 individuals who were cognitively healthy at baseline and clinically diagnosed with AD on follow-up with a minimum of 4 years of observation. We used mixed effects models with multiple change points to characterize person specific trajectories of decline in cognitive function. In those individuals who died and underwent brain autopsy, we ascertained pathological diagnoses of AD and other common conditions that can contribute to cognitive impairment and examined the relation of pathologic measures to trajectories of cognitive decline.
Methods
Participants
These analyses are based on participants in 2 ongoing longitudinal clinical-pathological cohort studies. The Religious Orders Study began in 1994 and involves older Catholic nuns, priests, and monks recruited from over 40 groups across the United States (Wilson, Bienias, Evans, & Bennett, 2004). The Rush Memory and Aging Project began in 1997 and involves older lay persons recruited from retirement communities, subsidized housing facilities, churches, and social service agencies in the Chicago metropolitan area (Bennett, Schneider, Buchman, et al., 2005). Persons in both studies agreed to annual clinical evaluations and brain autopsy at death. Written informed consent was obtained in each study after procedures were fully explained, and each was approved by the institutional review board of Rush University Medical Center.
Eligibility for these analyses required absence of cognitive impairment at baseline, completion of at least 5 annual clinical evaluations, and a clinical diagnosis of AD during follow-up. These criteria were designed to identify individuals whose cognitive trajectories spanned as much of the spectrum from intact function to dementia as possible. As shown in Table 1, at the time of these analyses 2,642 individuals had enrolled in the two parent studies. We excluded 836 with cognitive impairment at baseline (679 with mild cognitive impairment, 157 with dementia) and 116 who had no opportunity to be followed (due to death or recent enrollment) leaving 1,690 eligible for follow-up. Of these, 1,641 (97.1%) completed at least one follow-up evaluation and 1,239 were followed at least 4 years, making them eligible for the present analyses. During follow-up, 226 of these 1,239 individuals developed AD and primary analyses are based on them. They had a mean age at study entry of 79.9 (SD = 6.2) and a mean of 16.8 years of education (SD = 3.7); 77.0% were women and 96.5% were white and non-Hispanic. They were followed a mean of 10.2 years (SD = 3.5). The mean Mini-Mental State Examination score was 28.3 (SD = 1.7) at study entry, 21.1 (SD = 5.3) when AD was clinically diagnosed, and 16.3 (SD = 7.9) at the last clinical evaluation. There were 27 participants who developed AD but were ineligible for analyses due to insufficient follow-up. These 27 individuals did not differ from the 226 eligible participants in age (at baseline; 82.0 versus 79.9; t[251] = 1.6, p = 0.104), education (15.6 versus 16.8; t[251] = 1.6, p = 0.122), proportion of women (63.0% versus 77.0%; χ2[1] = 2.6, p = 0.109), or Mini-Mental State Examination score at AD diagnosis (18.3 vs 21.1; t[28.0] = 1.8, p = 0.079) or the last evaluation (17.0 versus 16.3; t[251] = 0.5, p = 0.652).
Table 1.
Summary of How the Analytic Groups Were Composed
| Religious Orders Study | Memory and Aging Project | Combined Studies | |
|---|---|---|---|
| Total number of participants | 1,160 | 1,482 | 2,642 |
| No cognitive impairment at baseline | 796 | 1,010 | 1,806 |
| Died before first follow-up | 18 | 22 | 40 |
| Enrolled < 1 year | 7 | 69 | 76 |
| Eligible for follow-up | 771 | 919 | 1,690 |
| Follow-up ≥1 year | 758 | 883 | 1,641 |
| Follow-up ≥ 4 years | 646 | 593 | 1,239 |
| Developed Alzheimer's disease | 134 | 92 | 226 |
| Died | 97 | 46 | 143 |
| Had brain autopsy | 95 | 42 | 137 |
| Neuropathologic examination completed | 88 | 38 | 126 |
Clinical Evaluation
At baseline and annually thereafter, participants had a uniform clinical evaluation that included a structured medical history, neurological examination, and cognitive performance testing. On the basis of this evaluation (but blinded to data from previous evaluations), an experienced clinician classified individuals with respect to mild cognitive impairment, dementia, and AD as described elsewhere (Wilson, Bienias, et al., 2004; Bennett, Schneider, Buchman, et al., 2005; Bennett et al., 2006; Bennett et al., 2002). A key issue in these diagnostic decisions is determining whether functioning in different cognitive domains is impaired. To maximize uniformity in these decisions over time and diagnosticians, an algorithm was developed to rate impairment in 5 cognitive domains (i.e., orientation, attention, memory, language, perception) based on educational attainment and scores on 11 of the cognitive tests (Bennett et al., 2002). After reviewing all cognitive test data and information on education and potential impediments to cognitive performance, a neuropsychologist agreed or disagreed with each algorithmic rating and assigned a new cognitive domain rating in the event of disagreement. The diagnosis of dementia and AD was based on the criteria of the joint working group of the National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer's Disease and Related Disorders Association (McKhann et al., 1984) which require a history of cognitive decline and impairment in at least 2 cognitive domains, 1 of which must be memory for a diagnosis of AD. Persons who did not meet dementia criteria but had 1 or more impaired cognitive domains were classified as mild cognitive impairment. These mild cognitive impairment criteria have been associated with intermediate levels of cognitive decline (Bennett et al., 2002; Boyle, Wilson, Aggarwal, Tang, & Bennett, 2006; Wilson, Aggarwal, et. al, 2010), mortality (Bennett et al., 2002; Wilson et al., 2009) and AD pathology (Bennett, Schneider, Bienias, Evans, & Wilson, 2005) relative to dementia and no cognitive impairment.
Assessment of Cognitive Function
A battery of 18 cognitive performance tests was administered as part of the annual clinical evaluations in each study. The Mini-Mental State Examination was used only for descriptive purposes. The remaining 17 tests included 7 measures of episodic memory: Word List Memory, Word List Recall, Word List Recognition, and immediate and delayed recall of the East Boston story and Story A from Logical Memory. Semantic memory was assessed with a 15-item form of the Boston Naming Test, Verbal Fluency, and a word recognition test. Digit Ordering and Digit Span Forward and Backward assessed working memory; Symbol Digit Modalities Test and Number Comparison assessed perceptual speed; and a 15-item version of Judgment of Line Orientation and an 11-item version of Standard Progressive Matrices assessed visuospatial ability.
To make use of all cognitive data and minimize floor and ceiling artifacts and other sources of measurement error, a composite measure of global cognition based on all 17 tests was used in analyses. Raw scores on individual tests were converted to z scores, using the baseline mean and SD of all participants in both studies, and the z scores were averaged to yield the composite score. Further information on the individual tests and the derivation of the composite measure of global cognition has been previously published (Wilson et al., 2002; Wilson, Barnes, & Bennett, 2003; Wilson et al., 2005).
Neuropathologic Examination
The protocol for brain removal, tissue sectioning and preservation, and quantifying pathologic findings has been previously described (Schneider, Wilson, Bienias, Evans, & Bennett, 2004; Schneider, Arvanitakis, Bang, & Bennett, 2007; Schneider, Arvanitakis, Leurgans, & Bennett, 2009). Neuritic plaques, diffuse plaques, and neurofibrillary tangles were counted in 4 regions (entorhinal cortex, midfrontal cortex, middle temporal cortex, inferior parietal cortex) using a modified Bielschowsky silver stain. The pathologic diagnosis of AD was made by a board-certified neuropathologist blinded to all clinical data. Each case was classified according to National Institute on Aging-Reagan criteria (1997) as “no AD,” “low likelihood AD,” “intermediate likelihood AD,” or high likelihood AD” based on levels of neuritic plaques, as expressed by a Consortium to Establish a Registry for AD score (Mirra et al., 1991), and neurofibrillary tangles, as expressed by the Braak score (Braak & Braak, 1991). A pathologic diagnosis of AD required an intermediate or high likelihood of disease. We also used a previously established continuous measure of AD pathologic burden in some analyses. Raw counts of neuritic plaques, diffuse plaques, and neurofibrillary tangles in each brain region were standardized and averaged to yield the composite measure (Bennett et al., 2003).
Cerebral infarcts visible to the naked eye were noted and their age was estimated as acute, subacute, or chronic. We examined 1 hemisphere for microscopic infarctions using hematoxylin-eosin stain in 6 cortical regions, 2 subcortical regions, and the midbrain. Chronic microinfarcts were either cavitated or incomplete infarcts, with few remaining macrophages and fibrillary gliosis. Analyses were based on the total number of gross plus microscopic chronic infarcts (i.e., at least 6 months of age), expressed on a 3-point ordinal scale, with no infarcts, 1 infarct, and ≥ 1 infarct categories. Hematoxylin-eosin stain was also used to identify hippocampal sclerosis, defined as severe neuronal loss and gliosis in CA1 or subiculum at the level of the lateral geniculate. Sclerosis that was mild or located elsewhere in the hippocampus was also noted. Antibodies to alpha-synuclein were used to identify Lewy bodies in substantia nigra, 2 limbic regions (entorhinal cortex, anterior cingulate cortex), and 3 neocortical regions (midfrontal cortex, middle temporal cortex, interior parietal cortex) (Schneider et al., 2007; Wilson, Yu, et al., 2011). Because they were relatively infrequent, both hippocampal sclerosis and Lewy bodies were treated as present or absent.
Data Analysis
We used mixed effects change point models (Hall, Ying, Kuo, & Lipton, 2003) to characterize change in cognitive function. Model estimation was done with a Bayesian Monte Carlo Markov Chain approach (Gelman, Carlin, Stern, & Rubin, 2003) implemented in OpenBugs software (Lunn, Spiegelhalter, Thomas, & Best, 2009). We initially constructed models that allowed rate of cognitive decline to accelerate at 1 or 2 points. Each model included random effects for the intercept, slope terms, and change point(s) to allow for individual differences in the level of cognition at AD diagnosis, rate of cognitive change, and the timing of the change point(s). We subsequently repeated the 2 change point model without assuming random effects were unrelated; with more stringent eligibility criteria; with terms added for age (at diagnosis) and education; and with an indicator for having postmortem data. In the subgroup with postmortem data, we repeated the original 2 change point model, first with a term added for the continuous measure of AD pathologic burden and then again with a term for cerebral infarction.
Results
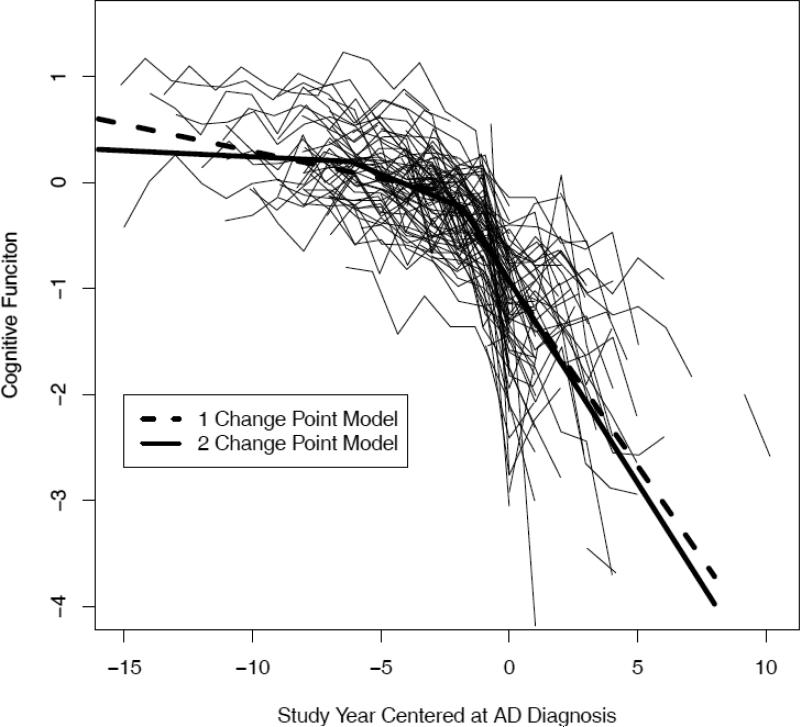
At baseline, scores on the composite measure of global cognition were approximately normally distributed with a mean of 0.124 (SD = 0.388, skewness = -0.145) and higher values indicating better cognitive performance. We characterized change in this cognitive measure in 2 models. One model allowed rate of cognitive decline to accelerate once during the observation period and the other model allowed rate of decline to accelerate twice. The model allowing 2 change points fit the data better than the 1 change point model, as indicated by having a lower Deviance Information Criterion (1,369 versus 1,468).
The model results are summarized in Table 2 (model A). The estimated annual rate of cognitive change in the first part of the trajectory did not differ from zero, as shown by the term for slope 1 in the table, indicating stable cognitive functioning. A mean of about 7.5 years before AD was diagnosed, global cognitive function began to decline with an average loss of 0.087-unit per year (slope 2 of model A in Table 2). Approximately 5.5 years later, a mean of 2.0 years before dementia onset, rate of global cognitive decline more than quadrupled to a mean annual loss of 0.370-unit per year (slope 3 of model A in Table 2). The random effects in the table indicate that there were significant individual differences in level of function at AD diagnosis, rates of cognitive decline, and the timing of the change points.
Table 2.
Estimated Paths of Global Cognitive Change in Alzheimer's Disease
| Model A | Model B | ||||
|---|---|---|---|---|---|
| Effect | Model Term | Estimate | 95% CI | Estimate | 95% CI |
| Fixed | Intercept | -0.928* | -0.985,-0.870 | -0.945* | -1.011,-0.883 |
| Slope 1 | 0.003 | -0.012,0.020 | 0.043* | 0.020,0.074 | |
| Slope 2 | -0.087* | -0.099,-0.073 | -0.093* | -0.108,-0.077 | |
| Slope 3 | -0.370* | -0.417,-0.334 | -0.389* | -0.433,-0.350 | |
| Change point 1 | -7.456* | -8.268,-6.726 | -7.700* | -8.748,-6.950 | |
| Change point 2 | -1.965* | -2.243,-1.654 | -1.849* | -2.214,-1.554 | |
| Random | Intercept | 0.184* | 0.147.0.227 | 0.220* | 0.178,0.276 |
| Slope 1 | 0.0004* | 0.0002,0.0008 | 0.004* | 0.002,0.006 | |
| Slope 2 | 0.001* | 0.0004,0.002 | 0.003* | 0.002.0.005 | |
| Slope 3 | 0.036* | 0.026,0.053 | 0.048* | 0.034,0.067 | |
| Change point 1 | 0.029* | 0.001,0.697 | 0.190* | 0.016,1.642 | |
| Change point 2 | 0.089* | 0.002,0.320 | 0.052* | 0.015,0.192 | |
| Error | 0.088* | 0.082,0.095 | 0.085* | 0.079,0.091 | |
Note. Results were estimated from 2 separate mixed-effects change point models. Fixed effects indicate the estimated group mean and random effects indicate person specific deviation from the group mean. Model terms were assumed to be uncorrelated in Model A but not in Model B. CI, confidence interval.
p < 0.05.
Prior to the clinical diagnosis of AD, mild cognitive impairment was diagnosed in 181 (80.0%). In this subgroup, the diagnosis of mild cognitive impairment was made a mean of 3.5 years (SD = 3.3) after the first change point which was a mean of 4.3 years (SD = 2.8) before the clinical diagnosis of AD.
In the interests of parsimony, the model assumed that the random effects were not correlated. To determine whether this constraint affected results, we dropped it and repeated the analysis. There were moderate positive correlations among the intercept and some slope terms (r = .51 [95% CI: .32, .67] for intercept – slope 1; r = .73 [95% CI: .63, 81] for intercept – slope 3; r = .31[95% CI: .07, .51] for slope 1 – slope 2; and r = .35 [95% CI: .12, .55] for slope 2 – slope 3). As shown in Table 2, however, the overall results of this analysis (model B) were similar to the original analysis (model A).
The original analysis was based on individuals with 4 to 17 years of follow-up. To see if including individuals in this group with less follow-up affected estimates of the temporal course of cognitive decline in the disease, we repeated the analysis four times, with eligibility restricted to persons followed for at least 5 years (n=210, first column of Table 3), 6 years (n=192, second column of Table 3), 7 years (n=170, third column of Table 3), or 8 years (n=143, fourth column of Table 3). Results of these analyses were comparable to the original model.
Table 3.
Estimated Paths of Global Cognitive Change in Alzheimer's Disease Subgroups with Different Durations of Follow-up*
| Alzheimer's Disease Subgroups | ||||||
|---|---|---|---|---|---|---|
| Effects | Model Term | Model Output | ≥ 6 Visits (n = 210) | ≥ 7 Visits (n = 192) | ≥ 8 Visits (n = 170) | ≥ 9 Visits (n = 143) |
| Fixed | Intercept | Estimate | -0.933* | -0.925* | -0.915* | -0.908* |
| 95% CI | -0.996,-0.871 | -0.993, -0.861 | -0.984, -0.841 | -0.993, -0.827 | ||
| Slope 1 | Estimate | 0.004 | 0.004 | 0.006 | 0.004 | |
| 95% CI | -0.013, 0.022 | -0.011, 0.022 | -0.007, 0.023 | -0.009, 0.020 | ||
| Slope 2 | Estimate | -0.088* | -0.089* | -0.087* | -0.094* | |
| 95% CI | -0.102, -0.075 | -0.101, -0.076 | -0.101, -0.074 | -0.110, -0.078 | ||
| Slope 3 | Estimate | -0.377* | -0.373* | -0.377* | -0.403* | |
| 95% CI | -0.417, -0.340 | -0.416, -0.335 | -0.423, -0335 | -0.460, -0.350 | ||
| Ch. point 1 | Estimate | -7.473* | -7.469* | -7.519* | -7.226* | |
| 95% CI | -8.365, -6.757 | -8.151, -6.750 | -8.418, -6.882 | -8.081, -6.561 | ||
| Ch. point 2 | Estimate | -1.864* | -1.850* | -1.850* | -1.666* | |
| 95% CI | -2.144, -1.584 | -2.190, -1.600 | -2.204, -1.565 | -2.017, -1.391 | ||
| Random | Intercept | Estimate | 0.190* | 0.196* | 0.208* | 0.214* |
| 95% CI | 0.153, 0.236 | 0.155, 0.246 | 0.168, 0.262 | 0.165. 0.281 | ||
| Slope 1 | Estimate | 0.0004* | 0.0004* | 0.0004* | 0.0004* | |
| 95% CI | 0.0002, 0.0009 | 0.0002, 0.0009 | 0.0002, 0.0009 | 0.0002, 0.0009 | ||
| Slope 2 | Estimate | 0.001* | 0.001* | 0.001* | 0.001* | |
| 95% CI | 0.001, 0.002 | 0.001, 0.002 | 0.001, 0.002 | 0.001, 0.003 | ||
| Slope 3 | Estimate | 0.042* | 0.043* | 0.047* | 0.060* | |
| 95% CI | 0.030, 0.059 | 0.030, 0.062 | 0.032, 0.068 | 0.041, 0.090 | ||
| Ch. point 1 | Estimate | 0.027* | 0.022* | 0.028* | 0.025* | |
| 95% CI | 0.001, 0.889 | 0.001, 0.788 | 0.001, 0.968 | 0.001, 0.740 | ||
| Ch. point 2 | Estimate | 0.115* | 0.138* | 0.182* | 0.166* | |
| 95% CI | 0.015, 0.316 | 0.003, 0.378 | 0.002, 0.538 | 0.002, 0.597 | ||
| Error | Estimate | 0.085* | 0.082* | 0.081* | 0.077* | |
| 95% CI | 0.079, 0.092 | 0.075, 0.088 | 0.075, 0.088 | 0.071, 0.084 | ||
Note. Results were estimated from 5 separate mixed-effects change point models. Fixed effects indicate the estimated group mean and random effects indicate person specific deviation from the group mean. CI, confidence interval.
p <0.05
Because of evidence that age (Wilson, Gilley, Bennett, Beckett, & Evans, 2000) and education (Wilson, Aggarwal, et al., 2004; Hall et al., 2007) may be related to cognitive decline in AD, we repeated the original analysis in all 226 affected persons with terms added for age at the time of clinical diagnosis and education. As shown in Table 4, older age at clinical diagnosis was associated with lower level of cognitive function at AD diagnosis, a later initial change point, and slightly more rapid decline following it, as shown by the terms for slope 2 and slope 3. Higher level of education was associated with a later second change point followed by more rapid cognitive decline.
Table 4.
Relation of Age at Clinical Diagnosis and Education to Paths of Global Cognitive Change in Alzheimer's Disease*
| Age at Diagnosis | Education | |||
|---|---|---|---|---|
| Model Term | Estimate | 95% CI | Estimate | 95% CI |
| Intercept | -0.188* | -0.318,-0.055 | 0.022 | -0.098,0.154 |
| Slope 1 | -0.013 | -0.035,0.007 | -0.016 | -0.40,0.006 |
| Slope 2 | -0.023* | -0.042,-0.005 | -0.013 | -0.030,0.003 |
| Slope 3 | -0.119* | -0.024,-0.003 | -0.180* | -0.294,-0.070 |
| Change point 1 | 1.160* | 0.341,2.116 | 0.108 | -0.671,0.824 |
| Change point 2 | 0.017 | -0.316,0.306 | 0.305* | 0.049,0.590 |
Note. Results were estimated from a mixed-effects change point model. Results show the fixed effect of a 1-unit increase in age at diagnosis and education. CI, confidence interval.
p <0.05.
To better understand the heterogeneity in the paths of cognitive decline, we examined postmortem data. A total of 143 individuals died during the observation period of whom 137 (95.8%) underwent a brain autopsy and uniform neuropathologic examination, the results of which were available at the time of these analyses in 126 (Table 1). The 126 with postmortem data completed fewer annual clinical evaluations than the 100 subjects without postmortem data (mean of 9.2 versus 10.7; t[190.0] = 3.5, p < 0.001) and were older at baseline (81.0 versus 78.4; t [224] = 3.2, p = 0.002), but the subgroups did not differ in age at AD diagnosis (87.7 versus 86.7; t [224] = 1.2, p = 0.243), proportion of women (72.2% versus 83.0% χ2 [1] = 3.7, p = 0.056), education (17.1 versus 16.4; t [224] = 1.5, p = 0.138), or Mini-Mental State Examination score at AD diagnosis (20.6 versus 21.7; t [214.7] = 1.5, p = 0.126) or the last evaluation (15.4 versus 17.5; t [224] = 1.9, p = 0.053). To determine whether the subgroups had different trajectories of change in cognitive function, we repeated the original analysis with a term added for presence or absence of postmortem data. This term was not associated with the intercept, slopes, or first change point but was associated with the second change point which occurred a mean of 0.622-year later (95% CI: 0.190, 1.061) in those with postmortem data compared to those without it.
A total of 101 individuals (80.2%) met National Institute on Aging-Reagan criteria for AD (40 high likelihood, 61 intermediate likelihood), 57 (45.2%) had chronic cerebral infarcts (27 [21.4%] with 1, 30 [23.8%] with > 1), 36 (28.6%) had Lewy bodies, and 21 (16.7%) had hippocampal sclerosis (9 [7.1%] severe, 12 [9.5%] mild). The pathologic measures were not correlated with one another, but many persons had more than one condition. Of the 101 with pathologic AD, 34 (33.7%) had AD alone, 43 (42.6%) had AD plus one other condition (cerebral infarction 27, Lewy bodies 12, hippocampal sclerosis 4), 21 had AD plus two other conditions (cerebral infarction and Lewy bodies 7, cerebral infarction and hippocampal sclerosis 7, Lewy bodies and hippocampal sclerosis 7), and 3 had AD plus three other conditions. Of the 25 individuals who did not meet pathologic criteria for AD, 9 had no significant pathologic findings, 8 had one pathologic finding (cerebral infarction 5, Lewy bodies 2, hippocampal sclerosis 1) and 8 had two pathologic findings (cerebral infarction and Lewy bodies 5, cerebral infarction and hippocampal sclerosis 3).
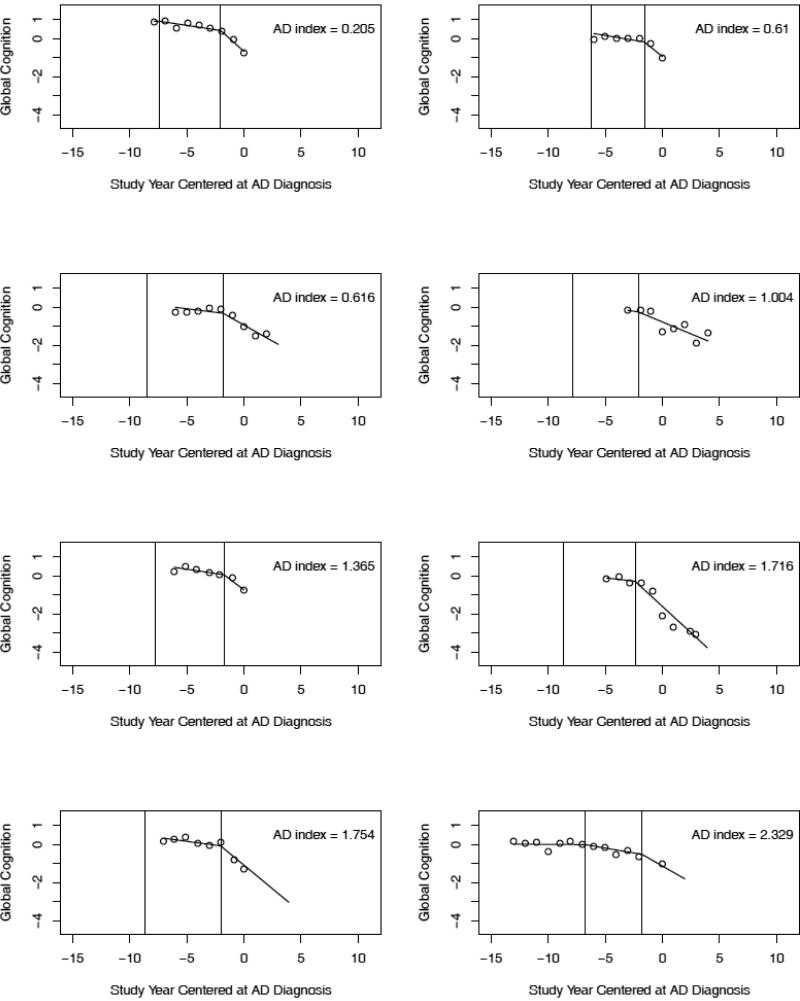
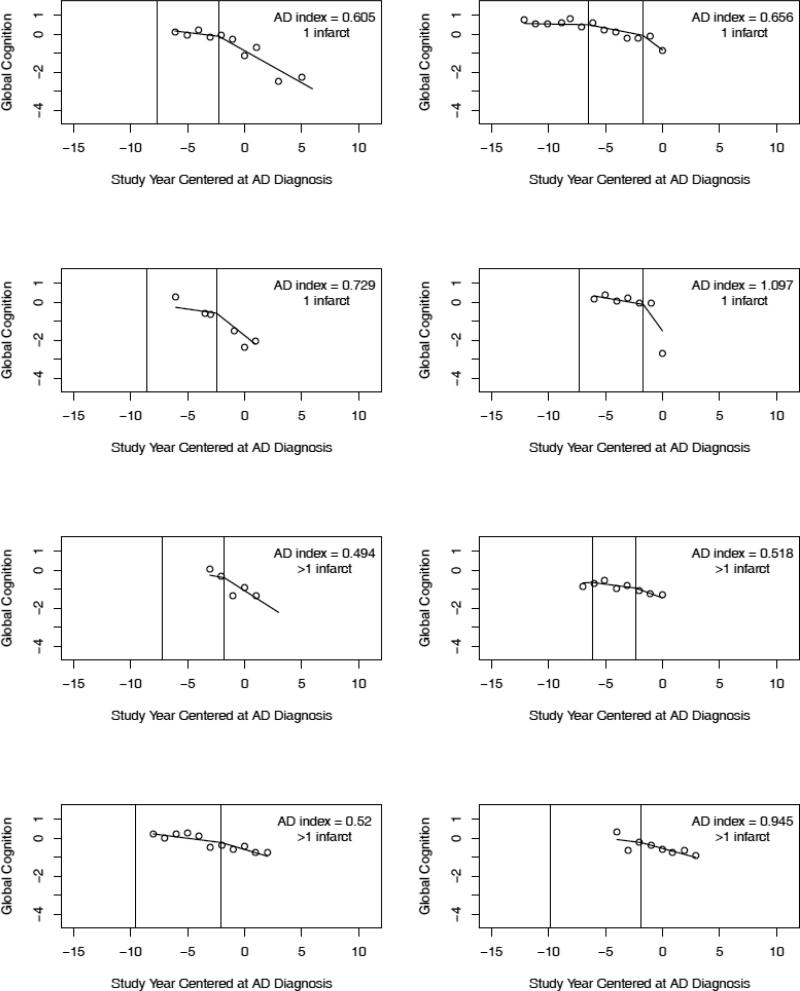
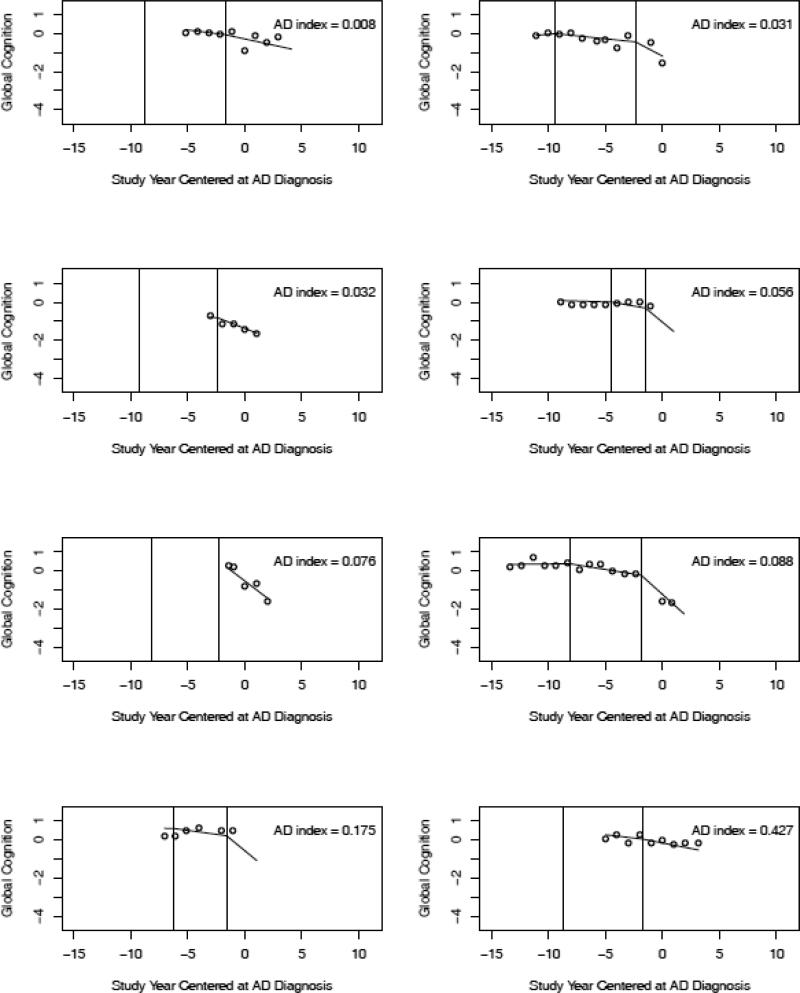
To link the pathologic findings to the cognitive data, we examined individual trajectories predicted by the model. We randomly selected trajectories of individuals with AD and no other pathologic condition (n = 8; Figure 2), AD plus cerebral infarction (n = 8; Figure 3), and no pathologic findings (n = 8; Figure 4). No clear differences between the pathologic subgroups are evident. To further investigate cognitive-pathologic correlations, we conducted analyses of the postmortem subgroup (Table 5). In an initial model, higher level of AD pathology was associated with lower level of cognitive function at AD diagnosis (i.e., intercept) but not with other trajectory components. In a subsequent model, cerebral infarction was associated with a later initial change point but no other trajectory components. Lewy bodies and hippocampal sclerosis were too infrequent to support analyses.
Figure 2.
Crude data points (open circles) and paths of cognitive change predicted by the model for 8 individuals with pathologic AD and no other pathologic conditions (randomly selected from 34) arranged in order of AD pathologic burden. The predicted path reflects both group means and person specific deviations from those means.
Figure 3.
Crude data points (open circles) and paths of cognitive change predicted by the model for 8 individuals with pathologic AD and 1 (upper 4 plots in order of AD pathologic burden) or >1 (lower 4 plots in order of AD pathologic burden) cerebral infarcts (randomly selected from 27). The predicted path reflects both group means and person specific deviations from those means.
Figure 4.
Crude data points (open circles) and paths of cognitive change predicted by the model for 8 individuals without pathologic AD or other pathologic conditions arranged in order of AD pathologic burden. The predicted path reflects both group means and person specific deviations from those means.
Table 5.
Relation of Alzheimer's Disease Pathology and Chronic Cerebral Infarction to Paths of Global Cognitive Change*
| Alzheimer's Disease | Cerebral Infarction | |||
|---|---|---|---|---|
| Model Term | Estimate | 95% CI | Estimate | 95% CI |
| Intercept | -0.124* | -0.205,-0.037 | -0.020 | -0.104,0.060 |
| Slope 1 | -0.014 | -0.049,0.037 | -0.009 | -0.115,0.024 |
| Slope 2 | -0.021 | -0.046,0.002 | -0.009 | -0.029,0.011 |
| Slope 3 | -0.040 | -0.091,0.014 | 0.029 | -0.023,0.083 |
| Change point 1 | 0.605 | -2.122,2.123 | 1.386* | 0.098,3.218 |
| Change point 2 | 0.016 | -0.202,0.263 | -0.178 | -0.429,0.055 |
Note. Results were estimated from 2 separate mixed-effects change point models. Results show the fixed effect of a 1-unit change in the pathologic measures. CI, confidence interval.
p <0.05.
Discussion
During an average of 10.2 years of annual observation, we assessed change in cognitive function in old people who developed an AD-like dementia. Global cognitive function began to decline a mean of 7.5 years before dementia onset and the rate of decline accelerated again a mean of 5.5 years later. The finding that cognitive decline in AD is nonlinear and precedes dementia onset by several years is broadly consistent with prior longitudinal cognitive studies of the disease before (Hall et al., 2000; Amieva et al., 2008; Johnson et al., 2009; Small & Bäckman, 2007; Grober et al., 2008) and after (Wilson, Leurgans, et al., 2011; Wilson et al., 1999; Wilson, Aggarwal, et al., 2010) dementia is diagnosed.
A longstanding question about the course of cognitive decline in AD is whether the rate of decline continues to increase (accelerating model [Hall et al., 2003]) or pauses at some point (plateau model [Twamley, Ropacki, & Bondi, 2006]). A plateau has been reported after the appearance of cognitive impairment and before dementia is diagnosed (Haxby, Raffele, Gillette, Schapiro, & Rapoport, 1992; Bäckman, Small, & Fratiglioni, 2001; Smith et al., 2007), but these reports were based on few subjects (Haxby et al., 1992; Bäckman et al., 2001) or a subset of cognitive measures (Smith et al., 2007), and all included persons who already had mild cognitive impairment at baseline. The present results support an accelerating model of cognitive decline in AD, consistent with several prior studies (Wilson, Leurgans, et al., 2011; Hall, Lipton, Sliwinski, & Stewart, 2000; Wilson, Aggarwal, et al., 2004; Grober et al., 2008; Johnson, Storandt, Morris, & Galvin, 2009). At the same time, however, these data show that cognitive decline in AD may not be very rapid until about 2 years before dementia is diagnosed and therefore may be difficult to distinguish from a flat trajectory.
Mild cognitive impairment is widely viewed as a precursor to dementia in AD. Consistent with this idea, we observed a period of mild to moderate cognitive decline lasting a mean of 5.5 years, preceded by cognitive stability and followed by precipitous cognitive decline. However, the data also illustrate measurement challenges that confront observational and intervention research on mild cognitive impairment. In particular, the wide confidence intervals around the change points attest to the difficulty in pinpointing the temporal boundaries of this period. In addition, mild cognitive impairment was usually diagnosed several years after the first change point and in some cases was not diagnosed before dementia. The basic problem is that continuous pathologic processes likely underly disease progression. As a result, dividing the disease course into categories by either diagnostic or statistical methods is likely to involve error, especially early in the disease course when its clinical manifestations are subtle and hard to detect.
In those who died and underwent a brain autopsy and uniform neuropathologic examination, the pathologic diagnosis of AD agreed with the clinical diagnosis of AD in 80% which is comparable to rates of concordance previously reported in these (Bennett et al., 2006) and other (Kukull et al., 1990; Galasko et al., 1994; Victoroff, Mack, Lyness, & Chui, 1995; Gearing et al., 1995) cohorts. Most of those who met pathologic criteria for AD had at least one additional pathologic condition, consistent with previous observations that most clinically diagnosed AD reflects multiple pathologic processes (Schneider et al., 2009). Pathologic measures of AD burden and cerebral infarction were not strongly related to trajectories of cognitive decline. Subdivision of cognitive trajectories into multiple components may have limited our power to detect an association of individual components with pathology. However, we have previously observed associations between these same pathologic measures and cognitive decline when people with and without dementia are included in analyses (Wilson, Leurgans, Boyle, Schneider, & Bennett, 2010; Wilson, Krueger, Boyle, & Bennett, 2010; Wilson, Segawa, Hizel, Boyle, & Bennett, in press). This suggests that the neuropathologic processes traditionally associated with dementia are primarily associated with dementia risk rather than dementia progression. A dissociation of this sort could explain why factors such as age that are robustly related to risk of dementia are not strongly related to how rapidly it progresses.
Strengths of this study should be noted. Clinical classification of mild cognitive impairment, dementia, and AD was based on a uniform clinical evaluation and widely accepted criteria applied by an experienced clinician, minimizing diagnostic error. The availability of a psychometrically sound measure of cognition, multiple evenly spaced cognitive assessments in each individual, and high rates of participation in follow-up and autopsy enhanced our ability to reliably estimate person-specific trajectories of cognitive decline and link them to postmortem findings.
The main limitations of the study are that the cohorts in both parent studies are selected and that only a subset of those with incident AD was eligible for analyses. Therefore, the generalizability of these findings remains to be established. In addition, we may have undersampled individuals whose disease progressed too slowly to be identified within the observation period or too rapidly to meet eligibility criteria, leading to an underestimation of the heterogeneity in cognitive trajectories.
Figure 1.
Crude paths of change in global cognition (thin black lines) and the mean path predicted by the one (thick dashed line) and two (thick solid line) change point models.
Acknowledgments
The authors thank the many Illinois residents who have participated in the Rush Memory and Aging Project and the many Catholic clergy members who have participated in the Religious Orders Study; Tracy Colvin, MPH, for coordinating the studies; Woojeong Bang, MS, for statistical programming; and John Gibbons, MS, and Greg Klein, MS, for data management. This research was supported by National Institute on Aging grants P30AG10161, R01AG15819, RO1AG17917, R01AG34374, and R01AG33678 and by the Illinois Department of Public Health. The funding organizations had no role in the design or conduct of the study; collection, management, analysis, or interpretation of the data; or preparation, review, or approval of the manuscript.
References
- Amieva H, LeGoff M, Millet X, Orgogozo JM, Pérés K, Barberger-Gateau P, Jacqmin-Gadda H, Dartigues JF. Prodromal Alzheimer's disease: Successive emergence of clinical symptoms. Annals of Neurology. 2008;64:492–498. doi: 10.1002/ana.21509. [DOI] [PubMed] [Google Scholar]
- Bäckman L, Small BJ, Fratiglioni L. Stability of the preclinical episodic memory deficit in Alzheimer's disease. Brain. 2001;124:96–102. doi: 10.1093/brain/124.1.96. [DOI] [PubMed] [Google Scholar]
- Bennett DA, Schneider JA, Aggarwal NT, Arvanitakis Z, Shah RC, Kelly JF, Fox JH, Cochran EJ, Arends D, Treinkman AD, Wilson RS. Decision rules guiding the clinical diagnosis of Alzheimer's disease in two community-based cohort studies compared to standard practice in a clinic-based cohort study. Neuroepidemiology. 2006;27:169–176. doi: 10.1159/000096129. [DOI] [PubMed] [Google Scholar]
- Bennett DA, Schneider JA, Bienias JL, Evans DA, Wilson RS. Mild cognitive impairment is related to Alzheimer disease pathology and cerebral infarctions. Neurology. 2005;64:834–841. doi: 10.1212/01.WNL.0000152982.47274.9E. [DOI] [PubMed] [Google Scholar]
- Bennett DA, Schneider JA, Buchman AS, Mendes de Leon C, Bienias JL, Wilson RS. The Rush Memory and Aging Project: Study design and baseline characteristics of the study cohort. Neuroepidemiology. 2005;25:163–175. doi: 10.1159/000087446. [DOI] [PubMed] [Google Scholar]
- Bennett DA, Wilson RS, Schneider JA, Evans DA, Aggarwal NT, Arnold SE, Cochran EJ, Berry-Kravis E, Bienias JL. Apolipoprotein E є4 allele, AD pathology, and the clinical expression of Alzheimer's disease. Neurology. 2003;60:245–252. doi: 10.1212/01.wnl.0000042478.08543.f7. [DOI] [PubMed] [Google Scholar]
- Bennett DA, Wilson RS, Schneider JA, Evans DA, Beckett LA, Aggarwal NT, Barnes LL, Fox JH, Bach J. Natural history of mild cognitive impairment in older persons. Neurology. 2002;59:198–205. doi: 10.1212/wnl.59.2.198. [DOI] [PubMed] [Google Scholar]
- Boyle PA, Wilson RS, Aggarwal NT, Tang Y, Bennett DA. Mild cognitive impairment: risk of Alzheimer disease and rate of cognitive decline. Neurology. 2006;67:441–445. doi: 10.1212/01.wnl.0000228244.10416.20. [DOI] [PubMed] [Google Scholar]
- Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathologica. 1991;82:239–259. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- Galasko D, Hansen LA, Katzman R, Wiederholt W, Masliah E, Terry R, Hill LR, Lessin P, Thal LJ. Clinical-neuropathological correlations in Alzheimer's disease and related dementias. Archives of Neurology. 1994;51:888–895. doi: 10.1001/archneur.1994.00540210060013. [DOI] [PubMed] [Google Scholar]
- Gearing M, Mirra SS, Hedreen JC, Sumi SM, Hansen LA, Heyman A. The Consortium to Establish a Registry for Alzheimer's Disease (CERAD). X. Neuropathology confirmation of the clinical diagnosis of Alzheimer's disease. Neurology. 1995;45:461–466. doi: 10.1212/wnl.45.3.461. [DOI] [PubMed] [Google Scholar]
- Gelman A, Carlin JB, Stern HS, Rubin DB. Bayesian data analysis. 2nd ed. Chapman & Hall; Boca Ratan, FL: 2003. [Google Scholar]
- Grober E, Hall CB, Lipton RB, Zonderman AB, Resnick SM, Kawas C. Memory impairment, executive dysfunction, and intellectual decline in preclinical Alzheimer's disease. Journal of the International Neuropsychological Society. 2008;14:266–278. doi: 10.1017/S1355617708080302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall CB, Derby C, LeValley A, Katz MJ, Verghese J, Lipton RB. Education delays accelerated decline on a memory test in persons who develop dementia. Neurology. 2007;69:1657–1664. doi: 10.1212/01.wnl.0000278163.82636.30. [DOI] [PubMed] [Google Scholar]
- Hall CB, Lipton RB, Sliwinski M, Stewart WF. A change point model for estimating the onset of cognitive decline in preclinical Alzheimer's disease. Statistics in Medicine. 2000;19:1555–1566. doi: 10.1002/(sici)1097-0258(20000615/30)19:11/12<1555::aid-sim445>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Hall CB, Ying J, Kuo L, Lipton RB. Bayesian and profile likelihood change point methods for modeling cognitive function over time. Computational Statistics & Data Analysis. 2003;42:91–109. [Google Scholar]
- Haxby JV, Raffaele K, Gillette J, Schapiro MB, Rapoport SI. Individual trajectories of cognitive decline in patients with dementia of the Alzheimer type. Journal of Clinical and Experimental Neuropsychology. 1992;14:575–592. doi: 10.1080/01688639208402846. [DOI] [PubMed] [Google Scholar]
- Johnson DK, Storandt M, Morris JC, Galvin JE. Longitudinal study of the transition from healthy aging to Alzheimer disease. Archives of Neurology. 2009;66:1254–1259. doi: 10.1001/archneurol.2009.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kukull WA, Larson EB, Reifler BV, Lampe TH, Yerby MS, Hughes JP. The validity of 3 clinical diagnostic criteria for Alzheimer's disease. Neurology. 1990;40:1364–1369. doi: 10.1212/wnl.40.9.1364. [DOI] [PubMed] [Google Scholar]
- Lunn D, Spiegelhalter D, Thomas A, Best N. The BUGS project: evolution, critique and future directions. Statistics in Medicine. 2009;28:3049–3067. doi: 10.1002/sim.3680. [DOI] [PubMed] [Google Scholar]
- McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer's disease: Report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- Mirra SS, Heyman A, McKeel D, Sumi SM, Crain BJ, Brownlee LM, Vogel FS, Hughes JP, van Belle G, Berg L. The Consortium to Establish a Registry for Alzheimer's Disease (CERAD). Part II: Standardization of the neuropathologic assessment of Alzheimer's disease. Neurology. 1991;41:479–486. doi: 10.1212/wnl.41.4.479. [DOI] [PubMed] [Google Scholar]
- Mungas D, Beckett L, Harvey D, Farias ST, Reed B, Carmichael O, Olichney J, Miller J, DeCarli C. Heterogeneity of cognitive trajectories in diverse older persons. Psychology and Aging. 2010;25:606–619. doi: 10.1037/a0019502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The National Institute on Aging, and Reagan Institute Working Group on Diagnostic Criteria for the Neuropathological Assessment of Alzheimer's Disease Consensus recommendations for the postmortem diagnosis of Alzheimer's disease. Neurobiology of Aging. 1997;18:S1–2. [PubMed] [Google Scholar]
- Schneider JA, Arvanitakis Z, Bang W, Bennett DA. Mixed brain pathologies account for most dementia cases in community-dwelling older persons. Neurology. 2007;69:2197–2204. doi: 10.1212/01.wnl.0000271090.28148.24. [DOI] [PubMed] [Google Scholar]
- Schneider JA, Arvanitakis Z, Leurgans SE, Bennett DA. The neuropathology of probable Alzheimer's disease and mild cognitive impairment. Annals of Neurology. 2009;66:200–208. doi: 10.1002/ana.21706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider JA, Wilson RS, Bienias JL, Evans DA, Bennett DA. Cerebral infarctions and the likelihood of dementia from Alzheimer disease pathology. Neurology. 2004;62:1148–1155. doi: 10.1212/01.wnl.0000118211.78503.f5. [DOI] [PubMed] [Google Scholar]
- Small BJ, Bäckman L. Longitudinal trajectories of cognitive change in preclinical Alzheimer's disease: A growth mixture modeling analysis. Cortex. 2007;43:826–834. doi: 10.1016/s0010-9452(08)70682-8. [DOI] [PubMed] [Google Scholar]
- Smith GE, Pankratz VS, Negash S, Machulda MM, Peterson RC, Boeve BF, Ivnik RJ. A plateau in pre-Alzheimer memory decline: evidence for compensatory mechanisms? Neurology. 2007;69:133–139. doi: 10.1212/01.wnl.0000265594.23511.16. [DOI] [PubMed] [Google Scholar]
- Storandt M, Grant EA, Miller JP, Morris JC. Rates of progression in mild cognitive impairment and early Alzheimer disease. Neurology. 2002;59:1034–1041. doi: 10.1212/wnl.59.7.1034. [DOI] [PubMed] [Google Scholar]
- Twamley EW, Ropacki SAL, Bondi MW. Neuropsychological and neuroimaging changes in preclinical Alzheimer's disease. Journal of the International Neuropsychological Society. 2006;12:707–735. doi: 10.1017/S1355617706060863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Victoroff J, Mack WJ, Lyness SA, Chui HC. Multicenter clinicopathological correlation in dementia. American Journal of Psychiatry. 1995;152:1476–1484. doi: 10.1176/ajp.152.10.1476. [DOI] [PubMed] [Google Scholar]
- Wilson RS, Aggarwal NT, Barnes LL, Bienias JL, Mendes de Leon CF, Evans DA. Biracial population study of mortality in mild cognitive impairment and AD. Archives of Neurology. 2009;66:767–772. doi: 10.1001/archneurol.2009.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson RS, Aggarwal NT, Barnes LL, Mendes de Leon CF, Hebert LC, Evans DA. Cognitive decline in incident Alzheimer disease in a community population. Neurology. 2010;74:951–955. doi: 10.1212/WNL.0b013e3181d64786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson RS, Barnes LL, Bennett DA. Assessment of lifetime participation in cognitively stimulating activities. Journal of Clinical and Experimental Neuropsychology. 2003;25:634–642. doi: 10.1076/jcen.25.5.634.14572. [DOI] [PubMed] [Google Scholar]
- Wilson RS, Barnes LL, Krueger KR, Hoganson G, Bienias JL, Bennett DA. Early and late life cognitive activity and cognitive systems in old age. Journal of the International Neuropsychological Society. 2005;11:400–407. [PubMed] [Google Scholar]
- Wilson RS, Beckett LA, Barnes LL, Schneider JA, Bach J, Evans DA, Bennett DA. Individual differences in rates of change in cognitive abilities of older persons. Psychology and Aging. 2002;17:179–193. [PubMed] [Google Scholar]
- Wilson RS, Beckett LA, Bennett DA, Albert MS, Evans DA. Change in cognitive function in older persons from a community population: Relation to age and Alzheimer disease. Archives of Neurology. 1999;56:1274–1279. doi: 10.1001/archneur.56.10.1274. [DOI] [PubMed] [Google Scholar]
- Wilson RS, Bienais JL, Evans DA, Bennett DA. Religious Orders Study: Overview and change in cognitive and motor speed. Aging, Neuropsychology, and Cognition. 2004;11:280–303. [Google Scholar]
- Wilson RS, Gilley DW, Bennett DA, Beckett LA, Evans DA. Person-specific paths of cognitive decline in Alzheimer's disease and their relation to age. Psychology and Aging. 2000;15:18–28. doi: 10.1037//0882-7974.15.1.18. [DOI] [PubMed] [Google Scholar]
- Wilson RS, Krueger KR, Boyle PA, Bennett DA. Loss of basic lexical knowledge in old age. Journal of Neurology Neurosurgery and Psychiatry. 2010;82:369–372. doi: 10.1136/jnnp.2010.212589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson RS, Leurgans SE, Boyle PA, Bennett DA. Cognitive decline in prodromal Alzheimer's disease and mild cognitive impairment. Archives of Neurology. 2011;68:251–356. doi: 10.1001/archneurol.2011.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson RS, Leurgans SE, Boyle PA, Schneider JA, Bennett DA. Neurodegenerative basis of age-related cognitive decline. Neurology. 2010;75:1070–1078. doi: 10.1212/WNL.0b013e3181f39adc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson RS, Li Y, Aggarwal NT, Barnes LL, McCann JJ, Gilley DW, Evans DA. Education and the course of cognitive decline in Alzheimer's disease. Neurology. 2004;63:1198–1202. doi: 10.1212/01.wnl.0000140488.65299.53. [DOI] [PubMed] [Google Scholar]
- Wilson RS, Segawa E, Hizel LP, Boyle PA, Bennett DA. Terminal dedifferentiation of cognitive abilities. Neurology. doi: 10.1212/WNL.0b013e31824f7ff2. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson RS, Yu L, Schneider JA, Arnold SE, Buchman AS, Bennett DA. Lewy bodies and olfactory dysfunction in old age. Chemical Senses. 2011;36:367–373. doi: 10.1093/chemse/bjq139. [DOI] [PMC free article] [PubMed] [Google Scholar]