Abstract
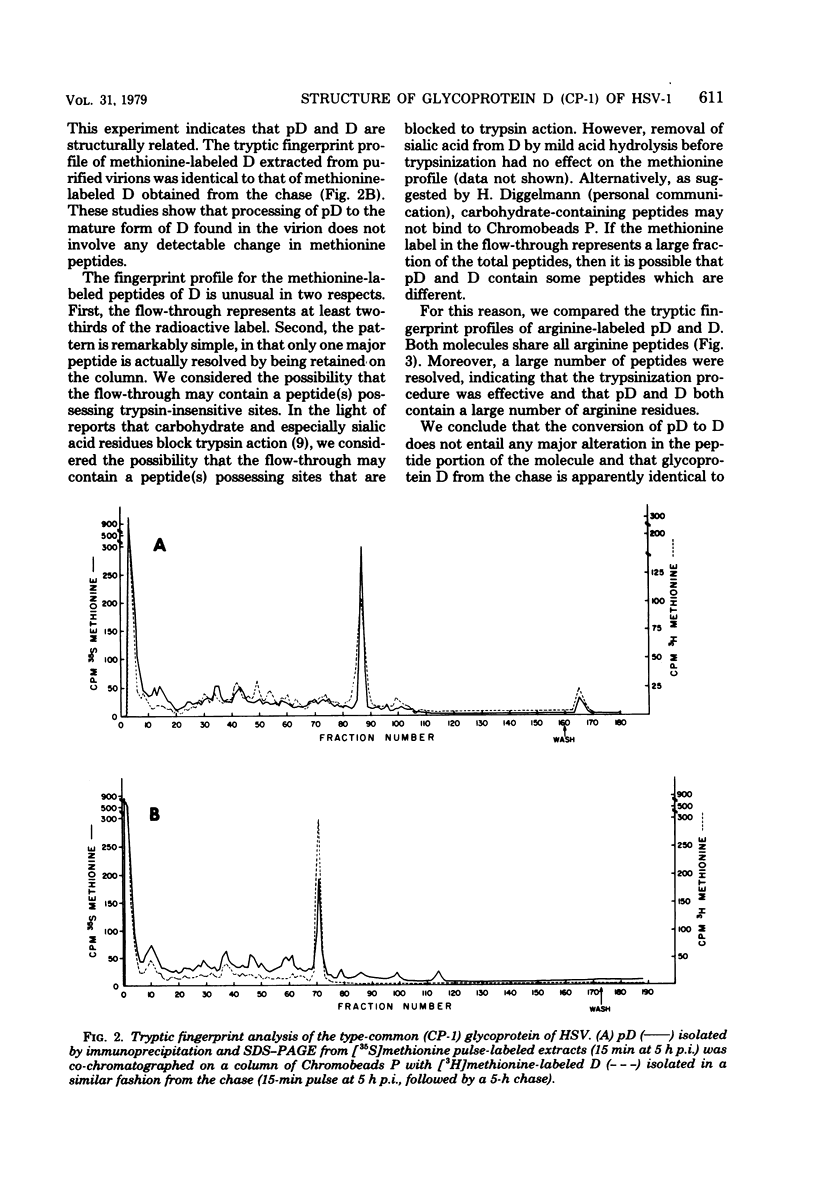
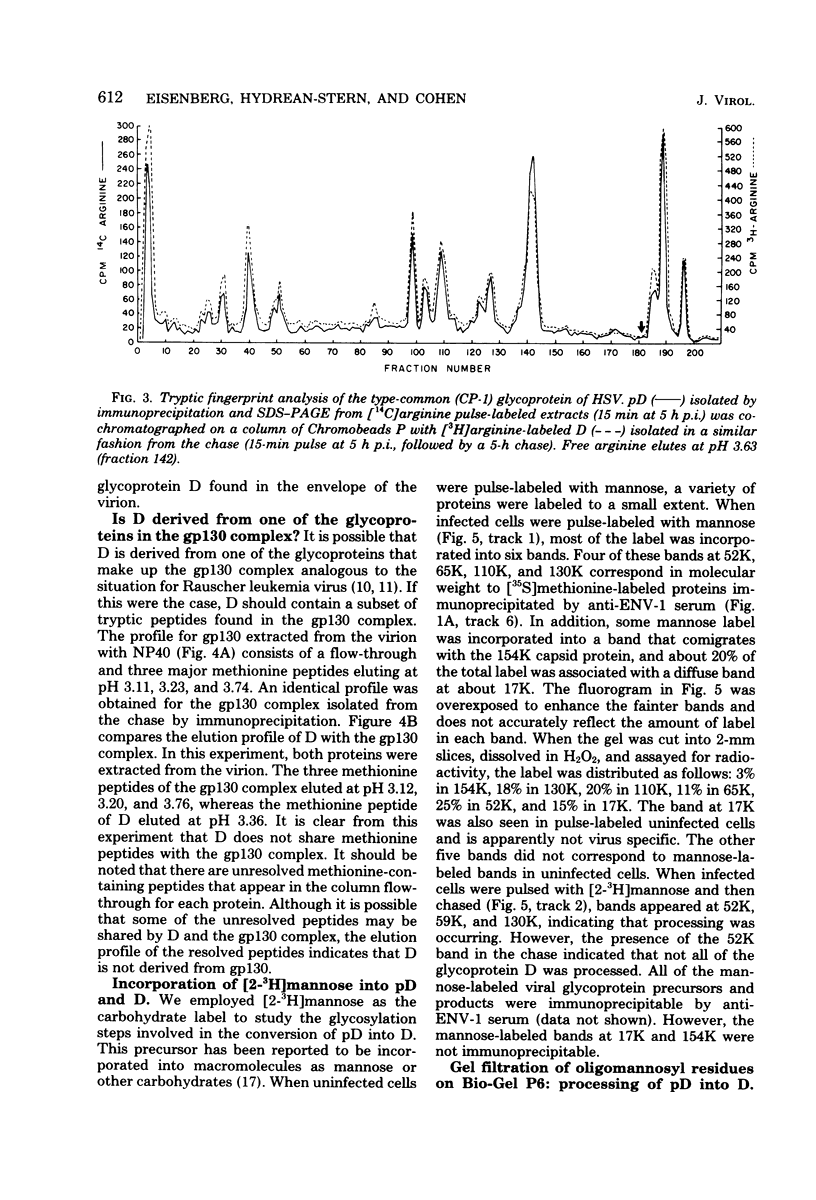
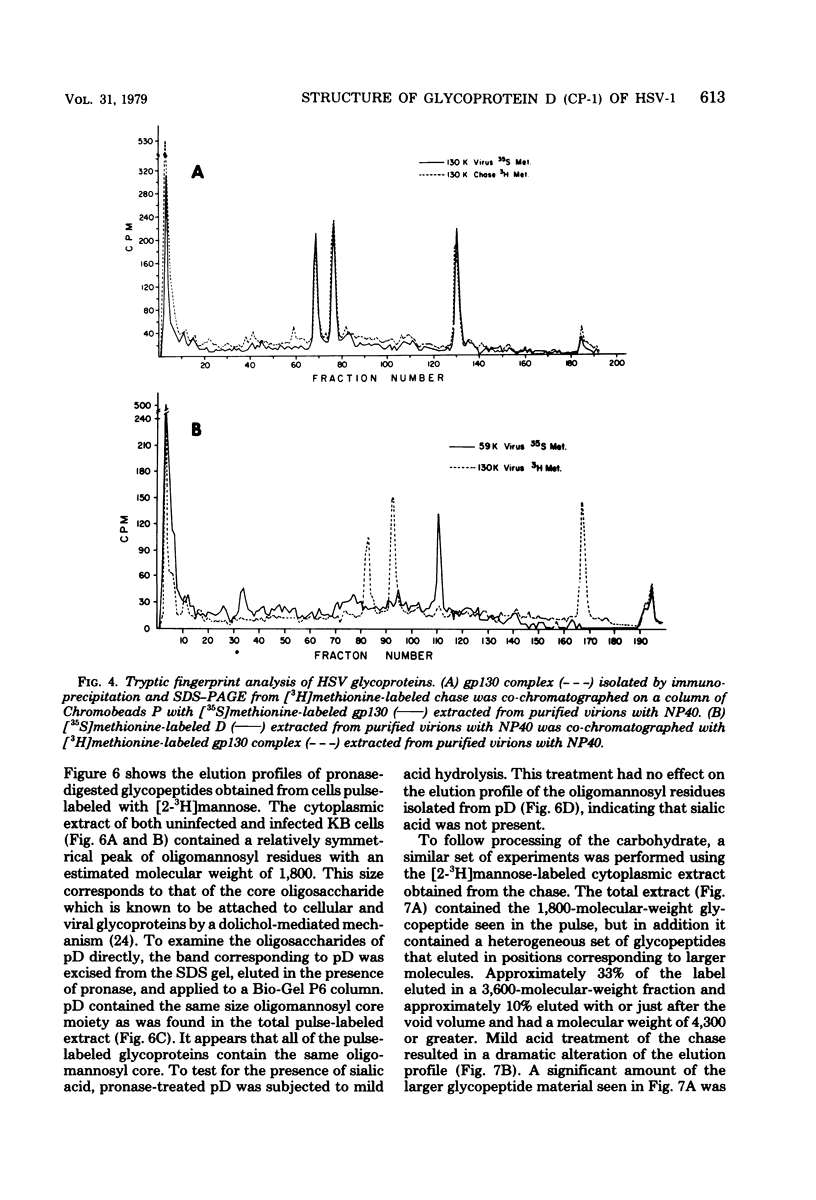
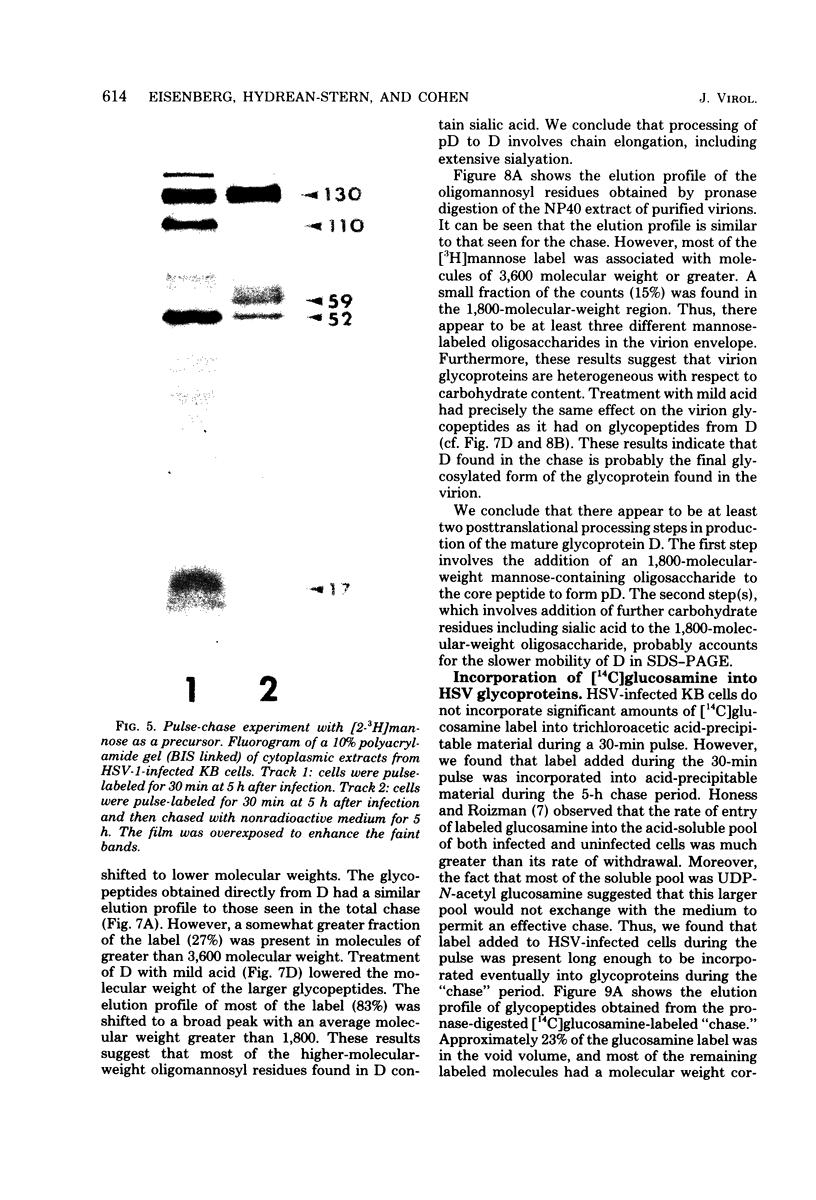
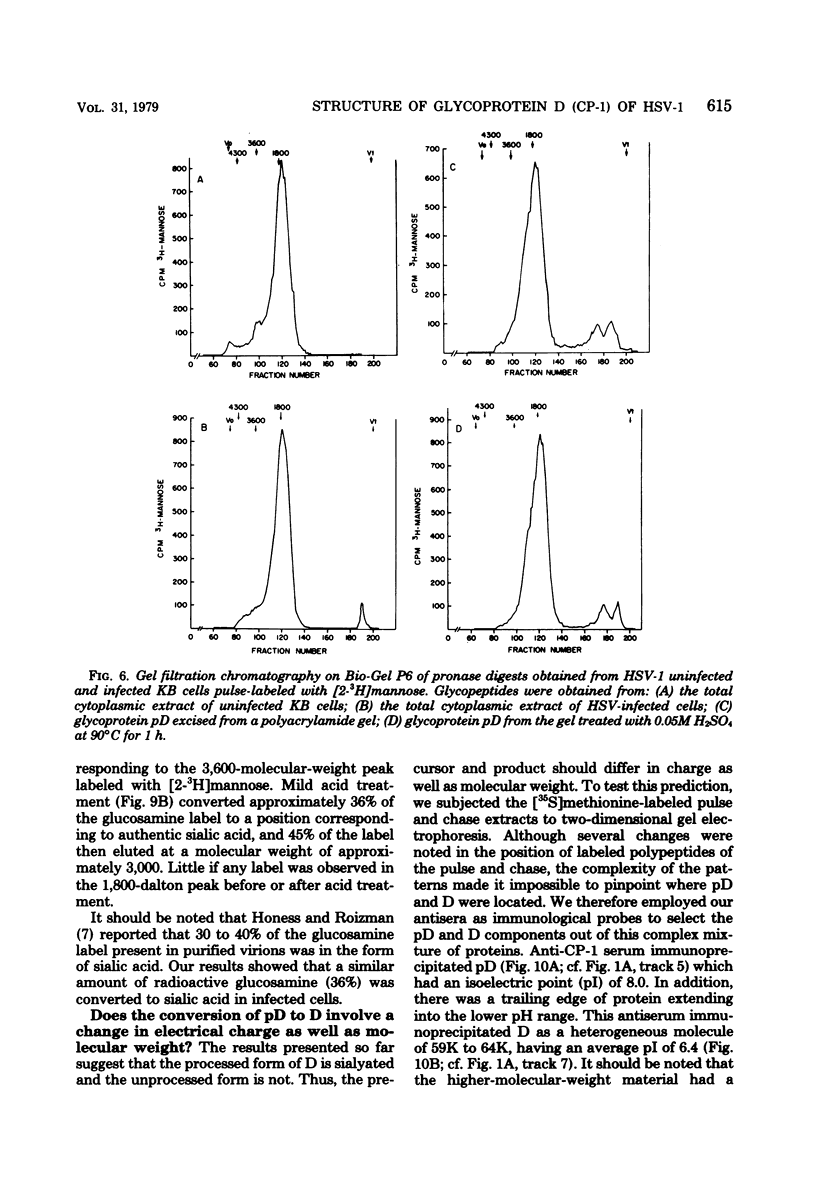
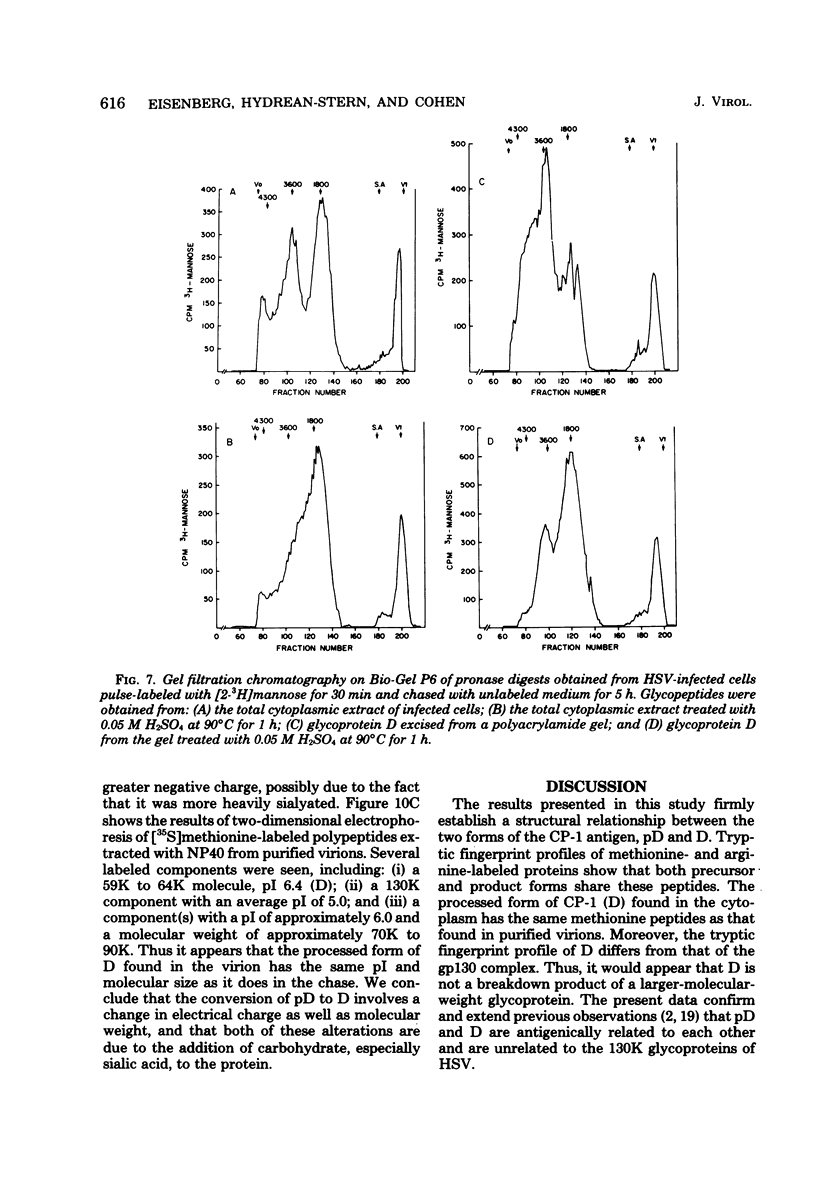
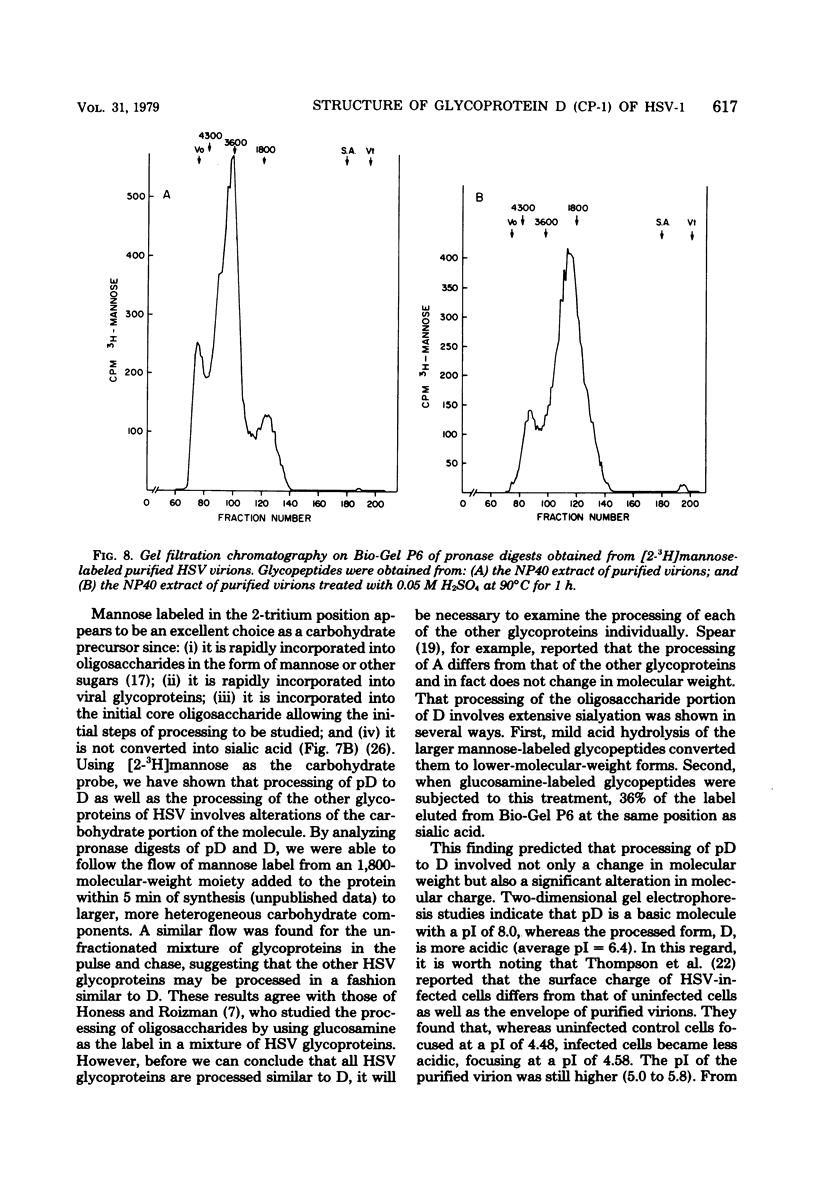
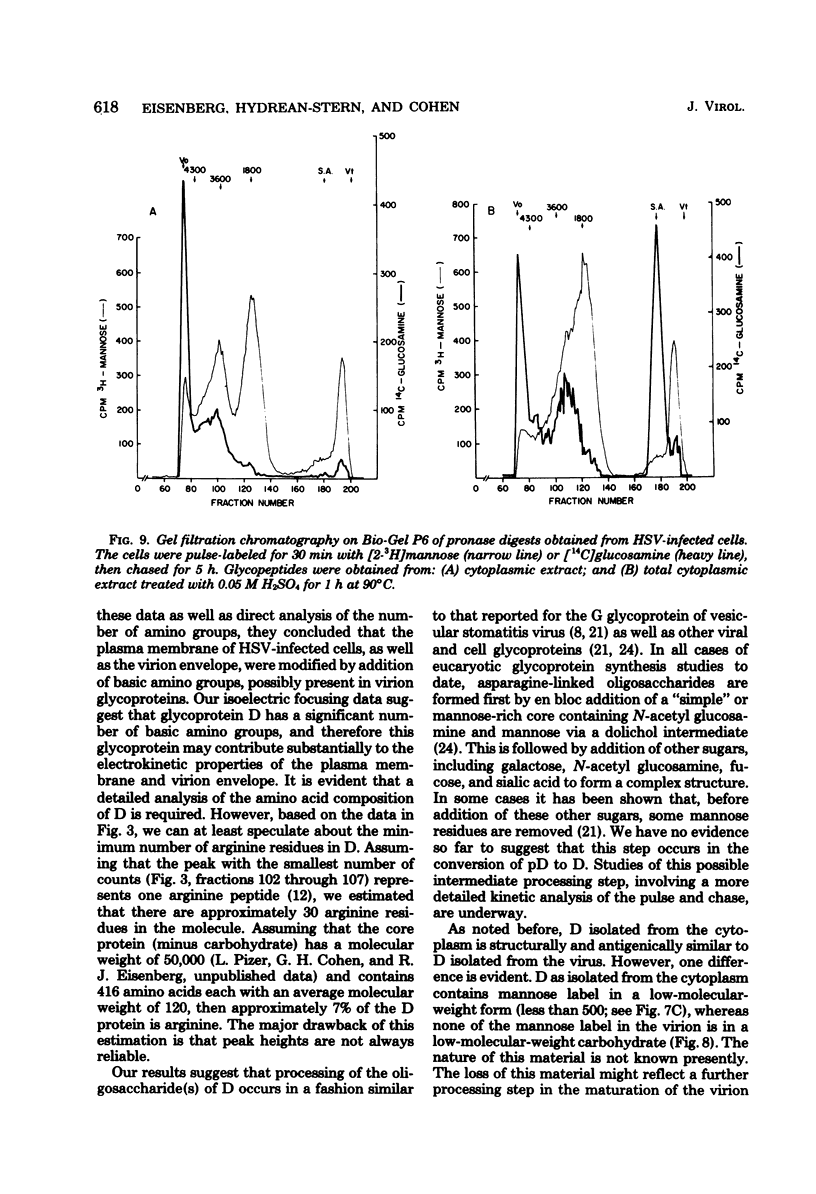
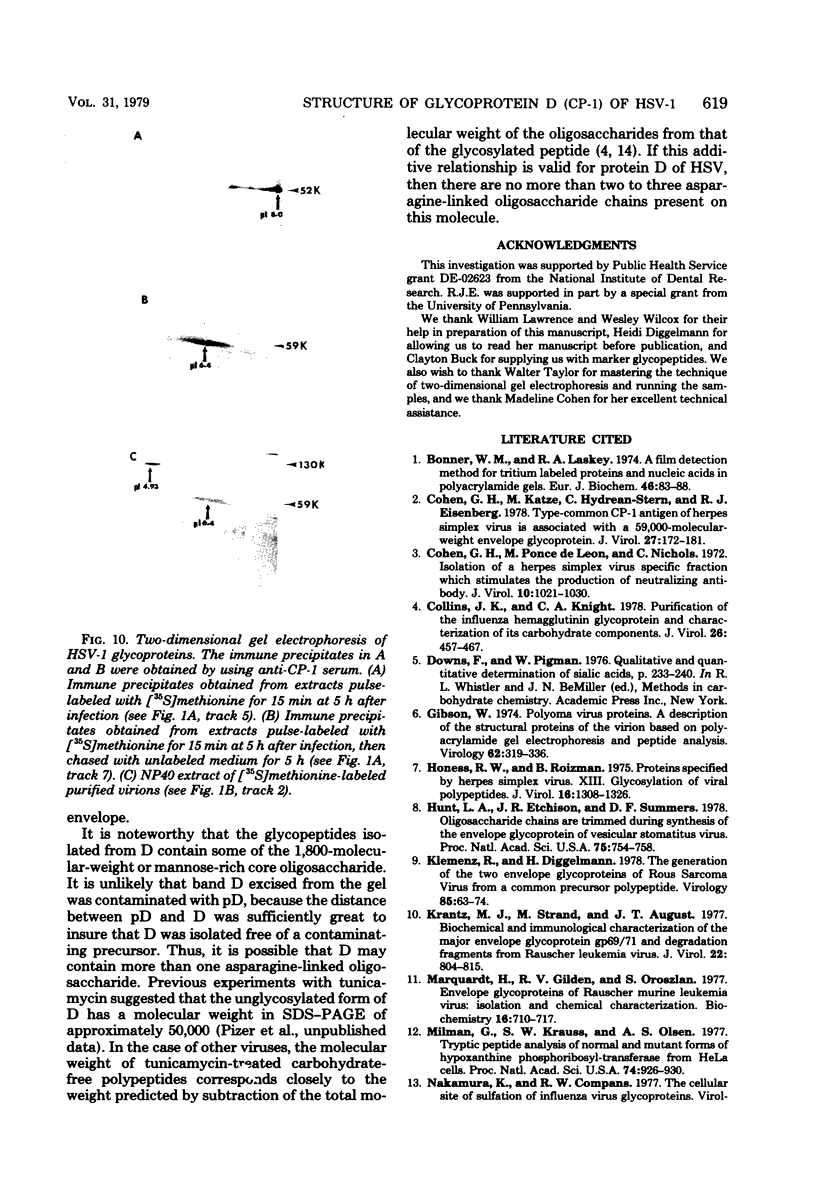
The type-common CP-1 antigen of herpes simplex virus type 1 (HSV-1) is associated in the infected cell with two components, a 52,000-molecular-weight glycoprotein (gp52 or pD) and a 59,000-molecular-weight glycoprotein (gp59 or D). The larger form (D) is also found in the virion envelope. It was postulated that pD is a precursor of D. We found that pD shared methionine and arginine tryptic peptides with D isolated from infected cell extracts. D isolated from infected extracts had the same trypric methionine peptide profile as D isolated from the virion envelope. Thus, processing of pD to D does not involve any major alterations in polypeptide structure. Furthermore, D did not share tryptic methionine peptides with the other major glycoproteins of HSV-1. Using [2-3H]mannose as a specific glycoprotein label, we found that pD, which is a basic protein (isoelectric point = 8.0) contained a 1,800-molecular-weight oligomannosyl core moiety and was processed by further glycosylation and sialyation to a more acidic and heterogeneous molecule D, which as a molecular weight of at least 59,000.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Cohen G. H., Katze M., Hydrean-Stern C., Eisenberg R. J. Type-common CP-1 antigen of herpes simplex virus is associated with a 59,000-molecular-weight envelope glycoprotein. J Virol. 1978 Jul;27(1):172–181. doi: 10.1128/jvi.27.1.172-181.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen G. H., Ponce de Leon M., Nichols C. Isolation of a herpes simplex virus-specific antigenic fraction which stimulates the production of neutralizing antibody. J Virol. 1972 Nov;10(5):1021–1030. doi: 10.1128/jvi.10.5.1021-1030.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins J. K., Knight C. A. Purification of the influenza hemagglutinin glycoprotein and characterization of its carbohydrate components. J Virol. 1978 May;26(2):457–467. doi: 10.1128/jvi.26.2.457-467.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson W. Polyoma virus proteins: a description of the structural proteins of the virion based on polyacrylamide gel electrophoresis and peptide analysis. Virology. 1974 Dec;62(2):319–336. doi: 10.1016/0042-6822(74)90395-x. [DOI] [PubMed] [Google Scholar]
- Honess R. W., Roizman B. Proteins specified by herpes simplex virus. XIII. Glycosylation of viral polypeptides. J Virol. 1975 Nov;16(5):1308–1326. doi: 10.1128/jvi.16.5.1308-1326.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt L. A., Etchison J. R., Summers D. F. Oligosaccharide chains are trimmed during synthesis of the envelope glycoprotein of vesicular stomatitis virus. Proc Natl Acad Sci U S A. 1978 Feb;75(2):754–758. doi: 10.1073/pnas.75.2.754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klemenz R., Diggelmann H. The generation of the two envelope glycoproteins of Rous sarcoma virus from a common precursor polypeptide. Virology. 1978 Mar;85(1):63–74. doi: 10.1016/0042-6822(78)90411-7. [DOI] [PubMed] [Google Scholar]
- Krantz M. J., Strand M., August J. T. Biochemical and immunological characterization of the major envelope glycoprotein gp69/71 and degradation fragments from Rauscher leukemia virus. J Virol. 1977 Jun;22(3):804–815. doi: 10.1128/jvi.22.3.804-815.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marquardt H., Gilden R. V., Oroszlan S. Envelope glycoproteins of Rauscher murine leukemia virus: isolation and chemical characterization. Biochemistry. 1977 Feb 22;16(4):710–717. doi: 10.1021/bi00623a024. [DOI] [PubMed] [Google Scholar]
- Milman G., Krauss S. W., Olsen A. S. Tryptic peptide analysis of normal and mutant forms of hypoxanthine phosphoribosyltransferase from HeLa cells. Proc Natl Acad Sci U S A. 1977 Mar;74(3):926–930. doi: 10.1073/pnas.74.3.926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura K., Compans R. W. Glycopeptide components of influenza viral glycoproteins. Virology. 1978 May 15;86(2):432–442. doi: 10.1016/0042-6822(78)90083-1. [DOI] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Ponce de Leon M., Hessle H., Cohen G. H. Separation of Herpes simplex virus-induced antigens by Concanavalin A affinity chromatography. J Virol. 1973 Oct;12(4):766–774. doi: 10.1128/jvi.12.4.766-774.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz R. T., Schmidt M. F., Anwer U., Klenk H. D. Carbohydrates of influenza virus. I. Glycopeptides derived from viral glycoproteins after labeling with radioactive sugars. J Virol. 1977 Aug;23(2):217–226. doi: 10.1128/jvi.23.2.217-226.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shively J. E., Kessler M. J., Todd C. W. Amino-terminal sequences of the major tryptic peptides obtained from carcinoembryonic antigen by digestion with trypsin in the presence of Triton X-100. Cancer Res. 1978 Aug;38(8):2199–2208. [PubMed] [Google Scholar]
- Spear P. G. Membrane proteins specified by herpes simplex viruses. I. Identification of four glycoprotein precursors and their products in type 1-infected cells. J Virol. 1976 Mar;17(3):991–1008. doi: 10.1128/jvi.17.3.991-1008.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spear P. G., Roizman B. Proteins specified by herpes simplex virus. V. Purification and structural proteins of the herpesvirion. J Virol. 1972 Jan;9(1):143–159. doi: 10.1128/jvi.9.1.143-159.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabas I., Schlesinger S., Kornfeld S. Processing of high mannose oligosaccharides to form complex type oligosaccharides on the newly synthesized polypeptides of the vesicular stomatitis virus G protein and the IgG heavy chain. J Biol Chem. 1978 Feb 10;253(3):716–722. [PubMed] [Google Scholar]
- Thompson C. J., Docherty J. J., Boltz R. C., Gaines R. A., Todd P. Electrokinetic alteration of the surface of herpes simplex virus infected cells. J Gen Virol. 1978 Jun;39(3):449–461. doi: 10.1099/0022-1317-39-3-449. [DOI] [PubMed] [Google Scholar]
- Vogt V. M., Eisenman R., Diggelmann H. Generation of avian myeloblastosis virus structural proteins by proteolytic cleavage of a precursor polypeptide. J Mol Biol. 1975 Aug 15;96(3):471–493. doi: 10.1016/0022-2836(75)90174-6. [DOI] [PubMed] [Google Scholar]
- Waechter C. J., Lennarz W. J. The role of polyprenol-linked sugars in glycoprotein synthesis. Annu Rev Biochem. 1976;45:95–112. doi: 10.1146/annurev.bi.45.070176.000523. [DOI] [PubMed] [Google Scholar]
- Yurchenco P. D., Ceccarini C., Atkinson P. H. Labeling complex carbohydrates of animal cells with monosaccharides. Methods Enzymol. 1978;50:175–204. doi: 10.1016/0076-6879(78)50019-0. [DOI] [PubMed] [Google Scholar]