Abstract
Background
Concerns about immune reconstitution inflammatory syndrome (IRIS) remain a barrier to antiretroviral therapy (ART) initiation during anti-tuberculosis treatment in co-infected patients.
Objective
We assessed IRIS incidence, severity, and outcomes relative to timing of ART initiation in patients with HIV-related tuberculosis (HIV-TB).
Setting
An outpatient clinic in Durban, South Africa
Patients
642 HIV-TB co-infected patients
Design
In a secondary analysis of the SAPiT trial, IRIS was assessed in patients randomized to initiate ART either within four weeks of tuberculosis treatment initiation (early integrated-treatment arm), within four weeks of completion of the intensive phase of tuberculosis treatment (late integrated-treatment arm) or within four weeks after tuberculosis therapy completion (sequential-treatment arm). IRIS was defined as new onset or worsening symptoms, signs or radiographic manifestations temporally related to treatment initiation accompanied by a treatment response. IRIS severity, hospitalization and time to resolution were monitored.
Results
IRIS incidence was 19.5 (n=43), 7.5 (n=18) and 8.1 (n=19) per 100 person-years in the early integrated-, late integrated-, and sequential-treatment arms, respectively; P < 0.001, and 45.5, 9.7 and 19.7 per 100 person-years in patients with baseline CD4+ counts <50 cells/mm3, P = 0.004. IRIS incidence was higher in the early integrated- compared to the late integrated- (incidence rate ratio (IRR) = 2.6, 95%confidence interval (CI): 1.5 to 4.8; P < 0.001) or sequential-treatment arm (IRR=2.4, 95%CI: 1.4 to 4.4; P < 0.001). IRIS cases in the early integrated-treatment arm were more severe (34.9% vs. 18.9%, P = 0.18); had significantly higher hospitalization rates (18/43 vs. 5/37; P = 0.01), and longer time to resolution (70.5 vs. 29.0 days; P = 0.001) compared to IRIS cases in the other two arms.
Limitation
IRIS could not be assessed, due to LTFU, withdrawal or death within 6 months of scheduled ART initiation, in more patients from the sequential treatment arm (n=74) than in the late integrated treatment arm (n=50) and in the early integrated treatment arm (n=32). This study did not assess IRIS risk in non-ambulant patients and in patients with extra-pulmonary and smear negative pulmonary tuberculosis.
Conclusion
Initiation of ART early during tuberculosis treatment resulted in significantly higher IRIS rates, with longer time to resolution, and more severe cases of IRIS requiring hospitalization. These findings, particularly relevant to patients initiating ART with CD4+ counts < 50 cells/mm3, need to be considered together with the increased survival benefit of early ART initiation in this group. Clintrials.gov: NCT00398996
INTRODUCTION
HIV-TB co-treatment is associated with an increased risk of immune reconstitution inflammatory syndrome (IRIS) (1, 2), overlapping side effects (3), potential drug interactions between rifampicin and antiretrovirals (4), high pill burden and programmatic challenges (5). Of these, IRIS, a paradoxical clinical deterioration in patients on effective treatment, remains the major obstacle to antiretroviral therapy (ART) initiation during tuberculosis treatment. IRIS results from the immune system’s restored ability to mount an inflammatory response following ART or tuberculosis treatment initiation (6). IRIS presents as two common clinical scenarios; unmasking IRIS; where a new infection is identified after ART initiation or, paradoxical IRIS, where despite effective treatment, clinical worsening of an infection occurs (6, 7). Clinical effects attributable to IRIS in TB HIV co-infected patients range from mild, self-limiting illness such as fever, return of cough, to more severe morbidity including lymph node enlargement, or recurrent, new, or deteriorating radiological manifestations and mortality (8).
Globally, an estimated 1.37 million people had HIV-related tuberculosis (HIV-TB) in 2009 (9). Recently published data provide compelling evidence for early ART initiation in HIV-TB patients. Data from the SAPIT(10,11), CAMELIA (10-13) and STRIDE(10-13) Trials, show substantial survival benefit with early ART initiation among HIV-TB co-infected patients with CD4+ count <50 cells/mm3, and the SAPIT and STRIDE studies both demonstrating no discernible difference in survival among patients with CD4+ count ≥50 cells/mm3(10-13). Thus far previous studies in HIV-TB co-infected patients have shown varying IRIS incidence rates of 11% to 71.4%(14-17) ART initiation proximal to tuberculosis treatment initiation, and in patients with baseline CD4+ counts <50 cells/mm3 have been associated with higher risk of IRIS (11-13, 16, 18-21).
Evidence for improved clinical outcomes is compelling in co-infected patients with CD4+ counts < 50 cells/mm3 (11-13). However, data from the SAPiT Trial show incidence of IRIS among patients with a CD4+ cell count < 50 cells/mm3 was 4.7 times higher in the early integrated-treatment arm as compared to the late integrated-treatment arm (P = 0.004), whereas among patients with a CD4+ ≥ 50 cells/mm3, the incidence of IRIS was 2.2 times (P = 0.01) higher in the early integrated-treatment arm as compared to the late integrated-treatment arm.
Prospective data for the systematic examination of incidence, severity, risk factors and outcome of IRIS events relative to timing of ART initiation in HIV-TB patients are limited. The purpose of this study was to assess IRIS risks and outcomes in patients initiating ART within a month of TB treatment initiation and compare this to patients initiating ART later to better guide patient-level decision-making about timing of initiation of ART in HIV-TB co-infected patients.
METHODS
Design Overview
The Starting Antiretroviral Therapy at Three Points in Tuberculosis (SAPIT) trial was a three-arm, randomized, open-label clinical trial in 642 patients from June 2005 to July 2010. The primary outcome of this study was to determine the optimal timing of ART initiation in tuberculosis HIV co-infected patients which has been reported elsewhere (10). In this paper, we present one of the secondary objectives of the SAPiT trial; an analysis of IRIS data by trial arm.
Setting and Participants
The study was conducted at the CAPRISA eThekwini HIV-tuberculosis clinic that adjoins the Prince Cyril Zulu Communicable Disease Centre, an out-patient tuberculosis facility in Durban, South Africa. HIV infected patients, ≥ 18 years old, with sputum smear positive pulmonary tuberculosis, and screening CD4+ count < 500 cells/mm3 were recruited and enrolled by study nurses and clinicians. All patients received standard cotrimoxazole prophylaxis and anti-tuberculosis therapy; the latter administered in a fixed drug combination consisting of rifampicin, isoniazid, ethambutol and pyrazinamide for two months (intensive phase) followed by isoniazid and rifampicin for four months (continuation phase). Patients with re-treatment tuberculosis also received streptomycin during a longer intensive phase of tuberculosis treatment as per South African treatment guidelines (22).
Randomization and interventions
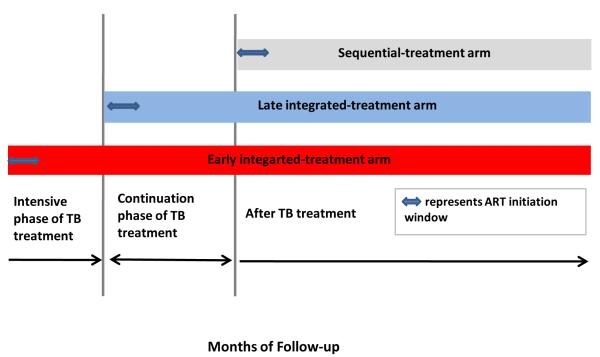
Patients were randomized to initiate ART within 4 weeks of tuberculosis treatment initiation (early integrated-treatment arm), within 4 weeks after completion of intensive phase of tuberculosis treatment (late integrated-treatment arm), or within 4 weeks after tuberculosis therapy completion (sequential-treatment arm) (Figure 1). A random allocation sequence was generated by the study statistician to randomly assign patients to one of the intervention arms. Patients were randomly assigned in a 1:1:1 ratio (with the use of sealed envelopes) to one of three study groups in permuted blocks of six or nine with no stratification. The standard first-line ART regimen comprised once daily Lamivudine (300mg), enteric coated Didanosine (250mg –if weight < 60kg and 400mg if weight > 60kg) and Efavirenz (600mg). Placebos were not used in this trial, hence study clinicians were not blind to treatment arm allocation when they assessed possible IRIS.
Figure 1.
SAPiT trial study schema
Outcomes and follow-up
Study patients, irrespective of arm allocation, were evaluated for features of suspected IRIS using a standardized set of criteria at every study visit. IRIS was defined as occurrence of new onset or worsening symptoms, signs or radiographic features temporally related to antiretroviral or tuberculosis treatment initiation; together with a CD4+ count increase and upon exclusion of confirmed tuberculosis or antiretroviral treatment failure, toxicity, non-adherence, or new concurrent opportunistic infections or other complication. This definition of IRIS used in this study is in accordance with other published case definitions (23, 24) in the following respects: IRIS occurred after the diagnosis of an underlying opportunistic infection (TB in this instance); the IRIS definition included an ART treatment response; presence of new onset or worsening clinical features consistent with an inflammatory process; timing of IRIS onset relative to TB and ART start, the exclusion of ART and TB treatment failure, toxicity and concurrent infections. The IRIS definition used in this study varied from published case definitions where it was not required that patients demonstrate an initial response to TB treatment, or conversion of Tuberculin Skin Test status from positive to negative.
Presentation of specific signs and symptoms indicative of IRIS as per standardised IRIS checklist triggered a detailed IRIS assessment, which included clinical examination, urine, sputum and blood-microscopy, culture and sensitivity, and chest x-ray evaluations. CD4+ count was not always done at the time of IRIS; however, CD4+ cell response to ART, in the presence of other protocol defining criteria of IRIS, was used when making the determination of suspected IRIS. All patients presenting with DAIDS clinical Grade 3 and 4 IRIS events, or IRIS events of a lower grade that warranted further investigation and management were referred for tertiary care to the Infectious Diseases Unit at a local hospital. All cases of IRIS identified during the trial were retrospectively assessed and found to meet the 2008 International Network for the Study of HIV-associated IRIS (INSHI) definition of one major or two minor clinical criteria (7). All IRIS events were followed-up, either until resolution or if unresolved to end of study follow-up. An experienced independent clinician conducted a detailed chart review of all suspected cases once all clinical and roentgenographic information became available, to verify the IRIS diagnosis, for inclusion in this analysis. Severity of IRIS was determined based on: IRIS associated deaths; life-threatening events; IRIS associated hospitalization and duration of hospitalization; number of events warranting steroid use; and proportion of IRIS events that did not resolve or resolved with sequelae at study conclusion. Every adverse event elicited by the IRS assessment tool was graded for severity using the Division of AIDS (NIAID/NIH) Table for Grading Adult and Paediatric Adverse Events, version 1.0, 28 December 2004.
Statistical Analysis
The sample size for the SAPiT trial was calculated as 649, which was based on the primary mortality outcome. The study was not powered for the secondary IRIS outcome. Following the second planned interim review on 1 September 2008, the study Safety Monitoring Committee (SMC) recommended, based on superior survival in the combined integrated-therapy groups, that all participants in the sequential-therapy group be initiated on ART as soon as possible and that the two integrated-therapy groups continue follow-up with no changes. The data presented in the 2010 NEJM publication (10) provided incomplete trial data from the September 2008 SMC review. The data presented in the 2011 NEJM publication (11), and in this analysis of IRIS data were conducted on the complete trial dataset. Additional deaths, AIDS defining illnesses, IRIS events and follow-up data to 18 months occurring since the SMC lock in 2008 are now included.
Statistical analyses were done using SAS (version 9.2; SAS Institute Inc., Cary, NC, USA). All statistical tests were two sided. Study duration was calculated as time from randomization to, IRIS event; withdrawal from study; death or 18 months post randomization, whichever occurred first. Retention rate was calculated as number of patients who completed study divided by the number randomized, minus deaths. Poisson approximations were used to calculate confidence intervals (CIs) for incidence rates. The CIs for the incidence rate ratios (IRRs) were calculated using the F-distribution. Fisher’s exact test or Fisher-Freeman-Halton test was used for analysis of categorical data. Wilcoxon two-sample, one way ANOVA, or the Kruskal-Wallis tests were used for the analysis of continuous data.
The trial was approved by the Biomedical Research Ethics Committee of the University of KwaZulu-Natal (E107/05), and the Medicines Control Council of South Africa (Ref: 20060157).
RESULTS
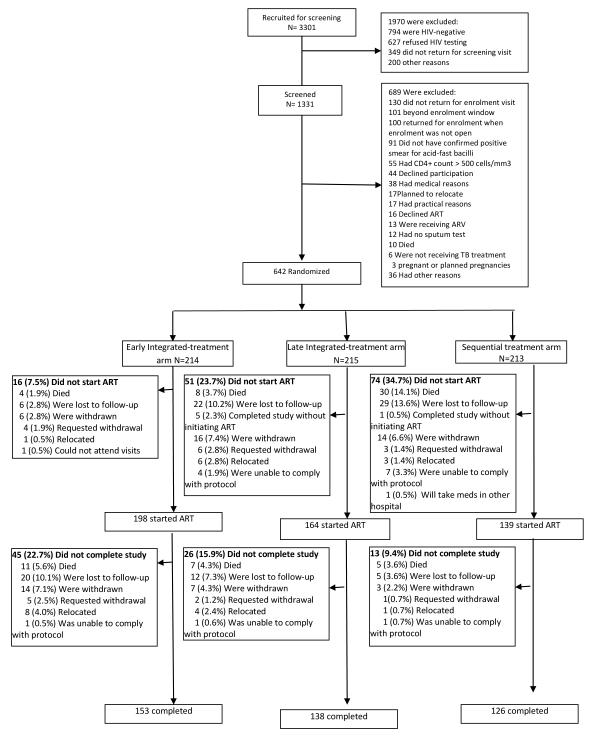
Of 1331 patients screened for eligibility, 642 were enrolled and randomized (Figure 2). Patients in the early integrated- (n=214), late integrated- (n=215), and sequential- (n=213) treatment arms had similar baseline demographic and clinical characteristics (Table 1). Retention rate (patients who completed their scheduled study exit visit divided by number of enrolled patients who did not die during follow-up) at 18 months was 76.9%, 71.5% and 70.9% in the early integrated-, late integrated- and sequential-treatment arms, respectively. While retention rates are similar across the three treatment arms, due to the timing of ART initiation in the 3 arms, a number of patients who could not be assessed for IRIS. These patients who exited from the study or died prior to 6 months post-ART initiation were 32 in the early integrated treatment arm, 50 in the late integrated treatment arm, and 74 in the sequential treatment arm. Overall, these 156 patients were younger, and the majority male (Appendix Table 1 and 2).
Figure 2.
SAPiT trial: Screening, randomization, and follow-up of study participants
Table 1.
Baseline characteristics of participants in the SAPiT trial
| Variable | Early integrated- treatment am (N=214) |
Late integrated- treatment arm (N=215) |
Sequential- treatment arm (N=213) |
P-value | Patients who developed IRIS (N=80) |
Patients who did not develop IRIS (N=562) |
P-value |
|---|---|---|---|---|---|---|---|
| Age in years (mean (SD)) | 34.3 (8.0) | 34.5 (8.7) | 33.9 (8.2) | 0.64 | 34.3 (6.4) | 34.2 (8.5) | 0.97 |
| Number of males (%) | 97 (45.3) | 112 (52.1) | 110 (51.6) | 0.30 | 39 (48.8) | 280 (49.8) | 0.91 |
| BMI (kg/m2) <18.5 (%) * | 25 (11.7) | 28 (13.0) | 29 (13.6) | 0.79 | 10 (12.5) | 72 (12.9) | 1.00 |
| History of tuberculosis (%) | 80 (37.4) | 68 (31.6) | 66 (31.0) | 0.31 | 31 (38.8) | 183 (32.6) | 0.31 |
| Extra pulmonary tuberculosis (%) † | 10 (4.7) | 9 (4.2) | 9 (4.3) | 1.00 | 5 (6.3) | 23 (4.1) | 0.38 |
| WHO stage 4(%) | 14 (6.5) | 11 (5.1) | 13 (6.1) | 0.80 | 6 (7.5) | 32 (5.7) | 0.46 |
| CD4+ count cells/mm3, median (IQR) | 154.5 (75-261) | 149 (77-244) | 140 (69-247) | 0.60 | 91 (36-176.5) | 155 (78-261) | <0.0001 |
| Number of patients with CD4+ count < 50 cells/mm (%) |
37 (17.3) | 35 (16.3) | 41 (19.2) | 0.71 | 26 (32.5) | 87 (15.5) | <0.001 |
| CD8 count cells/mm3, median (IQR) | 697(417-1030) | 659.5 (455-1084) | 663 (476-957) | 0.93 | 586 (371.5-987) | 676 (475-1007) | 0.09 |
| Log10 HIV RNA, copies/ml, mean (SD) ‡ | 5.0 ( 0.9) | 5.0 ( 0.9) | 5.1 (0.7) | 0.38 | 5.5 (0.7) | 5.0 (0.9) | <0.0001 |
IQR-Interquartile range SD-standard 2 deviation BMI-Body Mass Index IRIS–Immune Reconstitution Inflammatory Syndrome
5 patients in the sequential -treatment arm had missing BMI and missing data is not included in percentage calculation
1 patient in the late integrated- treatment arm and 3 patients in the sequential -treatment arm had missing extra pulmonary tuberculosis and missing data is not included in percentage calculation
Baseline viral load was not available for 16 patients in each of the early integrated- and late integrated -treatment arms and 12 in the sequential arm
IRIS incidence
Of the 642 patients evaluated at every study visit, there were 85 patients with suspected IRIS. Five patients with pulmonary infiltrates, respiratory symptoms, thoracic lymphadenopathy, cervical lymphadenopathy, abdominal pain and fever, subsequently found to have undiagnosed multi-drug resistant tuberculosis at the time of the IRIS event, were therefore not regarded as IRIS cases. Seventy-four of the remaining 80 patients with suspected IRIS demonstrated a CD4+ count increase. The remaining six (five - early integrated-; one - late integrated-), did not have available CD4+ count data at or after the IRIS diagnosis; since their exclusion did not materially change the results, these six patients were included in the analysis.
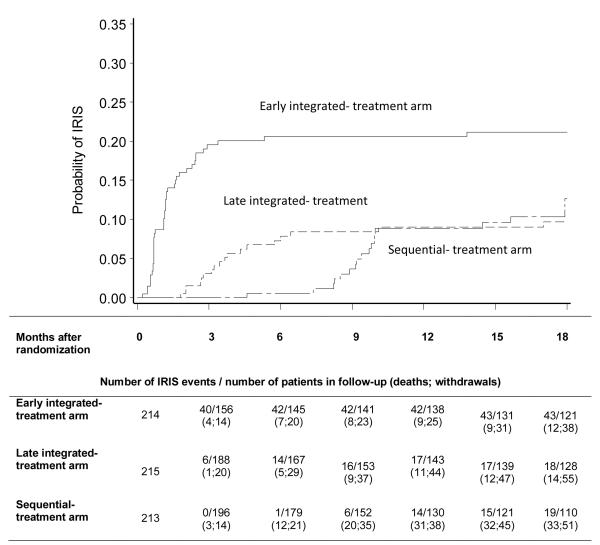
There were 43 patients with IRIS in the early integrated-, 18 in the late integrated- and 19 in the sequential-treatment arm (Table 2). IRIS incidence was significantly higher in the early integrated-: 19.5 compared to either late integrated-: 7.5, P < 0.001; or sequential-treatment arm: 8.1 per 100 person years; P < 0.001 (Figure 3, Table 2). Median (Interquartile range (IQR)) days to IRIS from ART initiation was 17.5 in the early integrated-treatment arm, 17 in the late integrated-treatment arm and 28 in the sequential-treatment arm P = 0.32. The median (IQR) CD4+ count (cells/mm3) at or near the IRIS event was 101; 116.5 and 132 in the early integrated-, late integrated- and sequential-treatment arms respectively; P = 0.52. Our results hold, even if the 6 cases that did not have CD4+ counts at or after the diagnosis of IRIS were excluded from the analysis (Appendix Table 3).
Table 2.
IRIS incidence in each of the study arms in the SAPiT trial
| Early integrated-treatment arm | Late integrated-treatment arm | Sequential -treatment arm | IRIS incidence rate ratio (95%CI) ; P- values |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. of IRIS event s |
No of death s or AIDS defini ng illnes s |
No. of person- years (n) |
IRIS incidence rate / 100 person- years(95% CI) |
No. of IRIS event s |
No of deaths or AIDS defining illness |
No. of person- years (n) |
IRIS incidence rate / 100 person- years (95% CI) |
No. of IRIS event s |
No of deaths or AIDS definin g illness |
No. of person- years (n) |
IRIS incidence rate / 100 person- years (95% CI) |
Early vs late integrated |
Early vs sequenti al |
Late vs sequentia l |
|
| All patie nts |
43 | 28 |
*219.8 (214) |
19.5 (14.2-26.4) |
18 | 26 |
*239.6 (215) |
7.5 (4.5-11.9) |
19 | 47 | 235.4 (213) |
8.1 (4.9-12.6) |
2.6 (1.5-4.8); <0.001 |
2.4 (1.4-4.4); <0.001 |
0.9 (0.5-1.9); 0.86 |
| <50 cells/ mm3 |
14 | 8 |
*30.8 (37) |
45.5 (24.9-76.4) |
4 | 11 |
*41.3 (35) |
9.7 (2.6-24.8) |
8 | 20 | 40.6 (41) |
19.7 (8.5-38.8) |
4.7 (1.5-19.6); 0.004 |
2.3 (0.9-6.4); 0.05 |
0.5 (0.1-1.8); 0.19 |
| ≥50 cells/ mm3 |
29 | 20 |
*189.0 (177) |
15.3 (10.3-22.0) |
14 | 15 |
*198.3 (180) |
7.1 (3.9-11.8) |
11 | 27 | 194.8 (172) |
5.6 (2.8-10.1) |
2.2 (1.1-4.5); 0.01 |
2.7 (1.3-6.0); 0.003 |
1.3 (0.5-3.0); 0.53 |
| Scenario 1: Assuming patients who dropped-out before ART initiation and within six months post ART initiation stayed in the study and that their IRIS rates were twice that of those who did not drop-out | |||||||||||||||
| All patie nts |
52 | 219.8 (214) |
23.7 (17.2-30.1) |
24 | 239.6 (215) |
10.0 (6.0-14.0) |
26 | 235.4 (213) |
11.0 (6.8-15.3) |
2.4 (1.5-3.8); <0.001 |
2.2 (1.3-3.4); 0.002 |
0.9 (0.5-1.6); 0.73 |
|||
| <50 cells/ mm3 |
16 | 30.8 (37) |
51.9 (26.3-76.9) |
4 | 41.3 (35) |
9.7 (2.6-24.8) |
10 | 40.6 (41) |
24.6 (9.3-39.5) |
5.4 (1.8-16.0); 0.003 |
2.1 (0.9-4.6); 0.06 |
0.4 (0.1-1.3); 0.11 |
|||
| ≥50 cells/ mm3 |
36 | 189.0 (177) |
19.0 (12.8-25.3) |
20 | 198.3 (180) |
10.1 (5.7-14.5) |
16 | 194.8 (172) |
8.2 (4.2-12.2) |
1.9 (1.1-3.3); 0.02 |
2.3 (1.3-4.2); 0.01 |
1.2 (0.6-2.4); 0.54 |
|||
| Scenario 2: Assuming patients who dropped-out before ART initiation and within six months post ART initiation stayed in the study and that their IRIS rates were five times that of those who did not drop-out | |||||||||||||||
| All patie nts |
65 | 219.8 (214) |
29.6 (22.4-36.7) |
33 | 239.6 (215) |
13.8 (9.1-18.4) |
36 | 235.4 (213) |
15.3 (10.3-20.3) |
2.1 (1.4-3.3); <0.001 |
1.9 (1.3-2.9); 0.001 |
0.9 (0.6-1.4); 0.66 |
|||
| <50 cells/ mm3 |
19 | 30.8 (37) |
61.7 (33.7-88.9) |
4 | 41.3 (35) |
9.7 (2.6-24.8) |
13 | 40.6 (41) |
32.0 (14.5-48.9) |
6.4 (2.2-18.7); <0.001 |
1.9 (0.9-3.9); 0.07 |
0.3 (0.1-0.9); 0.04 |
|||
| ≥50 cells/ mm3 |
46 | 189.0 (177) |
24.3 (17.3-31.4) |
29 | 198.3 (180) |
14.6 (9.3-20.0) |
23 | 194.8 (172) |
11.8 (7.0-16.6) |
1.7 (1.05-2.6); 0.03 |
2.1 (1.3-3.4); 0.005 |
1.2 (0.7-2.1); 0.44 |
|||
| Scenario 3: Assuming patients who dropped-out or died before ART initiation and within six months post ART initiation stayed in the study and that their IRIS rates were twice that of those who completed | |||||||||||||||
| All patie nts |
55 | 219.8 (214) |
25.0 (18.9-32.6) |
26 | 239.6 (215) |
10.9 (6.7-15.0) |
31 | 235.4 (213) |
13.2 (8.5-17.8) |
2.3 (1.5-3.7); <0.001 |
1.9 (1.2-3.0); 0.004 |
0.8 (0.5-1.4); 0.47 |
|||
| <50 cells/ mm3 |
17 | 30.8 (37) |
55.2 (28.8-80.9) |
5 | 41.3 (35) |
12.1 (1.6-22.9) |
14 | 40.6 (41) |
34.5 (16.2-52.2) |
4.6 (1.7-12.4); 0.003 |
1.6 (0.8-3.2); 0.19 |
0.4 (0.1-0.98); 0.04 |
|||
| ≥50 cells/ mm3 |
38 | 189.0 (177) |
20.1 (13.7-26.5) |
21 | 198.3 (180) |
10.6 (6.1-15.1) |
17 | 194.8 (172) |
8.7 (4.6-12.9) |
1.9 (1.1-3.2); 0.02 |
2.3 (1.3-4.1); 0.004 |
1.2 (0.6-2.3); 0.55 |
|||
| Scenario 4: Assuming patients who dropped-out or died before ART initiation and within six months post ART initiation stayed in the study and that their IRIS rates were five times that of those who completed | |||||||||||||||
| All patie nts |
74 | 219.8 (214) |
33.7 (26.0-41.3) |
37 | 239.6 (215) |
15.4 (10.4-20.4) |
43 | 235.4 (213) |
18.3 (12.8-23.8) |
2.2 (1.5-3.2); <0.001 |
1.8 (1.3-2.7); 0.001 |
0.8 (0.5-1.3); 0.45 |
|||
| <50 cells/ mm3 |
23 | 30.8 (37) |
74.7 (43.9- 104.5) |
6 | 41.3 (35) |
14.5 (2.9-26.3) |
25 | 40.6 (41) |
61.6 (37.1-84.9) |
5.1 (2.1-12.6); <0.001 |
1.2 (0.7-2.1); 0.50 |
0.2 (0.1-0.6); 0.002 |
|||
| ≥50 cells/ mm3 |
51 | 189.0 (177) |
27.0 (19.6-34.4) |
31 | 198.3 (180) |
15.6 (10.1-21.2) |
28 | 194.8 (172) |
14.4 (9.0-19.7) |
1.7 (1.1-2.7); 0.02 |
1.9 (1.2-3.0); 0.01 |
1.1 (0.7-1.8); 0.75 |
|||
| Scenario 5: Composite endpoint of death or IRIS | |||||||||||||||
| All patie nts |
55 | 219.8 (214) |
25.0 (18.9-32.6) |
32 | 239.6 (215) |
13.4 (9.1-18.9) |
52 | 235.4 (213) |
22.1 (16.5-29.0) |
1.9 (1.2-3.0); 0.003 |
1.1 (0.8-1.7); 0.44 |
0.6 (0.4-0.96); 0.03 |
|||
| <50 cells/ mm3 |
16 | 30.8 (37) |
51.9 (29.7-84.5) |
10 | 41.3 (35) |
24.2 (11.6-44.5) |
22 | 40.6 (41) |
54.2 (33.9-82.0) |
2.1 (0.9-5.3); 0.07 |
1.0 (0.5-1.9); 0.98 |
0.4 (0.2-0.9); 0.03 |
|||
| ≥50 cells/ mm3 |
39 | 189.0 (177) |
20.6 (14.7-28.2) |
22 | 198.3 (180) |
11.1 (7.0-16.8) |
30 | 194.8 (172) |
15.4 (10.4-22.0) |
1.9 (1.1-3.3); 0.01 |
1.3 (0.8-2.2); 0.20 |
0.7 (0.4-1.3); 0.28 |
|||
IRIS – Immune Reconstitution Inflammatory Syndrome
CI – Confidence Interval
Person years given in this table are slightly different from those reported in the NEJM 2011 paper due to some changes in the calendar time used for censoring.
Figure 3.
Kaplan-Meier estimates of cumulative probability of Immune Reconstitution Inflammatory Syndrome in the 3 SAPiT treatment arms
IRIS incidence in the subset of patients enrolled with CD4+ counts <50 cells/mm3 were 45.5 in the early integrated-; 9.7 in the late integrated-; and 19.7 per 100 person-years in the sequential-treatment arms respectively; P = 0.01 (Table 2). IRIS incidence in patients with CD4+ counts < 50 cells/mm3 was higher in the early integrated- compared to the late integrated-; P = 0.004 and sequential-treatment arms; P = 0.05. By comparison, in patients with CD4+ counts ≥ 50 cells/mm3, incidence of IRIS were 15.3; 7.1 and 5.6 per 100 person-years in the early integrated-, late integrated- and sequential- treatment arms respectively; P = 0.002. The incidence rate in patients enrolled with CD4+ counts ≥ 50 cells/mm3 was significantly higher in the early integrated- when compared to the late integrated-; P = 0.01 and sequential-treatment arms; P = 0.003 (Table 2). Overall, in patients with CD4+ counts < 50 cells/mm3, the median time to IRIS from ART initiation was double compared to patients with CD4+ counts ≥ 50 cells/mm3; 28 days (IQR 15 to 56 days) vs. 14 days (IQR 13 to 28 days), P = 0.01. The interaction between the three treatment arms and CD4+ count above and below 50 cells/mm3 on the risk of IRIS, was not statistically significant (P = 0.97) indicating homogeneity across the two CD4+ count strata in the effect of time to ART initiation on the risk of IRIS. Results from various sensitivity analyses hold with the assumption that patients who were LTFU, withdrew or died prior to six months post their scheduled ART initiation time-point, experienced IRIS rates 2 and 5 times the rates observed in those patients who completed follow-up. In a sensitivity analysis of the composite endpoint of death or IRIS, we find that our results hold for the comparison between the early integrated and late integrated treatment arm only (Table 2).
New onset or worsening respiratory symptoms (59/80) was the commonest clinical presentations of IRIS (Figure 4). Fever was uncommon (2/80), while 22.5% (18/80) of IRIS patients presented with new onset or worsening lymphadenopathy. In the sequential-treatment arm two participants, who had completed their tuberculosis treatment when ART was initiated, developed active TB within 3 months of ART initiation, and met the provisional INSHI case definition of unmasking tuberculosis-associated IRIS.
Figure 4.
Proportion of all Immune Reconstitution Inflammatory Syndrome (IRIS) patients who developed clinical signs, symptoms and radiographic features of IRIS.
Severity of IRIS events
Severe or life-threatening IRIS events occurred in 34.9%, 22.2%, and 15.8% of IRIS patients in the early integrated-, late integrated-, and sequential-treatment arms, respectively (Table 3). Forty-two per cent of patients with IRIS in the early integrated-treatment arm compared to 22% in the late integrated-treatment arm, and 5.0% in the sequential-treatment arm were hospitalized for IRIS related conditions; P = 0.01. A total of 34.9% (15/43) of IRIS cases in the early integrated-treatment arm compared to 18.9% (7/37) of the IRIS cases in the other two treatment arms were severe; P = 0.18. Median (IQR) duration of IRIS associated hospitalization was 9.5 (3–20 days) and 11.5 (6–23 days) in the early and late integrated-treatment arms respectively. In the sequential-treatment arm only one patient was hospitalized for 60 days, P = 0.28. Steroid therapy was initiated in eight IRIS patients; four- early integrated-; one-late integrated- and three in the sequential-treatment arm respectively; P = 0.69. Irrespective of baseline CD4+ count above or below 50 cells/mm3, medical visits were not increased due to the occurrence of IRIS. Seventy-two of the 80 patients with IRIS, had unscheduled medical visits at a median of 3 (range: 1–12) unscheduled medical visits compared to patients without IRIS, of whom 351/562 patients presented for unscheduled medical visits, at a median of 2 (range: 1–18) unscheduled medical visits. Unscheduled medical visits occurred at a median of 2 visits for both patients with CD4+count < 50 cells/mm3 (range: 1–16) and CD4+ count ≥ 50 cells/mm3, (range: 1–18), among the 80/113 patients and 343/529 patients that presented for unscheduled medical visits in each category respectively. In addition, there was no statistically significant difference in rates of single drug switching; p=0.54, or whole regimen change due to virologic failure, p=0.21, among patients with IRIS compared to those without IRIS.
Table 3.
Severity of immune reconstitution inflammatory syndrome
| Study arm | Early integrated- treatment am |
Late integrated- treatment arm |
Sequential- treatment arm |
|---|---|---|---|
| Number of patients with IRIS | 43 | 18 | 19 |
| Grade3/4* IRIS, n (%) | 15 (34.9) | 4 (22.2) | 3 (15.8) |
| Hospitalization for IRIS, n (%) | 18 (41.9) | 4 (22.2) | 1 (5.3) |
| Received steroids for IRIS, n (%) | 4 (9.3) | 1 (5.6) | 3 (15.8) |
|
Complete resolution of IRIS without
sequelae, n (%) |
38 (88.4) | 18 (100) | 16 (84.2) |
|
Time to IRIS resolution, median
(IQR), days |
70.5 (42-151) | 34.0 (24-118) | 23.5 (11.5-40.5) |
| IRIS associated deaths, n (%) | 2 (4.7) | 0 | 0 |
|
Time to IRIS from ART initiation, median (IQR), days |
17.5 (10-30) | 17 (14-28) | 28 (14-44) |
|
CD4 + count cells/mm3, median (IQR), days |
101 (36-172) | 116.5 (45-342) | 132 (33-251) |
IRIS – Immune Reconstitution Inflammatory Syndrome events were graded for severity using the Division of AIDS Table for Grading Adult and Pediatric Adverse Events, 2004
IQR- Interquartile range
Seventy two of the 80 IRIS events resolved completely during follow-up. Time to IRIS resolution was longer in the early integrated-, compared to the late integrated- and sequential-treatment arms, respectively; P = 0.001 (Table 3). Among the unresolved IRIS events, there were two deaths; both in the early integrated-treatment arm, due to respiratory complications. Two events, new onset pulmonary infiltrates (early integrated-treatment arm) and worsening papular pruritic eruption (sequential-treatment arm) did not resolve by study completion. Three IRIS events resolved with sequelae; tuberculosis meningitis and meningitis (both-early integrated-treatment arm) and herpes zoster (sequential-treatment arm). The outcome of IRIS in one patient was unknown.
Risk factors associated with IRIS
CD4+ cell count, viral load and WHO stage were associated with an increased risk of IRIS. IRIS incidence was 23.1 (95% CI: 15.1 to 33.8), 12.3 (95% CI: 8.7 to 16.8) and 5.6 (95% CI: 3.2 to 9.4) per 100 person years in patients with CD4+ counts <50 cells/mm3, between 50–200 cells/mm3 and >200 cells/mm3. Similarly, IRIS incidence was higher in patients with baseline viral load above 100 000 copies/ml compared to below 100 000 copies/ml (16.2 (95% CI: 12.4 to 20.8) vs. 6.0 (95% CI: 3.4 to 9.7)) per 100 person years (Appendix Table 4).
DISCUSSION
Patients with HIV-related TB who initiated ART in the first four weeks of tuberculosis treatment had a more than two-fold higher IRIS incidence than patients who initiated ART later. Importantly, IRIS occurring in patients who initiated ART early was more severe, took longer to resolve and required hospitalization more frequently.
Higher IRIS rates in patients starting ART early during tuberculosis treatment have been shown in clinical trials (11-13) as well as retrospective and observational studies (1, 25-27). In a study conducted in Cambodia, IRIS incidence rates were 3.76 vs 1.53 per 100 person months in the early vs late arms respectively (12). In another large multi-center trial (AIDS Clinical Trials Group (ACTG) 5221), IRIS rates of 11% with immediate ART vs 5% with early ART were reported (13), with 11.5% of patients with CD4+ count < 50 vs 5.4% of patients with CD4+ count ≥ 50 developing IRIS. This study also showed a substantial CD4+ count/ART arm interaction, P = 0.014. Time to IRIS from ART initiation was comparable across the STRIDE and SAPiT studies. The commonest clinical presentation of IRIS in the STRIDE study was lymphadenopathy followed by new onset constitutional symptoms; whereas fever followed by peripheral lymphadenopathy was the commonest clinical presenting features of IRIS in the CAMELIA study. In contrast, new onset or worsening respiratory symptoms then pulmonary infiltrates accounted for the most common presenting IRIS features in the SAPiT trial. These varied presentations of IRIS are likely due to the differing profile of patients in the 3 studies, i.e. patients in the SAPiT trial were all ambulant with smear positive pulmonary tuberculosis, patients in the STRIDE trial were a mix of ambulant and hospitalized patients that had all forms of TB, whereas patients from the CAMELIA trial were largely hospitalized with a clinically significantly lower baseline CD4+ counts than the other 2 trials.
Our study also showed that in severely immunocompromised patients (CD4+ counts <50 cells/mm3), risk of IRIS was almost 5 times higher in those initiating ART early. It is important to note that within this population, studies have demonstrated substantial decrease in risk of morbidity and mortality with early ART initiation (11-13). Patients with CD4+ count ≥50 cells/mm3 did not gain a survival benefit from ART initiation within the first 4 weeks compared to ART initiation at the start of the continuation phase of tuberculosis treatment, but experienced a two-fold (15.3 vs 7.1 per 100 person years) higher risk of developing IRIS (11). Similarly, no discernable survival or decreased morbidity benefit was evident with early ART initiation in patients with CD4+ counts ≥50 cells/mm3 in the ACTG 5221 study; the incidence rate of AIDS or death was 11.5% in patients who initiated ART within 2 weeks compared to 10.3% in those initiating ART within 8 - 12 weeks of tuberculosis treatment initiation (13).
Not only was IRIS more common, it was also more severe in patients initiating ART in the first 4 weeks of tuberculosis treatment. Those who initiated ART early had greater burden of IRIS related morbidity; longer duration of illness, more steroid use, and higher rates of hospitalization. Two-thirds of all severe or life-threatening IRIS associated adverse events occurred in early integrated-treatment arm patients. These patients experienced a disproportionate number of hospitalizations; accounting for approximately 80% of all IRIS-associated hospitalizations in the study. Consistent with previously published data (14, 20, 26, 28), low baseline CD4+ count and high baseline viral load were significant risk factors for IRIS in our study. Time to IRIS resolution in the early integrated-treatment arm was two-fold higher than the late integrated-treatment arm and three-fold higher than the sequential-treatment arm. Furthermore, 50% of all patients requiring steroids for management of IRIS were in the early integrated-treatment arm. Steroid therapy was prescribed in 10% of IRIS patients in this study to ameliorate the clinical course of IRIS where life-threatening space occupying lesions or danger of respiratory failure existed. The role of corticosteroids in the management of IRIS has however not been clearly defined. On one hand a study has demonstrated that steroids reduced the need for hospitalization and therapeutic procedures, and hastened improvement in IRIS symptoms, while other studies caution steroid use in IRIS as it has been shown to exacerbate underlying opportunistic infections including drug-resistant tuberculosis and Kaposi’s sarcoma (29, 30). There was no difference in the number of unscheduled medical visits, drug switching from toxicity or virologic failure among patients who developed IRIS compared to patients without IRIS. It is important to underscore that overall we found a relatively low IRIS associated death rate and a relatively benign nature of IRIS. These findings are clinically relevant on two levels; at an individual patient level these findings enhance confidence in co-administering TB and HIV treatment without fear of worsening morbidity and mortality due to IRIS; and, at a public health level these findings indicate that TB and HIV integration can occur without increasing the availability of resources for IRIS management especially in settings where TB and HIV are endemic.
In light of higher IRIS associated morbidity with early ART in tuberculosis treatment, the decision on timing of ART in co-infected patients should be influenced by baseline CD4+ count due to association between risk of IRIS as well as reported morbidity and mortality benefit by CD4+ count strata. Thus, in patients with CD4+ count <50 cells/mm3, balance of benefit/risk would favour initiation of ART within four weeks of TB treatment initiation. On the other hand, in patients with CD4+ counts ≥50 cells/mm3, the decision for early versus later initiation of ART during TB treatment must be weighed against availability of clinical capacity to diagnose and manage IRIS. Hence, careful consideration is required to assess, in each clinical setting, potential benefits versus risks of each strategy. Importantly, in patients with CD4+ count >50 cells/mm3, while ART initiation may be deferred until 8-12 weeks after tuberculosis treatment initiation, every effort should be made to initiate ART no later than 12 weeks after tuberculosis treatment initiation. Additionally, among patients with CD4+ count >50 cells/mm3 with clinical disease of major severity; organ system dysfunction; or low Karnofsky score, Body Mass Index (BMI), haemoglobin or albumin; early initiation of ART should be strongly considered, as these are associated with higher mortality rates.
We acknowledge several limitations to our study. Firstly, as we enrolled ambulant patients with sputum positive pulmonary TB, these results may not be directly generalizable to all forms and severity of tuberculosis in HIV infected patients. Though the differences in patient retention across the 3 study arms was not statistically significant, it is possible but unlikely that there was a greater ascertainment of IRIS in the early integrated-treatment arm compared to the other 2 arms as a result of more patients being retained in that arm.
In the absence of use of placebos in this trial, study clinicians were aware of when a given patient had begun ART, which could have biased the assessment of whether IRIS was present. We mitigated this bias to some extent by the use of standard checklists that were followed for clinical assessments and diagnosis of IRIS. It was not possible to prevent treating clinicians from knowing when patients initiated ART and this may have impacted their patient’s clinical management decision, including hospitalisation. We attempted to minimise this limitation by a standard procedure which required a second opinion on hospitalisation by a separate clinician. Decisions relating to steroid use were made by hospital clinicians unrelated to the study.
Further studies may be necessary to assess IRIS risk in non-ambulant patients and in patients with extra-pulmonary and smear negative pulmonary tuberculosis. While CD4+ count was a strong prognostic indicator of IRIS risk, CD4+ count assays are not always available in many settings. Decisions on timing of ART in individual patients need to be modified by clinical judgement of disease severity; and consideration of capacity to diagnose and manage IRIS. In the absence of a reliable diagnostic test for IRIS, it is possible that misclassification bias occurred. It is likely that milder forms of IRIS was missed, as IRIS diagnosis is dependent on patient self-report for specific symptoms and clinician awareness, especially where diagnostic radiography is not routinely available or inaccessible, even to symptomatic patients. This issue was addressed procedurally through use of a standardized IRIS evaluation checklist which was administered to every patient at each clinical visit. INSHI criteria for IRIS diagnosis was published three years after commencing our study (7). Despite lack of a standardized case definition for IRIS at the time, we implemented several steps in the design and conduct of the study to ensure consistency in reporting, recording and interpretation of suspected IRIS.
We address an important and current topic in the management of individuals co-infected with HIV and TB, by using data from a randomized clinical trial that had more cases of IRIS (80 patients) than prior reports, and where a single ART regimen was used in a well characterized smear-positive TB cohort. Since integration of tuberculosis and HIV treatment can reduce mortality by 56% (10), decisions on when to start ART during tuberculosis treatment should take into account the balance of risk and severity of IRIS and potential benefit in relation to morbidity and/or mortality. IRIS is substantially higher in patients starting ART earlier after TB treatment initiation. While patients with severe immunosuppression have a clear survival benefit from early ART initiation despite high IRIS risk (11-13) this balance of risks and benefits is different in patients with higher CD4+ counts. Deferring antiretroviral therapy initiation up to 12 weeks after tuberculosis treatment initiation may be an appropriate strategy in stable ambulant patients with CD4+ counts ≥ 50 cells/mm3 as this approach offers lower incidence and less severe IRIS without increasing risk of AIDS or mortality. Future research efforts need to focus on finding a reliable diagnostic marker of IRIS in routine clinical and laboratory settings. Further, a randomized placebo-controlled trial investigating whether the use of corticosteroids in patients with CD4+ counts < 50 cells/mm3 initiating HAART early in TB treatment reduces frequency and severity of IRIS events and need for hospitalization, is warranted. Additionally, validated clinical and laboratory tools to reliably diagnose IRIS will simplify clinical management decisions for TB-HIV co-infected patients.
Supplementary Material
Acknowledgements
We thank the patients for their participation in this study; Ms. Natasha Samsunder for laboratory analysis; Mrs Anneke Grobler for statistical support, Ms Tanuja Gengiah and Anushka Naidoo for pharmacy support, Prof Y M Moosa, Dr Aarthi Singh and Dr Munira Khan for additional clinical support, Mr. Faldie Burton for data management; and all other members of the SAPiT study team.
CAPRISA was established as part of the Comprehensive International Program of Research on AIDS (CIPRA) (grant # AI51794) from the US National Institutes of Health. The US President’s Emergency Plan for AIDS Relief (PEPfAR) funded the care of all the participants in the trial. The Global Fund to fight AIDS, Tuberculosis and Malaria funded the cost of the drugs used in the trial. The research infrastructure to conduct this trial, including the data management, laboratory and pharmacy cores were established through the CIPRA grant. Dr K Naidoo and Dr N Padayatchi were supported by the Columbia University-Southern African Fogarty AIDS International Training and Research Program (AITRP) funded by the Fogarty International Center, National Institutes of Health (grant # D43TW00231).
Role of the funding source The sponsor of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. All authors had access to data, commented on drafts, and approved the final report. The corresponding author had final responsibility for the decision to submit for publication.
Footnotes
Contributors The study was designed by KN, SSAK, WES, GF and NP. Data was gathered by KN, NJ, NP, NY, SG, GN, and SB. Data was analysed by NY. KN, SSAK and NY, vouch for data and analysis. KN, SSAK, and NY wrote the paper and KN decided to publish the paper.
Conflict of Interest: Dr. S. Abdool Karim reports being listed as a coinventor on two patents (2000/3437 and PCT/IB02/04550) that are part of the development of clade C HIV vaccines. No other potential conflict of interest relevant to this article was reported.
REFERENCES
- 1.Fishman JE, Saraf-Lavi E, Narita M, Hollender ES, Ramsinghani R, Ashkin D. Pulmonary tuberculosis in AIDS patients: transient chest radiographic worsening after initiation of antiretroviral therapy. AJR Am J Roentgenol. 2000;174(1):43–9. doi: 10.2214/ajr.174.1.1740043. [DOI] [PubMed] [Google Scholar]
- 2.Chien JW, Johnson JL. Paradoxical reactions in HIV and pulmonary TB. Chest. 1998;114:933–6. doi: 10.1378/chest.114.3.933. [DOI] [PubMed] [Google Scholar]
- 3.Girardi E, Palmieri F, Cingolani A, Ammassari A, Petrosillo N, Gillini L, et al. Changing clinical presentation and survival in HIV-associated tuberculosis after highly active antiretroviral therapy. Journal of acquired immune deficiency syndromes. 2001;26(4):326–31. doi: 10.1097/00126334-200104010-00006. [DOI] [PubMed] [Google Scholar]
- 4.Piscitelli SC, Gallicano KD. Interactions among drugs for HIV and opportunistic infections. N Engl J Med. 2001;344:984–96. doi: 10.1056/NEJM200103293441307. [DOI] [PubMed] [Google Scholar]
- 5.Abdool Karim SS, Abdool Karim Q, Friedland G, Lalloo U, El Sadr WM. Implementing antiretroviral therapy in resource-constrained settings: opportunities and challenges in integrating HIV and tuberculosis care. Aids. 2004;18:975–9. doi: 10.1097/00002030-200404300-00004. [DOI] [PubMed] [Google Scholar]
- 6.French MA. HIV/AIDS: immune reconstitution inflammatory syndrome: a reappraisal. Clin Infect Dis. 2009;48(1):101–7. doi: 10.1086/595006. [DOI] [PubMed] [Google Scholar]
- 7.Meintjes G, Lawn SD, Scano F, Maartens G, French MA, Worodria W, et al. Tuberculosis-associated immune reconstitution inflammatory syndrome: case definitions for use in resource-limited settings. Lancet Infect Dis. 2008;8(8):516–23. doi: 10.1016/S1473-3099(08)70184-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Elston JW, Thaker H. Immune reconstitution inflammatory syndrome. Int J STD AIDS. 2009;20(4):221–4. doi: 10.1258/ijsa.2008.008449. [DOI] [PubMed] [Google Scholar]
- 9.WHO . WHO Report 2009. Geneva, Switzerland: 2009. Global tuberculosis control - epidemiology, strategy, financing. [Google Scholar]
- 10.Naidoo K, Grobler A, Padayatchi N, Baxter C, Gray A, et al. Timing of initiation of antiretroviral drugs during tuberculosis therapy. N Engl J Med. 2010;362(8):697–706. doi: 10.1056/NEJMoa0905848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Abdool Karim SS, Naidoo K, Grobler A, Padayatchi N, Baxter C, Gray AL, et al. Integration of antiretroviral therapy with tuberculosis treatment. The New England journal of medicine. 2011;365(16):1492–501. doi: 10.1056/NEJMoa1014181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Blanc FX, Sok T, Laureillard D, Borand L, Rekacewicz C, Nerrienet E, et al. Earlier versus later start of antiretroviral therapy in HIV-infected adults with tuberculosis. The New England journal of medicine. 2011;365(16):1471–81. doi: 10.1056/NEJMoa1013911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Havlir DV, Kendall MA, Ive P, Kumwenda J, Swindells S, Qasba SS, et al. Timing of antiretroviral therapy for HIV-1 infection and tuberculosis. The New England journal of medicine. 2011;365(16):1482–91. doi: 10.1056/NEJMoa1013607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Burman W, Weis S, Vernon A, Khan A, Benator D, Jones B, et al. Frequency, severity and duration of immune reconstitution events in HIV-related tuberculosis. Int J Tuberc Lung Dis. 2007;11(12):1282–9. [PubMed] [Google Scholar]
- 15.French MA, Lenzo N, John M, Mallal SA, McKinnon EJ, James IR, et al. Immune restoration disease after the treatment of immunodeficient HIV-infected patients with highly active antiretroviral therapy. HIV Med. 2000;1(2):107–15. doi: 10.1046/j.1468-1293.2000.00012.x. [DOI] [PubMed] [Google Scholar]
- 16.Murdoch DM, Venter WD, Feldman C, Van Rie A. Incidence and risk factors for the immune reconstitution inflammatory syndrome in HIV patients in South Africa: a prospective study. AIDS. 2008;22(5):601–10. doi: 10.1097/QAD.0b013e3282f4a607. [DOI] [PubMed] [Google Scholar]
- 17.Ratnam I, Chiu C, Kandala NB, Easterbrook PJ. Incidence and risk factors for immune reconstitution inflammatory syndrome in an ethnically diverse HIV type 1-infected cohort. Clin Infect Dis. 2006;42(3):418–27. doi: 10.1086/499356. [DOI] [PubMed] [Google Scholar]
- 18.Breton G, Duval X, Estellat C, Poaletti X, Bonnet D, Mvondo Mvondo D, et al. Determinants of immune reconstitution inflammatory syndrome in HIV type 1-infected patients with tuberculosis after initiation of antiretroviral therapy. Clin Infect Dis. 2004;39(11):1709–12. doi: 10.1086/425742. [DOI] [PubMed] [Google Scholar]
- 19.Huruy K, Mulu A, Mengistu G, Shewa-Amare A, Akalu A, Kassu A, et al. Immune reconstitution inflammatory syndrome among HIV/AIDS patients during highly active antiretroviral therapy in Addis Ababa, Ethiopia. Jpn J Infect Dis. 2008;61(3):205–9. [PubMed] [Google Scholar]
- 20.Lawn SD, Myer L, Bekker LG, Wood R. Tuberculosis-associated immune reconstitution disease: incidence, risk factors and impact in an antiretroviral treatment service in South Africa. AIDS. 2007;21(3):335–41. doi: 10.1097/QAD.0b013e328011efac. [DOI] [PubMed] [Google Scholar]
- 21.Shelburne SA, Visnegarwala F, Darcourt J, Graviss EA, Giordano TP, White AC, Jr., et al. Incidence and risk factors for immune reconstitution inflammatory syndrome during highly active antiretroviral therapy. Aids. 2005;19(4):399–406. doi: 10.1097/01.aids.0000161769.06158.8a. [DOI] [PubMed] [Google Scholar]
- 22.DoH., South, Africa National Tuberculosis Management Guidelines 2008. 2008 http://www.sasohn.org.za/images/TBGUIDELINES2008SFINALFORPRINTINGMARCH09.pdf.
- 23.Colebunders R, John L, Huyst V, Kambugu A, Scano F, Lynen L. Tuberculosis immune reconstitution inflammatory syndrome in countries with limited resources. Int J Tuberc Lung Dis. 2006;10(9):946–53. [PubMed] [Google Scholar]
- 24.Shelburne SA, Montes M, Hamill RJ. Immune reconstitution inflammatory syndrome: more answers, more questions. J Antimicrob Chemother. 2006;57(2):167–70. doi: 10.1093/jac/dki444. [DOI] [PubMed] [Google Scholar]
- 25.Lawn SD, Bekker LG, Miller RF. Immune reconstitution disease associated with mycobacterial infections in HIV-infected individuals receiving antiretrovirals. Lancet Infect Dis. 2005;5(6):361–73. doi: 10.1016/S1473-3099(05)70140-7. [DOI] [PubMed] [Google Scholar]
- 26.Narita M, Ashkin D, Hollender ES, Pitchenik AE. Paradoxical worsening of tuberculosis following antiretroviral therapy in patients with AIDS. Am J Respir Crit Care Med. 1998;158(1):157–61. doi: 10.1164/ajrccm.158.1.9712001. [DOI] [PubMed] [Google Scholar]
- 27.Navas E, Martin-Davila P, Moreno L, Pintado V, Casado JL, Fortun J, et al. Paradoxical reactions of tuberculosis in patients with the acquired immunodeficiency syndrome who are treated with highly active antiretroviral therapy. Arch Intern Med. 2002;162(1):97–9. doi: 10.1001/archinte.162.1.97. [DOI] [PubMed] [Google Scholar]
- 28.Wendel KA, Alwood KS, Gachuhi R, Chaisson RE, Bishai WR, Sterling TR. Paradoxical worsening of tuberculosis in HIV-infected persons. Chest. 2001;120(1):193–7. doi: 10.1378/chest.120.1.193. [DOI] [PubMed] [Google Scholar]
- 29.Calligaro G, Meintjes G, Mendelson M. Pulmonary manifestations of the immune reconstitution inflammatory syndrome. Curr Opin Pulm Med. 2011;17(3):180–8. doi: 10.1097/MCP.0b013e328344f692. [DOI] [PubMed] [Google Scholar]
- 30.Meintjes G, Wilkinson RJ, Morroni C, Pepper DJ, Rebe K, Rangaka MX, et al. Randomized placebo-controlled trial of prednisone for paradoxical tuberculosis-associated immune reconstitution inflammatory syndrome. AIDS. 2010;24(15):2381–90. doi: 10.1097/QAD.0b013e32833dfc68. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.