Abstract
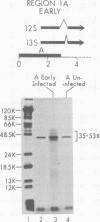
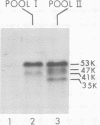
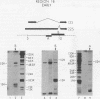
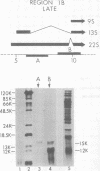
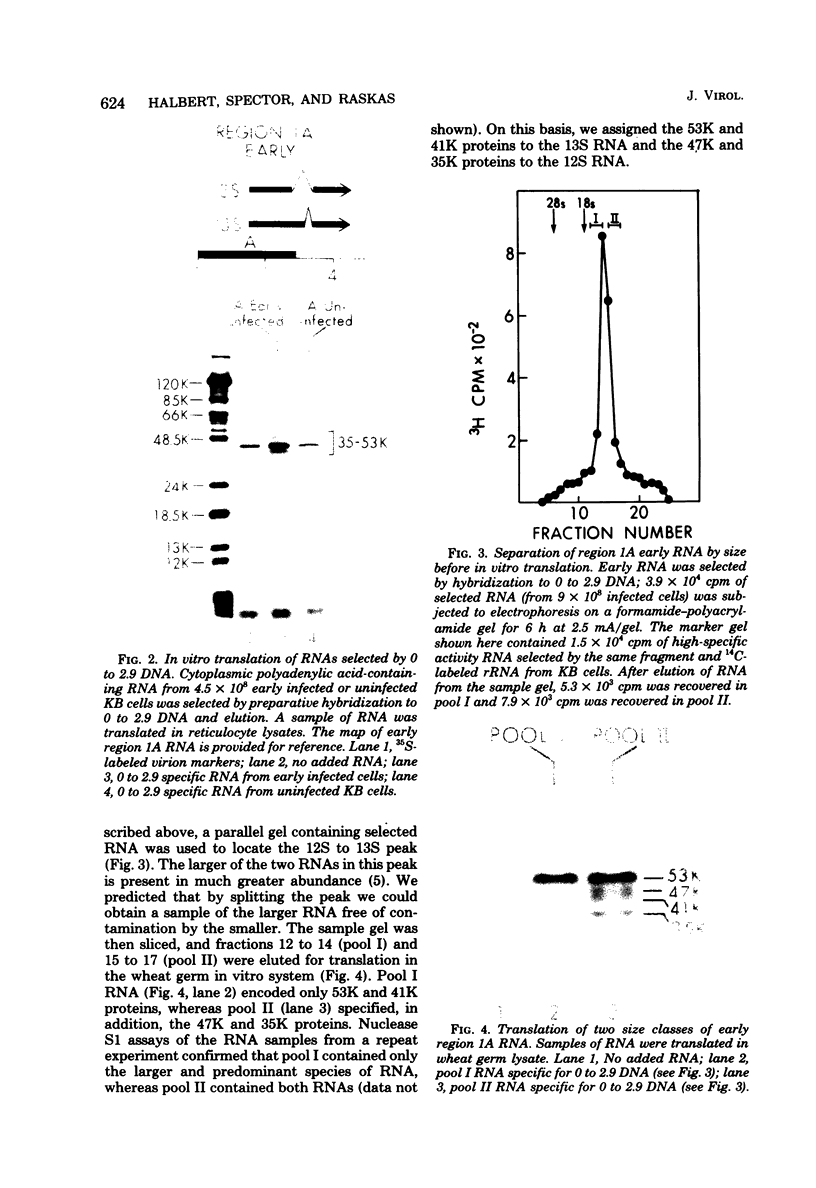
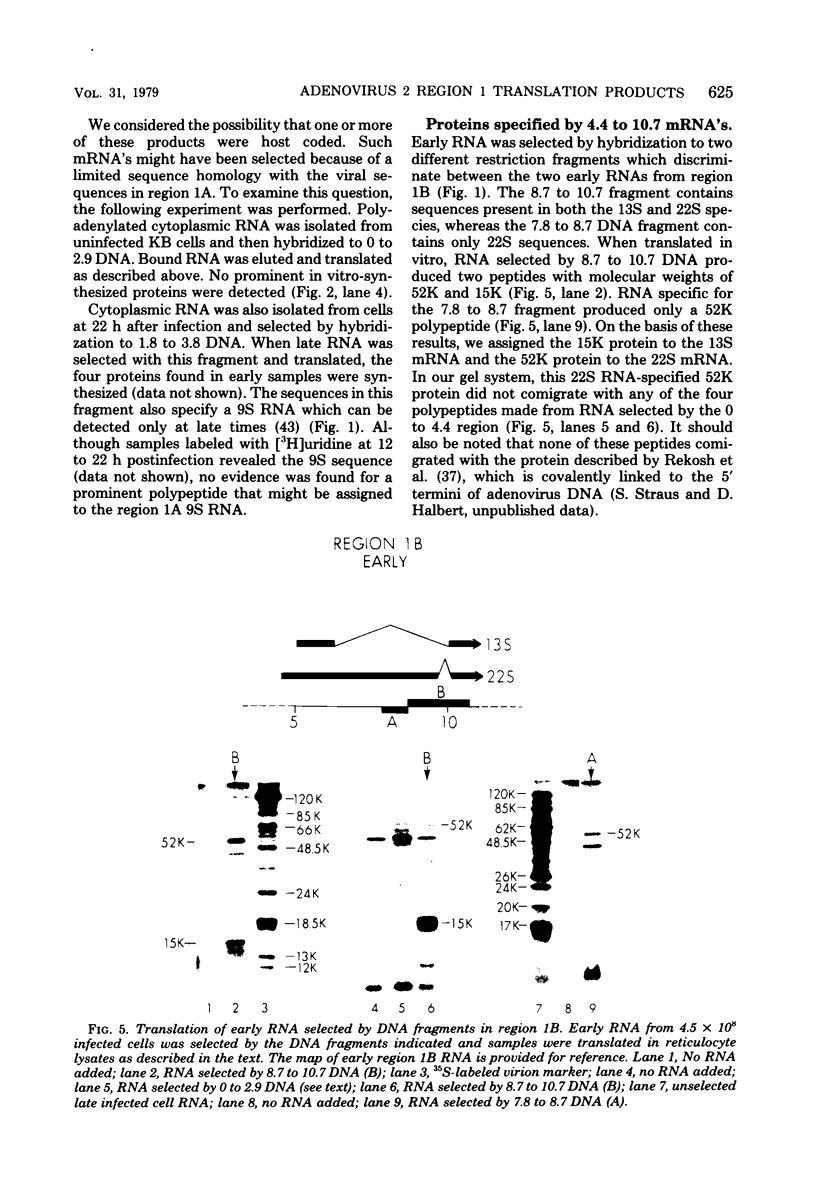
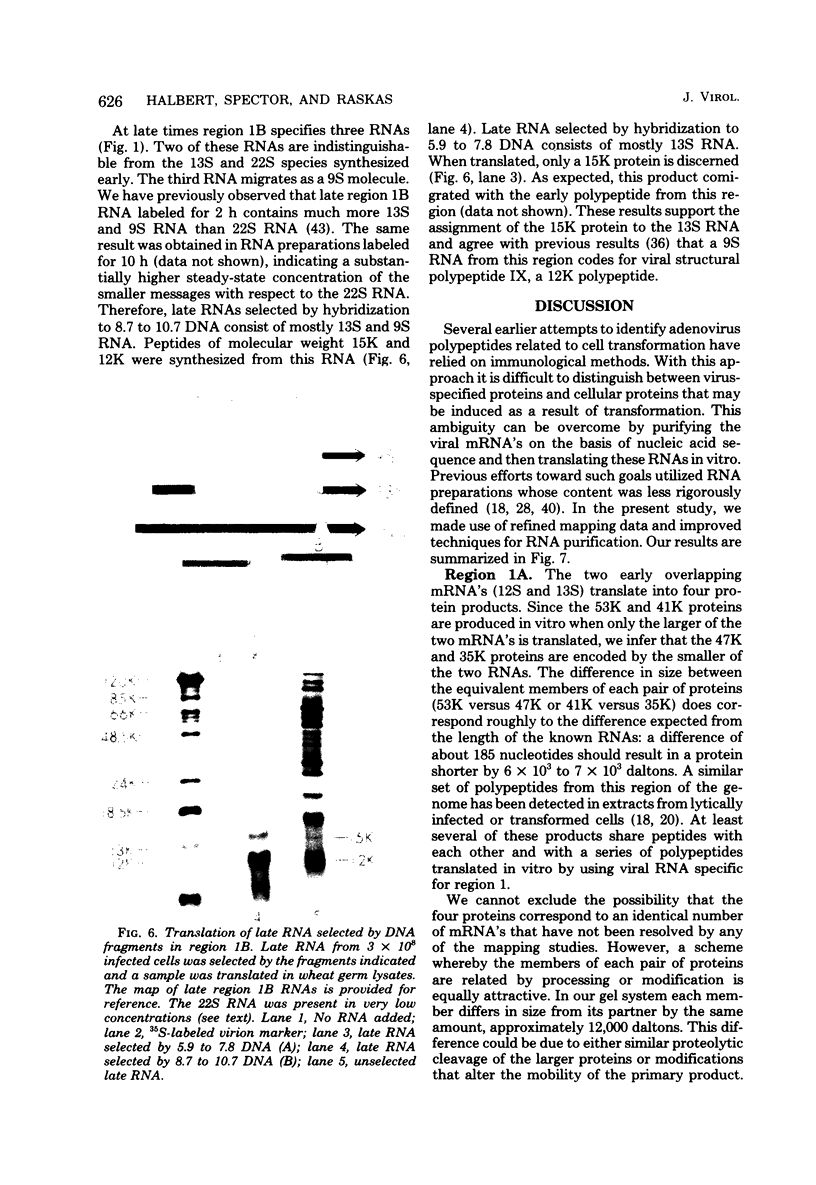
Region 1 DNA sequences (map positions 0 to 11% on the linear adenovirus 2 genome) are expressed both early and late in lytic infection and are required for transformation by the virus. During productive infection six distinct cytoplasmic RNAs are synthesized from this region. These RNAs comprise two families, each consisting of three size classes that share 3' sequences. Region 1 RNA's were purified by hybridization selection, using restriction fragments bound to nitrocellulose membranes, and by size fractionation. The isolated RNAs were then translated in cell-free systems derived from wheat germ and rabbit reticulocytes. The family of RNAs specified by 0 to 4.4 sequences includes two RNAs, which are 12S and 13S in size. These RNAs were partially separated by molecular weight and translated. The 13S RNA produced 53,000-dalton (53K) and 41K peptides, and the 12S RNA synthesized 47K and 35K products. The family of RNAs mapping from 4.4 to 11.0 encodes three separate polypeptides, each of which can be assigned to a specific RNA. A 12K product that comigrates with structural polypeptide IX is synthesized from the 9S RNA as previously reported (U. Pettersson and M. B. Mathews, Cell 12:741-750, 1977). The 13S RNA encodes a 15K polypeptide that corresponds to a 15K polypeptide in infected cell extracts. The 22s RNA encodes a 52K protein distinct from the 0 to 4.4 polypeptides.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berget S. M., Moore C., Sharp P. A. Spliced segments at the 5' terminus of adenovirus 2 late mRNA. Proc Natl Acad Sci U S A. 1977 Aug;74(8):3171–3175. doi: 10.1073/pnas.74.8.3171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berk A. J., Sharp P. A. Sizing and mapping of early adenovirus mRNAs by gel electrophoresis of S1 endonuclease-digested hybrids. Cell. 1977 Nov;12(3):721–732. doi: 10.1016/0092-8674(77)90272-0. [DOI] [PubMed] [Google Scholar]
- Berk A. J., Sharp P. A. Spliced early mRNAs of simian virus 40. Proc Natl Acad Sci U S A. 1978 Mar;75(3):1274–1278. doi: 10.1073/pnas.75.3.1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berk A. J., Sharp P. A. Structure of the adenovirus 2 early mRNAs. Cell. 1978 Jul;14(3):695–711. doi: 10.1016/0092-8674(78)90252-0. [DOI] [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Chow L. T., Broker T. R. The spliced structures of adenovirus 2 fiber message and the other late mRNAs. Cell. 1978 Oct;15(2):497–510. doi: 10.1016/0092-8674(78)90019-3. [DOI] [PubMed] [Google Scholar]
- Chow L. T., Gelinas R. E., Broker T. R., Roberts R. J. An amazing sequence arrangement at the 5' ends of adenovirus 2 messenger RNA. Cell. 1977 Sep;12(1):1–8. doi: 10.1016/0092-8674(77)90180-5. [DOI] [PubMed] [Google Scholar]
- Chow L. T., Roberts J. M., Lewis J. B., Broker T. R. A map of cytoplasmic RNA transcripts from lytic adenovirus type 2, determined by electron microscopy of RNA:DNA hybrids. Cell. 1977 Aug;11(4):819–836. doi: 10.1016/0092-8674(77)90294-x. [DOI] [PubMed] [Google Scholar]
- Craig E. A. Analysis of early adenovirus 2 RNA using Eco R-R1 viral DNA fragments. J Virol. 1975 May;15(5):1202–1213. doi: 10.1128/jvi.15.5.1202-1213.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig E. A., Raskas H. J. Nuclear transcripts larger than the cytoplasmic mRNAs are specified by segments of the adenovirus genome coding for early functions. Cell. 1976 Jun;8(2):205–213. doi: 10.1016/0092-8674(76)90004-0. [DOI] [PubMed] [Google Scholar]
- Duesberg P. H., Vogt P. K. Gel electrophoresis of avian leukosis and sarcoma viral RNA in formamide: comparison with other viral and cellular RNA species. J Virol. 1973 Sep;12(3):594–599. doi: 10.1128/jvi.12.3.594-599.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flint S. J., Sambrook J., Williams J. F., Sharp P. A. Viral nucleic acid sequences in transformed cells. IV. A study of the sequences of adenovirus 5 DNA and RNA in four lines of adenovirus 5-transformed rodent cells using specific fragments of the viral genome. Virology. 1976 Jul 15;72(2):456–470. doi: 10.1016/0042-6822(76)90174-4. [DOI] [PubMed] [Google Scholar]
- Gallimore P. H. Viral DNA in transformed cells. II. A study of the sequences of adenovirus 2 DNA IN NINE LINES OF TRANSFORMED RAT CELLS USING SPECIFIC FRAGMENTS OF THE VIRAL GENOME;. J Mol Biol. 1974 Oct 15;89(1):49–72. doi: 10.1016/0022-2836(74)90162-4. [DOI] [PubMed] [Google Scholar]
- Gelinas R. E., Myers P. A., Weiss G. H., Roberts R. J., Murray K. A specific endonuclease from Brevibacterium albidum. J Mol Biol. 1977 Aug 15;114(3):433–440. doi: 10.1016/0022-2836(77)90260-1. [DOI] [PubMed] [Google Scholar]
- Gilead Z., Jeng Y. H., Wold W. S., Sugawara K., Rho H. M., Harter M. L., Green M. Immunological identification of two adenovirus 2-induced early proteins possibly involved in cell transformation. Nature. 1976 Nov 18;264(5583):263–266. doi: 10.1038/264263a0. [DOI] [PubMed] [Google Scholar]
- Graham F. L., Abrahams P. J., Mulder C., Heijneker H. L., Warnaar S. O., De Vries F. A., Fiers W., Van Der Eb A. J. Studies on in vitro transformation by DNA and DNA fragments of human adenoviruses and simian virus 40. Cold Spring Harb Symp Quant Biol. 1975;39(Pt 1):637–650. doi: 10.1101/sqb.1974.039.01.077. [DOI] [PubMed] [Google Scholar]
- Graham F. L., van der Eb A. J., Heijneker H. L. Size and location of the transforming region in human adenovirus type 5 DNA. Nature. 1974 Oct 25;251(5477):687–691. doi: 10.1038/251687a0. [DOI] [PubMed] [Google Scholar]
- Harter M. L., Lewis J. B. Adenovirus type 2 early proteins synthesized in vitro and in vivo: identification in infected cells of the 38,000- to 50,000- molecular-weight protein encoded by the left end of the adenovirus type 2 genome. J Virol. 1978 Jun;26(3):736–749. doi: 10.1128/jvi.26.3.736-749.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson K., Persson H., Lewis A. M., Pettersson U., Tibbetts C., Philipson L. Viral DNA sequences and gene products in hamster cells transformed by adenovirus type 2. J Virol. 1978 Sep;27(3):628–639. doi: 10.1128/jvi.27.3.628-639.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitchingman G. R., Lai S. P., Westphal H. Loop structures in hybrids of early RNA and the separated strands of adenovirus DNA. Proc Natl Acad Sci U S A. 1977 Oct;74(10):4392–4395. doi: 10.1073/pnas.74.10.4392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klessig D. F. Two adenovirus mRNAs have a common 5' terminal leader sequence encoded at least 10 kb upstream from their main coding regions. Cell. 1977 Sep;12(1):9–21. doi: 10.1016/0092-8674(77)90181-7. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Laskey R. A., Mills A. D. Quantitative film detection of 3H and 14C in polyacrylamide gels by fluorography. Eur J Biochem. 1975 Aug 15;56(2):335–341. doi: 10.1111/j.1432-1033.1975.tb02238.x. [DOI] [PubMed] [Google Scholar]
- Levinson A. D., Levine A. J. The group C adenovirus tumor antigens: identification in infected and transformed cells and a peptide map analysis. Cell. 1977 Aug;11(4):871–879. doi: 10.1016/0092-8674(77)90298-7. [DOI] [PubMed] [Google Scholar]
- Levinson A., Levine A. J. The isolation and identification of the adenovirus group C tumor antigens. Virology. 1977 Jan;76(1):1–11. doi: 10.1016/0042-6822(77)90275-6. [DOI] [PubMed] [Google Scholar]
- Lewis J. B., Anderson C. W., Atkins J. F. Further mapping of late adenovirus genes by cell-free translation of RNA selected by hybridization to specific DNA fragments. Cell. 1977 Sep;12(1):37–44. doi: 10.1016/0092-8674(77)90183-0. [DOI] [PubMed] [Google Scholar]
- Lewis J. B., Atkins J. F., Baum P. R., Solem R., Gesteland R. F., Anderson C. W. Location and identification of the genes for adenovirus type 2 early polypeptides. Cell. 1976 Jan;7(1):141–151. doi: 10.1016/0092-8674(76)90264-6. [DOI] [PubMed] [Google Scholar]
- Marcu K., Dudock B. Characterization of a highly efficient protein synthesizing system derived from commercial wheat germ. Nucleic Acids Res. 1974 Nov;1(11):1385–1397. doi: 10.1093/nar/1.11.1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGrogan M., Raskas H. J. Species identification and genome mapping of cytoplasmic adenovirus type 2 RNAs synthesized late in infection. J Virol. 1977 Aug;23(2):240–249. doi: 10.1128/jvi.23.2.240-249.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGrogan M., Raskas H. J. Two regions of the adenovirus 2 genome specify families of late polysomal RNAs containing common sequences. Proc Natl Acad Sci U S A. 1978 Feb;75(2):625–629. doi: 10.1073/pnas.75.2.625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGrogan M., Spector D. J., Goldenberg C. J., Halbert D., Raskas H. J. Purification of specific adenovirus 2 RNAs by preparative hybridization and selective thermal elution. Nucleic Acids Res. 1979 Feb;6(2):593–607. doi: 10.1093/nar/6.2.583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nevins J. R., Darnell J. E. Groups of adenovirus type 2 mRNA's derived from a large primary transcript: probable nuclear origin and possible common 3' ends. J Virol. 1978 Mar;25(3):811–823. doi: 10.1128/jvi.25.3.811-823.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelham H. R., Jackson R. J. An efficient mRNA-dependent translation system from reticulocyte lysates. Eur J Biochem. 1976 Aug 1;67(1):247–256. doi: 10.1111/j.1432-1033.1976.tb10656.x. [DOI] [PubMed] [Google Scholar]
- Persson H., Pettersson U., Mathews M. B. Synthesis of a structural adenovirus polypeptide in the absence of viral DNA replication. Virology. 1978 Oct 1;90(1):67–79. doi: 10.1016/0042-6822(78)90334-3. [DOI] [PubMed] [Google Scholar]
- Pettersson U., Mathews M. B. The gene and messenger RNA for adenovirus polypeptide IX. Cell. 1977 Nov;12(3):741–750. doi: 10.1016/0092-8674(77)90274-4. [DOI] [PubMed] [Google Scholar]
- Rekosh D. M., Russell W. C., Bellet A. J., Robinson A. J. Identification of a protein linked to the ends of adenovirus DNA. Cell. 1977 Jun;11(2):283–295. doi: 10.1016/0092-8674(77)90045-9. [DOI] [PubMed] [Google Scholar]
- Roberts R. J., Myers P. A., Morrison A., Murray K. A specific endonuclease from Arthrobacter luteus. J Mol Biol. 1976 Mar 25;102(1):157–165. doi: 10.1016/0022-2836(76)90079-6. [DOI] [PubMed] [Google Scholar]
- Saborio J. L., Oberg B. In vivo and in vitro synthesis of adenovirus type 2 early proteins. J Virol. 1976 Mar;17(3):865–875. doi: 10.1128/jvi.17.3.865-875.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp P. A., Pettersson U., Sambrook J. Viral DNA in transformed cells. I. A study of the sequences of adenovirus 2 DNA in a line of transformed rat cells using specific fragments of the viral genome. J Mol Biol. 1974 Jul 15;86(4):709–726. doi: 10.1016/0022-2836(74)90348-9. [DOI] [PubMed] [Google Scholar]
- Spector D. J., McGrogan M., Raskas H. J. Regulation of the appearance of cytoplasmic RNAs from region 1 of the adenovirus 2 genome. J Mol Biol. 1978 Dec 15;126(3):395–414. doi: 10.1016/0022-2836(78)90048-7. [DOI] [PubMed] [Google Scholar]
- Tibbetts C., Johansson K., Philipson L. Hydroxyapatite chromatography and formamide denaturation of adenovirus DNA. J Virol. 1973 Aug;12(2):218–225. doi: 10.1128/jvi.12.2.218-225.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Ormondt H., Maat J., De Waard A., Van der Eb A. J. The nucleotide sequence of the transforming HpaI-E fragment of adenovirus type 5 DNA. Gene. 1978 Dec;4(4):309–328. doi: 10.1016/0378-1119(78)90048-3. [DOI] [PubMed] [Google Scholar]
- Westphal H., Lai S. P. Quantitative electron microscopy of early adenovirus RNA. J Mol Biol. 1977 Nov 5;116(3):525–548. doi: 10.1016/0022-2836(77)90082-1. [DOI] [PubMed] [Google Scholar]