Abstract
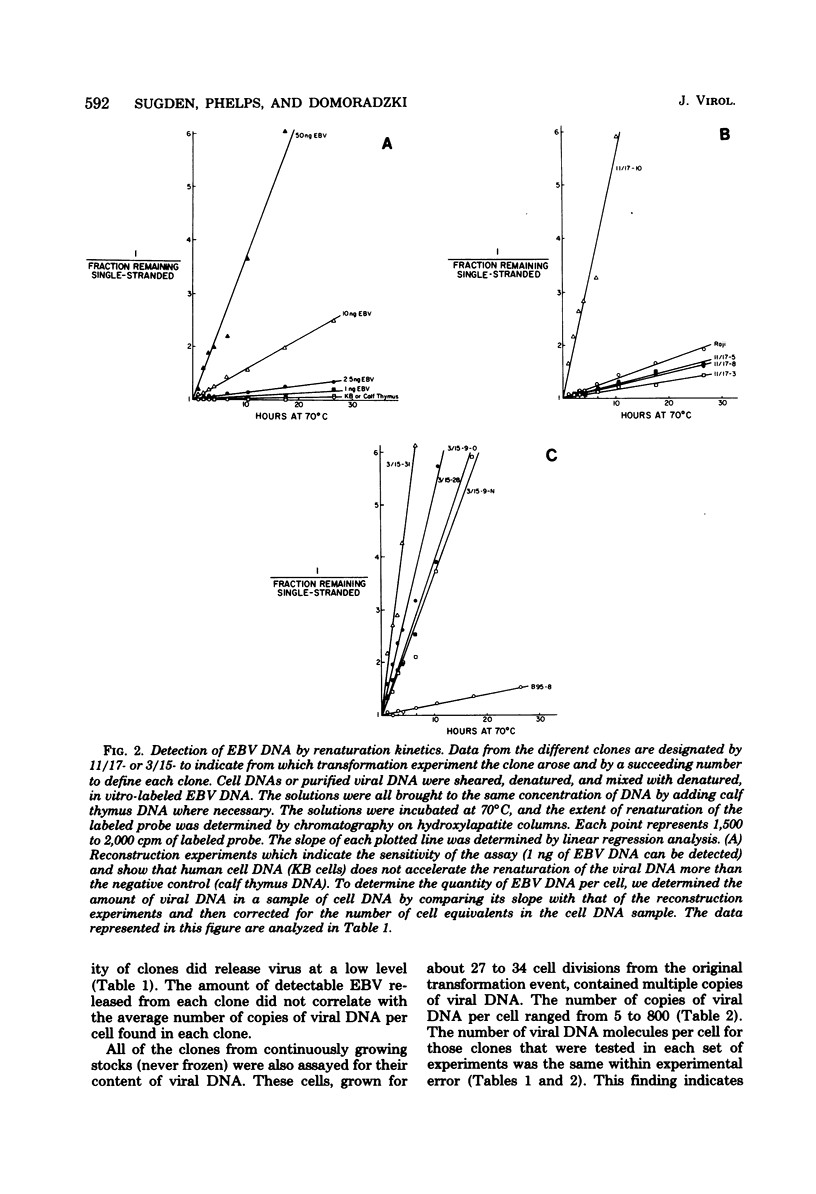
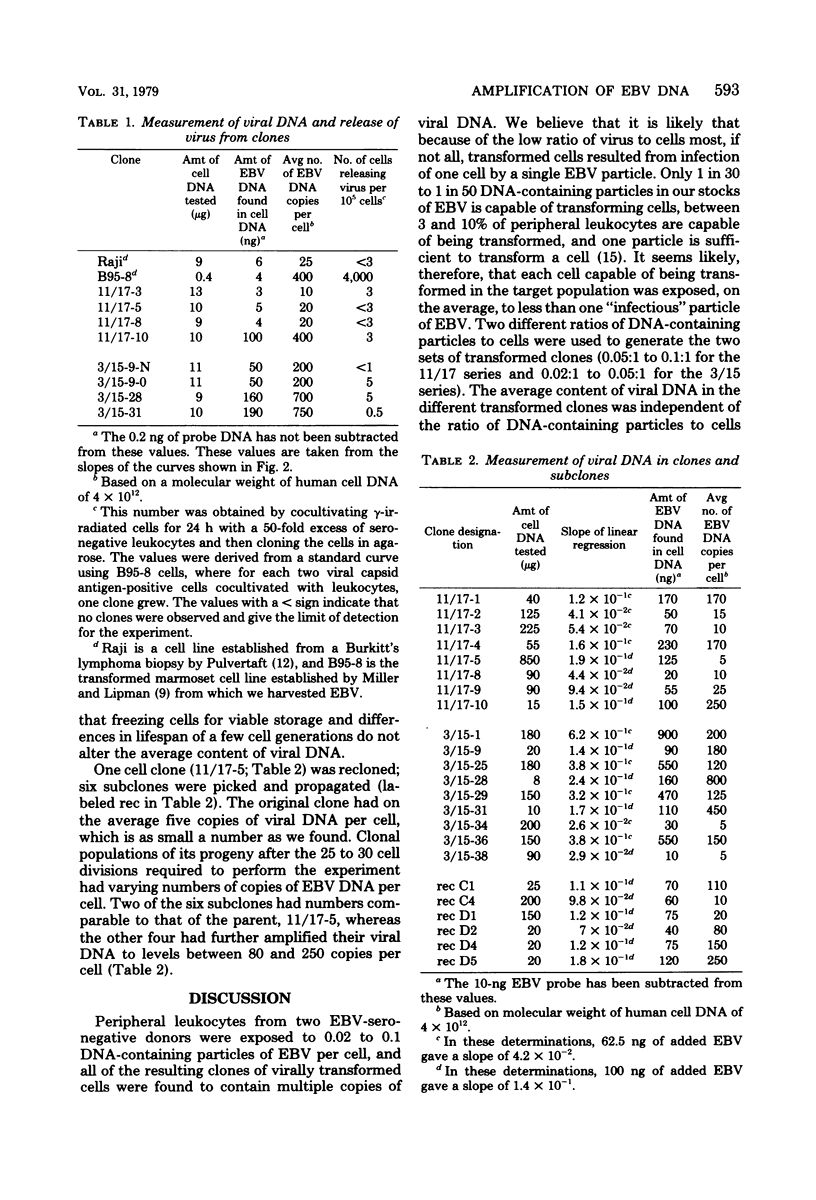
Leukocytes isolated from two adult donors who lacked detectable antibodies to antigens associated with Epstein-Barr virus were exposed to an average of 0.02 to 0.1 DNA-containing particles of Epstein-Barr virus per cell and immediately clones in agarose. Within about 30 generations all transformed cell clones contained between 5 and 800 copies of viral DNA per cell. Only 1 in 10(4) to less than 1 in 10(5) of the cells of each clone release virus, and the frequency of release did not correlate with the average number of copies of viral DNA in the cells of each clone. One clone that had an average of five copies of viral DNA per cell was recloned, and the average number of copies in four of six subclones increased 15-to 50-fold while the subclones were being propagated sufficiently to study them. These results indicate that Epstein-Barr virus DNA can undergo amplification relative to cell DNA at different times after it transforms cells.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams A. Concentration of Epstein-Barr virus from cell culture fluids with polyethylene glycol. J Gen Virol. 1973 Sep;20(3):391–394. doi: 10.1099/0022-1317-20-3-391. [DOI] [PubMed] [Google Scholar]
- Botchan M., McKenna G., Sharp P. A. Cleavage of mouse DNA by a restriction enzyme as a clue to the arrangement of genes. Cold Spring Harb Symp Quant Biol. 1974;38:383–395. doi: 10.1101/sqb.1974.038.01.041. [DOI] [PubMed] [Google Scholar]
- Chang R. S., Golden H. D. Transformation of human leucocytes by throat washing from infectious mononucleosis patients. Nature. 1971 Dec 10;234(5328):359–360. doi: 10.1038/234359a0. [DOI] [PubMed] [Google Scholar]
- Given D., Kieff E. DNA of Epstein-Barr virus. IV. Linkage map of restriction enzyme fragments of the B95-8 and W91 strains of Epstein-Barr Virus. J Virol. 1978 Nov;28(2):524–542. doi: 10.1128/jvi.28.2.524-542.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaschka-Dierich C., Falk L., Bjursell G., Adams A., Lindahl T. Human lymphoblastoid cell lines derived from individuals without lymphoproliferative disease contain the same latent forms of Epstein-Barr virus DNA as those found in tumor cells. Int J Cancer. 1977 Aug 15;20(2):173–180. doi: 10.1002/ijc.2910200203. [DOI] [PubMed] [Google Scholar]
- Lindahl T., Adams A., Bjursell G., Bornkamm G. W., Kaschka-Dierich C., Jehn U. Covalently closed circular duplex DNA of Epstein-Barr virus in a human lymphoid cell line. J Mol Biol. 1976 Apr 15;102(3):511–530. doi: 10.1016/0022-2836(76)90331-4. [DOI] [PubMed] [Google Scholar]
- Lindahl T., Klein G., Reedman B. M., Johansson B., Singh S. Relationship between Epstein-Barr virus (EBV) DNA and the EBV-determined nuclear antigen (EBNA) in Burkitt lymphoma biopsies and other lymphoproliferative malignancies. Int J Cancer. 1974 Jun 15;13(6):764–772. doi: 10.1002/ijc.2910130605. [DOI] [PubMed] [Google Scholar]
- Miller G., Lipman M. Release of infectious Epstein-Barr virus by transformed marmoset leukocytes. Proc Natl Acad Sci U S A. 1973 Jan;70(1):190–194. doi: 10.1073/pnas.70.1.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niederman J. C., Miller G., Pearson H. A., Pagano J. S., Dowaliby J. M. Infectious mononucleosis. Epstein-Barr-virus shedding in saliva and the oropharynx. N Engl J Med. 1976 Jun 17;294(25):1355–1359. doi: 10.1056/NEJM197606172942501. [DOI] [PubMed] [Google Scholar]
- Nonoyama M., Pagano J. S. Separation of Epstein-Barr virus DNA from large chromosomal DNA in non-virus-producing cells. Nat New Biol. 1972 Aug 9;238(84):169–171. doi: 10.1038/newbio238169a0. [DOI] [PubMed] [Google Scholar]
- PULVERTAFT J. V. A STUDY OF MALIGNANT TUMOURS IN NIGERIA BY SHORT-TERM TISSUE CULTURE. J Clin Pathol. 1965 May;18:261–273. doi: 10.1136/jcp.18.3.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherberg N. H., Refetoff S. The preparation of carrier-free iodine isotope-substituted cytosine nucleotides. Biochim Biophys Acta. 1974 Apr 10;340(4):446–451. doi: 10.1016/0005-2787(74)90065-3. [DOI] [PubMed] [Google Scholar]
- Sugden B. Comparison of Epstein-Barr viral DNAs in Burkitt lymphoma biopsy cells and in cells clonally transformed in vitro. Proc Natl Acad Sci U S A. 1977 Oct;74(10):4651–4655. doi: 10.1073/pnas.74.10.4651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugden B., Mark W. Clonal transformation of adult human leukocytes by Epstein-Barr virus. J Virol. 1977 Sep;23(3):503–508. doi: 10.1128/jvi.23.3.503-508.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugden B., Summers W. C., Klein G. Nucleic acid renaturation and restriction endonuclease cleavage analyses show that the DNAs of a transforming and a nontransforming strain of Epstein-Barr virus share approximately 90% of their nucleotide sequences. J Virol. 1976 May;18(2):765–775. doi: 10.1128/jvi.18.2.765-775.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zech L., Haglund U., Nilsson K., Klein G. Characteristic chromosomal abnormalities in biopsies and lymphoid-cell lines from patients with Burkitt and non-Burkitt lymphomas. Int J Cancer. 1976 Jan 15;17(1):47–56. doi: 10.1002/ijc.2910170108. [DOI] [PubMed] [Google Scholar]
- zur Hansen H., Diehl V., Wolf H., Schulte-Holthausen H., Schneider U. Occurrence of Epstein-Barr virus genomes in human lymphoblastoid cell lines. Nat New Biol. 1972 Jun 7;237(75):189–190. doi: 10.1038/newbio237189a0. [DOI] [PubMed] [Google Scholar]



