Abstract
Objective
Given the importance of fatigue in cancer, stroke and HIV, we sought to assess the measurement properties of a single, well-described fatigue scale in these populations. We hypothesized that the psychometric properties of the Functional Assessment of Chronic Illness Therapy – Fatigue (FACIT-F) subscale would be favorable and that the scale could serve as a useful indicator of fatigue in these populations.
Methods
Patients were eligible for the study if they were outpatients, aged 18 or older, with a diagnosis of cancer (n=297), stroke (n=51), or HIV/AIDS (n=51). All participants were able to understand and speak English. Patients answered study-related questions, including the FACIT-F using a touch-screen laptop, assisted by the research assistant as necessary. Clinical information was abstracted from patients’ medical records.
Results
Item-level statistics on the FACIT-F were similar across the groups and internal consistency reliability was uniformly high (α>0.91). Correlations with performance status ratings were statistically significant across the groups (range r=−0.28 to −0.80). Fatigue scores were moderately to highly correlated with general quality of life (range r=0.66–0.80) in patients with cancer, stroke, and HIV. Divergent validity was supported in low correlations with variables not expected to correlate with fatigue.
Conclusions
Originally developed to assess cancer-related fatigue, the FACIT-F has utility as a measure of fatigue in other populations, such as stroke and HIV. Ongoing research will soon allow for comparison of FACIT-F scores to those obtained using the fatigue measures from the Patient-Reported Outcomes Measurement Information System (PROMIS®; www.nihpromis.org) initiative.
Keywords: fatigue, assessment, psychometrics, cancer, stroke, HIV
Fatigue is the most prevalent symptom among individuals with cancer and may be due to the disease itself, its treatment, and/or psychosocial variables.[1] Depending on the patient population and means of measuring fatigue, prevalence estimates among cancer patients are generally high, ranging from 60 to over 90%.[1] Patients may describe their experience of fatigue in terms of being exhausted, tired, weak, or slowed. Furthermore, in a large sample of patients with advanced cancer who have received chemotherapy, fatigue was spontaneously endorsed and ranked as the most important symptom that should be monitored.[2] Although common, cancer-related fatigue remains poorly understood.[3] In clinical practice, fatigue may be neglected or under-detected due to the fact that it is a subjective experience that is assessed by patient self-report. Treatment of cancer-related fatigue is further complicated by its multifactorial clinical manifestations, involving both psychological and physical components.
Stroke is the leading cause of disability in adults and often results in reduced functional status, impaired psychological well-being, and economic hardship.[4] Persistent fatigue is a common symptom following stroke, [5] with prevalence estimates ranging from 23 to 75%, likely reflecting variations in measurement and sampling approaches. [5,6] The frequency of self-reported fatigue is roughly twice as high in patients post stroke as it is in matched controls, and 27% of stroke survivors experience fatigue every day.[7] Little research has focused on how best to measure post-stroke fatigue quantitatively.
People with HIV infection have reported fatigue as one of their most frequent complaints, regardless of how advanced their HIV infection or their use of Highly Active Antiretroviral Therapy.[8] For example, a study of 317 men and women who had been diagnosed with HIV for several years found that the three most frequently reported symptoms, using the Memorial Symptom Assessment Scale, were all fatigue-related: “lack of energy” (65%); “feeling drowsy” (57%); and “difficulty sleeping” (56%).[9] Furthermore, fatigue has been shown to affect the physical, social, familial, and psychological aspects of the lives of individuals with HIV. [10] Women and older persons with HIV infection have reported more fatigue than men and younger persons with HIV.[11] Despite the prevalence and impact of fatigue in the lives of people with HIV, family and physicians often do not acknowledge fatigue as a significant concern.[12]
Fatigue may develop for different reasons in cancer, stroke, and HIV. However, given the importance of fatigue across these three chronic conditions, we sought to assess the measurement properties of a single, well-described fatigue scale in these populations. We hypothesized that the psychometric of the Functional Assessment of Chronic Illness Therapy – Fatigue subscale[13] would be favorable and that the scale could serve as a useful indicator of fatigue in clinical research across these populations.
Method
Assessment of fatigue
All participants completed the Functional Assessment of Chronic Illness Therapy – Fatigue (FACIT-F) subscale.[13] The FACIT-F is a unidimensional,[14] 13-item scale that asks respondents to rate statements regarding their fatigue experience and its impact on their daily life. Sample items include: “I feel fatigued;” “I feel weak all over;” and “I feel listless (washed out)”. All items are rated using a 5-point intensity rating scale. By scoring convention, after appropriate reverse scoring of 11 items, lower scores on the FACIT Fatigue subscale indicate greater levels of fatigue. (A scoring template is available at www.facit.org.) Originally developed for use with cancer patients,[15,16] the scale has been successfully administered in a variety of other populations, including rheumatoid arthritis,[17,18] Parkinson’s disease,[19] systemic lupus erythematosis,[20] chronic anemia associated with aging,[21] as well as the general United States population.[3] To enhance the clinical usefulness of the FACIT-F subscale, Cella and colleagues[16] estimated a minimum clinically meaningful difference of 3 points by using both anchor- and distribution-based methods. Additionally, Eastern Cooperative Oncology Group (ECOG) performance ratings were available for all cancer patients and hemoglobin values, obtained within 30 days of fatigue ratings, were available for a subset of 430 cancer patients.
Patient Eligibility and Recruitment
Patients were eligible for the study if they were outpatients, aged 18 or older, with a diagnosis of cancer, stroke, or HIV/AIDS. All recruited patients were able to understand and speak English and could interact with a touch-screen computer with minimal assistance. While there were no general restrictions regarding disease severity or treatment status; stroke patients were required to score higher than 23 on the Folstein Mini-Mental Status Exam.[22]
Cancer patients were recruited from the Robert H. Lurie Comprehensive Cancer Center of Northwestern University. Additional details on the cancer population can be found in Lai et al.[23] Patients with HIV/AIDS were recruited from Northwestern Memorial Hospital in Chicago and stroke patients were recruited from the Rehabilitation Institute of Chicago.
Patients were approached for participation at the respective recruitment sites by trained research assistants and were also informed that they would be asked questions about their fatigue and degree of tiredness. All patients answered study-related questions using a touch-screen laptop, assisted by the research assistant as necessary. Clinical information was abstracted from patients’ medical records. Study procedures were approved by the institutional review boards of the respective recruitment sites and all patients provided informed consent.
Results
Sociodemographic and clinical descriptions of the samples can be found in the Appendix. Patients with cancer, stroke, and HIV were all middle-aged or older. There was variability in the gender distribution within the samples, with HIV patients more likely to be male. Most participants were Caucasian (50–82.5%), with the second largest group being African-Americans (9.4–43.1%).
Appendix.
Sociodemographic and clinical description of samples
| Cancer (n = 297)
|
Stroke (n = 51)
|
HIV (n = 51)
|
|
|---|---|---|---|
| Sociodemographic Information | |||
| Female (%) | 64.3 | 51.0 | 11.8 |
| Age (M(SD) years) | 58.1 (13.5) | 62.6 (13.9) | 40.2 (6.9) |
| Race (%) | |||
| Caucasian | 82.5 | 62.7 | 50.0 |
| Hispanic | 3.7 | 2.0 | 17.6 |
| African-American | 9.4 | 31.4 | 43.1 |
| Asian | 4.0 | 3.9 | |
| Pacific Island | 0.7 | ||
| Other | 1.0 | 4.0 | 2.0 |
| Marital Status (%) | |||
| Never Married | 12.5 | 17.6 | 68.6 |
| Married | 64.0 | 47.1 | 7.8 |
| Living with Partner | 1.7 | 17.6 | |
| Separated | 1.0 | 5.9 | 2.0 |
| Divorced | 9.4 | 13.7 | 3.9 |
| Widowed | 11.4 | 15.7 | |
| Living Situation (%) | |||
| Alone | 22.9 | 35.3 | 45.1 |
| With other adult(s), no dependent | 53.9 | 56.9 | 51.0 |
| With other adult(s), and dependents | 21.5 | 7.8 | 3.9 |
| With dependents only | 1.3 | ||
| Institution or Retirement Home | 0.3 | ||
| Education (%) | |||
| ≤ High school diploma | 18.2 | 27.5 | 25.5 |
| Some college | 28.6 | 29.4 | 29.4 |
| College degree | 31.0 | 29.4 | 35.3 |
| Advanced degree | 22.2 | 13.7 | 9.8 |
| Occupational Status (%) | |||
| Homemaker | 6.8 | 7.8 | 2.0 |
| Unemployed | 2.4 | 5.9 | 3.9 |
| Retired | 33.1 | 51.0 | |
| On disability | 13.5 | 23.5 | 47.1 |
| On leave of absence | 4.7 | ||
| FT employed | 28.4 | 7.8 | 47.1 |
| PT employed | 11.1 | 3.9 | |
|
| |||
| Clinical Information | |||
| Cancer Type (%) | |||
| Breast | 34.0 | ||
| Colorectal | 12.5 | ||
| Non-Hodgkins Lymphoma | 8.5 | ||
| Ovarian | 7.1 | ||
| Lung | 6.5 | ||
| Prostate | 5.1 | ||
| Cancer Stage (%) | |||
| 0 | 1.6 | ||
| 1 | 12.8 | ||
| 2 | 24.9 | ||
| 3 | 23.3 | ||
| 4 | 19.5 | ||
| Extent of Disease (%) | |||
| NED | 12.0 | ||
| Local | 17.2 | ||
| Regional | 4.0 | ||
| Metastasis | 51.8 | ||
| N/A | 15.0 | ||
| Mini-Mental Status Exam (%)* | |||
| 30 | 27.5 | ||
| 29–28 | 43.1 | ||
| 27–26 | 23.5 | ||
| 25–24 | 5.9 | ||
| Type of Stroke | |||
| % Infarct | 70.0 | ||
| Subtype of Stroke (%) | |||
| Intracerebral hemorrhage (ICH) | 36.4 | ||
| Subarachnoic hemorrhage (SAH) | 12.1 | ||
| Thrombotic | 36.4 | ||
| Embolic | 15.2 | ||
| Location (%) | |||
| Superficial/cortical | 27.3 | ||
| Subcortical | 56.8 | ||
| Combination or Other | 15.9 | ||
| % with Previous Stroke | 27.5 | ||
| Current Stroke Treatment (%) | |||
| Physical Therapy | 51.0 | ||
| Speech Therapy | 17.6 | ||
| Vocational Therapy | 3.9 | ||
| Psychological Intervention | 3.9 | ||
| Occupational Therapy | 35.3 | ||
| CD4+ T cell Count | |||
| Mean (SD) | 456 (315) | ||
| Minimum | 6 | ||
| Maximum | 1248 | ||
| HIV Viral Load (%) | |||
| Undetectable | 43.1 | ||
| < 5000 mL | 31.4 | ||
| 5000 – 49,000 mL | 7.8 | ||
| 50,000 – 100,000 mL | 7.8 | ||
| > 100,000 mL | 9.8 | ||
Most cancer patients had breast (34%) or colorectal (12.5%) cancer, with nearly equal numbers of patients with stage III or IV disease as those with less advanced cancers. Most participants with stroke sustained an infarct (70%) that was subcortical (56.8%) in location. Over a quarter (27.5%) of the stroke sample had experienced a previous stroke. Most patients with HIV had a CD4 exact count of 456, with a viral load that was either undetectable (43.1%) or < 5000 mL (31.4%).
Table 1 shows that that item-level statistics for the FACIT-Fatigue are similar across the three patient samples. The FACIT-Fatigue subscales scores (means ± SDs) for the three sample groups were similar, on average, with higher scores indicating lower levels of fatigue: cancer 36.0 ± 12.1, stroke 38.1 ± 9.6; HIV 34.0 ± 12.6. These three means were also worse than the FACIT-F score (=43) suggested to distinguish between the US general population and anemic cancer patients.[3] The internal consistency reliabilities were also similar across the groups with Cronbach alphas > 0.91.
Table 1.
FACIT-Fatigue subscale properties across chronic illness populations
| Item | CANCER (n = 297)
|
STROKE (n = 51)
|
HIV (n = 51)
|
Item | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Subscale M (SD) = 36.0 (12.1) | Subscale M (SD) = 38.1 (9.6) | Subscale M (SD) = 34.0 (12.6) | |||||||||||
| Chronbach α = 0.96
|
Chronbach α = 0.91
|
Chronbach α = 0.97
|
|||||||||||
| M | SD | Item-total correlation | α if item deleted | M | SD | Item-total correlation | α if item deleted | M | SD | Item-total correlation | α if item deleted | ||
| HI7 | 2.46 | 1.18 | 0.84 | 0.95 | 2.78 | 1.08 | 0.64 | 0.90 | 2.14 | 1.10 | 0.86 | 0.96 | HI7 |
| HI12 | 2.98 | 1.20 | 0.84 | 0.95 | 3.28 | 1.01 | 0.78 | 0.90 | 2.80 | 1.15 | 0.83 | 0.96 | HI12 |
| AN1 | 2.80 | 1.21 | 0.85 | 0.95 | 3.06 | 1.18 | 0.74 | 0.90 | 2.80 | 1.11 | 0.83 | 0.96 | AN1 |
| AN2 | 2.53 | 1.15 | 0.87 | 0.95 | 2.76 | 1.08 | 0.84 | 0.89 | 2.29 | 1.12 | 0.82 | 0.96 | AN2 |
| AN3 | 2.82 | 1.18 | 0.83 | 0.95 | 3.02 | 1.12 | 0.85 | 0.89 | 2.55 | 1.15 | 0.85 | 0.96 | AN3 |
| AN4 | 2.76 | 1.18 | 0.83 | 0.95 | 2.96 | 1.12 | 0.85 | 0.89 | 2.53 | 1.21 | 0.90 | 0.96 | AN4 |
| AN5 | 2.18 | 1.12 | 0.74 | 0.95 | 2.10 | 1.03 | 0.60 | 0.90 | 2.18 | 1.01 | 0.81 | 0.96 | AN5 |
| AN7 | 2.38 | 1.21 | 0.69 | 0.96 | 2.06 | 1.27 | 0.49 | 0.91 | 2.55 | 1.10 | 0.71 | 0.97 | AN7 |
| AN8 | 2.89 | 1.06 | 0.62 | 0.96 | 2.96 | 1.23 | 0.15 | 0.92 | 2.63 | 1.25 | 0.81 | 0.96 | AN8 |
| AN12 | 3.58 | 0.76 | 0.62 | 0.96 | 3.72 | 0.67 | 0.46 | 0.91 | 3.33 | 0.91 | 0.70 | 0.97 | AN12 |
| AN14 | 3.20 | 1.07 | 0.68 | 0.96 | 3.04 | 1.03 | 0.38 | 0.91 | 3.12 | 1.03 | 0.81 | 0.96 | AN14 |
| AN15 | 2.73 | 1.27 | 0.84 | 0.95 | 3.26 | 0.94 | 0.75 | 0.90 | 2.57 | 1.38 | 0.90 | 0.96 | AN15 |
| AN16 | 2.72 | 1.28 | 0.82 | 0.95 | 3.12 | 1.06 | 0.77 | 0.90 | 2.47 | 1.21 | 0.86 | 0.96 | AN16 |
Notes:
- Item means, item-total correlations, and alpha are based on reverse-scoring rules for the FACIT-Fatigue subscale (http://www.facit.org).
- All 13 items are rated on a 0 to 4 scale.
- By convention, lower scores on the FACIT-Fatigue indicate more fatigue.
As seen in Table 2, FACIT-Fatigue scores were significantly correlated with patient-rated ECOG performance status rating for all three samples — cancer (r = −0.55, p = 0.001), stroke (r = −0.28, p = .04), and HIV (r = −0.80, p < 0.001). Similarly, fatigue scores were highly associated with overall quality of life, as measured by the FACT-General and its subscales, across all three samples — cancer (r = 0.78, p <0.001), stroke (r = 0.66, p <0.001), and HIV (r = 0.80, p < 0.001). Although FACIT-Fatigue scores were correlated with patient performance status and self-reported well-being, the association of fatigue scores with clinical factors not usually correlated with fatigue (cancer stage, r = 0.02; Mini-Mental State Examination scores in stroke, r = 0.17; viral load in HIV, r = 0.07; were small and not statistically significant (p>0.05).
Table 2.
Correlations of FACIT-Fatigue subscale scores with other clinical variables
| Cancer (n = 297)
|
Stroke (n = 51)
|
HIV (n = 51)
|
||||
|---|---|---|---|---|---|---|
| t (df) or correl | p | t (df) or correl | p | t (df) or correl | p | |
| Sex | −0.87 (295) | 0.23 | −0.65 (49) | 0.52 | 0.30 (49) | 0.77 |
| Ethnicity (Caucasian vs. Other) | 0.45 (295) | 0.66 | −1.14 (49) | 0.26 | −1.04 (48) | 0.30 |
| Age | 0.04 | 0.48 | 0.21 | 0.15 | −0.02 | 0.87 |
| Patient-rated ECOG performance status rating | −0.66 | <0.001 | −0.28 | <0.04 | −0.80 | <0.001 |
| General quality of life (FACT-G) | 0.78 | <0.001 | 0.66 | <0.001 | 0.80 | <0.001 |
| FACT physical well-being (PWB) | 0.82 | <0.001 | 0.76 | <0.001 | 0.88 | <0.001 |
| FACT social well-being (SWB) | 0.31 | <0.001 | 0.43 | 0.001 | 0.32 | <0.03 |
| FACT emotional well-being (EWB) | 0.52 | <0.001 | 0.47 | <0.001 | 0.58 | <0.001 |
| FACT functional well-being (FWB) | 0.73 | <0.001 | 0.43 | <0.001 | 0.75 | <0.001 |
| Stage of illness | 0.02 | 0.74 | ||||
| Extent of illness (exc. N/A) | −0.11 | 0.09 | ||||
| MMSE | 0.17 | 0.24 | ||||
| Type of stroke (hemorrhagic vs. infarct) | −0.91 (48) | 0.37 | ||||
| CD4 exact count | 0.07 | 0.61 | ||||
| Viral load | 0.02 | 0.90 | ||||
Notes:
- As expected, FACIT-Fatigue subscale shows association with performance status and different aspects of well-being, but not clinical variables not generally associated with fatigue.
Table 3 describes the association between patient fatigue and performance status, as a function of diagnosis. The performance status scores of cancer, stroke and HIV groups (n = 399) were compared along the four response options: “0 [PS value], normal activities without symptoms” (n = 121), “1, some symptoms, but do not require bed rest during the day” (n = 183), “2, require bed rest for less than 50% of waking day” and “3, require bed rest for more than 50% of waking day” (n = 95). To account for low cell size in the more severe PS categories, we combined performance status ratings of 2 and 3 into a single category.
Table 3.
FACIT Fatigue subscale scores by disease and ECOG Performance Status Rating
| Patient-rated ECOG Performance Status | Cancer (n = 297)
|
Stroke (n = 51)
|
HIV (n = 51)
|
p-value | planned contrasts | ||||
|---|---|---|---|---|---|---|---|---|---|
| n | M (SD) | n | M (SD) | n | M (SD) | ||||
| 0, normal activities without symptoms | 89 | 44.9 (7.0) | 11 | 43.6 (5.2) | 21 | 43.8 (5.0) | 0.70 | C = S = H | |
| 1, some symptoms, but do not require bed rest during the waking day | 138 | 37.0 (8.9) | 30 | 37.3 (10.2) | 15 | 34.7 (9.0) | 0.62 | C = S = H | |
| 2, require bed rest for less than 50% of waking day |
|
70 | 22.9 (11.2) | 10 | 33.9 (9.1) | 15 | 19.5 (8.7) | < 0.01 | S > C = H (S less fatigue) |
| 3, require bed rest for more than 50% of waking day | |||||||||
Notes:
- Main effect for diagnosis – F(2, 398) = 4.56, p < 0.05
- Main effect for ECOG PS – F(2, 398) = 60.9, p < 0.001
- Diag x ECOG interaction – F(4, 398) = 3.15, p = 0.05
- By convention, lower scores on the FACIT-Fatigue indicate more fatigue.
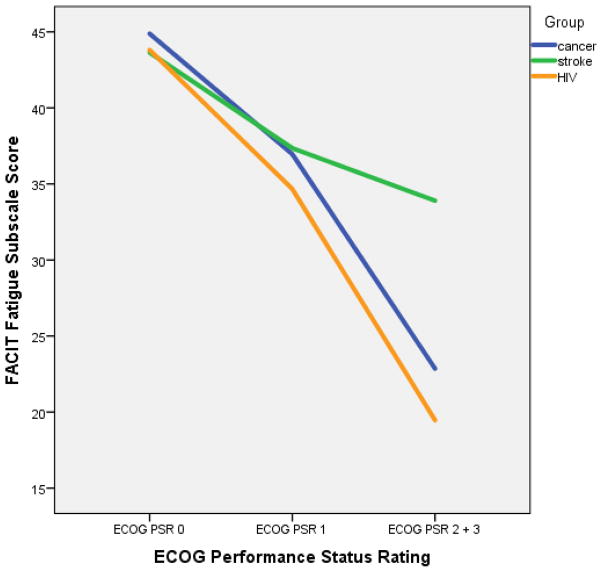
As expected, there was a main effect for performance status, with fatigue worsening with greater needs for bed rest (F[2, 398] = 60.9, p <0.001). We also found a main effect for diagnosis (F[2, 398] =4.56, p <0.05), which should be interpreted with caution, given the differential distribution of performance status ratings across the patient groups. A test for a diagnosis-ECOG interaction revealed a marginally significant interaction (F[4, 398] = 3.15, p = 0.05). As predicted, the patient groups reported similar levels of fatigue at the performance status ratings 0 and 1 (p>0.05). However, at the more severe ECOG PS, we found that patients with stroke experienced significantly less fatigue (33.9 ± 9.1) than those diagnosed with cancer (22.9 ± 11.2) or HIV (19.5 ± 8.7), p < 0.01.
Figure 1 depicts FACIT-Fatigue scores as a function of performance status ratings across the three patient groups: Fatigue worsens as performance status worsens. The trend is remarkably consistent for the cancer and HIV populations. For patients with stroke, those in the worst performance status category did not report as much fatigue as those with cancer or HIV.
Figure 1. Fatigue as a function of performance status rating.
By convention, lower scores on the FACIT-Fatigue indicate more fatigue.
Discussion
Fatigue is a common concern for patients with a variety of chronic illnesses. Having a common metric that can be used across clinical studies has the potential advantage of increasing comparability across studies, while improving our understanding of mechanisms and potential interventions for this symptom. As an initial step towards that goal, we tested the reliability and validity of fatigue, as measured by the FACIT-Fatigue scale in samples of patients with cancer, HIV, and stroke. Our results were promising and suggest that the scale may have utility for assessment of fatigue in populations outside of cancer (for which the scale was originally developed).
While this study did not ensure that the items of the FACIT-F capture every aspect of fatigue experienced by patients with HIV and stroke, it does provide reassurance that the set of FACIT-F questions are perceived as relevant and responsive to fatigue caused by a variety of conditions. The purpose of this article was not to extend or even claim content validity in stroke and HIV patients, but to evaluate the performance of this well-tested instrument in two new clinical populations. In that regard, key aspects of reliability and validity were demonstrated. Internal consistency reliabilities for the FACIT-F were uniformly high across the samples. Fatigue was correlated with general quality of life and performance status ratings, in expected ways. Evidence for divergent validity of the FACIT-F was similar across the samples – fatigue was not associated with sex, ethnicity, age, or specific clinical indicators. Fatigue ratings were lowest for stroke survivors; additionally, fatigue does not seem to characterize the most disabled stroke survivors to the extent that it does for patients with cancer or HIV. Unlike in cancer or HIV, both systemic diseases, worse performance status in stroke may reflect physical disability more than fatigue or low energy. This hypothesis has recently been supported in the stroke literature, albeit in relatively small samples.[24–26]
While the cancer sample was relatively large, this study included a limited number of patients with stroke or HIV. Readers should be cautious in generalizing conclusions based on association of clinical variables with fatigue; however, it is unlikely that a larger sample of patients would result in significant changes in the reliability of the scales or their validity in terms of general quality of life or performance status.
In summary, the FACIT-F is a brief, easy to administer, patient-reported instrument to assess fatigue. Originally developed to assess cancer-related fatigue, the scale has utility as a measure of fatigue across a number of chronic conditions. There are other instruments to choose from to measure fatigue in HIV and stroke. The value of demonstrating the validity of the FACIT-F in these patient groups is the ability to compare fatigue across groups without having to switch from one disease-specific instrument to another. This approach capitalizes on the common rather than unique elements of fatigue in these populations (to the extent there is a unique ground), and more readily allows for cross-disease comparisons of symptom reporting, for example.
Additional study of the scale’s psychometric properties in stroke and HIV may help improve our understanding of symptom onset, trajectory, and treatment. Ongoing research will soon allow for comparison of FACIT-Fatigue scores to those obtained using the fatigue measures from the Patient-Reported Outcomes Measurement Information System (PROMIS®; www.nihpromis.org) initiative.[27] The aim of this multi-center, collaborative project is to improve and standardize the measurement of clinically relevant symptoms, such as fatigue. PROMIS fatigue measures offer flexibility to researchers to measure over a broad spectrum of fatigue using dynamic computerized adaptive testing (CAT). FACIT-F items contributed to the PROMIS item banks;[28] creation of a linkage or look-up table to convert FACIT and PROMIS scores would allow for more direct comparison of completed and future studies that use these instruments.[29]
Acknowledgments
Deepa Rao’s contribution is supported by NIH grant # K23 MH 084551.
Footnotes
Competing Interest Statement
The authors have no competing interests to report.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Wagner LI, Cella D. Fatigue and cancer: causes, prevalence and treatment approaches. Br J Cancer. 2004 Aug 31;91(5):822–8. doi: 10.1038/sj.bjc.6602012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Butt Z, Rosenbloom SK, Abernethy AP, Beaumont JL, Paul D, Hampton D, et al. Fatigue is the most important symptom for advanced cancer patients who have had chemotherapy. J Natl Compr Canc Netw. 2008 May;6(5):448–55. doi: 10.6004/jnccn.2008.0036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cella D, Lai JS, Chang CH, Peterman A, Slavin M. Fatigue in cancer patients compared with fatigue in the general United States population. Cancer. 2002 Jan 15;94(2):528–38. doi: 10.1002/cncr.10245. [DOI] [PubMed] [Google Scholar]
- 4.Feigin VL, Lawes CM, Bennett DA, Anderson CS. Stroke epidemiology: a review of population-based studies of incidence, prevalence, and case-fatality in the late 20th century. Lancet Neurol. 2003 Jan;2(1):43–53. doi: 10.1016/s1474-4422(03)00266-7. [DOI] [PubMed] [Google Scholar]
- 5.Barker-Collo S, Feigin VL, Dudley M. Post stroke fatigue--where is the evidence to guide practice? N Z Med J. 2007;120(1264):U2780. [PubMed] [Google Scholar]
- 6.Choi-Kwon S, Kim JS. Poststroke fatigue: an emerging, critical issue in stroke medicine. Int J Stroke. 2011 Aug;6(4):328–36. doi: 10.1111/j.1747-4949.2011.00624.x. [DOI] [PubMed] [Google Scholar]
- 7.Ingles JL, Eskes GA, Phillips SJ. Fatigue after stroke. Arch Phys Med Rehabil. 1999 Feb;80(2):173–8. doi: 10.1016/s0003-9993(99)90116-8. [DOI] [PubMed] [Google Scholar]
- 8.Henderson M, Safa F, Easterbrook P, Hotopf M. Fatigue among HIV-infected patients in the era of highly active antiretroviral therapy. HIV Med. 2005 Sep;6(5):347–52. doi: 10.1111/j.1468-1293.2005.00319.x. [DOI] [PubMed] [Google Scholar]
- 9.Lee KA, Gay C, Portillo CJ, Coggins T, Davis H, Pullinger CR, et al. Symptom experience in HIV-infected adults: a function of demographic and clinical characteristics. J Pain Symptom Manage. 2009 Dec;38(6):882–93. doi: 10.1016/j.jpainsymman.2009.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Harmon JL, Barroso J, Pence BW, Leserman J, Salahuddin N. Demographic and illness-related variables associated with HIV-related fatigue. J Assoc Nurses AIDS Care. 2008 Mar-Apr;19(2):90–7. doi: 10.1016/j.jana.2007.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Voss JG. Predictors and Correlates of Fatigue in HIV/AIDS. J Pain Symptom Manage. 2005 Feb;29(2):173–84. doi: 10.1016/j.jpainsymman.2004.05.006. [DOI] [PubMed] [Google Scholar]
- 12.Jenkin P, Koch T, Kralik D. The experience of fatigue for adults living with HIV. J Clin Nurs. 2006 Sep;15(9):1123–31. doi: 10.1111/j.1365-2702.2006.01343.x. [DOI] [PubMed] [Google Scholar]
- 13.Vogelzang NJ, Breitbart W, Cella D, Curt GA, Groopman JE, Horning SJ, et al. Patient, caregiver, and oncologist perceptions of cancer-related fatigue: results of a tripart assessment survey. The Fatigue Coalition. Semin Hematol. 1997 Jul;34(3 Suppl 2):4–12. [PubMed] [Google Scholar]
- 14.Cella D, Lai JS, Stone A. Self-reported fatigue: one dimension or more? Lessons from the Functional Assessment of Chronic Illness Therapy – Fatigue (FACIT-F) questionnaire. Support Care Cancer. 2011 Sep;19(9):1441–50. doi: 10.1007/s00520-010-0971-1. [DOI] [PubMed] [Google Scholar]
- 15.Yellen SB, Cella DF, Webster K, Blendowski C, Kaplan E. Measuring fatigue and other anemia-related symptoms with the Functional Assessment of Cancer Therapy (FACT) measurement system. J Pain Symptom Manage. 1997 Feb;13(2):63–74. doi: 10.1016/s0885-3924(96)00274-6. [DOI] [PubMed] [Google Scholar]
- 16.Prue G, Rankin J, Allen J, Gracey J, Cramp F. Cancer-related fatigue: A critical appraisal. Eur J Cancer. 2006 May;42(7):846–63. doi: 10.1016/j.ejca.2005.11.026. [DOI] [PubMed] [Google Scholar]
- 17.Cella D, Yount S, Sorensen M, Chartash E, Sengupta N, Grober J. Validation of the Functional Assessment of Chronic Illness Therapy Fatigue Scale relative to other instrumentation in patients with rheumatoid arthritis. J Rheumatol. 2005 May;32(5):811–9. [PubMed] [Google Scholar]
- 18.Yount S, Sorensen MV, Cella D, Sengupta N, Grober J, Chartash EK. Adalimumab plus methotrexate or standard therapy is more effective than methotrexate or standard therapies alone in the treatment of fatigue in patients with active, inadequately treated rheumatoid arthritis. Clin Exp Rheumatol. 2007 Nov-Dec;25(6):838–46. [PubMed] [Google Scholar]
- 19.Hagell P, Hoglund A, Reimer J, Eriksson B, Knutsson I, Widner H, et al. Measuring fatigue in Parkinson’s disease: a psychometric study of two brief generic fatigue questionnaires. J Pain Symptom Manage. 2006 Nov;32(5):420–32. doi: 10.1016/j.jpainsymman.2006.05.021. [DOI] [PubMed] [Google Scholar]
- 20.Lai JS, Beaumont JL, Ogale S, Brunetta P, Cella D. Validation of the functional assessment of chronic illness therapy-fatigue scale in patients with moderately to severely active systemic lupus erythematosus, participating in a clinical trial. J Rheumatol. 2011 Apr;38(4):672–9. doi: 10.3899/jrheum.100799. [DOI] [PubMed] [Google Scholar]
- 21.Agnihotri P, Telfer M, Butt Z, Jella A, Cella D, Kozma CM, et al. Chronic anemia and fatigue in elderly patients: results of a randomized, double-blind, placebo-controlled, crossover exploratory study with epoetin alfa. J Am Geriatr Soc. 2007 Oct;55(10):1557–65. doi: 10.1111/j.1532-5415.2007.01357.x. [DOI] [PubMed] [Google Scholar]
- 22.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975 Nov;12(3):189–98. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 23.Lai JS, Cella D, Dineen K, Bode R, Von Roenn J, Gershon RC, et al. An item bank was created to improve the measurement of cancer-related fatigue. J Clin Epidemiol. 2005 Feb;58(2):190–7. doi: 10.1016/j.jclinepi.2003.07.016. [DOI] [PubMed] [Google Scholar]
- 24.Hoang CL, Salle JY, Mandigout S, Hamonet J, Macian-Montoro F, Daviet JC. Physical factors associated with fatigue after stroke: an exploratory study. Top Stroke Rehabil. 2012 Sep-Oct;19(5):369–76. doi: 10.1310/tsr1905-369. [DOI] [PubMed] [Google Scholar]
- 25.Crosby GA, Munshi S, Karat AS, Worthington E, Lincoln NB. Fatigue after stroke: frequency and effect on daily life. Disabil Rehabil. 2012;34(8):633–7. doi: 10.3109/09638288.2011.613517. [DOI] [PubMed] [Google Scholar]
- 26.Park JY, Chun MH, Kang SH, Lee JA, Kim BR, Shin MJ. Functional outcome in poststroke patients with or without fatigue. Am J Phys Med Rehabil. 2009 Jul;88(7):554–8. doi: 10.1097/PHM.0b013e3181a0dae0. [DOI] [PubMed] [Google Scholar]
- 27.Cella D, Riley W, Stone A, Rothrock N, Reeve B, Yount S, et al. The Patient-Reported Outcomes Measurement Information System (PROMIS) developed and tested its first wave of adult self-reported health outcome item banks: 2005–2008. J Clin Epidemiol. 2010 Nov;63(11):1179–94. doi: 10.1016/j.jclinepi.2010.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Junghaenel DU, Christodoulou C, Lai JS, Stone AA. Demographic correlates of fatigue in the US general population: results from the patient-reported outcomes measurement information system (PROMIS) initiative. 2011 Sep;71(3):117–23. doi: 10.1016/j.jpsychores.2011.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Smith E, Lai JS, Cella D. Building a measure of fatigue: the functional assessment of Chronic Illness Therapy Fatigue Scale. PM & R : the journal of injury, function, and rehabilitation. 2010 May;2(5):359–63. doi: 10.1016/j.pmrj.2010.04.017. [DOI] [PubMed] [Google Scholar]