Abstract
Context
An ethnobotany-based approach in the selection of raw plant materials to study was implemented.
Objective
To acquire raw plant materials using ethnobotanical field interviews as starting point to discover new bioactive compounds from medicinal plants of the Lao People’s Democratic Republic.
Methods
Using semi-structured field interviews with healers in the Lao PDR, plant samples were collected, extracted, and bio-assayed to detect bioactivity against cancer, HIV/AIDS, TB, malaria. Plant species demonstrating activity were recollected and the extracts subjected to a bioassay-guided isolation protocol to isolate and identify the active compounds.
Results
Field interviews with 118 healers in 15 of 17 provinces of Lao PDR yielded 753 collections (573 species) with 955 plant samples. Of these 955, 50 extracts demonstrated activity in the anticancer, 10 in the anti-HIV, 30 in the anti-TB, and 52 in the antimalarial assay. Recollection of actives followed by bioassay-guided isolation processes yielded a series of new and known in vitro-active anticancer and antimalarial compounds from 5 species.
Discussion
Laos has a rich biodiversity, harboring an estimated 8000–11,000 species of plants. In a country highly dependent on traditional medicine for its primary health care, this rich plant diversity serves as a major source of their medication.
Conclusions
Ethnobotanical survey has demonstrated the richness of plant-based traditional medicine of Lao PDR, taxonomically and therapeutically. Biological assays of extracts of half of the 955 samples followed by in-depth studies of a number of actives have yielded a series of new bioactive compounds against the diseases of cancer and malaria.
Keywords: Laos, biodiversity, ethnobotanical survey, interview methods, medicinal plants, bioactive compounds, cancer, HIV/AIDS, tuberculosis, malaria
Introduction
As one of the major components of an International Cooperative Biodiversity Group (ICBG) project based at the University of Illinois at Chicago (UIC), Studies on Biodiversity of Vietnam and Laos, also known as the “Vietnam-Laos ICBG” (Soejarto et al., 1999, 2004, 2006, 2007), an ethnobotanical survey was conducted in the Lao People’s Democratic Republic (Lao PDR; Laos; these terms are used interchangeably in this paper). This survey was part of the design of the drug discovery goal of this ICBG, namely, the discovery of bioactive compounds from plants of Vietnam and Laos as potential candidates for pharmaceutical development. Two approaches were used in the selection of plants to be investigated (Soejarto et al., 1999, 2002, 2006). The first was a biodiversity-based approach intended to maximize taxonomic diversity, hence, chemical diversity, focused primarily on the plant diversity of Cuc Phuong National Park, Vietnam. The second was an ethnobotany-driven drug discovery approach, performed by researchers based at the Institute of Traditional Medicine (ITM) of Laos in Vientiane, focused primarily on traditional medicinal plants of Laos. In the implementation of the project, plant samples collected were extracted in the ICBG host institutions in Vietnam and Laos, and the extracts dispatched to the laboratories of the Program for Collaborative Research in the Pharmaceutical Sciences (PCRPS), Department of Medicinal Chemistry and Pharmacognosy at the College of Pharmacy, UIC. In this project, no raw plant materials (“plant samples”) were shipped from either Vietnam or Laos to UIC.
This paper reports on the results of ethnobotany-driven survey of the medicinal plants of Laos through field interviews with healers in 15 provinces and the Vientiane Prefecture. The paper also reports on the results of follow-up laboratory analyses of the medicinal plants collected during the interview session.
Why Laos?
Lao People’s Democratic Republic is a landlocked country located in South East Asia, bordered by Thailand to the west, Myanmar to the northwest, China (Yunnan Province) to the north, Vietnam to its immediate east, and Cambodia to the south (Figure 1). It has a population of 6.47 million (Central Intelligence Agency, 2011). The country has a landmass of 236,800 square km, of which about 41% is covered by forest (Vongsiharath, 2008; Earth Times, 2010). Inadequate medical facilities that are often too far from communities living in isolated villages, and insufficient access to the most current pharmaceuticals on the market or to most current disease therapies, make it difficult to successfully treat certain health conditions in Laos (WHO, 2009). Malaria, viral infections, and a high infant mortality rate are relatively common throughout the country (WHO, 2009, 2011). Seventy-three percent of the population lives in rural areas of the country (WHO, 2009), where access to conventional medicine is limited or non-existent, either because of its high cost or because it is not available. In such rural areas, usage of Lao Traditional Medicine for primary health care is very high, much higher than in urban areas, resulting in a wider availability of traditional healers in rural villages and towns. All of these factors resulted in a high dependence of the Lao population on traditional medicines in their primary health care.
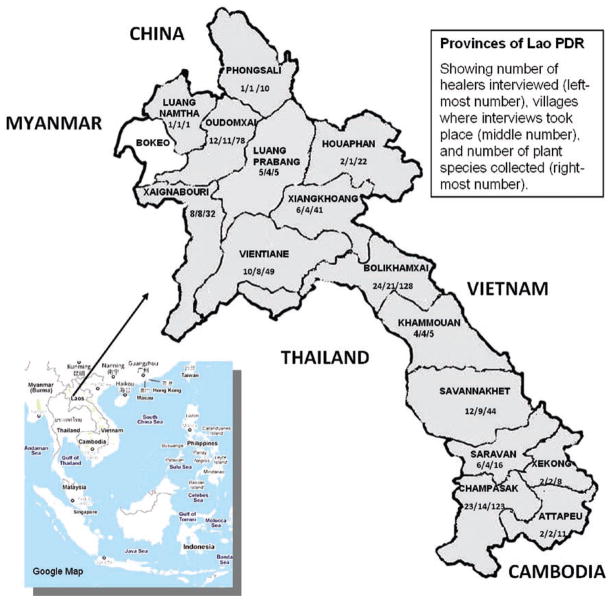
Figure 1.
Map of Lao PDR, showing provinces with numbers of healers interviewed (by province). The three numbers separated by a slash indicate the number of healers interviewed, the villages where the interviews took place, and the number of plant species collected. For example: Bolikhamsai Province 24/21/128 = 24 healers interviewed, 21 villages where interviews took place, 128 plant species were collected.
Recognizing the importance of traditional medicine in Laos, the Ministry of Health of Lao PDR created the Research Institute of Medicinal Plants (RIMP) in 1976, which was re-named as the Traditional Medicine Research Center (TMRC) in 2000, and further re-named as the Institute of Traditional Medicine (ITM) in 2010. The mission of ITM is to gather and catalogue indigenous traditional medicine treatments, the greater part of which are herbal remedies, and to adapt and undertake research on all Lao traditional medicines, including medicinal plants used by Lao people across the country’s provinces (presently–16 provinces plus Vientiane Prefecture and Vientiane capital city). Further, ITM has the mission to disseminate information on Lao medicinal plants, as well as to research medicinal plants for their safety of consumption, toxicity, and efficacy. Thus, ITM collects and documents medicinal plant use by conducting ethnobotanical interviews with village healers and then collecting the plants using field collection methods. ITM has a small herbarium collection holding, and offices, located in Vientiane, along with a medicinal plant garden on its grounds. Through the mandate of the Ministry of Health, ITM established daughter institutions, initially in 10 provinces in Lao PDR, called Provincial Traditional Medicine Stations (TMS). Each of the TM Stations acts as an ITM in miniature. Some have a small herbarium for storing voucher specimens of herbs used in traditional medicine practices. Additionally, there is equipment located on-site for drying, storing, and processing materia medica into remedies for household use. Also located on-site are patient rooms and benches for performing herbal massage and acupressure on patients, and a separate enclosed room that is used as an herbal sauna. Large, gallon drums, located behind a separate room, are filled with water set atop a heat source and aromatic herbs. As the water in the drums boil, herb-infused steam and vapor is piped into the enclosed room itself through the metal ducting connecting the output of the drums. Herbal saunas are used for a variety of chronic ailments, including the treatment of internal pains. The TM Stations play a significant role in the primary health care delivery of the rural population in Laos. The TM Station Heads, who are often trained in both clinical medicine, as well as traditional medicine, possess greater knowledge of the medicinal plants used to treat symptoms of diseases and illnesses. Malaria, yellow fever, tuberculosis, upper respiratory infections, gastrointestinal problems, peptic ulcers, and hepatitis are some of the more common ailments that TM Station personnel treat, and accordingly, collect materia medica throughout the year.
Through the confluence of the goal of the Vietnam-Laos ICBG and the mission of ITM and TMSs in the promotion, documentation, and study of Lao traditional medicine, a partnership came into being between the ICBG project and ITM (Soejarto et al., 1999, 2009).
Methods
Ethnobotanical field interviews
Study site and the protocol executed in the performance of field interviews
Considering the great cultural and ethnolinguistic diversity of the people of Laos (Chape, 1996; Chazee, 1999), and the fact that medicinal plant knowledge has been shown to have a link with geographical orientation (Libman, 2009), field interviews were designed to cover as broad an area of the country as possible, in order to maximize the diversity of knowledge and the plant species employed in traditional therapy. Thus, ethnobotanical field interviews were conducted in the following provinces of Lao PDR (from north to south; Figure 1): Luang Namtha, Phongsali, Oudomxai, Luang Prabang, Xaignabouri, Houaphan, Vientiane, Vientiane Prefecture, Xiangkhoang (sometimes spelled “Xiengkhuang”, but “Xiangkhoang is used in this paper), Bolikhamxai, Khammouan, Savannakhet, Saravan, Xekong, Champasak, and Attapeu. It should be noted that there are other English versions of the spelling of these provinces (National Geographic, 1996–2011; Statoids, 2011), and that the Vientiane province as depicted in Figure 1 includes the former Xaisomboun Special Zone (east portion of the province), which was dissolved in 2006 and annexed to the Vientiane province. In the present paper, for purposes of compiling statistics, interview data for the Vientiane Prefecture have been submerged with data from the Vientiane province. Due to some logistical issues, no interviews were performed in Bokeo Province.
For each interview session, communication between the staff of ITM and the office of the Provincial Health Department (PHD) preceded the actual field trip. Upon receiving communication from the ITM, staff of the PHD notified the Head of the TMS, to announce the upcoming field research visit of the ITM team and to request the support and collaboration of the TMS staff throughout the period of the field trip. On arrival in the province, where an interview session was to take place, the ITM team went directly to the TMS headquarters to meet with the Head of the TMS and discuss the plan of work. The ITM team normally stayed in the TMS facilities for the duration of the field trip or in a local guesthouse, if the TMS did not have adequate facilities to accommodate the visitors. Upon the recommendation of the TMS Head, the team headed to the village, where a healer or healers had been identified. However, before meeting with the healer, the team first met with the Head of the village to obtain clearance for the visit and to explain the purpose of the visit. Once a clearance was given, the team, and the TMS Head visited the house of the healer in that village. One or more healers may live in the same village. In this context, healers are defined as individuals in a community (male or female) recognized as the person to whom one comes to seek medical treatment in the event of health problems. The TMS Head accompanied the TMS team and assisted the team in explaining the purpose of the visit (ICBG research), and in obtaining permission for the conduct of the interview. The entire chain of permissions and clearances involved, including the ITM, the PHD, the TMS and the village head, support the authenticity of the prior informed consent of Lao people with interests in traditional medicine to the ICBG project. Healers were remunerated for their services, namely, the time and effort in collaborating with the interviewing team. A broader design of benefit-sharing with the communities is described in previous papers by Soejarto et al. (2004; 2006; 2007; 2009).
Field interview methods
Semi-structured interview was conducted in the Lao language in a relaxed, informal setting, with interviewee and interviewer sitting face-to-face, normally on an elevated floor of the healer’s house. Members of the healer’s family may or may not have been present, while the ITM team consisted of two or three persons (one, the field trip leader, served as the interviewer), occasionally with a UIC scientist present, but always with the presence of the Head of the TMS. Figure 2 provides an example of an Interview Guide used in the ICBG project. All interviews were performed under a research protocol approved by the Institutional Review Board (IRB) of the University of Illinois at Chicago: IRB Research Protocol #H-97-1056/1999 (1999–2003) and #2003-0636/2003 (2003–2008). A specific informed consent procedure was carried out with each healer under this protocol. An IRB research protocol is subject to a continuing annual review by the UIC IRB. In the process of the interviews, the interviewer and his/her team recorded the information in a field notebook in front of the healer. A typical interview session started with recording data on the demographics of the healer (name, age, gender, education, ethnicity, length of practice as a healer, name of village, district, and province). Once this demographic section was completed, the following question was asked “What diseases do you know how to treat?” This question would eventually lead to a specific disease or diseases. In the case of malaria, the Interview Guide in Figure 2 applies.
Figure 2.
A sample of an interview guide used in the ethnobotanical survey. This particular form is specifically intended for interview on the treatment of MALARIA. Similar forms, each with the disease heading of TUBERCULOSIS, HERPES/COLD SORES, CANCER, respectively, were used for each of those diseases.
Plant collection documentation
At the end of an interview session, the healer led the interviewing team to the location or locations of the various plants mentioned during the interview. It may be a garden or a forested area. If enough plant materials were available, with the permission of the healer, approximately 500 g of plant sample (specifically, the part mentioned used for disease treatment) was collected for biological assays, using collecting methods described in previous papers (Soejarto, 1993; Soejarto et al., 1996), documented by at least 3 herbarium specimens. These specimens were later distributed to the Herbarium of ITM in Vientiane, the National Herbarium of Vietnam in Hanoi, and the John G. Searle Herbarium of the Field Museum in Chicago. Frequently the plant identified by the healer did not have flowers or fruits. If after a further search in the area no flowering specimens could be found, voucher herbarium specimens were prepared from the sterile specimen, using standard good botanical collecting practices. Attempts were made at a later date to search and re-collect fertile (flowering/fruiting) herbarium specimens in the same general area. This effort to acquire flowering and/or fruiting specimens of the sterile specimens continues to the present. Data elements recorded for each collection included: locality, habitat, altitude, GPS reading, field characters of the plant, collector, collection number, date of collection, medicinal use, method of preparation, route of administration, part used, part collected, sample code (Figure 3).
Figure 3.
A sample of a completed Field Interview Form. This is a sample of a completed Field Interview Data Sheet that has been transcribed from the field notes from an interview session that led to the collection of Gongronema napalense. These data were eventually entered into a NAPIS Database housed at ITM. See text [Results- Ethnobotanical survey- Medicinal plants collected] for details about NAPIS.
Taxonomic identification
The first attempt at taxonomic determination of the collected voucher herbarium specimens took place at the Herbarium of the Institute of Traditional Medicine in Vientiane. The book of Lao common plant names by Vidal (1959) was the primary reference used. In almost every case, the scientific name (the Latin binomial) of the plant at hand was given in this book, though oftentimes, two or more Latin binomials were referred, and an erroneous scientific name given. The next step was consulting the herbarium collection holdings of ITM, to carefully confirm the taxonomic identity of the specimen as given in Vidal’s book, with an identified specimen in the ITM Herbarium. An additional standard resource used was the 3-volume book “Cayco Vietnam” (Flora of Vietnam) by Pham Hoang Ho (1991, 1992, 1993, 1999). Other resources included the taxonomic revisions from the Flora of Thailand.
Identification was also made through the dispatch of a set of voucher herbarium specimens to the ICBG plant taxonomy team based in Hanoi at the National Herbarium (Institute of Ecology and Biological Resources at the Vietnam Academy of Science and Technology), who had access to an excellent herbarium collection and library resources for the identification of Indo-Chinese plants. A similar set of vouchers was sent to the John G. Searle Herbarium at the Field Museum in Chicago for identification and/or confirmation of taxonomic identification.
Experimental protocols
Sample extraction
The complete description of the plant extraction protocol used in the ICBG project has been published in various papers communicating results of isolation of active compounds from plants of Cuc Phuong National Park in Vietnam and from medicinal plants of Laos (Hoang et al, 2002; Zhang et al., 2004, 2005; Ma et al., 2008). In short, the extraction protocol was as follows: A 100 g sample of dried and milled plant material was macerated and percolated overnight with 90% methanol, concentrated in vacuo, and subsequently defatted with n-hexane and partitioned with CHCl3 to yield a CHCl3-soluble extract. The combined chloroform fraction from multiple extractions was then evaporated to dryness below 40°C in vacuo, and the remaining aqueous extract was lyophilized after the last traces of organic solvent had been removed. One-half of the dried chloroform-soluble fraction was submitted for anti-HIV, antimalarial, anti-TB, and anticancer and/or cancer chemoprevention assays, while the other half was set aside for future use. It should be noted that the cancer chemoprevention assays were performed for only a brief span of time on a limited number of samples.
A plant extract with activity in one or more bioassays was prioritized for recollection and for follow-up bioassay-guided isolation studies. Among the factors taken into consideration for the recollection priority were the biological potency and toxicity of the extract, and chemical dereplication by a review of the chemical and biological literature.
Bioassay-guided isolation protocol
The recollected plant material was extracted with MeOH to afford an alcoholic extract, which was subsequently chromatographed over a silica gel column to afford a number of fractions. These fractions were then evaluated for their biological activity, and the active fractions were subjected to further column chromatography to afford additional sub-fractions, which were further evaluated for their biological activity. As the last step, the active sub-fractions were subjected to further separation, including preparative HPLC, to isolate the biologically active compounds (Zhang et al., 2003a,b).
All laboratory experimental investigations of the medicinal plant collections of Laos in our Vietnam-Laos ICBG were performed by a team of biologists and chemists based at the Program for Collaborative Research in the Pharmaceutical Sciences (PCRPS) at the Department of Medicinal Chemistry and Pharmacognosy, the Institute of Tuberculosis Research (ITR), College of Pharmacy, and the Department of Microbiology and Immunology of the College of Medicine, University of Illinois at Chicago. A scientist from the Institute of Traditional Medicine who was trained at the PCRPS contributed to the chemical isolation of the anticancer compounds of one of the species investigated.
Anticancer evaluation
Initially, anticancer evaluation of plant extracts was performed using a panel of 4 cancer cell lines: Lu1 (lung), KB (human oral epidermoid carcinoma), Col-2 (ovarian) and LNCaP (prostate). As the project advanced, the number of cell lines was increased to 7 (KB, HL-60, LNCaP, MCF-7, Col2, Lu1, HUVEC), though it eventually stabilized at 6.
Briefly, bioassay procedures involved treating cells with various concentrations of the test substance, and assessing cell growth after 72 h. A standard protocol for the assessment of cellular toxicity measured the ability of cultured cells to proliferate in the presence of a test extract or pure compound, and subsequently quantitated for total protein content with sulforhodamine B dye as a measure of the percentage of surviving cells. The results were expressed as an ED50 (concentration required to inhibit cell growth by 50%), and the criteria for activity, as established by the National Cancer Institute (NCI) (ED50 values <20 μg/ml for extracts, and <4 μg/ml for pure compounds). In the event of activity, bioassay-guided isolation of active compounds from the recollected plant extract was performed using the cell line in which activity was observed. During the course of the project, certain modifications of the protocol were introduced as the situation warranted. Follow-up animal studies were performed using a hollow fiber assay method. The hollow fiber assay was developed and introduced by the NCI as an intermediate screen through which compounds must pass before full evaluation in xenograft tumor models (Hollingshead et al., 1995).
Anti-HIV evaluation
The inhibition of HIV-1 infectivity was conducted using green fluorescent protein (GFP) reporter cell lines HOG. R5 (Tan et al., 1997). This reporter cell line for the quantitative assay of HIV infectivity was developed using HOS (human osteosarcoma) cells rendered susceptible to HIV infection by the transfection of genes for CD4 and CCR5 as the co-receptor for M-tropic HIV-1 isolates. This microtiter assay is based on the transactivation of a stably integrated HIV-1 LTR-GFP transcription unit. Upon HIV-1 entry into these HOS target cells, Tat expression increased the HIV LTR-directed transcription of the GFP gene as demonstrated by the increased fluorescence of detergent lysates of infected cells relative to that of uninfected controls. By virtue of the low level expression of endogenous CXCR4, HOG.R5 cells also efficiently supported infection by a diverse array of T-tropic viruses, in addition to M-tropic and clinical isolates.
Anti-TB evaluation
At the start of the project, the anti-TB assay was performed using a non-virulent strain of Mycobacterium tuberculosis. Following completion of a BSL-3 (Biosafety Level 3) facility at the Institute of Tuberculosis Research (ITR) at the College of Pharmacy, a virulent strain of M. tuberculosis was used. Extracts were screened against this virulent, but drug-sensitive strain (H37Rv), and bacterial viability was determined by the Microplate Alamar Blue assay (MABA), following a one-week incubation with 50 μg/ml extracts at 37°C (Collins & Franzblau, 1997). Reduction of the Alamar Blue reagent was measured fluorometrically in a microplate fluorometer using excitation of 530 nm and emission of 590 nm. Extract activity was scored as percent inhibition of fluorescence relative to control wells with bacteria, but without extract. Extracts effecting a reduction in bacterial viability (fluorescence) of 90% or greater were considered to have an MIC of ≤ 50 μg/ml. These extracts were re-tested at multiple concentrations to determine the actual minimum inhibitory concentration (MIC).
Antimalarial evaluation
The antimalarial activity of plant extracts and pure compounds was assessed utilizing the in vitro radioisotope-incorporation method of Desjardins et al. (1979) with minor modifications. All test compounds were assayed in the presence of a suspension of P. falciparum-infected erythrocytes (0.5–1.0% parasitemia, 1.0% cell hematocrit), over a concentration range of 14 ng/ml to 10 μg/ml, while extracts were pre-screened at a maximum concentration of 10 μg/ml. In addition, the known antimalarial drugs, quinine, chloroquine, mefloquine, and artemisinin were tested as controls in each experiment over a range of 0.3–250 ng/ml. Microtiter plates were incubated for 24 h at 37°C in a sealed chamber under an atmosphere of 5% CO2, 5% O2 and 90% N2. After this incubation period, 0.5 μCi of [3H(G)]hypoxanthine was added to each well, and the microtiter plate returned to the sealed chamber at 37°C for an additional 18 h incubation. The assay was terminated by harvesting the contents of each microtiter plate onto a glass fiber filtermat (Filtermat A) (Wallac) using a Tomtec 96-well harvester (Tomtec, Orange, Conn.). Filtermats were dried and immersed in minimal nonaqueous scintillation fluid in a small heat-sealed plastic pouch. The radioactivity in the wells was counted using the 1450 MicroBeta™ Liquid Scintillation Counter (Wallac Oy, Turku, Finland). Concentrations of both test compounds and positive controls which inhibited parasite-specific incorporation of (3H)hypoxanthine by 50% (IC50) were determined by non-linear regression analysis. Drug-free controls define 100% incorporation. During the last year of the project, the antimalarial assay was assessed using an in vitro Microplate Fluorometric Detection method of Smilkstein et al. (2004) and was performed at the Institute of Natural Products Chemistry, Vietnam Academy of Science and Technology, in Hanoi, against two clones of Plasmodium falciparum parasite, namely the chloroquine-sensitive and chloroquine-resistant clones.
In the event of activity, isolation of the biologically active compounds followed, using the bioassay-guided isolation protocol.
For more complete discussions on the experimental protocols used in the Vietnam-Laos ICBG project, readers are referred to previously published papers (Soejarto et al., 2002; Tan et al, 2006).
Results
Ethnobotanical survey
Healers interviewed
A total of 118 healers from 94 villages in 15 provinces of Lao PDR were visited and interviewed. Healers are trusted members of the community. They are often older persons and are respected because of the vast knowledge they possess regarding all matters of life, including illness and treatment. Their age ranges from 40 to 82 (Figure 4). Most of the interviewed healers were male; only 5 of the 118 were female. The number of plants focused on during an interview session varied, from one to several species. An interview session lasted 1–2 h, and in a single field trip, 1–5 interview sessions with the same or different healers in the same or different villages, were conducted. Each interview was followed by a trip to the field, guided by the healer, to collect voucher herbarium specimens and the bioassay samples. Throughout the period of our ICBG project, 37 field trips to 94 villages in 15 provinces and the Vientiane Prefecture were carried out. Supporting Data #1 presents the complete list of the 118 healers, their age, and the name of the villages visited in each province. Figure 1 presents the distribution map of the healers, villages visited, and the number of species collected in each province as a result of the interviews.
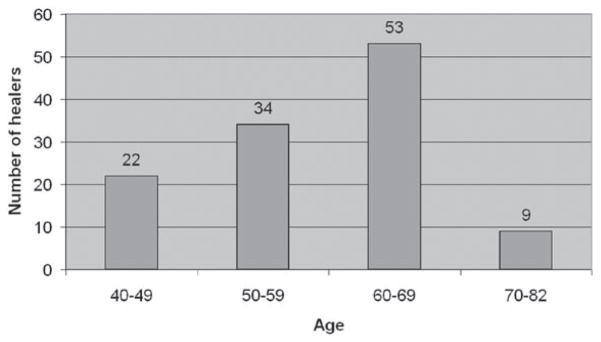
Figure 4.
Age distribution of the healers interviewed. A total of 118 healers (see Supporting Data #1) in 94 villages, in 15 provinces of Laos, and the Vientiane Prefecture, were interviewed. The age of the healers ranges from 42 to 82, with the majority aged 60–69. Of the 118 healers, only 5 were female.
Medicinal plants collected
Data from each interview were recorded in a field notebook, which were later transferred (at ITM) into an interview data sheet upon completion of the field trip. Figure 3 provides an example of a completed interview data sheet for Gongronema napalense (name of healer has been deleted). These interview data were eventually entered into a database system called NAPIS (Natural Product Information System), developed by Mr. Gregg Dietzman of White Point Systems (http://www.wps2.com/; http://www.conservationgis.org/whitepoint/napis.html;http://www.wps2.com/products.htm), which was later adopted for use by the ICBG project. This database is installed at the Institute of Traditional Medicine for the purpose of archiving data derived from this ICBG project, and also as an ITM effort in safeguarding and protecting the indigenous intellectual property rights of Lao traditional medicine. The legal framework for this measure has been presented in papers by Riley (2001), Sydara et al. (2005), and Riley et al. (2009). Thus, data on plant species collected are made available only through special arrangements and agreement with ITM.
Taxonomic identification of plants collected
One challenge we faced in this medicinal plant survey effort was the taxonomic identification of the plants collected. Many voucher herbarium collections were in sterile condition (lacking flowers and fruits), since the living specimens which healers pointed out, or to which the investigators were guided to in their collection, were not in flower or fruit at the time of the interview - even after some considerable effort to search for them during the field outing with the healer. As stated above, initial taxonomic determination was performed at the Herbarium of ITM in Vientiane by the ITM staff, through comparison with identified specimens and also with the use of the book by Vidal (1959) and Pham Hoang Ho (1991, 1992, 1993, 1999). Additionally, further determination or confirmation of identification was also performed at the National Herbarium of Vietnam in Hanoi by the Vietnamese ICBG taxonomy team, as well as at the John G. Searle Herbarium of the Field Museum by the project’s personnel. A total of 990 collections fully documented by voucher herbarium specimens were made, accompanied by 955 primary screening samples. No bioassay samples were collected for a limited number of collections, while there were a small number of multiple-sample collections. A subset of 753 collections, with determinations of at least to the genus level, is used as basis for analysis in the present paper. Of these 753 collections, however, 28 were identified only to the genus level and have been eliminated for further discussions, while the remaining 725 collections were identified to the species level, representing 573 unicate species in 362 genera and 110 families of angiosperms. Figure 5 presents the distribution of the 573 identified species among the 15 provinces and the Vientiane Prefecture.
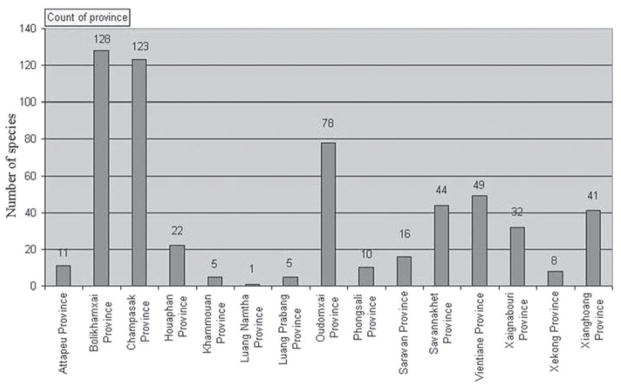
Figure 5.
Statistics on the total number of unique species collected. The high numbers of species from Bolikhamxai (128 species), Champasak (123 species) and Oudomxai (78 species) is partly due to a bias caused by good logistics and good facilities, as well as good communications with healers within the territory of the Traditional Medicine Stations located in these provinces.
Plant samples collected
As stated above, 990 collections were accompanied by 955 primary screening samples, each in the amount of 300–800 g dry weight. A larger sample (3–5 kg dry weight each) was re-collected for those that had shown activity, and hence was prioritized, in any one of the disease systems of this ICBG. All effort was made to ascertain the taxonomic identity of the species, prior to the actual recollection work. A total of 10 plant samples from 10 species (see below under “Summary of assays performed” subheading) were recollected for bioassay-guided isolation studies in all four of the disease systems, but to date in-depth studies of only five species from Laos have been completed: Asparagus cochinchinensis, Diospyros quaesita, Gongronema napalense, Nauclea orientialis, and Rourea minor. It must be noted that, due to the greater number of plant samples collected from Vietnam (Cuc Phuong National Park) in this Vietnam-Laos ICBG project, a greater portion of effort in chemical isolation studies by the biologists and chemists of our Vietnam-Laos ICBG had been targeted to the Vietnamese collections.
Diversity of diseases treated and epidemiology
In our database, each of the 990 plant collections is linked with medicinal use(s) as stated by the healer who gave that information. One or more diseases may be stated for a particular plant. As a result, 2353 “ethno disease” treatments are recorded for the 990 collections (extreme left column in Supporting Data #2). Each ethno disease is converted to an “assay category” equivalent for the purpose of identifying and correlating disease treatments stated by the healer, in terms of biological activity. Filtering/elimination of duplicates from the list of 2353 in both “ethno diseases” and “assay categories” results in 235 traditional (ethno) diseases (Supporting Data #3), and 121 assay categories (Supporting Data #4), respectively.
The total number of occurrence of a particular ethno disease across all provinces is indicated in the last-column (extreme-right column/grand total) in Supporting Data #3, while the total occurrence of an assay category across all provinces is indicated in the last-column (extreme-right column/grand total) in Supporting Data #4. A summary of the assay categories (diseases) in Supporting Data #4, across the 15 provinces (data for Vientiane Prefecture were merged with Vientiane Province), is presented in Table 1 and in Figure 6.
Table 1.
Highest frequencies (scoring over 50) of 8 diseases expressed as “bioassay categories” across 15 provinces of Laos.a,b
| Assay categories | ATT | BOL | CHA | HOU | KHA | LUP | LUN | OUD | PHO | SAR | SAV | VIE | XAI | XEK | XIA | Total |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cancer | 2 | 18 | 10 | 4 | 18 | 4 | 1 | 58 | ||||||||
| CNS | 4 | 37 | 43 | 9 | 10 | 10 | 14 | 65 | 30 | 46 | 71 | 33 | 11 | 35 | 440 | |
| Gastrointestinal disorder | 22 | 22 | 5 | 15 | 1 | 14 | 6 | 7 | 6 | 3 | 101 | |||||
| Infection | 42 | 74 | 14 | 1 | 35 | 3 | 25 | 17 | 20 | 12 | 10 | 9 | 262 | |||
| Inflammation | 1 | 63 | 82 | 20 | 2 | 1 | 2 | 57 | 5 | 18 | 42 | 34 | 34 | 6 | 9 | 376 |
| Malaria | 7 | 28 | 60 | 2 | 3 | 1 | 21 | 24 | 8 | 15 | 4 | 5 | 4 | 182 | ||
| Tuberculosis | 31 | 82 | 2 | 24 | 1 | 5 | 16 | 48 | 14 | 3 | 226 | |||||
| Viral | 2 | 26 | 36 | 3 | 5 | 25 | 4 | 2 | 18 | 20 | 15 | 1 | 2 | 159 | ||
| Grand total | 16 | 267 | 409 | 55 | 20 | 11 | 18 | 246 | 36 | 105 | 179 | 218 | 120 | 39 | 65 | 1804 |
Province code: ATT = Attapeu; BOL = Bolikhamxai; CHA = Champasak; HOU = Houaphan; KHA = Khammouan; LUP = Luang Prabang; LUN = Luang Namtha; OUD = Oudomxai; PHO = Phongsali; SAR = Saravan; SAV = Savannakhet; VIE = Vientiane; XAI = Xaignabouri; XEK = Xekong; XIA = Xiangkhoang.
These numbers were derived from Supporting Data #4, as explained in details in the text (in Results, under the subheading “Diversity of diseases treated and epidemiology”). Data for Vientiane Prefecture were submerged with Vientiane province.
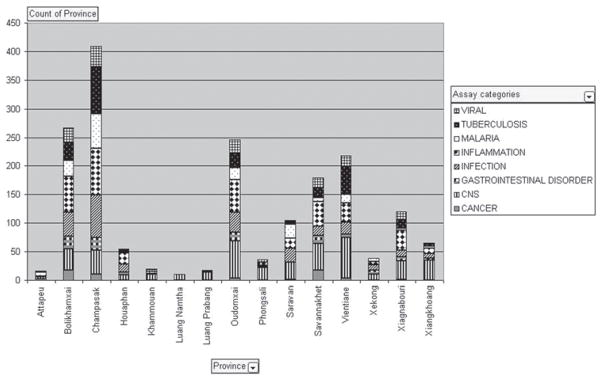
Figure 6.
Highest frequencies of assay categories (= diseases) across the 15 provinces of Laos. In this histogram, which is a graphic rendition of data in Table 1, the high frequency of occurrence of the highest-scoring disease [= assay] categories in the provinces of Bolikhamsai, Champasak, Oudomxai, Savannakhet, Vientiane, and Xaignabouri are very obvious. For the provinces of Attapeu, Khammouan, Luang Namtha, Luang Prabang, Phongsali, Xekong and Xiangkhoang, due to the small numbers of frequency of occurrence of the disease [= assay] categories, the separation of the bars is not obvious.
The total number of occurrence (frequency) of each assay category in the last-column (extreme-right column/grand total) in Supporting Data #4 shows 8 assay categories (diseases) scoring 50 or higher: cancer (58), CNS (440), gastrointestinal disorder (101), infection (262), inflammation (376), malaria (182), tuberculosis (226), and viral (159) (Table 1). Of interest to note here is the fact that cancer, malaria, tuberculosis, and HIV/AIDS (viral) are the four disease foci of our ICBG. Since interviews did not specifically target these 4 diseases at the start of an interview or during the course of each interview session (see Interview Guide in Figure 2), the high frequencies of these four “ICBG disease targets” was not a reflection or a bias of interview technique. The findings that these four diseases are the most commonly treated health problems is, in general terms, in agreement with the present status of health issues in Laos (WHO at http://www.who.int/countries/lao/en/).
Diversity of types of plant parts used in disease treatment
A great diversity of types of parts and combinations of parts of a plant were mentioned as the ingredients of a traditional plant-based remedy by the healers interviewed. Of the 990 collections, a total of 1142 plant parts and combination of parts used to prepare the medicines are mentioned (Table 2). This number includes data from collections, for which no bioassay samples were collected. As seen in this table, a total of 54 types of plant parts and combinations of parts were recorded (Table 2).
Table 2.
Plant parts and combination of parts used by healers in preparing their traditional remedies.a
| Parts/combination of parts | Frequency | Parts combination of parts | Frequency |
|---|---|---|---|
| Aged LF | 2 | Plant-no roots | 1 |
| BK | 25 | RH | 22 |
| BK+ST | 3 | RT | 114 |
| BK+SW | 1 | RT of ST | 3 |
| BK+WD | 2 | RT or TW | 1 |
| BR | 5 | RT+ST | 15 |
| BR or T\V | 1 | RT+SW | 8 |
| BR+ST | 1 | RT+TR | 1 |
| BULB | 1 | RT+TW+WD | 1 |
| CREEPER + LF | 1 | RT+YN | 1 |
| EX+ST | 1 | SB | 31 |
| FL+TW | 1 | SB+SW | 1 |
| FR | 3 | SB+YN | 1 |
| LF | 25 | SB+WD | 2 |
| LF 01 RT | 1 | ST | 252 |
| LF+RT | 2 | ST or VN | 1 |
| LF+RT+TW | 1 | ST+RT | 3 |
| LF^ST | 4 | ST+TW | 39 |
| LF+TW | 6 | SW | 84 |
| LF+YN | 3 | tr|tw | 1 |
| LF+WD | 2 | TRUNK | 4 |
| LN | 34 | TU | 2 |
| LN+RT | 1 | t\v | 15 |
| LN+RT+TW | 1 | TW+WD | 9 |
| PARAS ITE+TW | 1 | VN | 93 |
| PL | 101 | WD | 15 |
| PL of RH | 2 | WD or PARASITE | 1 |
These numbers represent all plant parts stated by healers interviewed as ingredients of remedies in all the 990 collections. There are 54 part types and combination of parts.
To explore the relationship between the plant samples collected and the plant parts that the samples consist of, data on bioassay samples linked to plant parts in Table 2 were retrieved. Based on similarities of the plant parts and combination of parts used, a clumping into 7 categories shows that the stem of the plant represents the most commonly used plant part in preparing traditional remedies, followed by underground parts, and whole plant (Figure 7). For three sample collections (SL7806, SL7820, SL7821) the plant part identity was described as “unknown”, and were excluded from Figure 7. Indeed, perusal of the list of the 573 identified species of the collections show that there is an abundance of shrub, vine and liana, and herbaceous species among the plants employed by the healers. It is interesting to speculate on how the healers identified and established which part(s) of the plant possess healing properties and power.
Figure 7.
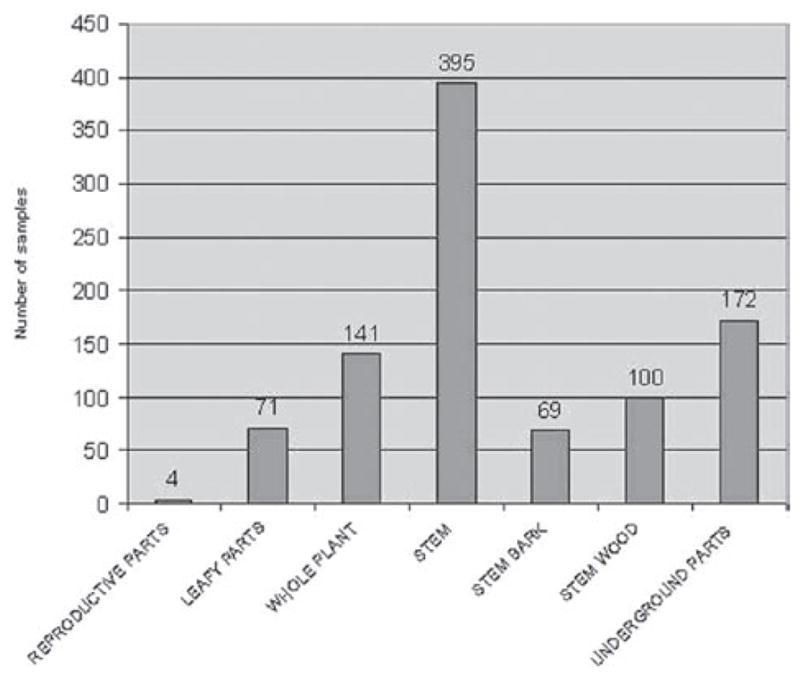
Frequency distribution of plant parts of the plant samples collected. The data used to structure this histogram were derived from data in Table 2. The discrepancy (3 plant parts) between the total number of samples (955) and the number of plant parts (952) of the samples collected is due to the fact that the plant parts of three samples were described as “unknown” and have been excluded from the Figure 7 (see text for further details).
Biological evaluation and chemical isolation studies
Summary of assays performed
The results of biological evaluation of medicinal plants of Laos collected in the present survey are summarized in Table 3. It should be noted that the ICBG database on the biological evaluation of plants of Cuc Phuong National Park and on the medicinal plants of Laos (Soejarto et al., 2006) is a dynamic database, which is evolving and continuously updated as new data are generated. Thus, the data presented in Table 3 are a mere cross-section in time. Only half (489) of the extracts of the 955 primary plant samples of medicinal plants of Laos collected had been submitted to bioassays as of December, 2010.
Table 3.
Summary of plant samples collected, their taxonomic identity, and the primary bioassays performed.a
| Number of collections | Number of samples | Number of identified species with samples | ||
| 990 | 955 [= 753 collections] | 573 | ||
| Number of samples | Number bioassayed | Number not bioassayed | ||
| 955 | 489 | 466 | ||
| Total primary bioassays performed: 3859 | ||||
| Anticancer (7 assays) | Anti-HIV (one assay) | Anti-TB (one assay) | Antimalaria (two assays) | |
| Total # assays performed: | 2900 | 249 | 256 | 454 |
| # Samples assayed: | 423 | 249 | 256 | 423 |
| A: 50 (12%) | A: 10 (4%) | A: 30 (12%) | A: 27(6%) | |
| [34 spp., 11 indet. 5 dup.] | [9 spp., 1 dup.] | [I9 spp., 11 indet.] | [21 spp. 6 indet.] | |
| Q: 5 | Q: 30 | Q: – | Q: 29 | |
| 1: 368 | 1: 209 | 1: 226 | 1: 367 | |
| A = Active | Q = Questionable | I = Inactive | ||
Of the 990 collections, 753 were taxonomically identified. Since 28 of the 753 collections were identified only to the genus level, and eliminated from further discussions, the actual number of collections used for analysis in the present paper is 725 collections, represented by 573 unicate species. Only half of the extracts (489) of the 955 samples have been submitted to bioassays. The results of the bioassays against the four disease targets are expressed in statistics of the extracts (samples) tested.
The numbers presented in Table 3 demonstrate very encouraging results for the biological evaluations of the extracts: 50 actives (12%), using 7 types of anticancer (cell line) assays; 10 actives (4%), using one type of anti-HIV assay; 30 actives, using one type of anti-TB assay (12%); and 27 actives, using two types (chloro-quinesensitive and chloroquine-resistant strains) of antimalarial assays (6%). Recollections of 10 of these 117 active species were completed and extracts submitted to bioassay-guided isolation studies. It should be noted that the taxonomic identity of the 10 active species (SL7006, SL7018, SL7037, SL7062, SL7105, SL7120, SL7287, SL7393, SL7407, and SL7530) that have been recollected had been unambiguously identified taxonomically, based on the voucher herbarium collections, as well as, based on fertile specimens (flowering/fruiting) of recollected individuals. To date, isolation studies of five (see below) of the 10 recollected species have yielded a series of new and in vitro-active anticancer and antimalarial compounds.
Species investigated
Asparagus cochinchinensis (Lour.) Merr. (Asparagaceae)
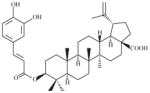
A twining herbaceous plant (Plate 1; voucher specimen Laos_037/K. Sydara 37) collected in Saravan Province, the root in a mixture with other plants, is used (Healer 95) to treat chronic fever. The plant also has a long history of use for treating fever, cough, kidney diseases, and benign breast tumors in China (Jiangsu Medical College, 1985). The Lao healer prepared the remedy as follows: Boil 15–20 g of dried plant with 20 g of plant no. 2, and 20 g of plant #3, in 1.5 L of water for 15–20 min. Five to seven pieces of the root may also be macerated in a glass of water for 3–4 h. The remedy is taken by mouth. Laboratory studies of the root sample collected from the same population of the initial sample (initial sample SL7037; recollected sample SLA7037) yielded 6 compounds (a new spirostanol saponin asparacoside and others) that showed cytotoxic activity in 5 cancer cell lines (Table 4; Zhang et al., 2004).
Table 4.
Biologically active compounds isolated from medicinal plants of Laos.
|
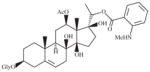
Gongronema napalense (Wall.) Decne (Asclepiadaceae) (SLA7530) (Vine) (Libman et al., 2008)
| |||||||
|---|---|---|---|---|---|---|---|
| Sample code | Chemical name of compound | Chemical structure of compound | Bioactivity of sample: ED50/IC50 (ng/mL)
|
||||
| KB
|
D6
|
W2
|
|||||
| ED50 | IC50 | SI | IC50 | SI | |||
| SLA7530-K001 | Gongroneside A |
|
>20000 | 2330 | >8.5 | 2020 | >13.7 |
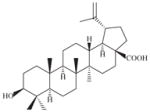
| Diospyros quaesita Thw. (Ebenaceae) (SLA7062) (LF+ST) (Ma et al., 2008) | |||||||
| SLA7062-K001 | Betulinic acid 3β-caffeate |
|
2500 | 870 | 2.9 | 607 | 4.1 |
| SLA7062-K001a | Betulinic acid |
|
>40000 | 3696 | >10.8 | 3776 | >10.5 |
| SLA7062-K001b | Betulinic acid 3β-bisacetylcaffeate |
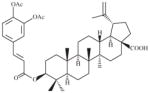
|
2070 | 480 | 4.3 | 443 | 4.7 |
| SLA7062-K004 | Pinoresinol |
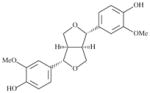
|
>40000 | 1361 | >29.3 | 3150 | >12.6 |
| Nauclea orientalis (L.) L. (Rubiaceae) (SLA7018) (TW) (He et al., 2005) | |||||||
| SLA7018-K002 | epi-Methoxy-naucleaorine |
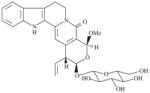
|
>20000 | 6535 ± 151 | >3.1 | 6979 ± 8 | >2.9 |
| SLA7018-K003 | Oleanolic acid |
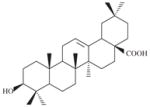
|
21000 | 2116 ± 308 | 9.9 | 2335 ± 13 | 9.0 |
| SLA7018-K006 | Naucleaorine |
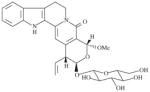
|
20000 | 3610 ± 595 | 5.5 | 4192 ± 8 | 4.8 |
| SLA7018-K008 | 3α,23-Dihydroxy-urs-12-en-28-oic acid |
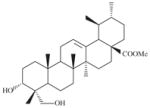
|
>20000 | 4567 ± 702 | >4.4 | 6006 ± 2581 | >3.3 |
| Rourea minor Leenh. (Connaraceae) (SLA7006) (Vine) (He et al., 2006) | |||||||
| SLA7006-K005 | Rouremin |
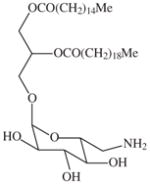
|
>20000 | 4000 | >5 | 3500 | >5 |
| SLA7006-K008 | Rourinoside |
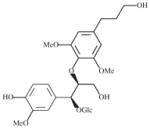
|
>20000 | 2100 | >9 | 1200 | >16 |
|
Asparagus cochinchinensis (Lour.) Merr. (Liliaceae) (SLA7037) (RT) (Zhang et al., 2004)
| ||||||||
|---|---|---|---|---|---|---|---|---|
| Sample code | Chemical name of compound | Chemical structure of compound | Bioactivity of sample: IC50(μg/ml)
|
|||||
| KB | Col-2 | LNCaP | LU-1 | HUVEC | hTERT | |||
| SLA7037-K001 | Asparacosin A |
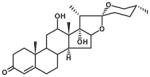
|
10.7 | >20 | >20 | >20 | >20 | >20 |
| SLA7037-K003 | Asparacoside |
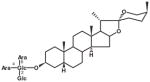
|
4.8 | 5.4 | 10.1 | 4.2 | 4.1 | 4.3 |
| SLA7037-K004 | 3′-Methoxy-asparenydiol |
|
12.0 | >20 | >20 | 19.7 | >20 | 0.3 |
| SLA7037-K006 | 3″-Methoxy-nyasol |
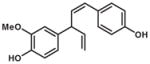
|
9.0 | 6.3 | 6.6 | 4.5 | 6.7 | 1.2 |
| SLA7037-K007 | 3″-Hydroxy-4″-methoxy-4″-dehyroxy-nyasol |
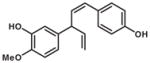
|
9.0 | 11.7 | 11.6 | 7.2 | 16.4 | 0.2 |
| SLA7037-K010 | Asparenydiol |
|
2.4 | >20 | >20 | 19.8 | >20 | 0.2 |
Diospyros quaesita Thw. (Ebenaceae)
A small to medium-sized tree (Plate 1; voucher specimen Laos_062/Bouamanivong 62) collected in Tana village in Xaignabouri Province, the leaf and stem sample is used (Healer 64) to treat cases of alcohol and drug dependence, as well as, to treat headache. The remedy is prepared by boiling 30 g of leaf and stem in 1.5 L of water for 15–20 min. The cool liquid is drunk. Laboratory studies of leafy branches recollected from the same plant in its original location, but in fruting condition (initial sample SL7062; recollected sample SLA7062), showed the presence of betulinic acid 3-caffeate, an in vitro-active antimalarial compound against both strains of malaria parasite (chloroquine-sensitive and chloroquine-resistant strains) (Table 4; Ma et al., 2008).
Gongronema napalense (Wall.) Decne. (Asclepiadaceae)
A vine (Figure 3 and Plate 1; voucher specimen Laos_541/Bountham et al. 37) that exudes milky latex when cut, collected in Champasak Province, the vine (entire plant) is used to treat polio. The remedy is prepared (Healer 66) by boiling a handful of an equal amount of the plant together with the wood of another plant (Kok mak kham) in 2 L of water until the water goes down to about 500 ml. Then the liquid is taken by mouth as needed for 7–15 days. The patient should not eat pork, while under this treatment. Decoction of this plant has also been recorded in the literature to treat leucorrhea, blennorrhea, and traumatic injury (Li et al., 1995). Laboratory studies of the whole flowering vine (initial sample SL7530; recollected sample SLA7530, 8 kg) yielded a new steroidal glycoside gongroneside A, in vitro-active in both strains of the malaria parasites (Table 4; Libman et al., 2008).
Nauclea orientalis (L.) L. (Rubiaceae)
A small tree (Plate 1; voucher specimen Laos_018/Bouamanivong 18), collected in Keng Sa Dok Village of Paksan District in Bolikhamxai Province, the dried stem (chipping of stem wood + stem bark) is used to treat cases of fatigue. The remedy is prepared (Healer 108) by boiling 15–20 g of dried plant/stem with 20 g each of two other plants in 1.5 L of water for 15–20 min. The remedy is taken by mouth. Laboratory studies of the dried stem collected from plant of the same population as the original tree (initial sample SL7018; recollected sample SLA7018, 5 kg) resulted in the isolation of two novel tetrahydro-β-carboline monoterpene alkaloid glucosides, naucleaorine and epimethoxynaucleaorine, as well as, a series of known compounds. Both naucleaorine and epimethoxynaucleaorine showed in vitro activities against the malaria parasites (Table 4; He et al., 2005).
Rourea minor (Gaertn.) Aubl. (Connaraceae)
A liana (woody vine) (Plate 1; voucher specimen Laos_006/Bouamanivong 6) collected in Pakkadan Village, Paksan District of Bolikhamxai Province, the dried stem is used to treat dengue fever. The remedy is prepared (Healer 77) by boiling 30 g of the dried stem together with three other plants in 2 L of water for 15–20 min, and the cooled liquid is taken by mouth. Bioassay-guided isolation of the dried stem of recollected plant in fruit as the original sample (initial sample SL7006; recollected sample SLA7006) resulted in the isolation of two new in vitro-active antimalarial glycosides, rourinoside and rouremin and a series of 5 other known compounds (Table 4; He et al., 2006).
Discussion
The Institute of Traditional Medicine of Lao PDR has been active in gathering information on medicinal plants of Laos through periodic field interviews and plant collection throughout the country as part of its mandate from the Ministry of Health. There are also a number of grant-supported projects to gather information on medicinal plants of Laos at ITM. However, the present ICBG-supported survey of medicinal plants of Laos is the largest of any effort to catalogue Lao medicinal plants through direct field interviews with Lao healers in recent years. This and other recent surveys have resulted in the continuing enrichment of our knowledge of medicinal plants of Laos, originally initiated and built in the early 1950s by the French ethnobotanist Alfred Petelot (Petelot, 1952, 1953, 1954). Data from the present survey are stored in an electronic format called NAPIS Database housed at ITM. These data are available only through special arrangement with the Director of the Institute of Traditional Medicine of Lao PDR, Ministry of Health, Vientiane, in an effort to safeguard the Intellectual Property Rights of the Lao healers and Lao people.
Our ethnobotanical survey demonstrates the richness of plant-based traditional medicine of Lao PDR, taxonomically and therapeutically. One hundred-eighteen healers provided the information on medicinal plants prescribed in their traditional practices to our team of scientists, resulting in the collection of 990 medicinal plants from 15 provinces (plus the Vientiane Prefecture). Due to the large number of plant specimens collected in sterile condition (lack of flowering/fruiting materials), not every specimen collected was fully identified at the writing of this paper. Of the 990 collections, 753 have been taxonomically identified and are used as the basis for analysis for this paper. However, 28 of the collections were only identified to the genus level, and have been eliminated for further discussions, while the remaining 725 were identified with confidence to the level of species, with many species duplicated among the collection, within and between provinces. Elimination of these duplicates left us with 573 unique species. As seen in Figure 1 and Figure 5, the greater number of these 573 species of medicinal plants was collected in Bolikhamsai (128) and Champasak (123) provinces. This was a result of the excellent transportation system to access these provinces from Vientiane, the base of the interview team, and by the fact that these two provinces have excellent facilities and healer communications in their Traditional Medicine Stations (TMS). Similar situations exist in the following provinces, where large numbers of species have also been collected: Oudomxai (78), Vientiane province and prefecture (49), Savannakhet (44), Xiangkhoang (41), and Xaignabouri (32). Unfavorable logistics and other conditions prevented interviews and collection trip to Bokeo Province. An important finding from this survey is the small percentage of duplication that exists between lots of species from different provinces. In a sense, this is in agreement with the results of a previous study (Libman et al. 2009), in which it was shown that knowledge of medicinal plants is a function of geographic location, rather than cultural diversity.
Another important finding is the great diversity of diseases (2353 different times ethno/traditional diseases are mentioned; Supporting Data #2), which the 118 healers use to treat diseases throughout Laos, and the fact that four of the diseases (“assay categories”) with a high frequency of use (more than 50) correspond to our ICBG project diseases of interest (cancer, HIV/AIDS, tuberculosis, and malaria; Table 1 and Figure 6). This is not a cause-and-effect situation, since the semi-structured interview methods do not lead the healer into focusing on those diseases from the start of the interview (see Interview Guide in Figure 2).
The finding that the four ICBG target diseases are among the most common health problems treated by traditional practitioners, in general terms, agrees with the present status of health issues in Laos (WHO at http://www.who.int/countries/lao/en/). Also of significance is the collection of plant samples belonging to a highly diverse medicinal plant species, and the great diversity of plant parts used by the healers (reflected in the diversity of parts in the bioassay samples collected). This diversity should provide a pool of chemical diversity as the starting point for the discovery of new bioactive molecules.
The goal of our Laos-based ICBG project at inception was to discover bioactive compounds with specific activities guided by field ethnobotanical interviews. Originally, the plan was to submit a plant sample extract with a history of use to treat malaria for only the antimalarial assay. Soon after the start of the project, however, it was realized that new discoveries would be missed if we restricted testing that extract against only that particular disease system. Four assay systems (cancer, HIV/AIDS, tuberculosis, malaria) were in operation from the start of the project. Thus, this led to a change in strategy to test every extract derived from medicinal plants of Laos in all our ICBG disease systems (cancer, HIV/AIDS, tuberculosis, malaria) in the assay systems and methods already established. These assay systems (described in the methodology section) continued in force throughout the period of the project.
Conclusions
Laos is a mega-diverse country belonging to the Indo-Burma biodiversity hotspot with an estimated 8000–11,000 species of plants found in the country (FAO, 2008; Conservation International, 2011; Department of Plant Sciences, Oxford University, 2011). The 990 documented collections of medicinal plants acquired, represent less than 10% of the flora of Laos, but these species were selected from those with a history of use in disease therapy. In other words, the use of these plants by the interviewed healers since time immemorial, through generations of healers, provides a strong suggestion of the potential for these plants to exhibit biological activity and provide new chemical constituents that may serve as candidates for medicines.
Biological assays of half (489) of the 955 sample extracts demonstrated in vitro activities against cancer, HIV/AIDS, tuberculosis and malaria disease systems (Table 3). In-depth studies of active samples to date have yielded a series of new bioactive compounds from 5 species, against the diseases of cancer and malaria. Therefore, further work with the remaining samples not assayed, as well as further in-depth studies of those with demonstrated activities in the preliminary assays, should lead us to discover many more new and active molecules.
Further support on the potential of Lao plants as a source of new medicines is provided by the results of a preliminary literature search performed using the NAPRALERT Database resource (http://www.napralert.org/) (Napralert, 2011). A randomly picked 100 of the 573 species were submitted to NAPRALERT. The results show (Supporting Data #5) that only about half of the plants we picked have been studied experimentally: 49 (49%) of the species have both biology/pharmacology data; 12 (12%) have chemistry data only; 2 (2%) have biology/pharmacology data only; and 37 (37%) have no chemistry and no biology/pharmacology data. It is clear that these 573 species had a high potential to provide us with new biomedical discoveries.
It is hopeful that one of these newly discovered compounds may serve as a candidate for development into a new pharmaceutical to alleviate human sufferings on our planet.
Acknowledgments
We wish to express our deepest thanks and appreciation to all healers of Laos who have graciously collaborated with us and made their knowledge available to our research in the present ICBG-sponsored research, for the period, 1999–2007. We also thank the Ministry of Health of Lao People’s Democratic Republic for its support and auspices in the planning and implementation of this research. Permit for the collection of plant materials for this research was granted by the Ministry of Agriculture and Forestry, for which we express our thanks. Special thanks go to Harry H.S. Fong, Professor Emeritus, Department of Medicinal Chemistry and Pharmacognosy, College of Pharmacy, and Lijun Rong, Associate Professor of Microbiology at the Department of Microbiology and Immunology, College of Medicine, University of Illinois at Chicago, for their advice and support. We also wish to thank the late Professor Norman Farnsworth, Distinguished University Professor Emeritus, Department of Medicinal Chemistry and Pharmacognosy, College of Pharmacy, UIC, for generous access to the NAPRALERT Database. Thanks are also due to Rohan Deshpande, Research Specialist at the Department of Medicinal Chemistry and Pharmacognosy for his assistance in the preparation of the manuscript.
This research was supported by NIH Grants 1-UO1-TW-001015-1 and 2-UO1-TW001015-06, International Cooperative Biodiversity Groups (ICBG), through funds from the National Institutes of Health, National Science Foundation, and the US Department of Agriculture Foreign Agricultural Service.
Footnotes
Declaration of interest
There are no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Central Intelligence Agency. [Accessed on 27 June 2011];East & Southeast Asia: Laos. 2011 Available online at https://www.cia.gov/library/publications/the-world-factbook/geos/la.html.
- Chape S. Biodiversity Conservation, Protected Areas and the Development Imperative in Lao PDR: Forging the Links. Vientiane: IUCN The World Conservation Union; 1996. [Google Scholar]
- Chazee L. The Peoples of Laos: Rural and Ethnic Diversities. Bangkok: White Lotus Press; 1999. [Google Scholar]
- Collins L, Franzblau SG. Microplate Alamar Blue assay versus BACTEC 460 system for high-throughput screening of compounds against Mycobacterium tuberculosis and Mycobacterium avium. Antimicrob Agents Chemother. 1997;41:1004–1009. doi: 10.1128/aac.41.5.1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conservation International. [Accessed on 2 July 2011];Indo-Burma Biodiversity Hotspots. 2011 Available online at http://www.biodiversityhotspots.org/xp/hotspots/indo_burma/Pages/default.aspx.
- Department of Plant Sciences, University of Oxford. [Accessed on 2 July 2011];Flore du Laos. 2011 Available online at http://dps.plants.ox.ac.uk/bol/HNL/Home/Index.
- Desjardins RE, Canfield CJ, Haynes JD, Chulay JD. Quantitative assessment of antimalarial activity in vitro by a semiautomated microdilution technique. Antimicrob Agents Chemother. 1979;16:710–718. doi: 10.1128/aac.16.6.710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Earth Times. [Accessed 3 July 2011];Laos To Increase Forest Coverage To Preserve Hydropower Potential. 2010 Available online at http://www.earthtimes.org/articles/news/359767,coverage-preserve-hydropower-potential.html.
- FAO. [Accessed on 2 July 2011];Agricultural Biodiversity in Lao PDR. 2008 Available online at http://www.fao.org/docrep/010/ai759e/ai759e00.htm; ftp://ftp.fao.org/docrep/fao/010/ai759e/ai759e00.pdf.
- He ZD, Ma CY, Zhang HJ, Tan GT, Tamez P, Sydara K, Bouamanivong S, Southavong B, Soejarto DD, Pezzuto JM, Fong HH. Antimalarial constituents from Nauclea orientalis (L.) L. Chem Biodivers. 2005;2:1378–1386. doi: 10.1002/cbdv.200590110. [DOI] [PubMed] [Google Scholar]
- He ZD, Ma CY, Tan GT, Sydara K, Tamez P, Southavong B, Bouamanivong S, Soejarto DD, Pezzuto JM, Fong HH, Zhang HJ. Rourinoside and rouremin, antimalarial constituents from Rourea minor. Phytochemistry. 2006;67:1378–1384. doi: 10.1016/j.phytochem.2006.04.012. [DOI] [PubMed] [Google Scholar]
- Hoang VD, Tan GT, Zhang HJ, Tamez PA, Hung NV, Cuong NM, Soejarto DD, Fong HH, Pezzuto JM. Natural anti-HIV agents-part I: (+)-demethoxyepiexcelsin and verticillatol from Litsea verticillata. Phytochemistry. 2002;59:325–329. doi: 10.1016/s0031-9422(01)00454-x. [DOI] [PubMed] [Google Scholar]
- Hollingshead MG, Alley MC, Camalier RF, Abbott BJ, Mayo JG, Malspeis L, Grever MR. In vivo cultivation of tumor cells in hollow fibers. Life Sci. 1995;57:131–141. doi: 10.1016/0024-3205(95)00254-4. [DOI] [PubMed] [Google Scholar]
- Jiangsu Medical College. A Dictionay of Traditional Chinese Medicines. Shanghai Science and Technology Publishers; Shanghai: 1985. Zhongyaodacidian; p. 318. [Google Scholar]
- Li B, Gilbert MG, Stevens WG. Zhengyi W, Raven PH, editors. Asclepiadaceae. [Accessed on 28 June 2011];Flora of China. 1995 16:189–270. Available online at http://www.efforas.org/florataxon.aspx?flora_id=2&taxon_id=10066.
- Libman A, Zhang HJ, Ma C, Southavong B, Sydara K, Bouamanivong S, Tan GT, Fong HHS, Soejarto DD. An antimalarial steroidal glycoside from Gongronema napalense. Asian J Trad Med. 2008;3:203–210. [PMC free article] [PubMed] [Google Scholar]
- Libman A, Southavong B, Sydara K, Bouamanivong S, Gyllenhaal C, Riley MC, Soejarto DD. The Influence of Cultural Tradition and Geographic Location on the Level of Medicinal Plant Knowledge Held by Various Cultural Groups in Laos. In: Compton CJ, Hartman JF, Sysamouth V, editors. Contemporary Lao Studies: Research on Development, Culture, Language, and Traditional Medicine; San Francisco: De Kalb, Illinois: Center for Laos Studies; Center for Southeast Asian Studies, Norhern Illinois University; 2009. pp. 277–291. [Google Scholar]; Selected Papers from the First International Conference on Lao Studies; De Kalb, Illinois. May 20–22, 2005. [Google Scholar]
- Ma CY, Musoke SF, Tan GT, Sydara K, Bouamanivong S, Southavong B, Soejarto DD, Fong HH, Zhang HJ. Study of antimalarial activity of chemical constituents from Diospyros quaesita. Chem Biodivers. 2008;5:2442–2448. doi: 10.1002/cbdv.200890209. [DOI] [PubMed] [Google Scholar]
- NAPRALERT. [Accessed on 2 July 2011];Natural Products Alert. 2011 Available online at http://www.napralert.org/
- National Geographic. Laos Map ©National Geographic Society, 1996–2011. 1996–2011 Available online at http://travel.nationalgeographic.com/travel/countries/laos-map/ Visited on July 5, 2011.
- Petelot A. Les Plantes Medicinales du Cambodge, du Laos et du Vietnam. Vol. 1. Centre National de Recherches Scientifiques et Techniques; Paris: 1952. p. 406. [Google Scholar]
- Petelot A. Les Plantes Medicinales du Cambodge, du Laos et du Vietnam. Vol. 2. Centre National de Recherches Scientifiques et Techniques; Paris: 1953. p. 284. [Google Scholar]
- Petelot A. Les Plantes Medicinales du Cambodge, du Laos et du Vietnam. Vol. 3. Centre National de Recherches Scientifiques et Techniques; Paris: 1954. p. 347. [Google Scholar]
- Petelot A. Les Plantes Medicinales du Cambodge, du Laos et du Vietnam. Vol. 4. Centre National de Recherches Scientifiques et Techniques; Paris: 1954. p. 307. [Google Scholar]
- Pham Hoang Ho. An Illustrated Flora of Vietnam. part 1 and part 2. Vol. 1. Santa Ana, CA: Mekong Printing; 1991. Cayco Vietnam; pp. 1–1249. [Google Scholar]
- Pham Hoang Ho. Cayco Vietnam. part 1. Vol. 2. Santa Ana, CA: Mekong Printing; 1992. pp. 1–1174. (1992) and part 2 (1993) [Google Scholar]
- Pham Hoang Ho. Cayco Vietnam. part 1 and part 2. Vol. 3. Santa Ana, CA: Mekong Printing; 1993. pp. 1–1276. [Google Scholar]
- Pham Hoang Ho. Cayco Vietnam. 1–3. Nha Xuat Bhan Tre, Ho Chi Minh City; Vietnam: 1999. pp. 1–991.pp. 1–951.pp. 1–1020. (vol. 2) (vol. 2) (vol. 3) [Google Scholar]
- Riley MC. Traditional Medicine Research Center (TMRC): A potential tool for protecting traditional and tribal medicinal knowledge in Laos. Cultural Survival Quart. 2001;24:21–24. [Google Scholar]
- Riley MC, Dietzman GR, Southavong B, Kadushin MR, Sydara K, Libman A, Bouamanivong S, Gyllenhaal C, Soejarto DD. The Lao Traditional Medicine Mapping Project (Lao TMMP) In: Compton CJ, Hartman JF, Sysamouth V, editors. Contemporary Lao Studies: Research on Development, Culture, Language, and Traditional Medicine. 2009. pp. 293–306. [Google Scholar]; Selected Papers from the First International Conference on Lao Studies; De Kalb, Illinois. May 20–22, 2005. [Google Scholar]
- Smilkstein M, Sriwilaijaroen N, Kelly JX, Wilairat P, Riscoe M. Simple and inexpensive fluorescence-based technique for high-throughput antimalarial drug screening. Antimicrob Agents Chemother. 2004;48:1803–1806. doi: 10.1128/AAC.48.5.1803-1806.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soejarto DD. Logistics and politics in plant drug discovery: The other end of the spectrum. In: Kinghorn AD, Balandrin M, editors. Human Medicinal Agents from Plants. American Chemical Society; Washington, DC: 1993. pp. 98–111. [Google Scholar]
- Soejarto DD, Gyllenhaal C, Ashton PS, Sohmer HS. Plant explorations in Asia under the sponsorship of the National Cancer Institute, 1986–1991: An overview. In: Balick MJ, Elisabetsky E, Laird SA, editors. Medicinal Plant Resources of the Tropical Forest. ch 21. Columbia University Press; New York: 1996. pp. 284–310. [Google Scholar]
- Soejarto DD, Gyllenhaal C, Regalado JC, Pezzuto JM, Fong HHS, Tan GT, Hiep NT, Xuan LT, Binh DQ, Hung NV, Bich TQ, Thin NN, Loc PK, Vu BM, Southavong BH, Sydara K, Bouamanivong S, O’Neill MJ, Lewis J, Xie X, Dietzman G. Studies on biodiversity of Vietnam and Laos: A UIC-based ICBG Program. Pharm Biol. 1999;37 (Supplement):1–14. [Google Scholar]
- Soejarto DD, Gyllenhaal C, Regalado JC, Pezzuto JM, Fong HHS, Tan GT, Hiep NT, Xuan LT, Hung NV, Bich TQ, Loc PK, Vu BM, Southavong BH, Sydara K, Bouamanivong S, O’Neill MJ, Dietzman G. An international collaborative program to discover new drugs from tropical biodiversity of Vietnam and Laos. Nat Prod Sci. 2002;8:1–15. [Google Scholar]
- Soejarto DD, Gyllenhaal C, Fong HH, Xuan LT, Hiep NT, Hung NV, Bich TQ, Southavong B, Sydara K, Pezzuto JM. The UIC ICBG (University of Illinois at Chicago International Cooperative Biodiversity Group) Memorandum of Agreement: A model of benefit-sharing arrangement in natural products drug discovery and development. J Nat Prod. 2004;67:294–299. doi: 10.1021/np0304363. [DOI] [PubMed] [Google Scholar]
- Soejarto DD, Zhang HJ, Fong HH, Tan GT, Ma CY, Gyllenhaal C, Riley MC, Kadushin MR, Franzblau SG, Bich TQ, Cuong NM, Hiep NT, Loc PK, Xuan le T, Hai NV, Hung NV, Chien NQ, Binh le T, Vu BM, Ly HM, Southavong B, Sydara K, Bouamanivong S, Pezzuto JM, Rose WC, Dietzman GR, Miller BE, Thuy TV. Studies on biodiversity of Vietnam and Laos 1998–2005: Examining the impact. J Nat Prod. 2006;69:473–481. doi: 10.1021/np058107t. [DOI] [PubMed] [Google Scholar]
- Soejarto DD, Gyllenhaal C, Tarzian Sorensen JA, Fong HHHS, Xuan LT, Binh LT, Hiep NT, Hung NV, Bich TQ, Vu BM, Southavong B, Sydara K, Pezzuto JM, Riley MC. Bioprospecting Arrangements: Cooperation Between the North and the South. In: Krattiger A, Mahoney RT, Nelsen L, et al., editors. Intellectual Property Management in Health and Agricultural Innovation: A Handbook of Best Practices. MIHR; Oxford, U.K: PIPRA; Davis, U.S.A: 2007. [Accessed on 3 July 2011]. Available online at www.ipHandbook.org (Topics #16.5) [Google Scholar]
- Soejarto DD, Southavong B, Sydara K, Bouamanivong S, Riley MC, Libman A, Kadushin MR, Gyllenhaal C. Studies on Medicinal Plants of Laos: A Collaborative Program between the University of Illinois at Chicago (UIC) and the Traditional Medicine Research Center (TMRC), Lao PDR: Accomplishments. In: Compton CJ, Hartman JF, Sysamouth V, editors. Contemporary Lao Studies: Research on Development, Culture, Language, and Traditional Medicine. Center for Laos Studies; San Francisco: Center for Southeast Asian Studies, Norhern Illinois University; De Kalb, Illinois: 2009. pp. 307–323. [Google Scholar]; Selected Papers from the First International Conference on Lao Studies; De Kalb, Illinois. May 20–22, 2005. [Google Scholar]
- Statoids. [Accessed on 5 July 2011];Provinces of Laos. 2011 Available online at http://www.statoids.com/ula.html [last updated on June 20, 2011]
- Sydara K, Gneunphonsavath S, Wahlström R, Freudenthal S, Houamboun K, Tomson G, Falkenberg T. Use of traditional medicine in Lao PDR. [Accessed on June, 2011];Complem Ther Med. 2005 13:199–205. doi: 10.1016/j.ctim.2005.05.004. Available online at http://ki.se/content/1/c6/06/43/79/UseTraditional.pdf. [DOI] [PubMed] [Google Scholar]
- Tan GT, Honnen WJ, Kayman SC, Pinter A. A sensitive microtiter infectivity assay for macrophage-tropic and primary isolates of HIV-1 based on the transactivation of a stably integrated gene for the green fluorescent protein. The 9th National Cooperative Vaccine Development Group (NCVDG) Meeting: Advances in AIDS Pathogenesis and Preclinical Vaccine Development; NIH, Bethesda, Maryland. May 4–7, 1997.1997. [Google Scholar]
- Tan G, Gyllenhaal C, Soejarto DD. Biodiversity as a source of anticancer drugs. Curr Drug Targets. 2006;7:265–277. doi: 10.2174/138945006776054942. [DOI] [PubMed] [Google Scholar]
- Vidal J. Extrait du Bulletin de l’Ecole Francaise d’Extrême-Orient, Tome XLIX, fascicule 2. Paris: l’Ecole Francaise d’Extrême-Orient; 1959. Noms Vernaculaires de Plantes (Lao, Mèo, Kha) en Usage au Laos. [Google Scholar]
- Vongsiharath V. [Accessed on 3 July 2011];Forest cover and Land-use Changes in Lao PDR according to the National Forest Reconnaissance Survey. 2008 Full document online at ftp://ftp.fao.org/docrep/fao/012/i1067e/i1067e01.pdf.
- WHO. [Accessed on 27 June 2011];WHO Country Cooperation Strategy (CCS) in the Lao People’s Democratic Republic 2009–2011. 2009 Available online at http://www.who.int/countryfocus/cooperation_strategy/ccs_lao_en.pdf.
- WHO. [Accessed on 27 June 2011];Lao People’s Democratic Republic. 2011 Available online at http://www.who.int/countryfocus/cooperation_strategy/ccs_lao_en.pdf.
- Zhang HJ, Tan GT, Hoang VD, Hung NV, Cuong NM, Soejarto DD, Pezzuto JM, Fong HHS. Natural anti-HIV agents. Part III. Litseaverticillols A-H, novel sesquiterpenes from Litsea verticillata. Tetrahedron. 2003a;59:141–148. [Google Scholar]
- Zhang HJ, Tamez P, Aydogmus Z, Tan GGT, Saikawa Y, Hashimoto K, Nakata M, Hung NV, Xuan LT, Cuong NM, Soejarto DD, Pezzuto JM, Fong HHS. Antimalarial agents from plants. III. Trichothecenes from Ficus flstulosa and Rhaphidophora decursiva. Planta Med. 2003b;68:1088–1091. doi: 10.1055/s-2002-36350. [DOI] [PubMed] [Google Scholar]
- Zhang HJ, Sydara K, Tan GT, Ma C, Southavong B, Soejarto DD, Pezzuto JM, Fong HHS. Bioactive constituents from Asparagus cochinchinensis. J Nat Prod. 2004;67:194–200. doi: 10.1021/np030370b. [DOI] [PubMed] [Google Scholar]
- Zhang HJ, Nguyen VH, Nguyen MC, Soejarto DD, Pezzuto JM, Fong HHS, Tan GT. Sesquiterpenes and butenolides, natural anti-HIV constituents from Litsea verticillata. Planta Med. 2005;71:452–457. doi: 10.1055/s-2005-864142. [DOI] [PubMed] [Google Scholar]