Abstract
Prostate-specific membrane antigen (PSMA) is a notable biomarker for diagnostic and therapeutic applications in prostate cancer. Gold nanoparticles (AuNPs) provide an attractive nanomaterial platform for combining a variety of targeting, imaging, and cytotoxic agents into a unified device for biomedical research. In this study, we present the generation and evaluation of the first AuNP system functionalized with a small molecule phosphoramidate peptidomimetic inhibitor for the targeted delivery to PSMA-expressing prostate cancer cells. The general approach involved the conjugation of streptavidin-coated AuNPs with a biotin-linked PSMA inhibitor (CTT54) to generate PSMA-targeted AuNPs. In vitro evaluations of these targeted AuNPs were conducted to determine PSMA-mediated and time-dependent binding to PSMA-positive LNCaP cells. The PSMA-targeted AuNPs exhibited significantly higher and selective binding to LNCaP cells compared to control non-targeted AuNPs, thus demonstrating the feasibility of this approach.
Keywords: gold nanoparticles, streptavidin, biotin, prostate-specific membrane antigen, prostate cancer
Prostate cancer is the second most common cancer diagnosed in men globally1 and remains the second leading cause of cancer mortality in men in the United States.2 Early stage primary prostate tumors are often successfully treated through standard techniques (e.g., radical prostatectomy, radiation, anti-androgen therapy). However, advanced stage and metastatic prostate cancer generally have poorer treatment prognoses, emphasizing a critical need to develop new techniques to improve patient outcomes. Prostate-specific membrane antigen (PSMA), also known as glutamate carboxypeptidase II (GCPII), is a classic type-II membrane glycoprotein and possesses ideal characteristics as an enzyme-biomarker target due to its unique expression in primary and metastatic prostate cancer cells3–6 and its proclivity to internalize upon binding targeting ligands.7–9 Of the chemical scaffolds used for targeting PSMA in prostate cancer research,10–15 our group developed phosphoramidate peptidomimetic inhibitors of PSMA to deliver an array of imaging8, 16–20 and therapeutic21–23 agents to prostate cancer cells in vitro and in vivo. Of these, the CTT54 inhibitor core is particularly efficacious as a PSMA targeting molecule due to its high affinity (14 nM), pseudo-irreversible mode of binding,8, 18 rapid uptake, and internalization in PSMA-positive (PSMA+) prostate cancer cells.8, 17, 19, 20, 24
Nanoparticles represent an emerging technology in medicinal applications due their unique pharmacokinetic properties, amenability for multi-functionalization, and high loading capacities. Because of these features, nanoparticles are attractive platforms for the development of multimodal theranostic agents.25, 26 Gold nanoparticles (AuNPs) in particular possess distinct and controllable physicochemical properties which offer advantages over other nanoparticle platforms. The gold core is biocompatible and has been directly utilized in imaging (e.g., optical contrast27 and computed tomography28) and therapeutic (e.g., radiotherapy,29 photothermal ablation,30, 31 mechanical disruption32) applications. Additionally, the gold surface can be modified by soft donors (e.g., thiols) tethered to reporting, therapeutic, targeting, or biological stabilizing molecules to generate multifunctional devices for in vitro and in vivo use.33–36
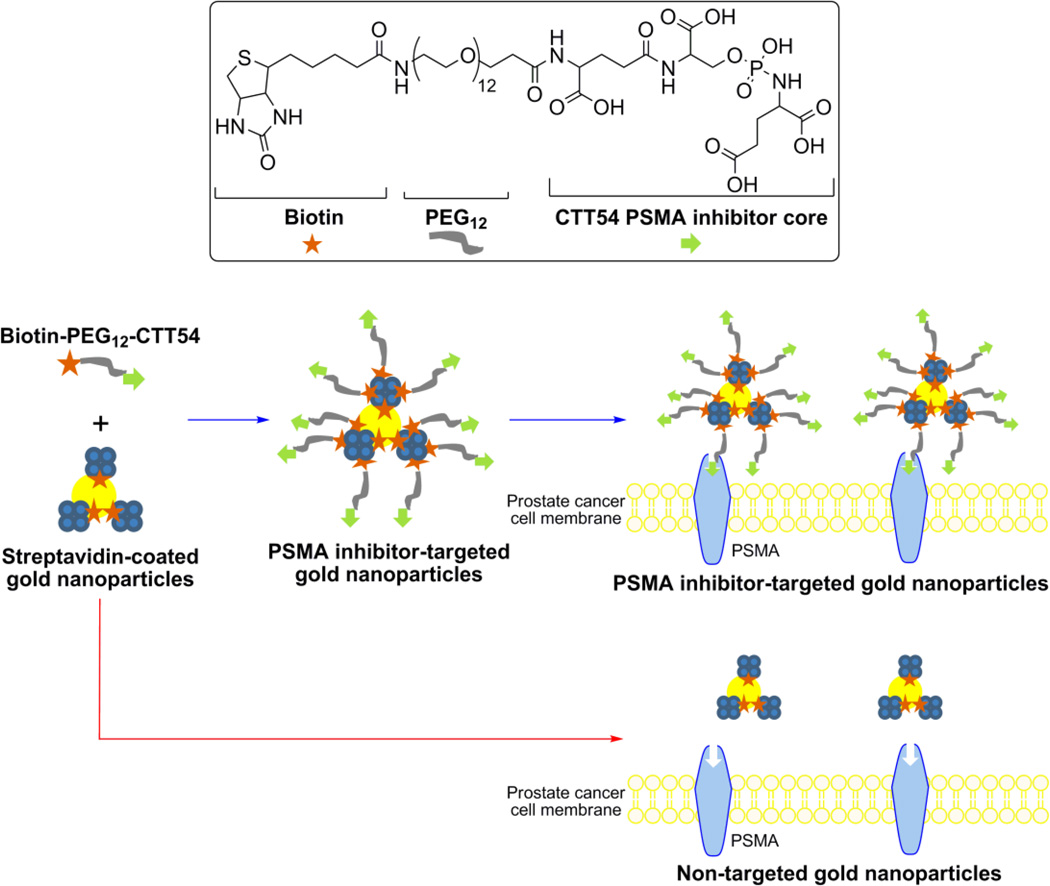
The combination of a nanoparticle platform with targeting ligands for tumor cell-surface biomarkers is a promising architecture for achieving selective delivery and uptake into target cells. With respect to PSMA targeting, several types of nanoparticles have been outfitted with various types targeting agents (e.g., antibodies, aptamers, urea inhibitors) demonstrating the utility of this biomarker for in vitro and in vivo prostate cancer applications.27, 31, 37–44 Although the PSMA-targeted delivery of AuNPs has been pioneered using anti-PSMA aptamers and antibodies,27, 31, 39 the employment of AuNPs with small molecule inhibitors of PSMA has not previously been reported. Employment of small molecules may offer several advantages over larger platforms in generating targeted AuNPs including low immunogenicity and reduced scale-up costs. Compared to antibodies,45 small molecules may be conjugated to nanoparticle surfaces in controllable orientations which do not compromise affinity for the biochemical target. Furthermore, antibodies bound to surface antigens present a barrier for subsequent binding of antibodies at neighboring surface antigens46, 47 and may partially limit the effectiveness of multifunctionlized antibody-targeted nanoparticle platforms. The focus of this study was to explore the feasibility of using a small molecule phosphoramidate peptidomimetic PSMA inhibitor for mediating the delivery of AuNPs to prostate cancer cells. The 1st-generation PSMA-targeted AuNP platform developed for this work employed facile biotin-streptavidin coupling48–50 to functionalize the nanoparticles.51–57 We have recently used a biotinylated PSMA inhibitor (CTT54) to promote the PSMA-mediated delivery of other macromolecular conjugates (e.g., streptavidin tetramers24 and streptavidin-coated magnetic beads58) to PSMA+ LNCaP cells. As an extension of this previous work to a nanoparticle system, streptavidin-coated AuNPs were outfitted with biotinylated-CTT54 in the current study (Figure 1).
Figure 1.
General scheme showing the structure of the biotin-PEG12-CTT54 inhibitor, AuNP functionalization strategy, and PSMA-mediated binding of targeted AuNPs to prostate cancer cells.
The synthesis of the PSMA-targeted AuNP platform was achieved by incubating the biotinylated PSMA inhibitor, biotin-PEG12-CTT54,24 with commercially available 5 nm AuNPs coated with streptavidin (AuNP-streptavidin; Figure 1). Following centrifugal filtration to remove excess biotin-PEG12-CTT54, the PSMA-targeted nanoparticles (AuNP-streptavidin:biotin-PEG12-CTT54) were re-suspended and characterized by transmission electron microscopy (TEM). TEM analysis showed monodisperse particles prior to and following conjugation (Figure S1), indicating the particles remained stable and free from aggregation during preparation.
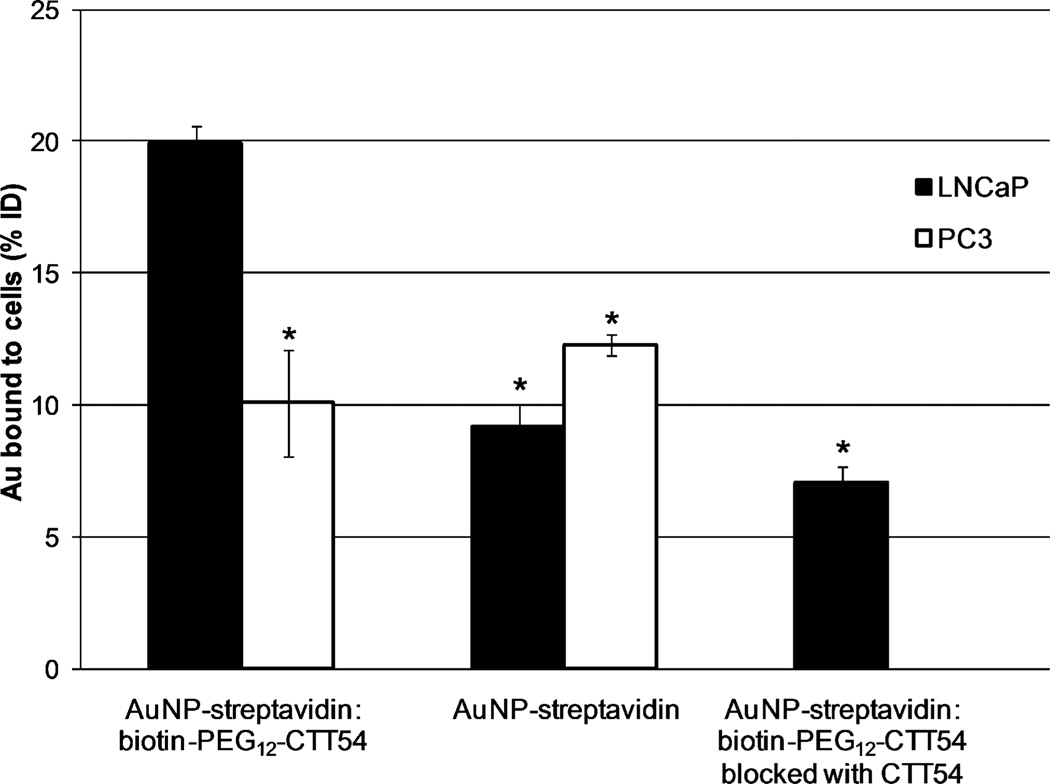
In vitro assessment of PSMA inhibitor-mediated binding was conducted by incubating the AuNP-streptavidin: biotin-PEG12-CTT54 with PSMA+ LNCaP cells and PSMA-negative (PSMA−) PC3 cells at 37 °C for 1 h followed by removal of excess nanoparticles by washing and centrifugation. The resultant pelleted cells were lysed with aqua regia to dissolve the AuNPs followed by removal of cellular debris by centrifugation. The resulting supernatant was analyzed by inductively coupled plasma optical emission spectroscopy (ICP-OES) to quantify the gold concentration bound to the cells as the percentage of the injected dose (% ID) initially added to the cells (procedural details available in the Supplementary Data). To test for non-specific cellular interactions inherent to the AuNP, non-targeted AuNP-streptavidin nanoparticles were also examined under the same conditions. The PSMA-targeted nanoparticles exhibited significantly greater binding to LNCaP cells compared to non-targeted AuNP-streptavidin nanoparticles after incubation at 37 °C for 1 h (Figure 2). These results supported the concept that small molecule inhibitors of PSMA could mediate the enhanced delivery of AuNPs to prostate cancer cells. In PC3 cells, both AuNP-streptavidin:biotin-PEG12-CTT54 and non-targeted AuNP-streptavidin showed significantly lower levels of binding compared to AuNP-streptavidin: biotin-PEG12-CTT54 to LNCaP cells. These findings suggested that the enhanced delivery of the inhibitor-targeted AuNPs observed in the LNCaP cells was due to inhibitor-mediated PSMA binding rather than to non-specific cell interactions. Further confirmation of the PSMA-specific binding of the PSMA-targeted AuNPs to LNCaP cells was provided by performing a competitive blocking study in which LNCaP cells were saturated first with free CTT54 prior to addition of AuNP-streptavidin:biotin-PEG12-CTT54 to the cells. As expected, the targeted binding of AuNP-streptavidin:biotin-PEG12-CTT54 to LNCaP cells was significantly reduced when first blocked by the unconjugated PSMA inhibitor CTT54 and was similar to that observed for non-targeted AuNPs (Figure 2).
Figure 2. Quantification of AuNP bound to LNCaP and PC3 cells in vitro.
Cells were incubated at 37 °C for 1 h with 4.0 nM targeted AuNP-streptavidin: biotin-PEG12-CTT54, 6.6 nM non-targeted AuNP-streptavidin, or 4.0 nM AuNP-streptavidin:biotin-PEG12-CTT54 blocked with CTT54. The total amount of AuNP bound to the cells was quantified by ICP-OES and expressed as the percentage of the injected dose (% ID). Values are the averages of one to two individual experiments (two to three replicate samples per experiment) with the standard deviations represented by error bars. *Indicates a significant difference (P < 0.05) compared to AuNP-streptavidin:biotin-PEG12-CTT54 in LNCaP cells.
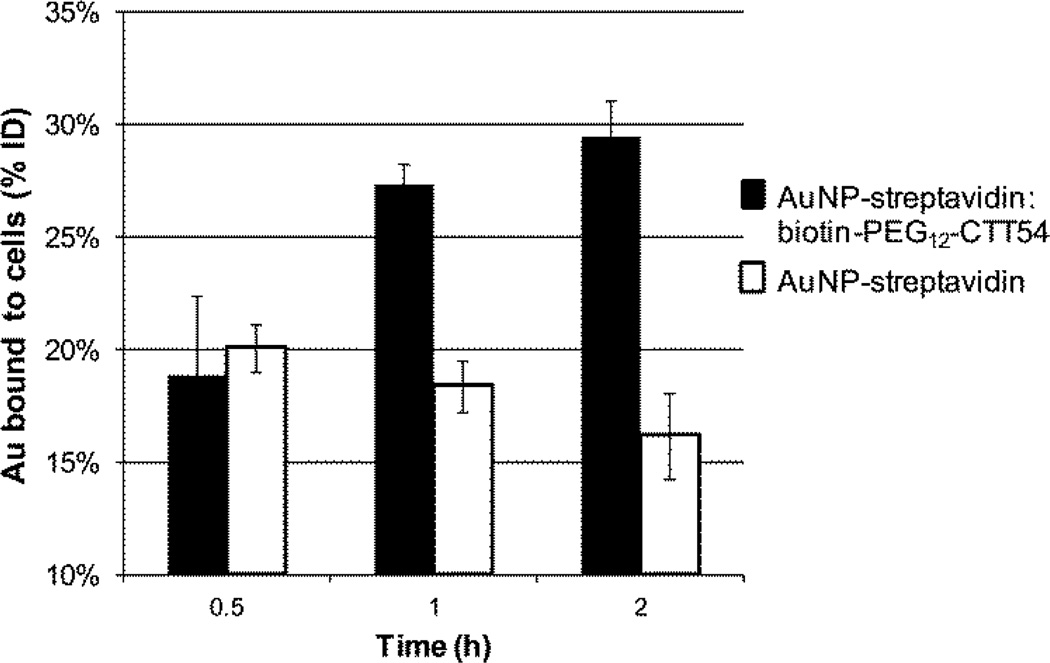
Based on the selective binding observed for the PSMA-targeted AuNPs in PSMA+ cells, the time-dependent delivery of both targeted and non-targeted AuNPs was examined over 2 h in LNCaP cells. Analysis at three time points (0.5, 1, and 2 h) confirmed an observable increase in the percent of AuNP-streptavidin: biotin-PEG12-CTT54 bound to the cells with the highest uptake at 2 h (Figure 3). This increased PSMA inhibitor-mediated binding to LNCaP cells is consistent with the trend observed previously with other CTT54 conjugates.19, 20, 24 In contrast, the percent of non-targeted AuNP-streptavidin bound to the cells gradually decreased over 2 h. A separate experiment demonstrated that residual biotin in LNCaP cells was not responsible for the levels of cell binding observed for the non-targeted AuNPs. AuNP-streptavidin nanoparticles which had been incubated with excess biotin (AuNP-streptavidin:biotin) showed similar levels of binding to LNCaP cells as the initial AuNP-streptavidin nanoparticles (Figure S2). These observations suggest that non-targeted AuNPs may exhibit weak and non-specific interactions with cells that may be transient and decrease over time. Overall, our results support the use of small molecule PSMA inhibitors to effectively deliver AuNPs to PSMA+ prostate cancer cells.
Figure 3. Time-dependent binding of AuNPs to LNCaP cells in vitro.
Cells were incubated at 37 °C for 0.5–2 h with 4.7 nM targeted AuNP-streptavidin: biotin-PEG12-CTT54 or 7.0 nM non-targeted AuNP-streptavidin. The total amount of AuNP bound to the cells was quantified by ICP-OES and expressed as the % ID (percentage of the injected dose). Values are the averages of 3–4 replicates with standard deviations represented by error bars. The difference in binding for the targeted and non-targeted AuNPs was significant at 1 h and 2 h (P < 0.05).
At present, the only reports of PSMA-mediated delivery of AuNPs to LNCaP cells have employed aptamers27, 39 or combinations of aptamers and antibodies31 as targeting ligands. The relative ratio of PSMA inhibitor-targeted AuNPs to non-targeted AuNPs bound to LNCaP cells observed in this study is consistent with the relative ratio observed previously between PSMA aptamer-targeted AuNPs and non-targeted AuNPs when the total amount of gold delivered to the cells was quantified.39 However, the inhibitor based PSMA-targeting of AuNPs in this study was achieved through small molecules nearly 1/100th the molecular mass of antibodies. These small molecules with their unique binding properties provide considerable advantages in terms of atom economy and scale-up potential, and thus represent a unique motif for targeting AuNPs to PSMA+ prostate cancer.
In summary, the results herein demonstrate for the first time that AuNPs can be functionalized to selectively target the prostate cancer tumor biomarker PSMA through the deployment of small molecule phosphoramidate peptidomimetic inhibitors. The in vitro results illustrate the significant and specific delivery of PSMA-targeted AuNPs over non-targeted AuNPs to PSMA+ LNCaP cells. These encouraging observations provide the basis for further exploration of CTT54-functionalized AuNPs for PSMA-mediated delivery of imaging and therapeutic combinations.
Supplementary Material
Acknowledgements
The authors extend their gratitude for technical assistance with ICP-OES to J. Lessmann, as well as C. Davitt and V. Lynch-Holm at the WSU Franceschi Microscopy and Imaging Center. This work was supported by the National Institutes of Health (5R01CA140617-02 and T32GM008336).
Abbreviations
- AuNPs
gold nanoparticles
- GCPII
glutamate carboxypeptidase II
- ICP-OES
inductively coupled plasma optical emission spectroscopy
- PEG
polyethylene glycol
- PSMA
prostate-specific membrane antigen
- PSMA−
PSMA-negative
- PSMA+
PSMA-positive
- TEM
transmission electron microscopy
- % ID
percentage of the injected dose
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Statement
C.E. Berkman is the inventor of a patent on the PSMA inhibitor described in this report and presently serves as the CSO of Cancer Targeted Technology.
Supplementary data
Supplementary data describes the experimental details for AuNP functionalization, TEM analysis, in vitro cell assays, and ICP-OES quantification.
References
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. CA: Cancer. J. Clin. 2011;61:69. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Siegel R, Ward E, Brawley O, Jemal A. CA: Cancer J. Clin. 2011;61:212. doi: 10.3322/caac.20121. [DOI] [PubMed] [Google Scholar]
- 3.Israeli RS, Powell CT, Fair WR, Heston WDW. Cancer Res. 1993;53:227. [PubMed] [Google Scholar]
- 4.Carter RE, Feldman AR, Coyle JT. Proc. Natl. Acad. Sci. U.S.A. 1996;93:749. doi: 10.1073/pnas.93.2.749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pinto JT, Suffoletto BP, Berzin TM, Qiao CH, Lin S, Tong WP, May F, Mukherjee B, Heston WD. Clin. Cancer Res. 1996;2:1445. [PubMed] [Google Scholar]
- 6.Bacich DJ, Pinto JT, Tong WP, Heston WD. Mamm. Genome. 2001;12:117. doi: 10.1007/s003350010240. [DOI] [PubMed] [Google Scholar]
- 7.Liu H, Rajasekaran AK, Moy P, Xia Y, Kim S, Navarro V, Rahmati R, Bander NH. Cancer research. 1998;58:4055. [PubMed] [Google Scholar]
- 8.Liu T, Wu LY, Kazak M, Berkman CE. Prostate. 2008;68:955. doi: 10.1002/pros.20753. [DOI] [PubMed] [Google Scholar]
- 9.Liu J, Kopeckova P, Buhler P, Wolf P, Pan H, Bauer H, Elsasser-Beile U, Kopecek J. Mol. Pharmaceutics. 2009;6:959. doi: 10.1021/mp8002682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ding P, Helquist P, Miller MJ. Org. Biomol. Chem. 2007;5:826. doi: 10.1039/b615603g. [DOI] [PubMed] [Google Scholar]
- 11.Aggarwal S, Singh P, Topaloglu O, Isaacs JT, Denmeade SR. Cancer Res. 2006;66:9171. doi: 10.1158/0008-5472.CAN-06-1520. [DOI] [PubMed] [Google Scholar]
- 12.Zhou J, Neale JH, Pomper MG, Kozikowski AP. Nat. Rev. Drug Discovery. 2005;4:1015. doi: 10.1038/nrd1903. [DOI] [PubMed] [Google Scholar]
- 13.Tsukamoto T, Wozniak KM, Slusher BS. Drug Discovery Today. 2007;12:767. doi: 10.1016/j.drudis.2007.07.010. [DOI] [PubMed] [Google Scholar]
- 14.Liu T, Toriyabe Y, Kazak M, Berkman CE. Biochemistry. 2008;47:12658. doi: 10.1021/bi801883v. [DOI] [PubMed] [Google Scholar]
- 15.Wu LY, Anderson MO, Toriyabe Y, Maung J, Campbell TY, Tajon C, Kazak M, Moser J, Berkman CE. Bioorg. Med. Chem. 2007;15:7434. doi: 10.1016/j.bmc.2007.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lapi SE, Wahnishe H, Pham D, Wu LY, Nedrow-Byers JR, Liu T, Vejdani K, VanBrocklin HF, Berkman CE, Jones EF. J. Nucl. Med. 2009;50:2042. doi: 10.2967/jnumed.109.066589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu T, Wu LY, Hopkins MR, Choi JK, Berkman CE. Bioorg. Med. Chem. Lett. 2010;20:7124. doi: 10.1016/j.bmcl.2010.09.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu T, Nedrow-Byers JR, Hopkins MR, Berkman CE. Bioorg. Med. Chem. Lett. 2011;21:7013. doi: 10.1016/j.bmcl.2011.09.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nedrow-Byers JR, Jabbes M, Jewett C, Ganguly T, He H, Liu T, Benny P, Bryan JN, Berkman CE. Prostate. 2012;72:904. doi: 10.1002/pros.21493. [DOI] [PubMed] [Google Scholar]
- 20.Nedrow-Byers JR, Moore AL, Ganguly T, Hopkins MR, Fulton MD, Benny PD, Berkman CE. Prostate. doi: 10.1002/pros.22575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu T, Wu LY, Choi JK, Berkman CE. Prostate. 2009;69:585. doi: 10.1002/pros.20909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu T, Wu LY, Choi JK, Berkman CE. Int. J. Oncol. 2010;36:777. doi: 10.3892/ijo_00000553. [DOI] [PubMed] [Google Scholar]
- 23.Liu T, Wu LY, Berkman CE. Cancer Lett. 2010;296:106. doi: 10.1016/j.canlet.2010.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu T, Nedrow-Byers JR, Hopkins MR, Wu LY, Lee J, Reilly PTA, Berkman CE. Bioorg. Med. Chem. Lett. 2012;22:3931. doi: 10.1016/j.bmcl.2012.04.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kelkar SS, Reineke TM. Bioconjugate Chem. 2011;22:1879. doi: 10.1021/bc200151q. [DOI] [PubMed] [Google Scholar]
- 26.Xie J, Lee S, Chen X. Adv. Drug Delivery Rev. 2010;62:1064. doi: 10.1016/j.addr.2010.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Javier DJ, Nitin N, Levy M, Ellington A, Richards-Kortum R. Bioconjugate Chem. 2008;19:1309. doi: 10.1021/bc8001248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim D, Park S, Lee JH, Jeong YY, Jon S. J. Am. Chem. Soc. 2007;129:7661. doi: 10.1021/ja071471p. [DOI] [PubMed] [Google Scholar]
- 29.Zhang X, Xing JZ, Chen J, Ko L, Amanie J, Gulavita S, Pervez N, Yee D, Moore R, Roa W. Clin. Invest. Med. 2008;31:E160. doi: 10.25011/cim.v31i3.3473. [DOI] [PubMed] [Google Scholar]
- 30.Lal S, Clare SE, Halas NJ. Acc. Chem. Res. 2008;41:1842. doi: 10.1021/ar800150g. [DOI] [PubMed] [Google Scholar]
- 31.Lu W, Singh AK, Khan SA, Senapati D, Yu H, Ray PC. J. Am. Chem. Soc. 2010;132:18103. doi: 10.1021/ja104924b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lukianova-Hleb EY, Oginsky AO, Samaniego AP, Shenefelt DL, Wagner DS, Hafner JH, Farach-Carson MC, Lapotko DO. Theranostics. 2011;1:3. doi: 10.7150/thno/v01p0003. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 33.Li X, Guo J, Asong J, Wolfert MA, Boons G-J. J. Am. Chem. Soc. 2011;133:11147. doi: 10.1021/ja2012164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Paciotti GF, Kingston DGI, Tamarkin L. Drug Dev. Res. 2006;67:47. [Google Scholar]
- 35.Qian X, Peng X-H, Ansari DO, Yin-Goen Q, Chen GZ, Shin DM, Yang L, Young AN, Wang MD, Nie S. Nat. Biotechnol. 2008;26:83. doi: 10.1038/nbt1377. [DOI] [PubMed] [Google Scholar]
- 36.Park J-A, Kim H-K, Kim J-H, Jeong S-W, Jung J-C, Lee G-H, Lee J, Chang Y, Kim T-J. Bioorg. Med. Chem. Lett. 2010;20:2287. doi: 10.1016/j.bmcl.2010.02.002. [DOI] [PubMed] [Google Scholar]
- 37.Gao X, Cui Y, Levenson RM, Chung LWK, Nie S. Nat. Biotechnol. 2004;22:969. doi: 10.1038/nbt994. [DOI] [PubMed] [Google Scholar]
- 38.Chandran SS, R. Banerjee S, C. Mease R, Pomper MG, R. Denmeade S. Cancer Biol. Ther. 2008;7:974. doi: 10.4161/cbt.7.6.5968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kim D, Jeong YY, Jon S. ACS Nano. 2010;4:3689. doi: 10.1021/nn901877h. [DOI] [PubMed] [Google Scholar]
- 40.Sanna V, Pintus G, Roggio AM, Punzoni S, Posadino AM, Arca A, Marceddu S, Bandiera P, Uzzau S, Sechi M. J. Med. Chem. 2011;54:1321. doi: 10.1021/jm1013715. [DOI] [PubMed] [Google Scholar]
- 41.Dhar S, Kolishetti N, Lippard SJ, Farokhzad OC. Proc. Natl. Acad. Sci. U.S.A. 2011;108:1850. doi: 10.1073/pnas.1011379108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kamaly N, Xiao Z, Valencia PM, Radovic-Moreno AF, Farokhzad OC. Chem. Soc. Rev. 2012;41:2971. doi: 10.1039/c2cs15344k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yang H-W, Hua M-Y, Liu H-L, Tsai R-Y, Chuang C-K, Chu P-C, Wu P-Y, Chang Y-H, Chuang H-C, Yu K-J, Pang S-T. ACS Nano. 2012;6:1795. doi: 10.1021/nn2048526. [DOI] [PubMed] [Google Scholar]
- 44.Chen Z, Penet M-F, Nimmagadda S, Li C, Banerjee SR, Winnard PT, Artemov D, Glunde K, Pomper MG, Bhujwalla ZM. ACS Nano. 2012;6:7752. doi: 10.1021/nn301725w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zajac A, Song D, Qian W, Zhukov T. Colloids Surf. B. 2007;58:309. doi: 10.1016/j.colsurfb.2007.02.019. [DOI] [PubMed] [Google Scholar]
- 46.Stoldt HS, Aftab F, Chinol M, Paganelli G, Luca F, Testori A, Geraghty JG. Eur. J. Cancer. 1997;33:186. doi: 10.1016/s0959-8049(96)00477-7. [DOI] [PubMed] [Google Scholar]
- 47.Juweid M, Neumann R, Paik C, Perez-Bacete MJ, Sato J, van Osdol W, Weinstein JN. Cancer Res. 1992;52:5144. [PubMed] [Google Scholar]
- 48.Chaiet L, Wolf FJ. Arch. Biochem. Biophys. 1964;106:1. doi: 10.1016/0003-9861(64)90150-x. [DOI] [PubMed] [Google Scholar]
- 49.Hendrickson WA, Pahler A, Smith JL, Satow Y, Merritt EA, Phizackerley RP. Proc. Natl. Acad. Sci. U.S.A. 1989;86:2190. doi: 10.1073/pnas.86.7.2190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lesch HP, Kaikkonen MU, Pikkarainen JT, Yla-Herttuala S. Expert Opin. Drug Delivery. 2010;7:551. doi: 10.1517/17425241003677749. [DOI] [PubMed] [Google Scholar]
- 51.Artemov D, Mori N, Okollie B, Bhujwalla ZM. Magn. Reson. Med. 2003;49:403. doi: 10.1002/mrm.10406. [DOI] [PubMed] [Google Scholar]
- 52.Lagerholm BC, Wang M, Ernst LA, Ly DH, Liu H, Bruchez MP, Waggoner AS. Nano Lett. 2004;4:2019. [Google Scholar]
- 53.Prow T, Smith JN, Grebe R, Salazar JH, Wang N, Kotov N, Lutty G, Leary J. Mol. Vision. 2006;12:606. [PubMed] [Google Scholar]
- 54.Suci PA, Kang S, Young M, Douglas T. J. Am. Chem. Soc. 2009;131:9164. doi: 10.1021/ja9035187. [DOI] [PubMed] [Google Scholar]
- 55.Kim E-M, Oh J-S, Ahn I-S, Park K-I, Jang J-H. Biomaterials. 2011;32:8654. doi: 10.1016/j.biomaterials.2011.07.075. [DOI] [PubMed] [Google Scholar]
- 56.Lai G, Wu J, Ju H, Yan F. Adv. Funct. Mater. 2011;21:2938. [Google Scholar]
- 57.Chen LQ, Xiao SJ, Hu PP, Peng L, Ma J, Luo LF, Li YF, Huang CZ. Anal. Chem. 2012;84:3099. doi: 10.1021/ac202810b. [DOI] [PubMed] [Google Scholar]
- 58.Wu LY, Liu T, Hopkins MR, Davis WC, Berkman CE. Prostate. 2012;72:1532. doi: 10.1002/pros.22508. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.