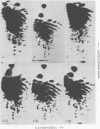
Abstract
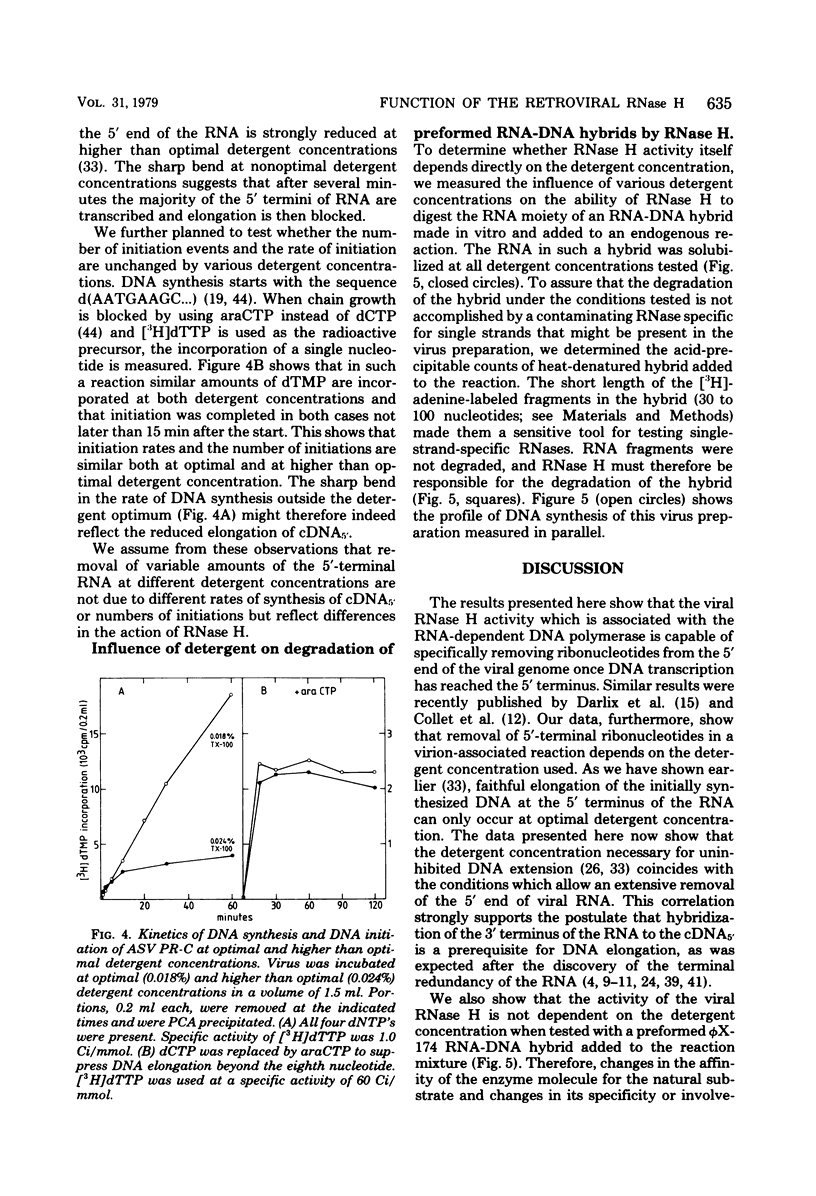
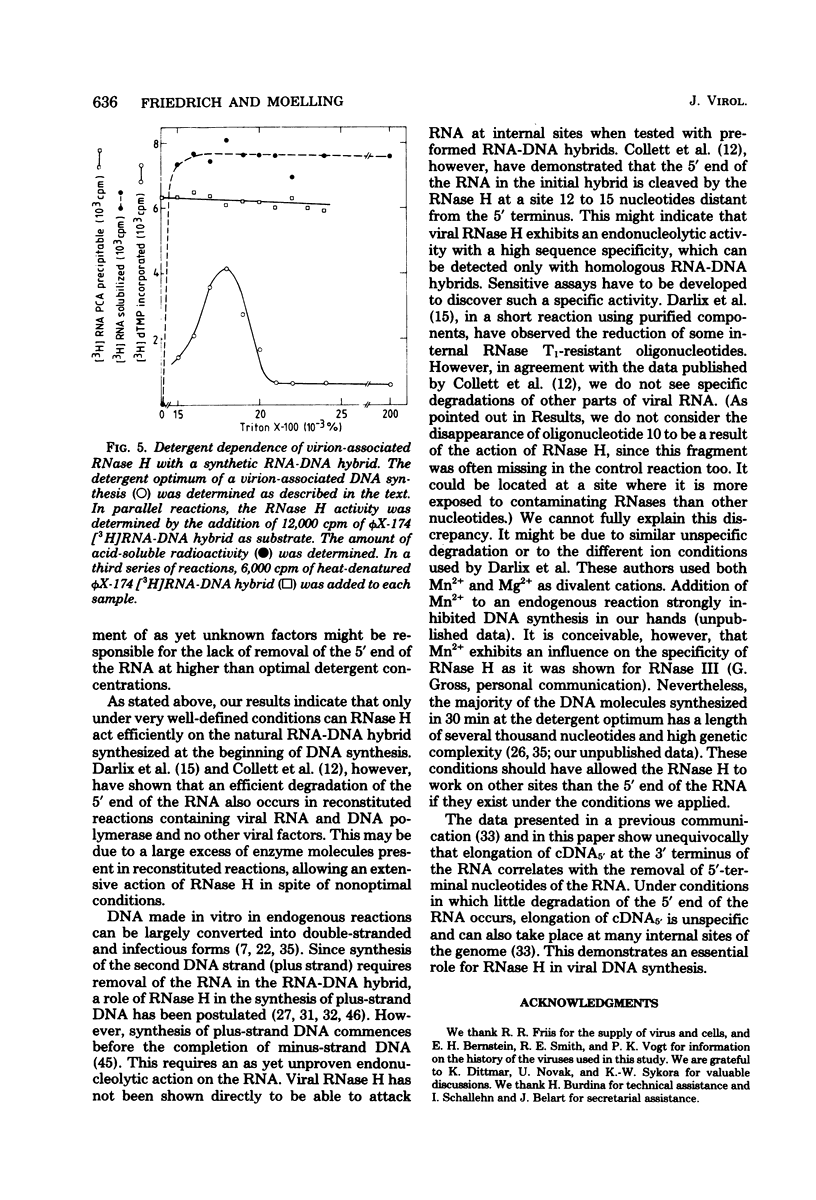
We investigated the influence of viral RNase H on the transcription of the avian sarcoma virus RNA in a virion-associated reaction. The ability of RNase H to degrade the RNA moiety of the initially formed RNA-DNA hybrid at the 5' end of the viral genome was found to be greatly dependent on the exact concentration of nonionic detergent used to activate the reaction. At a detergent concentration optimal for extensive and faithful in vitro transcription of avian sarcoma virus RNA by the virion-associated RNA-dependent DNA polymerase, most of the 5' terminus of the RNA was digested in 30 min at 41 degrees C. At higher than optimal detergent concentrations, however, little of that RNA was digested. We conclude that removal of the 5'-terminal redundancy in the RNA after its transcription into DNA is a prerequisite for base pairing of the DNA to the 3'-terminal redundant sequence. Lack of removal of this sequence leads to incorrect elongation and substantial reduction of DNA synthesis. When tested with a synthetic RNA-DNA hybrid, virion-associated RNase H did not reveal a detergent dependence.
Full text
PDF

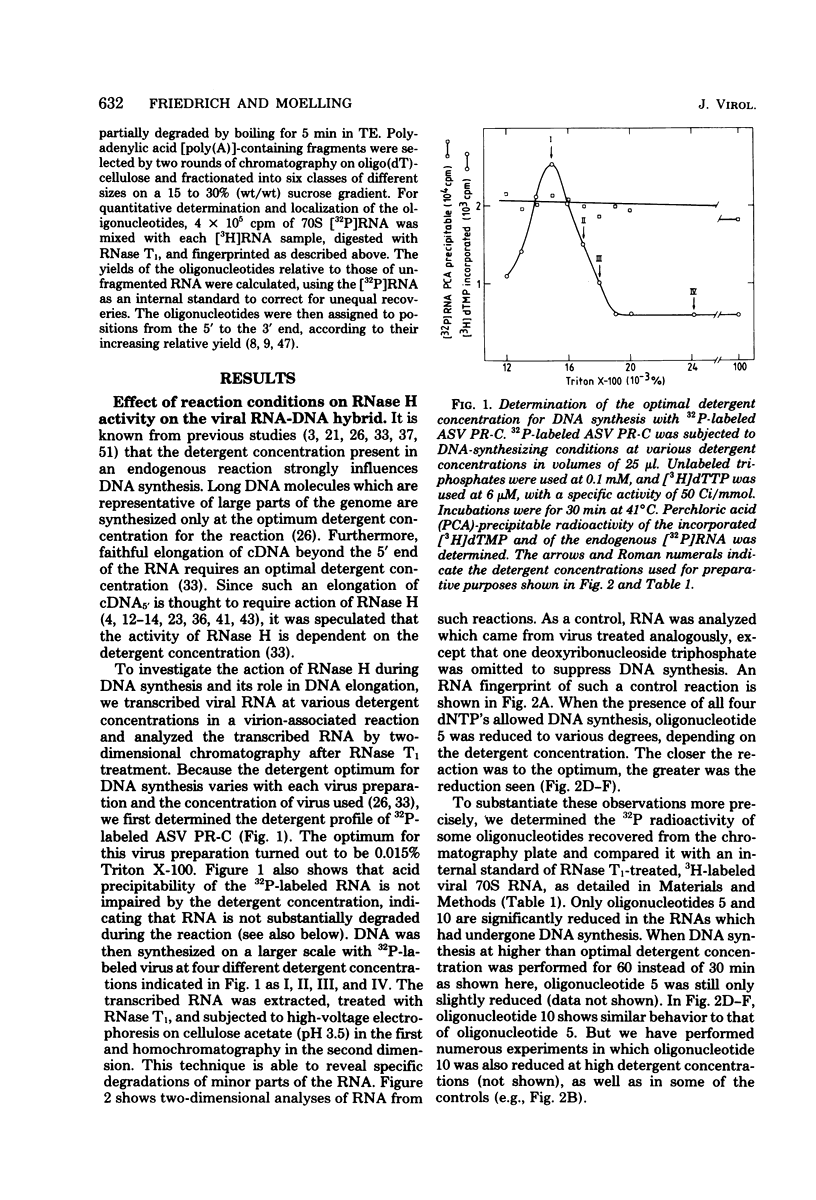
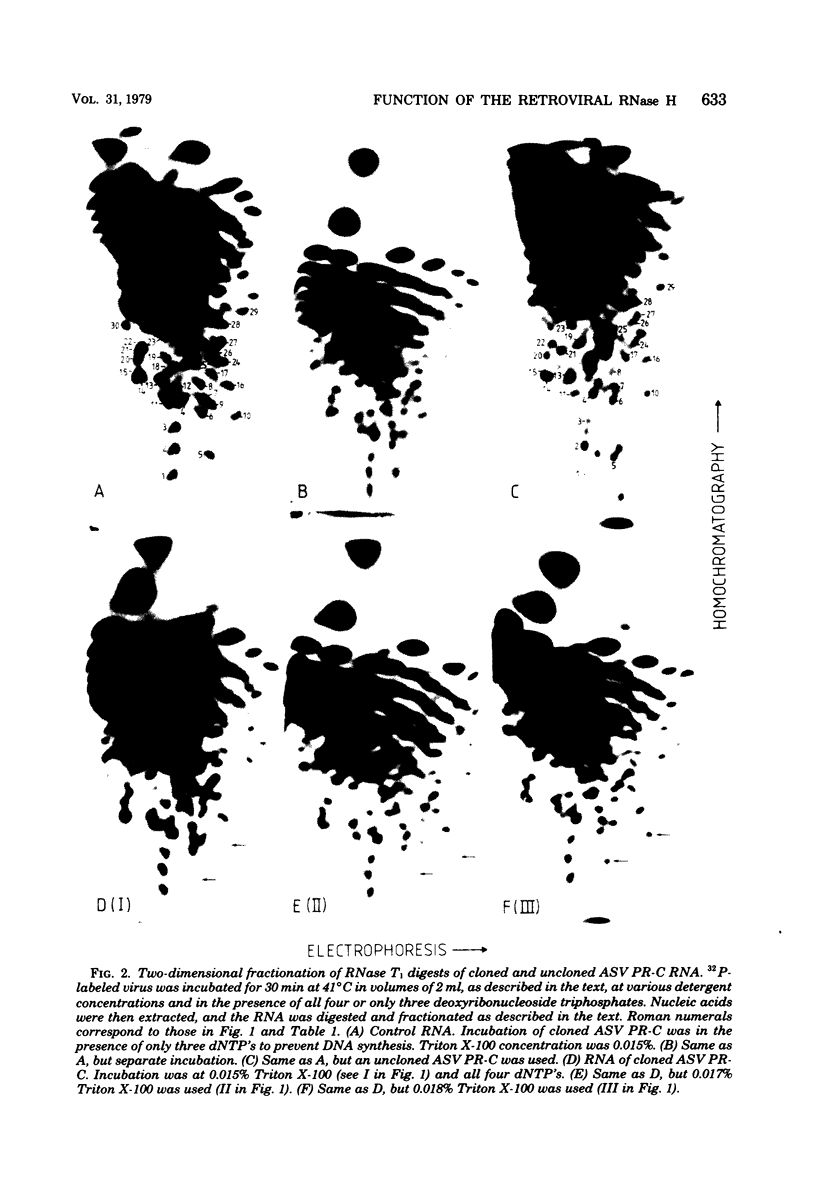
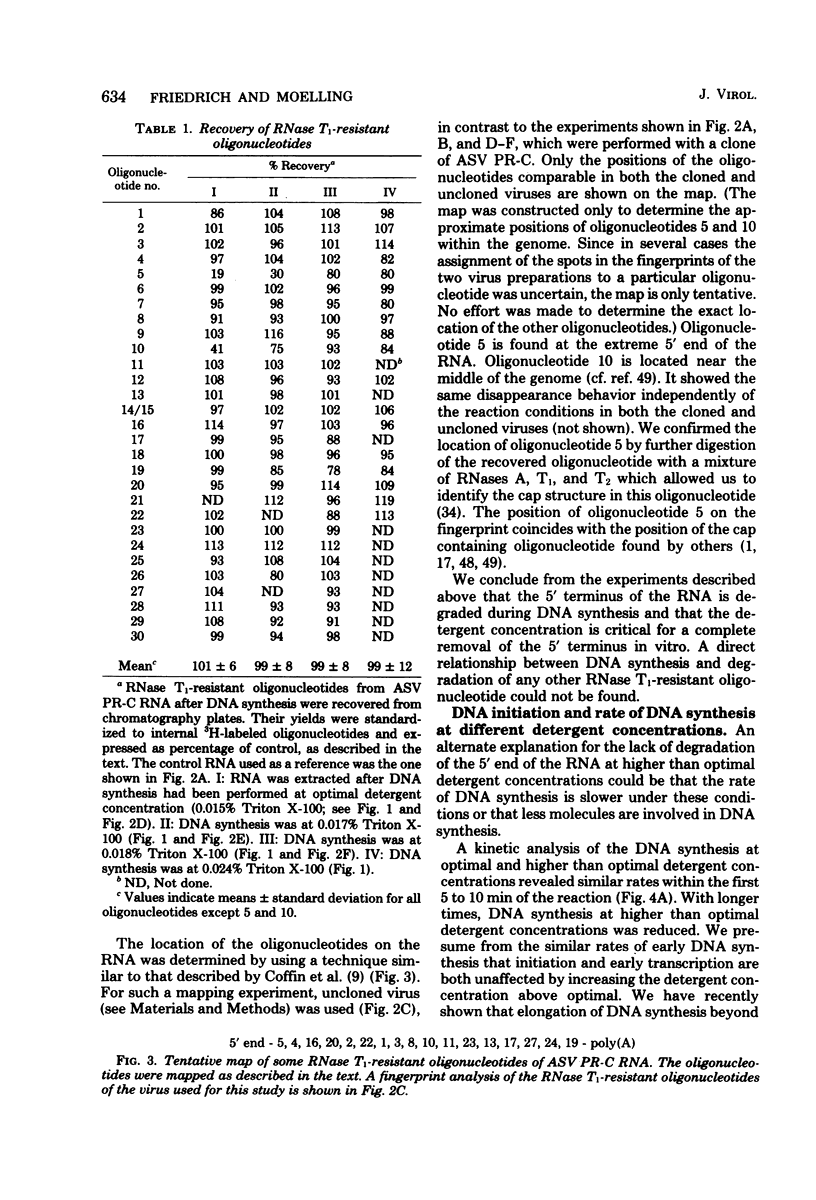


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beemon K., Duesberg P., Vogt P. Evidence for crossing-over between avian tumor viruses based on analysis of viral RNAs. Proc Natl Acad Sci U S A. 1974 Oct;71(10):4254–4258. doi: 10.1073/pnas.71.10.4254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop D. H., Ruprecht R., Simpson R. W., Spiegelman S. Deoxyribonucleic acid polymerase of Rous sarcoma virus: reaction conditions and analysis of the reaction product nucleic acids. J Virol. 1971 Nov;8(5):730–741. doi: 10.1128/jvi.8.5.730-741.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop J. M., Levinson W. E., Quintrell N., Sullivan D., Fanshier L., Jackson J. The low molecular weight RNAs of Rous sarcoma virus. I. The 4 S RNA. Virology. 1970 Sep;42(1):182–195. doi: 10.1016/0042-6822(70)90251-5. [DOI] [PubMed] [Google Scholar]
- Bishop J. M. Retroviruses. Annu Rev Biochem. 1978;47:35–88. doi: 10.1146/annurev.bi.47.070178.000343. [DOI] [PubMed] [Google Scholar]
- Brownlee G. G., Sanger F. Chromatography of 32P-labelled oligonucleotides on thin layers of DEAE-cellulose. Eur J Biochem. 1969 Dec;11(2):395–399. doi: 10.1111/j.1432-1033.1969.tb00786.x. [DOI] [PubMed] [Google Scholar]
- Clayman C. H., Mosharrafa E., Faras A. J. In vitro synthesis of infectious transforming DNA by the avian sarcoma virus reverse transcriptase. J Virol. 1979 Jan;29(1):242–249. doi: 10.1128/jvi.29.1.242-249.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffin J. M., Billeter M. A. A physical map of the Rous sarcoma virus genome. J Mol Biol. 1976 Jan 25;100(3):293–318. doi: 10.1016/s0022-2836(76)80065-4. [DOI] [PubMed] [Google Scholar]
- Coffin J. M., Hageman T. C., Maxam A. M., Haseltine W. A. Structure of the genome of Moloney murine leukemia virus: a terminally redundant sequence. Cell. 1978 Apr;13(4):761–773. doi: 10.1016/0092-8674(78)90226-x. [DOI] [PubMed] [Google Scholar]
- Coffin J. M., Haseltine W. A. Terminal redundancy and the origin of replication of Rous sarcoma virus RNA. Proc Natl Acad Sci U S A. 1977 May;74(5):1908–1912. doi: 10.1073/pnas.74.5.1908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collett M. S., Dierks P., Cahill J. F., Faras A. J., Parsons J. T. Terminally repeated sequences in the avian sarcoma virus RNA genome. Proc Natl Acad Sci U S A. 1977 Jun;74(6):2389–2393. doi: 10.1073/pnas.74.6.2389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collett M. S., Dierks P., Parsons J. T., Faras A. J. RNase H hydrolysis of the 5' terminus of the avian sarcoma virus genome during reverse transcription. Nature. 1978 Mar 9;272(5649):181–184. doi: 10.1038/272181a0. [DOI] [PubMed] [Google Scholar]
- Collett M. S., Faras A. J. Avian retrovirus RNA-directed DNA synthesis: transcription at the 5' terminus of the viral genome and the functional role for the viral terminal redundancy. Virology. 1978 May 15;86(2):297–311. doi: 10.1016/0042-6822(78)90072-7. [DOI] [PubMed] [Google Scholar]
- Collett M. S., Faras A. J. Evidence for circularization of the avian oncornavirus RNA genome during proviral DNA synthesis from studies of reverse transcription in vitro. Proc Natl Acad Sci U S A. 1976 Apr;73(4):1329–1332. doi: 10.1073/pnas.73.4.1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darlix J. L., Bromley P. A., Spahr P. F. Extensive in vitro transcription of rous sarcoma virus RNA by avian myeloblastosis virus DNA polymerase and concurrent activation of the associated RNase H. J Virol. 1977 Sep;23(3):659–668. doi: 10.1128/jvi.23.3.659-668.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duesberg P. H., Vogt P. K. RNA species obtained from clonal lines of avian sarcoma and from avian leukosis virus. Virology. 1973 Jul;54(1):207–219. doi: 10.1016/0042-6822(73)90130-x. [DOI] [PubMed] [Google Scholar]
- Ehrenberg L., Fedorcsak I., Solymosy F. Diethyl pyrocarbonate in nucleic acid research. Prog Nucleic Acid Res Mol Biol. 1976;16:189–262. doi: 10.1016/s0079-6603(08)60758-8. [DOI] [PubMed] [Google Scholar]
- Eiden J. J., Bolognesi D. P., Langlois A. J., Nichols J. L. The initial nucleotide sequence of DNA transcribed from avian myeloblastosis virus 70 S RNA by RNA-dependent DNA polymerase. Virology. 1975 May;65(1):163–172. doi: 10.1016/0042-6822(75)90016-1. [DOI] [PubMed] [Google Scholar]
- Friedrich R., Morris V. L., Goodman H. M., Bishop J. M., Varmus H. E. Differences between genomes of two strains of mouse mammary tumor virus as shown by partial RNA sequence analysis. Virology. 1976 Jul 15;72(2):330–340. doi: 10.1016/0042-6822(76)90162-8. [DOI] [PubMed] [Google Scholar]
- Garapin A. C., McDonnell J. P., Levinson W., Quintrell N., Fanshier L., Bishop J. M. Deoxyribonucleic acid polymerase associated with Rous sarcoma virus and avian myeloblastosis virus: properties of the enzyme and its product. J Virol. 1970 Nov;6(5):589–598. doi: 10.1128/jvi.6.5.589-598.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilboa E., Goff S., Shields A., Yoshimura F., Mitra S., Baltimore D. In vitro synthesis of a 9 kbp terminally redundant DNA carrying the infectivity of Moloney murine leukemia virus. Cell. 1979 Apr;16(4):863–874. doi: 10.1016/0092-8674(79)90101-6. [DOI] [PubMed] [Google Scholar]
- Haseltine W. A., Kleid D. G., Panet A., Rothenberg E., Baltimore D. Ordered transcription of RNA tumor virus genomes. J Mol Biol. 1976 Sep 5;106(1):109–131. doi: 10.1016/0022-2836(76)90303-x. [DOI] [PubMed] [Google Scholar]
- Haseltine W. A., Maxam A. M., Gilbert W. Rous sarcoma virus genome is terminally redundant: the 5' sequence. Proc Natl Acad Sci U S A. 1977 Mar;74(3):989–993. doi: 10.1073/pnas.74.3.989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jay E., Bambara R., Padmanabhan R., Wu R. DNA sequence analysis: a general, simple and rapid method for sequencing large oligodeoxyribonucleotide fragments by mapping. Nucleic Acids Res. 1974 Mar;1(3):331–353. doi: 10.1093/nar/1.3.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Junghans R. P., Duesberg P. H., Knight C. A. In vitro synthesis of full-length DNA transcripts of Rous sarcoma virus RNA by viral DNA polymerase. Proc Natl Acad Sci U S A. 1975 Dec;72(12):4895–4899. doi: 10.1073/pnas.72.12.4895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kacian D. L., Myers J. C. Anticomplementary nature of smaller DNA produced during synthesis of extensive DNA copies of poliovirus RNA. Proc Natl Acad Sci U S A. 1976 Oct;73(10):3408–3412. doi: 10.1073/pnas.73.10.3408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laskey R. A., Mills A. D. Enhanced autoradiographic detection of 32P and 125I using intensifying screens and hypersensitized film. FEBS Lett. 1977 Oct 15;82(2):314–316. doi: 10.1016/0014-5793(77)80609-1. [DOI] [PubMed] [Google Scholar]
- Leis J. P., Smith R. E., Dierks P., Parsons J. T., Collett M. S., Faras A. J. In vitro transcription of reconstituted 35s RNA.tRNAtrp template.primer complexes by the avian oncornavirus DNA polymerase. Effect of temperature on the size of the DNA transcripts. Virology. 1978 Mar;85(1):28–42. doi: 10.1016/0042-6822(78)90409-9. [DOI] [PubMed] [Google Scholar]
- Maniatis T., Jeffrey A., van deSande H. Chain length determination of small double- and single-stranded DNA molecules by polyacrylamide gel electrophoresis. Biochemistry. 1975 Aug 26;14(17):3787–3794. doi: 10.1021/bi00688a010. [DOI] [PubMed] [Google Scholar]
- Myers J. C., Spiegelman S. Sodium pyrophosphate inhibition of RNA.DNA hybrid degradation by reverse transcriptase. Proc Natl Acad Sci U S A. 1978 Nov;75(11):5329–5333. doi: 10.1073/pnas.75.11.5329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novak U., Friedrich R., Moelling K. Elongation of DNA complementary to the 5' end of the avian sarcoma virus genome by the virion-associated RNA-dependent DNA polymerase. J Virol. 1979 May;30(2):438–452. doi: 10.1128/jvi.30.2.438-452.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose J. K. Heterogneeous 5'-terminal structures occur on vesicular stomatitis virus mRNAs. J Biol Chem. 1975 Oct 25;250(20):8098–8104. [PubMed] [Google Scholar]
- Rothenberg E., Smotkin D., Baltimore D., Weinberg R. A. In vitro synthesis of infectious DNA of murine leukaemia virus. Nature. 1977 Sep 8;269(5624):122–126. doi: 10.1038/269122a0. [DOI] [PubMed] [Google Scholar]
- Schwartz D. E., Zamecnik P. C., Weith H. L. Rous sarcoma virus genome is terminally redundant: the 3' sequence. Proc Natl Acad Sci U S A. 1977 Mar;74(3):994–998. doi: 10.1073/pnas.74.3.994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scolnick E., Rands E., Aaronson S. A., Todaro G. J. RNA-dependent DNA polymerase activity in five RNA viruses: divalent cation requirements. Proc Natl Acad Sci U S A. 1970 Dec;67(4):1789–1796. doi: 10.1073/pnas.67.4.1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sedmak J. J., Grossberg S. E. A rapid, sensitive, and versatile assay for protein using Coomassie brilliant blue G250. Anal Biochem. 1977 May 1;79(1-2):544–552. doi: 10.1016/0003-2697(77)90428-6. [DOI] [PubMed] [Google Scholar]
- Smith R. E., Bernstein E. H. Production and purification of large amounts of Rous sarcoma virus. Appl Microbiol. 1973 Mar;25(3):346–353. doi: 10.1128/am.25.3.346-353.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoll E., Billeter M. A., Palmenberg A., Weissmann C. Avian myeloblastosis virus RNA is terminally redundant: implications for the mechanism of retrovirus replication. Cell. 1977 Sep;12(1):57–72. doi: 10.1016/0092-8674(77)90185-4. [DOI] [PubMed] [Google Scholar]
- Swanstrom R., Shank P. R. X-Ray Intensifying Screens Greatly Enhance the Detection by Autoradiography of the Radioactive Isotopes 32P and 125I. Anal Biochem. 1978 May;86(1):184–192. doi: 10.1016/0003-2697(78)90333-0. [DOI] [PubMed] [Google Scholar]
- Taylor J. M. An analysis of the role of tRNA species as primers for the transcription into DNA of RNA tumor virus genomes. Biochim Biophys Acta. 1977 Mar 21;473(1):57–71. doi: 10.1016/0304-419x(77)90007-5. [DOI] [PubMed] [Google Scholar]
- Taylor J. M., Garfin D. E., Levinson W. E., Bishop J. M., Goodman H. M. Tumor virus ribonucleic acid directed deoxyribonucleic acid synthesis: nucleotide sequence at the 5' terminus of nascent deoxyribonucleic acid. Biochemistry. 1974 Jul 16;13(15):3159–3163. doi: 10.1021/bi00712a024. [DOI] [PubMed] [Google Scholar]
- Varmus H. E., Heasley S., Kung H. J., Oppermann H., Smith V. C., Bishop J. M., Shank P. R. Kinetics of synthesis, structure and purification of avian sarcoma virus-specific DNA made in the cytoplasm of acutely infected cells. J Mol Biol. 1978 Mar 25;120(1):55–82. doi: 10.1016/0022-2836(78)90295-4. [DOI] [PubMed] [Google Scholar]
- Verma I. M. The reverse transcriptase. Biochim Biophys Acta. 1977 Mar 21;473(1):1–38. doi: 10.1016/0304-419x(77)90005-1. [DOI] [PubMed] [Google Scholar]
- Wang L. H., Duesberg P. H., Robins T., Yokota H., Vogt P. K. The terminal oligonucleotides of avian tumor virus RNAs are genetically linked. Virology. 1977 Oct 15;82(2):472–492. doi: 10.1016/0042-6822(77)90020-4. [DOI] [PubMed] [Google Scholar]
- Wang L. H., Duesberg P., Beemon K., Vogt P. K. Mapping RNase T1-resistant oligonucleotides of avian tumor virus RNAs: sarcoma-specific oligonucleotides are near the poly(A) end and oligonucleotides common to sarcoma and transformation-defective viruses are at the poly(A) end. J Virol. 1975 Oct;16(4):1051–1070. doi: 10.1128/jvi.16.4.1051-1070.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L., Galehouse D., Mellon P., Duesberg P., Mason W. S., Vogt P. K. Mapping oligonucleotides of Rous sarcoma virus RNA that segregate with polymerase and group-specific antigen markers in recombinants. Proc Natl Acad Sci U S A. 1976 Nov;73(11):3952–3956. doi: 10.1073/pnas.73.11.3952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson K. F., Mölling K., Bauer H. Ribonuclease H activity present in purified DNA polymerase from avian myeloblastosis virus. Biochem Biophys Res Commun. 1973 Mar 5;51(1):232–240. doi: 10.1016/0006-291x(73)90533-0. [DOI] [PubMed] [Google Scholar]
- Wu A. M., Cetta A. On the stimulation of viral DNA polymerase activity by nonionic detergent. Biochemistry. 1975 Feb 25;14(4):789–795. doi: 10.1021/bi00675a022. [DOI] [PubMed] [Google Scholar]